Abstract
Inadequate efficacy, high toxicity and drug resistance associated with existing chemotherapeutic agents mandate a need for novel therapeutic strategies for highly aggressive pancreatic cancer (PC). Guggulsterone (GS) exhibits potent anti-proliferative effects against various cancer cells and has emerged as an attractive candidate for use in complementary or preventive cancer therapies. However, the knowledge regarding the therapeutic potential of GS in PC is still limited and needs to be explored. We studied the effect of GS on PC cell growth, motility and invasion and elucidated the molecular mechanisms associated with its anti-tumor effects. Treatment of Capan1 and CD18/HPAF PC cells with GS resulted in dose- and time-dependent growth inhibition and decreased colony formation. Further, GS treatment induced apoptosis and cell cycle arrest as assessed by Annexin-V assay and FACS analysis. Increased apoptosis following GS treatment was accompanied with Bad dephosphorylation and its translocation to the mitochondria, increased Caspase-3 activation, decreased Cyclin D1, Bcl-2 and xIAP expression. Additionally, GS treatment decreased motility and invasion of PC cells by disrupting cytoskeletal organization, inhibiting activation of FAK and Src signaling and decreased MMP9 expression. More importantly, GS treatment decreased mucin MUC4 expression in Capan1 and CD18/HPAF cells through transcriptional regulation by inhibiting Jak/STAT pathway. In conclusion, our results support the utility of GS as a potential therapeutic agent for lethal PC.
1. Introduction
Pancreatic Cancer (PC) is the 10th most commonly diagnosed cancer and 4th leading cause of cancer deaths in the United States with a median 5-year survival of only about 6% [1, 2]. PC is often diagnosed at an advanced stage that is highly resistant to conventional chemo-radiation therapy and is difficult to treat [3]. Standard chemotherapy for PC produces only a modest survival benefit in patients with advanced disease and is associated with high toxicity and drug resistance [4]. Hence, effective yet non-toxic therapeutic agents capable of inhibiting the proliferation and metastasis of PC are urgently needed.
Naturally occurring bioactive phytochemicals, due to their non-toxic nature have emerged as promising options for the development of effective alternatives or adjuncts for conventional cytotoxic therapies. Guggulsterone (GS), [4, 17(20)-pregnadiene- 3,16-dione], a plant polyphenol derived from the exudates of plant Commiphora mukkul, has been used in traditional medicine for treating several ailments including obesity, hyperlipidemia, atherosclerosis, diabetes and osteoarthritis [5]. Recent studies indicate therapeutic and anti-proliferative activity of GS against several human cancers including head and neck, prostate, lung, breast, colon and ovarian cancer, with no apparent signs of toxicity on normal human fibroblast cells, immortalized esophageal cells, non-transformed prostate and colon epithelial cells [6–14]. Besides inhibiting cell proliferation and inducing apoptosis, GS inhibits cell motility and invasion of cancer cells in vitro and angiogenesis and metastasis in vivo [7, 9, 12, 14]. GS has also been reported to inhibit invasion and metastasis of PC cells through antagonizing Farnesoid X receptor [15] . Further, GS has been shown to increase the efficacy of gemcitabine in gall bladder cancer and PC cells, reverse the multi-drug resistance in breast cancer MCF7 cells [16–18] and enhance radiosensitivity [19].
GS inhibits the activation of transcription factors NF-κB and STAT3 in cancer cells [6, 20, 21], decreases production of reactive oxygen species (ROS), suppresses inflammation and modulates anti-apoptotic and cell cycle–regulatory proteins [10, 12, 13, 17, 20, 22, 23]. Besides affecting NF-κB and STAT3 activation, GS binds and modulates the activity of several steroid receptors like FXR, estrogen receptor alpha (Erα), progesterone receptor (PR), and pregnane X receptor (PXR) [24, 25]. Although the anticancer effects of GS have been documented in various cancers including PC, molecular mechanisms of GS mediated effects on PC are still inadequately understood.
Given the evidence for the anti-tumor effects of GS, we assessed the effect of GS on PC cells and investigated the underlying molecular mechanisms. Our results showed that GS inhibits proliferation, decreases motility and invasion and induces apoptosis in PC cells. These anti-tumor effects of GS possibly involve multiple networks including inhibition of FAK, Src, and Jak/STAT signaling, alteration in BAD phosphorylation, reorganization of actin cytoskeleton, and down-regulation of MUC4.
2. Materials and Methods
2.1 Chemicals and antibodies
Purified Guggulsterone (GS) and MTT [4, 5-dimethyl-2-yl]-2, 5-diphenyl tetrazolium bromide), were purchased from Sigma Chemical Co. (St. Louis, MO, USA) and Annexin-V conjugated AlexaFluor488 Apoptosis Detection Kit from Molecular Probes, Inc. (Eugene, OR). The protein assay kit was from Bio-Rad (Hercules, CA, USA). MUC4 monoclonal antibody (8G7) was developed in our laboratory [26]. The rabbit polyclonal antibodies against cleaved caspase-9 (Asp330), pSTAT3 (Ser705)/STAT3, pSTAT1 (Ser-727)/ STAT1, pFAK (Tyr 925, Tyr 576/577)/tFAK, pSrc/Src (Tyr 416), xIAP were obtained from Cell Signaling (St. Louis, MO, USA). Mouse monoclonal antibodies against Bcl2 (sc-492), cyclin D1 (sc-8396), survivin (sc-17779); rabbit polyclonal antibodies against 14-3-3ζ (sc-1019), were obtained from Santa Cruz Biotechnology (Santa Cruz, CA, USA). The polyclonal antibodies against STAT1, STAT3 were obtained from BD Laboratories (Bedford, MA, USA) and rabbit IgG from Vector Laboratories (Burlingame, CA, USA). β-actin antibody was obtained from Sigma-Aldrich (St. Louis, MO, USA). Horseradish peroxidase conjugated anti-mouse and anti-rabbit IgG were procured from GE Healthcare Biosciences (Uppsala, Sweden) and FITC-conjugated anti-mouse IgG was obtained from Invitrogen (California, U.S.A.).
2.2 Cell lines and cell culture conditions
The human PC highly aggressive cell lines- CD18/HPAF and Capan1 cells were procured from American Type Culture Collection (ATCC), and cultured in Dulbecco’s Modified Eagles Medium (DMEM, Sigma Aldrich, St. Louis, MO, USA) supplemented with 10% fetal bovine serum and antibiotics (100 µg/mL penicillin and streptomycin) [27]. Cells were grown at 37°C with 5% CO2 in a humidified atmosphere.
2.3 Cytotoxicity assay
The effect of GS on the viability of PC cells was determined using MTT assay as described previously [28]. Briefly, Capan1 and CD18/HPAF cells (5×103/well) were plated in a 96-well plate for 24 hrs and incubated in triplicates in the presence of medium containing GS (0 – 100 µM) or 0.02% of DMSO which served as a vehicle control in a final volume of 100 µl for 24–72 hrs at 37°C. Cell death was measured by adding MTT at 37°C for 3–4 hrs followed by addition of 100 µl of dimethyl sulphoxide (DMSO) to dissolve formazan crystals so formed. Optical density (OD) was measured at λ=570 nm and the percentage cell death was calculated [28]. The experiment was repeated thrice.
2.4 Cell cycle analysis and apoptosis assay
CD18/HPAF and Capan1 PC cells were treated with GS for 24 and 48 h, collected and washed with phosphate buffer saline (PBS, pH = 7.4). The cells were fixed in 70% ethanol for 30 min at −20oC and resuspended in buffer containing PBS-EDTA (0.5 M, pH = 8.0), Triton X-100 (0.05 %), RNAse A (50 µg/ml) and propidium iodide (PI, 100 µg/ml) for flow cytometry analysis. The PI-labeled cells were analyzed by flow cytometry using CantoTM flow cytometer (Becton Dickinson, Mountain, View, CA). Results were analyzed with ModFit LT™ software (Verity Software House, Topsham, ME). Apoptosis or necrosis was measured by Annexin-V FITC staining as described previously [29].
2.5 Immunoblot analysis and immunoprecipitation
Immunobloting was carried out as described previously [29]. Thirty to sixty µg of protein from the cell extracts were resolved by electrophoresis on either a 2.0% SDS-agarose gel (for MUC4) or a 10% SDS polyacrylamide gel (for other proteins) and transferred to polyvinylidene difluoride membrane (PVDF). After blocking, blots were incubated with specific antibodies as per manufacturer’s recommended protocol at 4°C overnight followed by incubation with horseradish peroxidase-labeled appropriate secondary immunoglobulin and the signal was detected using an electro chemiluminescence reagent kit (Amersham Pharmacia, Piscataway, NJ). β-actin was used as a control for protein loading.
For BAD and 14-3-3ζ interaction, cells with or without GS treatment for various time points were washed with ice-cold PBS and whole cell lysates were prepared as described previously [30]. Equal amount of protein (500 µg) was cleared with protein A/G-plus agarose beads (Oncogene Research, Boston, MA, U.S.A.) on a rotating platform. BAD or 14-3-3ζ proteins were immunoprecipitated from cleaned lysates using specific antibodies followed by the addition of protein A/G-plus agarose beads (50 µL) for 4hrs at 4°C. Immunoprecipitates were washed, and subsequently subjected to SDS PAGE followed by immunoblotting using BAD and 14-3-3ζ antibodies.
2.6 RNA isolation and RT-PCR
Cells were treated with GS (50µM) for different time points. Total RNA isolation and cDNA synthesis by reverse transcription was done as described previously [29]. Quantitative real-time PCR was performed using 1µl of a 1:5 dilution of first-strand cDNA using the SYBR Premix Ex Taq kit (Takara Bio, Madison, WI, USA) and specific primers (Table 1) for various genes, using Roche Light Cycler 480 system (Roche Diagnostics, Mannheim, Germany). Each reaction was done in triplicate and the level of mRNA expression of each gene was normalized to that of β-actin.
2.7 Chromatin immunoprecipitation analysis
Chromatin immunoprecipitation (ChIP) analysis was carried out as described previously [31]. Briefly, cross-linked chromatin was prepared from CD18/HPAF cells either untreated or treated with GS, sheared to an average length of 0.6 kb. After immunoprecipitation with antibodies against anti-STAT1 and STAT3 for 16 hrs at 4°C, the fragmented DNA was purified and the STAT1 and STAT3 binding sequences on MUC4 promoter were amplified by PCR using specific sets of primers (Table 1) [31, 32].
2.8 Motility and invasion assay
The effect of GS on the motility and invasion of PC cells was assessed by the Transwell migration assay. CD18/HPAF and Capan1 cells were treated with GS for 24h, trypsinized and resuspended in serum-free medium and plated (2.5×105) in the top chamber of polyethylene terephthalate membranes (pore size 8µm) (Becton Dickinson) for motility and (1 × 106) Capan1 cells were seeded on Matrigel-coated membrane inserts (BD Biosciences, Bedford, MA) for invasion assay. Cells were allowed to migrate for 24 hrs using 10% serum-containing media as a chemo attractant. After incubation, non-migrated cells were removed by scraping the membrane with a cotton swab. The migrated cells on the lower side of the membrane were stained with Diff-Quick cell stain kit (Dade-Behring Inc, Newark, DE, U.S.A.) and counted under the light microscope at high power (X100 magnification in 10 random fields). Each experiment was performed in triplicate. The results were expressed as the mean (±SE) number of cells migrating per high power field.
