Abstract
An early repolarization (ER) pattern in the ECG, consisting of J point elevation, distinct J wave with or without ST segment elevation or slurring of the terminal part of the QRS, was long considered a benign electrocardiographic manifestation. Experimental studies a dozen years ago suggested that an ER is not always benign, but may be associated with malignant arrhythmias. Validation of this hypothesis derives from recent case-control and population-based studies showing that an ER pattern in inferior or infero-lateral leads is associated with increased risk for life-threatening arrhythmias, termed early repolarization syndrome (ERS). Because accentuated J waves characterize both Brugada syndrome (BrS) and ERS, these syndromes have been grouped under the heading of J wave syndromes. BrS and ERS appear to share common ECG characteristics, clinical outcomes, risk factors as well as a common arrhythmic platform related to amplification of Ito-mediated J waves. However, they differ with respect to the magnitude and lead location of abnormal J waves and can be considered to represent a continuous spectrum of phenotypic expression. Recent studies support the hypothesis that BrS and ERS are caused by a preferential accentuation of the AP notch in right or left ventricular epicardium, respectively, and that this repolarization defect is accentuated by cholinergic agonists. Quinidine, cilostazol and isoproterenol exert ameliorative effects by reversing these repolarization abnormalities. Identifying subjects truly at risk is the challenge ahead. Our goal here is to review the clinical and genetic aspects as well as the cellular and molecular mechanisms underlying the J wave syndromes.
Keywords: Cardiac arrhythmias, Sudden cardiac death, Early repolarization syndrome, Brugada syndrome, Idiopathic Ventricular fibrillation
J Wave Syndromes: Clinical characteristics
The electrocardiographic J wave was first described in 19381 in an ECG recorded from an accidentally frozen human. It was referred to as the Osborn wave for many years after being reported by Osborn in hypothermic dogs in 1953.2 The appearance of prominent J wave in humans is encountered in cases of hypothermia,3-5 hypercalcemia6, 7 and more recently has been suggested as a marker for a substrate capable of generating life-threatening ventricular arrhythmias.8 In humans, the J wave more commonly appears as a J point elevation, with part of the J wave buried inside the QRS.
An early repolarization (ER) pattern on the ECG was first described in 1936 by Shipley and Hallaran , who studied four-lead ECGs of 200 healthy young men and women and described J deflection as slurring or notching of the terminal part of QRS complex and considered it as a normal variant.9 In subsequent years, ST segment elevation was added to these electrocardiographic manifestations and the complex was designated “early repolarization” based on the presumption that early repolarization was responsible,10 although no data were available to support this assertion. Experimental data in support of the hypothesis was first advanced with the identification of the cellular basis for the J wave in 1996.11
An ER pattern in the ECG, consisting of a distinct J wave or J point elevation, a notch or slur of the terminal part of the QRS and an ST segment elevation, is generally found in healthy young males and has traditionally been viewed as benign.12, 13 In 2000, we challenged this view on the basis of experimental data showing that an ER pattern in the canine coronary-perfused wedge preparation predisposes to the development of polymorphic ventricular tachycardia and fibrillation (VT/VF).8, 14, 15
Validation of this hypothesis was provided eight years later in the New England Journal of Medicine by Haïssaguerre et al.,16 and a letter to the editor by Nam et al.17 These reports together with numerous additional case control and population based studies provided clinical evidence that there is an increased prevalence of ER pattern, particularly in the inferior and infero-lateral leads, among patients with a history of idiopathic ventricular fibrillation, thus confirming a link between ER pattern in the ECG and life-threatening cardiac arrhythmias. (see also18, 19 for review).
The poorer prognosis of subjects with inferior or infero-lateral ER was confirmed in numerous population based studies. (see also18, 19 for references). We recently suggested a classification scheme for ER (Table 1).8 An ER pattern manifest exclusively in the lateral precordial leads was designated as Type 1; this form is prevalent among healthy male athletes and is thought to be associated with a relatively low level of risk for arrhythmic events. ER pattern in the inferior or infero-lateral leads was designated as Type 2; this form is thought to be associated with a moderate level of risk. Finally, an ER pattern appearing globally in the inferior, lateral and right precordial leads was labeled Type 3; this form is associated with the highest level of risk and in some cases has been associated with electrical storms.8 Type 3 ER may at times be very similar to that of Type 2, exhibiting infero-lateral ER, except for brief periods immediately before the development of VT/VF when pronounced J waves are also observed in the right precordial leads (see20 for an example). BrS represents a fourth variant in which ER is limited to the right precordial leads.
