Abstract
Glycogen synthase kinase 3 (GSK3) is a serine/threonine protein kinase that is involved in the multiple signaling processes of a cell. Increasing evidence suggests that GSK3β plays a key role in multiple cellular processes in the progression of diabetes, obesity, Alzheimer’s disease (AD), Parkinson’s disease (PD), inflammatory diseases, schizophrenia, bipolar and several mood disorders, and mitochondrial diseases. Recent research has found that increased GSK3β activity is linked to the pathogenesis of AD through amyloid beta (Aβ), phosphorylated tau and mitochondrial dysfunction. Recent research has also revealed that GSK3β is elevated in AD-affected tissues and is critically involved in dissociating the voltage-dependent anion channel 1 (VDAC1) protein from hexokinases, and causing disrupted glucose metabolism, mitochondrial dysfunction and activating apoptotic cell death. The purpose of this article is to review recent research that is elucidating the role of GSK3β in AD pathogenesis. We discuss the involvement of GSK3β in the phosphorylation of VDAC1 and dissociation of VADC1 with hexokinases in AD neurons.
Introduction
Alzheimer’s disease (AD) is the most common mental illness, characterized by deficits in cognition and memory, as well as changes in personality and behavior [1]. Currently, 5.4 million American suffer from AD, and this number is expected to increase as elderly individuals live longer [2]. Histopathological examination of postmortem brains from AD patients revealed that extracellular amyloid beta (Aβ) plaques and intracellular neurofibrillary tangles (NFTs) are the major pathological hallmarks of AD [3]. However, these pathological changes occur late in the disease process, and they are unlikely to represent the primary cause of clinical symptoms. Several other morphological and cellular changes have been identified in the etiology of AD, including inflammatory responses, synaptic damage, defects in the cholinergic system, abnormalities in the cell cycle, and mitochondrial structural and functional abnormalities [4–12].
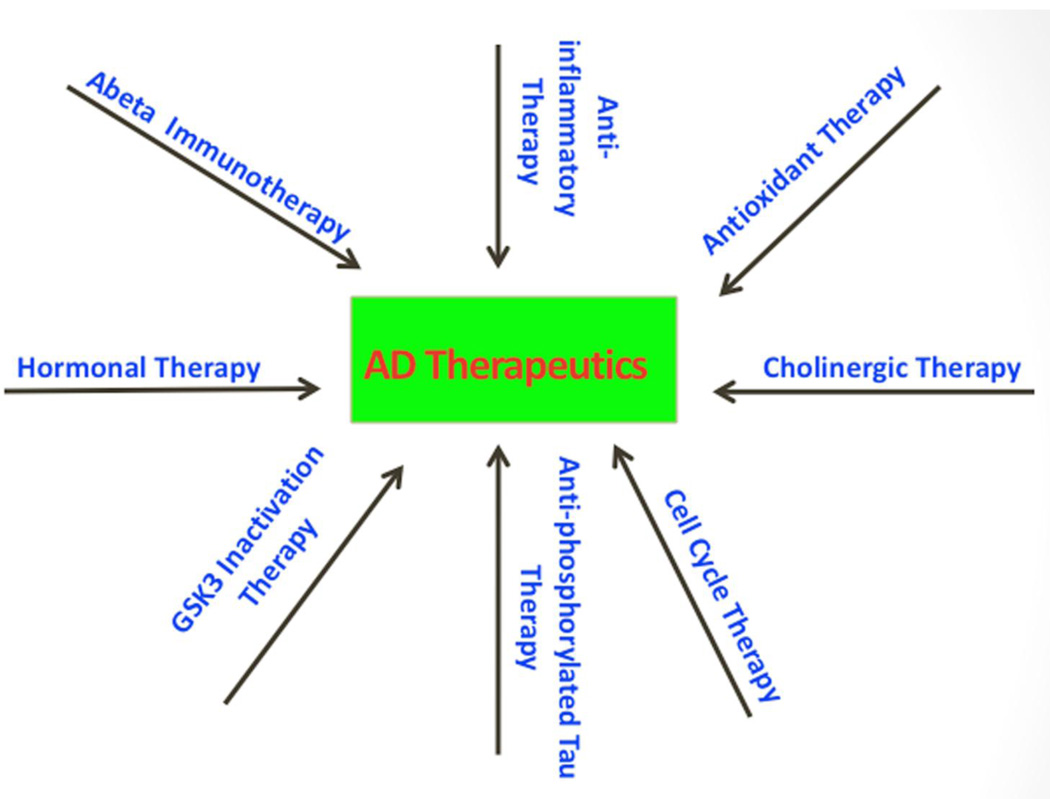
Recent research on glycogen synthase kinase 3 beta (GSK3β) revealed that elevated GSK3β activity is directly linked to increased levels of Aβ production and Aβ deposits, tau hyperphosphorylation, and synaptic damage in AD patients and AD animal models [13–17]. It is possible that elevation of GSK3β activity in AD brains and brain tissues from AD mouse models may occur due to Aβ association with insulin, wnt signaling or NMDA receptors [18]. Based on reported multiple cellular and pathological changes, several therapeutic strategies have been used to test agents and drugs on experimental rodent models, and on AD patients, including: Aβ-immunotherapy [19–21], anti-inflammatory therapy [22–25], antioxidant therapy [26–36], cholinergic therapy [37–44], cell cycle therapy [45–47], hormonal therapy [48–50], and inhibition of GSK3β activity [13–17] (Fig. 1). Although tremendous progress has been made in understanding the AD progression and pathogenesis, and in developing therapeutic strategies, we still not have agents or drugs that can slow or prevent AD progression.
Figure 1.
Therapeutic strategies in Alzheimer’s disease. Based on these cellular and pathological changes, multiple therapeutic strategies have been developed, including Aβ-immunotherapy, anti-phosphorylated tau therapy, anti-inflammatory therapy, antioxidant therapy, cholinergic therapy, cell cycle therapy, hormonal therapy, and inhibition of GSK3β activity. GSK3β is a multiple functional protein and inhibition of GSK3β activity may affect several pathways that are involved in Alzheimer’s disease pathogenesis.
The purpose of this article is to review the latest developments of GSK3β involvement in AD pathogenesis, particularly its association with mitochondria in causing mitochondrial dysfunction and neuronal damage. We also review and discuss GSK3β involvement in phosphorylation of VDAC1 and dissociating VADC1 with hexokinases in AD neurons.
Mitochondrial Dysfunction and Alzheimer’s Disease
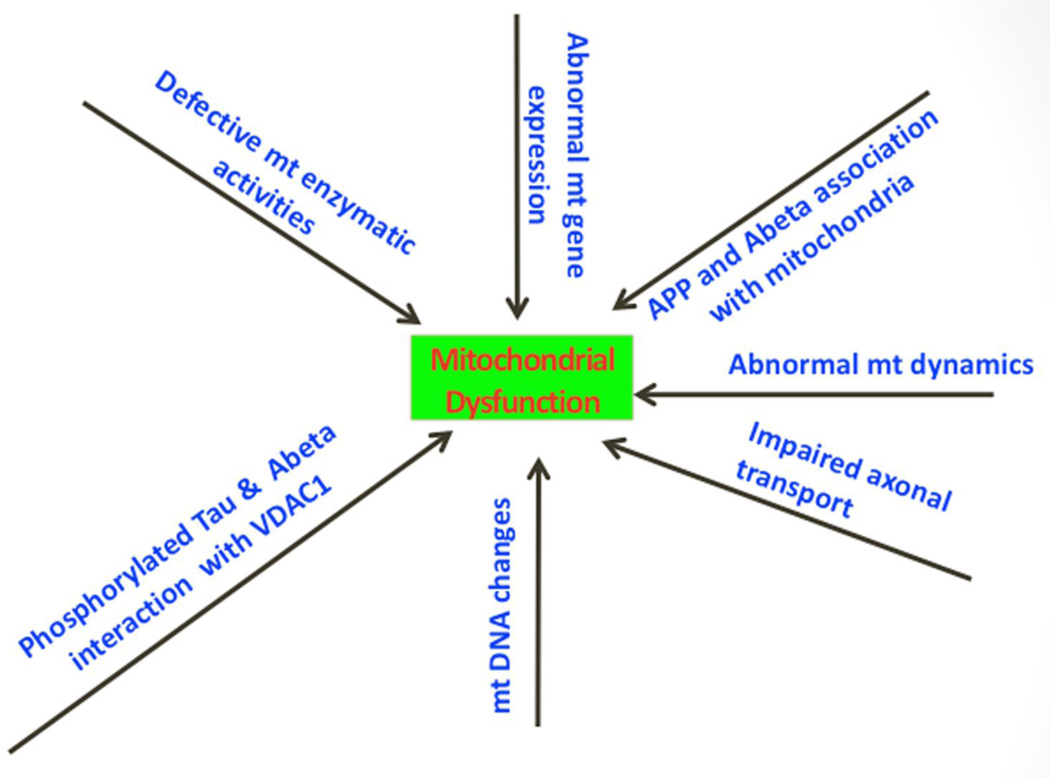
Mitochondrial dysfunction is a prominent and early cellular change in AD pathogenesis, but the precise mechanism underlying this dysfunction is still not completely understood. Mitochondrial abnormalities and oxidative stress have been extensively described in AD pathogenesis [2,11–12] (Fig. 2). Research on mitochondrial function revealed increased free radical production, lipid peroxidation, mitochondrial fission-linked GTPase activity, oxidative DNA, protein damage, and reduced ATP production and cytochrome oxidase activity in postmortem AD-affected brain tissues [51–56]. Further, using biochemical, molecular, gene expression, and electron microscopy studies, and postmortem AD brains and brains from AβPP mice, several studies found Aβ is associated with mitochondrial dysfunction and neuronal damage [51, 57–67]. Recent research also revealed that phosphorylated tau is critically involved in defective axonal transport of mitochondria, synaptic deprivation, oxidative stress, and abnormal mitochondrial dynamics in AD pathogenesis [68–69]. A recent study reported that, in postmortem AD brains and brain tissues from AβPP mice, Aβ (monomers and oligomers) and phosphorylated tau interacted with the mitochondrial outer membrane protein VDAC1 [62], suggesting that Aβ and/or phosphorylated tau may block the transport of organelles between mitochondria and the cytoplasm possibly causing defects in oxidative phosphorylation and mitochondrial ATP synthesis. It is unclear how Aβ and phosphorylated tau each interact with VDAC1, and how these interactions may lead to oxidative phosphorylation defects and the reduction of ATP synthesis in neurons affected by AD.
Figure 2.
Mitochondrial abnormalities in Alzheimer’s disease pathogenesis. Multiple mitochondrial abnormalities have been identified in Alzheimer’s disease pathogenesis, including abnormal mitochondrial gene expression, defective mitochondrial enzymatic activities, accumulation of somatic mitochondrial DNA changes, abnormal mitochondrial dynamics, impaired mitochondrial axonal transport, amyloid bet and amyloid precursor protein association with mitochondria, and amyloid beta and phosphorylated tau interaction with mitochondrial outermembrane protein, VDAC1. Research on mitochondrial function revealed increased free radical production, lipid peroxidation, mitochondrial fission-linked GTPase activity, oxidative DNA, protein damage, and reduced ATP production and cytochrome oxidase activity in postmortem Alzheimer’s disease-affected brain tissues and tissues from Alzheimer’s disease mouse models and peripheral tissues and cell models of Alzheimer’s disease.