2.9 Wound healing assay
To further confirm the effect of GS on the migration ability of PC cells, we performed an in vitro wound healing assay as described previously [28, 29]. Briefly, CD18/HPAF and Capan1 cells were grown to 90–100% confluency in 60-mm petri-plates and a series of linear wounds were made using a sterile 100 µl pipette tip. The cells were washed once with PBS to remove the dead and un-adhered cells at the site of the wound. The wound was then photographed before GS addition as baseline. DMEM with or without GS was added to the cells and incubated at 37°C. Photographs of the wound were taken at 0, 12, 24 and 36 hrs of treatment and motility of the GS treated cells was compared with untreated control cells. An average of eight wounds was taken to determine the average rate of migration at a given time point.
2.10 Anchorage dependent and independent clonogenecity assay
To examine the effect of GS on the clonogenic potential of PC cells, we performed an in vitro colony formation assay as described earlier [33]. Briefly, after GS treatment for 24 hrs, cells were washed and trypsinized. 2 × 103 cells were seeded in triplicate in a 100-mm petri plate and incubated for 2 weeks. Thereafter, the colonies formed were fixed in methanol and stained with 0.5% crystal violet. The colonies were counted and compared with untreated cells.
For anchorage-independent proliferation assay, 1 × 103 cells after GS treatment for 24h were plated in six-well plates, with a bottom layer of 0.5% agar and a top layer of 0.35% agar containing the cells. After 2 weeks of incubation at 37°C, colonies so formed were visualized under microscope, photographed and counted.
2.11 Immunofluorescence microscopy
Expression and localization of MUC4 protein was studied using confocal laser scan microscopy as described previously [28, 29]. For cytoskeleton re-organization, Rhodamine-conjugated phalloidin (Invitrogen) was used for staining actin filaments as described by us previously [29]. Briefly cells were treated with GS for 24 hrs and fixed with formaldehyde. Cells were incubated with Rhodamine conjugated Phalladoin for 30 min at room temperature, washed with PBS and mounted using Vector shield medium. Immunostaining was observed under a Zeiss confocal laser-scanning microscope (Carl Zeiss Microimaging, Thornwood, NY), and representative photographs were captured digitally at a magnification of ×63 using the 510 LSM software.
2.12 Subcellular fractionation: Isolation of cytoplasm and mitochondria
Cytoplasmic and mitochondrial fractions were prepared as previously described [30]. Briefly, CD18/HPAF (2 ×107) cells with or without GS treatment were harvested, resuspended in isotonic mitochondrial buffer (210mM mannitol, 70 mM sucrose, 1 mM EGTA, 10 mM Hepes, pH 7.5, 0.1% BSA) and homogenized with a polytron homogenizer. After a brief centrifuge at 2000g for 3 min of the homogenate to pellet the nuclei, supernatant was again centrifuged at 13,000g for 10 min to pellet mitochondria. The supernatant was further centrifuged at 15,000g to pellet light membranes. The resulting supernatant contained the cytosolic fractions. The mitochondria were washed with mitochondrial buffer twice, resuspended with 1% Nonidet P-40 lysis buffer, rocked for 60 min, and then centrifuged at 13,000 X g for 10 min at 4°C. The supernatant containing mitochondrial proteins was collected. Protein (100µg) from each fraction was subjected to 12% SDS-PAGE and analyzed by western blot analysis using antibodies against 14-3-3ζ and BAD. The purity of the fractions was confirmed by assessing localization of fraction-specific proteins including Tom20 for mitochondria.
2.13 Statistical analysis
Statistical analysis was carried out using the Medcalc software for Windows version 9.6.4.0 (MedCalc Software). Statistical significance was determined using the paired, two-tailed Student’s t-test. The criterion for statistical significance was *P <0.05, **P <0.01 and ***P <0.005. Each assay was repeated at least 3 times (unless otherwise specified), to check the validity of the results obtained. The fold change in the mRNA expression was calculated by δδCt method as a 2−δδCt. The mean values ± standard error is presented in figures.
3. Results
3.1 GS inhibits the growth of PC cells
To determine the cytotoxic effect of GS, highly aggressive Capan1 and CD18/HPAF PC cells were treated with varying concentrations of GS (5–100 µM) for 24–72 hrs. MTT assay revealed dose and time dependent increase in the cytotoxicity of GS in Capan1 and CD18/HPAF cells as shown in Fig. 1a and 1b, respectively. Treatment of Capan1 and CD18/HPAF PC cells with GS produced a cytotoxic effect with an inhibitory concentration at 50% (IC50) around 50 µM, which was used for all subsequent experiments. We have analyzed the effect of GS on less aggressive PC cells like MiaPaCa and ASPC1 (data not included) and observed the similar effects on cell proliferation
Fig. 1.
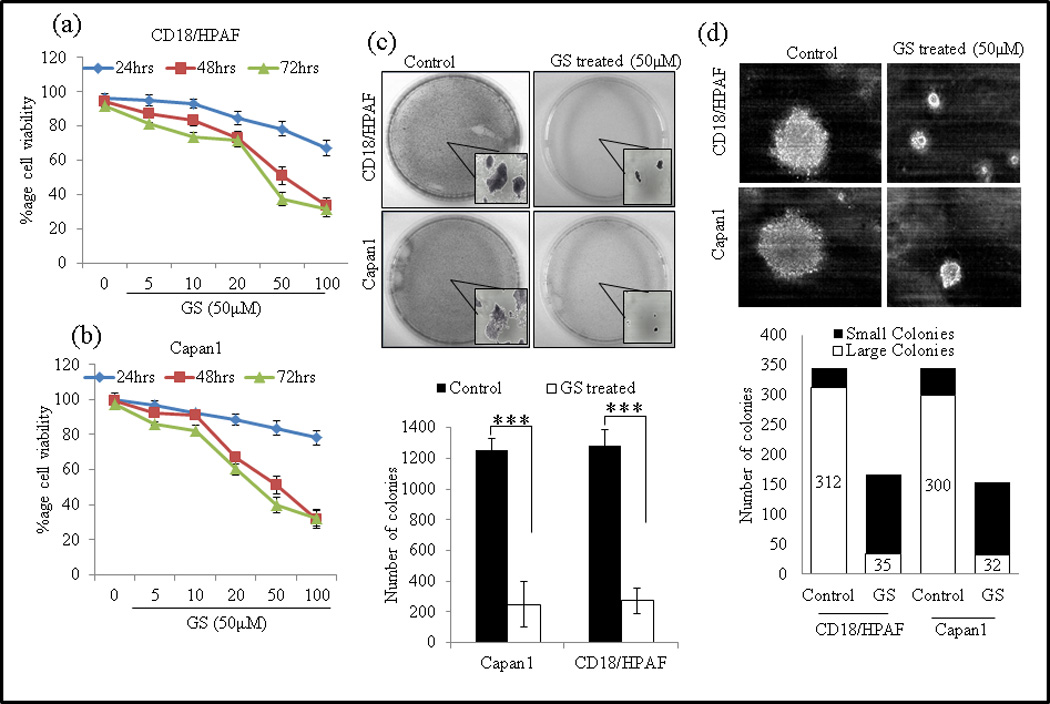
GS decreases proliferation of PC cell lines. PC cells Capan1 (a) and CD18/HPAF (b) were treated with different doses of GS for 24, 48 and 72 hrs and viable cell number was assayed by MTT assay. (c) GS reduces colony formation of PC cells. Capan1 and CD18/HPAF cells were incubated with GS (50µM) for 24 h and equal number of cells (1×103) cells were seeded in triplicate in 10% DMEM in a 12-well plate, and allowed them to grow in 10% DMEM for 2 weeks. At the end of the incubation period, the colonies formed were washed with PBS, fixed in methanol and then stained with 0.1% Crystal violet in PBS. The number of colonies (per well) were counted with the automatic colony counting tool of the Quantity One Imaging software. The graphs represent the mean (±SE) number of colonies. (d) GS reduces anchorage-independent growth of PC cells in soft agar. GS treated or untreated Capan1 and CD18/HPAF/HPAF cells were incubated with GS (50µM) for 24 hrs and equal number of cells (2×103) cells were seeded in triplicate with 0.35% agarose over the layer of 0.5% agarose and allowed them to grow 14 days. The colonies were counted and captured. The experiment were repeated twice (* p<0.05).
3.2 GS inhibits colony-forming ability of PC cells
Since the ability of tumor cells to form colonies in vitro is a reliable indicator of their tumorigenic potential in vivo, we investigated the effect of GS on of long-term colony formation. Exposure of PC Capan1 and CD18/HPAF cells to GS was associated with significant repression of colony-forming ability (Fig. 1c). At 50 µM of GS, the size and number of colonies was significantly reduced (P < 0.01), from 1252 to 250 in Capan1 cells, and from 1282 to 272 in CD18/HPAF cells.
Furthermore, we determined the anchorage-independent growth of GS treated Capan1 and CD18/HPAF cells on semi-solid medium. After culturing the cells for 2 weeks, both GS treated Capan1 and CD18/HPAF cells exhibited significantly reduced anchorage independent growth as indicated by fewer and smaller colonies compared to untreated control cells (Fig. 1d). The smaller colonies formed by GS treated Capan1 and CD18/HPAF cells might result from its decreased growth rate, and indicates the inhibitory activity of GS against tumorigenesis.
3.3 GS induces apoptosis in PC cells
To determine the effect of GS on PC cells, CD18/HPAF and Capan1 cells were either left untreated for 48 hrs or treated with GS (50 µM) for 12, 24 and 48 hrs and examined under phase contrast microscope (x100) and photographed (Fig. 2a). We observed a time dependent increase in small and round cells on GS treatment suggesting that GS induces cell death in CD18/HPAF and Capan1 cells. Furthermore, flow cytometric analysis of indicated significant increase in proportion of Sub G0 cells in 24–48 hrs indicating time dependent increase in cell death in GS (50µM) treated Capan1 (<p=0.04) and CD18/HPAF (<p=0.05) cells compared to untreated controls (Fig. 2b and 2c).
Fig. 2.