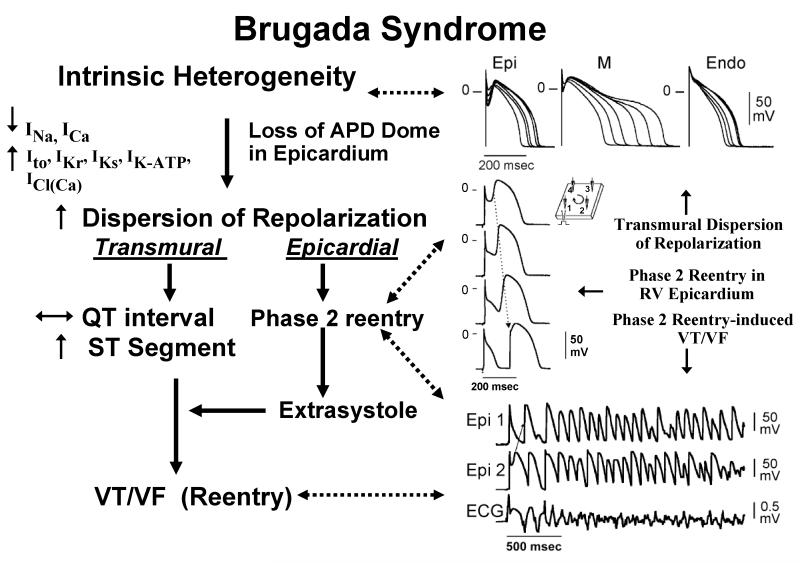
Figure 1.
Proposed mechanism for the Brugada syndrome. A shift in the balance of currents serves to amplify existing heterogeneities by causing loss of the action potential dome at some epicardial, but not endocardial sites. A vulnerable window develops as a result of the dispersion of repolarization and refractoriness within epicardium as well as across the wall. Epicardial dispersion leads to the development of phase 2 reentry, which provides the extrasystole that captures the vulnerable window and initiates VT/VF via a circus movement reentry mechanism. Modified from 76, with permission.
Genetics Basis for the J wave Syndromes
BrS has been associated with mutations in twelve different genes, accounting for approximately 40% of probands. Greater than 300 mutations in SCN5A (Nav1.5, BrS1) have been reported by centers worldwide accounting for 11-28% of BrS probands. Mutations in CACNA1C (Cav1.2, BrS3), CACNB2b (Cavβ2b, BrS4) and CACNA2D1 (Cavα2Δ , BrS9) are reportedly found in ~13% of probands. Mutations in glycerol-3-phophate dehydrogenase 1-like enzyme gene (GPD1L, BrS2), SCN1B (β1-subunit of Na channel, BrS5), KCNE3 (MiRP2; BrS6), SCN3B (β3-subunit of Na channel, BrS7), KCNJ8 (BrS8) and KCND3 (BrS10) are more rare. Mutations in these genes lead to loss of function in INa and ICa, as well as to a gain of function in Ito or IK-ATP. MOG1 was recently described as a new partner of NaV1.5, playing a role in its regulation, expression and trafficking. A missense mutation in MOG1 was also associated with BrS (BrS11). Mutations in sarcolemmal membrane-associated protein (SLMAP), a protein of unknown function localizing at T-tubules and sarcoplasmic reticulum, has recently been associated with BrS (BrS12). Preliminary reports indicate an important association with SCN10A, a neuronal sodium channel that co-associates with SCN5A, with a yield as high as 20%. Mutations in KCNH2 and KCNE5, although not causative, have been identified as capable of modulating the substrate for the development of BrS. Loss-of-function mutations in HCN4 causing a reduction in the pacemaker current, If, have the potential to unmask BrS by reducing heart rate.21 (see 18 for references).
