Abstract
Vasotocin/vasopressin is a neuropeptide that regulates social and reproductive behaviors in a variety of animals including fish. Arginine vasotocin (AVT) is expressed by cells in the ventral hypothalamic and preoptic areas in the diencephalon during embryogenesis in zebrafish suggesting that vasotocin might mediate other functions within the CNS prior to the development of social and reproductive behaviors. In order to examine potential early roles for vasotocin we cloned two zebrafish vasotocin receptors homologous to AVPR1a. The receptors are expressed primarily in the CNS in similar but generally non-overlapping patterns. Both receptors are expressed in the forebrain, midbrain and hindbrain by larval stage. Of note, AVTR1a-expressing neurons in the hindbrain appear to be contacted by the axons of preoptic neurons in the forebrain that include avt+ neurons and from sensory axons in the lateral longitudinal fasciculus (LLF). Furthermore, AVTR1a-expressing hindbrain neurons extend axons into the medial longitudinal fasciculus (MLF) that contains axons of many neurons thought to be involved in locomotor responses to sensory stimulation. One hypothesis consistent with this anatomy is that AVT signaling mediates or gates sensory input to motor circuits in the hindbrain and spinal cord.
1. Introduction
Signaling within the CNS via the nonapeptide, arginine vasopressin (AVP) in mammals and its homolog arginine vasotocin (AVT) in nonmammalian vertebrates, regulates social and reproductive behaviors in a wide variety of species (reviewed in Donaldson and Young, 2008). Dysfunction of signaling by AVP and oxytocin, another nonapeptide implicated in social behaviors, is thought to contribute to psychiatric disorders such as autism, affective disorders, obsessive-compulsive disorder, posttraumatic stress disorder and schizophrenia (reviewed in Heinrichs et al., 2009). Furthermore, AVP/AVT released into the circulation by the posterior pituitary in response to sexual stimulation, stress and dehydration mediates a variety of peripheral effects including antidiuretic activity by the kidney (Leng and Bicknell, 1986; Nishimura and Fan, 2003).
AVP is expressed by neurons of the supraoptic, paraventricular and suprachiasmatic nuclei of the hypothalamus in mammals (Brownstein et al., 1980; Young and Gainer, 2003) and AVT primarily by neurons of the preoptic area in fish (Venkatesh and Brenner, 1995; Acher et al., 1997). Additionally AVP is expressed by the bed nucleus of the stria terminalis and amygdala in the mammalian brain (DeVries and Buijs, 1983; DeVries et al., 1985). There are 3 AVP receptors in mammals with the AVPR1a (V1a) and AVPR1b (V1b) receptors expressed primarily in the CNS and the AVPR2 (V2) receptor in the periphery (Caldwell et al., 2008). AVT receptors have been identified in a number of teleosts as well (Mahlmann et al., 1994; Conklin et al., 1999; Warne, 2001; An et al., 2008). In the pupfish and perhaps other teleosts there appear to be two V1a receptors and a V2 receptor for AVT (Lema, 2010). Like the mammalian V1a receptor, RT-PCR found that the pupfish V1a receptors are widely expressed throughout the CNS.
AVT is expressed by cells in the ventral hypothalamus and the preoptic area of the diencephalon, and isotocin in the preoptic area (Tessmar-Raible et al., 2007; Eaton et al., 2008; Blechman et al., 2011) during embryogenesis in zebrafish prior to the development of social or reproductive behaviors. During embryogenesis behaviors exhibited by zebrafish embryos are restricted to simple motor responses such as escape swimming evoked by sensory stimulation (Saint-Amant and Drapeau, 1998). The embryonic expression of AVT suggests that it may participate in the development of sensory and/or motor circuits early in development. As a first step in examining the function of AVT signaling in the embryonic CNS, we cloned two V1a type receptors in zebrafish and determined their expression patterns during early stages of development. The expression pattern of the V1a receptors is concordant with the hypothesis that AVT signaling may play a role in early sensory/motor function.
2. Results
2.1. Zebrafish contain two AVT receptors homologous to AVPR1a
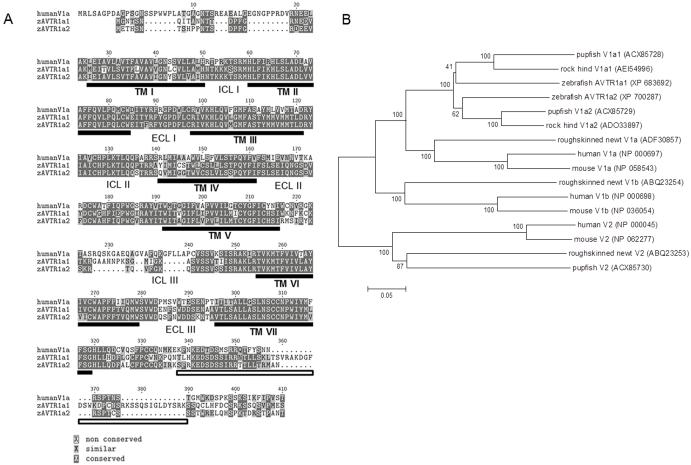
We cloned two AVT receptors by RT-PCR from adult zebrafish brain tissue, AVTR1a1 and AVTR1a2, that were 60% and 62% identical at the amino acid level with human V1a receptor and 54% and 52% identical with human V1b receptor, respectively (blastp, www.ncbi.nlm.nih.gov) (Fig. 1A). A phylogenetic analysis of zebrafish AVTR1a1 and AVTR1a2 was consistent with the assignment of the zebrafish receptors as V1a type (Fig. 1B). These findings are consistent with previous findings of a duplicated V1a receptor in teleosts (Lema, 2010).
Fig. 1.
Zebrafish AVTR1a1 and AVTR1a2. (A) Amino acid alignment for human V1a (NP_000697), zebrafish AVTR1a1, and zebrafish AVTR1a2. There is a high degree of amino acid conservation between human V1a and zebrafish AVTRs. The dark gray boxes indicate amino acid identity and light gray boxes similarity. The amino acid number corresponds to that for zebrafish AVTR1a1. The black bars mark the transmembrane domains (TMs) for zebrafish AVTR1a1. The white box indicates the DUF1856 domains conserved in the Ctermini of various AVP receptors. The locations of the intracellular loops (ICL) and extracellular loops (ECL) are also indicated. (B) The phylogeny of AVP/AVT receptors.
2.2. avtr1a1 and avtr1a2 are expressed primarily in the CNS in early stage zebrafish
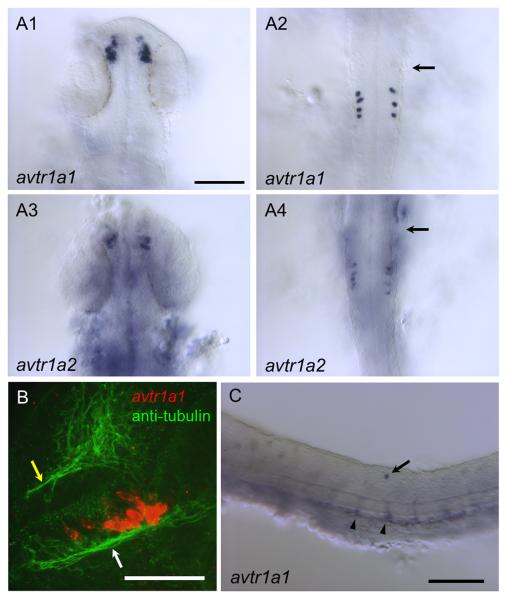
By 25 hours postfertilization (hpf) avtr1a1 and avtr1a2 are expressed by a cluster of cells in the forebrain and by a small number of discrete cells in the hindbrain (Fig. 2A). The earliest expression seen via in situ hybridization was avtr1a1 in the forebrain and hindbrain at 22 hpf (not shown). Examination of avtr1a1 expressing cells in embryos in which all axons are labeled with anti-acetylated -tubulin found that the forebrain cells are in apparent contact with the postoptic commissure (POC) and/or tract of the postoptic commissure (TPOC) (Fig. 2B; Chitnis and Kuwada, 1990), which is consistent with these cells projecting axons into the POC/TPOC. These avtr1a1+ neurons as the putative avtr1a1+ epiphyseal and nucleus of the posterior commissure neurons (see below) may also express HNK-1 since the pattern of early neurons/axons labeled with anti-HNK-1 and anti-acetylated -tubulin are similar (Wilson et al., 1990). Additionally there are irregularly spaced, occasional dorsal cells in the spinal cord of unknown identity that express avtr1a1 (Fig. 2C).