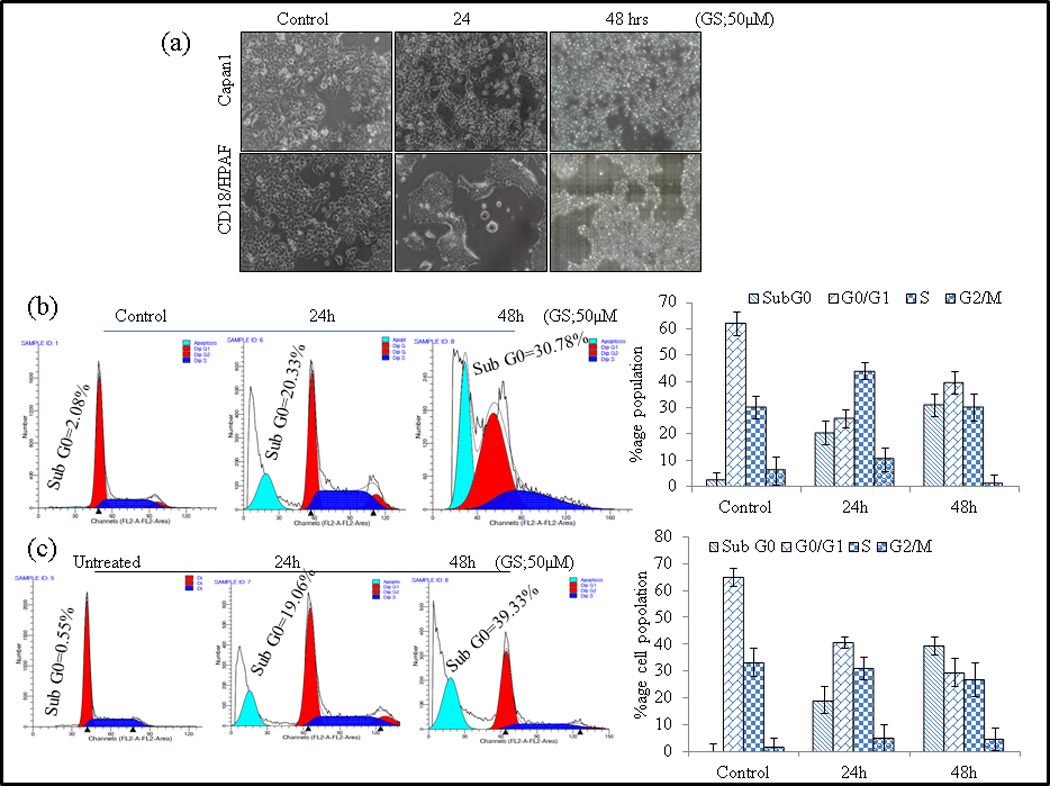
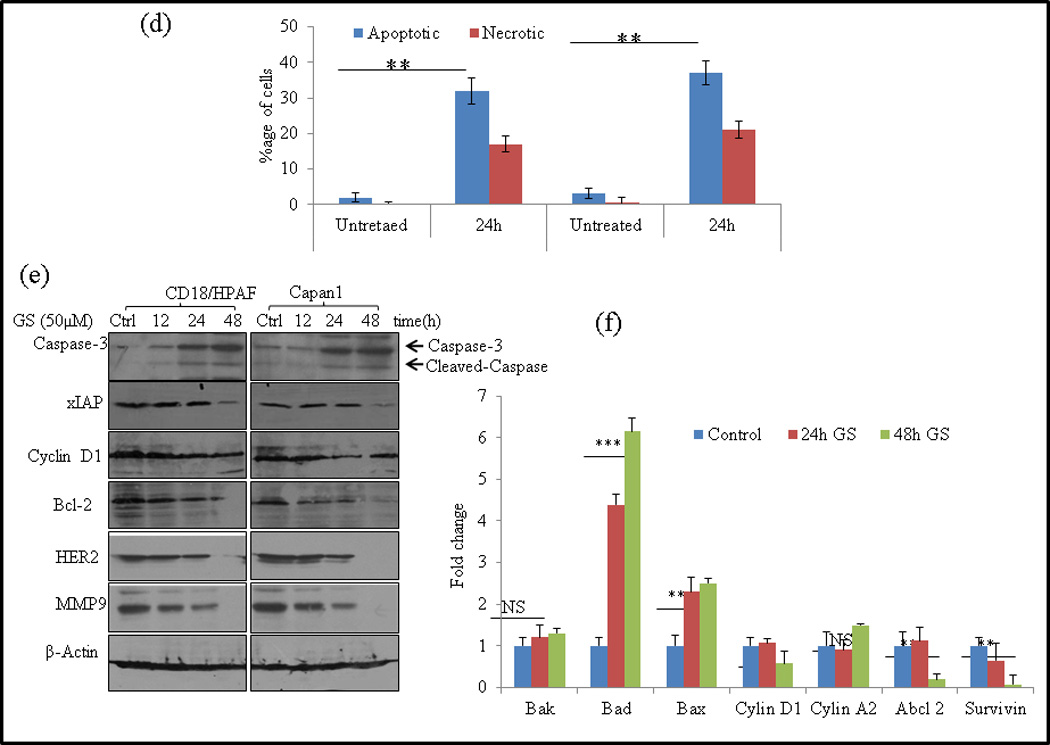
GS induces cell death in PC cells. (a) CD18/HPAF and Capan1 cells were either left untreated (48 h) or treated with GS for 12, 24 and 48 hrs and examined under -phase-contrast microscope (x100) and photographed. (b–c) CD18/HPAF (b) and Capan1 (c) were synchronized by overnight incubation with 1% serum containing media and then either treated with GS (24 and 48 hrs) or left untreated. Cells were fixed and stained with propidium iodide and DNA content was determined by flow cytometry. Representative profiles of the distribution of cells in sub-G0, G0/G1, G2/M and S phases are depicted on the left while the mean distribution of cells in various phases of cell cycle [± standard deviation (SD)] of 3 independent experiments are shown in the right panels. (d) Analysis of apoptotic cells Annexin V staining and flow cytometry. The quantification of apoptotic or necrotic cells was done using the dual staining with Annexin V and propidium iodide. (Apoptotic cells Annecin V+/P− staining; Necrotic cells V+/P+ stating). Columns, mean (n=3); bars, SD. **, P<0.01, statistically significant compared with the vehicle treated control. GS modulates cell cycle regulatory and anti-apoptotic proteins. (e) Capan-1 and CD18/HPAF cells were treated with 50 µM GS for various time points as indicated and whole-cell lysates were analyzed by western blotting using antibodies against Cyclin D1, Bcl-2, xIAP, Caspase-3, HER2 and MMP9. β-actin was used as an internal control. (f) Quantitative RT-PCR analysis of Bak, Bad, Bax, Cyclin D1, Cyclin A2, Abcl2 and Survivin mRNA levels after GS (50 µM) treatment in CD18/HPAF cells for indicated time points. Columns, mean (n=3); bars. NS: non-significant. SD. *, P<0.05, **, P<0.01, ***, P<0.001 statistically significant compared to untreated control.
To examine whether the GS-induced loss of the cell viability and induction of Sub G0 phase arrest in PC cells was associated with the induction of apoptosis or necrosis, GS treated and control were stained with Alexa Fluor-488-conjugated Annexin V, PI and analyzed by flow cytometry. Treatment of Capan1, and CD18/HPAF cells with GS increased number of apoptotic (37% and 32%) and necrotic cells (27% and 23%) respectively compared to (2% and 3.1%) apoptotic cells in untreated control cells (Fig. 2d).
3.4 GS modulates cell cycle regulatory and anti-apoptotic proteins
CD18/HPAF and Capan1 cells were treated with GS for the indicated time points and then harvested for western blotting and real time PCR analysis. GS treatment resulted in downregulation of the xIAP, Bcl2 and Cyclin D1 expression and significantly upregulated the levels of cleaved Caspase-3 in a time dependent manner at protein level (Fig. 2e). Our real- time PCR analysis also revealed a significant shift in the expression of pro-apoptotic molecules BAD and Bax, with no change in expression of Bak molecule (Fig. 2f), indicating the involvement of intrinsic mitochondrial pathway of apoptosis. Furthermore, the real time PCR analysis also showed significant decrease in expression of cell cycle regulatory proteins- Cyclin D1, Abcl2 and Survivin in CD18/HPAF cells (Fig. 2f).
3.5 GS treatment inhibits motility and invasion of PC cells
Capan1 and CD18/HPAF PC cells are highly invasive both in vitro and in vivo [28]. GS has been reported to inhibit migration of head and neck and breast cancer cells [9, 34]. To evaluate GS as a potential drug in PC therapy, its effect on migration and invasive abilities of Capan1 and CD18/HPAF cells was analyzed by wound healing assay (qualitative), Transwell migration (quantitative) assay and Matrigel based Transwell invasion assay. Our results showed that GS treatment significantly inhibited the migration of both Capan1 (p=0.001) and CD18/HPAF (p=0.001) cells and this effect was consistent across both the wound healing (Fig. 3a) and Transwell migration assays (Fig. 3b). The wound-healing assay clearly shows that untreated cells (0µM) migrated across the wound much faster than cells incubated with GS (50µM) (Fig. 3a). Transwell assay results indicated a significant decrease in number of migrating Capan1 (P= 0.002) and CD18/HPAF (P= 0.002) cells compared to untreated control cells (Fig. 3b). In addition, the matrigel-based migration assay indicated that GS was able to inhibit significantly the cell invasion of Capan1 cells (p=0.001) (Fig. 3c).
Fig. 3.