The familial nature of ER pattern has been demonstrated in a number of studies.22-24 ER pattern and ERS have been associated with mutations in 6 genes. Consistent with the findings that IK-ATP activation can generate an ER pattern in canine ventricular wedge preparations, a rare variant in KCNJ8, responsible for the pore forming subunit of the IK-ATP channel, has been reported in a patients with ERS as well as BrS.25-27 Loss of function mutations in the α1 and β2 and α2δ subunits of the cardiac L-type calcium channel (CACNA1C, CACNB2, and CACNA2D1) have been uncovered in patients with ERS.28 The most recent addition to the genes associated with ERS is SCN5A, the gene that encodes the α subunit of the cardiac sodium channel.29 Interestingly, it appears that The SCN5A mutations are associated with a Type 3 ERS in which a J point or ST segment elevations is present in the right precordial leads as well as in the inferior and lateral leads under baseline conditions or following a sodium block challenge.29
It is noteworthy that only a small fraction of identified genetic variants in the numerous genes associated with BrS and ERS have been investigated functionally to elucidate establish causality or a plausible contribution to pathogenesis. Very few have been studied in genetically engineered animal model or native cardiac cell. Computational strategies have been developed to predict the functional consequences of mutations, but none of these methods have been rigorously tested. The lack of functional or biological validation of mutation effects remains the most severe limitation of genetic test interpretation.30 This limitation is further extended to those cases in which a susceptibility gene has been identified on the basis of a single proband and with the absence of familial segregation data.
Ionic and Cellular Mechanisms
The J wave in the ECG is inscribed as a consequence of the presence of a prominent Ito-mediated action potential notch in epicardium, but not endocardium giving rise to a transmural voltage gradient.31, 32 Direct evidence in support of this hypothesis derives from data first reported using the arterially-perfused canine ventricular wedge preparation.11
Factors that augment or reduce Ito or that speed or slow the kinetics of the current can importantly modify the manifestation of the J wave on the ECG. Whether augmented by exposure to hypothermia, ICa and INa blockers or Ito agonists such as NS5806 or reduced by Ito blockers such as 4-aminopyridine, quinidine or premature activation or, changes in the magnitude of the epicardial AP notch parallel those of the J wave.33, 34
Brugada Syndrome
The proposed cellular mechanism for the Brugada syndrome is summarized in Fig. 1. Most studies support the hypothesis that the Brugada syndrome results from amplification of heterogeneities intrinsic to the early phases of the action potential among the different transmural cell types. An increase in net repolarizing current due to either a decrease of inward currents such ands INa or ICa or an increase of outward current such as Ito or IK-ATP, accentuates the notch leading to augmentation of the J wave or appearance of ST segment elevation. A further increase in net repolarizing current can result in partial or complete loss of the action potential dome, leading to a transmural voltage gradient that manifests as an accentuated J wave or an ST segment elevation.15, 33, 34 In regions of the myocardium exhibiting a prominent Ito, such as the epicardium of the right ventricle, marked accentuation of the action potential notch gives rise to a coved-type ST segment elevation diagnostic of BrS. Additional outward shift of the net current active during the early phase of the AP can lead to loss of the AP dome (APD), thus creating a dispersion of repolarization between epicardium and endocardium as well as within epicardium, between the region at which the dome is lost and regions at which it is maintained. The transmural dispersion is responsible for the development of ST segment elevation and the creation of a vulnerable window across the ventricular wall, whereas the epicardial dispersion give to phase 2 reentry, which provides the extrasystole that captures the vulnerable window, thus precipitating VT/VF. The VT is usually polymorphic, resembling a very rapid form of Torsade de Pointes.
Recent studies have suggested that delayed conduction or abnormal depolarization in the right ventricular outflow tract provides the principal substrate of the ST segment elevation or J waves associated with BrS.35, 36 The repolarization vs. depolarization hypotheses controversy has been documented as a published debate.37 Our group has performed thousands of experiments in several different models of Brugada syndrome involving both loss of inward current and gain of outward currents. In none of these have we or anyone else working with similar models ever found evidence that the electrocardiographic or arrhythmic manifestations of BrS are based principally on conduction delay.
