Abstract
Structural abnormalities in temporal lobe, including the superior temporal gyrus (STG) and planum temporale (PT), have been reported in schizophrenia (SCZ) and bipolar disorder (BPD) patients. While most MRI studies have suggested gray matter volume and surface area reduction in temporal lobe regions, few have explored changes in laminar thickness in PT and STG in SCZ and BPD. ROI subvolumes of the STG from 94 subjects were used to yield gray matter volume, gray/white surface area and laminar thickness for STG and PT cortical regions. Morphometric analysis suggests there may be gender and laterality effects on the size and shape of the PT in BPD (n=36) and SCZ (n=31) with reduced laterality in PT in subjects with SCZ but not in BPD. In addition, PT surface area was seen to be larger in males, and asymmetry in PT surface area was larger in BPD. Subjects with SCZ had reduced thickness and smaller asymmetry in PT volume. Thus, the PT probably plays a more sensitive role than the STG in structural abnormalities seen in SCZ.
Keywords: laminar thickness, superior temporal gyrus, planum temporale, schizophrenia, bipolar disorder, laterality
1. Introduction
Previous neuroimaging studies of schizophrenia have focused on morphometric properties of the superior temporal gyrus (STG). Abnormalities in the posterior or caudal STG which includes the planum temporale (PT) have been associated with auditory hallucinations. Specifically, reduced laterality of the STG and PT (Barta et al., 1997; McCarley et al., 2002) has been replicated by several recent studies (Hasan et al., 2011; Oertel et al., 2010; Takahashi et al., 2009). However, the diagnosis of schizophrenia is often confused with that of psychotic bipolar disorder because of the presence of psychotic symptoms in the latter (Brown et al., 2011; Hulshoff Pol et al., 2012; Yu et al., 2010). While both disorders may have psychotic symptoms at onset (Ellison-Wright and Bullmore, 2010; Rimol et al., 2010; Takahashi et al., 2010; Vita et al., 2011; Yuksel et al., 2012), subsequent assessment of the symptom course usually results in a more clear-cut diagnosis with different treatments. Also the two illnesses exhibit different functional behaviour such as neural responses in auditory oddball tasks (Ethridge et al., 2012). Comparing schizophrenia and psychotic bipolar disorder in neuroimaging studies may shed light on any disparate or common aspects of their etiologies.
Schizophrenia is believed to be a neurodevelopmental disorder with embryonic origins resulting in subtle aberration in cortical properties such as area, thickness and volume in temporal and frontal regions (Palaniyappan and Liddle, 2012). In adulthood, these changes can be detected by magnetic resonance imaging (MRI) technology at the spatial resolution of 1 cubic millimeters (Hartberg et al., 2011; van Haren et al., 2011). The region of interest (ROI) approach focusing on temporal and frontal regions in large clinical populations has proved to be valuable (Giuliani et al., 2005; Palaniyappan et al., 2012), but female gender continues to be underrepresented (Sun et al., 2009). The ROI approach requires precise definitions of anatomical boundaries, which can be compounded by abnormalities in psychotic disorders (Perlini et al., 2012). This can be overcome by viewing the ROI as a laminar mantle composed of gray matter voxels and a gray/white cortical surface (Miller et al., 2000). This approach has been applied in clinical neuroimaging studies of the cingulate in subjects with Alzheimer's Disease (Miller et al., 2003) and schizophrenia (Calabrese et al., 2008; Wang et al., 2007) and of the prefrontal cortex in subjects with major depressive disorder (Ceyhan et al., 2011) and schizophrenia (Harms et al., 2010).
In addition, we recently observed variable cortical thickness in the left PT in three groups of age-matched and gender-matched controls and patients with schizophrenia and bipolar disorder (Qiu et al., 2008). That study was focused on the use of a novel surface mapping method rather than on the gross morphometric properties of the PT (volume, thickness and surface area). In the current study, we used an expanded sample with an increased female representation. We sought to analyze differences between schizophrenia and psychotic bipolar disorder at the gross level with respect to accurate anatomical delineation of the STG and PT gray/white cortical surface, and further demonstrate that the effect of psychotic disorders on the PT is more pronounced than on the STG. We hypothesized that the PT and STG would be different in the schizophrenia, bipolar disorder and control groups, and that the differences would be more pronounced in males than in females.
2. Methods
2.1 Subjects
A total of 124 subjects were enrolled from schizophrenia and bipolar disorder studies at the Division of Psychiatric Neuroimaging at Johns Hopkins University School of Medicine. The data was previously collected as part of NIH grant (R01 MH43775-09A1). The Johns Hopkins Medicine Institutional Review Board approved the study, and each person gave written informed consent to participate in the study. The diagnosis of bipolar disorder or schizophrenia patients was based on DSM-IV-R and was determined using a semi-structured interview and two instruments, either the MINI (Sheehan et al., 1998) and DIGS (Nurnberger et al., 1994) or the SCAN (Wing et al., 1990) and the CIDI-SF (Kessler et al., 1998). All diagnoses were made by consensus between a research psychiatrist and a research assistant. Bipolar disorder patients were considered to have psychotic symptoms if they had at least one such episode, including hallucinations or delusions in the context of an affective episode (manic or depression) in clear consciousness. All affected subjects were medicated; however none of the subjects with schizophrenia were on mood stabilizers. Exclusion criteria included a lifetime history of substance dependence, current substance abuse and non-right-handedness (Annett, 1970). The Hollingshead Scale was used to assess socioeconomic status (Hollingshead, 1975).
2.2 MRI
All subjects gave informed consent prior to MRI scanning. T1 weighted 3D volumes were acquired using a 1.5 T Philip MR system and MPRAGE sequence (repetition time = 13.40 ms, echo time = 4.6 ms, flip angle = 20, number of acquisition = 1, matrix 256 × 256), with 1 cubic millimeter isotropic resolution across the entire cranium. Using ANALYZE (Robb et al., 1989), the raw MR data were reformatted from signed 16-bit to unsigned 8-bit and then skull stripped via the watershed module. Intracranial volume (ICV) was calculated from Freesurfer 3.0.5 (Segonne et al., 2004). The large sample size of 94 subjects should outweigh the concerns about how FreeSurfer calculates ICV (Pengas et al., 2009).
2.3 Segmentation
As detailed in Ratananther et al. (2003) and Qiu et al. (2008), a 3D ROI subvolume encompassing the STG was masked in each hemisphere in each subject. Bayesian segmentation was used to label voxels in the subvolume as gray matter (GM), white matter (WM), or cerebrospinal fluid (CSF); a modification was that in some cases WM and GM tissues were fitted by two Gaussians as it was found that segmentation was improved by increasing the complexity of the mixtures (Lee et al., 2008).
2.4 Cortical Surface Reconstruction
Using the GM/WM threshold from the segmentation as the iso-intensity value, triangulated isosurfaces were generated at the GM/WM interface (Han et al., 2002; Han et al., 2001). Following Ratnanather et al. (2003), gyral and sulcal boundaries were defined by dynamic programming (DP) as curves with maximal and minimal mean curvature between initial and terminal landmarks on the surface.