VDAC1 and Alzheimer’s Disease
VDAC, which is ubiquitously located in the mitochondrial outer membrane, is generally thought to be the primary means by which metabolites diffuse in and out of mitochondria [70–72]. Three VDAC isoforms (VDAC1, VDAC2, and VDAC3) have been found in mammalian mitochondria. Of these isoforms, VDAC1 is the most widely expressed, followed by VDAC2, and then VDAC3 [73–74]. The relevance of VDAC2 and VDAC3 is minimal for neurodegenerative diseases such as AD, PD and HD because of their low expressions in neurons.
VDAC proteins perform several important functions in the cell, including maintaining synaptic plasticity, mitochondrial permeability transition (MPT) pore; and regulating the shape and structure of mitochondria, hexokinase interactions with mitochondria, and apoptosis signaling [75,76]. The change in mitochondrial permeability that is characteristic of apoptosis is mediated by the Bcl-2 family of proteins, which binds to VDAC and alters channel kinetics and conductance [75]. Recent research also revealed that VDAC is inhibited by the cytoskeletal protein tubulin, resulting in impairments in channel conductance [77]. In addition, several recent studies revealed that VDAC proteins and their binding partners are modified post-translationally due to VDAC phosphorylation and are involved in VDAC dysfunction [78,79]. However, the causal factors of VDA1phosphorylation in AD pathogenesis is not completely understood.
Di Pinto and colleagues [80] studied the role of alpha-helix of VDAC1 in pore activity. They synthesized the human VDAC1 N-terminal peptide Ac-AVPPTYADLGKSARDVFTK-NH2 (Prn2-20) and determined its structure by circular dichromism (CD) and nuclear magnetic resonance spectroscopy. CD studies showed that the Prn2-20 peptide exists in an aqueous solvent as an unstructured peptide without stable secondary structure. No ordered structure was observed in dodecyl beta-maltoside. Differential scanning calorimetric measurements were carried out in order to examine the membrane affinity of VDAC. Upon the interaction with the negatively charged 1,2 dipalmitoyl-sn-glycero-3-phosphoserine membrane, Prn2-20 exhibited distinctive behavior, suggesting that electrostatics may play an important role. Interaction between the peptide and artificial bilayers indicates that lies on the membrane surface. Recombinant HVDAC1 deletion mutants, devoid of N-terminal amino acid 7 or 19, were used to transfect eukaryotic cells. In studies of N-terminal VDAC structure, in which cells were transfected with human VDAC1 lacking amino acid 7 or 19, the over-expression of human VDAC1 increased the number of COS cells with depolarized mitochondria, which progressively reduced. The mitochondrial targeting of the deletion mutants was unaffected. This study concluded that the VDAC N-terminal peptide plays a role in the proper function of VDAC1 during apoptotic events.
Geula and colleagues [81] studied the location and translocation of the VDAC1 N-terminal domain, and its role in voltage-gating and as a target for anti-apoptotic proteins. They used site-directed mutagenesis and cysteine residue substitution, together with a thiol-specific cross-linker, to determine whether the VDAC1 N-terminal region exists in a dynamic equilibrium and is located fully within the pore or exposed outside the β-barrel. Using a single cysteine-residue-bearing VDAC1, they found that the N-terminal region lies within the pore. However, the region can be exposed outside the β-barrel where it dimerizes with the N-terminal domain of a second VDAC1 molecule. When the N-terminal region α-helix structure was perturbed, intra-molecular cross-linking was abolished and dimerization was enhanced. As a result of this structural change, the mutant form of VDAC1 also displayed reduced voltage-gating and reduced binding to hexokinase, but not to the anti-apoptotic proteins Bcl-2 and Bcl-xL. Replacing glycine residues in the N-terminal domain glycine-rich sequence yielded less intra-molecular cross-linked product cut more dimerization, suggesting that the glycine-rich sequence of VDAC1 provides the flexibility needed for N-terminal translocation from the internal pore to the channel face. N-terminal mobility may thus contribute to channel gating and interaction with anti-apoptotic proteins.
To determine the link between VDAC1 and AD, the Reddy research team studied VDAC1 protein levels in cortical tissues from postmortem AD brains at different stages of disease progression (early, definite, and severe) and cortical tissues from 6-, 12-, and 24-month-old AβPP mice. Progressively increased levels of VDAC1 protein were found in the postmortem AD brains relative to the control subjects, and progressively increased levels of VDAC1 were also found in the cerebral cortices of the 6-, 12-, and 24-month-old AβPP mice [62]. To determine the physical interaction between VDAC1 and Aβ, we recently performed co-IP analysis, using the VDAC1 antibody Aβ - 6E10 and immunoblotting analysis and protein lysates of cortical tissues from control subjects; from patients with early, definite, and severe AD; and from APP, APPxPS1, and 3XTg.AD mice. A 4 kDa Aβ was found in VDAC1-IP-elutes from AD patients and from APP, APPxPS1, and 3XTg.AD mice, indicating that Aβ interacts with VDAC1. Mitochondrial functional parameters were found to be defective, including reduced ATP and cytochrome oxidase activity, and levels of lipid peroxidation, free radicals production, and mitochondrial fission-linked GTPase activity were elevated [62].
Thinnes [83] (2011) proposed that the GxxxG motif of the N-terminal of VDAC1 might interact with the GxxxG motif of the C-terminal of Aβ peptide in AD neurons. Thinnes proposed that the GxxxG motifs are aggregation/membrane perturbation motifs and that Aβ, a C-terminal cleaved product from APP by beta-secretase BACE1 and gamma-secretase, may induce AD via apoptosis by opening type-1 porin/VDAC in cell membranes of hypometabolic neuronal cells [83]. Considering the ubiquitous expression nature of APP, beta-and gamma-secretases and VDAC1, apoptosis might play a role in all these proteins/motifs.
These research findings strongly support that the N-terminal VDAC1 is critical for VDAC dimerization, and mitochondrial pore gating activity. These findings also indicate that VDAC1 is elevated in AD progression. It is possible that Aβ and phosphorylated tau are strongly linked with N-terminal VDAC1 and may cause the blockage of mitochondrial pores, which in turn may disrupt the transport of proteins and metabolites between mitochondria and cytoplasm, leading to defects in oxidative phosphorylation, mitochondrial dysfunction, and neuronal damage.
GSK3 Structure and Function
In 1980, GSK3 was discovered as a regulatory kinase. It is encoded by 2 genes: GSK3α, located on chromosome 19, and GSK3β, located on chromosome 2. GSK3 is ubiquitously expressed in raging from yeast to mammals and is recognized as a kinase for a large number of proteins involved in multiple cellular pathways [84]. GSK3 is a serine/threonine protein kinase that mediates the addition of phosphate molecules onto serine and threonine amino acid residues
GSK3α is comprised a molecular mass of 51 kDa, and GSK3β, a molecular mass of 47 kDa. These 2 isoforms are highly homologous at the kinase domain, but are differentiated at the N- and C-terminal regions. GSK3α contains an extended glycine-rich, N-terminal region that may be responsible for cellular localization and interactions with other partners [85] (Fig. 3). The activities of GSK-3α and GSK-3 β are positively regulated by the phosphorylation of Tyr279 and Tyr216, and negatively regulated by the N-terminal phosphorylation of Ser21 and Ser9. Studies of GSK3β knockout mice have revealed that the total absence of GSK3β is embryonically lethal, suggesting that GSK3α may not compensate for the absence of GSK3β [86].
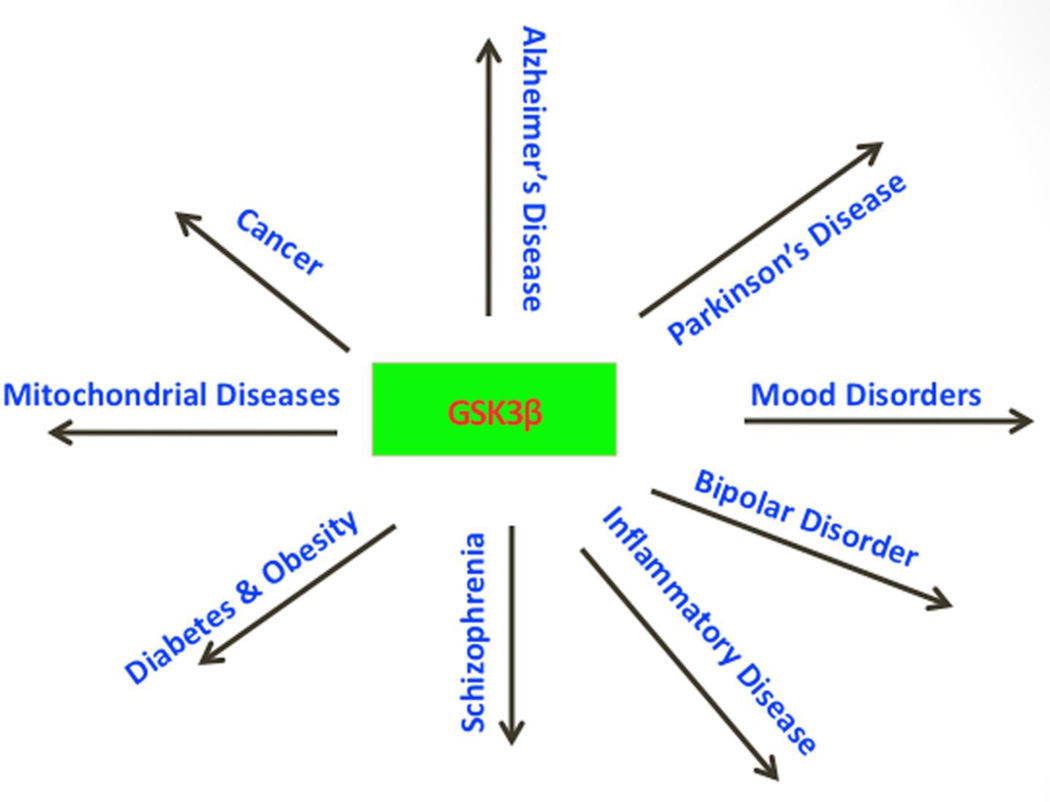
Figure 3.
Human diseases that are associated with glycogen synthase kinase 3.
Increasing evidence suggests that GSK3 dysregulation is implicated in a large number of diseases, including AD [13,16,17,87], PD [88], cancer [89–91], diabetes [92], inflammatory diseases [93], schizophrenia, bipolar and several mood disorders [94], and mitochondrial diseases [95] (Fig. 3). These diseases involve GSK3 activity in normal and disease process. In addition, GSK3 is also involved in the regulation of several cellular pathways, including cell migration [96], Wnt signaling [97], phosphatidylinositol 3-kinase, and neurotrophic pathways in cell survival [98]. Inactivation of GSK3 has been found to lead to cell senescence [99]. Further, increased activation of GSK3β was found to be pro-apoptotic, and its inhibition, anti-apoptotic [100–104]. Inhibition of GSK3β activity is suggested as a therapeutic strategy for several neurodegenerative diseases, including AD and PD.
