Abstract
Background
Data regarding the difference in the clinical course from metastasis to prostate cancer–specific mortality (PCSM) following radical prostatectomy (RP) compared with radiation therapy (RT) are lacking.
Objective
To examine the association between primary treatment modality and prostate cancer– specific survival (PCSS) after metastasis.
Design, setting, and participants
We used the Surveillance Epidemiology and End Results– Medicare linked database from 1994 to 2007 for patients diagnosed with localized prostate cancer (PCa). We used cancer stage and Gleason score to stratify patients into low and intermediate–high risks.
Intervention
Radical prostatectomy or radiation therapy.
Outcome measurements and statistical analysis
Our outcome is time from onset of metastases to PCSM. Propensity score matching and Cox regression were used to analyze the PCSM hazard for the RP group compared with the RT group.
Results and limitations
Our study consisted of 66 492 men diagnosed with PCa, 51 337 men receiving RT, and 15 155 men undergoing RP within 1 yr of cancer diagnosis. During the study period, 2802 men were diagnosed as having metastatic disease. A total of 916 men with metastases were included in the propensity-matched cohort; of these men, 186 died from PCa. During the follow-up, for the low-risk patients, the adjusted PCSS after metastasis was 86.2% and 79.3% in the RP and RT groups, respectively; for the intermediate–high-risk patients, the PCSS after metastasis was 76.3% and 63.3% in the RP and RT groups, respectively. The hazard ratios estimating the risk of PCSM between the RP and RT groups were 0.68 (95% confidence interval [CI], 0.38–1.22) and 0.51 (95% CI, 0.36–0.73) for the low- and intermediate–high-risk groups, respectively. Because of the nature of observational studies, the results may be affected by residual confounders and treatment indication.
Conclusions
Following the development of metastases, men who received primary RP have a longer PCSS than men who received primary RT. Our results may have implications for the timing and nature of local PCa treatment.
Keywords: Prostate cancer, Neoplasm metastasis, Radiation therapy, Prostatectomy
1. Introduction
Since the adoption of prostate-specific antigen (PSA) as a screening tool, more men have received a diagnosis of prostate cancer (PCa) and have undergone treatment earlier than in the pre-PSA era [1]. Given the prolonged natural history of PCa, management requires careful consideration of the severity of the disease, the health of the patient, and the benefits and risks of intervention. Radical prostatectomy (RP) and radiation therapy (RT) are two common interventions for localized PCa [2,3]. However, there is no conclusive evidence that either treatment is superior to the other in terms of cancer control or functional outcome [4]. Although retrospective studies have compared the two treatments in terms of rates of biochemical failure, metastasis-free survival, and PCa-specific survival (PCSS) [5–7], data regarding the difference in the clinical course from metastasis to death following RP compared with RT are lacking. Mortality [8] and morbidity precipitously increase once metastases develop, but the biologic processes that underlie the development of tumor metastasis and affect the natural history of disease afterward are not well understood. We undertook this study to examine the impact of primary treatment modality on PCSS after metastasis.
2. Methods
2.1. Study population
We used data from the Surveillance Epidemiology and End Results (SEER) database linked to Medicare claims. SEER provides a nearly representative sample of approximately 26% of the US population [9]. Our cohort included PCa patients aged 66–85 yr from 1994 to 2007. Data on patients with incomplete Medicare records during the study follow-up (ie, patients not continuously enrolled in both Medicare Part A and Part B and patients who enrolled in health maintenance organizations) were excluded. The sample was limited to 119 997 men diagnosed with incident localized PCa. We excluded men who were diagnosed as metastatic; who received palliative treatments; who had RP, RT, or androgen-deprivation therapy (ADT) treatment before PCa diagnosis (n = 23 040); who were without cancer grade (n = 3331); or who were without primary treatments (n = 21 889). We further excluded men who received RT with a modality other than brachytherapy, intensity-modulated radiotherapy (IMRT), three-dimensional conformal radiotherapy (3D CRT), or a combination (n = 4589) or who received both RP and RT during the follow-up (n = 656). After exclusion criteria, a total of 66 492 men were included in the study.
2.2. Outcome variables
The primary outcome was PCSS after metastases. We created an algorithm [10] to identify metastasis in men diagnosed with PCa from Medicare claims. A diagnosis of metastases had to meet the following conditions: (1) at least two claims with International Classification of Diseases, Ninth Revision (ICD-9), codes 198.5 (bone and bone marrow), 197.0 (lung), 197.7 (liver), or 198.3 (brain and spinal cord) and (2) two Medicare claims separated by 30 d to minimize false positives. We defined the date of metastasis as the earliest occurrence of one of the previously mentioned claims patterns at any time during follow-up. The occurrence of PCa-specific mortality (PCSM) was determined from SEER cause-of-death data through December 31, 2007.
2.3. Study covariates
The study population was divided into the following age cohorts: 66–69, 70–74, 75–79, and ≥80 yr at diagnosis. Clinical stage (extent of disease in SEER) was categorized into T1 or T2 using the American Joint Committee on Cancer classification system [11]. The SEER registry described cancer stage as well differentiated, moderately differentiated, and poorly differentiated based on a Gleason score of 2–4, 5–7, and 8–10, respectively, before 2003. Starting in 2003, Gleason 7 was reclassified from moderately differentiated to poorly differentiated. The Charlson score was derived from Medicare claims during the year prior to PCa diagnosis using a validated algorithm [12]. Participation of state buy-in was included in the study as a proxy for poverty. Because of the lack of PSA data before 2004, PSA was not used to classify risk levels. Patients with well-differentiated or moderately differentiated tumor and cancer stage ≤T2a were categorized as low risk. Patients who did not have low-risk cancer were grouped in the intermediate–high-risk category.
We searched for Medicare claim records of computed tomography, magnetic resonance imaging, and radionuclide bone scanning [13] from the last date of primary treatments to metastasis. We also abstracted records of chemotherapy and ADT from 180 d after primary treatments to metastasis and after.