2.4.1. STG
DP delineation of the STG was initiated with several landmarks. The posterior landmark of the STG boundary began at the intersection of the angular gyrus (AG) and the STG at the most posterior extent of the lateral fissure (LF). The anterior landmark of the STG boundary was located at the superior portion of the temporal pole at the ascending ramus of the LF. The inferior extent of the STG boundary followed from the posterior landmark along the superior temporal sulcus (STS) all the way to the anterior landmark. In the case that a connection between the medial temporal gyrus (MTG) and the STG interrupted the STS, none of this connection was included. The superior extent of the STG boundary followed from the anterior landmark along the LF to the posterior landmark. Identification and placement of these landmarks are illustrated in Supplementary Figure 1.
2.4.2 PT
The PT was extracted from the dorsal surface of the delineated STG surface. Using the rules first developed (Ratnanather et al., 2003), the surface was delineated along the lateral boundary of the STG and the anterior boundary from the STG to the retroinsular end of the Heschl's Sulcus (HS).
2.5 Validation
Sixty were used for validating the delineation of the STG surfaces. Specifically, the GM in the STG was manually segmented by applying the protocol initially developed for the PT (Honeycutt et al., 2000) and extended it for the STG (see table 1 in Ratnanather et al., 2003). Histograms of set distances from the delineated surface and the corresponding isosurface of the GM volume, i.e. shortest distance of vertices in one surface to one vertex in the other, were computed (Miller et al., 2000; Ratnanather et al., 2003).
Table 1.
Summary population data (for additional details see Mahon et al. (2012)): numbers expressed as mean (standard deviation). Distributions on age (F = 0.93,p = 0.4), sex (χ2= 0.69,p = 0.71) and intracranial volume (ICV) calculated from Freesurfer 3.0.5 (Segonne et al., 2004) were similar across the three groups.
| Characteristic | Psychotic Bipolar Disorder Patients (n=36) | Schizophrenia Patients (n=31) | Healthy Comparison Subjects (n=27) |
|---|---|---|---|
| Age, years | 39.9 (11.1) | 41.4 (9.5) | 44.0 (15.6) |
| Males (%) | 52.8 | 54.8 | 44.4 |
| Education level, years | 14.6 (2.5) | 12.5 (2.2) | 13.7 (2.6) |
| Socioeconomic status | 3.2 (1.0) | 3.9 (1.2) | 3.8 (1.0) |
| Duration of illness, years | 17.6 (12.7) | 19.3 (11.0) | NA |
2.6 Thickness, Surface Area and Volume
Surface area was calculated as the sum of the area of the triangulated faces of the delineated surface. The volume was calculated as the number of voxels from the 95th percentile of the Labeled Cortical Depth Map (LCDM), which is a histogram of distances of segmented gray matter voxels to the surface (Miller et al., 2000); the 95th percentile was chosen to reflect the uncertainty of the GM segmentation in the GM/CSF region. The corresponding laminar thickness was calculated as the ratio of the volume to the area.
2.7 Statistical Analysis
Following Mahon et al. (2012), data from a total of 94 subjects were included in the analysis. We examined the effect of diagnostic group on our three measures of interest, thickness, surface area and volume, for each region of interest (STG and PT). Thus, we tested a total of six responses using MANOVA models, one for each region and measure. MANOVA allows for testing the effect of a categorical predictor variable on multiple continuous dependent variables in a single model and does not hold sphericity as an assumption. In each of our models, we tested for the effect of diagnostic group on total region (left plus right) and laterality (left minus right) measures, and determined significance using Pillai's trace, which is considered to be the most robust of the common MANOVA test statistics (Olson, 1974). Using MANCOVA models including terms to adjust for the potential covariates of age, sex, ICV, education and socioeconomic status did not qualitatively change the results (not shown). We did not adjust for testing the three measures of interest as volume and surface area are highly correlated (Pearson's r is 0.9727), and laminar thickness is a direct ratio of the two, and thus these were not independent tests. When MANOVA results were significant, we performed Hotelling's T-squared statistics as post-hoc pairwise tests on the diagnostic groups using the same two dependent variables. Finally, where Hotelling's T-test was significant between two diagnostic groups, standard t-tests were used to compare the groups on each individual metric (total region and laterality. Analogously, Hotelling's T-test was used to compare genders on total region and laterality, with significant Hotelling's T-statistics followed by standard t-tests. Finally, using ANCOVA we tested for the presence of interactions between diagnostic group and the demographic variables of age and sex. Significant p-values are reported.
3. Results
Comparisons of demographic and clinical characteristics across study groups indicated that while there was a difference in years of education and socioeconomic status, there was no significant group difference by age, sex, duration of illness or total intracranial volume (all p>0.05, see Table 1 and Mahon et al. (2012)). Patients with schizophrenia had fewer years of education on average than those with bipolar disorder, but the latter group had lower socioeconomic status than other two groups.
3.1 Comparison of manually segmented STG and delineated STG surface
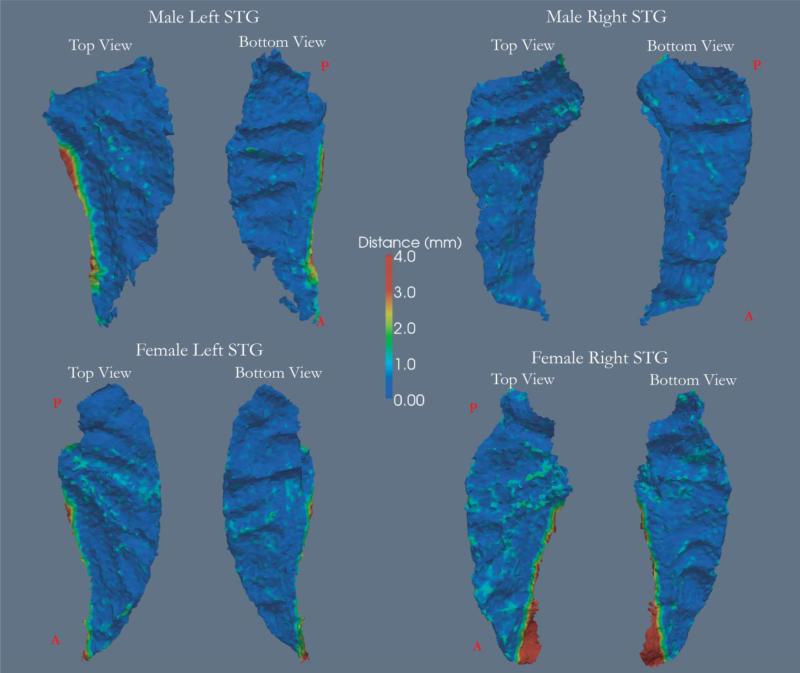
The gray/white boundary of isosurfaces encompassing the manually segmented STG and the delineated STG surfaces were observed to be closely aligned (Supplementary Figure 2). For left and right STGs, from 60 subjects based on groups (control: 11 males and 10 females; schizophrenia: 9 males and 11 females; bipolar disorder: 10 males and 9 females), 92.06 ± 3.48%, 95.85± 0.89% and 91.29 ± 3.81% (mean ± s.e.m.) of the vertices of the delineated left STG surface in controls, schizophrenia and bipolar disorder groups respectively were found to lie within 2 voxels of the manually segmented STG surface. Similarly for the right STG, 93.4 ± 3.83%, 97.69 ± 0.72% and 96.44 ± 0.63% of the vertices were found to lie within 2 voxels. Fig. 1 shows that upon closer inspection, the differences between the two surfaces were located along sulcal boundaries especially in the anterior boundary.