Fig. 2.
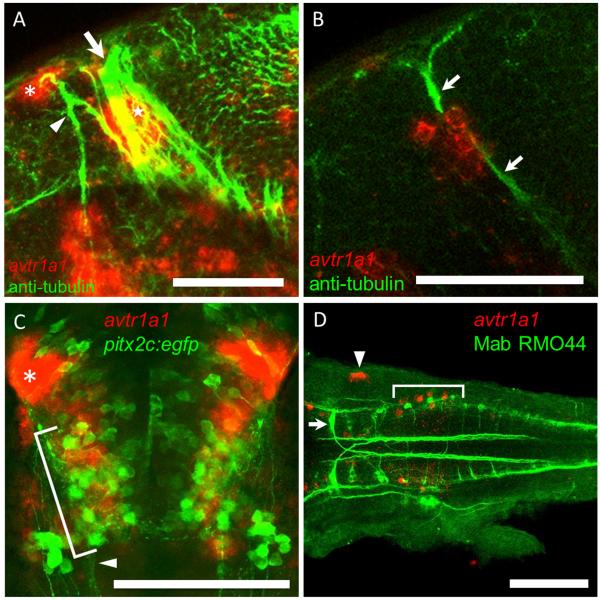
Vasotocin receptors (avtr1a1 and avtr1a2) are expressed in discrete populations of cells in the CNS of 24/28 hpf embryos. (A) Dorsal views (anterior up) of in situ hybridizations of 25 hpf embryos showing that both avtr1a1 and avtr1a2 are expressed in the forebrain (A1, A3) and the posterior hindbrain (A2, A4). Arrows denote position of the posterior border of the otocyst. Scale:100 m. (B) Combined in situ hybridization (red) and anti-acetylated tubulin labeling of axons (green) showing avtr1a1 expressing neurons in the forebrain appear to project axons into the postoptic commissure (white arrow) and tract of the postoptic commissure (28 hpf, lateral view with anterior right and dorsal up). Yellow arrow denotes the anterior commissure. Scale: 50 m. (C) Lateral view of trunk of 24 hpf embryo showing avtr1a1 expressing neuron in the dorsal spinal cord (arrow) and apparent endothelial cells forming blood vessels (arrowhead). Scale: 100 m.
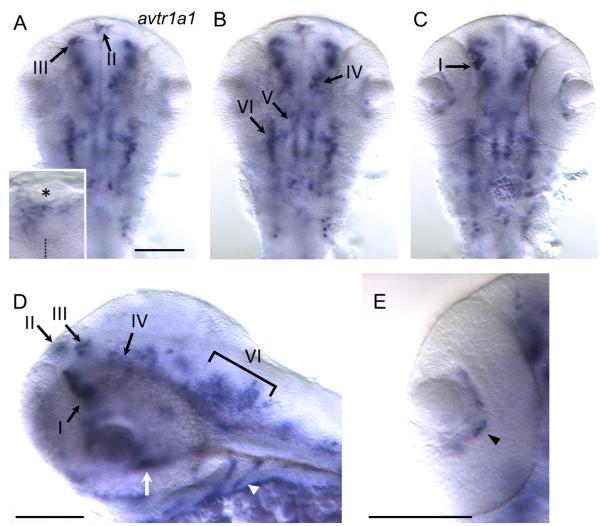
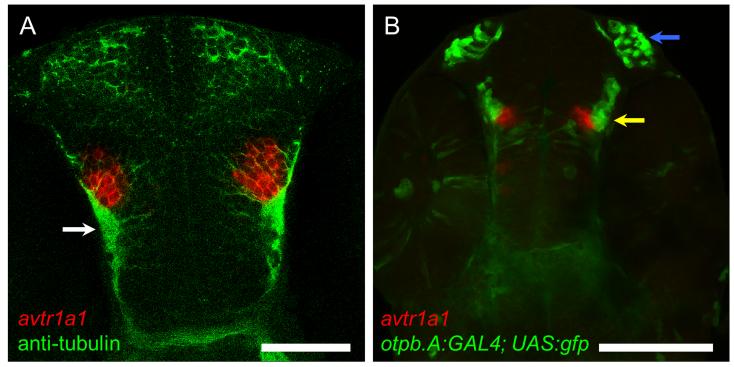
By 48 hpf avtr1a1 is expressed by a cluster of ventral forebrain cells (I), forebrain cells near the dorsal midline (II), dorsal cells located at the forebrain/tectum boundary (III) ventral cells near the forebrain/tegmentum border (IV), and two longitudinal stripes of cells in the midbrain/anterior hindbrain region (V and VI) when viewed from a dorsal perspective as well as the posterior hindbrain cells (Fig. 3A-C). Lateral views show that in the ventral forebrain cluster I is located anterior and ventral to cluster IV (Fig. 3D). Examination of cluster I avtr1a1+ cells in 48 hpf embryos with all axons labeled with antiacetylated tubulin showed that these neurons appear to project axons into the TPOC (Fig. 4A) suggesting that these are the POC/TPOC forebrain neurons observed earlier (Fig. 2B). Examination of forebrain avtr1a1+ cells in otpb.A:GAL4; UAS:gfp embryos that express GFP in preoptic neurons (Fujimoto et al., 2011) showed that cluster I avtr1a1+ cells are adjacent to the preoptic neurons at 48 hpf (Fig. 4B). Furthermore at 72 hpf avtr1a1 is expressed in the region immediately posterior to the preoptic region, either ventral or dorsal to the preoptic neurons (not shown). Thus preoptic neurons do not express avtr1a1 during the embryonic stages examined.
Fig. 3.
More neurons express avtr1a1 at 48 hpf. (A) View of the brain focused dorsally showing avtr1a1+ cells in the base of the epiphysis (II) and the forebrain/tectum boundary (III). Inset shows a higher magnification view of the cluster II avtr1a1+ cells with asterisk denoting the epiphysis and dotted line the midline. Scale: 100 m for (A-C). (B) Same view focused more ventrally than (A) showing the avtr1a1+ cells at the forebrain/tegmentum boundary (IV) and two rough stripes of cells that cross the midbrain/hindbrain boundary (V and VI). (C) Same view focused even more ventrally showing the anterior forebrain avtr1a1+ cells (I). (D) Lateral view showing the avtr1a1+ cells in the anterior forebrain (I), near the dorsal midline of the forebrain (II), at the forebrain/tectum boundary (III), at the forebrain/tegmentum boundary (IV) and the lateral stripe near the midbrain/hindbrain boundary (VI). Outside the brain the pharyngeal arches (arrowhead) and a midline structure in between the eyes (arrow) also express avtr1a1. Scale: 100 m. (E) View of eye showing avtr1a1+ cells (arrowhead) between the lens and the retina. Scale: 100 m.