2.4. Statistical methods
To compare the differences in proportions of baseline characteristics between RT and RP, χ2tests were used. The cumulative incidence of PCSM, treating other causes of death as a competing risk, was computed to estimate the PCSS [14]. The median follow-up time was computed using Kaplan-Meier methods [15].
We adopted the propensity score–matching method [16] to balance observed covariates between RT and RP. Propensity scores reflect the probability that a patient received RT or RP based on his baseline characteristics. We defined the logit of predicted probability of treatment as a propensity score using the following baseline characteristics: age, race, year of diagnosis, SEER region, state buy-in, comorbidity, and cancer grade/stage. Subjects receiving RT were matched on a one-to-one basis with subjects receiving RP. Matching was performed based on nearest-neighbor matching, and RP and RT patients were matched within their respective risk groups. With time from metastasis to PCSM as the response variable, the Cox regression method was used to analyze hazard ratios (HRs) and 95% confidence intervals (CIs) for PCSM for RP compared with RT. Finally, we performed a sensitivity analysis to measure the potential influence that an unmeasured confounder might have on the HR estimates.
Descriptive analysis and propensity score matching were performed using SAS statistical software v.9.2 (SAS Institute, Cary, NC, USA). Cox regressions were carried out using R v.2.13, (R Foundation for Statistical Computing, Vienna, Austria). Sensitivity analyses were conducted using Microsoft Excel. Statistical significance was set at 0.05, and all tests were two-tailed.
3. Results
3.1. Baseline characteristics of men at diagnosis
Among a total of 66 492 men, 51 337 men receiving RT and 15 155 men receiving RP within 1 yr of cancer diagnosis were included in the analysis (Table 1). The median follow-up is 7.3 yr (interquartile range [IQR]: 4.7–9.9) from diagnosis. Among these 66 492 men, 2802 were diagnosed with metastases during the follow-up. Propensity score matching was performed on these men with metastases, with a resultant 342 men in the low-risk group and 574 men in the intermediate–high-risk group. Table 2 and Table 3 demonstrate the differences in baseline characteristics between men with metastases who received primary RT compared with RP. These differences decreased substantially after propensity score matching.
Table 1.
Baseline characteristics of 66 492 men diagnosed with clinically localized (T1 or T2) prostate cancer, according to treatment
| Characteristic | Treatment |
|||
|---|---|---|---|---|
| Irradiation, no. (%) (n = 51 337) |
Radical prostatectomy, no. (%) (n = 15 155) |
p value | ||
| Age, yr | ||||
| 66–69 | 10 434 ( 20.3) | 7717 ( 50.9) | ||
| 70–74 | 19 608 ( 38.2) | 6053 ( 39.9) | <0.0001 | |
| 75–79 | 15 987 ( 31.1) | 1234 ( 8.1) | ||
| ≥80 | 5308 ( 10.3) | 151 ( 1.0) | ||
| Race | ||||
| White | 43 084 ( 83.9) | 12973 ( 85.6) | ||
| Black | 4718 ( 9.2) | 1157 ( 7.6) | <0.0001 | |
| Others | 3535 ( 6.9) | 1025 ( 6.8) | ||
| Year of diagnosis | ||||
| 2000 or earlier | 16 224 ( 31.6) | 6406 ( 42.3) | ||
| 2001 or later | 35 113 ( 68.4) | 8749 ( 57.7) | <0.0001 | |
| Region | ||||
| North central | 10 816 ( 21.1) | 2947 ( 19.5) | ||
| Northeast | 11 481 ( 22.4) | 1474 ( 9.7) | <0.0001 | |
| South | 7001 ( 13.6) | 1659 ( 10.9) | ||
| West | 22 039 ( 42.9) | 9075 ( 59.9) | ||
| Comorbidity | ||||
| 0 | 38 543 ( 75.1) | 12 998 ( 85.8) | ||
| 1 | 9124 ( 17.8) | 1719 ( 11.3) | <0.0001 | |
| ≥2 | 3670 ( 7.2) | 438 ( 2.9) | ||
| Cancer grade | ||||
| 1994–2002 | ||||
| Well differentiated | 1444 (5.4) | 470 ( 5.4) | ||
| Moderately differentiated | 20 262 ( 75.5) | 6899 ( 78.8) | <0.0001 | |
| Poorly differentiated | 5117 ( 19.1) | 1392 ( 15.9) | ||
| 2003 or later | ||||
| Well differentiated | 165 ( 0.7) | 49 ( 0.8) | ||
| Moderately differentiated | 12 343 ( 50.4) | 3140 ( 49.1) | 0.169 | |
| Poorly differentiated | 12 006 (48.9 ) | 3205 ( 50.1) | ||
| Cancer stage | ||||
| T1 | 22 828 (44.5) | 6803 ( 44.9) | 0.013 | |
| T2a | 7246 (14.1) | 1996 ( 13.2) | ||
| ≥T2b | 21 26 (41.4) | 6356 ( 41.9) | ||
Cancer grade: The Surveillance Epidemiology and End Results registry used a system of describing tumors as well differentiated, moderately differentiated, and poorly differentiated based on a Gleason score of 2–4, 5–7, and 8–10, respectively, before 2003. Starting in 2003, code 7 was reclassified from moderately differentiated to poorly differentiated.
Table 2.
