Abstract
The conventional mass spectrometry (MS)-based strategy is often inadequate for the comprehensive characterization of various size neuropeptides without assistance of genomic information. This study evaluated sequence coverage of different size neuropeptides in two crustacean species, blue crab Callinectes sapidus and Jonah crab Cancer borealis using conventional MS methodologies and revealed limitations to mid- and large-size peptide analysis. Herein we attempt to establish a multi-scale strategy for simultaneous and confident sequence elucidation of various sizes of peptides in the crustacean nervous system. Nine novel neuropeptides spanning a wide range of molecular weights (0.9-8.2 kDa) were fully sequenced from a major neuroendocrine organ, the sinus gland of the spiny lobster Panulirus interruptus. These novel neuropeptides included seven allatostatin (A- and B-type) peptides, one crustacean hyperglycemic hormone precursor-related peptide, and one crustacean hyperglycemic hormone. Highly accurate multi-scale characterization of a collection of varied size neuropeptides was achieved by integrating traditional data-dependent tandem MS, improved bottom-up sequencing, multiple fragmentation technique-enabled top-down sequencing, chemical derivatization, and in silico homology search. Collectively, the ability to characterize a neuropeptidome with vastly differing molecule sizes from a neural tissue extract could find great utility in unraveling complex signaling peptide mixtures employed by other biological systems.
Keywords: Neuropeptide, peptidomics, de novo sequencing, mass spectrometry, crustacean
INTRODUCTION
Neuropeptides, including endogenous peptide neuromodulators and hormones, mediate or modulate neuronal communication by acting on cell surface receptors and are involved in a broad range of physiological and behavioral processes [1-4]. Mass spectrometry (MS)-based neuropeptidomics aims to completely characterize the neuropeptides in a target organism as an important first step toward a better understanding of the structure and function of these complex signaling molecules [5-17]. A significant challenge to this goal is that many of these endogenous neuropeptides display large diversity at the molecular and cellular level, such as various molecular sizes [15], extensive and multiple post-translational modifications (PTMs) [18], different hydrophobicities [12], and a wide dynamic range of concentrations [19]. Because of this, a uniform approach for comprehensive neuropeptide characterization is difficult to engineer.
A neuropeptidome usually contains peptides of various sizes [1]. Although liquid chromatography coupled to tandem mass spectrometry (LC-MS/MS) with data-dependent acquisition is a powerful tool in peptidome research [5, 6, 11-13, 17], it lacks the capability for de novo sequencing of mid-size and large peptides due to inefficient fragmentation of peptides larger than 4 kDa [20]. Innovation of MS-based instrumentation has greatly advanced our capability to analyze complex peptide mixtures, including those large peptides and intact proteins with extensive modifications [21]. For example, emergence of ultra-high resolution Fourier transform mass spectrometers greatly facilitates the bioinformatics-assisted peptidome research, as mass accuracy is a critical factor in scoring algorithm for peptide sequence assignment and protein identification [12, 17]. The observed mass values of peptide precursors and their resulting fragment ions are matched to the theoretical values arising from a genome/cDNA sequence, and thus partial peptide sequence coverage from interpretation of MS/MS spectra can usually produce confident hits [10]. However, there are many valuable animal models whose genomes have not been sequenced yet, thus no genomic database or large cDNA database is available for database searching strategy commonly used [1, 3]. Peptide discovery in these model organisms would need to rely on obtaining full sequence coverage, including enhanced local identification confidence on each amino acid residue.
The California spiny lobster Panulirus interruptus has long served as an important animal model for many areas of research in endocrinology and neurobiology [22-24], but its genome has not been sequenced yet and there is no protein/cDNA database. In particular, its stomatogastric ganglion has been utilized as a powerful model system to understand the cellular mechanisms of rhythmic pattern generation in neuronal networks [22]. Many studies reported that neurotransmitters and neuropeptides regulate the functional output of these well-defined neuronal circuits [25-28]. Therefore, it is highly desirable to obtain accurate molecular information on neuropeptides in this species.
In a previous study [29], we established a strategy by combining bottom-up, off-line top-down, and on-line top-down MS methods for confident de novo sequencing of crustacean hyperglycemic hormone (CHH)-family neuropeptides with molecular weight (MW) around 8.4-9.2 kDa. The current work aims to discover and confidently identify various sizes of novel neuropeptides (MW 0.9-8.2 kDa) in the crustacean nervous system. We evaluate the possibility for improvement of current approaches on sequencing of small-, middle- and large-molecular sizes of neuropeptides. A multi-scale strategy is established by rational optimization of methodology and further validated by sequencing of nine novel neuropeptides in P. interruptus sinus gland, a major neuroendocrine structure that secretes peptide hormones to regulate many essential functions of the animal.
MATERIALS AND METHODS
Chemicals
Methanol, glacial acetic acid, borane pyridine and formaldehyde were obtained from Sigma-Aldrich (St. Louis, MO). Optima grade formic acid, acetonitrile (ACN), water, and methanol were purchased from Fisher Scientific (Pittsburgh, PA).
Animals and Tissue Dissection
Blue crabs Callinectes sapidus and Jonah crabs Cancer borealis were shipped from the Fresh Lobster Company (Gloucester, MA); and the California spiny lobsters were purchased from Catalina Offshore Products (San Diego, CA). Tissue dissection was performed according to our previous report [30]. Briefly, ten animals were anesthetized in ice, and the sinus glands were dissected and collected in chilled acidified methanol and stored in −80 °C freezer prior to further sample processing.
Tissue Extraction and LC Fractionation
The tissues were homogenized and extracted with 100 μL of acidified methanol (methanol:H2O:acetic acid, 90:9:1, v:v:v) [30, 31]. After centrifugation, supernatant fractions were combined and the extraction procedure was repeated three times. After dryness in a Savant SC 110 SpeedVac concentrator (Thermo Electron Corporation, CA), the sample was re-suspended in 100 μL of deionized water for further analysis. High-performance liquid chromatography (HPLC) separations were performed with a Waters Alliance HPLC system (Milford, MA) according to our previous report [31]. The mobile phases included solution A (water containing 0.1% formic acid) and solution B (ACN containing 0.1% formic acid). Approximately 50 μL of extract was injected onto a Phenomenex Gemini C18 column (2.1 mm i.d., 150 mm length, 5 μm particle size; Torrance, CA). The separations consisted of a 120 min gradient of 5–95% solution B. The flow rate was 0.2 mL/min. Fractions were automatically collected every 2 min, followed by drying in SpeedVac, re-suspended in 40 μL water, and stored in −80 °C.
Formaldehyde Labeling, and Peptide Digestion
For formaldehyde labeling [14], 1 μL of peptide fraction was mixed with 9 μL of ACN with 0.1% formic acid, followed by the addition of 1 μL of formaldehyde (4% in H2O) and 1 μL of borane pyridine (120 mM in methanol). The labeling reaction was allowed to take place for 10 min with mixing on Vortex. Excess formaldehyde was quenched via the addition of 1 μL of ammonium bicarbonate buffer (0.2 M). The resulting solution was stored at −20 °C before further analysis.