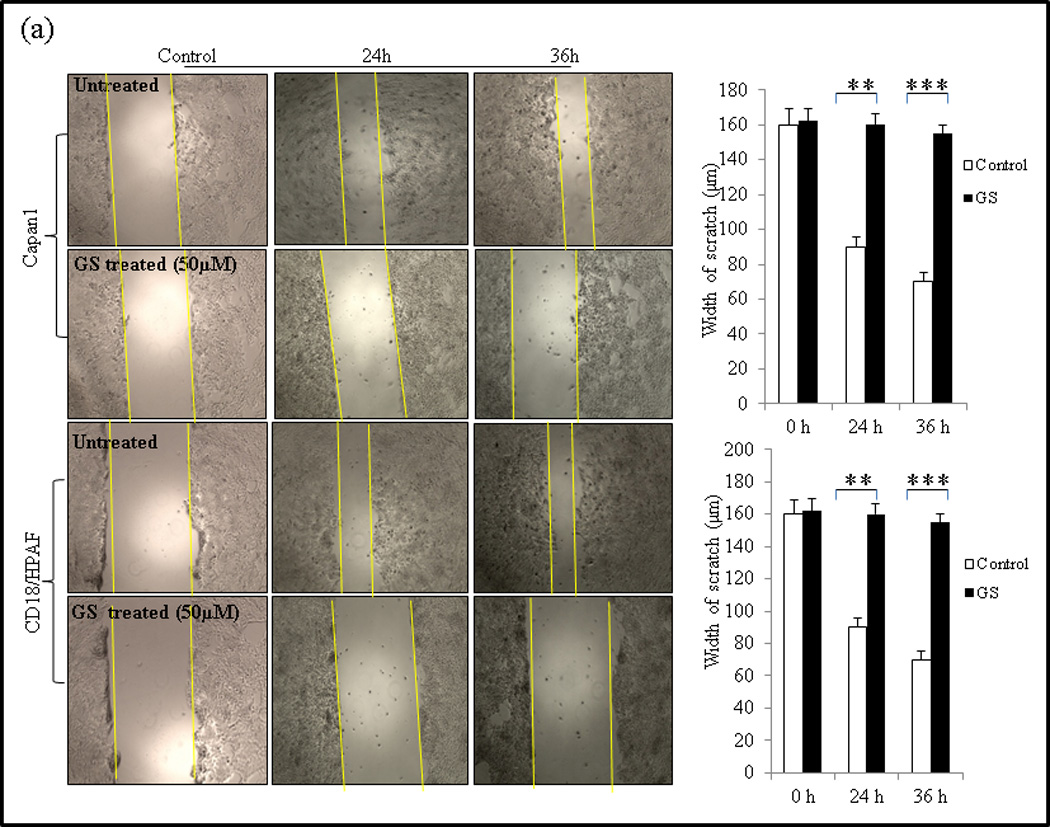
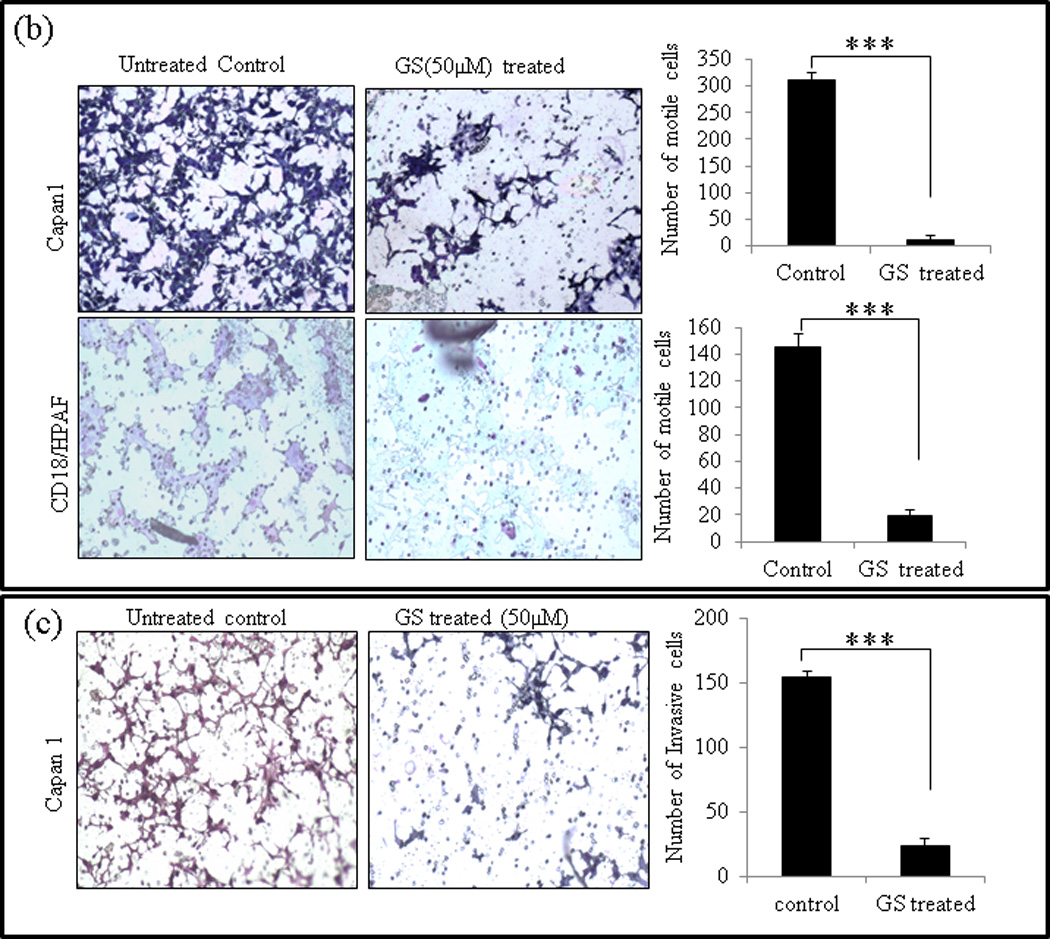
GS inhibits cell migration and invasion of PC cells. (a) Wound healing assay for evaluating the effect of GS on the motility of Capan1 and CD18/HPAF cells. Confluent monolayers of Capan1 and CD18/HPAF cells were scratched with a 200µl sterile pipette tip. Cells were carefully washed with 10% DMEM to remove the unattached cells and repair was monitored microscopically. Images were taken immediately (t = 0h) and after incubation with GS for 24 and 36 hrs. The representative photographs showed the same area at time zero and after 24 and 36h of incubation with or without GS. Columns, mean (n = 3); bars, SD. **, P<0.01, ***, P<0.001, statistically significant compared with the vehicle treated control. (b) Transwell migration assay showing the inhibitory effects of GS on Capan1 and CD18/HPAF cell migration. Equal number of GS treated PC cells (1 × 105 cells) were seeded into the upper chamber in 1 ml of serum-free DMEM and the lower compartments were filled with 1 ml of DMEM containing 10% serum. After 24 hrs at 37°C, non-invading cells on the upper surface of the filter were wiped out with a cotton swab, and the invaded cells on the lower surface of the filter were fixed and stained using Diff Kit and counted under a light microscope (magnification, x100). (c) Matrigel assay indicating the inhibitory effects of GS on PC cell invasion. Capan1 cells were treated with GS (50µM) for 24 hrs and equal number of the cells (1 × 106 cells) was seeded into the upper part of matrigel coated Transwell chamber as discussed above. The number of migrated cells through the 8µm pore of polyethylene terephtalate (PET) membrane was quantified in 10 random fields. Columns, mean of migrated and invasive cell number from three independent experiments; bars, SD. *, P<0.05, **, P<0.01, statistically significant.
To further analyze the effect of GS on motility and migration of PC cells, the expression levels of matrix metalloproteinase 9 (MMP9), which targets many extracellular proteins including adhesion molecules [35] during invasion and metastasis, was analyzed by western blot analysis. In agreement with the experiments discussed above, we observed that the expression levels of MMP9 was down regulated in GS-treated Capan1 and CD18/HPAF cells compared to the untreated control cells (Fig. 3a).
3.6 GS downregulates MUC4 expression in PC cells
Recent observations suggest that MUC4 is involved in PC cell motility, invasion and promotes resistance to apoptosis against various chemotherapeutic agents[36, 37]. These observations prompted us to determine the effect of GS on MUC4 expression in PC cells. CD18/HPAF and Capan1 PC cells were kept untreated for 48 hrs (used as control) or treated with GS (50µM) for 12, 24 and 48 hrs. Protein lysates and mRNA were collected and the expression of MUC4 was assessed by western blotting and RT-PCR analysis. GS decreased MUC4 expression in both Capan1 and CD18/HPAF cells in a time dependent manner with complete downregulation of MUC4 at 48 hrs (Fig. 4a). Decrease in MUC4 mRNA levels indicates transcriptional regulation of MUC4 using GS in PC cells. Our western blot and RT-PCR results were further confirmed by confocal microscopy, showing the similar MUC4 downregulation with GS treatment (Fig. 4b).
Fig. 4.
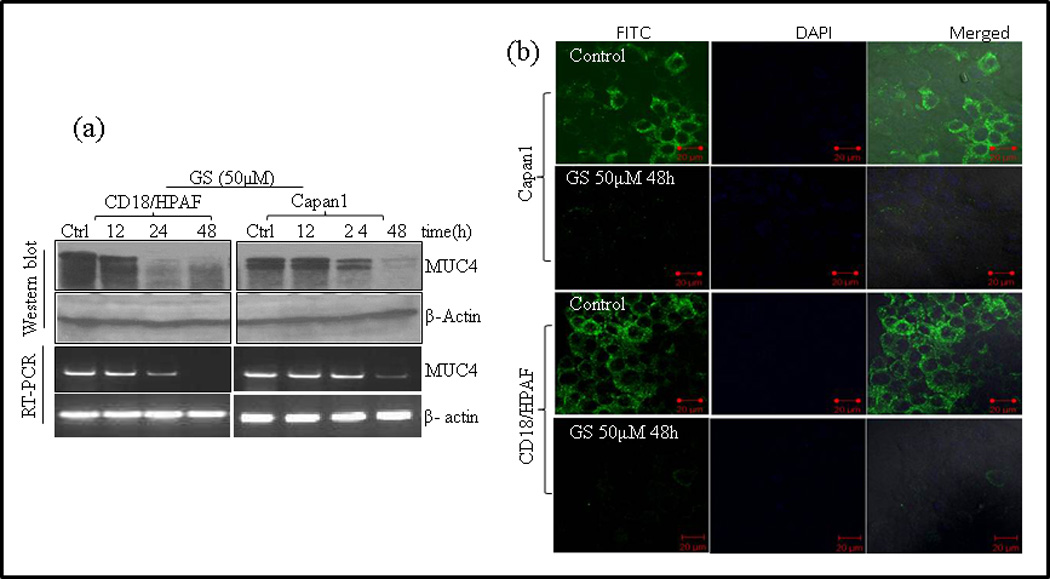
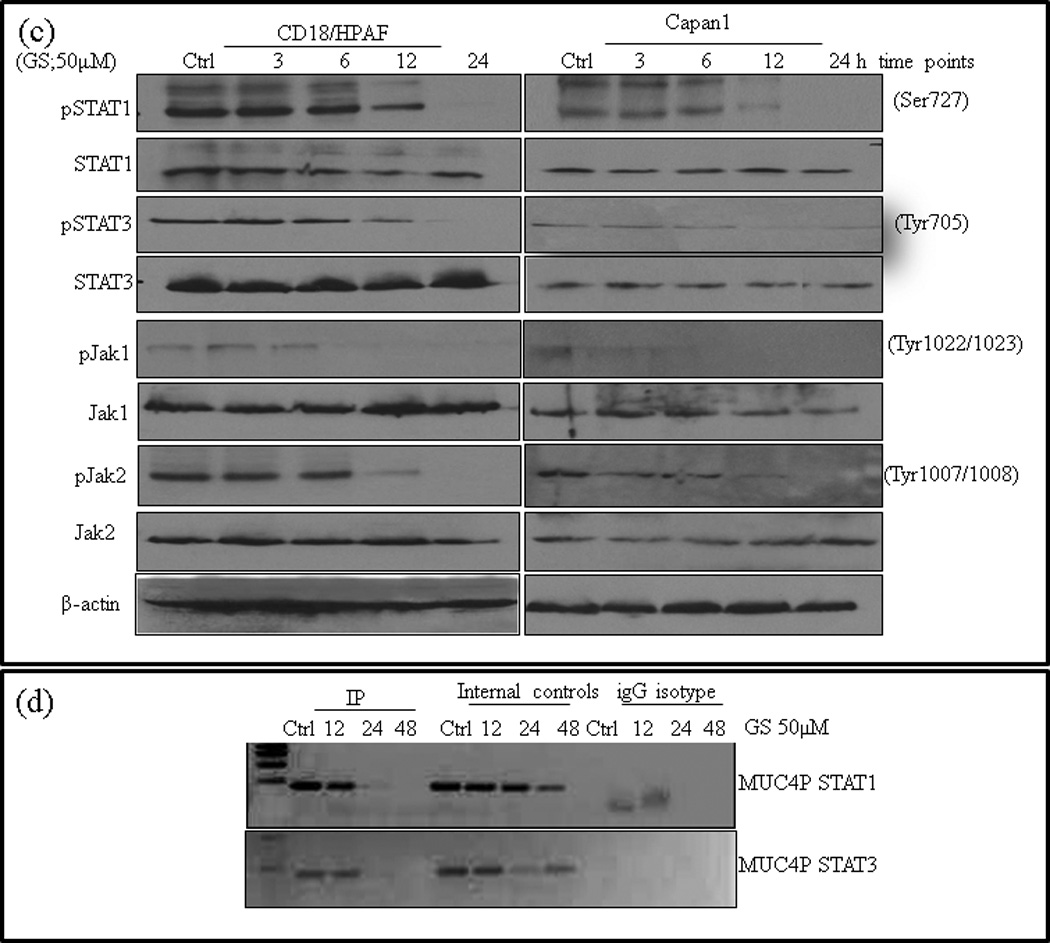
GS down regulates MUC4 expression in PC cells. MUC4 expressing Capan1 and CD18/HPAF cells were either left untreated for 48hrs or treated with GS for 12, 24 and 48 hrs followed by protein and RNA isolation. MUC4 expression was determined by (a) western blotting using 2% agarose gel and by RT-PCR. β-actin was used as a loading control for western blot analysis and for RT-PCR. GS down regulates MUC4 in both the cells lines in a time dependent manner with complete loss of MUC4 expression at 48 hrs of GS treatment. (b) Confocal microscopic images of MUC4 expression in CD18/HPAF and Capan1 cells after being incubated with GS for 48 hrs. Cells were stained with anti-MUC4 monoclonal antibody 8G7 and FITC-conjugated secondary antibody (green color). Nuclei were counterstained with DAPI (blue color). (Original Magnification x 63 for all photomicrographs). (c) GS inhibits Jak/Stat3 pathway in pancreatic cancer cells. Capan1 and CD18/HPAF cells were either left untreated for 24 hrs or treated with GS for 12 and 24 hrs and whole cell extract was prepared. Western blot analysis was done and protein expression was determined by probing with antibodies against phosphorylated and total STAT1, STAT3, Jak1 and Jak2 proteins. β-actin was used as loading control. (d) GS inhibits recruitment of STAT1 and STAT3 on MUC4 promoter. Cross-linked, sheared chromatin was prepared from CD18/HPAF cells treated with GS for indicated time points and subjected to immunoprecipitation with the STAT1 and STAT3 antibodies. The immunoprecipitated complexes were subjected to PCR analysis using primer pairs spanning the human MUC4 promoter. Chromatin obtained before immunoprecipitation was used as internal control.