Fig. 4.
At 48 hpf the avtr1a1+ cells in the anterior forebrain (I) are the POC/TPOC neurons and not preoptic neurons. (A) Dorsal perspective (anterior up) of an embryo labeled with avtr1a1 riboprobe (red) and anti-acetylated tubulin labeled axons (green) showing that the forebrain avtr1a1+ cells appear to extend axons in the TPOC (arrow). Scale: 50 m. (B) A ventral perspective of an otpb.A:GAL4; UAS:gfp embryo labeled with avtr1a1 riboprobe showing that the anterior forebrain avtr1a1+ neurons (red) are located just medial to the preoptic neurons (green, yellow arrow). Blue arrow denotes the olfactory neurons that express GFP in the transgenic embryos. Scale: 100 m.
The dorsal forebrain cells (II) are found at the lateral base of the epiphysis, which can be seen with DIC optics in 48 hpf embryos (Fig. 3A inset). These cells are likely neurons since they appear to extend axons in the dorsal-ventral diencephalic tract (DVDT) (Fig. 5A; Chitnis and Kuwada, 1990; Wilson et al., 1990). The cluster of neurons on the left side of the brain appears to be larger than that on the right side (Fig. 3A inset) in accord with the left-right asymmetry exhibited by the pineal complex in embryonic zebrafish (Concha et. al., 2000). The forebrain/tectum boundary cells (III) could be neurons of the nucleus of the posterior commissure (nucPC) since they appear to extend axons in the PC (Fig. 5B; Chitnis and Kuwada, 1990). The avtr1a1 expressing cells near the midbrain/hindbrain boundary (V) at approximately 48 hpf could be neurons of the nucleus of the Medial Longitudinal Fasciculus (nucMLF) since these neurons reside in this region. nucMLF neurons express pitx2c and are labeled by EGFP in pitx2c:egfp zebrafish (Wolman et al., 2008). However, in situ hybridization for avtr1a1 in these transgenic embryos showed that the avtr1a1 expressing cells were in the same region as the nucMLF neurons but did not express pitx2c:egfp suggesting they were not nucMLF neurons (Fig. 5C). It is noteworthy that the medial longitudinal stripe of cells (V) are also located in a position similar to that of the raphe serotonergic neurons (Lillesaar et. al., 2007).
Fig. 5.
The epiphyseal and nucPC neurons but not the nucMLF nor T reticular neurons express avtr1a1 at approximately 48 hpf. (A) Lateral view (anterior left, dorsal up) of a z-stack of confocal images of a embryo double labeled for avtr1a1 and anti-acetylated tubulin (axons) showing that the cluster II avtr1a1+ cells at the lateral base of the epiphysis (red, asterisk) project axons into the DVDT (green, arrowhead) and the avtr1a1+ cluster III cells (red, star) are adjacent to the PC (arrow). The avtr1a1+ cells seen ventrally are the neurons in the ventral forebrain and near the forebrain/tegmentum boundary (I and IV). Scale: 50 um. (B) A single focal plane seen in a lateral view showing that the cluster III avtr1a1+ cells (red) appear to extend axons in the PC (green, arrows). Scale: 50 m. (C) Ventral perspective of pitx2c:egfp embryos labeled with a avtr1a1 riboprobe showing that the nucMLF neurons (green, bracket) do not express avtr1a1 (red). Arrowhead denotes the MLF; asterisk denotes the forebrain avtr1a1+ neurons. Scale: 100 m. (D) Dorsal perspective (anterior left) of the hindbrain of embryo double labeled for avtr1a1 riboprobe (red, bracket) and MAb RMO44 (green) showing that the posterior hindbrain avtr1a1+ cells are not the T reticular neurons. The arrowhead indicates avtr1a1+ cells in the otocyst. Scale: 100 m.
Given that many neurons in the hindbrain are characterized by their stereotyped locations, morphology and molecular properties, we also examined the avtr1a1+ hindbrain cells in order to determine their identities. The caudal most avtr+ cells are located in the region of the T reticular neurons that project into the contralateral MLF and can be labeled with MAb RMO44 (Skromne et al., 2007), which recognizes a specific neurofilament expressed by these neurons. Double-labeling with RMO44 and in situ hybridization for avtr1a1 showed that the caudal avtr1a1 positive neurons are not T reticular neurons (Fig. 5D). Additionally, the double-labeled embryos demonstrated that the M cell which projects posteriorly into the contralateral MLF and are also labeled by RMO44 do not express avtr1a1.
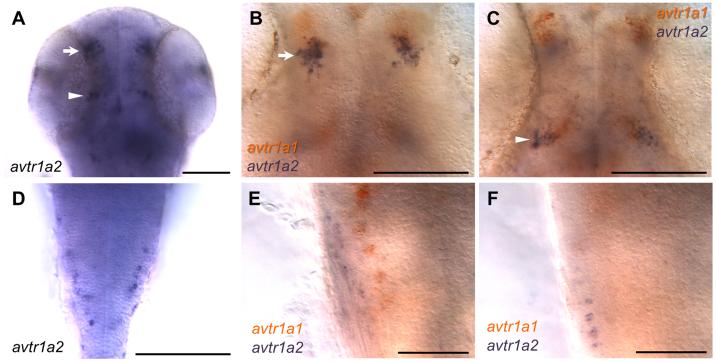
At 48 hpf avtr1a2 appears to be expressed by fewer cells in the midbrain/anterior hindbrain region compared with avtr1a1 while the forebrain expression of the receptors is similar to that for avtr1a1 (Fig. 6A,D). Since avtr1a1 and avtr1a2 are expressed in similar patterns in the brain, it is possible that they may be co-expressed in neurons. However, double in situ hybridization of 48 hpf embryos for both receptor genes found that the two receptors appeared generally not to be co-expressed by neurons (Fig. 6B,C,E,F).
Fig. 6.
avtr1a2 is expressed in a pattern similar to avtr1a1 but they are not coexpressed by neurons. Dorsal perspective showing that avtr1a2 is expressed in the brain (arrow and arrowhead) (A) and hindbrain (D) at 48 hpf. Double in situ hybridizations for avtr1a1 and avtr1a2 shows that brain neurons generally do not coexpress avtr1a1 and avtr1a2 (B and C) nor do posterior hindbrain neurons (E and F) at 48 hpf. B and C are the same field of view from the same embryo as are E and F. Scale for A-D: 100 m and for E-F: 50 m.
avtr1a1 is expressed by cells outside of the CNS with expression seen in the endothelial cells of the developing vasculature in the trunk at 24 hpf (Fig. 2C); the pharyngeal arches; a midline structure ventral to the brain between the eyes (Fig. 3D); and cells in the space between the lens and the retina by 48 hpf (Fig. 3E).
