Abstract
Background:
Brugada syndrome is an inherited disease associated with vulnerability to ventricular tachycardia and sudden cardiac death in young adults. Milrinone and cilostazol, oral phosphodiesterase (PDE) type III inhibitors, have been shown to increase ICa and modestly increase heart rate by elevating the level of intracellular cyclic AMP.
Objective:
The present study examines the effectiveness of these PDE inhibitors to suppress arrhythmogenesis in an experimental model of Brugada syndrome.
Methods:
Action potential (AP) and ECG recordings were obtained from epicardial and endocardial sites of coronary-perfused canine right ventricular wedge preparations. The Ito agonist NS5806 (5 μM) and Ca2+ channel blocker verapamil (2 μM) were used to pharmacologically mimic Brugada phenotype.
Results:
The combination induced all-or-none repolarization at some epicardial sites but not others, leading to ST-segment elevation as well as an increase in both epicardial and transmural dispersion of repolarization. Under these conditions, phase 2 reentry developed as the epicardial AP dome propagated from sites where it was maintained to sites at which it was lost, generating closely coupled extrasystoles and ventricular tachycardia. Addition of the PDE inhibitor milrinone (2.5 μM) or cilostazol (5-10 μM) to the coronary perfusate restored the epicardial AP dome, reduced dispersion and abolished phase 2 reentry—induced extrasystoles and ventricular tachycardia.
Conclusions:
Our study identifies milrinone as a more potent alternative to cilostazol for reversing the repolarization defects responsible for the electrocardiographic and arrhythmic manifestations of Brugada syndrome. Both drugs normalize ST segment elevation, and suppress arrhythmogenesis in experimental models of Brugada syndrome.
Keywords: Phosphodiesterase inhibitor, cardiac arrhythmias, sudden cardiac death, electrophysiology, pharmacology
Introduction
Brugada syndrome (BrS) is an inherited cardiac disease associated with vulnerability to ventricular tachycardia (VT) and sudden cardiac death in young adults with a structurally normal heart. The ECG pattern of BrS is characterized by a J point and ST segment elevation in the right precordial leads.1 The right ventricular (RV) manifestations of the disease are thought to be due to the prominence of Ito in RV vs. left ventricle (LV) epicardium2 Recent studies have suggested that a slowly conducting embryonic phenotype is maintained in the RVOT of the mouse heart,3 thus providing evidence in support of a conduction defect in RV as the basis for an alternative mechanism for BrS.4 The ECG pattern is often concealed, but can be unmasked or modulated by fever, vagal stimulation, and a number of pharmacological agents.5 On a molecular level, BrS has been linked to mutations causing decreased inward currents (peak sodium channel current, L-type calcium channel current) or increased outward currents (especially transient outward potassium current,6) during phase 1 of the epicardial (Epi) action potential (AP), thus accentuating the spike-and-dome morphology of the AP, most prominently in the epicardium of the right ventricular outflow tract (RVOT).5 The net outward shift of current balance can lead to loss of the dome or phase 2 of the action potential creating a dispersion of repolarization both within epicardium and between epicardium and endocardium, thus creating the substrate for phase 2 reentry and polymorphic VT.7
Milrinone and cilostazol, oral phosphodiesterase (PDE) type III inhibitors, have been shown to increase ICa and modestly increase heart rate by elevating the level of intracellular cyclic AMP.8-11 Previous reports have demonstrated the effectiveness of cilostazol in patients with BrS, nevertheless cilostazol has a class IIb recommendation on the brugadadrugs.org website.12-15 Abud et al. reported failure of this drug to prevent ventricular fibrillation in a patient with BrS.16 Cilostazol is known to inhibit platelet aggregation and to act as an arterial vasodilator, causing dilation of the arteries supplying blood to the legs and decreasing platelet coagulation.17, 18 Cilostazol is approved for the treatment of intermittent claudication and is often used off-label for treatment of intracranial atherosclerosis and secondary stroke prevention19 in addition to its use in the management of BrS.
Milrinone is also a PDE-3 inhibitor that is used principally in the management of patients with heart failure. Through its action to increase cAMP, milrinone increases contractility in a failing heart. It also works as a vasodilator, thus helping to alleviate increased pressure (afterload) in the heart. Milrinone has not been reported to be of benefit in BrS, although it was suggested as a drug of potential benefit by Marquez and co-workers.20 Milrinone is listed as a Class 3 recommendation on BrugadaDrugs.org due to the paucity of information available.
The present study tests the hypothesis that milrinone may be a useful alternative to cilostazol for the management of BrS. The principal focus of the study is to elucidate the cellular mechanisms responsible and test the hypothesis that both milrinone and cilostazol exert their ameliorative effects in BrS by reversing the repolarization defects associated with the development of the ECG and arrhythmic manifestations of BrS.
Methods
Wedge Preparations
All experiments were carried out in compliance with the Guide for Care and Use of Laboratory Animals published by the National Institutes of Health (NIH publication No 85-23, Revised 1996) and approved by the Institutional Animal Care and Use Committee. Detailed methods for isolation and recording of transmembrane activity from coronary-perfused canine RV wedge preparations have been reported previously,21, 22 and are briefly described in the Online Supplement.
Transmembrane APs were simultaneously recorded from two Epi (Epi 1 [distal] and Epi 2 [proximal]; Epi1-Epi2 distance was approx. 5-10 mm) and one Endo site with the use of floating microelectrodes. Impalements were obtained from the Epi and Endo surfaces of the preparation at positions approximating the transmural axis of the ECG recording.