Baseline characteristics for men diagnosed as having metastasis after treatment, low risk at cancer diagnosis
| Characteristic | Overall cohort | Propensity-matched cohort | ||||
|---|---|---|---|---|---|---|
| Irradiation, no. (%) (n = 708) |
Radical prostatectomy, no. (%) (n = 178) |
SD* | Irradiation, no. (%) (n = 171) |
Radical prostatectomy, no. (%) (n = 171) |
SD* | |
| Age, yr | ||||||
| 66–69 | 169 ( 23.9) | 78 ( 43.8) | 0.4313 | 70 ( 40.9) | 71 ( 41.5) | 0.0119 |
| 70–74 | 282 ( 39.8) | 89 ( 50.0) | 0.2055 | 90 ( 52.6) | 89 ( 52.1) | 0.0117 |
| 75–79 | 209 ( 29.5) | 10 ( 5.6) | 0.6615 | 10 ( 5.9) | 10 ( 5.9) | 0 |
| ≥80 | 48 ( 6.8) | 1 ( 0.6) | 0.3353 | 1 ( 0.6) | 1 ( 0.6) | 0 |
| Race | ||||||
| White | 590 ( 83.3) | 158 ( 88.8) | 0.1572 | 141 ( 82.5) | 152 ( 88.9) | 0.1844 |
| Black | 65 ( 9.2) | 11 ( 6.2) | 0.1129 | 17 ( 9.9) | 10 ( 5.9) | 0.1522 |
| Others | 53 ( 7.5) | 9 ( 5.1) | 0.1003 | 13 ( 7.6) | 9 ( 5.3) | 0.0955 |
| Year of diagnosis | ||||||
| 2000 or earlier | 409 ( 57.8) | 143 ( 80.3) | 0.5034 | 136 ( 79.5) | 136 ( 79.5) | 0 |
| 2001 or later | 299 ( 42.2) | 35 ( 19.7) | 0.5034 | 35 ( 20.5) | 35 ( 20.5) | 0 |
| Region | ||||||
| North central | 179 ( 25.3) | 41 ( 23.0) | 0.0526 | 41 ( 23.9) | 41 ( 23.9) | 0 |
| Northeast | 166 ( 23.5) | 13 ( 7.3) | 0.4592 | 13 ( 7.6) | 13 ( 7.6) | 0 |
| South | 99 ( 13.9) | 12 ( 6.7) | 0.2393 | 12 ( 7.0) | 12 ( 7.0) | 0 |
| West | 264 ( 37.3) | 112 ( 62.9) | 0.5304 | 105 ( 61.4) | 105 ( 61.4) | 0 |
| Comorbidity | ||||||
| 0 | 542 ( 76.6) | 160 ( 89.9) | 0.3626 | 153 ( 89.5) | 153 ( 89.5) | 0 |
| ≥1 | 166 ( 23.5) | 18 ( 10.1) | 0.3626 | 18 ( 10.5) | 18 ( 10.5) | 0 |
| Cancer stage | ||||||
| T1 | 462 (65.3) | 128 ( 71.9) | 0.1438 | 122 ( 71.4) | 121 ( 70.8) | 0.0129 |
| T2a | 246 (34.8) | 50 ( 28.1) | 0.1438 | 49 ( 28.7) | 50 ( 29.2) | 0.0129 |
| ≥T2b | NA | NA | ||||
| Cancer grade Well differentiated |
42 ( 5.9) | 19 ( 10.7) | 0.1725 | 20 ( 11.7) | 19 ( 11.1) | 0.0184 |
| Moderately differentiated State buy-in |
666 ( 94.1) | 159 ( 89.3) | 0.1725 | 151 ( 88.3) | 152 ( 88.9) | 0.0184 |
| No | 651 (91.9) | 168 (94.4) | 0.0965 | 157 (91.8) | 161 ( 94.2) | 0.0917 |
| Yes | 57 (8.1) | 10 (5.6) | 0.0965 | 14 ( 8.2) | 10 ( 5.9) | 0.0917 |
SD = standardized difference.
SD = |P1 - P2| / square root of (P1[1 – P1] + P2[1 – P2] / 2). SDs are the same for all categorical variables with two levels.
Cancer grade: The Surveillance Epidemiology and End Results registry used a system of describing tumors as well differentiated, moderately differentiated, and poorly differentiated based on a Gleason score of 2–4, 5–7, and 8–10, respectively, before 2003. Starting in 2003, code 7 was reclassified from moderately differentiated to poorly differentiated.
Table 3.
Baseline characteristics for men diagnosed as having metastasis after treatment, intermediate– high risk at cancer diagnosis
| Characteristic | Overall cohort | Propensity-matched cohort | ||||
|---|---|---|---|---|---|---|
| Irradiation, no. (%) (n = 1615) |
Radical prostatectomy, no. (%) (n = 301) |
SD* | Irradiation, no. (%) (n = 287) |
Radical prostatectomy, no. (%) (n = 287) |
SD* | |
| Age, yr | ||||||
| 66–69 | 315 ( 19.5) | 158 ( 52.5) | 0.7318 | 143 ( 49.8) | 144 ( 50.2) | 0.0070 |