3.7 GS inhibits recruitment of STAT1 and STAT3 to the MUC4 promoter
Our laboratory and others have reported that activated STAT1 and STAT3 transcription factors are potential regulators of MUC4 expression in PC involving JAK pathway [31, 38–40]. Similarly, hyper activated STAT3 has been correlated with increased murine Muc4 levels in gastric tumors of mice [41]. GS has been shown to modulate the activation of STAT transcription factors and their binding ability on gene promoters in HNSCC and colon cancers [6, 6, 8, 9, 21, 21], suggesting that STATs may be one the potential targets of GS mediated MUC4 downregulation. Immunoblot analysis of the lysates of GS-treated Capan1 and CD18/HPAF PC cells revealed a time dependent decrease in the phosphorylation of STAT1 and STAT3 proteins (Fig. 4c). GS-induced inhibition was observed as early as 6 hrs with complete inhibition observed at 24 hrs. We also observed similar effect of GS treatment on the expression of activated upstream STAT kinases, pJak1 and pJak2 (Fig. 4c), however, GS treatment did not alter the overall levels of STAT1, STAT3, Jak1 and Jak2 proteins levels till 12 h of GS treatment, but a slight decrease was observed at 24hrs of treatment (Fig. 4c). These results suggested that GS-reduced phosphorylated STAT1 and STAT3 levels by inhibiting the upstream kinases and also by reduction of total STAT levels.
To further establish the effect of GS on the association of STAT1 and STAT3 with MUC4 promoter, we performed ChIP assay using anti-STAT1 and -STAT3 antibodies. The occupancy of the promoter was analyzed using specific pairs of primers [31, 32] spanning the STAT1 and STAT3 binding motif of the MUC4 promoter. GS treatment significantly inhibited recruitment of STAT1 and STAT3 to the MUC4 promoter (Fig. 4d) suggesting that GS down regulates MUC4 expression at transcriptional level by modulating STAT binding on MUC4 gene promoter.
3.8 GS induces dissociation of BAD from 14-3-3ζ and its accumulation on mitochondria
Phosphorylation of BAD results in cytoplasmic sequestration by its interaction with 14-3-3ζ [42], inhibiting its pro apoptotic function at mitochondrial membrane. We have previously reported that MUC4 induced phosphorylation of BAD in CD18/HPAF cells [37]. GS induced BAD dephosphorylation and its dissociation from 14-3-3ζ has been reported in HNSCC [30]. Hence, we determined, whether decreased MUC4 expression by GS was accompanied by BAD dephosphorylation and its dissociation from 14-3-3ζ. Immunoblot analysis showed a time dependent decrease in the level of phosphorylated BAD (pBAD, Ser-136) following GS treatment in CD18/HPAF cells (Fig. 5a). Co-immunoprecipitation (co-IP) analysis revealed that dephosphorylation of BAD was accompanied by its dissociation from 14-3-3ζ (Fig. 5b), and its subsequent translocation to mitochondria (Fig. 5c), thus increasing its pro-apoptotic effect.
Fig. 5.
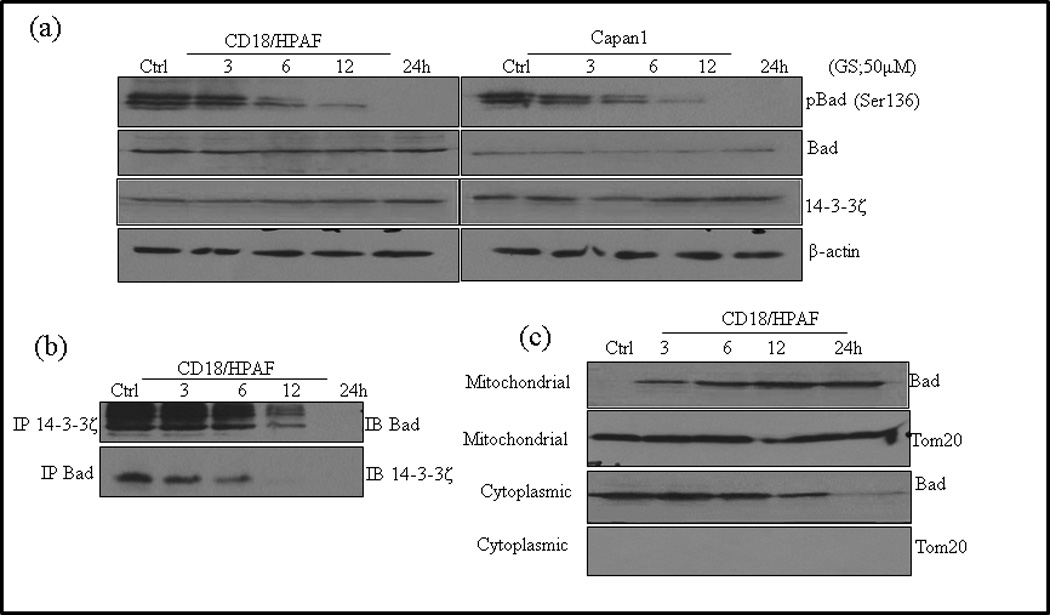
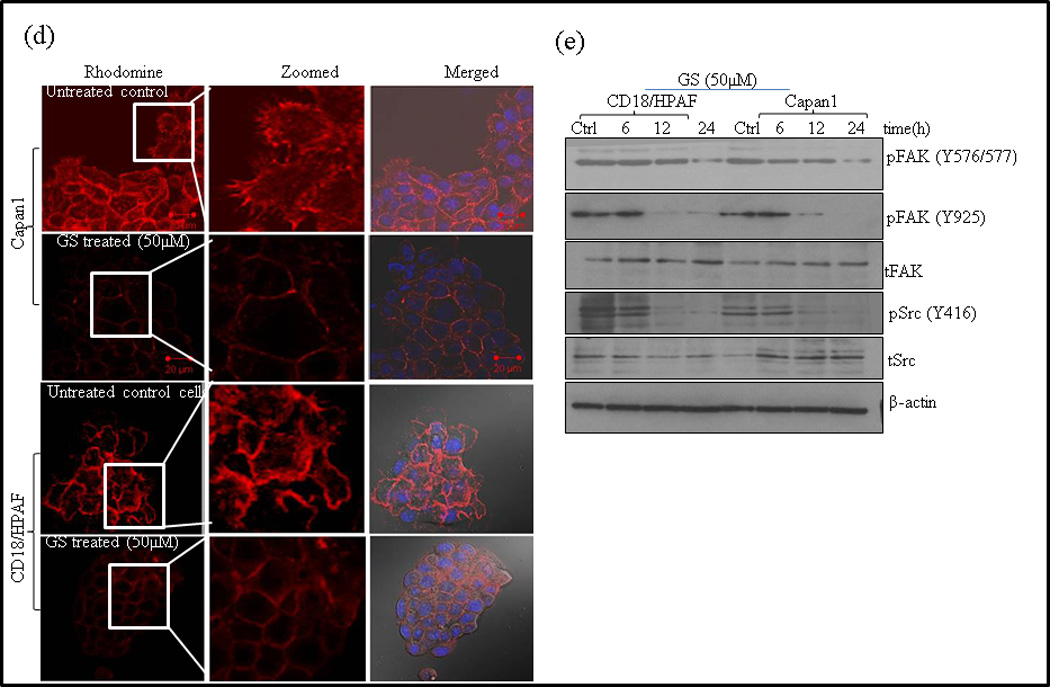
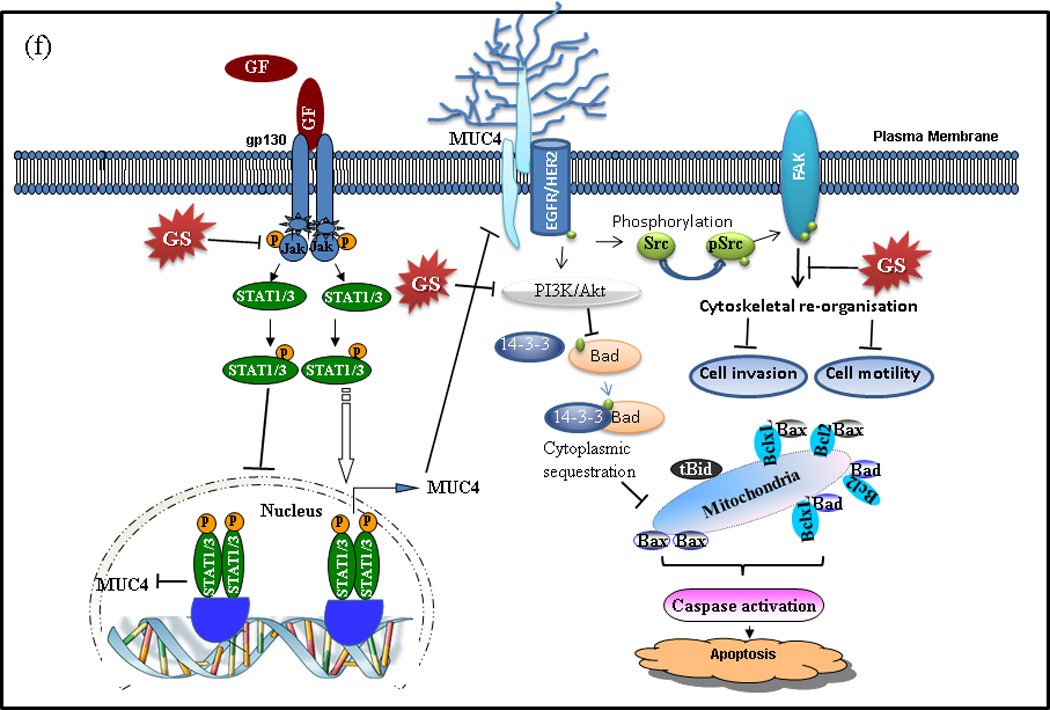
Mechanisms of GS induced apoptosis and inhibition of motility in PC cells. (a) Capan1 and CD18/HPAF cells were treated with 50µM GS for indicated time points and 50µg of protein were resolved on 12% SDS-PAGE gel and probed for pBAD, BAD and 14-3-3ζ antibodies. β -actin was used as a loading control. (b) Association of BAD and 14-3-3ζ was studied in the lysates of GS treated CD18/HPAF cells by immunoprecipitation and western blotting as described in Materials and Methods. 14-3-3-ζ was immunoprecipitated using specific antibody and the bound levels of BAD co-precipitated with 14-3-3ζ were determined by western blot analysis. Reverse immunoprecipitation assay were carried out in which BAD were immunoprecipitated followed by western blotting analysis of 14-3-3ζ. (c) Analysis of BAD in the cytoplasm and mitochondria following GS treatment. Cytoplasmic and mitochondrial fractions were prepared from GS treated CD18/HPAF cells. Western blotting of Tom20 was done to determine the purity of the sub-cellular fractions obtained. Representative images represented are of 3 independent experiments. Control for cytoplasmic proteins. (d) GS disrupts actin cytoskeleton involved in cell motility and cell invasion of PC cells. Actin filaments were stained with Rhodamine-conjugated phalloidin and visualized by confocal microscopy. The cell nucleus was stained with DAPI. Scale bars 20µM. (e) GS inhibits Fak/Src activation. Capan1 and CD18/HPAF cells were treated with GS for indicated time points. Protein lysates (50µg) were resolved by 10% SDS-PAGE and probed with antibodies against pFAK (y397, y576/577), total FAK, pSrc, total Src. β-actin was used as a loading control. GS inhibit in vitro clonogenic ability of PC cells. (f) Schematic diagram showing the effect of GS on PC cells. GS possibly induces anti-proliferative and anti-invasive effects via both MUC4-dependent and independent pathways. GS decreases STAT phosphorylation and therefore their translocation to the nucleus, hence inhibiting MUC4 expression. MUC4 down regulation inhibits the Her2/pSrc/FAK signaling thereby decreasing cell motility and invasion. GS also decreased BAD phosphorylation, its dissociation from 14-3-3ζ and its translocation to mitochondria. The decrease in BAD phosphorylation could either be due to MUC4 downregulation or by direct inhibition of PI3K signaling by GS. GF: growth factor; GS: Guggulsterone; FAK: focal adhesion kinase
3.9 GS induces rearrangement of the cytoskeleton of PC cells
Recently, we have observed that MUC4 knockdown decreases cell motility by inducing cytoskeletal reorganization as observed by reduced lammilopodia and filopodia [29]. We thus determined whether GS treatment resulted in actin cytoskeleton rearrangement to impact motility and invasion of PC cells. Phalloidin staining indicated a disruption of the cortical actin network with reduced lammilopodia and filopodia in GS treated cells compared to untreated control cells, suggesting that Capan1 and CD18/HPAF cells have a decreased motility following GS treatment (Fig. 5d).