Figure 1.
Four delineated STG cortical surfaces. The colormap represents the distance of each vertex from the manually segmented STG surface. In general, vertices further than 2mm away from the manually segmented surface were located along the LF. Anterior and posterior locations are marked respectively by A and P in red.
3.2 Cortical Analysis
Cortical analysis was performed and corrected by investigators blind to diagnosis prior to statistical analysis. All calculations were done in native space i.e. no inter-subject registration or resampling/deformation of the triangulated surfaces were performed. Processed data were checked for quality control. Delineated surfaces were examined for anatomical inconsistencies by viewing the surfaces embedded in the native MRI and segmented volumes; the LCDM histogram of gray matter distances to the corresponding surfaces were also examined for large distances. In this way, artifacts were identified and corrected. So the outliers noted in figures 2 and 3 are more likely to be a consequence of the anatomical definitions used in data generation. Table 2 and Figures 2 and 3 describe the summary statistics for the volume, surface area and laminar thickness for the STG and PT in the three groups.
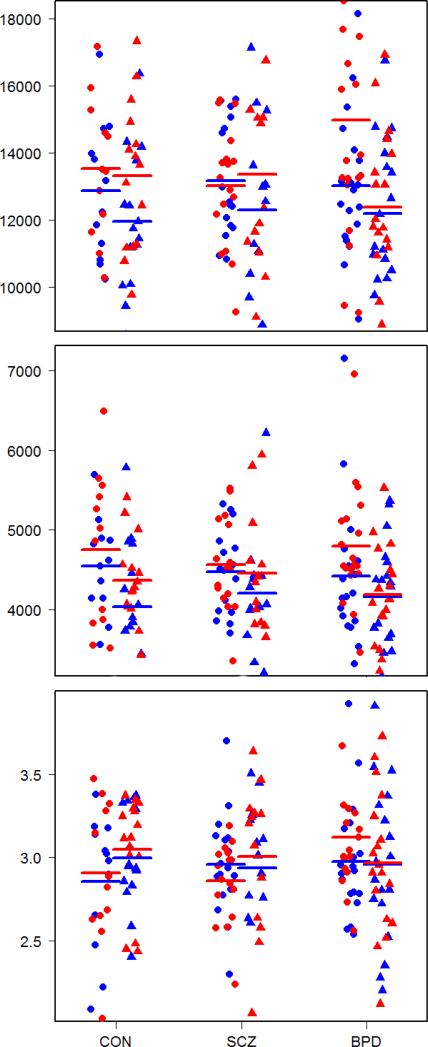
Figure 2.
STG volume in mm3 (top), surface area in mm3 (middle) and thickness in mm (bottom). Male and female gender is respectively indicated by circle and triangle while left and right side is respectively indicated by blue and red color. Means are indicated by thick lines.
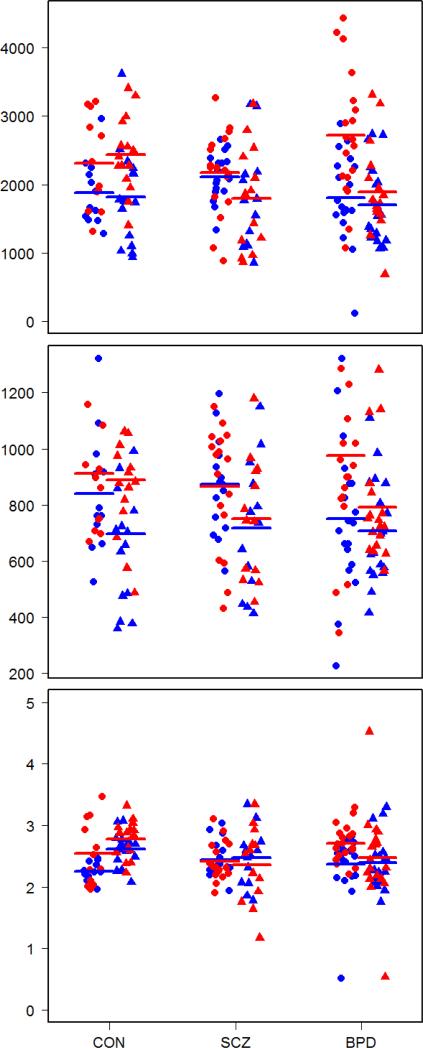
Figure 3.
PT volume in mm3 (top), surface area in mm3 (middle) and thickness in mm (bottom). Male and female gender is respectively indicated by circle and triangle while left and right side is respectively indicated by blue and red color. Means are indicated by thick lines.
Table 2.
Summary statistics – mean and standard deviation - for volume (mm3), surface area (mm2) and thickness (mm) of left PT (LPT), right PT (RPT), left STG (LSTG) and right STG (RSTG) in schizophrenia SCZ), bipolar disorder (BPD) and control (CON) groups.
| Volume | Surface area | Thickness | Volume | Surface area | Thickness | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| LPT | CON | M | 2309 (682.7) | 911.1 (239.1) | 2.55 (0.52) | RPT | CON | M | 1886 (478.3) | 841.2 (217.6) | 2.25 (0.15) |
| F | 2443 (551.3) | 889.5 (223.5) | 2.78 (0.30) | F | 1818 (725.5) | 697.0 (275.9) | 2.62 (0.28) | ||||
| all | 2384 (604.5) | 899.1 (226.3) | 2.67 (0.42) | all | 1848 (617.6) | 761.1 (257.6) | 2.45 (0.29) | ||||
| SCZ | M | 2171 (618.7) | 865.8 (219.5) | 2.56 (0.80) | SCZ | M | 2113 (347.7) | 872.8 (162.9) | 2.44 (0.29) | ||
| F | 1798 (768.6) | 752.0 (215.7) | 2.36 (0.62) | F | 1793 (745.1) | 718.6 (236.4) | 2.47 (0.46) | ||||
| all | 2010 (700.7) | 816.5 (221.7) | 2.47 (0.73) | all | 1975 (568.0) | 806.0 (209.3) | 2.46 (0.37) | ||||
| BPD | M | 2717 (926.3) | 976.4 (334.2) | 2.88 (0.90) | BPD | M | 1809 (642.1) | 751.5 (263.5) | 2.37 (0.52) | ||
| F | 1895 (634.8) | 792.2 (197.7) | 2.47 (0.75) | F | 1704 (594.8) | 706.1 (186.5) | 2.39 (0.43) | ||||
| all | 2317 (890.4) | 886.8 (288.1) | 2.68 (0.85) | all | 1758 (613.3) | 729.4 (227.3) | 2.38 (0.47) | ||||
| LSTG | CON | M | 13537 (2070) | 4750 (974.6) | 2.91 (0.43) | RSTG | CON | M | 12881 (2041) | 4543 (595.5) | 2.85 (0.41) |
| F | 13320 (2199) | 4367 (530.7) | 2.05 (0.33) | F | 11951 (2076) | 4029 (838.8) | 3.00 (0.29) | ||||
| all | 13416 (2105) | 4537 (768.9) | 2.99 (0.38) | all | 12365 (2075) | 4258 (772.5) | 2.94 (0.35) | ||||
| SCZ | M | 13022 (1790) | 4561 (572.6) | 2.86 (0.24) | SCZ | M | 13176 (1666) | 4469 (513.2) | 2.96 (0.31) | ||
| F | 13369 (2865) | 4456 (747.6) | 3.01 (0.45) | F | 12304 (2652) | 4203 (737.1) | 2.94 (0.44) | ||||
| all | 13172 (2279) | 4516 (644.2) | 2.92 (0.35) | all | 12798 (2153) | 4354 (623.1) | 2.95 (0.36) | ||||
| BPD | M | 14976 (3961) | 4795 (745.8) | 3.12 (0.68) | BPD | M | 13020 (2096) | 4418 (874.2) | 2.98 (0.35) | ||
| F | 12392 (2314) | 4186 (595.6) | 2.97 (0.42) | F | 12197 (2232) | 4157 (690.9) | 2.96 (0.47) | ||||
| all | 13719 (3476) | 4499 (735.3) | 3.05 (0.57) | all | 12620 (2173) | 4291 (790.6) | 2.97 (0.40) |
3.2.1 STG
Hotelling's T-test showed significant differences in surface area between males and females (T2 = 0.0921, p = 0.0123). Males had a significantly greater total surface area than females (pleft+right = 0.0032). No significant diagnostic group results were found in the STG.