| 70–74 | 633 ( 39.2) | 118 ( 39.2) | 0.0002 | 119 ( 41.5) | 118 ( 41.1) | 0.0071 |
| 75–79 | 471 ( 29.2) | 23 ( 7.6) | 0.5782 | 23 ( 8.0) | 23 ( 8.0) | 0 |
| ≥80 | 196 ( 12.1) | 2 ( 0.7) | 0.4821 | 2 (0.7) | 2 (0.7) | 0 |
| Race | ||||||
| White | 1389 ( 86.0) | 256 ( 85.1) | 0.0272 | 248 ( 86.4) | 244 ( 85.0) | 0.0398 |
| Black | 129 ( 7.9) | 33 ( 10.9) | 0.1017 | 30 ( 10.5) | 31 ( 10.8) | 0.0113 |
| Others | 97 ( 6.0) | 12 ( 3.9) | 0.0928 | 9 ( 3.1) | 12 ( 4.2) | 0.0557 |
| Year of diagnosis | ||||||
| 2000 or earlier | 758 (46.9) | 219 ( 72.8) | 0.5460 | 206 ( 71.8) | 205 (71.4) | 0.0077 |
| 2001 or later | 857 ( 53.1) | 82 ( 27.2) | 0.5460 | 81 ( 28.2) | 82 ( 28.6) | 0.0077 |
| Region | ||||||
| North central | 356 ( 22.0) | 65 ( 21.6) | 0.0109 | 65 ( 22.7) | 65 ( 22.7) | 0 |
| Northeast | 391 ( 24.2) | 30 ( 9.9) | 0.3854 | 32 ( 11.2) | 30 ( 10.5) | 0.0225 |
| South | 183 ( 11.3) | 34 ( 11.3) | 0.0011 | 32 ( 11.2) | 34 ( 11.9) | 0.0218 |
| West | 685 ( 42.4) | 172 ( 57.1) | 0.2978 | 158 ( 55.1) | 158 ( 55.1) | 0 |
| Comorbidity | ||||||
| 0 | 1246 ( 77.2) | 261 ( 86.7) | 0.2504 | 245 ( 85.4) | 247 ( 86.1) | 0.0199 |
| ≥1 | 369 ( 22.9) | 40 ( 13.3) | 0.2504 | 42 ( 14.6) | 40 ( 13.9) | 0.0199 |
| Cancer stage | ||||||
| T1 | 291 ( 18.0) | 55 ( 18.3) | 0.0066 | 42 ( 14.6) | 54 ( 18.8) | 0.1122 |
| T2a | 124 ( 7.7) | 25 ( 8.3) | 0.0231 | 14 ( 4.9) | 23 ( 8.0) | 0.1280 |
| ≥T2b | 1200 ( 74.3) | 221 ( 73.4) | 0.0201 | 231 ( 80.5) | 210 ( 73.2) | 0.1741 |
| Cancer grade Well differentiated |
27 (1.7) | 7 (2.3) | 0.0467 | 10 ( 3.5) | 7 ( 2.4) | 0.0617 |
| Moderately differentiated |
623 (38.6) | 132 (43.9) | 0.1074 | 138 ( 48.1) | 126 ( 43.9) | 0.0840 |
| Poorly differentiated State buy-in |
965 (59.8) | 162 (53.8) | 0.1200 | 139 ( 48.4) | 154 ( 53.7) | 0.1047 |
| No | 1466 (90.1) | 278 (92.4) | 0.0571 | 265 ( 92.3) | 266 ( 92.7) | 0.0132 |
| Yes | 149 (9.2) | 23 (7.6) | 0.0571 | 22 ( 7.7) | 21 ( 7.3) | 0.0132 |
SD = standardized difference.
SD = |P1 - P2| / square root of (P1[1 – P1] + P2[1 – P2] / 2). SDs are the same for all categorical variables with two levels.
Cancer grade: The Surveillance Epidemiology and End Results registry used a system of describing tumors as well differentiated, moderately differentiated, and poorly differentiated based on a Gleason score of 2–4, 5–7, and 8–10, respectively, before 2003. Starting in 2003, code 7 was reclassified from moderately differentiated to poorly differentiated.
3.2. Prostate cancer–specific survival
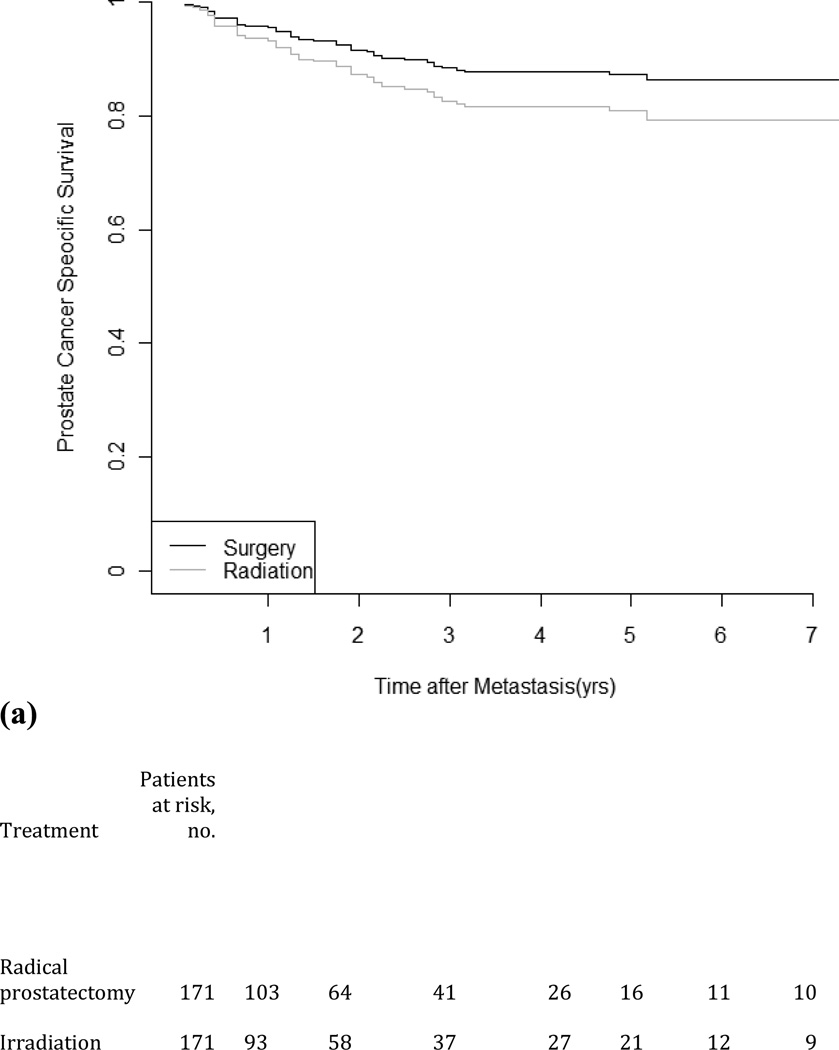
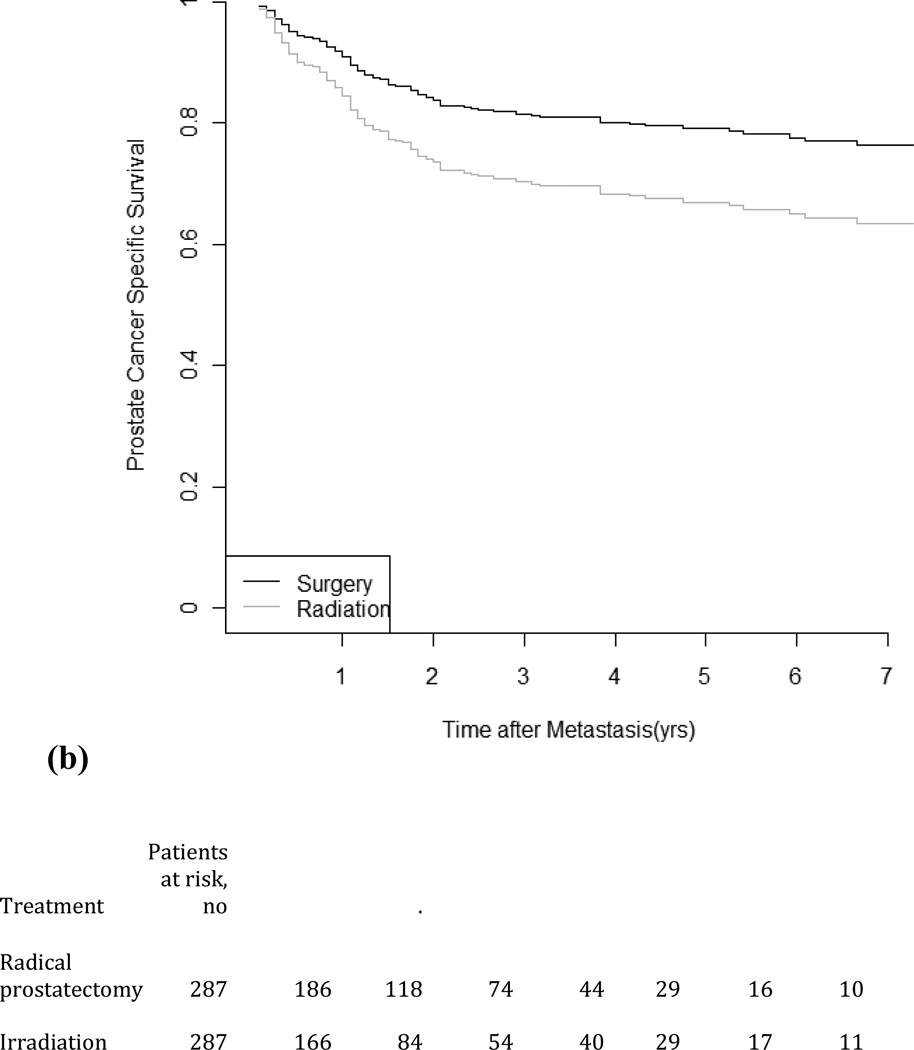
Figure 1 depicts time from metastasis to PCSM for patients receiving either primary RT or RP. The median follow-up is 33 mo (IQR: 19–59) from metastasis. Of the 916 men in the propensity score–matching cohort, 186 died from PCa during the study period (48 men in the low-risk group and 138 men in the intermediate–high-risk group). The median survival time from metastasis was 30 mo (IQR: 10–95) for RP and 26 mo (IQR: 8–101) for RT. While there was little difference in time to metastases between RT and RP, PCSS after metastasis for the follow-up period was 86.2% for RP and 79.3% for RT among the low-risk patients and was 76.3% for RP and 63.3% for RT among the intermediate–high-risk