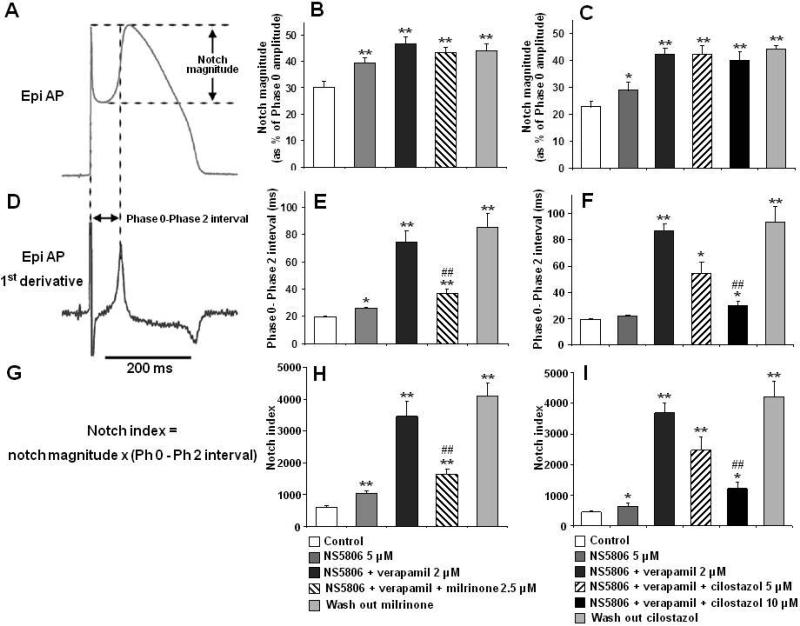
The Epi AP notch magnitude (NM); [phase 1 magnitude / phase 0 amplitude x 100], phase 0 to phase 2 interval; [time between the first 2 peaks of the derivative of the AP] as well as the notch index (NI); [NM × (Ph 0 to Ph 2 interval)] which approximates the area of the notch were measured as previously described.21
Statistical analysis
Results are presented as mean + S.E.M. (standard error of the mean) throughout the publication. Statistical comparisons were made using Student's t-test for paired and unpaired data.
Results
Using coronary-perfused canine right ventricular wedge preparations, we induced the Brugada phenotype by addition of 5 μM NS5806 (Ito activator) and 2 μM verapamil (Ca2+ channel blocker) to the coronary perfusate. NS5806 has previously been shown to increase Ito in isolated canine cardiomyocytes, resulting in augmentation of the notched appearance of the RV action potential, most notably in the epicardium.23 NS5806 (5 μM ) and verapamil (2 μM) accentuated the AP notch in right ventricular epicardium, leading to the development of a prominent J point and ST segment elevation, characteristic of the Brugada phenotype (Figures 1 and 2). Longer exposure caused all-or-none repolarization at the end of the Epi AP phase 1, leading to loss of the AP dome at some Epi sites. The voltage gradient between the abbreviated epicardium and the normal endocardium produces the ST segment elevation, and also provides a vulnerable window for reentrant arrhythmias. Propagation of the dome from regions at which it was maintained to regions at which it was lost, caused local re-excitation via a phase 2 re-entry mechanism, leading to the development of closely coupled extrasystoles and polymorphic VT/ventricular fibrillation (VT/VF) can result. Milrinone (2.5 μM) and cilostazol (5-10 μM) restored the Epi AP dome, normalized the ECG and terminated all arrhythmic activity. Figures 1 and 2 show representative recordings of APs from RV wedge preparations recorded under control conditions, after NS5806 (5 μM), +verapamil (2 μM), +PDE inhibitor (2.5 μM milrinone or 5-10 μM cilostazol) and after washout of the PDE inhibitors.
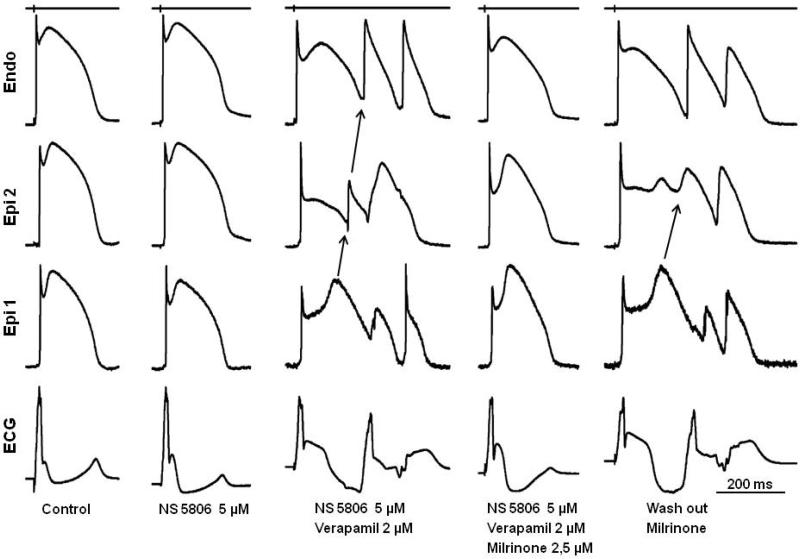
Figure 1.
Effect of milrinone to reverse the repolarization defects responsible for the electrocardiographic and arrhythmic manifestations of Brugada syndrome in a coronary-perfused right ventricular wedge model pharmacologically mimicking loss of function of ICa in the setting of a prominent Ito. Recordings obtained at a basic cycle length of 1000 ms. Each grouping represents the transmembrane action potentials (APs) recorded from 2 epicardial (Epi) sites and 1 endocardial (Endo) site together with an ECG, all simultaneously recorded. The Ito agonist NS5806 increases notch and J wave parameters but does not induce arrhythmic activity. The addition of verapamil leads to marked accentuation of the Epi AP notch, giving rise to a large J wave, appearing as an ST segment elevation. Loss of the dome at Epi 2 results in development of phase 2 reentry. Milrinone reverses these repolarization defects, restoring AP duration homogeneity, normalizing the ECG and abolishing all arrhythmic activity. The Brugada phenotype promptly reappears after washout of milrinone. Time calibrations for the recordings are indicated on the right of the figure. The top trace is a stimulus marker.