Production of GSK3β-Mediated Aβ and Phosphorylation of Tau
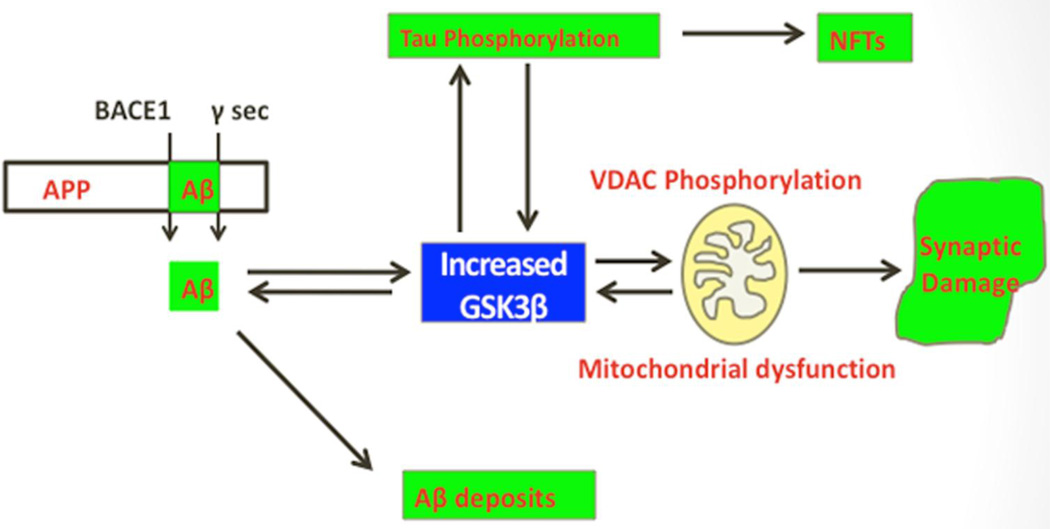
In the recent years, several studies have focused on elucidating the role of GSK3β in AD pathogenesis, mainly because of its known role in causing the phosphorylation of tau and in producing increased levels of Aβ and Aβ deposits in AD brains, AD mouse models, and AD fly models [13–17] (Fig. 4). They found that the inhibition of GSK3β reduce AD pathology. Additional research using AD models found ameliorated cognitive decline AD mice [13–14]. Together, these results suggest that inactivation of GSK3β may be useful as a therapeutic strategy to AD patients.
Figure 4.
Glycogen synthase kinase 3 beta and Alzheimer’s disease pathology. Amyloid beta-induced elevated GSK3β activity is a key event in abnormal APP processing, increased Aβ production, and phosphorylation of tau and synaptic pathology in AD. GSK3β is proposed to activate VDAC1 phosphorylation that ultimately leading to mitochondrial dysfunction and synaptic damage in AD
Hurtado and colleagues [15] studied the roles of GSK3α and GSK3β in AD pathogenesis, using novel viral and genetic approaches. They developed recombinant adeno-associated virus 2/1 short hairpin RNA constructs and injected them intraventricularly into newborn AD transgenic mice of Aβ plaques (PDAPP+ /−), both Aβ plaques and NFTs (PDAPP+ /−; PS19+ /−), or wild-type controls. They found that the reduced expression and the activity of either GSK3α or GSK3β. They also found that the knockdown of GSK3α, but not of GSK3β, resulted in the reduction of senile plaques in PDAPP+ /−, and PS19+ /−, and PDAPP+ /− transgenic mice. Moreover, they found that GSK3α and GSK3β knockdown in combination, reduced the phosphorylation and misfolding of tau in PS19+ /− and PDAPP+ /− mice.
To study the effects of GSK3α reduction on Aβ formation, Hurtado et al. [15] generated triple transgenic mice using the CaMKIIα-Cre (α-calcium/calmodulin-dependent protein kinase II-Cre) system to knockdown GSK3α in PDAPP+ /− mice. GSK3α KD significantly reduced Aβ and ameliorated memory deficits in PDAPP+ /− mice. Their results suggest that GSK3α contributes to both SP and NFT pathogenesis while GSK3β modulates NFT formation, suggesting common but also different targets for both isoforms [15].
In another study, Avrahami and colleagues [105] studied the effects of GSK3α and GSK3β in 5XFAD mice that co-express mutated APP and PS1 and produce massive cerebral Aβ deposits. They found that both GSK3α and GSK3β were hyperactive in this model. Nasal treatment of 5XFAD mice with a novel substrate of competitive GSK3 inhibitor, L803-mts, reduced Aβ deposits and ameliorated cognitive deficits. Studies of 5XFAD brain samples indicated that L803-mts restored the activity of mammalian targets of rapamycin and inhibited autophagy. Lysosomal acidification was impaired in the 5XFAD brains, indicated by reduced cathepsin D activity and decreased N-glycoyslation of the vacuolar ATPase subunit V0a1, a modification required for lysosomal acidification. Treatment with L803-mts restored lysosomal acidification in 5XFAD brains. Studies in SH-SY5Y cells confirmed that GSK3α and GSK3β, in combination, impaired lysosomal acidification and that the treatment with L803-mts enhanced the acidic lysosomal pool as demonstrated in LysoTracker Red-stained cells. Further, L803-mts was found to restore the impairment of lysosomal acidification that was caused by dysfunctional PS1. These researchers provide evidence that mTOR is a target activated by GSK3 but inhibited by impaired lysosomal acidification and elevation in amyloid precursor protein/Aβ loads and inhibition of GSK3 restores lysosomal acidification that in turn enables clearance of Aβ burdens and reactivation of mTOR [105].
Ly and colleagues [106] inhibited GSK3β to determine its protective effects. Using cell and molecular biology methods, they studied AD pathology in cell culture and APP23 transgenic mice. They found that the reduced GSK3β activity is involved in reducing BACE1-mediated cleavage of APP and Aβ production by decreasing BACE1 gene transcription and expression. They also found that the regulation of BACE1 gene expression by GSK3β was dependent on NF-κB signaling. Inhibition of GSK3 signaling markedly reduced Aβ deposits and NFTs, and rescued memory deficits in the APP23 transgenic mice. These data provide evidence that GSK β regulates BACE1 expression and AD pathogenesis, and that the inhibition of GSK3 signaling reduces Aβ neuropathology and alleviates memory deficits in the APP23 mice. These data suggest that interventions that specifically target the β-isoform of GSK3 may be an effective approach for AD patients.
DaRocha-Saouto et al. [13] studied the role of oligomeric assemblies of Aβ in GSK3β activity using primary neuronal cultures and APP/Tau mice. They found that increased activity of GSK3β after exposure to oligomeric Aβ in neurons in culture in the brain of double transgenic APP/tau mice and in AD brains. Activation of GSK3β, even in the absence of Aβ, is sufficient to produce a phenocopy of Aβ-induced dendritic spine loss in neurons in culture, while pharmacological inhibition of GSK3β prevents spine loss and increases expression of CREB-target genes like BDNF. Of note, in transgenic mice GSK3β inhibition ameliorated plaque-related neuritic changes and increased CREB-mediated gene expression. Moreover, GSK3β inhibition robustly decreased the oligomeric Aβ load in the mouse brain. All these findings support the idea that GSK3β is aberrantly activated by the presence of Aβ, and contributes, at least in part, to the neuronal anatomical derangement associated with Aβ plaques in AD brains and to Aβ pathology itself [13].
Leroy and colleagues [107] studied qualitative and quantitative phosphorylation of tau by GSK3β using in vitro assays and NMR spectroscopy. They found that three residues can be phosphorylated (Ser-396, Ser-400, and Ser-404) by GSK3β alone, without priming. Ser-404 is essential in this process, as its mutation to Ala prevents all activity of GSK3β. However, priming enhances the catalytic efficacy of the kinase, as initial phosphorylation of Ser-214 by the cAMP-dependent protein kinase leads to the rapid modification by GSK3β of four regularly spaced additional sites. Because the regular incorporation of negative charges by GSK3β leads to a potential parallel between phospho-Tau and heparin, they investigated its interaction with the heparin/low density lipoprotein receptor binding domain of human apolipoprotein E. They observed an interaction between the GSK3β-promoted regular phospho-pattern on Tau and the apolipoprotein E fragment but none in the absence of phosphorylation or the presence of an irregular phosphorylation pattern by the prolonged activity of cAMP-dependent protein kinase. Apolipoprotein E is therefore able to discriminate and interact with specific phosphorylation patterns of tau.
Using inducible gene expression system to express Arctic mutant Aβ42 specifically in adult neurons in drosophila model, Sofola and colleagues [14] studied GSK3 mediated Aβ42 accumulation. This fly model was used to examine the role of events during adulthood and early AD etiology. Expression of Aβ42 in adult neurons increased GSK3 activity, and inhibition of GSK3 (either genetically or pharmacologically by lithium treatment) rescued Aβ42 toxicity. Aβ42 pathogenesis was also reduced by removal of endogenous fly tau; but, within the limits of detection of available methods, tau phosphorylation did not appear to be altered in flies expressing Aβ42. The GSK3-mediated effects on Aβ42 toxicity appear to be at least in part mediated by tau-independent mechanisms, because the protective effect of lithium alone was greater than that of the removal of tau alone. Finally, Aβ42 levels were reduced upon GSK3 inhibition, pointing to a direct role of GSK3 in the regulation of Aβ42 peptide level, in the absence of APP processing. Their study points to the need both to identify the mechanisms by which GSK3 modulates Aβ42 levels in the fly and to determine if similar mechanisms are present in mammals, and it supports the potential therapeutic use of GSK3 inhibitors in AD.
Overall, these studies indicate that both GSK3α and GSK3β are involved in enhancing Aβ production and tau phosphorylation in AD pathogenesis. However, it is unclear the exact mechanism(s) of Aβ-induced GSK3α and GSK3β increased activities in AD. Further, it is unclear whether Aβ production activates GSK3β activity or vice versa in AD pathogenesis.
AKT, PIK3, GSK3β, and Alzheimer’s Disease
The serine/threonine kinase (Akt), also known as protein kinase B, has become a major focus of research because of its involvement in several cellular processes, including cancer, insulin metabolism, and AD [108]. The three isoforms of Akt – Akt1, Akt2, and Akt3 – are reported to involve in major intracellular signaling pathway that are associated with apoptosis [109]. Akt is known to interact with PIK3 to protect cells against several cellular insults, including oxidative stress and apoptotic cell death. PI3K (or phosphatidylinositide 3-kinases) is a family of enzymes involved in several cellular functions, including cell growth, proliferation, differentiation, motility, survival, and intracellular trafficking. Interestingly, PIK3 and Akt act negatively with GSK3β and protect cells against GSK3β toxicity.
As discussed above, GSK3β is highly expressed in brain tissue. It has been identified as an in vivo substrate of the Akt/PKB pathway. Phosphorylation of the N-terminal serine 9 residue of GSK3β by Akt/PKB is important for the inhibition of GSK3β during insulin-dependent glycogen synthesis and neuronal survival. The regulation of GSK3 by Akt is likely to affect other signaling events where GSK3β is important such as the hyperphosphorylation of tau.
Several studies have reported that increased activity of Akt protects cells against toxic insults of oxidative stress, Aβ, and DNA damage [110–112]. Several cell culture studies demonstrated that Akt and PIK3, in combination, protect against Aβ toxicity [111,113].
Using cell and molecular biology methods, Ryder and Ni [114] studied the Akt/PKB pathway sing kidney cells from AD patients and postmortem brain cells from AD patients. They found expressed the APP mutation and lymphoblast cells expressed the PS1 mutation. They also found reduced levels of Akt/PKB, increased GSK3β activity in AD neurons, and the colocalization of GSK3β and tau, suggesting a possible interaction between Akt/GSK3β and tau in vivo in AD.