patients. In the cohort, the HRs of PCSM for RP compared with RT estimated from the unadjusted model are 0.58 (95% CI, 0.37–0.92) and 0.68 (95% CI, 0.52–0.90) for low-risk and intermediate–high-risk groups, respectively. The HRs were nearly identical when cancer grade and cancer stage were included as covariates. In the propensity score–matched cohort, the HRs for RP compared with RT were 0.68 (95% CI, 0.38–0.1.22) and 0.51 (95% CI, 0.36–0.73) for the low-risk and intermediate–high-risk groups, respectively, in a multivariable model (Table 4).
Fig. 1.
Propensity score–matched prostate cancer–specific survival in men diagnosed as having metastases treatment by competing-risks models: (a) low risk; (b) intermediate– high risk.
Table 4.
Hazard ratios of risk of prostate cancer death after diagnosis of metastasis associated with radical prostatectomy compared with radiation therapy
| Cancer risk group | ||
|---|---|---|
| Characteristic | Low | Intermediate–high |
| Radical prostatectomy vs radiation therapy, HR (95% CI) |
Radical prostatectomy vs radiation therapy, HR (95% CI) |
|
| Overall cohort | ||
| Unadjusted model | 0.58 (0.37–0.92) | 0.68 (0.52–0.90) |
| Model adjusted for cancer stage and cancer grade* |
0.58 (0.37–0.92) | 0.67 (0.51–0.89) |
| Multivariate Cox** | 0.57 (0.35–0.91) | 0.59 (0.44–0.79) |
| Propensity-matching cohort | ||
| Unadjusted model* | 0.64 (0.36–1.12) | 0.55 (0.39–0.77) |
HR = hazard ratio; CI = confidence interval.
HRs were estimated from the Cox regression considering other causes of death as a competing risk.
Covariates included age, race, year of diagnosis, Surveillance Epidemiology and End Results region, comorbidity, cancer stage, and state buy-in.
3.3. Use of imaging scans and secondary cancer therapies
From the time of primary treatment to metastasis, the RT patients received slightly more imaging studies than the RP patients (low risk: 78.9% and 74.2%; intermediate–high risk: 82.9% and 80.5%). From 180 d after their primary treatments to metastasis, 13.3% of RT patients and 14.6% of RP patients received chemotherapy, and 17.8% of RT patients and 25.3% of RP patients received ADT. After metastasis, 35.4% of RT patients and 38.7% of RP patients received chemotherapy, and 9.4% of RT patients and 12.9% of RP patients received ADT.
3.4. Sensitivity analyses
We used the percentage of chemotherapy use and imaging studies performed after metastasis from our cohort to estimate the effects of potential unmeasured confounders on the HR of PCSM (Table 5). The sensitivity analyses are based on the base estimate for RP compared with RT (0.57; 95% CI, 0.45–0.73). In these sensitivity analyses, we used 15%, 40%, 75%, and 80% as examples of prevalence rates of an unmeasured confounder in the RP group. The hazard ratio (0.5–2.0) was presented in the analysis study as the risk estimate associated with unmeasured confounders. For example, 15% of RP patients received chemotherapy, and only 10% of RT patients received chemotherapy. We hypothesized that chemotherapy may extend the length of survival by 50% [17]. After adjustment for unmeasured confounders, the risk of PCSS is still significantly lower in RP patients (HR: 0.56; 95% CI, 0.36–0.71) compared with RT patients.