2.3 The morphology and location of hindbrain avtr1a+ neurons are consistent with a role in sensory/motor responses
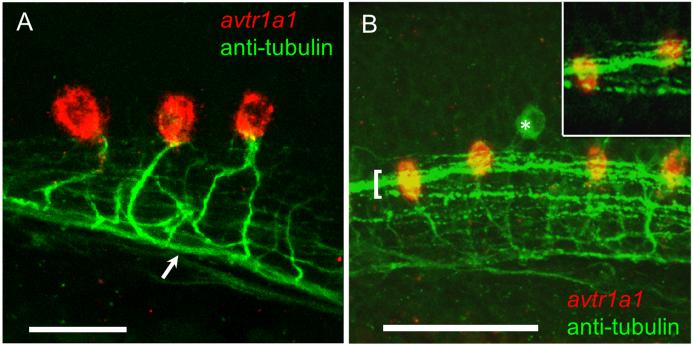
We determined the axonal trajectories of avtr1a1+ neurons by combined in situ hybridization and immunolabeling with anti-acetylated tubulin. We found that the hindbrain avtr1a1+ neurons project axons towards and into the ipsilateral MLF (Fig. 7A). The fact that these avtr1a1+ neurons project into the MLF indicates that these neurons may participate in regulating motor responses since many neurons with axons within the MLF are known to have a motor function in teleosts including zebrafish (Zottoli, 1977; Nissanov et al., 1990; Gahtan et al., 2002). A group of cells known as IC neurons are located in the caudal hindbrain and project axons in the ipsilateral MLF (Mendelson, 1986; Metcalfe et al., 1986). IC neurons are rhythmically active during spontaneous coiling of the body (Saint-Amant and Drapeau, 2001). At present there are no markers for these neurons so it is not possible to readily identify whether IC neurons are avtr+.
Fig. 7.
avtr1a1 expressing neurons in the caudal hindbrain project axons into the ipsilateral MLF and are in close proximity with the LLF at 28 hpf. (A) Lateral view (anterior left) showing caudal hindbrain, avtr1a1+ neurons (red) project axons (labeled with antiacetylated tubulin) into the ipsilateral MLF (arrow). Scale: 20 m. (B) Lateral view showing that the LLF (green, bracket) courses over the avtr1a1+ neurons (red). Asterisk denotes a RB sensory neuron. Inset: single confocal plane showing LLF axons coursing over avtr1a1+ hindbrain neurons. Scale: 50 m.
Many neurons that participate in motor responses in zebrafish receive sensory input (Gahtan et al., 2002; Kohashi and Oda, 2008). In the zebrafish, hindbrain sensory axons extend along the lateral longitudinal fasciculus (LLF). Axons in the LLF appear to course over the cell bodies of the avtr1a1-expressing neurons as seen in confocal stack images and in single focal planes (Fig. 7B), so potentially could make synapses with the avtr1a1+ neurons.
The finding that the axons of avtr1a1+ neurons project into the MLF and the proximity of their cell bodies to the RB sensory axons of the LLF are consistent with a role for avtr1a1+ neurons in motor responses of embryos to sensory stimulation. Further physiological and morphological analysis of avtr1a1+ neurons will be required to establish this hypothesis.
2.4 Preoptic neurons project axons in the vicinity of avtr1a1-positive neurons
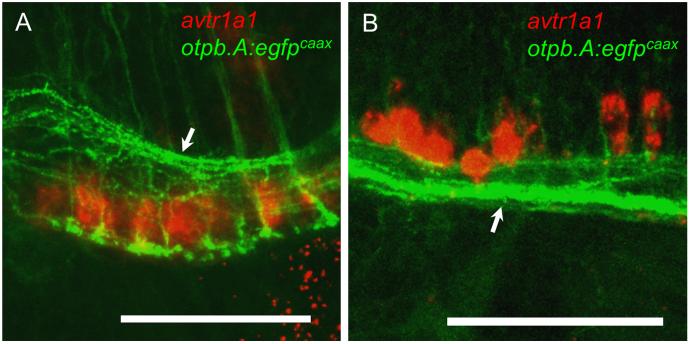
Since avt, avtr1a1, and avtr1a2 are expressed early in zebrafish, we wondered if the avt+ neurons innervate the receptor-expressing neurons in embryos. Unfortunately anti-AVT appears not to label AVT-expressing neurons until 5 dpf (Eaton et al., 2008), so we examined transgenic embryos in which avt+ neurons expressed GFP. Preoptic neurons express AVT and isotocin, an ortholog of mammalian oxytocin, and this requires expression of the homeobox transcription factor, orthopediab, in preoptic neurons (Blechman et al., 2007; Ryu et al., 2007; Eaton et al., 2008; Lohr et al., 2009). Preoptic neurons can be visualized in the otpb.A:egfp and otpb.A:GAL4; UAS:gfp transgenic zebrafish that express GFP in preoptic neurons (Fujimoto et al., 2011). To examine whether preoptic neurons could innervate avtr+ neurons, transgenic otpb.A:egfpcaax embryos that express membrane-targeted EGFP in preoptic neurons were examined in combination with in situ hybridization for avtr1a1. In otpb.A:egfpcaax embryos EGFP-labeled axons from preoptic neurons projected posteriorly. In confocal stack images the posteriorly-projecting axons extend in the medial longitudinal catecholaminergic tract (MLCT; Kastenhuber et al., 2010) in close proximity to avtr1a1 expressing neurons in the anterior and caudal hindbrain (Fig. 8). This could be seen in single focal planes as well (not shown). Thus preoptic neurons including those presumably expressing avt could be presynaptic to the avtr+ neurons. However, since preoptic neurons include those that express isotocin as well as vasotocin, physiological and anatomical examination of confirmed avt expressing neurons and avtr+ neurons will be required to establish synaptic interactions.
Fig. 8.
avtr1a1+ neurons are in close proximity to the axons of otpb expressing preoptic neurons at 49-51 hpf. (A) Lateral view (anterior left) in an otpb.A:egfpcaax embryo showing that the anterior hindbrain avtr1a1+ neurons (red) are in close proximity to the axons of preoptic neurons in the MLCT (arrow). Scale: 50 m. (B) Lateral view in an otpb.A:egfpcaax embryo showing that the avtr1a1+ neurons in the caudal hindbrain are in close proximity to the preoptic axons in the MLCT (arrow). Scale: 50 m.
3. Discussion
Although AVP/AVT signaling within the brain has been widely studied as an important factor for reproductive and social behaviors (reviewed in Stoop, 2012), relatively few investigations have examined the development of AVP/AVT signaling during CNS development. Indeed AVP and oxytocin binding sites are found in the mammalian neonatal spinal cord (Liu et al., 2003; Stoop, 2012). Furthermore, it is likely that these neonatal spinal neurons receive input from AVP expressing axons: AVP is expressed by paraventricular (PVN) and supraoptic hypothalamic neurons in rat embryos (Lipari et al., 2001), AVP expressing PVN cells innervate the spinal cord (Sawchenko and Swanson, 1982; Motawei et al., 1999; Hallbeck et al., 2001), and PVN axons project to the neonatal spinal cord (Leong et al., 1984; Kudo et al., 1993; Lakke, 1997). Our study demonstrates that AVT receptors are expressed in the CNS early in zebrafish development together with AVT (Eaton et al., 2008). This suggests that AVT signaling may regulate some aspect of CNS development (e.g. Boer et al., 1982a & b; Hammock and Levitt, 2012) and/or control early behaviors.