Apparently compelling data in support of delayed conduction in the RVOT as the basis for BrS was recently provided by Nademanee and co-workers.36 Using a bipolar electrogram applied to the epicardial surface of the RVOT, these authors recorded late potentials, in some cases appearing as a continuous fractionated electrogram. This activity was interpreted as indicating delayed conduction over the anterior aspect of the RVOT epicardium. Catheter ablation over this abnormal region of the RVOT resulted in normalization of the Brugada ECG pattern over a period of up to 3 months and prevented induction of VT/VF as well as spontaneous recurrence of VT/VF episodes. Signal averaged ECG (SAECG) recordings have also demonstrated late potentials in patients with the Brugada syndrome, especially in the anterior wall of the right ventricular outflow tract (RVOT).38-44
Although late potentials of the type described by Nademanee et al. are traditionally ascribed to conduction delays secondary to structural defects, in cases of BrS they are more likely to arise from a delayed second upstroke of the epicardial action potential in the RVOT or as a consequence of concealed phase 2 reentry.37, 40, 45 It is noteworthy that wall motion abnormalities have also been detected in BrS patients46. Although such contractile abnormalities are commonly considered pathognomonic of structural disease, in the setting of BrS, they are likely to be the result of loss of the action potential dome in the right ventricular epicardium.40, 47 Loss of the dome is expected to lead to contractile dysfunction because calcium entry into the cells is greatly diminished and sarcoplasmic reticulum calcium stores are depleted.
The rate-dependence of the ST segment elevation in BrS has been suggested to be helpful in discriminating between the repolarization and depolarization hypotheses.45 If the ST segment elevation is due predominantly to delayed conduction in the RVOT, acceleration of the rate would be expected to further aggravate conduction and thus accentuate the ST segment elevation in the ECG. If, on the other hand, ST segment elevation or the BrS phenotype is secondary to accentuation of the epicardial action potential notch, acceleration of the rate would be expected to normalize the ECG, by reducing the action potential notch and restoring the action potential dome in RV epicardium. This occurs because the transient outward current, which is at the heart of this mechanism, is slow to recover from inactivation and is less available at faster rates.
Although there are relatively few reports of the effects of pacing, most investigators in the field agree that there is a tendency for the Brugada ECG to normalize during an increase in heart rate consistent with the repolarization hypothesis.48-50 There are however also reports of ST segment elevation or J point elevation with exercise. Amin et al. recently reported J point elevation in BrS patients (both SCN5A+ and SCN5A−) during exercise.51 The principal difference between studies showing a decrease vs. increase of the J point in response to exercise appears to be the presence of a prominent ST segment elevation at baseline in the former.
In BrS cases in which ST segment elevation is accompanied by notching of the QRS, suggesting major conduction delay in the RV, exercise-induced acceleration of rate leads to further fragmentation of the QRS, but to a normalization of the ST segment elevation in all right precordial leads.37 These findings suggest that the ST segment elevation is due to a repolarization defect and not to the clearly apparent depolarization defect responsible for the fragmentation of the QRS. Fragmentation of the QRS is associated with increased mortality and arrhythmic events in patients with coronary artery disease as well as patients with arrhythmogenic right ventricular cardiomyopathy and Brugada syndrome.52
Finally, Kurita et al. placed monophasic action potential (MAP) electrodes on the epicardial and endocardial surfaces of the right ventricular outflow tract (RVOT) in patients with the Brugada syndrome and demonstrated an accentuated notch in the epicardial response, thus providing direct support for the repolarization hypothesis in humans.53, 54
Additional support for the repolarization hypothesis derives from the demonstration that quinidine normalizes ST segment elevation and suppresses late potentials recorded in patients with Brugada syndrome.55-57 These effects of the drug are presumably due to inhibition of Ito leading to reduction of the epicardial action potential notch and normalization of the repolarization heterogeneities. If the ST segment elevation or the late potentials were due to delayed conduction, quinidine-induced INa inhibition would be expected to accentuate their appearance.