Table 5.
Sensitivity analysis estimating the effect of an unmeasured confounder on the hazard ratio of death
| Prevalence in radical prostatectomy patients, % |
Prevalence in irradiation patients, % |
Cancer-specific mortality HR adjusted for unmeasured confounder (95% CI) |
||
|---|---|---|---|---|
| Unmeasured confounder, HR 0.5 |
Unmeasured confounder, HR 0.75 |
Unmeasured confounder, HR 2.00 |
||
| 15 | 5 | 0.55 (0.35–0.87) | 0.57 (0.36–0.90) | 0.64 (0.41–1.01) |
| 10 | 0.56 (0.36–0.90) | 0.57 (0.37–0.91) | 0.61 (0.39–0.96) | |
| 15 | 0.58 (0.37–0.92) | 0.58 (0.37–0.92) | 0.58 (0.37–0.92) | |
| 40 | 30 | 0.55 (0.35–0.87) | 0.56 (0.36–0.90) | 0.62 (0.40–0.99) |
| 40 | 0.58 (0.37–0.92) | 0.58 (0.37–0.92) | 0.58 (0.37–0.92) | |
| 50 | 0.62 (0.39–0.98) | 0.60 (0.38–0.95) | 0.54 (0.35–0.86) | |
| 75 | 80 | 0.60 (0.39–0.96) | 0.59 (0.38–0.93) | 0.56 (0.36–0.89) |
| 85 | 0.63 (0.40–1.00) | 0.60 (0.38–0.95) | 0.55 (0.35–0.87) | |
| 90 | 0.66 (0.42–1.05) | 0.61 (0.39–0.96) | 0.53 (0.34–0.85) | |
| 80 | 50 | 0.46 (0.30–0.74) | 0.53 (0.34–0.84) | 0.70 (0.44–1.10) |
| 60 | 0.50 (0.32–0.79) | 0.55 (0.35–0.87) | 0.65 (0.42–1.04) | |
| 70 | 0.54 (0.34–0.85) | 0.56 (0.36–0.89) | 0.61 (0.39–0.97) | |
HR = hazard ratio; CI = confidence interval.
4. Discussion
This is the first study demonstrating that primary treatment may make a difference with regard to survival time after metastasis in a large population-based cohort. In this study, men who received RP were less likely to die from PCa after metastasis than men who received RT. The risk of PCSM after metastasis is not associated with cancer risk at cancer diagnosis. These results should be considered hypothesis-generating and, it is hoped, will spur further investigations into how primary treatment may affect PCSS following metastases.
Metastases are responsible for most of the deaths among cancer patients, and few effective treatments are available. The factors regulating the development of metastases have not been fully elucidated, but the process of tumor self-seeding with circulating tumor cells (CTCs) may play an essential role [18]. Studies of tumor self-seeding suggest that CTCs are the intermediaries between primary tumors and metastases. Based on the self-seeding theory, CTCs return to, and grow in, the primary tumor sites from their derived metastases. Metastatic cells may affect the gene expression patterns of the parental tumor site during the metastatic colonization and accelerate tumor progression [19]. One possible explanation for the improved PCSS after metastasis in men receiving RP compared with men receiving RT is that tumor self-seeding of the irradiated prostate leads to increased metastatic potential of the cancer cells. Self-seeding may be more likely to occur in irradiated patients, in whom the intact prostate has a large volume to fuel this process and speed up the tumor progression, in contrast to surgical patients. Previous studies have shown that the presence and extent of residual tumor cells within the primary site after aggressive treatment may contribute to tumor progression and predict cancer-specific survival [20,21]. Clinically, surgical resection of primary tumors in patients with metastatic disease has become a well-established paradigm in the treatment of renal cell carcinoma [22,23]. This procedure may not be curative in all patients, but the possibility of cancer-specific survival increases after resection of primary tumor and isolated metastases. Our results add to the growing evidence in the literature that controlling the primary site may be important in patients with metastatic cancer.
In general, men who received primary RP have a better outcome after metastasis than men who received primary RT in our study. However, the survival benefit is statistically significant in the intermediate–high-risk group but not in the low-risk group. The possible explanation is that the 10- and 15-yr PCSS is 98% and 93%, respectively, in the most recent data [8]. The high survival rate leaves a narrow margin of effectiveness for any treatment, and it is particularly difficult to demonstrate any treatment effect in low-risk patients.
To elucidate the potential detection bias, we looked for claim records of imaging studies (computed tomography, magnetic resonance imaging, and radionuclide bone scanning) from the last date of primary treatment to metastasis. This percentage was slightly higher in RT patients than in RP patients. While we may not be able to exclude the effect of detection bias completely from the study, the slight difference in the rate of imaging is unlikely to account for the large difference in survival.
We explored whether confounders could explain our findings, beyond the difference in primary treatment and detection. One confounder could be the disparity in secondary treatments. In RP patients, secondary treatments were started earlier and were more prevalent than in RT patients, a finding that has been reported previously [6]. This finding may be related to the patients’ ability to tolerate secondary therapy and to ease of diagnosing biochemical recurrence after RP (PSA should be undetectable after RP). Our sensitivity analysis (Table 5) addressed how the imbalance in the use of secondary treatments may influence outcomes and found that our conclusion is relatively robust under various scenarios.