FAK, a non-receptor protein-tyrosine kinase, plays central role in integrin-mediated signaling, and is involved in cellular motility and protection against apoptosis [43]. Src-mediated phosphorylation and activation of FAK is required for actin stress fiber formation and focal adhesion assembly during cell adhesion and cell spreading [44, 45]. Therefore, we determined if the effects of GS on motility and actin remodeling are mediated through FAK/Src signaling. Western blot analysis of GS treatment showed decreased phosphorylation of both Src and FAK in a time dependent manner, both in CD18/HPAF and Capan1 cells (Fig. 5e).
4. Discussion
Several studies indicate that GS suppresses proliferation and induces apoptosis in a variety of tumor cells, including pancreas, breast, colon, oral, lung, melanoma, myeloma, leukemia, and prostate carcinoma [7, 9–11, 13, 14, 30]. Therefore, in the present study we evaluated the anti-tumor effects of GS in lethal PC cells and investigated the underlying mechanisms. The current study demonstrates that GS treatment inhibited the cell growth, increased apoptosis, decreased invasion and metastasis by effecting cytoskeletal re-organization, suppressed colony formation and downregulated MUC4 expression through inhibiting Jak/STAT signaling. Besides MUC4, GS also down regulated MUC1 and MUC16 expression in PC cells (data not shown). The overall effect of GS on PC cells is presented in the schematic diagram in Fig. 5f. To our knowledge, this is the first evidence demonstrating that GS can modulate mucin expression and assumes importance in view of the oncogenic role of MUC4 in PC and its potential as a therapeutic target for PC. These intriguing findings are relevant to a large number of PC patients in the view that we and others have reported significant over-expression of MUC4 in PC compared to the normal pancreas [46].
One of the most significant findings of our studies was the downregulation of MUC4 mucin. In accordance with the present findings, recent studies from our group demonstrated that chemopreventive agents, Thymoquinon (TQ), Graviola and Basil also resulted in MUC4 down-regulation, increased apoptosis and decreased invasion and metastasis of PC cells [28, 47, 48]. MUC4, a member of membrane bound mucin, is one of the top differentially overexpressed genes in PC [46, 49]. While undetectable in the normal pancreas, its expression increases progressively with the progression of the disease and is associated with poor prognosis. Our recent studies support the oncogenic role of MUC4 and indicate that MUC4 can induce neoplastic transformation of fibroblasts and promote survival, invasion and metastasis of PC cells [27, 33]. MUC4 also promotes resistance to apoptosis induced by chemotherapeutic agents like Gem, trastuzumab, and cisplatin in PC cells and down-regulation of MUC4 reverses chemoresistance of PC stem/progenitor cells and their progenies [2, 36, 37]. MUC4 also induces epithelial to mechenchymal transition (EMT) by stabilizing N-Cadherin expression in pancreatic cancer cells [29]. In addition to MUC4, other membrane-bound mucins MUC1 and MUC16 have are also overexpressed in PC and contribute to pathogenesis [50, 51]. GS treatment also resulted in significant down-regulation of MUC1 and MUC16 in both Capan1 and CD18/HPAF cells (data not shown) indicating that GS mediated effects may not be limited to MUC4 only, but may also involve other mucins. However, given the significance of MUC4 in PC pathogenesis, we focused our efforts to understand the mechanism of MUC4 down-regulation by GS.
Several cytokines including IL-4, IL-6, TNF-α and IFN-γ have been shown to be involved in deregulated overexpression of MUC4 [28, 31, 39, 41, 52], through JAK/STAT pathway, in particular STAT3 and to a minor extent STAT1 [53]. We and others have identified 5 STAT1 binding sites on MUC4 promoter, and two STAT3 binding sites on 5′-UTR of MUC4 [31, 32, 38], suggesting a possible role of STAT transcription factors in the transcriptional regulation of MUC4. Recently GS induced decrease in levels of phosphotyrosine STAT3 has been demonstrated in multiple myeloma and HNSCC cell lines [6, 9, 21]. Furthermore, GS has been reported to decrease IL-6 expression and subsequent STAT3 binding on the VEGF promoter, indicating the effect of GS on transcription factor binding on gene promoters [8, 21]. In accord with these reports, our ChIP analysis also indicated decreased binding of both STAT1 and STAT3 to the MUC4 promoter (Fig. 4d) and reduced MUC4 transcripts following GS treatment. Further, decreased binding of STATs was accompanied with decreased phosphorylation of both STAT1 and STAT3 (Fig. 4c) along with their upstream kinases Jak1and Jak2.
Apoptosis induction and inhibition of cell proliferation are important mechanisms for anti-cancer effects of many natural chemopreventive agents[10], and the mechanism involves the up-regulation of pro-apoptotic proteins and/or down-regulation of anti-apoptotic proteins. Our studies demonstrate that GS treatment resulted in a dose and time dependent growth inhibition of cell proliferation and increased apoptosis characterized by increased sub diploid cells (Fig. 2b and 2c), Caspase-3 activation, a time-dependent decrease in the levels of anti-apoptotic/proliferative genes Bcl-2, xIAP and a simultaneous increase in the pro-apoptotic proteins BAD and Bax (Fig. 2 e–f) indicating the involvement of intrinsic apoptotic pathway. MUC4 down regulation has been shown to induce intrinsic pathway of apoptosis and this may be a possible mechanism of GS induced apoptosis in PC cells. These results are in accord with earlier observations from our lab showing MUC4 knockdown in CD18/HPAF PC cells activates intrinsic mitochondrial apoptotic pathways and reduced resistance to gemcitabine treatment by effecting BAD phosphorylation, suggesting that MUC4-mediated increased BAD phosphorylation is sufficient to protect PC cells from apoptosis [37]. Pro-apoptotic function requires mitochondrial translocation of BAD and its interaction with Bcl2/Bcl-xl to release Bax and formation of ion pores to release cytochrome c into cytoplasm. However, in proliferating and cancerous cells, pro-apoptotic function of BAD is inhibited by its sustained phosphorylation (ser-136) and its interaction with 14-3-3 family of proteins, which results in its sequestration in the cytoplasm away from the mitochondria [30]. Consistent with these reports, we observed that GS induced MUC4 down regulation was accompanied with BAD de-phosphorylation. Co-immunoprecipitation assays using 14-3-3ζ specific antibody revealed dissociation of BAD from 14-3-3ζ and its subsequent translocation from cytoplasm to mitochondria (Fig. 5c).
Cell motility being the important requirement for tumor invasion, is an attractive therapeutic target for inhibiting advanced PC. GS impacts several signaling pathways regulating cell motility and invasion [9, 14]. Following GS treatment, we observed significant inhibition of PC cell motility and invasion. These results are in accord with earlier observations showing GS prevented the migration of metastatic human breast cancer MDA-MB-231 cells [34]. Further, we observed a significant decrease in number and length of both lammilopodia and filopodia suggesting reorganization of actin cytoskeleton following GS treatment (Fig. 5d). Cytoskeleton re-organization is a critical step for cell motility and invasion. Our previous studies have shown that MUC4 modulates cancer cell motility by altering morphology, actin-cytoskeleton and downstream signaling events [27, 54]. It is likely that the decreased cell motility and invasion following GS treatment could in part be due to MUC4 down-regulation by GS. Focal adhesion kinase is overexpressed in invasive and metastatic tumors and orchestrates cytoskeletal reorganization and MMPs regulation during cell motility and invasion [55]. MUC4 modulates HER2 downstream signaling by physical interaction leading to FAK, Akt and ERK activation implicated in cell survival and motility [54]. Recent studies demonstrated that FAK is linked to the downregulation of HER2 activity at the cell periphery, which appears to be important for the formation of motile cells [56, 57]. Our studies indicated that GS inhibits phosphorylation of FAK (tyrosines 925 and 576/577) and Src (tyrosine 416), while total FAK and Src levels remained unchanged (Fig. 5e). Furthermore, GS decreased HER-2 expression in both Capan1 and CD18/HPAF cells, suggesting that GS possibly decreases cell motility and invasion through MUC4 modulated Her-2/FAK/Src signaling.
An earlier report suggested that GS inhibits cell migration and invasion in less aggressive Mia-PaCa2 and PANC1 PC cells by targeting FXR expression [15]. To determine if GS modulated effects on migration and invasion in our studies are FXR mediated, we checked the expression of FXR in highly aggressive Capan1 and CD18/HPAF cells. We observed a faint expression of FXR in CD18/HPAF cells (data not shown). As against the earlier reports [15], we didn’t observe any expression of FXR in Capan1 cells (data not shown) signifying FXR independent effects of GS in our cell system. Together, the data presented in the manuscript and the published work of Ahn et al and Lee JY et al [15, 17], supports that GS exhibits anti-proliferative and pro-apoptotic effects against pancreatic cancer cell of various aggressiveness.