3.2.2 PT
Hotelling's T-test showed significant differences in surface area between males and females (T2 = 0.0839, p = 0.0186). Males had a significantly greater total surface area than females (pleft+right = 0.0048). Hotelling's T-test showed significant differences in volume between males and females (T2 = 0.0713, p = 0.0345). Males had a significant greater total PT volume than females (pleft+right = 0.0112). There was a statistically significant difference in volume by diagnostic group (ΛPillai = 0.077, F2,185 = 3.72, p = 0.0056). Hotelling's T-test showed significant differences in PT volume between controls and schizophrenic patients (T2 = 0.119, p = 0.0009) and between schizophrenic patients and bipolar patients (T2 = 0.0788, p = 0.0046). Hemispheric difference in volume was significantly lower in schizophrenic patients than in both bipolar patients (p = 0.0180) and controls (p = 0.0108).
There was a statistically significant difference in laminar thickness by diagnostic group (ΛPillai = 0.067, F2,185 = 3.21, p = 0.0132). Hotelling's T-test showed significant differences in volume between controls and schizophrenic patients (T2 = 0.118, p = 0.0009) and between schizophrenic patients and bipolar patients (T2 = 0.0522, p = 0.0298). Total laminar thickness was significantly greater in controls than in schizophrenic patients (p = 0.0237).
There was a statistically significant difference in surface area by diagnostic group (ΛPillai = 0.0517, F2,185 = 2.45, p = 0.0456). Hotelling's T-test showed significant differences in volume between controls and schizophrenic patients (T2 = 0.0611, p = 0.0303) and between schizophrenic patients and bipolar patients (T2 = 0.0603, p = 0.0170). Bipolar patients had a significantly higher difference in surface area than schizophrenic patients (p = 0.0441).
There was a significant diagnosis effect on hemispheric difference in volume (F = 3.58, p = 0.0320). There was a gender-diagnosis interaction for left side volume (p = 0.0472), a gender-diagnosis interaction for left side laminar thickness (p = 0.0039), and an age-diagnosis interaction for left side laminar thickness (p = 0.0028).
4. Discussion
In this paper, measurements of volume, surface area and laminar thickness were obtained separately for STG and PT in a differential analysis of schizophrenia and psychotic bipolar disorder in a medium sized population. Volume here is defined as the number of voxels at the 95th percentile of the LCDM, which is a histogram of the distances of the segmented gray matter voxels to the nearest vertex on the gray/white surface. This estimate is influenced by the boundary of the gray/white surface, which is delineated by curvature-based dynamic programming, a process which has been validated for the PT (Ratnanather et al., 2003) and the STG (Figure 1). The estimate is also dependent on ensuring that segmented gray matter voxels are correctly assigned to the cortical region and not a neighbouring or opposing one, hence the importance of accurate delineation of the cortical region. Surface area is logically the summation of the area of the triangular faces of the triangulated surface; it too is dependent on the accuracy of the delineated surface as well as the isovalue given by the gray/white threshold from the Bayesian segmentation. This delineation of surfaces has been found to be reliable for several cortical structures (Ratnanather et al., 2003; Ratnanather et al., 2001; Ratnanather et al., 2004). The laminar approximation of thickness, expressed as the ratio of the volume to surface area, is a simplification based on the assumption that thickness is uniform everywhere in the region.
The procedure for delineating the STG and PT cortical surfaces is dependent on reliable definitions of gyral and sulcal boundaries (Fig. 1). Differences with the surfaces obtained from manual segmentation and curvature based delineation, especially along the sulcal edges may be attributed to the frame of reference being adjusted to facilitate the manual segmentation. Care must be taken in delineating regions of the lateral fissure as well as connections between the STG and the middle temporal gyrus. The procedure can be applied to any triangulated surfaces generated by software such as FreeSurfer (Fischl, 2012) particularly in cases where there is uncertainty in the boundaries due to gross abnormalities in disease which can be masked by the partitioning in the pial view (Desikan et al., 2006; Fischl et al., 2004). So more precise delineation based on sulcal and gyral definitions could help account for variations in morphometric measures in neuroimaging studies of the STG and PT, such as the present study.
STG and PT morphometric data obtained in this study (Fig. 2 and 3) are consistent with other similar neuroimaging studies. For example, volumes are similar to those reported by Takahashi et al. (2010) for both STG and PT. Surface areas are slightly larger than those reported by Crespo-Facorro et al. (2004) for the PT and Goghari et al.(2007) for the STG. Thicknesses are similar to those reported by Rimol et al. (2010) for the STG and by Beasley et al. (2005) and Chance et al. (2004) for the PT postmortem.
It should be noted that the focus on the PT as the key sub-manifold i.e. triangulated subset of the STG, was motivated by previous work (Barta et al., 1997; Frangou et al., 1997; Petty et al., 1995). Thus we did not consider the Heschl's Gyrus (HG), although it being adjacent to the PT suggests that it could also be affected by schizophrenia (Hubl et al., 2010; Nenadic et al., 2010; Shinn et al., 2013; Smiley et al., 2009). It is also worth noting that stereological analysis by Chance et al. (2008) showed normal aging related differences in the PT but it was absent in schizophrenia as well as in HG.
Many differences between the results and reported data are likely attributed to the different methods used. The approach adopted here was to exploit the accuracy of the gray/white cortical surface and gray matter segmentation methods. Then, one obtains the volume from the distribution of distances of gray matter voxels relative to the surface. While more sophisticated analysis of these distributions will be pursued elsewhere, the ROI approach is more likely to be reliable than whole brain analysis (Perlini et al., 2012; Sun et al., 2009). The approach adopted here is also consistent with recent analyses suggesting that thickness and surface area provide more complete information, especially when dealing with potentially overlapping symptoms (Rimol et al., 2010; Rimol et al., 2012).