For trypsin digestion of CHHs, 1 μL of peptide fraction was reduced and alkylated by incubation in 2.5 mM Dithiothreitol (DTT) for 1 h at 37 °C followed by incubation in 7 mM iodoacetamide (IAA) in the dark at room temperature for 1 h. A 1 μL of peptide was digested at 37 °C overnight after addition of 50 mM ammonium bicarbonate buffer with 0.5 μg of trypsin (Promega, Madison, WI). For Lys-C digestion of CPRPs, 1 μL of peptide fraction was digested overnight at 37 °C after re-suspending in 10μL of 50 mM Tris-HCl, pH 8.0, and addition of 0.5 μg of Lys-C (Princeton Separations, Adelphia, NJ). Finally, each digest was quenched by the addition of formic acid to a final concentration of 0.5%, desalted on a C18 ZipTip (Millipore, Bedford, MA), and the eluent was further dried in SpeedVac.
Nano-LC-Electrospray (ESI)-Linear Trap Quadrupole (LTQ)-Orbitrap Elite
A 1 μL of crude tissue extract was reduced by incubation in 2.5 mM DTT for 1 h at 37 °C and desalted by C18 ZipTip and resuspended in 10 μL of water containing 0.1% formic acid. A 0.5 μL of peptide sample was injected onto an Ultimate 3000 RSLCnano system coupled to an Orbitrap Elite mass spectrometer (Thermo Fisher Scientific, Bremen, Germany) for data-dependent MS/MS analysis with electron-transfer dissociation (ETD) and higher-energy collisional dissociation (HCD) according to our previous report [29].
NanoLC-ESI-Quadrupole Time of Flight (QTOF)-MS/MS
Nanoscale LC-ESI-Q-TOF MS/MS was performed on a Waters nanoAcquity ultra performance LC system coupled to a Synapt G2 high-definition mass spectrometer (Waters Corp., Milford, MA). Chromatographic separations were performed on a Waters BEH 130Å C18 reversed phase capillary column (150 mm × 75 μm, 1.7 μm). The mobile phases used were: 0.1% formic acid in deionized water (A); 0.1% formic acid in ACN (B). An aliquot of 2.0 μL of HPLC fraction was injected and loaded onto the Waters NanoEase trap column using 95% mobile phase A and 5% mobile phase B at a flow rate of 10 μL/min for 3 min. For neuropeptides, the linear gradient was from 5 to 45% buffer B over 75 min. A data dependent acquisition was employed for the MS survey scan and the selection of three precursor ions and subsequent MS/MS of the selected parent ions. The MS scan range was from m/z 400–2000 and the MS/MS scan was from m/z 50–2000.
Top-down MS/MS on ESI-LTQ- Fourier Transform Ion Cyclotron Resonance (FTICR) mass spectrometer
A 0.5 μL of crustacean hyperglycemic hormone (CHH) peptide fraction was reduced by incubation in 2.5 mM DTT for 1 h at 37 °C and desalted by C18 ZipTip and resuspended in 10 μL of 50% ACN containing 2% formic acid. The DTT-reduced CHH sample was directly infused into a 7T LTQ-FTICR Ultra hybrid mass spectrometer (Thermo Scientific Inc., Bremen, Germany) equipped with an automated chip-based nano-ESI source (Triversa NanoMate; Advion BioSciences, Ithaca, NY) as described previously [29]. For collision-induced dissociation (CID) and electron-capture dissociation (ECD) fragmentation, individual charge states of peptide molecular ions were first isolated and then dissociated using 22–28% of normalized collision energy for CID or 4% electron energy for ECD with a 60 ms duration with no delay. Typically, 1000 transients were averaged to ensure high quality MS/MS spectra. All FTICR spectra were processed with Xtract Software (Xcalibur 2.1, Thermo Scientific Inc., Bremen, Germany) using a S/N threshold of 1.5 and fit factor of 40% and validated manually. The resulting mass lists were further assigned using the in-house developed “Ion Assignment” software [32]. The assigned ions were manually validated to ensure the quality and accuracy of assignments. The analysis of CHH-precursor related peptides (CPRPs) employed the same protocol of CHHs, except for no DTT reduction of disulfide bonds.
In Silico Homology Search
For small neuropeptides, the resulting sequence was put in NCBI tBLASTn search engine against nucleotide collection database (no. of sequence 16341740). Crustacean (taxid: 6657) was chosen as organism [16].
For mid-size neuropeptides, the raw data from ESI-LTQ-FTICR were deisotoped with Xtract v2.1 (ThermoFisher, Bremen, Germany) and uploaded in a custom 168-core ProSightPC 3.0 cluster [17, 20]. The data was searched in a “Sequence Tag Search” mode against a home-built crustacean neuropeptide database, in which minimum tag score was set at 0.01, and compiler tolerance was 10 ppm. A minimum tag size of 4 residues was required. The database was created with 693 previously identified crustacean neuropeptides.
For large neuropeptides, the LC-ESI-QTOF-MS/MS raw data were converted to peak list (.pkl) files using ProteinLynx software 2.4 (Waters). Peptides were identified by searching against an NCBInr protein database 20120519 (18132328 sequences; 6219145704 residues) using the Mascot v2.4 search engine. Trypsin was selected as enzyme allowing up to 2 missed cleavages. Carboxylmethyl cysteine was specified as fixed modifications, and methionine oxidation and pyro-Glu as variable modifications. Precursor and MS/MS tolerances were set within 0.5 Da and 0.5 Da for monoisotopic mass, respectively. Peptide charge states include 1+, 2+ and 3+ charged peptides.
RESULTS AND DISCUSSION
Attempts for Rational Improvement of Methodology
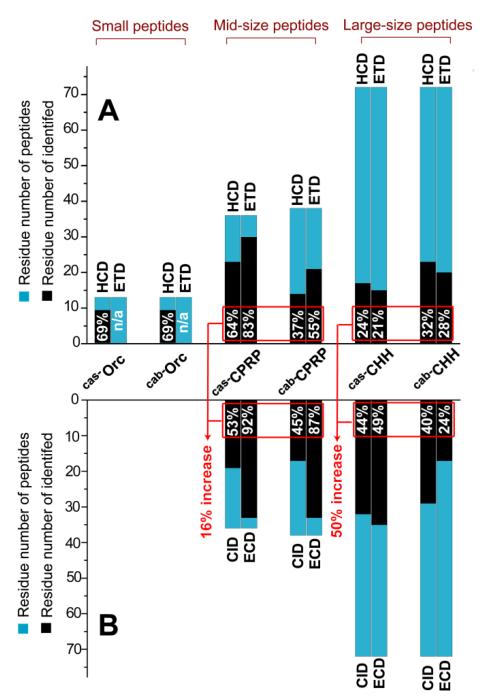
In the past decade, there has been a great success with mass spectrometry (MS)-based neuropeptidomics [1, 5-11, 15, 33, 34]. However, the majority of these published peptide lists show excellent coverage rates on small neuropeptides (< 2.5 kDa), but few identifications in the higher mass range because the traditional strategies are inefficient for de novo sequencing of mid-size (2.5-5 kDa) and large (>5 kDa) neuropeptides. The first aim of this study is to evaluate the performance of traditional strategies on their sequence coverage of crustacean neuropeptides from previously studied model organisms. The newly released Thermo LTQ-Orbitrap Elite instrument [35] has demonstrated powerful performance in both bottom-up and top-down proteomics. This instrument platform provides ultra-high resolving power and allows multiple complementary fragmentation methods (ETD, HCD, and CID) to be performed on liquid chromatographic time scales [35]. Therefore, it is tempting to employ such a state-of-the-art analytical platform for a comprehensive sequencing analysis of various sizes of neuropeptides using data-dependent LC-MS/MS with multiple fragmentation techniques. Here, we evaluated two neuropeptide extracts from the sinus glands of Callinectes sapidus [31] and Cancer borealis [15]. The former extract contained Cas−Orc (Callinectes sapidus, Cas; orcokinin, Orc; MW 1474.64 Da), Cas−CPRP (3836.99 Da), and Cas−CHH (8472.80 Da); and the latter sample yielded Cab−Orc (Cancer borealis, Cab; MW 1473.66 Da), Cab−CPRP (3976.06 Da), and Cab−CHH (8539.88 Da). Their sequences obtained on this new instrument platform agreed well with previously published sequences [15, 29-31, 36]. The two model samples were analyzed by LC-MS/MS with HCD and ETD, respectively. As shown in Figure 1A, 69% sequence coverage was obtained by HCD for the two small peptides orcokinins. However, for the two mid-size peptides, 64% and 37% were obtained by HCD, and 83% and 55% by ETD, respectively. For the large peptides, less than 32% of sequence was determined by either HCD or ETD. Because the mid- and large-sized peptide analysis yielded much lower sequence coverage, complete sequence elucidation or identification cannot be obtained in the absence of a genome or a cDNA database.