Tokutake et al [115] developed a novel cell co-culture system to assess the effects of physiologically relevant levels of extracellular Aβ in donor cells on the phosphorylation of tau in recipient cells. They demonstrated that physiologically relevant levels of secreted Aβ are sufficient to cause hyperphosphorylation of tau in recipient N2a cells expressing human tau and in primary culture neurons. Hyperphosphorylation of tau was inhibited by blocking Aβ production in donor cells. The expression of familial AD-linked PSEN1 mutants and APP ΔE693 mutant that induce the production of oligomeric Aβ in donor cells results in a similar hyperphosphorylation of Tau in recipient cells. The mechanism underlying the Aβ-induced tau hyperphosphorylation is mediated by the impaired insulin signal transduction because we demonstrated that the phosphorylation of Akt and GSK3β upon insulin stimulation is less activated under this condition. Treating cells with the insulin-sensitizing drug rosiglitazone, a peroxisome proliferator-activated receptor γ agonist, attenuates the Aβ-dependent hyperphosphorylation of tau. These findings suggest that the disturbed insulin signaling cascade may be implicated in the pathways through which soluble Aβ induces tau phosphorylation.
Baki et al [116] studied the role of presenilin-1 (PS1) in neuronal PI3K/Akt signaling using primary neuronal cultures from wild-type and PS1 null (PS1−/−) embryonic mouse brains. They found that in PS1−/− cultures, the onset of neuronal maturation coincides with a decrease in the PI3K-dependent phosphorylation-activation of Akt and phosphorylation-inactivation of glycogen synthase kinase-3 (GSK-3). Mature PS1−/− neurons show increased activation of apoptotic caspase-3 and progressive degeneration preceded by dendritic retraction. Expression of exogenous WT PS1 or constitutively active Akt in PS1−/− neurons stimulates PI3K signaling and suppresses both caspase-3 activity and dendrite retraction. The survival effects of PS1 are sensitive to inhibitors of PI3K kinase but insensitive to gamma-secretase inhibitors. Familial AD mutations suppress the ability of PS1 to promote PI3K/AKT signaling, prevent phosphorylation/inactivation of GSK3 and promote activation of caspase-3. These mutation effects are reversed upon coexpression of constitutively active Akt. These data indicate that the neuroprotective role of PS1 depends on its ability to activate the PI3K/Akt signaling pathway and that PS1 FAD mutations increase GSK3 activity and promote neuronal apoptosis by inhibiting the function of PS1. These observations suggest that stimulation of PI3K/Akt signaling may be beneficial to FAD patients.
Overall, these studies suggest that familial AD mutations may suppress the PI3K/Akt signaling pathway, which may in turn activate GSK3β activity, resulting neuronal damage in the neurons from AD patients.
GSK3β and Its Link with VDAC1 and Hexokinases
GSK3β is associated with mitochondrial dysfunction via the phosphorylation of VDAC1 in AD patients. GSK3β hyperactivity has been linked to Aβ production, Aβ deposits, hyperphosphorylated tau, and NFT formation [84,117]. GSK3β phosphorylates VDAC1 on threonine 51, resulting in the detachment of hexokinase from VDAC1 [118]. There are no published reports on VDAC2 and VDAC3 and their associations with GSK3 phosphorylation, this may be because of low levels of VDAC2 and VDAC3 expressions in the brain and other tissues of mammals including rodents, humans and nonhuman primates. Further research is needed to understand phosphorylation by GSK3β of VDAC2 or VDAC3 in neurodegenerative diseases such as AD, PD and HD and other mitochondrial diseases.
Pastorino et al. [118] reported that Akt mediates the binding of hexokinase 2 to mitochondria by negatively regulating the activity of GSK3β. On inhibition of Akt, GSK3β is activated and phosphorylates VDAC. Hexokinase 2 is unable to bind to GSK3b-phosphorylated VDAC1, resulting in the dissociation of hexokinase 2 from the mitochondria. The inhibition of Akt potentiates chemotherapy-induced cytotoxicity, an effect that is dependent on GSK3β activation and its ability to disrupt the binding of hexokinase 2 to mitochondria [118] (Fig. 5).
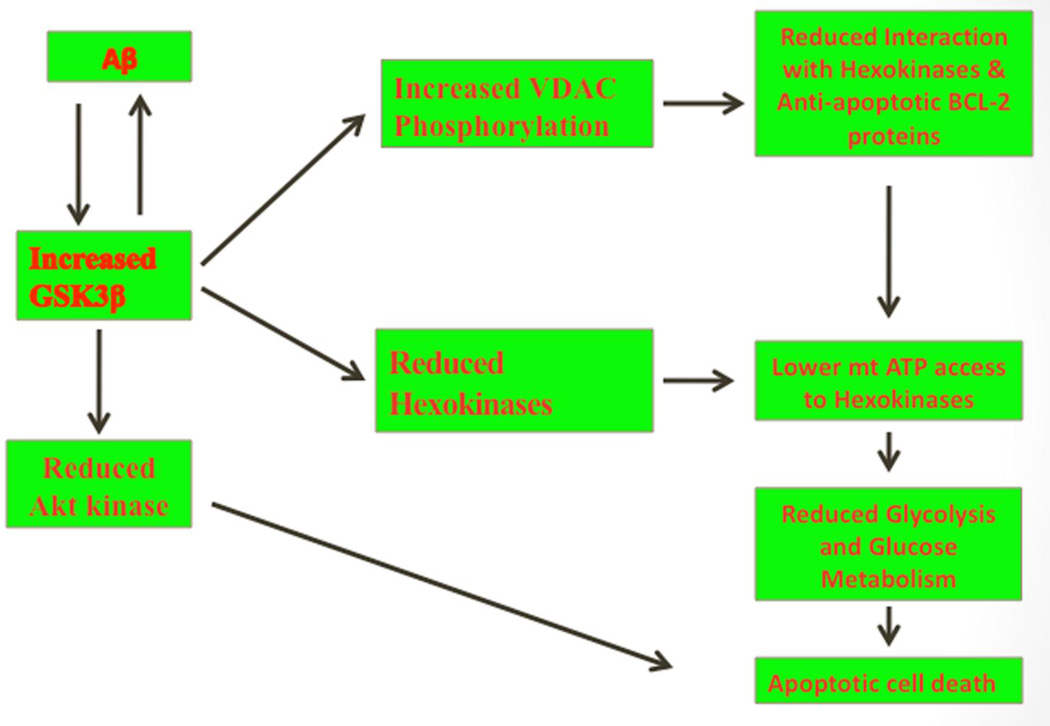
Figure 5.
A proposed model of amyloid beta-induced GSK3β and mitochondrial dysfunction in Alzheimer’s disease. Increasing evidence suggests that in Alzheimer’s disease pathogenesis, elevated GSK3β activity is a key event in abnormal APP processing, increased Aβ production, and hyperphosphorylation of tau. Amyloid beta-induced GSK3β is associated with mitochondrial dysfunction via the phosphorylation of VDAC1 and dissociation of heoxokinases with VDAC1 in Alzheimer’s disease. Elevated GSK3β further activate abnormal APP processing, leading to increased Aβ production, and hyperphosphorylation of tau, like vicious cycle. Further, GSK3β phosphorylates VDAC1 on threonine 51, resulting in the detachment of hexokinase from VDAC1 and anti-apoptotic Bcl2 family of proteins. The detached heoxikinase from mitochondria, may not be able to have access to mitochondria ATP and may not able to supply necessary ATP to glycolysis and glucose metabolism. Further, elevated GSK3β suppress Akt kinase, reduced Akt kinase may not prevent apoptotic cell death in Alzheimer’s disease neurons.
Given the capacity of GSK3β to phosphorylate VDAC1 in AD, VDAC1 might be phosphorylated on the putative GSK3β epitope in AD. It is possible that phosphorylated VDAC1 might result in the inability of hexokinase to interact with VDAC1. Increasing evidence also suggests that in AD pathogenesis, elevated GSK3β activity is a key event in abnormal APP processing, increased Aβ production, and hyperphosphorylation of tau [119,120]. However, the molecular interactions among Aβ, GSK3β, and VDAC1, and among phosphorylated tau, GSK3β, and VDAC1 in AD progression are unclear. Research is needed to understand molecular links among Aβ, phosphorylated tau, GSK3β, andVDAC1 phosphorylation. Additional research is also needed to elucidate how increased activity of GSK3β results in the reduction of hexokinase and the subsequent detachment of hexokinases from VDAC1 in AD neurons.
Hexokinase is a glycolytic enzyme that catalyzes the transfer of a high-energy phosphate group to a hexose the initial step in the cellular utilization of free hexoses in a glycolytic pathway. Hexokinases are expressed in various tissues, including the brain and liver, and hexokinase 1 is highly expressed in the brain [121]. Hexokinase consumes ATP in order to phosphorylate glucose in the glycolysis. Several studies have shown that VDAC1 interacts not only with hexokinase isoforms 1 and 2 but also with the Bcl2 family of proteins [85,118,122–126]. Further, hexokinase 1 and 2 have been found to bind to mitochondria via VDAC [85,118,122–126]. The binding of hexokinase with VDAC allows the direct access of hexokinase to mitochondrial ATP in the glycolytic pathway. Recent studies also revealed that hexokinase inhibits apoptosis by binding to VDAC and preventing the release of cytochrome c [85,123,126].
Several studies found increased VDAC1 in AD postmortem brains, APP mice, and cells treated with Aβ [62,127–128], suggesting that this elevation of VDAC1 may be because of Aβ-induction in AD process As discussed above, Aβ-induced GSK3β levels were found to be markedly increased in AD brains, AD mouse models, and cells treated with Aβ, and increased levels of Aβ-induced VDAC1 phosphorylation, reduced hexokinase, and leading to reduced VDAC1-hexokinase interaction. This reduced VDAC1-hexokinase interaction may in turn lead to low ATP availability in mitochondria, resulting in reduced glucose metabolism and uninhibited apoptosis in AD neurons. Based on PET scan and functional MRI studies, increasing evidence suggests that reduced glycolysis/glucose metabolism in the brains of AD patients [129–131]. It is possible that Aβ-induced elevated GSK3β activity progressively detach hexokinases from VDACs in AD neurons, leading to reduced glycolysis/glucose metabolism selectively in affected regions of the brains AD patients. However, further research is needed to confirm this notion.
Wang et al [132] studied neuroprotective effects of hesperidin, a bioactive flavonoid compound, on Aβ25-35-induced neurotoxicity in PC12 cells. They found that the hesperidin significantly inhibited Aβ25-35-induced apoptosis by reversing Aβ-induced mitochondrial dysfunction, including the mitochondrial permeability transition pore opening, intracellular free calcium increase and reactive oxygen species production. They also found reduced levels of hexokinase and increased GSK3β in Aβ25–35 peptide treated cells. However, in the hesperidin-pretreated cells, hexokinase levels were either normal or similar to the hexokinase levels in untreated Aβ25–35 cells. They also found that hesperidin activated Akt and inhibited GSK3β in cells pretreated with Aβ25–35. Their observations suggest a mechanistic link between GSK3β activation and mitochondrial damage in PC12 cells treated with Aβ25–35 [129].