There are some limitations to our study. First, although our algorithm to identify metastases has been carefully validated [10], the risk of misclassification cannot be completely avoided. Next, despite our efforts to address the selection bias in methods, our results may still be confounded by indication, which is natural in any observational study. Also, the SEER cancer grade was based on the highest grade recorded in the pathology report at the diagnosis, based on surgical specimens for patients undergoing RP and biopsy for patients without RP. This situation may cause some disparity in cancer grade between the RP and RT groups. However, our outcome of interest was disease progression after metastasis, which may be less prone to selection bias; although men receiving primary RT are more likely to have a higher grade of PCa at diagnosis, we did not find that cancer grade at diagnosis was associated with the outcome after metastasis. Our study revealed a large difference in disease progression after metastasis between RP and RT patients. Given the results of propensity score matching and sensitivity analysis, the difference in PCSS cannot be easily explained by unmeasured confounders. Another limitation is that SEER reclassified Gleason score 7 from the moderately differentiated to the poorly differentiated category in 2003. However, the change in definition affects both RT and RP patients; therefore, our interpretation of results should remain similar. Still another limitation is the lack of data in SEER-Medicare regarding the prevalence of performed pelvic lymph node dissection in the RP patients. Finally, the RT modalities used were heterogeneous and included brachytherapy, IMRT, 3D CRT, and combination therapy. Although there is no evidence of a survival difference between irradiation modalities in the literature, the heterogeneity in treatment modalities and total radiation dose may affect the outcomes.
Several strengths of this population-based study are worth noting. First, our study included patients derived from various health care settings, so the results may be more generalizable than those of previous studies from community-based urology groups or from an experienced surgeon in a single institution [5–7,24]. Differences in patient characteristics, experience of the surgeon, and hospital volume have all been shown to affect patient outcomes [25–27]. Second, men ≥65 yr are usually underrepresented in randomized controlled trials, although approximately 50% of men diagnosed with PCa are ≥65 yr [28–30]. Our study included only men ≥65 yr, so it fills a knowledge gap in the literature.
5. Conclusions
This population-based study suggests that primary treatment modality may affect PCSS after metastasis. Following the development of metastases, men who had received primary RP had a longer PCSS than men who had received primary RT. Our results may have potential implications for the timing and nature of localized PCa treatment.
Take-home message.
Men who underwent radical prostatectomy as the primary treatment for prostate cancer have a better prognosis than men who received radiation therapy after the development of metastasis. This finding may have implications for the timing and nature of local prostate cancer treatment.
Acknowledgment statement
The authors acknowledge the efforts of the Applied Research Branch, Division of Cancer Prevention and Population Science, National Cancer Institute (NCI); the Office of Information Services and the Office of Strategic Planning, Center for Medicare and Medicaid Services (CMS); Information Management Services (IMS), Inc.; and the Surveillance Epidemiology and End Results (SEER) program tumor registries in the creation of the SEER-Medicare database. We are grateful for the technical assistance in manuscript submission provided by Julia Sugumar.
Funding/Support and role of the sponsor: This study was supported by National Cancer Institute (NCI) Challenge Grant RC1CA145722, Robert Wood Johnson Foundation 60624, and CINJ Biometrics shared resource (NCI CA-72720–10). The NCI was not involved in the design or conduct of this study. The content is solely the responsibility of the authors and does not necessarily represent the official view of the NCI or the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author contributions: Grace L. Lu-Yao had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Shao, S. Kim, Stein, I.Y. Kim, Lu-Yao.
Acquisition of data:
Analysis and interpretation of data: Shao, S. Kim, Moore, Shih, Lin, Lu-Yao.
Drafting of the manuscript: Shao, S. Kim, Moore, Shih, Lin, Lu-Yao.
Critical revision of the manuscript for important intellectual content: Shao, Moore, Stein, I.Y.
Kim, Shih, Lin, Lu-Yao.
Statistical analysis: Shao.
Obtaining funding: None.
Administrative, technical, or material support: Shao.
Supervision: Lu-Yao.
Other (specify): None.
Financial disclosures: Grace L. Lu-Yao certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending) are the following: During the past 5 yr, Grace L. Lu-Yao has served as a consultant to the Merck Research Laboratory, which did not contribute funding or play any role whatsoever in the design, interpretation, or drafting of this manuscript.
References
- 1.Shao YH, Albertsen PC, Shih W, Roberts CB, Lu-Yao GL. The impact of PSA testing frequency on prostate cancer incidence and treatment in older men. Prostate Cancer Prostatic Dis. 2011;14:332–339. doi: 10.1038/pcan.2011.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shao YH, Albertsen PC, Roberts CB, et al. Risk profiles and treatment patterns among men diagnosed as having prostate cancer and a prostate-specific antigen level below 4.0 ng/ml. Arch Intern Med. 2010;170:1256–1261. doi: 10.1001/archinternmed.2010.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kawachi MH, Bahnson RR, Barry M, et al. NCCN clinical practice guidelines in oncology: prostate cancer early detection. J Natl Compr Canc Netw. 2010;8:240–262. doi: 10.6004/jnccn.2010.0016. [DOI] [PubMed] [Google Scholar]
- 4.Wilt TJ, MacDonald R, Rutks I, Shamliyan TA, Taylor BC, Kane RL. Systematic review: comparative effectiveness and harms of treatments for clinically localized prostate cancer. Ann Intern Med. 2008;148:435–448. doi: 10.7326/0003-4819-148-6-200803180-00209. [DOI] [PubMed] [Google Scholar]