The expression of AVT and its receptors correlate with the earliest behaviors of zebrafish embryos. These are spontaneous coiling of the body and motor responses to sensory stimulation, touch-induced fast escape response and swimming (Saint-Amant and Drapeau, 1998). Thus it is possible that AVT signaling may play a role in regulating these early motor behaviors. Several findings from our study are consistent with this hypothesis. First, AVT receptor expressing neurons in the hindbrain project axons into the MLF as do many other neurons known to regulate motor behaviors in zebrafish. Second, the morphological relationship between sensory axons of the LLF and the AVTR expressing hindbrain neurons suggests that sensory neurons could synaptically activate avtr+ neurons. Third, otpb expressing preoptic neurons, which include AVT expressing neurons, project axons into the hindbrain with the axons in close proximity to avtr+ neurons. Since isotocin, the fish homolog to oxytocin, is also expressed during embryogenesis (Eaton et al., 2007), it is possible that both AVT and isotocin may regulate motor behaviors in zebrafish embryos.
Our hypothesis assumes that the interaction between the avt+ preoptic neurons and the hindbrain avtr+ neurons is a synaptic one based upon release of neuropeptide from axon terminals. AVP and oxytocin, as are other neuropeptides, are found in large dense core vesicles in neurons and can be released in a Ca2+ dependent fashion directly from the cell bodies and dendrites (Brownstein et al., 1980; Ludwig and Leng, 2006). Recently, however, physiologically relevant synaptic release of oxytocin from axon terminals was elegantly demonstrated in the amygdala (Knobloch et al., 2012). In the case of zebrafish embryos the cell bodies of avt+ preoptic neurons are in the diencephalon while the avtr+ neurons in question are located in the hindbrain. The distance between the avt+ preoptic cell bodies and avtr+ hindbrain neurons and the physical proximity of preoptic axons and the hindbrain neurons is consistent with a synaptic release from axon terminals of preoptic neurons. However it is also possible that AVT could act in a humoral fashion via release into the early vascular system and/or ventricles.
We hypothesize that binding of AVT by the hindbrain avtr+ neurons increases the excitability of these neurons and thus regulates the responsiveness of the receptor expressing neurons to sensory stimulation in zebrafish (Fig. 9) much like the sensorimotor processing role proposed for AVP/AVT (Rose and Moore, 2002). In fact AVP and oxytocin depolarize motor neurons, interneurons and preganglionic neurons in the rodent spinal cord (Suzue et al., 1981; Kolaj and Renaud, 1998; Oz et al., 2001; Liu et al., 2003). Application of AVP onto facial motor neurons and oxytocin onto vagal motor neurons leads to a TTX insensitive, voltage-dependent persistent INa (Raggenbass et al., 1991) while in neurons in the lumbar spinal cord AVP suppresses resting conductance to K+ and enhances conductance to cations (Ogier et al., 2006). Furthermore, AVT induces an inward current in oocytes expressing flounder avtr (Warne, 2001). Finally AVP can induce rhythmic activity in the spinal cord by itself or in combination with serotonin suggesting that AVP can activate central pattern generators (Pearson et al., 2003). Interestingly, the invertebrate homologs of AVP and oxytocin, conopressin, hirudotocin and annetocin, induced activation of a central pattern generator for reproductive behaviors in the Medicinal leech (Wagenaar et al., 2010) suggesting that regulation of motor behaviors might be an evolutionarily ancient function for these neuropeptides.
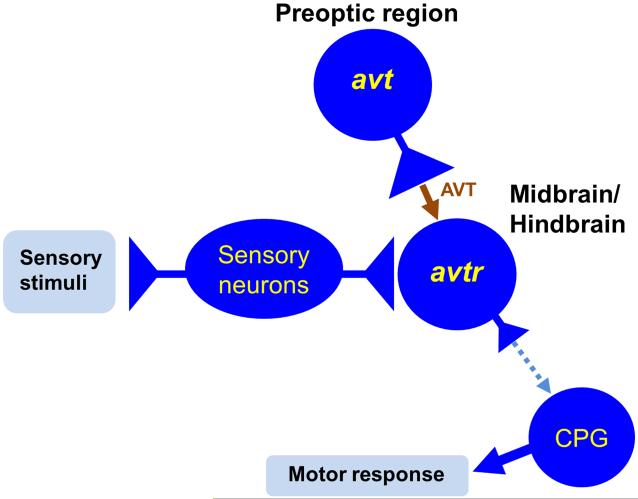
Fig. 9.
Model for the regulation of the responsiveness of avtr+ neurons in the hindbrain to sensory input in zebrafish embryos. In the model AVT is constitutively released at the synapse onto avtr+ neurons to enhance activation of these neurons by sensory stimulation and modulate motor responses mediated by the central pattern generator (CPG). Dashed arrow denotes hypothesized connection between the hindbrain avtr+ neurons and the CPG.
4. Materials and methods
Fish breeding and maintenance
Zebrafish (Danio rerio) were maintained in a breeding facility following the guidelines set forth by the University of Michigan Animal Care and Use protocols (Zhou et al., 2008). The fish were provided daily with either dry food (Micro pellets, Kyorin, Japan) or brine shrimp (Brine Shrimp Direct, Utah). Embryos were collected and incubated at 28.5 °C and staged according to hours post fertilization (hpf; Westerfield, 1995). For in situ hybridization of the embryos older than one day post-fertilization, the embryos were kept in 0.2 mM 1-Phenyl-2-thiourea (Sigma, P-7629) to keep them transparent until they were fixed at desired developmental stages. Transgenic lines used in this paper were otpb.A:GAL4 (Tg(otpb:GAL4-VP16, myl7:GFP)zc57) and Tg(UAS:GFP) (Fujimoto et al., 2011); otpb.A:egfpcaax (Tg(otpb:1EGFP)zc49) (Xing et al., 2012); and pitx2c:egfp (Tg(pitx2:EGFP)zy8) (Wolman et al., 2008).
cDNA cloning of zebrafish AVTRs
cDNA cloning for AVTRs was conducted following standard protocols (Zhou et al., 2008). Whole brains were dissected from male adult zebrafish (approximately 14 months post fertilization, N=4) and total RNA was extracted following the manufacture’s protocol for TRIzol® Reagent (Invitrogen, #15596-018). The RNA was reverse transcribed with oligo-dT primers (Invitrogen, #58862) following the manufacture’s protocol for SuperScript® II Reverse Transcriptase (Invitrogen, #18064-022). For the PCR of avtr1a1 cDNA, the following primers were used to amplify the coding sequence along with 5′-UTR and 3′-UTRs; forward primer, 5′-GCTCGCGCCTTTACGCATGA-3′, and reverse primer, 5′-ACAGGAGGGTAAATGCTTTTGACT-3′. To PCR avtr1a2, the following primers were used; forward primer, 5′-CGCGCGTATTTCCATCAATCAAGC-3′, and reverse primer, 5′-AATCGCTGTGCTTTCAGCTGGT-3′. The PCR products were purified and cloned into pGEM T-easy vector following the manufacture′s instructions (Promega, #A137A). The obtained clones were sequenced at the University of Michigan Sequencing Core.
Sequence analysis
Amino acid alignments for human V1a (NP_000697), zebrafish AVTR1a1 and AVTR1a2 (Fig. 1) were constructed with ClustalW (Biology Workbench, SCSD; http://seqtool.sdsc.edu). Default settings of the textshade option (Biology Workbench) were adopted except that amino acid identity (dark gray) and similarity (light gray) were color-coded. The amino acids were numbered corresponding to that for zebrafish AVTR1a1. The transmembrane domains for zebrafish AVTR1a1 (black bars) were determined using MEMSAT3 (http://bioinf.cs.ucl.ac.uk/psipred). The white box indicates the DUF1856 domains (unknown function) conserved in the C-termini of various AVP receptors (conserved domain search; http://www.ncbi.nlm.nih.gov/cdd) . The intracellular loops (ICL) and extracellular loops (ECL) are also indicated. Phylogenetic tree was constructed based on the alignment of amino acid sequences for vasopressin/vasotocin receptors across vertebrate species with the MEGA 4.0 software (neighbor-joining method with pairwise deletion and bootstrap values from 1000 replicates) as described by Lema (2010). GenBank accession numbers are provided in the parentheses.