It is noteworthy that experimental models displaying major conduction delays have been developed by exposing wedge preparations to global ischemia.58, 59 Progressive delay in these models leads to a gradual prolongation of the R wave and inversion of the T wave. The ECG at first glance resembles a BrS phenotype, but with closer inspection it is clear that the depolarization hypothesis does not lead to an ST segment elevation, but rather to an R wave prolongation. The marked conduction delay is due to a discontinuity in conduction in the deep subepicardium. Conduction under these conditions is exquisitely sensitive to rate, with acceleration leading to block and inexcitability. This is a common characteristic of ischemia-induced tombstone morphology of the ECG, induced by coronary spasm.60 but is not characteristic of BrS A similar phenomenon is observed in right ventricular wedge preparations following exposure to hyperkalemic conditions.61
Also of interest is the observation that magnetocardiograms recorded from patients with complete RBBB generate currents from the RVOT to the upper left chest that are opposite from those recorded in patients with Brugada syndrome.62
While the available data, both basic and clinical, point to the repolarization hypothesis, i.e., transmural voltage gradients that develop secondary to accentuation of the epicardial notch, at times leading to loss of the action potential dome, as the predominant mechanism underlying the Brugada syndrome ECG signature and arrhythmogenesis, there is no doubt that in some cases, particularly those associated with sodium channel loss of function, conduction slowing may contribute to the development of arrhythmias. There are also likely to be cases in which a conduction defect may predominate.63, 64
In patients with BrS, the appearance of prominent J waves is limited to the leads facing the right ventricular outflow tract where Ito is most prominent. The prominent Ito in right ventricular epicardium provides for a net outward current, which promotes the appearance of the J wave in this region of the ventricular myocardium. In the case of ERS, the appearance of prominent J waves is generally limited to the inferior and lateral precordial ECG leads, pointing to heterogeneities in left ventricular (LV) myocardium as the cause.
Early Repolarization Syndrome
Early repolarization pattern is characterized by J point elevation, J waves with and without ST segment elevation and slurring of the terminal part of the ECG in inferior limb and lateral precordial leads. These electrocardiographic manifestations of ER can be recapitulated in the coronary-perfused LV wedge preparation. Figure 2 illustrates the diversity of ECG phenotypes generated by different configurations of the epicardial action potential notch and varying degrees of transmural conduction in coronary-perfused canine left ventricular wedge preparations. These range from a J point elevation to slurring of the terminal part of the QRS, distinct J waves with and without ST segment elevation as well as gigantic J waves, appearing as an ST segment elevation, which often give rise to polymorphic VT. The distinctive ER patterns all result from “early repolarization” of the epicardial action potential and reflect the dynamicity that could be observed clinically in select patients with ER or ERS. These observations provide justification for the long-standing nomenclature and question the need for narrow or overly restrictive definitions of ER pattern.65-67
Figure 2.
Diverse manifestations of early repolarization pattern. Each panel shows transmembrane action potentials recorded from the epicardial and endocardial regions of arterially-perfused canine left ventricular wedge preparations and a transmural ECG simultaneously recorded. Under the conditions indicated, early repolarization of the epicardial action potential result in different configurations of the action potential notch giving rise to diverse electrocardiographic manifestations of ERP. The six panels illustrate the cellular basis for a J point elevation, a distinct J wave, slurring of the terminal part of the QRS, combined J wave, J point and ST segment elevation, and a gigantic J wave appearing as an ST segment elevation, which gives rise to polymorphic VT. Modified from 77, with permission.
The ionic and cellular mechanisms involved in generating ER patterns in the ECG appear to be similar to those responsible for BrS. A net outward shift of current due to reduction of ICa or INa or augmentation of IK-ATP have been shown to underlie ER, giving rise to J point elevation and distinct J waves with and without ST segment elevation.