Using proteomic analysis, western blotting, and immunohistochemical techniques, Cuadrado-Tejedor et al. [128] studied VDAC1 in AD postmortem brains, APP mice, and Aβ cell cultures. They found VDAC1 overexpressed in the hippocampus from AD transgenic mice models and overexpressed in postmortem brain tissues from AD patients at an advanced stage of AD. Interestingly, Aβ soluble oligomers induced the upregulation of VDAC1 in a human neuroblastoma cell line, supporting a correlation between Aβ levels and VDAC1 expression. In hippocampal extracts from transgenic mice, a significant increase in VDAC1 was observed. The levels of hexokinase I, which interacts with VDAC1 and affects its function, were decreased in mitochondrial samples from the APP mice. Elevated VDAC1 phosphorylation and reduced mitochondrial hexokinase levels may facilitate the release of proapoptotic factors, including bcl and bax, leading to defective function of VDAC channel in AD neurons.
Overall, these studies suggest that an Aβ-induced increase in GSK3β may be responsible for the inhibition of VDAC-hexokinase interactions. The inhibited interaction between VDAC1 and hexokinase may lead to low access of mitochondrial ATP to hexokinases in the glycolytic pathway in AD neurons. The mechanistic link between Aβ-induced VDAC1 phosphorylation and impaired interaction between VDAC1 and hexokinase in D neurons is not well understood, and it is still not clear whether the activation of AKT and/or the inhibition of GSK3β enhances hexokinases association with VDAC1 in AD neurons. Additional research is needed to address these issues.
Conclusions and Future Studies
Mounting evidence suggests that mitochondrial dysfunction and oxidative stress are involved in AD progression and pathogenesis. Further, recent research on AD postmortem brains and brain tissues from AβPP transgenic mice revealed that VDAC1 is increased in AD-affected brain tissues from AβPP transgenic mice. Recent research also revealed that VDAC1 interacts with Aβ and phosphorylated tau and that these interactions progressively increased with disease progression. The abnormal VDAC1 interaction with Aβ and phosphorylated tau ultimately leads to the blockage of MPT pores and a disruption in the transport of proteins and metabolites between mitochondria and the rest of cell
GSK3β activity was found to be elevated in AD postmortem brains and AD transgenic mice. This increase in GSK3β activity has been linked to Aβ production, Aβ deposits, hyperphosphorylated tau, and NFT formation. GSK3β is associated with mitochondrial dysfunction via VDAC1 phosphorylation in AD patients. GSK3β phosphorylates VDAC1 on threonine 51, resulting in the detachment of hexokinase from VDAC1. Recent research also revealed that Akt mediates the binding of hexokinase 2 to the mitochondria by negatively regulating the activity of GSK3β in the disease process.
To better understand the molecular bases among Aβ/phosphorylated tau, VDAC1, GSK3β, and hexokinase in AD pathogenesis, the following questions need to be addressed. (1) How do Aβ and phosphorylated tau each interact with VDAC1, and do these interactions lead to oxidative phosphorylation defects and the reduction of ATP synthesis in neurons affected by AD? (2) What are causal factors of VDAC1 phosphorylation in AD? (3) What is the mechanistic link between VDAC1 and Aβ, and what is the effect of the VDAC1-Aβ relationship on mitochondrial phenotypes, function, and neuronal damage? And (4) What is the mechanistic link between Aβ-induced VDAC1 phosphorylation and the consequent, impaired interaction between VDAC1 and hexokinase in AD?
Addressing these questions will improve our basic understanding of the AD process and may provide important information that can be used in the development of therapeutic strategies to treat AD patients.
Research Highlights.
GSK3 is a multifunctional serine/threonine protein kinase.
GSK3βplays a key role in multiple cellular processes in Alzheimer’s disease.
Aβ-induced GSK3βphosphorylates VDAC1, cause mitochondrial dysfunction.
The detached hexokinases from VDAC do not have access to ATP.
Acknowledgements
This research was supported by NIH grants AG028072, AG042178, and RR000163, and a grant from the Medical Research Foundation of Oregon.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Selkoe DJ. Alzheimer's disease: genes, proteins, and therapy. Physiol. Rev. 2001;81:741–766. doi: 10.1152/physrev.2001.81.2.741. [DOI] [PubMed] [Google Scholar]
- 2.Alzheimer Association. Alzheimer’s disease: Facts and Figures. 2013 [Google Scholar]
- 3.Reddy PH, Tripathi R, Troung Q, Tirumala K, Reddy TP, Anekonda V, Shirendeb UP, Calkins MJ, Reddy AP, Mao P, Manczak M M. Abnormal mitochondrial dynamics and synaptic degeneration as early events in Alzheimer's disease: Implications to mitochondria-targeted antioxidant therapeutics. Biochim Biophys Acta. 2012;1822:639–649. doi: 10.1016/j.bbadis.2011.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.LaFerla FM, Green KN, Oddo S. Intracellular amyloid-beta in Alzheimer's disease. Nat Rev Neurosci. 2007:499–509. doi: 10.1038/nrn2168. [DOI] [PubMed] [Google Scholar]
- 5.Mattson MP. Pathways towards and away from Alzheimer's disease. Nature. 2004;430:631–639. doi: 10.1038/nature02621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reddy PH, Beal MF. Are mitochondria critical in the pathogenesis of Alzheimer's disease? Brain Res Brain Res Rev. 2005;49:618–632. doi: 10.1016/j.brainresrev.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 7.Reddy PH, Manczak M, Mao P, Calkins MJ, Reddy AP, Shirendeb U U. Amyloid-beta and mitochondria in aging and Alzheimer's disease: implications for synaptic damage and cognitive decline. J Alzheimers Dis. 2010;20:S499–S512. doi: 10.3233/JAD-2010-100504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reddy PH. Mitochondrial dysfunction in aging and Alzheimer's disease: strategies to protect neurons. Antioxid Redox Signal. 2007;9:1647–1658. doi: 10.1089/ars.2007.1754. [DOI] [PubMed] [Google Scholar]
- 9.Reddy PH, MF Beal MF. Amyloid beta, mitochondrial dysfunction and synaptic damage: implications for cognitive decline in aging and Alzheimer's disease. Trends. Mol. Med. 2008;14:45–53. doi: 10.1016/j.molmed.2007.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Du H, Guo L, YaN SS. Synaptic mitochondrial pathology in Alzheimer's disease. Antioxid Redox Signal. 2012 Jun 15;16(12):1467–75. doi: 10.1089/ars.2011.4277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Swerdlow RH. Brain aging, Alzheimer's disease, and mitochondria. Biochim. Biophys. Acta. 2011;1812:1630–1639. doi: 10.1016/j.bbadis.2011.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhu X, Perry G, Smith MA, Wang X. Abnormal Mitochondrial Dynamics in the Pathogenesis of Alzheimer's Disease. J. Alzheimers. Dis. 2013;33(Suppl 1):S253–S262. doi: 10.3233/JAD-2012-129005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.DaRocha-Souto B, Coma M, Pérez-Nievas BG, Scotton TC, Siao M, Sánchez-Ferrer P, Hashimoto T, Fan Z, Hudry E, Barroeta I, Serenó L, Rodríguez M, Sánchez MB, Hyman BT, Gómez-Isla T. Activation of glycogen synthase kinase-3 beta mediates β-amyloid induced neuritic damage in Alzheimer's disease. Neurobiol Dis. 2012;45:425–437. doi: 10.1016/j.nbd.2011.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sofola O, Kerr F, Rogers I, Killick R, Augustin H, Gandy C, Allen MJ, Hardy J, Lovestone S, Partridge L. Inhibition of GSK-3 ameliorates Abeta pathology in an adult-onset Drosophila model of Alzheimer's disease. PLoS Genet. 2012;6 doi: 10.1371/journal.pgen.1001087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hurtado DE, Molina-Porcel L, Carroll JC, Macdonald C, Aboagye AK, Trojanowski JQ, Lee VM. Selectively silencing GSK-3 isoforms reduces plaques and tangles in mouse models of Alzheimer's disease. J. Neurosci. 2012;32:7392–7402. doi: 10.1523/JNEUROSCI.0889-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Martinez A, Perez DJ. GSK-3 inhibitors: a ray of hope for the treatment of Alzheimer's disease? J Alzheimers Dis. 2008;15:181–191. doi: 10.3233/jad-2008-15204. [DOI] [PubMed] [Google Scholar]
- 17.Kremer A, Louis JV, Jaworski T, Van Leuven F. GSK3 and Alzheimer's Disease: Facts and Fiction…. Front Mol Neurosci. 2011;4:17. doi: 10.3389/fnmol.2011.00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Inestrosa NV, Arenas E. Emerging roles of Wnts in the adult nervous system. Nat Rev Neurosci. 2010;11:77–86. doi: 10.1038/nrn2755. [DOI] [PubMed] [Google Scholar]
- 19.Schenk D, Barbour R, Dunn W, Gordon G, Grajeda H, Guido T, Hu K, Huang J, Johnson-Wood K, Khan K, Kholodenko D, Lee M, Liao Z, Lieberburg I, Motter R, Mutter L, Soriano F, Shopp G, Vasquez N, Vandevert C, Walker S, Wogulis M, Yednock T, Games D, Seubert P. Immunization with amyloid-beta attenuates Alzheimer-disease-like pathology in the PDAPP mouse. Nature. 1999;400:173–177. doi: 10.1038/22124. [DOI] [PubMed] [Google Scholar]
- 20.Morgan D, Diamond DM, Gottschall PE, Ugen KE, Dickey C, Hardy J, Duff K, Jantzen P, DiCarlo G, Wilcock D, Connor K, Hatcher J, Hope C, Gordon M, Arendash GW. A beta peptide vaccination prevents memory loss in an animal model of Alzheimer’s disease. Nature. 2000;408:982–985. doi: 10.1038/35050116. [DOI] [PubMed] [Google Scholar]
- 21.DeMattos RB, Bales KR, Cummins DJ, Dodart JC, Paul SM, Holtzman DM. Peripheral anti-A beta antibody alters CNS and plasma A beta clearance and decreases brain A beta burden in a mouse model of Alzheimer’s disease. Proc. Natl. Acad. Sci. U S A. 2001;98:8850–8855. doi: 10.1073/pnas.151261398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McGeer PL, McGeer EG. The inflammatory response system of brain: implications for therapy of Alzheimer and other neurodegenerative diseases. Brain. Res. Brain Res. Rev. 1995;21:195–218. doi: 10.1016/0165-0173(95)00011-9. [DOI] [PubMed] [Google Scholar]