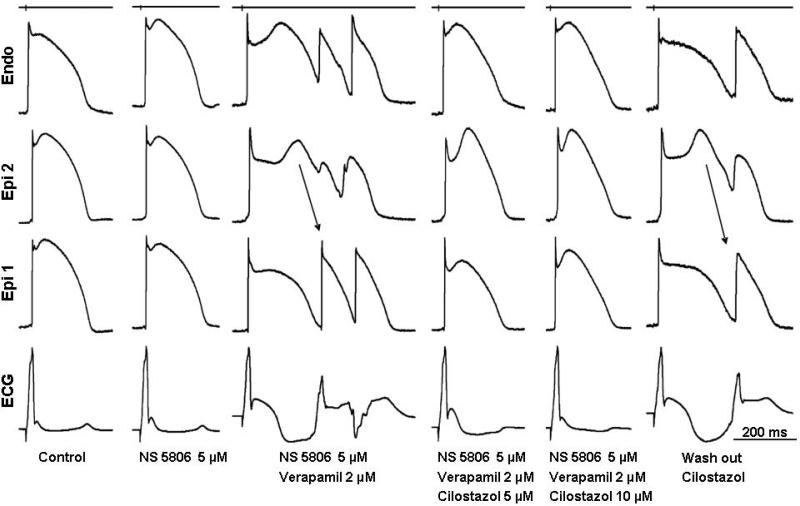
Figure 2.
Effect of cilostazol to reverse the repolarization defects underlying the electrocardiographic and arrhythmic manifestations of Brugada syndrome in a coronary-perfused right ventricular wedge model. Recordings were obtained at a basic cycle length of 1000 ms. Each shows transmembrane action potentials (APs) recorded from 2 epicardial (Epi) sites and 1 (Endo) endocardial site together with an ECG, all simultaneously recorded. The Ito agonist NS5806 increases notch and J wave parameters but does not induce arrhythmic activity. The addition of verapamil leads to marked accentuation of the Epi AP notch, giving rise to a large J wave, appearing as an ST segment elevation. Loss of the dome at Epi 2 results in development of phase 2 reentry. Cilostazol (5 μM) reverses these repolarization defects, restoring AP duration homogeneity, normalizing the ECG and abolishing all arrhythmic activity. Cilostazol (10 μM) further reduced notch and J wave parameters. The Brugada phenotype promptly reappears after washout of cilostazol. Time calibrations for the recordings are indicated on the right of the figure. The top trace is a stimulus marker.
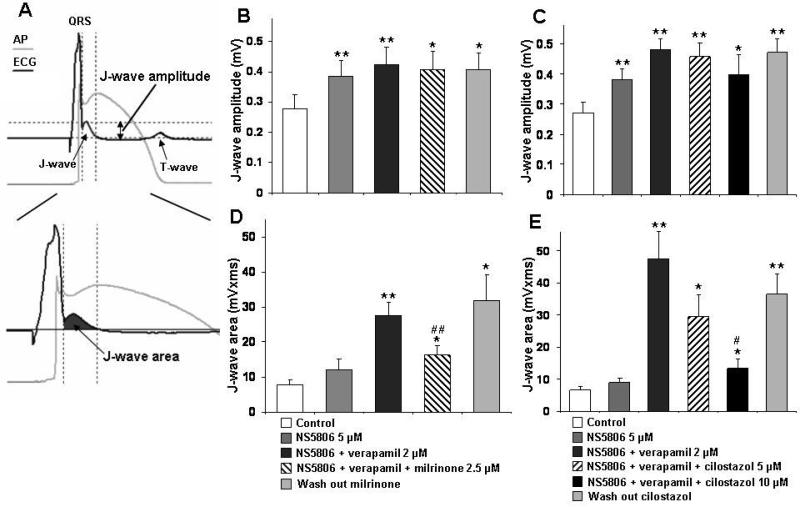
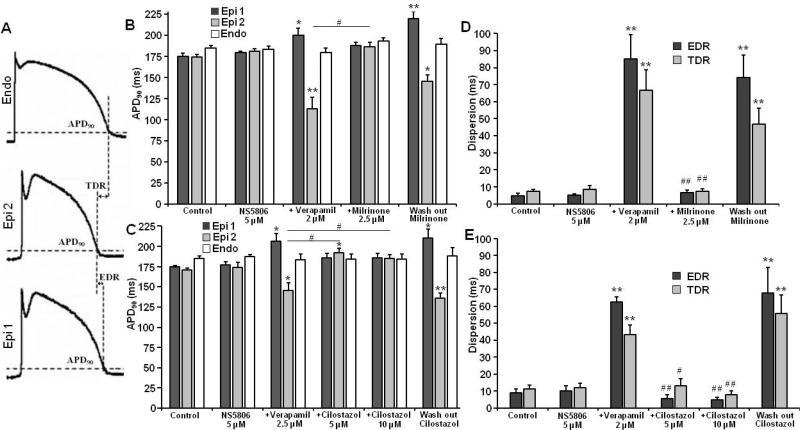
The effects of milrinone and cilostazol on notch magnitude, Phase 0 to Phase 2 interval (time between the first 2 peaks of the first derivative of the Epi AP) and notch index on RV wedges are summarized in Figure 3 and Table 1. The NS5806-induced increase in Ito caused an outward shift in the balance of current in the early phase of the Epi AP leading to a moderate increase in notch magnitude and notch index (approximation of notch area) in epicardium, but little change in endocardium. The greater accentuation of the AP notch in epicardium than endocardium gives rise to an increase in transmural voltage gradient causing an increase in the amplitude and area of the J wave (Figure 4 and Table 1). The addition of verapamil to the coronary perfusate caused a further outward shift in the balance of current leading to further accentuation of all of these parameters. Under these conditions, loss of the dome at some epicardial sites but not others caused a prominent increase in epicardial and transmural dispersion of repolarization, thus creating the substrate for the development of phase 2 reentry (Figure 5 and Table 2). Milrinone (2.5 μM) and cilostazol (10 μM) restored the AP dome, thus reducing notch magnitude and index, J wave amplitude and area, as well as epicardial and transmural dispersion of repolarization (Figure 3-5 and Table 1,2).