Our working hypothesis is that an outward shift in repolarizing current due to a decrease in sodium or calcium channel currents or an increase in Ito, IK-ATP, IK-ACh, or other outward currents gives rise to the J wave syndromes (Figure 3). The phenotype depends on the part of the heart that is principally affected and the ion channels involved. The J wave syndromes can be viewed as a spectrum of disorders involving accentuation of the epicardial action potential notch in different regions of heart, leading to the development of prominent J waves that predispose to the development phase 2 reentry, which serves to trigger VT/VF.8
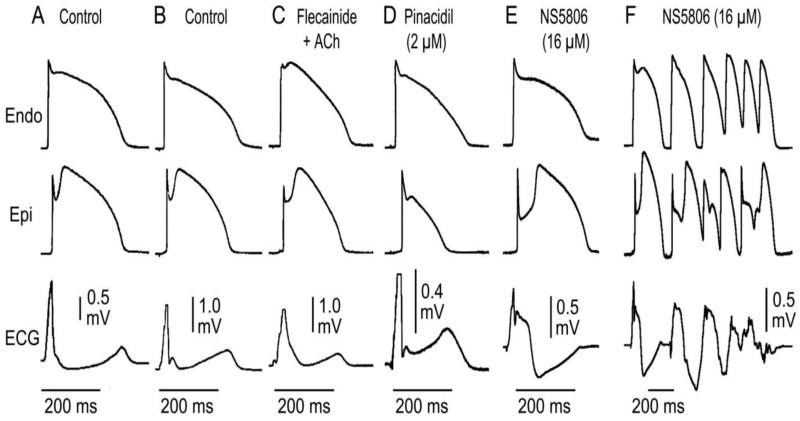
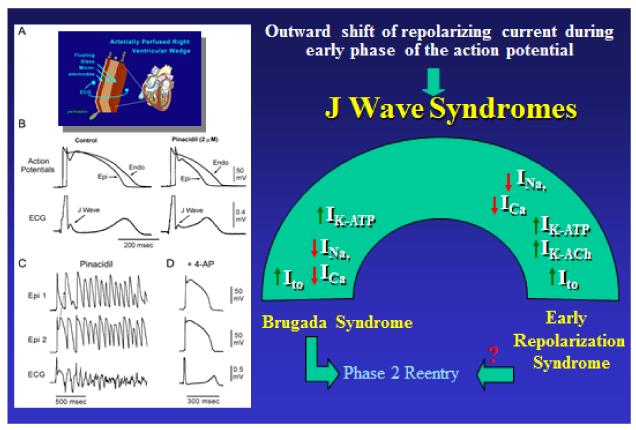
Figure 3.
Ionic and Cellular basis for the early repolarization syndrome. Left panel: A: Schematic of Coronary-perfused wedge preparation B: Simultaneous recording of transmembrane action potentials (APs) from epicardial (Epi) and endocardial (Endo) regions and a transmural ECG in an isolated arterially perfused canine ventricular wedge. A J wave in the transmural ECG is manifest due to the presence of an AP notch in epicardium but not endocardium. Pinacidil (2 μM), an ATP-sensitive potassium channel opener, causes depression of the AP dome in epicardium, resulting in ST segment elevation in the ECG resembling the ERS. C: IK-ATP activation in the canine right ventricular wedge preparation using 2.5 uM pinacidil produces heterogeneous loss of the AP dome in epicardium, resulting in ST segment elevation, phase 2 reentry and ventricular tachycardia or ventricular fibrillation (VT/VF) (BrS phenotype). D: The Ito blocker, 4-aminopyridine (4-AP), restored the Epi AP dome, reduced both transmural and Epi dispersion of repolarization, normalized the ST segment and prevented phase 2 reentry and VT/VF in the continued presence of pinacidil. (Modified from 8, with permission). Right panel: Schematic depicting our working hypothesis of the ionic mechanism underlying the J wave syndromes. An outward shift in repolarizing current due to a decrease in sodium or calcium channel currents or an increase in Ito, IK-ATP or IK-ACh, or other outward currents gives rise to accentuated J waves associated with the Brugada syndrome and early repolarization syndrome. Both are thought to be triggered by closely-coupled phase 2 reentrant extrasystoles, but in the case of ERS a Purkinje source of ectopic activity is also suspected. (Modified from 8, with permission)
Clinical Manifestations of J Wave Syndromes
In both BrS and ERS, the manifestation of the J wave or ER is dynamic, with the most prominent ECG changes appearing just before the onset of VT/VF. (see also18, 19 for references). Other ECG characteristics of ERS also closely match those of BrS, including the presence of accentuated J waves, ST segment elevation, pause and bradycardia-dependence, short coupled extrasystole-induced polymorphic VT/VF. Suppression of the ECG features by isoproterenol or pacing in ER patients further supports the notion that they share common underlying electrophysiologic abnormalities with BrS patients.20 However, salient diagnostic features of BrS such as provocation by sodium channel blockers or positive signal averaged ECG are rarely observed in these ERS patients.17, 20 An exception to this rule appears to apply to ERS associated with SCN5A mutations.29 Kawata and coworkers recently showed that sodium channel blockers attenuate ER in patients with both ERS apparently due to slowing of transmural conduction so that J point shifts to a lower position on the terminal part of the QRS.68 The principal difference between ERS and BrS is the myocardial region associated with the highest arrhythmogenic risk; it is the right ventricular outflow tract (RVOT) in the case of BrS and the inferior region of the left ventricle in ERS (Table 2).