The reduced thickness of the PT in SCZ is consistent with published data. There appears to be a gender effect. For females, negligible laterality in the PT might be a reflection of the effect of gestational production of testosterone on the PT formation (Castle and Murray, 1991). This further suggests that PT should be analyzed in addition to STG. In the PT, reduced laterality was observed in all three measures. It is thought that the degree of lateralization is strongly correlated with the severity of symptoms (Oertel et al., 2010). Thus area reduction in PT together with thickness could be an important factor in schizophrenia.
The biological mechanism for lateralization is unknown (Crow, 2000; Palaniyappan et al., 2012; Seldon, 2006). Stereological analysis by Chance et al. (2008) suggests that the surface area of the planum temporale depends partly on the proliferation and expansion of the cortical minicolumns in gestation as suggested by the classic model of the developing cortex from Rakic (1988). The larger surface area in males would be consistent with wider minicolumn spacing accruing from prolonged developmental proliferation. On the other hand, the smaller surface area in females would be consistent with narrow minicolumns accruing from a faster maturation rate. Thus it would seem reasonable to conclude that the size and asymmetry of the PT is linked closely with the minicolumn spacing. Furthermore assuming constant volume, PT thickness is also thought to be associated with the minicolumn spacing (Harasty et al., 2003; Seldon, 2006). Therefore the different effects on thickness and area may reflect different ontogenic and phylogenic control in cortical development.
For comparison with schizophrenia, the bipolar disorder subjects in this study were restricted to those with psychosis. This would allow for a better understanding of the different effect of the diseases by way of disturbances of a network of brain structures as implied by the Research Domain Criteria (RDoC) (Insel et al., 2010). The results reported here are consistent with gray matter loss being larger in schizophrenia than in bipolar disorder subjects, especially in the STG (Pridmore and Bowe, 2011).
A caveat is that changes in gray matter volume and thickness can be confounded by anti-psychotic medication. A limitation of this study is that detailed information on medication was not collected at the time of the scan. Participant self-report and review of past medical records confirms that subjects with schizophrenia and bipolar disorders were treated with different classes of medication. The majority of subjects with schizophrenia took or had taken antipsychotics (none took mood stabilizers) and subjects with bipolar disorder were treated with lithium and other mood stabilizers. Given that the classes of medication used to treat schizophrenia and bipolar disorder differed, it would have not been possible to adjust for medication in the analysis. Nor were individual psychopathology or cognitive scores collected. Thus, our results should be confirmed in future studies able to assess the effect of treatment and psychopathology on STG and PT morphometry.
The omission of a complete medication history represents a weakness of this study. It is, in general, difficult to obtain a reliable long-term history of medication use by any patient, particularly in psychiatric patients. Even in the presence of resources such as nation-wide drug history databases (which is only now beginning to emerge), adherence to neuroleptic medications tends to be on the order of 50 % adherence to the prescribed medication plan (e.g. Lacro et al., 2002). Furthermore, there is only weak to moderate concordance between self-reported measures of medication adherence and pharmacy refill records in general medicine patients with chronic conditions (Cook et al., 2005), suggesting that self-report is not likely to be that useful as a means of assessing adherence. It is difficult to imagine that the adherence of psychiatric patients would be better than the general medicine group (Cramer and Rosenheck, 1998).
At the time of the study, the resources to obtain a reasonably reliable assessment of past medication trials (despite having a list of the subjects’ current medications) were lacking. Thus a list of the subjects medication use at one time point (the time of the subject characterization and scan) does not represent data that is of much relevance to the extremely important question of whether medication side effects account for the findings. It should be noted that the majority of studies in the literature suffer from the same difficulties regarding medication history as this study, and that a definitive answer to the question would require a sophisticated prospective study design, which is not the case here.
In summary, an approach that calculates the three main morphometric parameters revealed reduced laterality in PT in subjects with schizophrenia while laterality was unaffected in subjects with bipolar disorders. Given the reliability of the procedure with respect to the gray/white surface and gray matter segmentation, the results are consistent with recently reported findings. The specificity of the PT in schizophrenia suggests that it is a major contributor to the structural abnormalities associated that have previously shown to be associated with functional abnormalities in the left temporal lobe (Ethridge et al., 2012; Hugdahl et al., 2009).
Supplementary Material
Acknowledgements
This work was supported by research grants from the National Institute of Health (R01 MH064838, R01 EB000975 and P41 EB015909).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributors
The paper and project were conceived by Tilak Ratnanather and Patrick Barta. The data was collected and reviewed by Nancy Honeycutt, Pamela Mahon and Patrick Barta. The data was analyzed by Nancy Honeycutt, Britni Crocker, Clare Poynton, Dominic Pisano, Elizabeth Postell, Shannon Cebron and Elvan Ceyhan.
Conflict of Interest
There are no conflicts of interest.
References
- Annett M. A classification of hand preference by association analysis. British journal of psychology. 1970;61(3):303–321. doi: 10.1111/j.2044-8295.1970.tb01248.x. [DOI] [PubMed] [Google Scholar]
- Barta PE, Pearlson GD, Brill LB, 2nd, Royall R, McGilchrist IK, Pulver AE, Powers RE, Casanova MF, Tien AY, Frangou S, Petty RG. Planum temporale asymmetry reversal in schizophrenia: replication and relationship to gray matter abnormalities. The American journal of psychiatry. 1997;154(5):661–667. doi: 10.1176/ajp.154.5.661. [DOI] [PubMed] [Google Scholar]