- 23.Launer LJ. Nonsteroidal anti-inflammatory drugs and Alzheimer disease: what’s next? JAMA. 2003;289:2865–2867. doi: 10.1001/jama.289.21.2865. [DOI] [PubMed] [Google Scholar]
- 24.Gasparini L, Ongini E, Wenk G. Non-steroidal anti-inflammatory drugs (NSAIDs) in Alzheimer’s disease: old and new mechanisms of action. J. Neurochem. 2004;91:521–536. doi: 10.1111/j.1471-4159.2004.02743.x. [DOI] [PubMed] [Google Scholar]
- 25.Gasparini L, Ongini E, Wilcock D, Morgan D. Activity of flurbiprofen and chemically related anti-inflammatory drugs in models of Alzheimer’s disease. Brain. Res. Brain Res. Rev. 2005;48:400–408. doi: 10.1016/j.brainresrev.2004.12.029. [DOI] [PubMed] [Google Scholar]
- 26.Nakashima H, Ishihara T, Yokota O O, et al. Effects of alphatocopherol on an animal model of tauopathies. Free. Rad. Biol. Med. 2004;37:176–186. doi: 10.1016/j.freeradbiomed.2004.04.037. [DOI] [PubMed] [Google Scholar]
- 27.Conte V, Uryu K, Fujimoto S, Yao Y, Rokach J, Longhi L, Trojanowski JQ, Lee VM, McIntosh TK, Praticò D. Vitamin E reduces amyloidosis and improves cognitive function in Tg2576 mice following repetitive concussive brain injury. J. Neurochem. 2004;90:758–764. doi: 10.1111/j.1471-4159.2004.02560.x. [DOI] [PubMed] [Google Scholar]
- 28.Sung S, Yao Y, Uryu K, Yang H, Lee VM, Trojanowski JQ, Pratico D. Early vitamin E supplementation in young but not aged mice reduces Abeta levels and amyloid deposition in a transgenic model of Alzheimer’s disease. FASEB J. 2004;18:323–325. doi: 10.1096/fj.03-0961fje. [DOI] [PubMed] [Google Scholar]
- 29.Matsubara E, Bryant-Thomas T, Pacheco Quinto J, et al. Melatonin increases survival and inhibits oxidative and amyloid pathology in a transgenic model of Alzheimer’s disease. J. Neurochem. 2003;85:1101–1108. doi: 10.1046/j.1471-4159.2003.01654.x. [DOI] [PubMed] [Google Scholar]
- 30.Stackman RW, Eckenstein F, Frei B, Kulhanek D, Nowlin J, Quinn JF. Prevention of agerelated spatial memory deficits in a transgenic mouse model of Alzheimer’s disease by chronic Ginkgo biloba treatment. Exp. Neurol. 2003;184:510–520. doi: 10.1016/s0014-4886(03)00399-6. [DOI] [PubMed] [Google Scholar]
- 31.Yang F, Lim GP, Begum AN, Ubeda OJ, Simmons MR, Ambegaokar SS, Chen PP, Kayed R, Glabe CG, Frautschy SA, Cole GM. Curcumin inhibits formation of amyloid beta oligomers and fibrils, binds plaques, and reduces amyloid in vivo. J. Biol. Chem. 2005;280:5892–5901. doi: 10.1074/jbc.M404751200. [DOI] [PubMed] [Google Scholar]
- 32.Morris MC, Beckett LA, Scherr PA, Hebert LE, Bennett DA, Field TS, Evans DA. Vitamin E and vitamin C supplement use and risk of incident Alzheimer disease. Alzheimer Disease and Associated Disorders. 1998;12:121–126. doi: 10.1097/00002093-199809000-00001. [DOI] [PubMed] [Google Scholar]
- 33.Morris MC, Evans DA, Bienias JL, Tangney CC, Bennett DA, Aggarwal N, Wilson RS, Scherr PA. Dietary intake of antioxidant nutrients and the risk of incident Alzheimer disease in a biracial community study. JAMA. 2002;287:3230–3237. doi: 10.1001/jama.287.24.3230. [DOI] [PubMed] [Google Scholar]
- 34.Morris MC, Evans DA, Tangney CC, Bienias JL, Wilson RS, Aggarwal NT, Scherr PA. Relation of the tocopherol forms to incident Alzheimer disease and to cognitive change. Am. J. Clin Nut. 2005;81:508–514. doi: 10.1093/ajcn.81.2.508. [DOI] [PubMed] [Google Scholar]
- 35.Grundman M, Petersen RC, Ferris SH, Thomas RG, Aisen PS, Bennett DA, Foster NL, Jack CR, Jr, Galasko DR, Doody R, Kaye J, Sano M, Mohs R, Gauthier S, Kim HT, Jin S, Schultz AN, Schafer K, Mulnard R, van Dyck CH, Mintzer J, Zamrini EY, Cahn-Weiner D, Thal LJ. Mild cognitive impairment can be distinguished from Alzheimer disease and normal aging for clinical trials. Arch. Neurol. 2004;61:59–66. doi: 10.1001/archneur.61.1.59. [DOI] [PubMed] [Google Scholar]
- 36.Zandi PP, Anthony JC, Khachaturian S, Stone SV, Gustafson D, Tschanz JT, Norton MC, Welsh-Bohmer KA, Breitner JC. Reduced risk of Alzheimer disease in users of antioxidant vitamin supplements: the Cache County Study. Arch. Neurol. 2004;61:82–88. doi: 10.1001/archneur.61.1.82. [DOI] [PubMed] [Google Scholar]
- 37.Clarke NA, Francis PT. Cholinergic and glutamatergic drugs in Alzheimer’s disease therapy. Exp. Rev. Neurothe. 2005;5:671–682. doi: 10.1586/14737175.5.5.671. [DOI] [PubMed] [Google Scholar]
- 38.Racchi M, Mazzucchelli M, Lenzken SC, Porrello E, Lanni C, Govoni S. Role of acetylcholinesterase inhibitors in the regulation of amyloid beta precursor protein (AbetaPP) metabolism. Chemico-Biol. Interc. 2005;157:335–338. doi: 10.1016/j.cbi.2005.10.099. [DOI] [PubMed] [Google Scholar]
- 39.Kaduszkiewicz H, Zimmermann T, Beck-Bornholdt HP, van den Bussche H. Cholinesterase inhibitors for patients with Alzheimer’s disease: systematic review of randomised clinical trials. B. Med. J. 331(331):321–327. doi: 10.1136/bmj.331.7512.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Greig NH, Utsuki T, Ingram DK, Wang Y, Pepeu G, Scali C, Yu QS, Mamczarz J, Holloway HW, Giordano T, Chen D, Furukawa K, Sambamurti K, Brossi A, Lahiri DK. Selective butyrylcholinesterase inhibition elevates brain acetylcholine, augments learning and lowers Alzheimer beta-amyloid peptide in rodent. Proc. Natl. Acad. Sci. U S A. 2005;102:17213–17218. doi: 10.1073/pnas.0508575102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ellis JM. Cholinesterase inhibitors in the treatment of dementia. J. Am. Osteo. Asso. 2005;105:145–158. [PubMed] [Google Scholar]
- 42.Zimmermann M, Borroni B, Cattabeni F, Padovani A, Di Luca M M. Cholinesterase inhibitors influence APP metabolism in Alzheimer disease patients. Neurobiol. Dis. 2005;19:237–242. doi: 10.1016/j.nbd.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 43.Wilkinson DG, Francis PT, Schwam E, Payne-Parrish J. Cholinesterase inhibitors used in the treatment of Alzheimer’s disease: the relationship between pharmacological effects and clinical efficacy. Drugs & Aging. 2004;21:453–478. doi: 10.2165/00002512-200421070-00004. [DOI] [PubMed] [Google Scholar]
- 44.Lahiri DK, Rogers JT, Greig NH, Sambamurti K. Rationale for the development of cholinesterase inhibitors as anti-Alzheimer agents. Cur. Pharma. Des. 2004;10:3111–3119. doi: 10.2174/1381612043383331. [DOI] [PubMed] [Google Scholar]
- 45.Zhu X, Raina AK, Perry G, Smith MA. Alzheimer’s disease: the two-hit hypothesis. Lancet Neurology. 2004;3:219–226. doi: 10.1016/S1474-4422(04)00707-0. [DOI] [PubMed] [Google Scholar]
- 46.Herrup K, Neve R, Ackerman SL, Copani A. Divide and die: cell cycle events as triggers of nerve cell death. J. Neurosci. 2004;24:9232–9239. doi: 10.1523/JNEUROSCI.3347-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Neve RL, McPhie DL. The cell cycle as a therapeutic target for Alzheimer’s disease. Pharmacology & Therapeutics. 2006;111:99–113. doi: 10.1016/j.pharmthera.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 48.Tang MX, Jacobs D, Stern Y, Marder K, Schofield P, Gurland B, Andrews H, Mayeux R. Effect of oestrogen during menopause on risk and age at onset of Alzheimer’s disease. Lancet. 1996;348:429–432. doi: 10.1016/S0140-6736(96)03356-9. [DOI] [PubMed] [Google Scholar]
- 49.Marlatt MW, Webber KM, Moreira PI, Lee HG, Casadesus G, Honda K, Zhu X, Perry G, Smith MA. Therapeutic opportunities in Alzheimer disease: one for all or all for one? Cur. Med. Chem. 2005;12:1137–1147. doi: 10.2174/0929867053764644. [DOI] [PubMed] [Google Scholar]
- 50.Casadesus G, Zhu X, Atwood CS, Webber KM, Perry G, Bowen RL, Smith MA. Beyond estrogen: targeting gonadotropin hormones in the treatment of Alzheimer’s disease. Cur. Drug Targets. CNS and Neurol. Dis. 2004;3:281–285. doi: 10.2174/1568007043337265. [DOI] [PubMed] [Google Scholar]
- 51.Devi L, Prabhu BM, Galati DF, Avadhani NG, Anandatheerthavarada HK. Accumulation of amyloid precursor protein in the mitochondrial import channels of human Alzheimer's disease brain is associated with mitochondrial dysfunction. J Neurosci. 2006;26:9057–9068. doi: 10.1523/JNEUROSCI.1469-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jr. Parker WD, Filley CM, Parks JK. Cytochrome oxidase deficiency in Alzheimer's disease. Neurology. 1990;40:1302–1303. doi: 10.1212/wnl.40.8.1302. [DOI] [PubMed] [Google Scholar]
- 53.Maurer I, Zierz S, Möller HJ. A selective defect of cytochrome c oxidase is present in brain of Alzheimer disease patients. Neurobiol. Aging. 2000;21:455–462. doi: 10.1016/s0197-4580(00)00112-3. [DOI] [PubMed] [Google Scholar]
- 54.Smith MA, Perry G, Richey PL, Sayre LM, Anderson VE, Beal MF, Kowall N. Oxidative damage in Alzheimer's. Nature. 1996;382:120–121. doi: 10.1038/382120b0. [DOI] [PubMed] [Google Scholar]
- 55.Wang J, Xiong S, Xie C, Markesbery WR, Lovell MA. Increased oxidative damage in nuclear and mitochondrial DNA in Alzheimer's disease. J. Neurochem. 2005;93:953–962. doi: 10.1111/j.1471-4159.2005.03053.x. [DOI] [PubMed] [Google Scholar]
- 56.Butterfield DA, Sultana R. Redox proteomics identification of oxidatively modified brain proteins in Alzheimer's disease and mild cognitive impairment: insights into the progression of this dementing disorder. J. Alzheimers. Dis. 2007;12:61–72. doi: 10.3233/jad-2007-12107. [DOI] [PubMed] [Google Scholar]
- 57.Reddy PH, McWeeney S, Park BS, Manczak M, Gutala RV, Partovi D, Jung Y, Yau V, Searles R, Mori M, Quinn J. Gene expression profiles of transcripts in amyloid precursor protein transgenic mice: up-regulation of mitochondrial metabolism and apoptotic genes is an early cellular change in Alzheimer's disease. Hum. Mol. Genet. 2004;13:1225–1240. doi: 10.1093/hmg/ddh140. [DOI] [PubMed] [Google Scholar]
- 58.Manczak M, Park BS, Jung Y, Reddy PH. Differential expression of oxidative phosphorylation genes in patients with Alzheimer's disease: implications for early mitochondrial dysfunction and oxidative damage. Neuromolecular Med. 2004;5:147–162. doi: 10.1385/NMM:5:2:147. [DOI] [PubMed] [Google Scholar]