Table 2. Features Common to Brugada and Early Repolarization Syndromes and Possible Underlying Mechanisms.
| BrS | ERS | Possible Mechanism(s) | |
|---|---|---|---|
| Region Associated with highest arrhythmic risk |
RVOT | Inferior myocardium |
Increased levels of Ito |
| Male Predominance | Yes (75%) | Yes (80%) | Testosterone modulation of ion currents underlying the epicardial AP notch |
| Average age of first event | ~35-40 | 42 | |
| Dynamicity of ECG | High | High | Autonomic modulation of ion channel currents underlying early phases of the epicardial AP |
| VT/VF trigger | Short-coupled PVC |
Short-coupled PVC |
Phase 2 reentry |
| Ameliorative response to quinidine |
Yes | Yes | Inhibition of Ito and possible vagolytic effect |
| Ameliorative response to Isoproterenol and cilostazol |
Yes | Yes | Increased ICa and faster heart rate |
| Ameliorative response to pacing |
Yes | Yes | Reduced availability of Ito due to slow recovery from inactivation |
| Vagally-mediated accentuation of ECG pattern |
Yes | Yes | Direct effect to inhibit ICa and indirect effect to increase Ito (due to slowing of heart rate) |
RVOT=right ventricular outflow tract, AP=action potential; PVC=premature ventricular contraction
Of note, experimental studies have also provided evidence in support of the hypothesis that accentuated J waves associated with the early phases of ischemia may also be due to an accentuation of the action potential notch in epicardium, but not endocardium.69
Differentiating J waves Caused by Depolarization vs. Repolarization Abnormalities
It is noteworthy that perturbation in the terminal part of the QRS that are generally referred to as J waves can arise from either repolarization or depolarization abnormalities. When due to depolarization defects, it often appears as a notch interrupting the descending limb of the QRS, with little or no ST segment elevation. Depolarization defects contributing to apparent J waves on the descending limb of the QRS or “terminal QRS distortion” can occur in the setting of bundle branch block, peri-infarction block, or severe ischemia in the Sclarovsky-Birnbaum system.70.
One way to distinguish between the two mechanisms is to examine the effect of rate or atrial premature responses. When due to delayed conduction, the notched appearance should become accentuated with acceleration of rate or prematurity, and when due to repolarization problems, the amplitude of the J wave should diminish. These different responses are due to the fact that delayed conduction almost invariably becomes more accentuated at faster rates or with prematurity, whereas the Ito-mediated APN diminishes due to insufficient time for the Ito to reactivate.