Whole-mount in situ hybridization & immunolabeling
To synthesize RNA probes for in situ hybridization for avtr genes, partial coding sequences (685 bp for avtr1a1 & 658 bp for avtr1a2) were cloned into pGEM T-Easy vectors using the following primers; forward primer (avtr1a1), 5′-CTTGGGAATGTTCGCGTCCACTTA-3′, reverse primer (avtr1a1), 5′-TTAGGCTGTTTCCCATGCTGGAAC-3′, forward primer (avtr1a2), 5′-GGACTTTCTGTGCAGGATCGTCAA-3′, and reverse primer (avtr1a2), 5′-TCCGCTGAACACCATGTAGATCCA-3′. The partial clones were sequenced at the University of Michigan Sequencing Core and confirmed to be identical to the complete AVTR cDNA clones described above (cDNA cloning of zebrafish AVTRs). The partial cDNA clones were used to synthesize, in vitro, digoxigenin (DIG)-labeled antisense probes with DIG RNA labeling mix (Roche) following the manufacture’s instructions for mMESSAGE mMACHINE Kit (Ambion, Life Technologies). The sense probe for avtr1a1 was used as a negative control and found to generate no significant labeling (not shown). The standard protocol was followed to conduct in situ hybridization (Zhou et al., 2008). Anti-DIG-AP Fab fragments (Roche) was used to carry out color reactions using NBT/BCIP as a substrate (Roche). For fluorescent double-labeling, Fast Red (Roche) was used as a substrate for in situ hybridization and immunolabeling was carried out with primary antibodies [antiacetylated tubulin (1:1000, Sigma-Aldrich), anti-GFP (1:1000, Torrey Pines Biolabs), anti-Neurofilament-M/RMO44 (1:500, Invitrogen, Life Technologies)] and Alexa488-anti-mouse/rabbit IgG (1:500, Invitrogen, Life Technologies). The labeled embryos were mounted in Vectashield mounting medium (Vector Laboratories) and fluorescent images were acquired with a Leica SP5 laser scanning confocal microscope.
For double in situ hybridization, dinitrophenol (DNP)-labeled anti-sense riboprobes for avtr1a1 were synthesized with DNP-11-UTP (PerkinElmer), and DIG-labeled riboprobes for avtr1a2 were synthesized using DIG RNA labeling mix (Roche). Prior to in situ hybridization, the embryos were treated with 2% hydrogen peroxide, and dextran sulfate was added to the hybridization reaction as described by Lauter et al. (2011). The alkaline phosphatase-mediated color reaction was first carried out for avtr1a2 using anti-DIG-AP Fab fragments (Roche) with NBT/BCIP (Roche) as a substrate. After anti-DIG-AP was inactivated by 0.1 M glycine-HCL (pH 2.2), the color reaction for avtr1a1 was conducted using anti-DNP-AP (Vector Laboratories) with INT/BCIP (Roche) as a substrate. The labeled embryos were mounted in 70 % glycerol to obtain bright-field microscope images.
Two vasotocin receptors are expressed in the CNS of zebrafish embryos as is vasotocin.
Vasotocin receptor neurons may be innervated by sensory and vasotocin neurons.
Vasotocin receptor expressing neurons may participate in locomotor responses.
Vasotocin signaling in the embryonic CNS may gate sensory input to motor circuits.
Acknowlegements
We thank M. Halloran and J. Yost for the pitx2c:egfp transgenic fish and A. Migda for the care of zebrafish. Research was supported by grants from NINDS (RO1NS54731) and NSF (0725976) to JYK.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errorsmaybe discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Acher R, Chauvet J, Chauvet MT, Michel G, Rouille Y. Molecular evolution of neurohypophysial hormones in relation to osmoregulation: the two fish options. Fish Physiol. Biochem. 1997;17:325–332. [Google Scholar]
- An KW, Kim NN, Choi CY. Cloning and expression of aquaporin 1 and arginine vasotocin receptor mRNAfrom the black porgy, Acanthopagrus schlegeli: effect of freshwater acclimation. Fish Physiol. Biochem. 2008;34:185–194. doi: 10.1007/s10695-007-9175-0. [DOI] [PubMed] [Google Scholar]
- Blechman J, Borodovsky N, Eisenberg M, Nabel-Rosen H, Grimm J, Levkowitz G. Specification of hypothalamic neurons by dual regulation of the homeodomain protein Orthopedia. Development. 2007;134:4417–4426. doi: 10.1242/dev.011262. [DOI] [PubMed] [Google Scholar]
- Blechman J, Amir-Zilberstein, Gutnik A, Ben-Dor S, Levkowitz G. The metabolic regulator PGC-1 directly controls the expression of the hypothalamic neuropeptide oxytocin. J. Neurosci. 2011;31:14835–14840. doi: 10.1523/JNEUROSCI.1798-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boer GJ, Uylings HB, Patel AJ, Boer K, Kragten R. The regional impairment of brain development in Brattleboro diabetes insipidus rat: some vasopressin supplemental studies. Ann. N.Y. Acad. Sci. 1982a;394:703–717. doi: 10.1111/j.1749-6632.1982.tb37488.x. [DOI] [PubMed] [Google Scholar]
- Boer GJ, van Rheenen-Verberg CMH, Uylings HBM. Impaired brain development of the diabetes insipidus Brattleboro rat. Dev. Brain Res. 1982b;3:557–575. doi: 10.1016/0165-3806(82)90054-2. [DOI] [PubMed] [Google Scholar]
- Brownstein MJ, Russell JT, Gainer H. Synthesis, transport, and release of posterior pituitary hormones. Science. 1980;207:373–378. doi: 10.1126/science.6153132. [DOI] [PubMed] [Google Scholar]
- Caldwell HK, Lee H-J, Macbeth AH, Young WS., 3rd Vasopressin: behavioral roles of an “original” neuropeptide. Prog. Neurobiol. 2008;84:1–24. doi: 10.1016/j.pneurobio.2007.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chitnis AB, Kuwada JY. Axonogenesis in the brain of zebrafish embryos. J. Neurosci. 1990;10:1892–1905. doi: 10.1523/JNEUROSCI.10-06-01892.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Concha ML, Burdine RD, Russell C, Schier AF, Wilson SW. A nodal signaling pathway regulates the laterality of neuroanatomical asymmetries in the zebrafish forebrain. Neuron. 2000;28:399–409. doi: 10.1016/s0896-6273(00)00120-3. [DOI] [PubMed] [Google Scholar]
- Conklin DJ, Smith MP, Olson KR. Pharmacological characterization of arginine vasotocin vascular smooth muscle receptors in the trout (Oncorhynchus mykiss) in vitro. Gen. Comp. Endocrinol. 1999;114:36–46. doi: 10.1006/gcen.1998.7233. [DOI] [PubMed] [Google Scholar]
- DeVries GJ, Buijs RM. The origin of the vasopressinergic and oxytocinergic innervation of the rat brain with special reference to the lateral septum. Brain Res. 1983;273:307–317. doi: 10.1016/0006-8993(83)90855-7. [DOI] [PubMed] [Google Scholar]