Currently there is no data available on pharmacokinetics, bioavailability and metabolism of GS in humans. However Verma N et al has shown the pharmacokinetics of Z-guggulsterone and its metabolite E-guggulsterone in the orally gavaged rats with 50 mg /kg body weight of guggulsterone and observed the maximal plasma concentration to be ~3.3µmol/L [58]. Moreover, a reasonably amount (~43%) of this z-guggulsterone was found to be bioavailable suggesting the possibility of achieving the desired concentrations of GS needed to inhibit tumor growth in humans [58]. However, the detailed knowledge of these basic parameters is very important and much needed for proper evaluation of the clinical benefits of GS. Therefore, future studies are required to evaluate those activities and the associated benefits in the prevention or treatment of cancer in humans.
The present study showing the down-regulation of MUC4 by GS treatment is novel and warrants further studies to determine the molecular mechanism of GS mediated down-regulation of mucins that are differentially up-regulated in PC. Given the high incidence of MUC4 expression (>80%) in PC and its functional involvement in chemo-resistance, the future studies may involve elucidating the Gemcitabine sensitization by targeting MUC4 with GS. Overall, GS, which has a role in MUC4 downregulation among other anti-tumorigenic properties, has potential for the development of novel therapies against PC, and further studies must be done to evaluate its therapeutic value in preclinical models.
Supplementary Material
Acknowledgements
The invaluable technical support from Kavita Mallya is greatly appreciated. We also thank Janice A. Tayor and James R. Talaska of the Confocal Laser Scanning Microscope Core Facility at UNMC, Victoria B. Smith and Megan Michalak of the UNMC Cell Analysis Core Facility, and the Eppley Cancer Center for their support of the core facilities. The authors of this work are supported by grants from the National Institutes of Health: P20 GM103480, R21 CA156037, R01 CA78590, R03 CA167342, EDRN U01 CA111294, R01 CA131944, R01 CA133774, R01 CA138791, R03 CA 139285, SPORE P50 CA127297 and TMEN U54163120.
Abbreviations
- PC
Pancreatic cancer
- MUC4
Mucin4
- GS
Guggulsterone
- STAT
Signal transduction and activator of transcription
- FXR
Farnesoid X receptor
- EMT
Epithelial to mechenchymal transition
- MTT
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
- HNSCC
Head and Neck Squamous Cell Carcinoma
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest Statement
There are no potential conflicts of interest involved with this work.
Reference List
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J. Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Mimeault M, Johansson SL, Senapati S, Momi N, Chakraborty S, Batra SK. MUC4 down-regulation reverses chemoresistance of pancreatic cancer stem/progenitor cells and their progenies. Cancer Lett. 2010;295:69–84. doi: 10.1016/j.canlet.2010.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hu G, Li F, Ouyang K, Xie F, Tang X, Wang K, Han S, Jiang Z, Zhu M, Wen D, Qin X, Zhang L. Intrinsic gemcitabine resistance in a novel pancreatic cancer cell line is associated with cancer stem cell-like phenotype. Int. J. Oncol. 2012;40:798–806. doi: 10.3892/ijo.2011.1254. [DOI] [PubMed] [Google Scholar]
- 4.Burris HA, III., Moore MJ, Andersen J, Green MR, Rothenberg ML, Modiano MR, Cripps MC, Portenoy RK, Storniolo AM, Tarassoff P, Nelson R, Dorr FA, Stephens CD, Von Hoff DD. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J. Clin. Oncol. 1997;15:2403–2413. doi: 10.1200/JCO.1997.15.6.2403. [DOI] [PubMed] [Google Scholar]
- 5.Shishodia S, Harikumar KB, Dass S, Ramawat KG, Aggarwal BB. Aggarwal, The guggul for chronic diseases: ancient medicine, modern targets. Anticancer Res. 2008;28:3647–3664. [PubMed] [Google Scholar]
- 6.Ahn KS, Sethi G, Sung B, Goel A, Ralhan R, Aggarwal BB. Aggarwal, Guggulsterone, a farnesoid X receptor antagonist, inhibits constitutive and inducible STAT3 activation through induction of a protein tyrosine phosphatase SHP-1. Cancer Res. 2008;68:4406–4415. doi: 10.1158/0008-5472.CAN-07-6696. [DOI] [PubMed] [Google Scholar]
- 7.An MJ, Cheon JH, Kim SW, Kim ES, Kim TI, Kim WH. Guggulsterone induces apoptosis in colon cancer cells and inhibits tumor growth in murine colorectal cancer xenografts. Cancer Lett. 2009;279:93–100. doi: 10.1016/j.canlet.2009.01.026. [DOI] [PubMed] [Google Scholar]
- 8.Kim ES, Hong SY, Lee HK, Kim SW, An MJ, Kim TI, Lee KR, Kim WH, Cheon JH. Guggulsterone inhibits angiogenesis by blocking STAT3 and VEGF expression in colon cancer cells. Oncol. Rep. 2008;20:1321–1327. [PubMed] [Google Scholar]
- 9.Leeman-Neill RJ, Wheeler SE, Singh SV, Thomas SM, Seethala RR, Neill DB, Panahandeh MC, Hahm ER, Joyce SC, Sen M, Cai Q, Freilino ML, Li C, Johnson DE, Grandis JR. Guggulsterone enhances head and neck cancer therapies via inhibition of signal transducer and activator of transcription-3. Carcinogenesis. 2009;30:1848–1856. doi: 10.1093/carcin/bgp211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shishodia S, Aggarwal BB. Guggulsterone inhibits NF-kappaB and IkappaBalpha kinase activation, suppresses expression of anti-apoptotic gene products, and enhances apoptosis. J. Biol. Chem. 2004;279:47148–47158. doi: 10.1074/jbc.M408093200. [DOI] [PubMed] [Google Scholar]
- 11.Shishodia S, Sethi G, Ahn KS, Aggarwal BB. Guggulsterone inhibits tumor cell proliferation, induces S-phase arrest, and promotes apoptosis through activation of c-Jun Nterminal kinase, suppression of Akt pathway, and downregulation of antiapoptotic gene products. Biochem. Pharmacol. 2007;74:118–130. doi: 10.1016/j.bcp.2007.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Singh SV, Zeng Y, Xiao D, Vogel VG, Nelson JB, Dhir R, Tripathi YB. Caspase-dependent apoptosis induction by guggulsterone, a constituent of Ayurvedic medicinal plant Commiphora mukul, in PC-3 human prostate cancer cells is mediated by Bax and Bak. Mol. Cancer Ther. 2005;4:1747–1754. doi: 10.1158/1535-7163.MCT-05-0223. [DOI] [PubMed] [Google Scholar]
- 13.Singh SV, Choi S, Zeng Y, Hahm ER, Xiao D. Guggulsterone-induced apoptosis in human prostate cancer cells is caused by reactive oxygen intermediate dependent activation of c-Jun NH2-terminal kinase. Cancer Res. 2007;67:7439–7449. doi: 10.1158/0008-5472.CAN-07-0120. [DOI] [PubMed] [Google Scholar]
- 14.Xiao D, Singh SV. z-Guggulsterone, a constituent of Ayurvedic medicinal plant Commiphora mukul, inhibits angiogenesis in vitro and in vivo. Mol. Cancer Ther. 2008;7:171–180. doi: 10.1158/1535-7163.MCT-07-0491. [DOI] [PubMed] [Google Scholar]
- 15.Lee JY, Lee KT, Lee JK, H Lee K, Jang KT, Heo JS, Choi SH, Kim Y, Rhee JC. Farnesoid X receptor, overexpressed in pancreatic cancer with lymph node metastasis promotes cell migration and invasion. Br. J. Cancer. 2011;104:1027–1037. doi: 10.1038/bjc.2011.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xu HB, Xu LZ, Li L, Fu J, Mao XP. Reversion of P-glycoprotein-mediated multidrug resistance by guggulsterone in multidrug-resistant human cancer cell lines. Eur. J. Pharmacol. 2012;694:39–44. doi: 10.1016/j.ejphar.2012.06.046. [DOI] [PubMed] [Google Scholar]
- 17.Ahn DW, Seo JK, Lee SH, Hwang JH, Lee JK, Ryu JK, Kim YT, Yoon YB. Enhanced antitumor effect of combination therapy with gemcitabine and guggulsterone in pancreatic cancer. Pancreas. 2012;41:1048–1057. doi: 10.1097/MPA.0b013e318249d62e. [DOI] [PubMed] [Google Scholar]
- 18.Yang MH, Lee KT, Yang S, Lee JK, Lee KH, Moon IH, Rhee JC. Guggulsterone enhances antitumor activity of gemcitabine in gallbladder cancer cells through suppression of NF-kappaB. J. Cancer Res. Clin. Oncol. 2012;138:1743–1751. doi: 10.1007/s00432-012-1254-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Choudhuri R, Degraff W, Gamson J, Mitchell JB, Cook JA. Guggulsterone-mediated enhancement of radiosensitivity in human tumor cell lines. Front Oncol. 1:19. doi: 10.3389/fonc.2011.00019. Epub;%2011 Jul 21. (2011) 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lv N, Song MY, Kim EK, Park JW, Kwon KB, Park BH. Guggulsterone, a plant sterol, inhibits NF-kappaB activation and protects pancreatic beta cells from cytokine toxicity. Mol. Cell Endocrinol. 2008;289:49–59. doi: 10.1016/j.mce.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 21.Macha MA, Matta A, Chauhan SS, Siu KW, Ralhan R. Guggulsterone (GS) inhibits smokeless tobacco and nicotine-induced NF-kappaB and STAT3 pathways in head and neck cancer cells. Carcinogenesis. 2011;32:368–380. doi: 10.1093/carcin/bgq278. [DOI] [PubMed] [Google Scholar]
- 22.Kapoor S. Guggulsterone: a potent farnesoid X receptor antagonist and its rapidly evolving role as a systemic anticarcinogenic agent. Hepatology. 2008;48:2090–2091. doi: 10.1002/hep.22601. [DOI] [PubMed] [Google Scholar]
- 23.Sarfaraz S, Siddiqui IA, Syed DN, Afaq F, Mukhtar H. Guggulsterone modulates MAPK and NF-kappaB pathways and inhibits skin tumorigenesis in SENCAR mice. Carcinogenesis. 2008;29:2011–2018. doi: 10.1093/carcin/bgn180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Burris TP, Montrose C, Houck KA, Osborne HE, Bocchinfuso WP, Yaden BC, Cheng CC, Zink RW, Barr RJ, Hepler CD, Krishnan V, Bullock HA, Burris LL, Galvin RJ, Bramlett K, Stayrook KR. The hypolipidemic natural product guggulsterone is a promiscuous steroid receptor ligand. Mol. Pharmacol. 2005;67:948–954. doi: 10.1124/mol.104.007054. [DOI] [PubMed] [Google Scholar]