Figure 1. Evaluation results for sequencing of various sizes of neuropeptides.
(A) On-line LC-MS/MS; (B) Off-line top-down MS/MS. The percentage of peptide sequence coverage is labeled in the black bar of every peptide. The percentage of increase in red is calculated by the following expression Σ identified residue number/ Σ peptide residue number. The MS/MS fragmentation maps of these model peptides are shown in Figure S-1 and S-2.
Compared with on-line LC-MS/MS, the off-line MS/MS method requires highly purified samples and lengthy data acquisition times, which lowers the analytical throughput. However, it can generate high quality MS/MS spectra for sequence interpretation [32]. Therefore, we evaluated whether or not off-line top-down MS could achieve complete sequencing of mid- and large-size peptides. The LC-purified Cas−CPRP, Cas−CHH, Cab−CPRP, and Cab−CHH were directly infused into an ESI-LTQ-FTICR mass spectrometer for top-down fragmentation by CID and ECD, respectively. Compared with the on-line LC-MS/MS results, this off-line strategy offers 16% increase of total sequence coverage for mid-size peptides and 50% increase for large peptides (Figure 1B). However, complete sequence coverage was not obtained by this off-line strategy, indicating its limitation. It should be noted that the off-line top-down MS can also be carried out on LTQ-Orbitrap using ETD and HCD with comparable results. In addition, because the CHHs contain disulfide bonds, the samples containing CHHs were reduced with DTT before analysis to obtain efficient MS fragmentation [29].
In this study, we established a multi-scale strategy for complete amino acid residue sequencing of various sizes of neuropeptides (Figure 2) based on the instrument platforms of LC-ESI-QTOF and ESI-LTQ-FTICR. We chose the P. interruptus sinus gland as a target neuroendocrine organ to explore the multi-scale strategy and peptide discovery. The sinus glands located in crustacean eyestalk synthesizes and secretes numerous peptide hormones regulating multiple physiological activities, such as molting, hemolymph glucose levels, osmoregulation, and integument color changes [37]. Therefore, the elucidation of novel neuropeptide content will facilitate their functional studies. Table 1 lists nine such novel neuropeptides including one large, one mid-size and seven small peptides from this understudied model organism.
Figure 2. Workflow of the multi-scale strategy.
The top panel shows reversed phase HPLC fractionation of P. interruptus sinus gland tissue extract showing the presence of various sizes of novel neuropeptides. The bottom panel outlines each specific strategy for discovery and identification of small, mid-size and large peptides. It should be noted that the retention behavior of peptides in the reversed phase HPLC is not only based on molecular size, but also the hydrophobicity and other sequence-dependent effects. Here, we show three different color regions in the LC profile for the purpose of illustration.
Table 1.
Novel neuropeptides identified in this study.
| Peptide | Sequence | Exptl MW a | Mass error (Da) a | Spectrum |
|---|---|---|---|---|
| Pai−AST-A1 | SRQYAF GLamide | 940.55 | 0.06 | Fig. 3A |
| Pai−AST-A2 | NRQYSFGLamide | 982.50 | 0.02 | Fig. S3 |
| Pai−AST-A3 | NRPYSFGLamide | 951.55 | 0.05 | Fig. S3 |
|
| ||||
| Pai−AST-B1 | ANWNKFHGSWamide | 1244.59 | 0.00 | Fig. 3B |
| Pai−AST-B2 | ADWNKFHGSWamide | 1245.60 | 0.02 | Fig. 3C |
| Pai−AST-B3 | GNWNKFHGSWamide | 1230.59 | 0.01 | Fig. S3 |
| Pai−AST-B4 | GDWNKFHGSWamide | 1231.57 | 0.01 | Fig. S3 |
|
| ||||
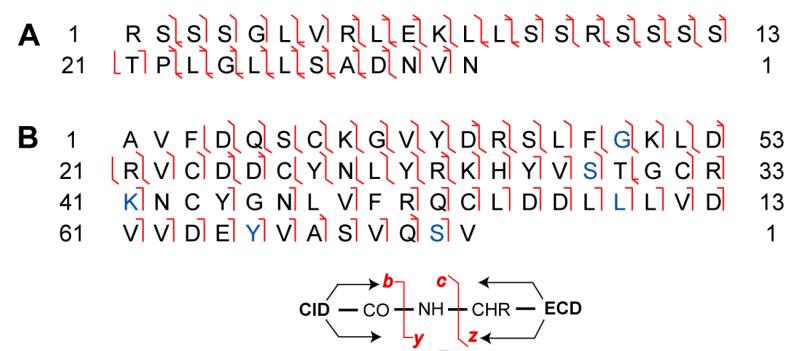
| Pai−CPRP | RSS SGLVRLEKLL SSRSS SSTPLGLLS ADHNVN |
3466.86 | 0.00 | Fig. 5 |
|
| ||||
| Pai−CHH | AVFDQSCKGVYDRSLFGKLDRVCDD CYNLYRKHYVSTGCRKNCYGNLVFR QCLDDLLLVDVVDEYVASVQSV b |
8271.12 | 0.19 | Fig. 7 |
Small peptides were measured on ESI-QTOF; mid-size and large peptides were analyzed on an LTQ-FTICR mass spectrometer.
Pai−CHH contains three disulfide bonds. The disulfide linkages are the same as the homologous CHH peptide(Ref. 46).