Figure 3.
Effect of milrinone and cilostazol on epicardial (Epi) notch parameters: notch magnitude (as % of Phase 0 amplitude), phase 0 to phase 2 (Ph 0–Ph 2) interval (time between the first 2 peaks of the derivative of the action potential (AP)) and notch index (notch magnitude x Ph 0 to Ph 2 interval). Notch parameters are measured form right ventricular APs in which the dome was maintained. Results are mean ± S.E.M. * p < 0.05 and ** p < 0.01 vs. control.## p < 0.01 vs. NS5806+verapamil combination (Brugada-model). n=7 for B, E and H, n=6 for C, F and I.
Table 1.
Effect of milrinone and cilostazol on right ventricular epicardial action potential notch and J wave parameters.
| Notch magnitude (% ofph 0 amp) |
Notch duration (ms) |
Notch index (NM*ND) |
J wave amplitude (mV) |
J wave area (mV*ms) |
|
|---|---|---|---|---|---|
| Control | 29.9 ± 2.5 | 19.8 ± 0.5 | 594.4 ± 51.7 | 0.279 ± 0.045 | 7.94 ± 1.22 |
|
NS5806
5 μM |
39.4 ± 2.1† | 26.0 ± 1.0* | 1021.7 ± 94.9† | 0.384 ± 0.050† | 11.96 ± 3.30 |
|
+Verapamil
2 μM |
46.6 ± 2.6† | 74.3 ± 8.5† | 3466.1 ± 459.8† | 0.424 ± 0.058† | 27.57 ± 3.64† |
|
+Milrinone
2.5 μM |
43.4 ± 1.9† | 37.2 ± 2.6†,‡ | 1624.1 ± 161.1†,‡ | 0.407 ± 0.059* | 16.28 ± 2.62*,‡ |
|
Wash out
Milrinone |
44.0 ± 2.† | 85.6 ± 9.9† | 4116.3 ± 402.1† | 0.405 ± 0.057* | 31.80 ± 7.30* |
|
| |||||
|
| |||||
| Notch magnitude (% ofph 0 amp) |
Notch duration (ms) |
Notch index (NM*ND) |
J wave amplitude (mV) |
J wave area (mV*ms) |
|
|
| |||||
| Control | 22.7 ± 1.9 | 19.2 ± 0.7 | 450.8 ± 40.6 | 0.272 ± 0.035 | 6.55 ± 1.02 |
|
NS5806
5 μM |
29.1 ± 2.9* | 21.9 ± 0.6 | 654.3 ± 85.3* | 0.382 ± 0.036† | 8.80 ± 1.40 |
|
+Verapamil
2 μM |
42.3 ± 2.4† | 87.2 ± 4.8† | 3689.3 ± 306.3† | 0.482 ± 0.034† | 47.45 ± 8.42† |
|
+Cilostazol
5 μM |
42.3 ± 3.3† | 54.3 ± 8.7* | 2470.8 ± 428.1† | 0.458 ± 0.045† | 29.38 ± 6.86* |
|
+Cilostazol
10 μM |
40.2 ± 2.8† | 29.5 ± 3.3*,‡ | 1226.3 ± 215.0*,‡ | 0.398 ± 0.067* | 13.34 ± 2.86*,† |
|
Wash out
Cilostazol |
44.2 ± 1.2† | 93.3 ± 11.9† | 4206.8 ± 499.7† | 0.472 ± 0.045† | 36.39 ±6.50† |
NM: notch magnitude (as % of Phase 0 amplitude); ND: notch duration (Phase 0 to Phase 2 interval); NI: notch index (NM × Ph 0 to Ph 2 interval). Notch parameters are measured form right ventricular action potentials in which the dome was maintained. Results are mean ± S.E.M.
p< 0.05 and
p< 0.01 vs. control.
p< 0.05 and
p< 0.01 vs. NS5806+verapamil combination (Brugada-model). n=7 for milrinone, n=6 for cilostazol. Basic cycle length=1000 ms.
Figure 4.
Effect of milrinone and cilostazol on J-wave amplitude and J wave area on canine arterially perfused right ventricular wedge. Results are mean ± S.E.M. * p < 0.05 and ** p < 0.01 vs. control. # p < 0.05 and ## p < 0.01 vs. NS5806+verapamil combination (Brugada-model). n=7 for B and D, n=6 for C and E. AP — action potential.
Figure 5.
Effect of milrinone and cilostazol on action potential duration at 90% repolarization (APD90), epicardial (EDR) and transmural (TDR) dispersion of repolarization in the canine arterially-perfused right ventricular wedge. The combination of NS5806 (5 μM) and verapamil (2 μM) induces heterogeneous loss of the epicardial (Epi) action potential (AP) dome, producing both EDR and TDR. Addition of milrinone (2.5 μM) or cilostazol (5-10 μM) reduces dispersion. APD90, EDR and TDR are measured in case of single stimulated beat with loss of the AP dome at EPI 2 site but not EPI 1. Results are mean ± S.E.M. * p < 0.05 and ** p < 0.01 vs. control. # p < 0.05 and ## p < 0.01 vs. NS5806+verapamil combination (Brugada-model). n=7 for B and D, n=6 for C and E. Endo — endocardial.
Table 2.