- 25.Brobst DE, Ding X, Creech KL, Goodwin B, Kelley B, Staudinger JL. Guggulsterone activates multiple nuclear receptors and induces CYP3A gene expression through the pregnane X receptor. J. Pharmacol. Exp. Ther. 2004;310:528–535. doi: 10.1124/jpet.103.064329. [DOI] [PubMed] [Google Scholar]
- 26.Moniaux N, Varshney GC, Chauhan SC, Copin MC, Jain M, Wittel UA, Andrianifahanana M, Aubert JP, Batra SK. Generation and characterization of anti-MUC4 monoclonal antibodies reactive with normal and cancer cells in humans. J. Histochem. Cytochem. 2004;52:253–261. doi: 10.1177/002215540405200213. [DOI] [PubMed] [Google Scholar]
- 27.Chaturvedi P, Singh AP, Moniaux N, Senapati S, Chakraborty S, Meza JL, Batra SK. MUC4 mucin potentiates pancreatic tumor cell proliferation, survival, and invasive properties and interferes with its interaction to extracellular matrix proteins. Mol. Cancer Res. 2007;5:309–320. doi: 10.1158/1541-7786.MCR-06-0353. [DOI] [PubMed] [Google Scholar]
- 28.Torres MP, Ponnusamy MP, Chakraborty S, Smith LM, Das S, Arafat HA, Batra SK. Effects of thymoquinone in the expression of mucin 4 in pancreatic cancer cells: implications for the development of novel cancer therapies. Mol. Cancer Ther. 2010;9:1419–1431. doi: 10.1158/1535-7163.MCT-10-0075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rachagani S, Macha MA, Ponnusamy MP, Haridas D, Kaur S, Jain M, Batra SK. MUC4 potentiates invasion and metastasis of pancreatic cancer cells through stabilization of fibroblast growth factor receptor 1. Carcinogenesis. 2012;33:1953–1964. doi: 10.1093/carcin/bgs225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Macha MA, Matta A, Chauhan S, Siu KM, Ralhan R. 14-3-3 zeta is a molecular target in guggulsterone induced apoptosis in head and neck cancer cells. BMC. Cancer. 2010;10:655. doi: 10.1186/1471-2407-10-655. 655-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Andrianifahanana M, Singh AP, Nemos C, Ponnusamy MP, Moniaux N, Mehta PP, Varshney GC, Batra SK. IFN-gamma-induced expression of MUC4 in pancreatic cancer cells is mediated by STAT-1 upregulation: a novel mechanism for IFN-gamma response. Oncogene. 2007;26:7251–7261. doi: 10.1038/sj.onc.1210532. [DOI] [PubMed] [Google Scholar]
- 32.Perrais M, Pigny P, Ducourouble MP, Petitprez D, Porchet N, Aubert JP, Van I S. Characterization of human mucin gene MUC4 promoter: importance of growth factors and proinflammatory cytokines for its regulation in pancreatic cancer cells. J. Biol. Chem. 2001;276:30923–30933. doi: 10.1074/jbc.M104204200. [DOI] [PubMed] [Google Scholar]
- 33.Bafna S, Singh AP, Moniaux N, Eudy JD, Meza JL, Batra SK. MUC4, a multifunctional transmembrane glycoprotein, induces oncogenic transformation of NIH3T3 mouse fibroblast cells. Cancer Res. 2008;68:9231–9238. doi: 10.1158/0008-5472.CAN-08-3135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Silva J, Dasgupta S, Wang G, Krishnamurthy K, Ritter E, Bieberich E. Lipids isolated from bone induce the migration of human breast cancer cells. J. Lipid Res. 2006;47:724–733. doi: 10.1194/jlr.M500473-JLR200. [DOI] [PubMed] [Google Scholar]
- 35.Zhao X, Guan JL. Focal adhesion kinase and its signaling pathways in cell migration and angiogenesis. Adv. Drug Deliv. Rev. 2011;63:610–615. doi: 10.1016/j.addr.2010.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nagy P, Friedlander E, Tanner M, Kapanen AI, Carraway KL, Isola J, Jovin TM. Decreased accessibility and lack of activation of ErbB2 in JIMT-1, a herceptin-resistant, MUC4-expressing breast cancer cell line. Cancer Res. 2005;65:473–482. [PubMed] [Google Scholar]
- 37.Bafna S, Kaur S, Momi N, Batra SK. Pancreatic cancer cells resistance to gemcitabine: the role of MUC4 mucin. Br. J. Cancer. 2009;101:1155–1161. doi: 10.1038/sj.bjc.6605285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kossow C, Jose D, Jaster R, Wolkenhauer O, Rateitschak K. Mathematical modelling unravels regulatory mechanisms of interferon-gamma-induced STAT1 serine-phosphorylation and MUC4 expression in pancreatic cancer cells. IET. Syst. Biol. 2012;6:73–85. doi: 10.1049/iet-syb.2011.0017. [DOI] [PubMed] [Google Scholar]
- 39.Mejias-Luque R, Peiro S, Vincent A, Van I S, de BC. IL-6 induces MUC4 expression through gp130/STAT3 pathway in gastric cancer cell lines. Biochim. Biophys. Acta. 2008;1783:1728–1736. doi: 10.1016/j.bbamcr.2008.05.020. [DOI] [PubMed] [Google Scholar]
- 40.Kunigal S, Ponnusamy MP, Momi N, Batra SK, Chellappan SP. Nicotine, IFN-gamma and retinoic acid mediated induction of MUC4 in pancreatic cancer requires E2F1 and STAT-1 transcription factors and utilize different signaling cascades. Mol. Cancer. 2012;11:24. doi: 10.1186/1476-4598-11-24. 24-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mejias-Luque R, Linden SK, Garrido M, Tye H, Najdovska M, Jenkins BJ, Iglesias M, Ernst M, de BC. Inflammation modulates the expression of the intestinal mucins MUC2 and MUC4 in gastric tumors. Oncogene. 2010;29:1753–1762. doi: 10.1038/onc.2009.467. [DOI] [PubMed] [Google Scholar]
- 42.Thomadaki H, Scorilas A. BCL2 family of apoptosis-related genes: functions and clinical implications in cancer. Crit Rev. Clin. Lab Sci. 2006;43:1–67. doi: 10.1080/10408360500295626. [DOI] [PubMed] [Google Scholar]
- 43.Gabarra-Niecko V, Schaller MD, Dunty JM. FAK regulates biological processes important for the pathogenesis of cancer. Cancer Metastasis Rev. 2003;22:359–374. doi: 10.1023/a:1023725029589. [DOI] [PubMed] [Google Scholar]
- 44.Verbeek BS, Vroom TM, Rijksen G. Overexpression of c-Src enhances cell-matrix adhesion and cell migration in PDGF-stimulated NIH3T3 fibroblasts. Exp. Cell Res. 1999;248:531–537. doi: 10.1006/excr.1999.4416. [DOI] [PubMed] [Google Scholar]
- 45.Kaplan KB, Swedlow JR, Morgan DO, Varmus HE. c-Src enhances the spreading of src−/− fibroblasts on fibronectin by a kinase-independent mechanism. Genes Dev. 1995;9:1505–1517. doi: 10.1101/gad.9.12.1505. [DOI] [PubMed] [Google Scholar]
- 46.Andrianifahanana M, Moniaux N, Schmied BM, Ringel J, Friess H, Hollingsworth MA, Buchler MW, Aubert JP, Batra SK. Mucin (MUC) gene expression in human pancreatic adenocarcinoma and chronic pancreatitis: a potential role of MUC4 as a tumor marker of diagnostic significance. Clin. Cancer Res. 2001;7:4033–4040. [PubMed] [Google Scholar]
- 47.Torres MP, Rachagani S, Purohit V, Pandey P, Joshi S, Moore ED, Johansson SL, Singh PK, Ganti AK, Batra SK. Graviola: a novel promising natural-derived drug that inhibits tumorigenicity and metastasis of pancreatic cancer cells in vitro and in vivo through altering cell metabolism. Cancer Lett. 2012;323:29–40. doi: 10.1016/j.canlet.2012.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shimizu T, Torres MP, Chakraborty S, Souchek JJ, Rachagani S, Kaur S, Macha M, Ganti AK, Hauke RJ, Batra SK. Holy Basil leaf extract decreases tumorigenicity and metastasis of aggressive human pancreatic cancer cells in vitro and in vivo: Potential role in therapy. Cancer Lett. 2013:10. doi: 10.1016/j.canlet.2013.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chauhan SC, Singh AP, Ruiz F, Johansson SL, Jain M, Smith LM, Moniaux N, Batra SK. Aberrant expression of MUC4 in ovarian carcinoma: diagnostic significance alone and in combination with MUC1 and MUC16 (CA125) Mod. Pathol. 2006;19:1386–1394. doi: 10.1038/modpathol.3800646. [DOI] [PubMed] [Google Scholar]
- 50.Hollingsworth MA, Swanson BJ. Mucins in cancer: protection and control of the cell surface. Nat. Rev. Cancer. 2004;4:45–60. doi: 10.1038/nrc1251. [DOI] [PubMed] [Google Scholar]
- 51.Haridas D, Chakraborty S, Ponnusamy MP, Lakshmanan I, Rachagani S, Cruz E, Kumar S, Das S, Lele SM, Anderson JM, Wittel UA, Hollingsworth MA, Batra SK. Pathobiological implications of MUC16 expression in pancreatic cancer. PLoS. One. 2011;6:e26839. doi: 10.1371/journal.pone.0026839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Damera G, Xia B, Sachdev GP. IL-4 induced MUC4 enhancement in respiratory epithelial cells in vitro is mediated through JAK-3 selective signaling. Respir. Res. 2006) 39;7:39. doi: 10.1186/1465-9921-7-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Heinrich PC, Behrmann I, Haan S, Hermanns HM, Muller-Newen G, Schaper F. Principles of interleukin (IL)-6-type cytokine signalling and its regulation. Biochem. J. 2003;374:1–20. doi: 10.1042/BJ20030407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ponnusamy MP, Singh AP, Jain M, Chakraborty S, Moniaux N, Batra SK. MUC4 activates HER2 signalling and enhances the motility of human ovarian cancer cells. Br. J. Cancer. 2008;99:520–526. doi: 10.1038/sj.bjc.6604517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chang YM, Kung HJ, Evans CP. Nonreceptor tyrosine kinases in prostate cancer. Neoplasia. 2007;9:90–100. doi: 10.1593/neo.06694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mitra SK, Mikolon D, Molina JE, Hsia DA, Hanson DA, Chi A, Lim ST, Bernard-Trifilo JA, Ilic D, Stupack DG, Cheresh DA, Schlaepfer DD. Intrinsic FAK activity and Y925 phosphorylation facilitate an angiogenic switch in tumors. Oncogene. 2006;25:5969–5984. doi: 10.1038/sj.onc.1209588. [DOI] [PubMed] [Google Scholar]
- 57.Mitra SK, Lim ST, Chi A, Schlaepfer DD. Intrinsic focal adhesion kinase activity controls orthotopic breast carcinoma metastasis via the regulation of urokinase plasminogen activator expression in a syngeneic tumor model. Oncogene. 2006;25:4429–4440. doi: 10.1038/sj.onc.1209482. [DOI] [PubMed] [Google Scholar]
- 58.Verma N, Singh SK, Gupta RC. Simultaneous determination of the stereoisomers of guggulsterone in serum by high-performance liquid chromatography. J. Chromatogr. B Biomed. Sci. Appl. 1998;708:243–248. doi: 10.1016/s0378-4347(97)00626-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