Small Neuropeptides
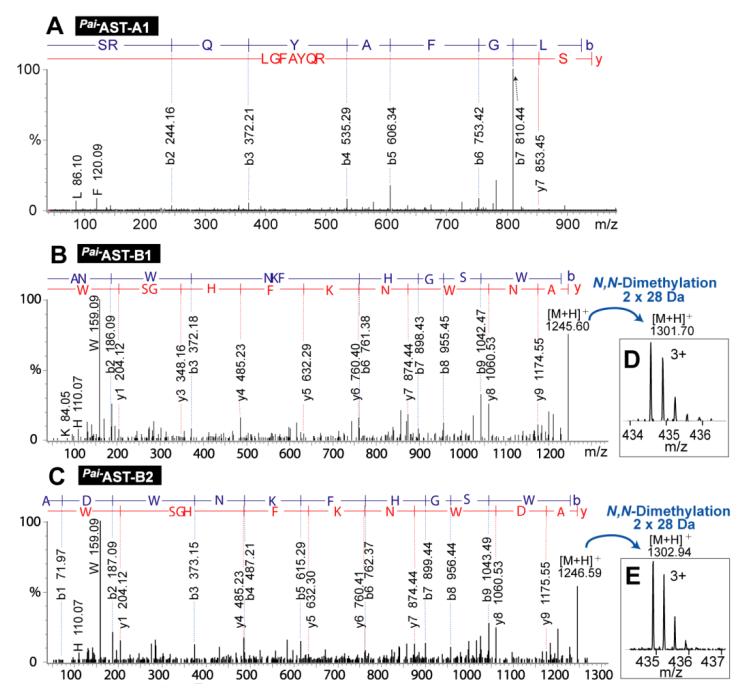
For de novo sequencing of small neuropeptides, we followed the conventional strategy [30]. The LC fractions #7, 8, and 9 (Figure 2) of P. interruptus sinus gland crude extract were analyzed by LC-MS/MS on an ESI-QTOF instrument. The resulting MS/MS spectra were processed by a software called Pepseq [31] for sequence interpretation, leading to identification of seven novel allatostatin (AST)-family neuropeptides listed in Table 1, Pai−AST-An (Pai, Panulirus interruptus; A-type; n=1, 2, 3) and Pai−AST-Bn (B-type; n=1, 2, 3, 4). A-type AST [15] is a peptide family possessing the C-terminal motif –YXFGLamide (X stands for a variable amino acid, and commonly either A or S), and the B-type AST-family peptides [38] possess the C-terminal motif −WX6Wamide (X6 are six variable amino acids). Figure 3 displays three representative de novo sequencing MS/MS spectra (others are shown in Figure S-3). To differentiate the isobaric (i.e., equal mass) residues (lysine vs. glutamine) in the four discovered B-type AST peptides, formaldehyde labeling [14, 39-41] reactions were performed to dimethylate the N-termini of the peptide chain and a putative lysine side chain. As shown in Figure 3D and 3E, there has 2×28 Da of mass increase after dimethylation, indicating that the fifth residue from N-terminus is lysine. Furthermore, the resulting peptide sequences were validated by BLAST homology search, with a match to autologous or homologous AST preprohomone genome (Table S-1). Interestingly, the Pai−AST-A1 and Pai−AST-A2 peptide were validated by the autologous AST preprohomone from P. interruptus with complete sequence match, confirming the sequence assignment and peptide identification. In addition, it should be noted that the MS approach used here could not differentiate leucine from isoleucine, so these residues were assigned according to homologous sequences by BLAST in this study.
Figure 3. De novo sequencing of AST-family neuropeptides.
MS/MS spectra of Pai−AST-A1 (A), Pai−AST-B1 (B), and Pai−AST-B2 (C). MS spectra of dimethylated Pai−AST-B1 (D), and Pai−AST-B2 (E). Panels A, B and C are deconvoluted spectra exported from Pepseq, and Panels D and E are original zoom-in mass spectra.
The AST neuropeptide was first discovered and named based on their function of inhibiting juvenile hormone production in the corpora allata of insects [42, 43]. In crustaceans, ASTs regulate a range of important processes and can act as inhibitors of endocrine function, as neuromodulators, on muscle, and directly on metabolic pathways [43]. The A- and B-type ASTs possess similar physiological functions but exhibit distinct sequence diversity, suggesting that their functions have undergone convergent evolution, highlighting the importance of allatostatic substances in crustaceans.
Mid-size Neuropeptides
CPRP is produced concurrent with the cleavage of CHH from its preprohormone [30, 31, 44]. CPRP is located between the signal peptide and the CHH sequence and is separated from the CHH by a dibasic cleavage site. There are currently no reports on the physiological roles played by CPRPs in crustaceans.
In LC fraction #13 (Figure 2), a peptide candidate with MW 3468.68 was directly infused into ESI-LTQ-FTICR mass spectrometer for top-down ECD and CID fragmentation. Sequence Tag Search in ProSight is a powerful tool for homology search of mid-size peptide [20]. Specifically, sequence tags were obtained from continuous fragment ions in top-down MS/MS spectra, followed by subsequent matching against the homologous sequences in peptide database. Here, the ECD data of this putative peptide was searched against a home-built crustacean neuropeptide database, and resulted matches to several CPRP peptides from other crustacean species (Figure S-4), suggesting that this peptide belongs to CPRP family.
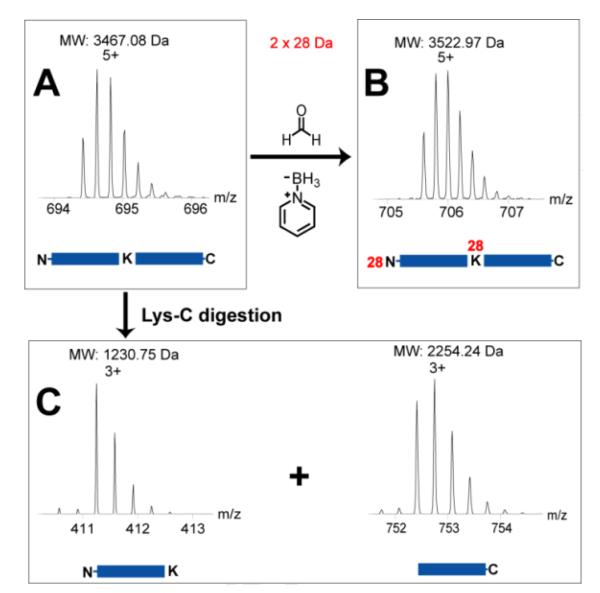
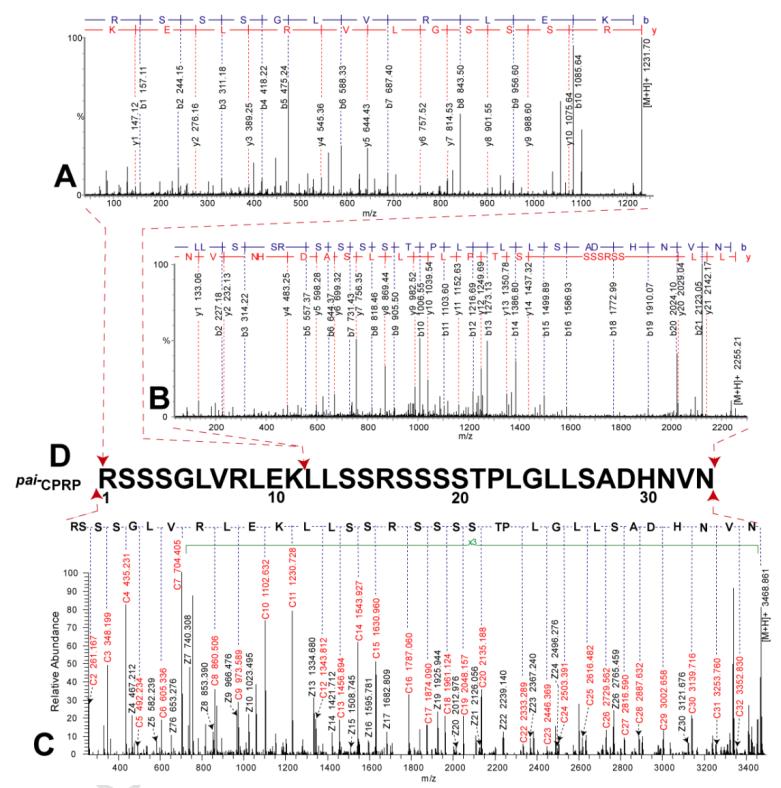