Effect of milrinone and cilostazol on action potential duration (APD90), maximal EDR, TDR and incidence of Phase 2 reentrant and polymorphic VT arrhythmias.
| Epi 1 APD90
(ms) |
Epi 2 API) 90
(ms) |
Endo APD90
(ms) |
EDR (ms) |
TDR (ms) |
Phase 2 reentry |
Polymorphic VT |
|
|---|---|---|---|---|---|---|---|
| Control | 174.7 ± 3.6 | 173.9 ± 3.3 | 184.7 ± 3.1 | 4.8 ± 1.4 | 7.3 ± 1.1 | 0/7 | 0/7 |
|
NS5806
5 μM |
179.2 ± 1.9 | 180.6 ± 3.4 | 183.1 ± 3.5 | 5.3 ± 0.4 | 8.7 ± 2.0 | 1/7 | 1/7 |
|
+Verapamil
2 μM |
201.1 ± 8.4* | 112.8 ± 13.5† | 179.7 ± 4.9 | 84.9 ± 14.4† | 66.9±11.8† | 7/7 | 7/7 |
|
+Milrinone
2.5 μM |
187.8 ± 3.5 | 186.5 ± 4.8‡ | 193.0 ± 4.0 | 6.5 ± 1.7§ | 7.5 ±1.3§ | 1/7 | 0/7 |
|
Wash out
Milrinone |
219.2 ± 8.5† | 145.1 ± 7.6* | 189.2 ± 6.9 | 74.2 ± 13.3† | 46.7 ± 9.5† | 7/7 | 6/7 |
|
| |||||||
|
| |||||||
| Epi 1 APD90
(ms) |
Epi 2 APD90
(ms) |
Endo APD90
(ms) |
EDR (ms) |
TDR (ms) |
Phase 2 reentry |
Polymorphic VT |
|
|
| |||||||
| Control | 174.7 ± 2.0 | 170.5 ± 3.1 | 184.8 ± 3.7 | 9.1 ± 2.3 | 11.0 ± 2.3 | 0/6 | 0/6 |
|
NS5806
5 μM |
177.3 ± 3.6 | 174.1 ± 5.9 | 187.1 ± 2.4 | 9.9 ± 3.0 | 12.1 ± 2.7 | 0/6 | 0/6 |
|
+Verapamil
2 μM |
206.7 ± 9.4* | 145.8 ± 9.2* | 183.3 ± 7.3 | 62.5 ± 3.1† | 43.3 ± 5.7† | 6/6 | 4/6 |
|
+Cilostazol
5 μM |
186.0 ± 5.3 | 191.9 ± 5.3*,‡ | 183.8 ± 6.4 | 5.6 ± 2.3§ | 12.9 ± 4.1‡ | 0/6 | 0/6 |
|
+Cilostazol
10 μM |
185.9 ± 5.3 | 185.1 ± 5.1‡ | 183.9 ± 6.4 | 4.8 ± 1.6§ | 7.8 ± 2.1§ | 0/6 | 0/6 |
|
Wash out
Cilostazol |
210.2 ± 11.2* | 135.8 ± 6.4† | 188.3 ± 9.9 | 67.8 ± 15.1† | 55.5 ± 11.2† | 6/6 | 4/6 |
APD90: action potential durations at 90% of repolarization; TDR: transmural dispersion of repolarization; EDR: epicardial dispersion of repolarization. APD90, EDR and TDR are measured in case of single stimulated beat with loss of the AP dome at EPI 2 site but not EPI 1. Results are mean ± S.E.M.
p < 0.05 and
p < 0.01 vs. control.
p < 0.05 and
p < 0.01 vs. NS5806+verapamil combination (Brugada-model). n=7 for milrinone, n=6for cilostazol. Basic cycle length=1000 ms.
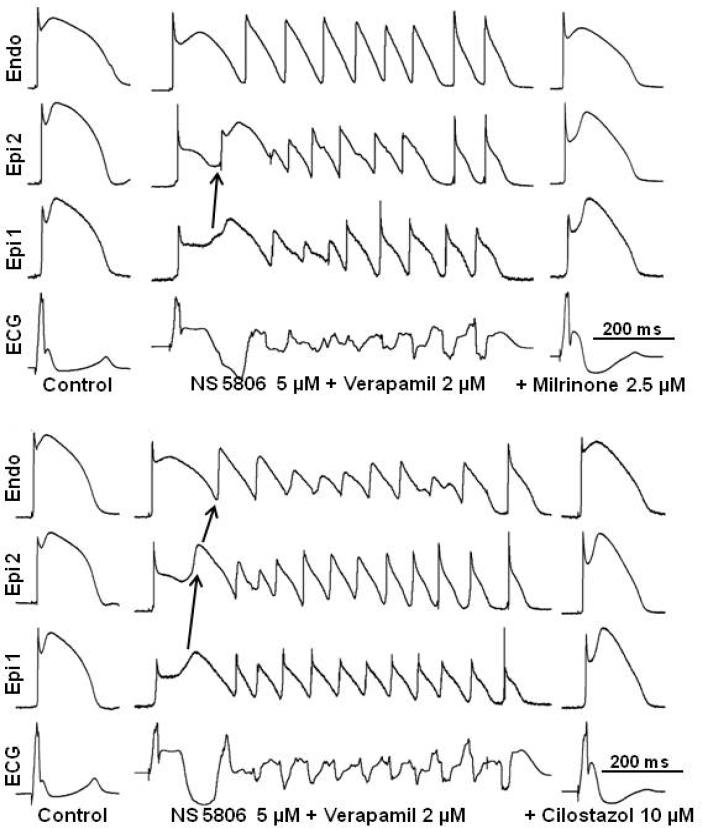
Figure 6 illustrates the development of polymorphic VT following exposure of the RV wedge to NS5806 and verapamil and the effect of milrinone and cilostazol to normalize the ECG and abort all arrhythmic activity. The combination of NS5806 and verapamil generated a closely coupled phase 2 reentrant extrasystole that captured the vulnerable window created by the transmural dispersion of repolarization, leading to the development of polymorphic VT. Milrinone (2.5 μM) and cilostazol (10 μM) promptly restored the action potential dome, reduced the ST segment elevation (J wave), and abolished phase 2 reentry as well as VT/VF (Figure 6). Milrinone (2.5 μM) abolished VT/VF in 7 out of 7 preparations, whereas cilostazol (5-10 μM) abolished VT/VF in 6 of 6 preparations (Table 2). Milrinone also was more potent than cilostazol in reducing action potential notch parameters (compare data for milrinone (2.5 μM) and cilostazol (5 μVI)-Table 1). Thus, a smaller concentration of milrinone than of cilostazol was needed to reverse the repolarization defect and exert an ameliorative effect.