- 59.Manczak M, Anekonda TS, Henson E, Park BS, Quinn J, Reddy PH. Mitochondria are a direct site of A beta accumulation in Alzheimer's disease neurons: implications for free radical generation and oxidative damage in disease progression. Hum. Mol. Genet. 2006;15:1437–1449. doi: 10.1093/hmg/ddl066. [DOI] [PubMed] [Google Scholar]
- 60.Manczak M, Mao P, Calkins MJ, Cornea A, Reddy AP, Murphy MP, Szeto HH, Park B, Reddy PH. Mitochondria-targeted antioxidants protect against amyloid-beta toxicity in Alzheimer's disease neurons. J Alzheimers Dis. 2010;20(Suppl 2):S609–S631. doi: 10.3233/JAD-2010-100564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Manczak M, Calkins MJ, Reddy PH. Impaired mitochondrial dynamics and abnormal interaction of amyloid beta with mitochondrial protein Drp1 in neurons from patients with Alzheimer's disease: implications for neuronal damage. Hum. Mol. Genet. 2011;20:2495–2509. doi: 10.1093/hmg/ddr139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Manczak M, Reddy PH. Abnormal interaction of VDAC1 with amyloid beta and phosphorylated tau causes mitochondrial dysfunction in Alzheimer's disease. Hum. Mol. Genet. 2012;21:5131–5146. doi: 10.1093/hmg/dds360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yao J, Irwin RW, Zhao L, Nilsen J, Hamilton RT, Brinton RD. Mitochondrial bioenergetic deficit precedes Alzheimer's pathology in female mouse model of Alzheimer's disease. Proc Natl Acad Sci USA. 2009;106:14670–14675. doi: 10.1073/pnas.0903563106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Caspersen C, Wang N, Yao J, Sosunov A, Chen X, Lustbader JW, Xu HW, Stern D, McKhann G, Yan SS. Mitochondrial Abeta: a potential focal point for neuronal metabolic dysfunction in Alzheimer's disease. FASEB J. 2005;19:2040–1. doi: 10.1096/fj.05-3735fje. [DOI] [PubMed] [Google Scholar]
- 65.Devi L, Ohno M. Mitochondrial dysfunction and accumulation of the β-secretase-cleaved C-terminal fragment of APP in Alzheimer's disease transgenic mice. Neurobiol Dis. 2012;45:417–424. doi: 10.1016/j.nbd.2011.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hansson Petersen CA, Alikhani N, Behbahani H, Wiehager B, Pavlov PF, Alafuzoff I, Leinonen V, Ito A, Winblad B, Glaser E, Ankarcrona M. The amyloid beta-peptide is imported into mitochondria via the TOM import machinery and localized to mitochondrial cristae. Proc Natl Acad Sci U S A. 2008;105:13145–13150. doi: 10.1073/pnas.0806192105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Crouch PJ, Blake R, Duce JA, Ciccotosto GD, Li QX, Barnham KJ, Curtain CC, Cherny RA, Cappai R, Dyrks T, Masters CL, Trounce IA IA. Copper-dependent inhibition of human cytochrome c oxidase by a dimeric conformer of amyloid-beta1-42. J. Neurosci. 2005;25:672–679. doi: 10.1523/JNEUROSCI.4276-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Reddy PH. Abnormal tau, mitochondrial dysfunction, impaired axonal transport of mitochondria, and synaptic deprivation in Alzheimer's disease. Brain. Res. 2011;1415:136–148. doi: 10.1016/j.brainres.2011.07.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Duboff B, Feany M, Götz J. Why size matters - balancing mitochondrial dynamics in Alzheimer's disease. Trends Neurosci. 2013 Apr 11; doi: 10.1016/j.tins.2013.03.002. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 70.Hodge T, Colombini M M. Regulation of metabolite flux through voltage-gating of VDAC channels. J Membr Biol. 1997;157:271–279. doi: 10.1007/s002329900235. [DOI] [PubMed] [Google Scholar]
- 71.Rostovtseva T, Colombini M M. VDAC channels mediate and gate the flow of ATP: implications for the regulation of mitochondrial function. Biophys J. 1997;72:1954–1962. doi: 10.1016/S0006-3495(97)78841-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Colombini M. VDAC structure, selectivity, and dynamics. Biochim Biophys Acta. 2012;1818:1457–1465. doi: 10.1016/j.bbamem.2011.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Craigen WJ, Graham BH BH. Genetic strategies for dissecting mammalian and Drosophila voltage-dependent anion channel functions. J. Bioenerg. Biomembr. 2008;40:207–212. doi: 10.1007/s10863-008-9146-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yamamoto T, Yamada A, Watanabe M, Yoshimura Y, Yamazaki N, Yoshimura Y, Yamauchi T, Kataoka M, Nagata T, Terada H, Shinohara Y. VDAC1, having a shorter N-terminus than VDAC2 but showing the same migration in an SDS-polyacrylamide gel, is the predominant form expressed in mitochondria of various tissues. J. Proteome. Res. 2006;5:3336–3344. doi: 10.1021/pr060291w. [DOI] [PubMed] [Google Scholar]
- 75.Raghavan A, Sheiko T, Graham BH, Craigen WJ WJ. Voltage-dependant anion channels: Novel insights into isoform function through genetic models. Biochim Biophys Acta. 2012;1818:1477–1485. doi: 10.1016/j.bbamem.2011.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Shimizu S, Narita M. Tsujimoto Y, Bcl-2 family proteins regulate the release of apoptogeniccytochrome c by the mitochondrial channel VDAC. Nature. 1993;399:483–487. doi: 10.1038/20959. [DOI] [PubMed] [Google Scholar]
- 77.Rostovtseva TK, Bezrukov SM. VDAC inhibition by tubulin and its physiological implications. Biochim Biophys Acta. 2012;1818:1526–1535. doi: 10.1016/j.bbamem.2011.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kerner J, Lee K, Tandler B, Hoppel CL. VDAC proteomics: Post-translation modifications. Biochim Biophys Acta. 2012;1818:1520–1525. doi: 10.1016/j.bbamem.2011.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lemasters JJ, Holmuhamedov EL, Czerny C, Zhong Z, Maldonado EN EN. Regulation of mitochondrialfunction by voltage dependent anion channels in ethanol metabolism and the Warburg effect. Biochim Biophys Acta. 2012;1818:1536–1544. doi: 10.1016/j.bbamem.2011.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.De Pinto V, Tomasello FF, Messina A, Guarino F, Benz R, La Mendola D, Magrì A, Milardi D, Pappalardo G. Determination of the conformation of the human VDAC1 N-terminal peptide, a protein moiety essential for the functional properties of the pore. Chembiochem. 2007;8:744–756. doi: 10.1002/cbic.200700009. [DOI] [PubMed] [Google Scholar]
- 81.Geula S, Ben-Hail D, Shoshan-Barmatz V. Structure-based analysis of VDAC1: N-terminus location, translocation, channel gating and association with anti-apoptotic proteins. Biochem. J. 2012;444:475–485. doi: 10.1042/BJ20112079. [DOI] [PubMed] [Google Scholar]
- 83.Thinnes FP. Apoptogenic interactions of plasmalemmal type-1 VDAC and Aβ peptides via GxxxG motifs induce Alzheimer's disease - a basic model of apoptosis? Wien Med Wochenschr. 2011;161:274–276. doi: 10.1007/s10354-011-0887-5. [DOI] [PubMed] [Google Scholar]
- 84.Jope RS, Johnson GV. The glamour and gloom of glycogen synthase kinase-3. Trends Biochem Sci. 2004;29:95–102. doi: 10.1016/j.tibs.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 85.Azoulay-Zohar H, Israelson A, Abu-Hamad S, Shoshan-Barmatz V. In self-defence: hexokinase promotes voltage-dependent anion channel closure and prevents mitochondria-mediated apoptotic cell death. Biochem. J. 2004;377:347–355. doi: 10.1042/BJ20031465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hoeflich KP, Luo J, Rubie EA, Tsao MS, Jin O, Woodgett JR. Requirement for glycogen synthase kinase-3beta in cell survival and NF-kappaB activation. Nature. 2000;406:86–90. doi: 10.1038/35017574. [DOI] [PubMed] [Google Scholar]
- 87.Hernandez F, Lucas JJ, Avila J. GSK3 and tau: two convergence points in Alzheimer's disease. J. Alzheimers. Dis. 2013;33(Suppl 1):S141–S144. doi: 10.3233/JAD-2012-129025. [DOI] [PubMed] [Google Scholar]
- 88.Al Sweidi S, Sánchez MG, Bourque M, Morissette M, Dluzen D, Di Paolo T. Oestrogen receptors and signalling pathways: implications for neuroprotective effects of sex steroids in Parkinson's disease. J Neuroendocrinol. 2012;24:48–61. doi: 10.1111/j.1365-2826.2011.02193.x. [DOI] [PubMed] [Google Scholar]
- 89.Mishra R. Glycogen synthase kinase 3 beta: can it be a target for oral cancer. Mol Cancer. 2010;9:144. doi: 10.1186/1476-4598-9-144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Fung TK, Gandillet A, So CW. Selective treatment of mixed-lineage leukemia leukemic stem cells through targeting glycogen synthase kinase 3 and the canonical Wnt/β-catenin pathway. Curr Opin Hematol. 2012;19:280–286. doi: 10.1097/MOH.0b013e3283545615. [DOI] [PubMed] [Google Scholar]
- 91.Mills CN, Nowsheen S, Bonner JA, Yang ES. Emerging roles of glycogen synthase kinase 3 in the treatment of brain tumors. Front. Mol. Neurosci. 2011;4:47. doi: 10.3389/fnmol.2011.00047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Gao C, Hölscher C, Liu Y, Li L. GSK3: a key target for the development of novel treatments for type 2 diabetes mellitus and Alzheimer disease. Rev. Neurosci. 2011;23:1–11. doi: 10.1515/rns.2011.061. [DOI] [PubMed] [Google Scholar]
- 93.Wang H, Brown J, Martin M. Glycogen synthase kinase 3: a point of convergence for the host inflammatory response. Cytokine. 2011;53:130–140. doi: 10.1016/j.cyto.2010.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Bersani FS, Iannitelli A, Pacitti F, Bersani G. Sleep and biorythm disturbances in schizophrenia, mood and anxiety disorders: a review. Riv. Psichiatr. 2012;47:365–375. doi: 10.1708/1175.13027. [DOI] [PubMed] [Google Scholar]
- 95.Rasola A, Sciacovelli M, Pantic B, Bernardi P. Signal transduction to the permeability transition pore. FEBS. Lett. 2010;584:1989–1996. doi: 10.1016/j.febslet.2010.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Sun T, Rodriguez M, Kim L. Glycogen synthase kinase 3 in the world of cell migration. Dev Growth Differ. 2009;51:735–742. doi: 10.1111/j.1440-169X.2009.01141.x. [DOI] [PubMed] [Google Scholar]