Deducing the peptide sequence solely based on the top-down ECD data may cause misidentification, as shown in the evaluation data acquired on LTQ-Orbitrap as described above. Because CPRP-family peptides contain multiple arginine and lysine, trypsin digestion produces too short tryptic peptides to be detected by MS, and also it is challenging to assemble multiple tryptic peptides from a mixture. Bonet-Costa et al. [45] reported an integrated top-down and bottom-up strategy to characterize histone proteins, where similar challenges exist in that the target proteins contain multiple basic amino acid residues. In our study, to sequence the mid-size CPRP peptides with multiple basic amino acid residues, we introduced a modified bottom-up de novo sequencing method. The Pai−CPRP candidate peptide was allowed to react with formaldehyde [39], yielding a mass increase of 2×28 Da due to dimethylation on both the N-terminus and lysine side chain (Figure 4A and B), which indicated that this Pai−CPRP contained one lysine. Consequently, after Lys-C digestion, the peptide is broken into two parts, and the proteolytic peptides can be identified by assembling peptide pair which yields total mass of 3467.08 + 18.01 = 3485.09 Da. As we expected, two proteolytic peptides were observed with MW 1230.75 and 2254.24 Da (sum = 3484.99 Da). Interpretation of their MS/MS spectra by Pepseq generated amino acid sequences as 1RSSSGLVRLEK11 (Figure 5A) and 12LLSSRSSSSTPLGLLSADHNVN33 (Figure 5B). Accordingly, the top-down ECD spectrum in Figure 5C was confidently annotated, resulting in assembly of this Pai−CPRP as shown in Figure 5D. Figure 8A illustrates the fragmentation maps by both top-down ECD and CID showing 91% of sequence coverage, which further confirms the sequence resulting from the modified bottom-up method. Subsequently, this novel Pai−CPRP was aligned with other four CPRPs [15, 30, 31] from related crustacean species showing a high degree of homology across multiple species (Figure S-5).
Figure 4. Strategy for de novo sequencing of a mid-sized CPRP neuropeptide.
Zoom-in MS spectra of (A) intact Pai−CPRP, (B) dimethylated Pai−CPRP, and (C) proteolytic peptides of Pai−CPRP after Lys-C digestion. The 28 Da of mass increase of peptides arises from N,N-dimethylation.
Figure 5. De novo sequencing MS/MS spectra of Pai−CPRP.
Bottom-up MS/MS spectra of (A) Pai−CPRP [1-11] and (B) Pai−CPRP [12-33]. (C) Top-down ECD spectra of intact Pai−CPRP exported from Xtract after charge deconvolution. (D) Sequence assembly of Pai−CPRP.
Figure 8. Fragmentation maps of Pai−CPRP (A) and Pai−CHH (B) from off-line top-down MS/MS.
In Panel B, the residues different from the reference sequence Jal−CHH are highlighted in blue.
Large Neuropeptides
CHH family is a group of structurally related peptide hormones that play multifunctional roles during the development and throughout the entire life cycle of crustaceans [44]. They regulate carbohydrate metabolism and also exert an inhibitory effect on molting, reproduction, and on osmoregulatory functions [37]. Characterization of CHHs family neuropeptides is challenging, as they contain 70-80 amino acids and complex disulfide bridge connections.
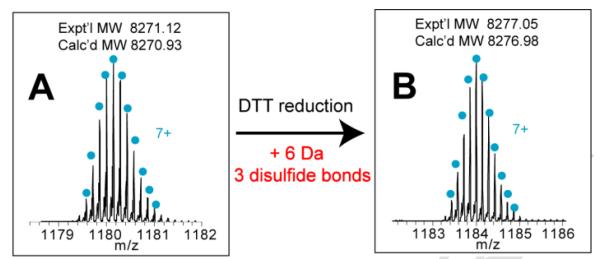
In LC fraction #18 (Figure 2), accurate mass measurement showed a peptide candidate with MW 8271.12 Da, and further DTT reduction of this peptide produced 6 Da of mass increase (Figure 6). These observations matched the two unique features of the CHH neuropeptide family, i.e., MW ranging from 8-10 kDa and PTMs containing thee disulfide bonds, so this peptide was tentatively assigned as the CHH candidate in P. interruptus (termed as Pai−CHH).
Figure 6. Determination of disulfide bond numbers in Pai−CHH.
High resolution zoom-in spectra of (A) intact Pai−CHH and (B) DTT-reduced Pai−CHH.
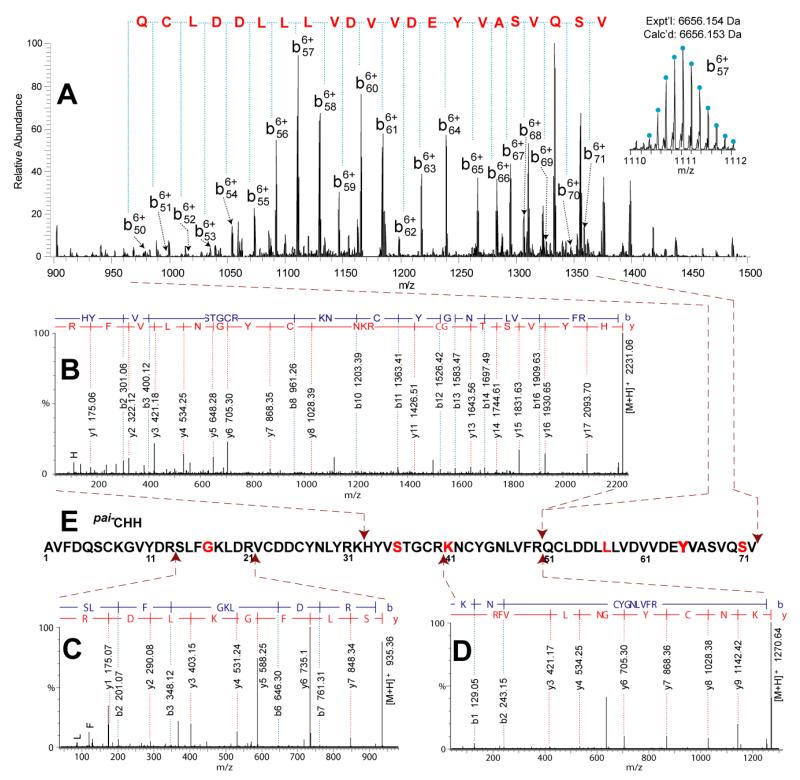
Different from the mid-size peptides, tryptic digestion of large CHH can produce tryptic segments matching to the homologous sequences. Here, the homology search of CHH-family neuropeptides was performed by bottom-up proteomic method. The tryptic digest of the Pai−CHH candidate was analyzed by LC-MS/MS on an ESI-QTOF mass spectrometer, followed by Mascot searching against a NCBI protein database. The first hit was the intact Jal−CHH peptide from Jasus lalandii [46] with 45% homology match (Figure S-6), which was further used as reference sequence to conduct the bottom-up de novo sequencing.