Figure 6.
Effect of milrinone and cilostazol to suppress polymorphic ventricular tachycardia (VT) in a right ventricle wedge model of Brugada syndrome. The combination of NS5806 (5 μM) and verapamil (2 μM) induces heterogeneous loss of the epicardial (Epi) action potential dome. Phase 2 reentry occurs as the dome propagates from Epi 1 to Epi 2, triggering an episode of polymorphic VT. Addition of 2.5 μM milrinone (upper panel) or 10 μM cilostazol (lower panel) abolishes phase 2 reentry and polymorphic VT. Endo — endocardial.
Washout of the PDE inhibitors led to reappearance of arrhythmic activity in all preparations.
Discussion
It is well established that mutations leading to a decrease in inward currents (INa, or ICaL) or increase in outward currents (Ito and IK-ATP) are capable of causing BrS in humans.6,24-27 In the present study, we used the Ito agonist NS5806 (5 μM) and the ICa antagonist verapamil (2 μM) to pharmacologically model the BrS genotypes responsible for a loss of function of ICa (BrS 3,4 and 9)28 and a gain of function of Ito (BrS 5, 6 and 10)6,29-31 so as to induce the Brugada phenotype in the coronary-perfused canine RV wedge preparation.
The combination of NS5806 (5 μM) and verapamil (2 μM) caused all or no repolarization, leading to loss of the AP dome at some epicardial sites. The spike-and-dome AP morphology was maintained at other epicardial sites, resulting in an epicardial dispersion of repolarization (EDR). Conduction of the AP dome from sites at which it was maintained to sites at which it was lost caused local re-excitation via a phase 2 re-entry mechanism, leading to the development of closely coupled extrasystoles (Figure 1, 2 and 6). The loss of the dome in the epicardium also created a transmural dispersion of repolarization (TDR). The combination of EDR and TDR created a vulnerable window within the preparation (Figure 5 and Table 2), which when captured by a closely coupled extrasystole induced short VT. Phase 2 of the action potential could be restored by boosting the inward calcium current.
At therapeutically relevant concentrations,32,33 milrinone (2.5 μM) and cilostazol (5-10 μM) were found to be very effective in normalizing ST segment elevation and preventing the occurrence of VT/VF (Figure 1, 2 and 6 and Table 2). The ameliorative action of cilostazol and milrinone are likely attributable to the effect of these PDE inhibitors to increase cAMP and thus boost ICa, thus leading to a reversal of the repolarization defect permitting the development of the ECG and arrhythmic manifestations of BrS and suppression of all arrhythmic activity. It is noteworthy that both milrinone and cilostazol exert a positive inotropic and chronotropic effects. The increase in heart rate would also be expected to indirectly reduce Ito. Moreover cilostazol may exert a direct effect to block Ito, especially at higher concentrations.34
These actions of the PDE inhibitors to suppress the BrS phenotype is consistent with the ameliorative effects of β-adrenergic agonists such as isoproterenol in the clinic, particularly in controlling electrical storm in BrS patients.35,36
Conclusion
The present study identifies, for the first time, milrinone as a more potent PDE inhibitor than cilostazol for the suppression of BrS in an experimental model of the disease. Both PDE inhibitors are shown to work at the cellular level by restoring the action potential dome (due to an increase in ICa) in right ventricular epicardium, abolishing repolarization abnormalities, thus restoring electrical homogeneity of the RV myocardium where the substrate of BrS most commonly develops. Our results might prove helpful in the identification of other pharmacological agents useful in the approach to therapy of BrS. Whereas the utility of cilostazol is documented,12-14 studies are needed to establish the clinical usefulness of milrinone for prevention of VT related to BrS.
Study limitations
As with all data derived from experimental animal models, extrapolation of coronary-perfused wedge data to the clinic must be done with great care. This cautionary note notwithstanding, it important to point out that the wedge preparation identified quinidine for the treatment of BrS in 1999,7 which is used worldwide for the treatment of BrS. In that same paper, we identified an increase of ICa as another therapeutic measure using isoproterenol, which then led to the use of cilostazol. All three agents are used today for primary prevention and as adjunct therapy. Thus, the ventricular wedge preparation has been highly predictive of the clinical efficacy of all pharmacologic agents thus far tested.
The effects of milrinone and cilostazol were only evaluated in BrS models involving loss of function of inward currents, representing the majority of BrS probands thus far identified. In preliminary experiments, we have observed qualitatively similar effects of these two agents in BrS models involving a gain of function of Ito using NS5806.
Finally, the pharmacological models of BrS used m the present study, while they mimic the loss or gain of function of ICa and Ito caused by BrS mutations, do not fully recapitulate all of the gating defects encountered with the various mutations responsible for BrS. Nonetheless, these models closely recapitulate both the electrocardiographic and arrhythmic manifestations of BrS.
Acknowledgments
We are grateful to José Di Diego, MD for continuous support and personal guidance and to Serge Sicouri, MD and Vladislav Nesterenko, PhD for helpful discussions and support. We also gratefully acknowledge the technical assistance of Judy Hefferon, Rebecca Warren and Robert Goodrow.