- 5.Kibel AS, Ciezki JP, Klein EA, et al. Survival among men with clinically localized prostate cancer treated with radical prostatectomy or radiation therapy in the prostate specific antigen era. J Urol. 2012;187:1259–1265. doi: 10.1016/j.juro.2011.11.084. [DOI] [PubMed] [Google Scholar]
- 6.Zelefsky MJ, Eastham JA, Cronin AM, et al. Metastasis after radical prostatectomy or external beam radiotherapy for patients with clinically localized prostate cancer: a comparison of clinical cohorts adjusted for case mix. J Clin Oncol. 2010;28:1508–1513. doi: 10.1200/JCO.2009.22.2265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cooperberg MR, Vickers AJ, Broering JM, Carroll PR. Comparative risk-adjusted mortality outcomes after primary surgery, radiotherapy, or androgen-deprivation therapy for localized prostate cancer. Cancer. 2010;116:5226–5234. doi: 10.1002/cncr.25456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 9.Warren JL, Klabunde CN, Schrag D, Bach PB, Riley GF. Overview of the SEER-Medicare data: content, research applications, and generalizability to the United States elderly population. Med Care. 2002;40 doi: 10.1097/01.MLR.0000020942.47004.03. IV-3-18. [DOI] [PubMed] [Google Scholar]
- 10.Dolan M, Kim S, Shao Y-H, Lu-Yao G. Authentication of algorithm to detect metastases in men with prostate cancer using ICD-9 codes. Epidemiol Res Intl. doi: 10.1155/2012/970406. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.American Joint Committee on Cancer. Manual for staging of cancer. ed. 5. Philadelphia, PA: JB Lippincott; 1997. [Google Scholar]
- 12.Klabunde CN, Potosky AL, Legler JM, Warren JL. Development of a comorbidity index using physician claims data. J Clin Epidemiol. 2000;53:1258–1267. doi: 10.1016/s0895-4356(00)00256-0. [DOI] [PubMed] [Google Scholar]
- 13.Makarov DV, Desai R, Yu JB, et al. Appropriate and inappropriate imaging rates for prostate cancer go hand in hand by region, as if set by thermostat. Health Aff (Millwood) 2012;31:730–740. doi: 10.1377/hlthaff.2011.0336. [DOI] [PubMed] [Google Scholar]
- 14.Putter H, Fiocco M, Geskus RB. Tutorial in biostatistics: competing risks and multi-state models. Stat Med. 2007;26:2389–2430. doi: 10.1002/sim.2712. [DOI] [PubMed] [Google Scholar]
- 15.Schemper M, Smith TL. A note on quantifying follow-up in studies of failure time. Control Clin Trials. 1996;17:343–346. doi: 10.1016/0197-2456(96)00075-x. [DOI] [PubMed] [Google Scholar]
- 16.D’Agostino RB., Jr. Propensity score methods for bias reduction in the comparison of a treatment to a non-randomized control group. Stat Med. 1998;17:2265–2281. doi: 10.1002/(sici)1097-0258(19981015)17:19<2265::aid-sim918>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 17.Petrylak DP, Tangen CM, Hussain MH, et al. Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer. N Engl J Med. 2004;351:1513–1520. doi: 10.1056/NEJMoa041318. [DOI] [PubMed] [Google Scholar]
- 18.Kim MY, Oskarsson T, Acharyya S, et al. Tumor self-seeding by circulating cancer cells. Cell. 2009;139:1315–1326. doi: 10.1016/j.cell.2009.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chaffer CL, Weinberg RA. A perspective on cancer cell metastasis. Science. 2011;331:1559–1564. doi: 10.1126/science.1203543. [DOI] [PubMed] [Google Scholar]
- 20.Crook JM, Malone S, Perry G, et al. Twenty-four-month postradiation prostate biopsies are strongly predictive of 7-year disease-free survival. Cancer. 2009;115:673–679. doi: 10.1002/cncr.24020. [DOI] [PubMed] [Google Scholar]
- 21.Tzelepi V, Efstathiou E, Wen S, et al. Persistent, biologically meaningful prostate cancer after 1 year of androgen ablation and docetaxel treatment. J Clin Oncol. 2011;29:2574–2581. doi: 10.1200/JCO.2010.33.2999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Flanigan RC, Salmon SE, Blumenstein BA, et al. Nephrectomy followed by interferon alfa-2b compared with interferon alfa-2b alone for metastatic renal-cell cancer. N Engl J Med. 2001;345:1655–1659. doi: 10.1056/NEJMoa003013. [DOI] [PubMed] [Google Scholar]
- 23.Alt AL, Boorjian SA, Lohse CM, Costello BA, Leibovich BC, Blute ML. Survival after complete surgical resection of multiple metastases from renal cell carcinoma. Cancer. 2011;117:2873–2882. doi: 10.1002/cncr.25836. [DOI] [PubMed] [Google Scholar]
- 24.Boorjian SA, Thompson RH, Tollefson MK, et al. Long-term risk of clinical progression after biochemical recurrence following radical prostatectomy: the impact of time from surgery to recurrence. Eur Urol. 2011;59:893–899. doi: 10.1016/j.eururo.2011.02.026. [DOI] [PubMed] [Google Scholar]
- 25.Vickers AJ, Bianco FJ, Serio AM, et al. The surgical learning curve for prostate cancer control after radical prostatectomy. J Natl Cancer Inst. 2007;99:1171–1177. doi: 10.1093/jnci/djm060. [DOI] [PubMed] [Google Scholar]
- 26.Klein EA, Bianco FJ, Serio AM, et al. Surgeon experience is strongly associated with biochemical recurrence after radical prostatectomy for all preoperative risk categories. J Urol. 2008;179:2212–2216. doi: 10.1016/j.juro.2008.01.107. discussion 2216−7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ellison LM, Heaney JA, Birkmeyer JD. The effect of hospital volume on mortality and resource use after radical prostatectomy. J Urol. 2000;163:867–869. [PubMed] [Google Scholar]
- 28.Shao YH, Demissie K, Shih W, et al. Contemporary risk profile of prostate cancer in the United States. J Natl Cancer Inst. 2009;101:1280–1283. doi: 10.1093/jnci/djp262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lane JA, Hamdy FC, Martin RM, Turner EL, Neal DE, Donovan JL. Latest results from the UK trials evaluating prostate cancer screening and treatment: the CAP and ProtecT studies. Eur J Cancer. 2010;46:3095–3101. doi: 10.1016/j.ejca.2010.09.016. [DOI] [PubMed] [Google Scholar]
- 30.Wilt TJ, Brawer MK, Barry MJ, et al. The Prostate Cancer Intervention Versus Observation Trial:VA/NCI/AHRQ Cooperative Studies Program #407 (PIVOT): design and baseline results of a randomized controlled trial comparing radical prostatectomy to watchful waiting for men with clinically localized prostate cancer. Contemp Clin Trials. 2009;30:81–87. doi: 10.1016/j.cct.2008.08.002. [DOI] [PubMed] [Google Scholar]