Bottom-up de novo sequencing was carried out using Pepseq to interpret the tryptic peptide sequences. However, it is challenging to de novo sequence the homologous tryptic peptides which contain different residues from the reference sequence Jal−CHH. We tried Spider Homology Search in PEAKS [47], where the bottom-up MS/MS data was searched against a home-built database containing Jal−CHH. However, no hit was found. Alternatively, we manually sequenced the homologous tryptic peptides. The tryptic peptides were selected according to two criteria, i.e., MW < reference tryptic peptide ±129 Da (based on the assumption that a single amino acid substitution may occur from the reference sequence and the largest amino acid residue difference between tryptophan and glycine equals 129 Da) and ion intensity > 200 counts (based on typical precursor ion threshold for obtaining decent MS/MS spectra), and then submitted to Pepseq for sequence interpretation. Five new tryptic peptides were determined: CHH[14-21], CHH[14-32], CHH[32-50], CHH[33-50], and CHH [41-50] (sequences shown in Table 2), which contains residues different from the reference sequence Jal−CHH. By this bottom-up sequencing method, the first 50 amino acid portion AA1-AA50 was determined. The sequence assembly is shown in Figure 7E, and all the tryptic peptides are listed in Table 2.
Table 2.
Tryptic peptides of CHH identified by bottom-up de novo sequencing method.
| Exptl MW | Calcd MW | Sequence a | Tryptic peptide | MS/MS spectrum b |
|---|---|---|---|---|
| 1543.50 | 1543.71 | AVFDQSCKGVYDR | CHH[1-13] | Fig. S7 |
| 2075.66 | 2076.01 | AVFDQSCKGVYDRSLFGK | CHH[1-18] | – |
| 934.36 | 934.52 | SLFGKLDR | CHH[14-21] | Fig. 7C |
| 2420.79 | 2421.15 | SLFGKLDRVCDDCYNLYRK | CHH[14-32] | – |
| 1760.49 | 1760.76 | LDRVCDDCYNLYR | CHH[19-31] | Fig. S7 |
| 1888.53 | 1888.85 | LDRVCDDCYNLYRK | CHH[19-32] | – |
| 1376.35 | 1376.55 | VCDDCYNLYR | CHH[22-31] | Fig. S7 |
| 1504.39 | 1504.64 | VCDDCYNLYRK | CHH[22-32] | Fig. S7 |
| 2358.16 | 2358.15 | KHYVSTGCRKNCYGNLVFR | CHH[32-50] | – |
| 2230.06 | 2230.05 | HYVSTGCRKNCYGNLVFR | CHH[33-50] | Fig. 7B |
| 1269.64 | 1269.63 | KNCYGNLVFR | CHH[41-50] | Fig. 7D |
| 1141.35 | 1141.53 | NCYGNLVFR | CHH[42-50] | Fig. S7 |
Fixed carbamidomethyl modification is on cysteine. The residues different from the homologous sequence are highlighted with underline.
Data is selectively shown for covering the sequence CHH[1-50]. CHH[51-71] was determined by top-down de novo sequencing.
Figure 7. De novo sequencing MS/MS spectra of Pai−CHH.
(A) Top-down CID spectra of DTT-reduced Pai−CHH. Bottom-up MS/MS spectra of (B) Pai−CHH[33-50], (C) Pai−CHH[14-21] and (D) Pai−CHH[41-50]. (E) Sequence assembly of Pai−CHH. The dipeptide segments 38GC39, 45GN46, and 49FR50 were assigned according to the homologous sequence Jal−CHH.
Top-down MS-based sequencing was then performed to determine the remaining residues AA51-AA72. The Pai−CHH peptide was treated with DTT to reduce disulfide bonds according to our previous report [29], and then was directly infused into ESI-LTQ-FTICR for CID and ECD fragmentation. In Figure 7A, a set of continuous b ions, b50 – b71, was clearly detected, leading to confident identification of the remaining residues as 51QCLDDLLLVDVVDEYVASVQSV72. Figure 8B are the ECD and CID fragmentation maps of Pai−CHH, which further confirms the bottom-up MS results described above, especially on the different residues from the reference sequence. For example, the Gly9 residue was confirmed by observation of c8, c9 and z63, z64 ion pairs. The sequence assembly is illustrated in Figure 7E, and further sequence alignment of CHHs from homologous species was shown in Figure S-8. A combination of the bottom-up and top-down methods not only provided complementary sequence interpretation, but also increased the local identification confidence on each amino acid residue of this large peptide hormone.
CONCLUSIONS
This study systematically evaluated limitations and several improvements of current approaches to the discovery of neuropeptides of various sizes, which facilitates the rational design of methodology for comprehensive characterization of neuropeptides in the crustacean nervous system. A multi-scale strategy was established enabling accurate identification of nine novel neuropeptides spanning a wide range of molecular sizes in P. interruptus sinus gland. The results provide a foundation for future functional and mechanistic studies of these novel neuropeptides.
Supplementary Material
Highlights.
Sequence coverage of neuropeptide analysis revealed limitations to mid- and large-size peptide analysis.
A multi-scale strategy was established for elucidation of various sizes of peptides.
Nine novel neuropeptides (MW 0.9-8.2 kDa) were fully sequenced in the spiny lobster P. interruptus.
SIGNIFICANCE.
Mass spectrometry (MS)-based neuropeptidomics aims to completely characterize the neuropeptides in a target organism as an important first step toward a better understanding of the structure and function of these complex signaling molecules. Although liquid chromatography coupled to tandem mass spectrometry (LC-MS/MS) with data-dependent acquisition is a powerful tool in peptidomic research, it often lacks the capability for de novo sequencing of mid-size and large peptides due to inefficient fragmentation of peptides larger than 4 kDa. This study describes a multi-scale strategy for complete and confident sequence elucidation of various sizes of neuropeptides in the crustacean nervous system. The aim is to fill a technical gap where the conventional strategy is inefficient for comprehensive characterization of a complex neuropeptidome without assistance of genomic information. Nine novel neuropeptides in a wide range of molecular weights (0.9-8.2 kDa) were fully sequenced from a major neuroendocrine organ of the spiny lobster, Panulirus interruptus. The resulting molecular information extracted from such multi-scale peptidomic analysis will greatly accelerate functional studies of these novel neuropeptides.