- DeVries GJ, Buijs RM, Van Leeuwen FW, Caffe AR, Swab DF. The vasopressinergic innervation of the brain in normal and castrated rats. J. Comp. Neurol. 1985;233:236–254. doi: 10.1002/cne.902330206. [DOI] [PubMed] [Google Scholar]
- Donaldson ZR, Young LJ. Oxytocin, vasopressin, and the neurogenetics of sociality. Science. 2008;322:900–904. doi: 10.1126/science.1158668. [DOI] [PubMed] [Google Scholar]
- Eaton JL, Glasgow E. Zebrafish orthopedia (otp) is required for isotocin cell development. Dev. Genes. Evol. 2007;217:149–158. doi: 10.1007/s00427-006-0123-2. [DOI] [PubMed] [Google Scholar]
- Eaton JL, Holmqvist B, Glasgow E. Ontogeny of vasotocin-expressing cells in zebrafish: Selective requirement fo the transcriptional regulators orthopedia and singleminded 1 in the preoptic area. Dev. Dyn. 2008;237:995–1005. doi: 10.1002/dvdy.21503. [DOI] [PubMed] [Google Scholar]
- Fujimoto E, Stevenson TJ, Chien C, Bonkowsky JL. Identification of a dopaminergic enhancer indicates complexity in vertebrate dopamine neuron phenotype specification. Dev. Biol. 2011;352:393–404. doi: 10.1016/j.ydbio.2011.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gahtan E, Sankrithi N, Campos JB, O’Malley DM. Evidence for a widespread brain stem escape network in larval zebrafish. J Neurophysiol. 2002;87:608–14. doi: 10.1152/jn.00596.2001. [DOI] [PubMed] [Google Scholar]
- Hallbeck M, Larhammar D, Blomqvist A. Neuropeptide expression in rat paraventricular hypothalamic neurons that project to the spinal cord. J. Comp. Neurol. 2001;433:222–238. doi: 10.1002/cne.1137. [DOI] [PubMed] [Google Scholar]
- Hammock EA, Levitt P. Modulation of parvalbumin interneuron number by developmentally transient neocortical vasopressin receptor 1a (V1aR) Neurosci. 2012;222:20–28. doi: 10.1016/j.neuroscience.2012.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinrichs M, von Dawans B, Domes G. Oxytocin, vasopressin, and human social behavior. Frontiers Neuroendocrinol. 2009;30:548–557. doi: 10.1016/j.yfrne.2009.05.005. [DOI] [PubMed] [Google Scholar]
- Kastenhuber E, Kratochwil CF, Ryu S, Schweitzer J, Driever W. Genetic dissection of dopaminergic and noradrenergic contributions to catecholaminergic tracts in early larval zebrafish. J. Comp. Neurol. 2010;518:439–458. doi: 10.1002/cne.22214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knobloch HS, Charlet A, Hoffmann LC, Eliava M, Khrulev S, Cetin AH, Osten P, Schwarz MK, Seeburg PH, Stoop R, Grinevich V. Evoked axonal oxytocin release in the central amygdala attenuates fear response. Neuron. 2012;73:553–566. doi: 10.1016/j.neuron.2011.11.030. [DOI] [PubMed] [Google Scholar]
- Kohashi T, Oda Y. Initiation of Mauthner- or non-Mauthner-mediated fast escape evoked by different modes of sensory input. J. Neurosci. 2008;28:10641–10653. doi: 10.1523/JNEUROSCI.1435-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolaj M, Renaud LP. Vasopressin-induced currents in rat neonatal spinal lateral horn neurons are G-protein mediated and involve two conductances. J. Neurophysiol. 1998;80:1900–1910. doi: 10.1152/jn.1998.80.4.1900. [DOI] [PubMed] [Google Scholar]
- Kudo N, Furukawa F, Okado N. Development of descending fibers to the rat embryonic spinal cord. Neurosci. Res. 1993;16:131–141. doi: 10.1016/0168-0102(93)90080-a. [DOI] [PubMed] [Google Scholar]
- Lakke EA. The projections to the spinal cord of the rat during development: a timetable of descent. Adv. Anat. Embryol. Cell Biol. 1997;135:1–143. doi: 10.1007/978-3-642-60601-4. [DOI] [PubMed] [Google Scholar]
- Lauter G, Soll I, Hauptmann G. Two-color fluorescent in situ hybridization in the embryonic zebrafish brain using differential detection systems. BMC Developmental Biology. 2011;11:43. doi: 10.1186/1471-213X-11-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lema SC. Identification of multiple vasotocin receptor cDNAs in teleost fish: Sequences, phylogenetic analysis, sites of expression, and regulation in the hypothalamus and gill in response to hyperosmotic challenge. Mol. Cell. Endocrinol. 2010;321:215–230. doi: 10.1016/j.mce.2010.02.015. [DOI] [PubMed] [Google Scholar]
- Leng G, Bicknell RJ. The neurohypophysis. In: Lightman SL, Everett BJ, editors. Neuroendocrinology. Blackwell Scientific Publications; Boston: 1986. pp. 177–206. [Google Scholar]
- Leong SK, Shieh JY, Wong WC. Localizing spinal-cord-projecting neurons in adult albino rats. J. Comp. Neurol. 1984;228:1–17. doi: 10.1002/cne.902280103. [DOI] [PubMed] [Google Scholar]
- Lillesaar C, Tannhauser B, Stigloher C, Kremmer E, Bally-Cuif L. The serotonergic phenotype is acquired by converging genetic mechanisms within the zebrafish central nervous system. Dev. Dyn. 2007;236:1072–1084. doi: 10.1002/dvdy.21095. [DOI] [PubMed] [Google Scholar]
- Lipari EF, Lipari D, Gerbino A, Di Liberto D, Bellafiore M, Catalano M, Valentino B. The hypothalamic magnocellular neurosecretory system in developing rats. Eur. J. Histochem. 2001;45:163–168. doi: 10.4081/1626. [DOI] [PubMed] [Google Scholar]
- Liu X, Tribollet E, Ogier R, Barberis C, Raggenbass M. Presence of functional vasopressin receptors in spinal ventral horn neurons of young rats: a morphological and electrophysiological study. Eur. J. Neurosci. 2003;17:1833–1846. doi: 10.1046/j.1460-9568.2003.02625.x. [DOI] [PubMed] [Google Scholar]
- Lohr H, Ryu S, Driever W. Zebrafish diencephalic A11-related dopaminergic neurons share a conserved transcriptional network with neuroendocrine cell lineages. Development. 2009;136:1007–1017. doi: 10.1242/dev.033878. [DOI] [PubMed] [Google Scholar]
- Ludwig M, Leng G. Dendritic peptide release and peptide dependent behaviours. Nat. Rev. Neurosci. 2006;7:126–136. doi: 10.1038/nrn1845. [DOI] [PubMed] [Google Scholar]
- Mahlmann S, Meyerhof W, Hausmann H, Heierhorst J, Schönrock C, Zwiers H, Lederis K, Richter D. Structure, function, and phylogeny of [Arg8]vasotocin receptors from telesot fish and toad. Proc. Natl. Acad. Sci. U.S.A. 1994;91:1342–1345. doi: 10.1073/pnas.91.4.1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendelson B. Development of reticulospinal neurons of the zebrafish. II. Early axonal outgrowth and cell body position. J. Comp. Neurol. 1986;251:172–184. doi: 10.1002/cne.902510204. [DOI] [PubMed] [Google Scholar]
- Metcalfe WK, Mendelson B, Kimmel CB. Segmental homologies among reticulospinal neurons in the hindbrain of the zebrafish larvae. J. Comp. Neurol. 1986;251:147–159. doi: 10.1002/cne.902510202. [DOI] [PubMed] [Google Scholar]