- Beasley CL, Chana G, Honavar M, Landau S, Everall IP, Cotter D. Evidence for altered neuronal organisation within the planum temporale in major psychiatric disorders. Schizophrenia research. 2005;73(1):69–78. doi: 10.1016/j.schres.2004.08.011. [DOI] [PubMed] [Google Scholar]
- Brown GG, Lee JS, Strigo IA, Caligiuri MP, Meloy MJ, Lohr J. Voxel- based morphometry of patients with schizophrenia or bipolar I disorder: a matched control study. Psychiatry research. 2011;194(2):149–156. doi: 10.1016/j.pscychresns.2011.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calabrese DR, Wang L, Harms MP, Ratnanather JT, Barch DM, Cloninger CR, Thompson PA, Miller MI, Csernansky JG. Cingulate gyrus neuroanatomy in schizophrenia subjects and their non-psychotic siblings. Schizophrenia research. 2008;104(1-3):61–70. doi: 10.1016/j.schres.2008.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castle DJ, Murray RM. The Neurodevelopmental Basis of Sex-Differences in Schizophrenia. Psychological medicine. 1991;21(3):565–575. doi: 10.1017/s0033291700022194. [DOI] [PubMed] [Google Scholar]
- Ceyhan E, Hosakere M, Nishino T, Alexopoulos J, Todd RD, Botteron KN, Miller MI, Ratnanather JT. Statistical Analysis of Cortical Morphometrics Using Pooled Distances Based on Labeled Cortical Distance Maps. Journal of mathematical imaging and vision. 2011;40(1):20–35. doi: 10.1007/s10851-010-0240-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chance SA, Casanova MF, Switala AE, Crow TJ. Auditory cortex asymmetry, altered minicolumn spacing and absence of ageing effects in schizophrenia. Brain : a journal of neurology. 2008;131(Pt 12):3178–3192. doi: 10.1093/brain/awn211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chance SA, Tzotzoli PM, Vitelli A, Esiri MM, Crow TJ. The cytoarchitecture of sulcal folding in Heschl's sulcus and the temporal cortex in the normal brain and schizophrenia: lamina thickness and cell density. Neuroscience letters. 2004;367(3):384–388. doi: 10.1016/j.neulet.2004.06.041. [DOI] [PubMed] [Google Scholar]
- Cook CL, Wade WE, Martin BC, Perri M., 3rd Concordance among three self-reported measures of medication adherence and pharmacy refill records. Journal of the American Pharmacists Association : JAPhA. 2005;45(2):151–159. doi: 10.1331/1544345053623573. [DOI] [PubMed] [Google Scholar]
- Cramer JA, Rosenheck R. Compliance with medication regimens for mental and physical disorders. Psychiatric services. 1998;49(2):196–201. doi: 10.1176/ps.49.2.196. [DOI] [PubMed] [Google Scholar]
- Crespo-Facorro B, Kim JJ, Chemerinski E, Magnotta V, Andreasen NC, Nopoulos P. Morphometry of the superior temporal plane in schizophrenia: relationship to clinical correlates. The Journal of neuropsychiatry and clinical neurosciences. 2004;16(3):284–294. doi: 10.1176/jnp.16.3.284. [DOI] [PubMed] [Google Scholar]
- Crow TJ. Schizophrenia as the price that homo sapiens pays for language: a resolution of the central paradox in the origin of the species. Brain research. Brain research reviews. 2000;31(2-3):118–129. doi: 10.1016/s0165-0173(99)00029-6. [DOI] [PubMed] [Google Scholar]
- Desikan RS, Segonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, Buckner RL, Dale AM, Maguire RP, Hyman BT, Albert MS, Killiany RJ. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. NeuroImage. 2006;31(3):968–980. doi: 10.1016/j.neuroimage.2006.01.021. [DOI] [PubMed] [Google Scholar]
- Ellison-Wright I, Bullmore E. Anatomy of bipolar disorder and schizophrenia: a meta-analysis. Schizophrenia research. 2010;117(1):1–12. doi: 10.1016/j.schres.2009.12.022. [DOI] [PubMed] [Google Scholar]
- Ethridge LE, Hamm JP, Shapiro JR, Summerfelt AT, Keedy SK, Stevens MC, Pearlson G, Tamminga CA, Boutros NN, Sweeney JA, Keshavan MS, Thaker G, Clementz BA. Neural activations during auditory oddball processing discriminating schizophrenia and psychotic bipolar disorder. Biological psychiatry. 2012;72(9):766–774. doi: 10.1016/j.biopsych.2012.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B. FreeSurfer. NeuroImage. 2012;62(2):774–781. doi: 10.1016/j.neuroimage.2012.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, van der Kouwe A, Destrieux C, Halgren E, Segonne F, Salat DH, Busa E, Seidman LJ, Goldstein J, Kennedy D, Caviness V, Makris N, Rosen B, Dale AM. Automatically parcellating the human cerebral cortex. Cerebral cortex. 2004;14(1):11–22. doi: 10.1093/cercor/bhg087. [DOI] [PubMed] [Google Scholar]
- Frangou S, Sharma T, Sigmudsson T, Barta P, Pearlson G, Murray RM. The Maudsley Family Study. 4. Normal planum temporale asymmetry in familial schizophrenia. A volumetric MRI study. The British journal of psychiatry : the journal of mental science. 1997;170:328–333. doi: 10.1192/bjp.170.4.328. [DOI] [PubMed] [Google Scholar]
- Giuliani NR, Calhoun VD, Pearlson GD, Francis A, Buchanan RW. Voxel-based morphometry versus region of interest: a comparison of two methods for analyzing gray matter differences in schizophrenia. Schizophrenia research. 2005;74(2-3):135–147. doi: 10.1016/j.schres.2004.08.019. [DOI] [PubMed] [Google Scholar]
- Goghari VM, Rehm K, Carter CS, MacDonald AW. Sulcal thickness as a vulnerability indicator for schizophrenia. The British journal of psychiatry : the journal of mental science. 2007;191:229–233. doi: 10.1192/bjp.bp.106.034595. [DOI] [PubMed] [Google Scholar]
- Han X, Xu C, Braga-Neto U, Prince JL. Topology correction in brain cortex segmentation using a multiscale, graph-based algorithm. Medical Imaging, IEEE Transactions on. 2002;21(2):109–121. doi: 10.1109/42.993130. [DOI] [PubMed] [Google Scholar]
- Han X, Xu C, Prince JL. A topology preserving deformable model using level sets, Computer Vision and Pattern Recognition, 2001. CVPR 2001. Proceedings of the 2001 IEEE Computer Society Conference on. 2001;762:II–765-II-770. [Google Scholar]
- Harasty J, Seldon HL, Chan P, Halliday G, Harding A. The left human speech-processing cortex is thinner but longer than the right. Laterality. 2003;8(3):247–260. doi: 10.1080/13576500244000175. [DOI] [PubMed] [Google Scholar]
- Harms MP, Wang L, Campanella C, Aldridge K, Moffitt AJ, Kuelper J, Ratnanather JT, Miller MI, Barch DM, Csernansky JG. Structural abnormalities in gyri of the prefrontal cortex in individuals with schizophrenia and their unaffected siblings. The British journal of psychiatry : the journal of mental science. 2010;196(2):150–157. doi: 10.1192/bjp.bp.109.067314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartberg CB, Sundet K, Rimol LM, Haukvik UK, Lange EH, Nesvag R, Dale AM, Melle I, Andreassen OA, Agartz I. Brain cortical thickness and surface area correlates of neurocognitive performance in patients with schizophrenia, bipolar disorder, and healthy adults. Journal of the International Neuropsychological Society : JINS. 2011;17(6):1080–1093. doi: 10.1017/S1355617711001081. [DOI] [PubMed] [Google Scholar]