ACKNOWLEGMENTS
The authors wish to thank Prof. Neil L. Kelleher’s group at Northwestern University for access to an LTQ-Orbitrap Elite system and Drs. Adam D. Catherman and Paul M. Thomas from the Kelleher group for instrument assistance. We would also like to thank Prof. Deborah Baro from the Georgia State University for providing some of the spiny lobsters used in this study. We are also grateful to Prof. Ying Ge at UW-Madison for helpful suggestions on top-down MS analysis and Matt Lawrence at the UW Human Proteomics Program for experimental assistance with the LTQ-FTICR instrument. This work is supported in part by the National Institutes of Health grant (R01DK071801 to LL) and the National Science Foundation grant (CHE-0967784 to LL). LL acknowledges an H. I. Romnes Faculty Research Fellowship. CJ thanks an Oversea Training Fellowship and UW Vilas Conference Presentation Funds. CBL acknowledges an NIH-supported Chemistry Biology Interface Training Program Predoctoral Fellowship (grant number T32-GM008505) and an NSF predoctoral graduate fellowship.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Li L, Sweedler JV. Peptides in the brain: mass spectrometry-based measurement approaches and challenges. Annu Rev Anal Chem. 2008;1:451–83. doi: 10.1146/annurev.anchem.1.031207.113053. [DOI] [PubMed] [Google Scholar]
- [2].Mykles DL, Adams ME, Gade G, Lange AB, Marco HG, Orchard I. Neuropeptide action in insects and crustaceans. Physiol Biochem Zool. 2010;83:836–46. doi: 10.1086/648470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Christie AE, Stemmler EA, Dickinson PS. Crustacean neuropeptides. Cell Mol Life Sci. 2010;67:4135–69. doi: 10.1007/s00018-010-0482-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Hummon AB, Richmond TA, Verleyen P, Baggerman G, Huybrechts J, Ewing MA, et al. From the genome to the proteome: uncovering peptides in the Apis brain. Science. 2006;314:647–9. doi: 10.1126/science.1124128. [DOI] [PubMed] [Google Scholar]
- [5].Che FY, Yuan Q, Kalinina E, Fricker LD. Peptidomics of Cpe fat/fat mouse hypothalamus: effect of food deprivation and exercise on peptide levels. J Biol Chem. 2005;280:4451–61. doi: 10.1074/jbc.M411178200. [DOI] [PubMed] [Google Scholar]
- [6].Dowell JA, Heyden WV, Li L. Rat neuropeptidomics by LC-MS/MS and MALDI-FTMS: Enhanced dissection and extraction techniques coupled with 2D RP-RP HPLC. J Proteome Res. 2006;5:3368–75. doi: 10.1021/pr0603452. [DOI] [PubMed] [Google Scholar]
- [7].Fricker LD, Lim J, Pan H, Che FY. Peptidomics: identification and quantification of endogenous peptides in neuroendocrine tissues. Mass Spectrom Rev. 2006;25:327–44. doi: 10.1002/mas.20079. [DOI] [PubMed] [Google Scholar]
- [8].Jimenez CR, Spijker S, de Schipper S, Lodder JC, Janse CK, Geraerts WP, et al. Peptidomics of a single identified neuron reveals diversity of multiple neuropeptides with convergent actions on cellular excitability. J Neurosci. 2006;26:518–29. doi: 10.1523/JNEUROSCI.2566-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Fricker LD. Neuropeptidomics to study peptide processing in animal models of obesity. Endocrinology. 2007;148:4185–90. doi: 10.1210/en.2007-0123. [DOI] [PubMed] [Google Scholar]
- [10].Falth M, Skold K, Svensson M, Nilsson A, Fenyo D, Andren PE. Neuropeptidomics strategies for specific and sensitive identification of endogenous peptides. Mol Cell Proteomics. 2007;6:1188–97. doi: 10.1074/mcp.M700016-MCP200. [DOI] [PubMed] [Google Scholar]
- [11].Hatcher NG, Atkins N, Jr., Annangudi SP, Forbes AJ, Kelleher NL, Gillette MU, et al. Mass spectrometry-based discovery of circadian peptides. Proc Natl Acad Sci U S A. 2008;105:12527–32. doi: 10.1073/pnas.0804340105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Zhang X, Petruzziello F, Zani F, Fouillen L, Andren PE, Solinas G, et al. High Identification Rates of Endogenous Neuropeptides from Mouse Brain. J Proteome Res. 2012;11:2819–27. doi: 10.1021/pr3001699. [DOI] [PubMed] [Google Scholar]
- [13].Shen Y, Tolic N, Xie F, Zhao R, Purvine SO, Schepmoes AA, et al. Effectiveness of CID, HCD, and ETD with FT MS/MS for degradomic-peptidomic analysis: comparison of peptide identification methods. J Proteome Res. 2011;10:3929–43. doi: 10.1021/pr200052c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Chen R, Hui L, Cape SS, Wang J, Li L. Comparative Neuropeptidomic Analysis of Food Intake via a Multi-faceted Mass Spectrometric Approach. ACS Chem Neurosci. 2010;1:204–14. doi: 10.1021/cn900028s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Ma M, Wang J, Chen R, Li L. Expanding the Crustacean neuropeptidome using a multifaceted mass spectrometric approach. J Proteome Res. 2009;8:2426–37. doi: 10.1021/pr801047v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Ma M, Bors EK, Dickinson ES, Kwiatkowski MA, Sousa GL, Henry RP, et al. Characterization of the Carcinus maenas neuropeptidome by mass spectrometry and functional genomics. Gen Comp Endocrinol. 2009;161:320–34. doi: 10.1016/j.ygcen.2009.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Lee JE, Atkins N, Jr., Hatcher NG, Zamdborg L, Gillette MU, Sweedler JV, et al. Endogenous peptide discovery of the rat circadian clock: a focused study of the suprachiasmatic nucleus by ultrahigh performance tandem mass spectrometry. Mol Cell Proteomics. 2010;9:285–97. doi: 10.1074/mcp.M900362-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Bai L, Romanova EV, Sweedler JV. Distinguishing endogenous D-amino acid-containing neuropeptides in individual neurons using tandem mass spectrometry. Anal Chem. 2011;83:2794–800. doi: 10.1021/ac200142m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Chen R, Ma M, Hui L, Zhang J, Li L. Measurement of neuropeptides in crustacean hemolymph via MALDI mass spectrometry. J Am Soc Mass Spectrom. 2009;20:708–18. doi: 10.1016/j.jasms.2008.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Tran JC, Zamdborg L, Ahlf DR, Lee JE, Catherman AD, Durbin KR, et al. Mapping intact protein isoforms in discovery mode using top-down proteomics. Nature. 2011;480:254–8. doi: 10.1038/nature10575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].McLafferty FW. A century of progress in molecular mass spectrometry. Annu Rev Anal Chem. 2012;4:1–22. doi: 10.1146/annurev-anchem-061010-114018. [DOI] [PubMed] [Google Scholar]
- [22].Thuma JB, White WE, Hobbs KH, Hooper SL. Pyloric neuron morphology in the stomatogastric ganglion of the lobster, Panulirus interruptus. Brain Behav Evol. 2009;73:26–42. doi: 10.1159/000202988. [DOI] [PubMed] [Google Scholar]