- Motawei K, Pyner S, Ranson RN, Kamel M, Coote JH. Terminals of paraventricular spinal neurones are closely associated with adrenal medullary sympathetic preganglionic neurones: immunocytochemical evidence for vasopressin as a possible neurotransmitter in this pathway. Exp. Brain Res. 1999;126:68–76. doi: 10.1007/s002210050717. [DOI] [PubMed] [Google Scholar]
- Nishimura H, Fan Z. Regulation of water movement across vertebrate renal tubes. Comp. Biochem. Physiol. A: Mol. Integr. Physiol. 2003;136:479–498. doi: 10.1016/s1095-6433(03)00162-4. [DOI] [PubMed] [Google Scholar]
- Nissanov J, Eaton RC, DiDomenico R. The motor output of the Mauthner cell, a reticulospinal command neuron. Brain Res. 1990;517:88–98. doi: 10.1016/0006-8993(90)91012-6. [DOI] [PubMed] [Google Scholar]
- Ogier R, Tribollet E, Suarez P, Raggenbass M. Identified motoneurons involved in sexual and eliminative functions in the rat are powerfully excited by vasopressin and tachykinins. J. Neurosci. 2006;26:10717–10726. doi: 10.1523/JNEUROSCI.3364-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oz M, Kolaj M, Renaud LP. Electrophysiological evidence forvasopressin V(1) receptors on neonatal motoneurons, premotor and other ventral horn neurons. J. Neurophysiol. 2001;86:1202–1210. doi: 10.1152/jn.2001.86.3.1202. [DOI] [PubMed] [Google Scholar]
- Pearson SA, Mouihate A, Pittman QJ, Whelan PJ. Peptidergic activation of locomotor pattern generators in the neonatal spinal cord. 2003;23:10154–10163. doi: 10.1523/JNEUROSCI.23-31-10154.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raggenbass M, Goumaz M, Sermasi E, Tribollet E, Dreifuss JJ. Vasopressin generates a persistent voltage-dependent sodium current in a mammalian motoneuron. J. Neurosci. 1991;11:1609–1616. doi: 10.1523/JNEUROSCI.11-06-01609.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose JD, Moore FL. Behavioral neuroendocrinology of vasotocin and vasopressin and the sensorimotor processing hypothesis. Front. Neuroendocrinol. 2002;23:317–341. doi: 10.1016/s0091-3022(02)00004-3. [DOI] [PubMed] [Google Scholar]
- Ryu S, Mahler J, Acampora D, Holzschuh J, Erhardt S, Omodei D, Simeone A, Driever W. Orthopedia homeodomain protein is essential for diencephalic dopaminergic neuron development. Curr. Biol. 2007;17:873–880. doi: 10.1016/j.cub.2007.04.003. [DOI] [PubMed] [Google Scholar]
- Saint-Amant L, Drapeau P. Time course of the development of motor behaviors in the zebrafish embryo. J. Neurobiol. 1998;37:622–632. doi: 10.1002/(sici)1097-4695(199812)37:4<622::aid-neu10>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Saint-Amant L, Drapeau P. Synchronization of an embryonic network of identified spinal interneurons solely by electrical coupling. Neuron. 2001;31:1035–1046. doi: 10.1016/s0896-6273(01)00416-0. [DOI] [PubMed] [Google Scholar]
- Sawchenko PE, Swanson LW. The organization of noradrenergic pathways from the brainstem to the paraventricular and supraoptic nuclei in the rat. Brain Res. 1982;257:275–325. doi: 10.1016/0165-0173(82)90010-8. [DOI] [PubMed] [Google Scholar]
- Skromne I, Thorsen D, Hale M, Prince VE, Ho RK. Repression of the hindbrain developmental program by Cdx factors is required for the specification of the vertebrate spinal cord. Develop. 2007;134:2147–2158. doi: 10.1242/dev.002980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoop R. Neuromodulation by oxytocin and vasopressin. Neuron. 2012;76:142–159. doi: 10.1016/j.neuron.2012.09.025. [DOI] [PubMed] [Google Scholar]
- Suzue Suzue T, Yanaihara N, Otsuka M. Actions of vasopressin, gastrin releasing peptide and other peptides on neurons on newborn rat spinal cord in vitro. Neurosci. Lett. 1981;26:137–142. doi: 10.1016/0304-3940(81)90339-6. [DOI] [PubMed] [Google Scholar]
- Tessmar-Raible K, Raible F, Christodoulou F, Guy K, Rembold M, Hausen H, Arendt D. Conserved sensory-neurosecretory cell types in annelid and fish forebrain: Insights into hypothalamus evolution. Cell. 2007;129:1389–1400. doi: 10.1016/j.cell.2007.04.041. [DOI] [PubMed] [Google Scholar]
- Venkatesh B, Brenner S. Structure and organization of the isotocin and vasotocin genes from teleosts. Adv Exp Med Biol. 1995;395:629–638. [PubMed] [Google Scholar]
- Wagenaar DA, Hamilton MS, Huang T, Kristan WB, French KA. A hormoneactivated central pattern generator for courtship. Curr. Biol. 2010;20:487–495. doi: 10.1016/j.cub.2010.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warne JM. Cloning and characterization of an arginine vasotocin receptor from the euryhaline flounder Platichthys flesus. Gen.Comp. Endocrinol. 2001;122:312–319. doi: 10.1006/gcen.2001.7644. [DOI] [PubMed] [Google Scholar]
- Westerfield M. The zebrafish book. University of Oregon; Eugene, OR: 1995. [Google Scholar]
- Wolman MA, Sittaramane VK, Essner JJ, Yost HJ, Chandrasekhar A, Halloran MC. Transient axonal glycoprotein-1 (TAG-1) and laminin-1 regulate dynamic growth cone behaviors and initial axon direction in vivo. Neur. Dev. 2008;3:1–14. doi: 10.1186/1749-8104-3-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson SW, Ross LS, Parrett T, Easter SS. The development of a simple scaffold of axon tracts in the brain of the embryonic zebrafish, Brachydanio rerio. Development. 1990;108:121–145. doi: 10.1242/dev.108.1.121. [DOI] [PubMed] [Google Scholar]
- Xing L, Hoshijima K, Grunwald DJ, Fujimoto E, Quist TS, Sneddon J, Chien CB, Stevenson TJ, Bonkowsky JL. Zebrafish foxP2 zinc finger nuclease mutant has normal axon pathfinding. PLoS One. 2012;7:e43968. doi: 10.1371/journal.pone.0043968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young WS, 3rd, Gainer H. Transgenesis and the study of expression, cellular targeting and function of oxytocin, vasopressin and their receptors. Neuroendocrinology. 2003;78:185–203. doi: 10.1159/000073702. [DOI] [PubMed] [Google Scholar]
- Zhou W, Horstick EJ, Hirata H, Kuwada JY. Identification and expression of voltage-gated calcium channel β subunits in Zebrafish. Dev. Dyn. 2008;237:3842–3852. doi: 10.1002/dvdy.21776. [DOI] [PubMed] [Google Scholar]
- Zottoli SJ. Correlation of the startle reflex and Mauthner cell auditory responses in unrestrained goldfish. J Exp Biol. 1977;66:243–254. doi: 10.1242/jeb.66.1.243. [DOI] [PubMed] [Google Scholar]