- 97.Wu D, Pan W. GSK3: a multifaceted kinase in Wnt signaling. Trends Biochem Sci. 2010;35:161–168. doi: 10.1016/j.tibs.2009.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kim YT, Hur EM, Snider WD, Zhou FQ. Role of GSK3 Signaling in Neuronal Morphogenesis. Front Mol. Neurosci. 2011;4:48. doi: 10.3389/fnmol.2011.00048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kim YM, Seo YH, Park CB, Yoon SH, Yoon G. Roles of GSK3 in metabolic shift toward abnormal anabolism in cell senescence. Ann N Y Acad Sci. 2010;1201:65–71. doi: 10.1111/j.1749-6632.2010.05617.x. [DOI] [PubMed] [Google Scholar]
- 100.Pap M, Cooper GM. Role of glycogen synthase kinase-3 in the phosphatidylinositol 3-Kinase/Akt cell survival pathway. J. Biol. Chem. 1998;273:19929–19932. doi: 10.1074/jbc.273.32.19929. [DOI] [PubMed] [Google Scholar]
- 101.Maggirwar SB, Tong N, Ramirez S, Gelbard HA, Dewhurst S. HIV-1 Tat-mediated activation of glycogen synthase kinase-3beta contributes to Tat-mediated neurotoxicity. J. Neurochem. 1999;73:578–586. doi: 10.1046/j.1471-4159.1999.0730578.x. [DOI] [PubMed] [Google Scholar]
- 102.Hetman M, Cavanaugh JE, Kimelman D, Xia Z. Role of glycogen synthase kinase-3beta in neuronal apoptosis induced by trophic withdrawal. J. Neurosci. 2000;20:2567–2574. doi: 10.1523/JNEUROSCI.20-07-02567.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Bijur GN, De Sarno P, Jope RS. Glycogen synthase kinase-3beta facilitates staurosporine- and heat shock-induced apoptosis. Protection by lithium. J. Biol. Chem. 2000;275:7583–7590. doi: 10.1074/jbc.275.11.7583. [DOI] [PubMed] [Google Scholar]
- 104.Bijur GN, Jope RS. Proapoptotic stimuli induce nuclear accumulation of glycogen synthase kinase-3 beta. J. Biol. Chem. 2001;276:37436–37442. doi: 10.1074/jbc.M105725200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Avrahami L, Farfara D, Shaham-Kol M, Vassar R, Frenkel D, Eldar-Finkelman H. Inhibition of glycogen synthase kinase-3 ameliorates β-amyloid pathology and restores lysosomal acidification and mammalian target of rapamycin activity in the Alzheimer disease mouse model: in vivo and in vitro studies. J. Biol. Chem. 2013;288:1295–306. doi: 10.1074/jbc.M112.409250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ly PT, Wu Y, Zou H, Wang R, Zhou W, Kinoshita A, Zhang M, Yang Y, Cai F, Woodgett J, Song W. Inhibition of GSK3β-mediated BACE1 expression reduces Alzheimer-associated phenotypes. J. Clin. Invest. 2013;123:224–235. doi: 10.1172/JCI64516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Leroy A, Landrieu I, Huvent I, Legrand D, Codeville B, Wieruszeski JM, Lippens G. Spectroscopic studies of GSK3{beta} phosphorylation of the neuronal tau protein and its interaction with the N-terminal domain of apolipoprotein E. J. Biol. Chem. 2010;285:33435–33444. doi: 10.1074/jbc.M110.149419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Vivanco I, Sawyers CL. The phosphatidylinositol 3-Kinase AKT pathway in human cancer. Nat Rev Cancer. 2002;2:489–501. doi: 10.1038/nrc839. [DOI] [PubMed] [Google Scholar]
- 109.Brazil DP, Hemmings BA. Ten years of protein kinase B signalling: a hard Akt to follow. Trends Biochem Sci. 2001;26:657–664. doi: 10.1016/s0968-0004(01)01958-2. [DOI] [PubMed] [Google Scholar]
- 110.Chong ZZ, Li F, Maiese K. Oxidative stress in the brain: novel cellular targets that govern survival during neurodegenerative disease. Prog. Neurobiol. 2005;75:207–246. doi: 10.1016/j.pneurobio.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 111.Martín D, Salinas M, López-Valdaliso R, Serrano E, Recuero M, Cuadrado A. Effect of the Alzheimer amyloid fragment Abeta(25–35) on Akt/PKB kinase and survival of PC12 cells. J. Neurochem. 2001;78:1000–1008. doi: 10.1046/j.1471-4159.2001.00472.x. [DOI] [PubMed] [Google Scholar]
- 112.Henry MK, Lynch JT, Eapen AK, Quelle FW. DNA damage-induced cell-cycle arrest of hematopoietic cells is overridden by activation of the PI-3 kinase/Akt signaling pathway. Blood. 2001;98:834–841. doi: 10.1182/blood.v98.3.834. [DOI] [PubMed] [Google Scholar]
- 113.Cedazo-Mínguez A, Popescu BO, Blanco-Millán JM, Akterin S, Pei JJ, Winblad B, Cowburn RF. Apolipoprotein E and beta-amyloid (1–42) regulation of glycogen synthase kinase-3beta. J. Neurochem. 2003;87:1152–1164. doi: 10.1046/j.1471-4159.2003.02088.x. [DOI] [PubMed] [Google Scholar]
- 114.Ryder J, Su Y, Ni B. Akt/GSK3beta serine/threonine kinases: evidence for a signalling pathway mediated by familial Alzheimer's disease mutations. Cell. Signal. 2004;16:187–200. doi: 10.1016/j.cellsig.2003.07.004. [DOI] [PubMed] [Google Scholar]
- 115.Tokutake T, Kasuga K, Yajima R, Sekine Y, Tezuka T, Nishizawa M, Ikeuchi T. Hyperphosphorylation of Tau induced by naturally secreted amyloid-β at nanomolar concentrations is modulated by insulin-dependent Akt-GSK3β signaling pathway. J. Biol. Chem. 2012;287:35222–35233. doi: 10.1074/jbc.M112.348300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Baki L, Neve RL, Shao Z, Shioi J, Georgakopoulos A, Robakis NK NK. Wild-type but not FAD mutant presenilin-1 prevents neuronal degeneration by promoting phosphatidylinositol 3-kinase neuroprotective signaling. J. Neurosci. 2008;28:483–490. doi: 10.1523/JNEUROSCI.4067-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Jope RS, Yuskaitis CJ, Beurel E. Glycogen synthase kinase-3 (GSK3): inflammation, diseases, and therapeutics. Neurochem Res. 2007;32:577–595. doi: 10.1007/s11064-006-9128-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Pastorino JG, Hoek JB, Shulga N. Activation of glycogen synthase kinase 3beta disrupts the binding of hexokinase II to mitochondria by phosphorylating voltage-dependent anion channel and potentiates chemotherapy-induced cytotoxicity. Cancer Res. 2005;65:10545–10554. doi: 10.1158/0008-5472.CAN-05-1925. [DOI] [PubMed] [Google Scholar]
- 119.Jo J, Whitcomb DJ, Olsen KM, Kerrigan TL, Lo SC, Bru-Mercier G, Dickinson B, Scullion S, Sheng M, Collingridge G, Cho K. Aβ(1–42) inhibition of LTP is mediated by a signaling pathway involving caspase-3, Akt1 and GSK-3β. Nat. Neurosci. 2011;14:545–547. doi: 10.1038/nn.2785. [DOI] [PubMed] [Google Scholar]
- 120.Jimenez S, Torres M, Vizuete M, Sanchez-Varo R, Sanchez-Mejias E, Trujillo-Estrada L, Carmona-Cuenca I, Caballero C, Ruano D, Gutierrez A, Vitorica J. Age-dependent accumulation of soluble amyloid beta (Abeta) oligomers reverses the neuroprotective effect of soluble amyloid precursor protein-alpha (sAPP(alpha)) by modulating phosphatidylinositol 3-kinase (PI3K)/Akt-GSK-3beta pathway in Alzheimer mouse model. J. Biol. Chem. 2011;286:18414–1825. doi: 10.1074/jbc.M110.209718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Wilson JE. Hexokinases. Rev. Physiol. Biochem. Pharmacol. 1995;126:65–198. doi: 10.1007/BFb0049776. [DOI] [PubMed] [Google Scholar]
- 122.Nakashima RA, Paggi MG, Scott LJ, Pedersen PL. Purification and characterization of a bindable form of mitochondrial bound hexokinase from the highly glycolytic AS-30D rat hepatoma cell line. Cancer Res. 1988;48:913–919. [PubMed] [Google Scholar]
- 123.Abu-Hamad S, Zaid H, Israelson A, Nahon E, Shoshan-Barmatz V. Hexokinase-I protection against apoptotic cell death is mediated via interaction with the voltage-dependent anion channel-1: mapping the site of binding. J Biol Chem. 2008;283:13482–13490. doi: 10.1074/jbc.M708216200. [DOI] [PubMed] [Google Scholar]
- 124.Majewski N, Nogueira V, Bhaskar P, Coy PE, Skeen JE, Gottlob K, Chandel NS, Thompson CB, Robey RB, Hay N. Hexokinase-mitochondria interaction mediated by Akt is required to inhibit apoptosis in the presence or absence of Bax and Bak. Mol. Cell. 2004;16:819–830. doi: 10.1016/j.molcel.2004.11.014. [DOI] [PubMed] [Google Scholar]
- 125.Pastorino JG, Shulga N, Hoek JB. Mitochondrial binding of hexokinase II inhibits Bax-induced cytochrome c release and apoptosis. J Biol Chem. 2002;277:7610–7618. doi: 10.1074/jbc.M109950200. [DOI] [PubMed] [Google Scholar]
- 126.Zaid H, Abu-Hamad S, Israelson A, Nathan I, Shoshan-Barmatz V. The voltage-dependent anion channel-1 modulates apoptotic cell death. Cell. Death. Differ. 2005;12:751–760. doi: 10.1038/sj.cdd.4401599. [DOI] [PubMed] [Google Scholar]
- 127.Yoo BC, Fountoulakis M, Cairns N, Lubec G. Changes of voltage-dependent anion-selective channel proteins VDAC1 and VDAC2 brain levels in patients with Alzheimer's disease and Down syndrome. Electrophoresis. 2001;22:172–179. doi: 10.1002/1522-2683(200101)22:1<172::AID-ELPS172>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 128.Cuadrado-Tejedor M, Vilariño M, Cabodevilla F, Del Río J, Frechilla D, Pérez-Mediavilla A. Enhanced expression of the voltage-dependent anion channel 1 (VDAC1) in Alzheimer's disease transgenic mice: an insight into the pathogenic effects of amyloid-β. J. Alzheimers Dis. 2011;23:195–206. doi: 10.3233/JAD-2010-100966. [DOI] [PubMed] [Google Scholar]
- 129.Hunt A, Schonknecht P, Henze M, Seidl U, Haberkorn U, Schroder J. Reduced cerebral glucose metabolism in patients at risk for Alzheimer's disease. Psychiatry Research: Neuroimaging. 2007;155:147–154. doi: 10.1016/j.pscychresns.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 130.Liu F, Shi J, Tanimukai H, Gu J, Gu J, Grundke-Iqbal I, Iqbal K, Gong CX. Reduced O-GlcNAcylation links lower brain glucose metabolism and tau pathology in Alzheimer's disease. Brain. 2009;132:1820–1832. doi: 10.1093/brain/awp099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Mosconi L, Brys M, Switalski R, Mistur R, Glodzik L, Pirraglia E, Tsui W, De Santi S, de Leon MJ. Maternal family history of Alzheimer's disease predisposes to reduced brain glucose metabolism. Proc Natl Acad Sci U S A. 2007;104:19067–19072. doi: 10.1073/pnas.0705036104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Wang DM, Li SQ, Zhu XY, Wang Y, Wu WL, Zhang XP. Protective Effects of Hesperidin Against Amyloid-β (Aβ) Induced Neurotoxicity Through the Voltage Dependent Anion Channel 1 (VDAC1)-Mediated Mitochondrial Apoptotic Pathway in PC12 Cells. Neurochem Res. 2013;38:1034–1044. doi: 10.1007/s11064-013-1013-4. [DOI] [PubMed] [Google Scholar]