- Hasan A, Kremer L, Gruber O, Schneider-Axmann T, Guse B, Reith W, Falkai P, Wobrock T. Planum temporale asymmetry to the right hemisphere in first-episode schizophrenia. Psychiatry research. 2011;193(1):56–59. doi: 10.1016/j.pscychresns.2011.02.008. [DOI] [PubMed] [Google Scholar]
- Hollingshead AB. Four factor index of social status. Yale University; New Haven, CT: 1975. [Google Scholar]
- Honeycutt NA, Musick A, Barta PE, Pearlson GD. Measurement of the planum temporale (PT) on magnetic resonance imaging scans: temporal PT alone and with parietal extension. Psychiatry research. 2000;98(2):103–116. doi: 10.1016/s0925-4927(00)00043-3. [DOI] [PubMed] [Google Scholar]
- Hubl D, Dougoud-Chauvin V, Zeller M, Federspiel A, Boesch C, Strik W, Dierks T, Koenig T. Structural analysis of Heschl's gyrus in schizophrenia patients with auditory hallucinations. Neuropsychobiology. 2010;61(1):1–9. doi: 10.1159/000258637. [DOI] [PubMed] [Google Scholar]
- Hugdahl K, Loberg EM, Nygard M. Left temporal lobe structural and functional abnormality underlying auditory hallucinations in schizophrenia. Frontiers in neuroscience. 2009;3(1):34–45. doi: 10.3389/neuro.01.001.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulshoff Pol HE, van Baal GC, Schnack HG, Brans RG, van der Schot AC, Brouwer RM, van Haren NE, Lepage C, Collins DL, Evans AC, Boomsma DI, Nolen W, Kahn RS. Overlapping and segregating structural brain abnormalities in twins with schizophrenia or bipolar disorder. Arch Gen Psychiatry. 2012;69(4):349–359. doi: 10.1001/archgenpsychiatry.2011.1615. [DOI] [PubMed] [Google Scholar]
- Insel T, Cuthbert B, Garvey M, Heinssen R, Pine DS, Quinn K, Sanislow C, Wang P. Research domain criteria (RDoC): toward a new classification framework for research on mental disorders. The American journal of psychiatry. 2010;167(7):748–751. doi: 10.1176/appi.ajp.2010.09091379. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Andrews G, Mroczek D, Ustun B, Wittchen H-U. The World Health Organization Composite International Diagnostic Interview short-form (CIDI-SF). International Journal of Methods in Psychiatric Research. 1998;7(4):171–185. [Google Scholar]
- Lacro JP, Dunn LB, Dolder CR, Leckband SG, Jeste DV. Prevalence of and risk factors for medication nonadherence in patients with schizophrenia: a comprehensive review of recent literature. The Journal of clinical psychiatry. 2002;63(10):892–909. doi: 10.4088/jcp.v63n1007. [DOI] [PubMed] [Google Scholar]
- Lee NA, Priebe CE, Miller MI, Ratnanather JT. Validation of alternating Kernel mixture method: application to tissue segmentation of cortical and subcortical structures. Journal of biomedicine & biotechnology. 2008;2008:346129. doi: 10.1155/2008/346129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahon PB, Eldridge H, Crocker B, Notes L, Gindes H, Postell E, King S, Potash JB, Ratnanather JT, Barta PE. An MRI study of amygdala in schizophrenia and psychotic bipolar disorder. Schizophrenia research. 2012;138(2-3):188–191. doi: 10.1016/j.schres.2012.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarley RW, Salisbury DF, Hirayasu Y, Yurgelun-Todd DA, Tohen M, Zarate C, Kikinis R, Jolesz FA, Shenton ME. Association between smaller left posterior superior temporal gyrus volume on magnetic resonance imaging and smaller left temporal P300 amplitude in first-episode schizophrenia. Arch Gen Psychiatry. 2002;59(4):321–331. doi: 10.1001/archpsyc.59.4.321. [DOI] [PubMed] [Google Scholar]
- Miller MI, Hosakere M, Barker AR, Priebe CE, Lee N, Ratnanather JT, Wang L, Gado M, Morris JC, Csernansky JG. Labeled cortical mantle distance maps of the cingulate quantify differences between dementia of the Alzheimer type and healthy aging. Proceedings of the National Academy of Sciences of the United States of America. 2003;100(25):15172–15177. doi: 10.1073/pnas.2136624100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller MI, Massie AB, Ratnanather JT, Botteron KN, Csernansky JG. Bayesian construction of geometrically based cortical thickness metrics. NeuroImage. 2000;12(6):676–687. doi: 10.1006/nimg.2000.0666. [DOI] [PubMed] [Google Scholar]
- Nenadic I, Smesny S, Schlosser RG, Sauer H, Gaser C. Auditory hallucinations and brain structure in schizophrenia: voxel-based morphometric study. The British journal of psychiatry : the journal of mental science. 2010;196(5):412–413. doi: 10.1192/bjp.bp.109.070441. [DOI] [PubMed] [Google Scholar]
- Nurnberger JI, Blehar MC, Kaufmann CA, York-Cooler C, Simpson SG, Harkavy-Friedman J, Severe JB, Malaspina D, Reich T. Diagnostic interview for genetic studies: Rationale, unique features, and training. Archives of General Psychiatry. 1994;51(11):849–859. doi: 10.1001/archpsyc.1994.03950110009002. [DOI] [PubMed] [Google Scholar]
- Oertel V, Knochel C, Rotarska-Jagiela A, Schonmeyer R, Lindner M, van de Ven V, Haenschel C, Uhlhaas P, Maurer K, Linden DE. Reduced laterality as a trait marker of schizophrenia--evidence from structural and functional neuroimaging. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2010;30(6):2289–2299. doi: 10.1523/JNEUROSCI.4575-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson CL. Comparative Robustness of 6 Tests in Multivariate-Analysis of Variance. J Am Stat Assoc. 1974;69(348):894–908. [Google Scholar]
- Palaniyappan L, Balain V, Radua J, Liddle PF. Structural correlates of auditory hallucinations in schizophrenia: a meta-analysis. Schizophrenia research. 2012;137(1-3):169–173. doi: 10.1016/j.schres.2012.01.038. [DOI] [PubMed] [Google Scholar]
- Palaniyappan L, Liddle PF. Differential effects of surface area, gyrification and cortical thickness on voxel based morphometric deficits in schizophrenia. NeuroImage. 2012;60(1):693–699. doi: 10.1016/j.neuroimage.2011.12.058. [DOI] [PubMed] [Google Scholar]
- Pengas G, Pereira JM, Williams GB, Nestor PJ. Comparative reliability of total intracranial volume estimation methods and the influence of atrophy in a longitudinal semantic dementia cohort. Journal of neuroimaging : official journal of the American Society of Neuroimaging. 2009;19(1):37–46. doi: 10.1111/j.1552-6569.2008.00246.x. [DOI] [PubMed] [Google Scholar]
- Perlini C, Bellani M, Brambilla P. Structural imaging techniques in schizophrenia. Acta psychiatrica Scandinavica. 2012;126(4):235–242. doi: 10.1111/j.1600-0447.2012.01868.x. [DOI] [PubMed] [Google Scholar]
- Petty RG, Barta PE, Pearlson GD, McGilchrist IK, Lewis RW, Tien AY, Pulver A, Vaughn DD, Casanova MF, Powers RE. Reversal of asymmetry of the planum temporale in schizophrenia. The American journal of psychiatry. 1995;152(5):715–721. doi: 10.1176/ajp.152.5.715. [DOI] [PubMed] [Google Scholar]