Funding sources: This study was supported by grant HL47678 from NHLBI (CA), NYSTEM grant #C026424 (CA) and the Masons of New York, Florida, Massachusetts, Connecticut, Maryland and Rhode Island.
Abbreviations and Acronyms
- AP
action potential
- APD90
action potential durations at 90% of repolarization
- BCL
basic cycle length
- BrS
Brugada syndrome
- ECG
electrocardiogram
- EDR
epicardial dispersion of repolarization
- Endo
endocardial
- Epi
epicardial
- LV
left ventricle
- NI
notch index
- NM
notch magnitude
- PDE
phosphodiesterase
- RV
right ventricle
- RVOT
right ventricular outflow tract
- TDR
transmural dispersion of repolarization
- VF
ventricular fibrillation
- VT
ventricular tachycardia
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest: None.
References
- 1.Brugada P, Brugada J. Right bundle branch block, persistent ST segment elevation and sudden cardiac death: a distinct clinical and electrocardiographic syndrome: a multicenter report. J Am Coll Cardiol. 1992;20:1391–6. doi: 10.1016/0735-1097(92)90253-j. [DOI] [PubMed] [Google Scholar]
- 2.Di Diego JM, Cordeiro JM, Goodrow RJ, et al. Ionic and cellular basis for the predominance of the Brugada syndrome phenotype in males. Circulation. 2002;106:2004–11. doi: 10.1161/01.cir.0000032002.22105.7a. [DOI] [PubMed] [Google Scholar]
- 3.Boukens BJ, Sylva M, de Gier-de VC, et al. Reduced Sodium Channel Function Unmasks Residual Embryonic Slow Conduction in the Adult Right Ventricular Outflow Tract. Circ Res. 2013 doi: 10.1161/CIRCRESAHA.113.301565. [DOI] [PubMed] [Google Scholar]
- 4.Wilde AA, Postema PG, Di Diego JM, et al. The pathophysiological mechanism underlying Brugada syndrome: depolarization versus repolarization. J Mol Cell Cardiol. 2010;49:543–53. doi: 10.1016/j.yjmcc.2010.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Antzelevitch C. Brugada syndrome. PACE. 2006;29:1130–59. doi: 10.1111/j.1540-8159.2006.00507.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Delpón E, Cordeiro JM, Núñez L, et al. Functional effects of KCNE3 mutation and its role in the development of Brugada syndrome. Circ Arrhythm Electrophysiol. 2008;1:209–18. doi: 10.1161/CIRCEP.107.748103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yan GX, Antzelevitch C. Cellular basis for the Brugada syndrome and other mechanisms of arrhythmogenesis associated with ST segment elevation. Circulation. 1999;100:1660–6. doi: 10.1161/01.cir.100.15.1660. [DOI] [PubMed] [Google Scholar]
- 8.Endoh M, Yanagisawa T, Taira N, Blinks JR. Effects of new inotropic agents on cyclic nucleotide metabolism and calcium transients in canine ventricular muscle. Circulation. 1986;73:III117–III133. [PubMed] [Google Scholar]
- 9.Rapundalo ST, Grupp I, Grupp G, Abdul MM, Solaro RJ, Schwartz A. Myocardial actions of milrinone: characterization of its mechanism of action. Circulation. 1986;73:III134–III144. [PubMed] [Google Scholar]
- 10.Atarashi H, Endoh Y, Saitoh H, Kishida H, Hayakawa H. Chronotropic effects of cilostazol, a new antithrombotic agent, in patients with bradyarrhythmias. J Cardiovasc Pharmacol. 1998;31:534–9. doi: 10.1097/00005344-199804000-00010. [DOI] [PubMed] [Google Scholar]
- 11.Matsui K, Kiyosue T, Wang JC, Dohi K, Arita M. Effects of pimobendan on the L-type Ca2+ current and developed tension in guinea-pig ventricular myocytes and papillary muscle: comparison with IBMX, milrinone, and cilostazol. Cardiovasc Drugs Ther. 1999;13:105–13. doi: 10.1023/a:1007779908346. [DOI] [PubMed] [Google Scholar]
- 12.Tsuchiya T, Ashikaga K, Honda T, Arita M. Prevention of ventricular fibrillation by cilostazol, an oral phosphodiesterase inhibitor, in a patient with Brugada syndrome. J Cardiovasc Electrophysiol. 2002;13:698–701. doi: 10.1046/j.1540-8167.2002.00698.x. [DOI] [PubMed] [Google Scholar]
- 13.Kanlop N, Shinlapawittayatorn K, Sungnoon R, Weerateerangkul P, Chattipakorn S, Chattipakorn N. Cilostazol attenuates ventricular arrhythmia induction and improves defibrillation efficacy in swine. Can J Physiol Pharmacol. 2010;88:422–8. doi: 10.1139/y09-127. [DOI] [PubMed] [Google Scholar]
- 14.Kanlop N, Chattipakorn S, Chattipakorn N. Effects of cilostazol in the heart. J Cardiovasc Med (Hagerstown) 2011;12:88–95. doi: 10.2459/JCM.0b013e3283439746. [DOI] [PubMed] [Google Scholar]
- 15.Postema PG, Wolpert C, Amin AS, et al. Drugs and Brugada syndrome patients: review of the literature, recommendations, and an up-to-date website ( www.brugadadmgs.org) Heart Rhythm. 2009;6:1335–41. doi: 10.1016/j.hrthm.2009.07.002. www.brugadadmgs.org [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Abud A, Bagattin D, Goyeneche R, Becker C. Failure of cilostazol in the prevention of ventricular fibrillation in a patient with Brugada syndrome. J Cardiovasc Electrophysiol. 2006;17:210–2. doi: 10.1111/j.1540-8167.2005.00290.x. [DOI] [PubMed] [Google Scholar]