- [23].Thuma JB, Harness PI, Koehnle TJ, Morris LG, Hooper SL. Muscle anatomy is a primary determinant of muscle relaxation dynamics in the lobster (Panulirus interruptus) stomatogastric system. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2007;193:1101–13. doi: 10.1007/s00359-007-0261-7. [DOI] [PubMed] [Google Scholar]
- [24].Ouyang Q, Goeritz M, Harris-Warrick RM. Panulirus interruptus Ih-channel gene PIIH: modification of channel properties by alternative splicing and role in rhythmic activity. J Neurophysiol. 2007;97:3880–92. doi: 10.1152/jn.00246.2007. [DOI] [PubMed] [Google Scholar]
- [25].Hopkins PM. The eyes have it: A brief history of crustacean neuroendocrinology. Gen Comp Endocrinol. 2012;175:357–66. doi: 10.1016/j.ygcen.2011.12.002. [DOI] [PubMed] [Google Scholar]
- [26].Marder E. Neuromodulation of neuronal circuits: back to the future. Neuron. 2012;76:1–11. doi: 10.1016/j.neuron.2012.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Selverston AI, Szucs A, Huerta R, Pinto R, Reyes M. Neural mechanisms underlying the generation of the lobster gastric mill motor pattern. Front Neural Circuits. 2009;3:1–12. doi: 10.3389/neuro.04.012.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Marder E, Bucher D. Understanding circuit dynamics using the stomatogastric nervous system of lobsters and crabs. Annu Rev Physiol. 2007;69:291–316. doi: 10.1146/annurev.physiol.69.031905.161516. [DOI] [PubMed] [Google Scholar]
- [29].Jia C, Hui L, Cao W, Lietz CB, Jiang X, Chen R, et al. High-definition De Novo Sequencing of Crustacean Hyperglycemic Hormone (CHH)-family Neuropeptides. Mol Cell Proteomics. 2012;11:1951–64. doi: 10.1074/mcp.M112.020537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Fu Q, Goy MF, Li L. Identification of neuropeptides from the decapod crustacean sinus glands using nanoscale liquid chromatography tandem mass spectrometry. Biochem Biophys Res Commun. 2005;337:765–78. doi: 10.1016/j.bbrc.2005.09.111. [DOI] [PubMed] [Google Scholar]
- [31].Hui L, Cunningham R, Zhang Z, Cao W, Jia C, Li L. Discovery and characterization of the Crustacean hyperglycemic hormone precursor related peptides (CPRP) and orcokinin neuropeptides in the sinus glands of the blue crab Callinectes sapidus using multiple tandem mass spectrometry techniques. J Proteome Res. 2011;10:4219–29. doi: 10.1021/pr200391g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Ge Y, Rybakova IN, Xu Q, Moss RL. Top-down high-resolution mass spectrometry of cardiac myosin binding protein C revealed that truncation alters protein phosphorylation state. Proc Natl Acad Sci U S A. 2009;106:12658–63. doi: 10.1073/pnas.0813369106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Petruzziello F, Fouillen L, Wadensten H, Kretz R, Andren PE, Rainer G, et al. Extensive characterization of Tupaia belangeri neuropeptidome using an integrated mass spectrometric approach. J Proteome Res. 2012;11:886–96. doi: 10.1021/pr200709j. [DOI] [PubMed] [Google Scholar]
- [34].Clynen E, Baggerman G, Huybrechts J, Vanden Bosch L, De Loof A, Schoofs L. Peptidomics of the locust corpora allata: identification of novel pyrokinins (-FXPRLamides) Peptides. 2003;24:1493–500. doi: 10.1016/j.peptides.2003.10.006. [DOI] [PubMed] [Google Scholar]
- [35].Michalski A, Damoc E, Lange O, Denisov E, Nolting D, Muller M, et al. Ultra High Resolution Linear Ion Trap Orbitrap Mass Spectrometer (Orbitrap Elite) Facilitates Top Down LC MS/MS and Versatile Peptide Fragmentation Modes. Mol Cell Proteomics. 2012;11:1–11. doi: 10.1074/mcp.O111.013698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Ma M, Chen R, Ge Y, He H, Marshall AG, Li L. Combining bottom-up and top-down mass spectrometric strategies for de novo sequencing of the crustacean hyperglycemic hormone from Cancer borealis. Anal Chem. 2009;81:240–7. doi: 10.1021/ac801910g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Webster SG, Keller R, Dircksen H. The CHH-superfamily of multifunctional peptide hormones controlling crustacean metabolism, osmoregulation, moulting, and reproduction. Gen Comp Endocrinol. 2012;175:217–33. doi: 10.1016/j.ygcen.2011.11.035. [DOI] [PubMed] [Google Scholar]
- [38].Szabo TM, Chen R, Goeritz ML, Maloney RT, Tang LS, Li L, et al. Distribution and physiological effects of B-type allatostatins (myoinhibitory peptides, MIPs) in the stomatogastric nervous system of the crab Cancer borealis. J Comp Neurol. 2011;519:2658–76. doi: 10.1002/cne.22654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Boersema PJ, Raijmakers R, Lemeer S, Mohammed S, Heck AJ. Multiplex peptide stable isotope dimethyl labeling for quantitative proteomics. Nat Protoc. 2009;4:484–94. doi: 10.1038/nprot.2009.21. [DOI] [PubMed] [Google Scholar]
- [40].Yang SJ, Nie AY, Zhang L, Yan GQ, Yao J, Xie LQ, et al. A novel quantitative proteomics workflow by isobaric terminal labeling. J Proteomics. 2012;75:5797–806. doi: 10.1016/j.jprot.2012.07.011. [DOI] [PubMed] [Google Scholar]
- [41].Hsu JL, Huang SY, Chow NH, Chen SH. Stable-isotope dimethyl labeling for quantitative proteomics. Anal Chem. 2003;75:6843–52. doi: 10.1021/ac0348625. [DOI] [PubMed] [Google Scholar]
- [42].Audsley N, Matthews HJ, Price NR, Weaver RJ. Allatoregulatory peptides in Lepidoptera, structures, distribution and functions. J Insect Physiol. 2008;54:969–80. doi: 10.1016/j.jinsphys.2008.01.012. [DOI] [PubMed] [Google Scholar]
- [43].Stay B, Tobe SS. The role of allatostatins in juvenile hormone synthesis in insects and crustaceans. Annu Rev Entomol. 2007;52:277–99. doi: 10.1146/annurev.ento.51.110104.151050. [DOI] [PubMed] [Google Scholar]
- [44].Chung JS, Zmora N, Katayama H, Tsutsui N. Crustacean hyperglycemic hormone (CHH) neuropeptides family: Functions, titer, and binding to target tissues. Gen Comp Endocrinol. 2009;166:447–54. doi: 10.1016/j.ygcen.2009.12.011. [DOI] [PubMed] [Google Scholar]
- [45].Bonet-Costa C, Vilaseca M, Diema C, Vujatovic O, Vaquero A, Omenaca N, et al. Combined bottom-up and top-down mass spectrometry analyses of the pattern of post-translational modifications of Drosophila melanogaster linker histone H1. J Proteomics. 2012;75:4124–38. doi: 10.1016/j.jprot.2012.05.034. [DOI] [PubMed] [Google Scholar]
- [46].Marco HG, Hansen IA, Scheller K, Gade G. Molecular cloning and localization of a cDNA encoding a crustacean hyperglycemic hormone from the South African spiny lobster, Jasus lalandii. Peptides. 2003;24:845–51. doi: 10.1016/s0196-9781(03)00168-2. [DOI] [PubMed] [Google Scholar]
- [47].Zhang J, Xin L, Shan BZ, Chen WW, Xie MJ, Yuen D, et al. PEAKS DB: De Novo Sequencing Assisted Database Search for Sensitive and Accurate Peptide Identification. Mol Cell Proteomics. 2012;11:1–8. doi: 10.1074/mcp.M111.010587. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.