- Pridmore S, Bowe G. Neuroimaging in the field of psychoses. The Malaysian journal of medical sciences : MJMS. 2011;18(1):6–11. [PMC free article] [PubMed] [Google Scholar]
- Qiu A, Vaillant M, Barta P, Ratnanather JT, Miller MI. Region-of-interest-based analysis with application of cortical thickness variation of left planum temporale in schizophrenia and psychotic bipolar disorder. Human brain mapping. 2008;29(8):973–985. doi: 10.1002/hbm.20444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakic P. Specification of cerebral cortical areas. Science. 1988;241(4862):170–176. doi: 10.1126/science.3291116. [DOI] [PubMed] [Google Scholar]
- Ratnanather JT, Barta PE, Honeycutt NA, Lee N, Morris HM, Dziorny AC, Hurdal MK, Pearlson GD, Miller MI. Dynamic programming generation of boundaries of local coordinatized submanifolds in the neocortex: application to the planum temporale. NeuroImage. 2003;20(1):359–377. doi: 10.1016/s1053-8119(03)00238-6. [DOI] [PubMed] [Google Scholar]
- Ratnanather JT, Botteron KN, Nishino T, Massie AB, Lal RM, Patel SG, Peddi S, Todd RD, Miller MI. Validating cortical surface analysis of medial prefrontal cortex. NeuroImage. 2001;14(5):1058–1069. doi: 10.1006/nimg.2001.0906. [DOI] [PubMed] [Google Scholar]
- Ratnanather JT, Wang L, Nebel MB, Hosakere M, Han X, Csernansky JG, Miller MI. Validation of semiautomated methods for quantifying cingulate cortical metrics in schizophrenia. Psychiatry research. 2004;132(1):53–68. doi: 10.1016/j.pscychresns.2004.07.003. [DOI] [PubMed] [Google Scholar]
- Rimol LM, Hartberg CB, Nesvag R, Fennema-Notestine C, Hagler DJ, Jr., Pung CJ, Jennings RG, Haukvik UK, Lange E, Nakstad PH, Melle I, Andreassen OA, Dale AM, Agartz I. Cortical thickness and subcortical volumes in schizophrenia and bipolar disorder. Biological psychiatry. 2010;68(1):41–50. doi: 10.1016/j.biopsych.2010.03.036. [DOI] [PubMed] [Google Scholar]
- Rimol LM, Nesvag R, Hagler DJ, Jr., Bergmann O, Fennema-Notestine C, Hartberg CB, Haukvik UK, Lange E, Pung CJ, Server A, Melle I, Andreassen OA, Agartz I, Dale AM. Cortical volume, surface area, and thickness in schizophrenia and bipolar disorder. Biological psychiatry. 2012;71(6):552–560. doi: 10.1016/j.biopsych.2011.11.026. [DOI] [PubMed] [Google Scholar]
- Robb RA, Hanson DP, Karwoski RA, Larson AG, Workman EL, Stacy MC. Analyze: a comprehensive, operator-interactive software package for multidimensional medical image display and analysis. Computerized medical imaging and graphics : the official journal of the Computerized Medical Imaging Society. 1989;13(6):433–454. doi: 10.1016/0895-6111(89)90285-1. [DOI] [PubMed] [Google Scholar]
- Segonne F, Dale AM, Busa E, Glessner M, Salat D, Hahn HK, Fischl B. A hybrid approach to the skull stripping problem in MRI. NeuroImage. 2004;22(3):1060–1075. doi: 10.1016/j.neuroimage.2004.03.032. [DOI] [PubMed] [Google Scholar]
- Seldon HL. Cortical laminar thickness and column spacing in human temporal and inferior parietal lobes: intra-individual anatomical relations. Laterality. 2006;11(3):226–250. doi: 10.1080/13576500500489162. [DOI] [PubMed] [Google Scholar]
- Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, Hergueta T, Baker R, Dunbar GC. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. The Journal of clinical psychiatry 59 Suppl. 1998;20:22–33. quiz 34-57. [PubMed] [Google Scholar]
- Shinn AK, Baker JT, Cohen BM, Ongur D. Functional connectivity of left Heschl's gyrus in vulnerability to auditory hallucinations in schizophrenia. Schizophrenia research. 2013;143(2-3):260–268. doi: 10.1016/j.schres.2012.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smiley JF, Rosoklija G, Mancevski B, Mann JJ, Dwork AJ, Javitt DC. Altered volume and hemispheric asymmetry of the superficial cortical layers in the schizophrenia planum temporale. The European journal of neuroscience. 2009;30(3):449–463. doi: 10.1111/j.1460-9568.2009.06838.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun J, Maller JJ, Guo L, Fitzgerald PB. Superior temporal gyrus volume change in schizophrenia: a review on region of interest volumetric studies. Brain research reviews. 2009;61(1):14–32. doi: 10.1016/j.brainresrev.2009.03.004. [DOI] [PubMed] [Google Scholar]
- Takahashi T, Wood SJ, Yung AR, Soulsby B, McGorry PD, Suzuki M, Kawasaki Y, Phillips LJ, Velakoulis D, Pantelis C. Progressive gray matter reduction of the superior temporal gyrus during transition to psychosis. Arch Gen Psychiatry. 2009;66(4):366–376. doi: 10.1001/archgenpsychiatry.2009.12. [DOI] [PubMed] [Google Scholar]
- Takahashi T, Wood SJ, Yung AR, Walterfang M, Phillips LJ, Soulsby B, Kawasaki Y, McGorry PD, Suzuki M, Velakoulis D, Pantelis C. Superior temporal gyrus volume in antipsychotic-naive people at risk of psychosis. The British journal of psychiatry : the journal of mental science. 2010;196(3):206–211. doi: 10.1192/bjp.bp.109.069732. [DOI] [PubMed] [Google Scholar]
- van Haren NE, Schnack HG, Cahn W, van den Heuvel MP, Lepage C, Collins L, Evans AC, Hulshoff Pol HE, Kahn RS. Changes in cortical thickness during the course of illness in schizophrenia. Arch Gen Psychiatry. 2011;68(9):871–880. doi: 10.1001/archgenpsychiatry.2011.88. [DOI] [PubMed] [Google Scholar]
- Vita A, De Peri L, Turrina C, Sacchetti E. Brain Morphological Abnormalities at the Onset of Schizophrenia and Other Psychotic Disorders: A Review of the Evidence. In: Ritsner MS, editor. Handbook of Schizophrenia Spectrum Disorders. Vol. 1. Springer; Netherlands: 2011. pp. 431–443. [Google Scholar]
- Wang L, Hosakere M, Trein JC, Miller A, Ratnanather JT, Barch DM, Thompson PA, Qiu A, Gado MH, Miller MI, Csernansky JG. Abnormalities of cingulate gyrus neuroanatomy in schizophrenia. Schizophrenia research. 2007;93(1-3):66–78. doi: 10.1016/j.schres.2007.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wing JK, Babor T, Brugha T, Burke J, Cooper JE, Giel R, Jablenski A, Regier D, Sartorius N. Scan: Schedules for clinical assessment in neuropsychiatry. Archives of General Psychiatry. 1990;47(6):589. doi: 10.1001/archpsyc.1990.01810180089012. [DOI] [PubMed] [Google Scholar]
- Yu K, Cheung C, Leung M, Li Q, Chua S, McAlonan G. Are Bipolar Disorder and Schizophrenia Neuroanatomically Distinct? An Anatomical Likelihood Meta-analysis. Frontiers in human neuroscience. 2010;4:189. doi: 10.3389/fnhum.2010.00189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuksel C, McCarthy J, Shinn A, Pfaff DL, Baker JT, Heckers S, Renshaw P, Ongur D. Gray matter volume in schizophrenia and bipolar disorder with psychotic features. Schizophrenia research. 2012;138(2-3):177–182. doi: 10.1016/j.schres.2012.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.