- 17.Dawson DL, Cutler BS, Meissner MH, Strandness DE., Jr. Cilostazol has beneficial effects in treatment of intermittent claudication: results from a multicenter, randomized, prospective, double-blind trial. Circulation. 1998;98:678–86. doi: 10.1161/01.cir.98.7.678. [DOI] [PubMed] [Google Scholar]
- 18.Pratt CM. Analysis of the cilostazol safety database. Am J Cardiol. 2001;87:28D–33D. doi: 10.1016/s0002-9149(01)01719-2. [DOI] [PubMed] [Google Scholar]
- 19.Kwon SU, Cho YJ, Koo JS, et al. Cilostazol prevents the progression of the symptomatic intracranial arterial stenosis: the multicenter double-blind placebo-controlled trial of cilostazol in symptomatic intracranial arterial stenosis. Stroke. 2005;36:782–6. doi: 10.1161/01.STR.0000157667.06542.b7. [DOI] [PubMed] [Google Scholar]
- 20.Marquez M, Salica G, Hermosillo AG, et al. Ionic basis of pharmacological therapy in Brugada syndrome. J Cardiovasc Electrophysiol. 2007;18:234–40. doi: 10.1111/j.1540-8167.2006.00681.x. [DOI] [PubMed] [Google Scholar]
- 21.Fish JM, Welchons DR, Kim YS, Lee SH, Ho WK, Antzelevitch C. Dimethyl lithospermate B, an extract of danshen, suppresses arrhythmogenesis associated with the Brugada syndrome. Circulation. 2006;113:1393–400. doi: 10.1161/CIRCULATIONAHA.105.601690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Di Diego JM, Sicouri S, Myles RC, Burton FL, Smith GL, Antzelevitch C. Optical and electrical recordings from isolated coronary-perfused ventricular wedge preparations. J Mol Cell Cardiol. 2013;54:53–64. doi: 10.1016/j.yjmcc.2012.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Calloe K, Cordeiro JM, Di Diego JM, et al. A transient outward potassium current activator recapitulates the electrocardiographic manifestations of Brugada syndrome. Cardiovasc Res. 2009;81:686–94. doi: 10.1093/cvr/cvn339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Antzelevitch C, Pollevick GD, Cordeiro JM, et al. Loss-of-function mutations in the cardiac calcium channel underlie a new clinical entity characterized by ST-segment elevation, short QT intervals, and sudden cardiac death. Circulation. 2007;115:442–9. doi: 10.1161/CIRCULATIONAHA.106.668392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen Q, Kirsch GE, Zhang D, et al. Genetic basis and molecular mechanisms for idiopathic ventricular fibrillation. Nature. 1998;392:293–6. doi: 10.1038/32675. [DOI] [PubMed] [Google Scholar]
- 26.Watanabe H, Koopmann TT, Le Scouarnec S, et al. Sodium channel b1 subunit mutations associated with Brugada syndrome and cardiac conduction disease in humans. J Clin Invest. 2008;118:2260–8. doi: 10.1172/JCI33891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.London B, Michalec M, Mehdi H, et al. Mutation in glycerol-3-phosphate dehydrogenase 1 like gene (GPD1-L) decreases cardiac Na+ current and causes inherited arrhythmias. Circulation. 2007;116:2260–8. doi: 10.1161/CIRCULATIONAHA.107.703330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Burashnikov E, Pfeiffer R, Barajas-Martinez H, et al. Mutations in the cardiac L-type calcium channel associated J wave syndrome and sudden cardiac death. Heart Rhythm. 2010;7:1872–82. doi: 10.1016/j.hrthm.2010.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hu D, Barajas-Martinez H, Medeiros-Domingo A, et al. A novel rare variant in SCN1Bb linked to Brugada syndrome and SIDS by combined modulation of Na(v)1.5 and K(v)4.3 channel currents. Heart Rhythm. 2012;9:760–9. doi: 10.1016/j.hrthm.2011.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nakajima T, Wu J, Kaneko Y, et al. KCNE3 T4A as the genetic basis of brugada-pattern electrocardiogram. Circ J. 2012;76:2763–72. doi: 10.1253/circj.cj-12-0551. [DOI] [PubMed] [Google Scholar]
- 31.Giudicessi JR, Ye D, Kritzberger CJ, et al. Novel mutations in the KCND3-encoded Kv4.3 K+ channel associated with autopsy-negative sudden unexplained death. Hum Mutat. 2012;33:989–97. doi: 10.1002/humu.22058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bilginer B, Oal B, Yigitkanli K, et al. Treatment of cerebral vasospasm with cilostazol in subarachnoid haemorrhage model. Acta Neurochir Suppl. 2008;104:291–5. [Google Scholar]
- 33.He GW. Yang CQ: Inhibition of vasoconstriction by phosphodiesterase III inhibitor milrinone in human conduit arteries used as coronary bypass grafts. J Cardiovasc Pharmacol. 1996;28:208–14. doi: 10.1097/00005344-199608000-00005. [DOI] [PubMed] [Google Scholar]
- 34.Xiao GS, Liao YH. Effect of colostazol on transient outward potassium current in human atrial myocytes. Zhongguo Ying Yong Sheng Li Xue Za Zhi. 2004;20:238–41. [PubMed] [Google Scholar]
- 35.Suzuki H, Torigoe K, Numata O, Yazaki S. Infant case with a malignant form of Brugada syndrome. J Cardiovasc Electrophysiol. 2000;11:1277–80. doi: 10.1046/j.1540-8167.2000.01277.x. [DOI] [PubMed] [Google Scholar]
- 36.Tanaka H, Kinoshita O, Uchikawa S, et al. Successful prevention of recurrent ventricular fibrillation by intravenous isoproterenol in a patient with Brugada syndrome. PACE. 2001;24:1293–4. doi: 10.1046/j.1460-9592.2001.01293.x. [DOI] [PubMed] [Google Scholar]