Summary and Conclusion
Idiopathic ventricular tachycardia and fibrillation (VT/VF) have been associated with the appearance of prominent J waves for over three decades. Accentuated J waves characterize both Brugada and early repolarization syndromes prompting their designation as J wave syndromes. An early repolarization (ER) pattern, characterized by J point elevation, slurring of the terminal part of the QRS and in some cases ST segment elevation, although considered a benign electrocardiographic manifestation until a decade ago, has more recently been shown to be associated with increased risk for life-threatening arrhythmias, named early repolarization syndrome (ERS). Experimental data as well as numerous clinical case-control and population-based association studies have advanced evidence that an ER pattern in the inferior or infero-lateral leads is associated with increased risk for development of life-threatening arrhythmias. ERS and Brugada syndrome (BrS) share similar electrocardiographic features, clinical outcomes, risk factors as well as a common arrhythmic platform related to amplification of Ito-mediated J waves. Although BrS and ERS differ with respect to the magnitude and lead location of abnormal J wave manifestation, they are thought to represent a continuous spectrum of phenotypic expression, termed J wave syndromes. The two syndromes have been shown to be associated with gain of function mutations in genes that encode outward currents, such as Ito and IK-ATP, or in loss of function mutations in genes that encode inward currents such as ICa and INa, thus producing an outward shift in the balance of current active during the early phases of the action potential. In the case of epicardium, this results in an accentuation of the action potential notch, which underlies the manifestation of J waves and apparent ST segment elevation. Although the vast majority of subjects presenting with ER patterns are not believed to be at increased risk, the challenge ahead is to identify the small subset of patients who are at risk.
Table 1. J-wave Syndromes: Similarities and Differences.
| J Wave Syndromes | ||||||
|---|---|---|---|---|---|---|
| Inherited | Acquired | |||||
| ER in lateral leads ERS Type 1 |
ER in inferior or infero-lateral leads ERS Type 2 |
Global ER ERS Type 3 |
Brugada Syndrome |
Ischemia- mediated VT/VF |
Hypothermia- mediated VT/VF |
|
| Anatomic Location | Antero-lateral left ventricle |
Inferior left ventricle | Left and right ventricles |
Right ventricle | Left and right ventricles |
Left and right ventricles |
|
Leads Displaying J
point/ J-wave |
I, V4-V6 | II, III, aVF | Global | V1-V3 | Any of 12 leads | Any of 12 leads |
|
Response of J wave /ST
Elevation to: Bradycardia or pause Na+ channel blockers |
|
|
|

|
N/A N/A |
N/A N/A |
| Sex Dominance | Male | Male | Male | Male | Male71,72 | Either gender |
| VT/VF | Rare Common in healthy athletes12, 13, 34 |
Yes16,73 | Yes, Electrical storms17, 74 |
Yes | Yes | Yes |
|
Response of to
Quinidine: J wave /ST elevation VT/VF |

|

|

|

|
Limited data |
 75
75
|
|
Response of to
Isoproterenol: J wave /ST elevation VT/VF |

|

|
Limited data |

|
N/A | N/A |
ER= early repolarization; ERS=early repolarization syndrome; N/A=not available; VF=ventricular fibrillation; VT=ventricular tachycardia. Modified from 8, with permission.
Acknowledgments
Support: Supported by grants HL47678 from NHLBI, NIH, C026424 from NYSTEM, and the Masons of New York State, Florida, Massachusetts Connecticut, Maryland, Rhode Island and Wisconsin
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest: There are no conflicts of interest to disclose.
Reference
- 1.Tomaszewski W. Changement electrocardiographiques observes chez un homme mort de froid. Arch Mal Coeur Vaiss. 1938;31:525–528. [Google Scholar]
- 2.Osborn JJ. Experimental hypothermia: respiratory and blood pH changes in relation to cardiac function. Am J Physiol. 1953;175:389–398. doi: 10.1152/ajplegacy.1953.175.3.389. [DOI] [PubMed] [Google Scholar]
- 3.Clements SD, Hurst JW. Diagnostic value of ECG abnormalities observed in subjects accidentally exposed to cold. Am J Cardiol. 1972;29:729–734. doi: 10.1016/0002-9149(72)90178-6. [DOI] [PubMed] [Google Scholar]
- 4.Thompson R, Rich J, Chmelik F, Nelson WL. Evolutionary changes in the electrocardiogram of severe progressive hypothermia. J Electrocardiol. 1977;10:67–70. doi: 10.1016/s0022-0736(77)80034-4. [DOI] [PubMed] [Google Scholar]
- 5.Eagle K. Images in clinical medicine. Osborn waves of hypothermia. N Engl J Med. 1994:10–680. doi: 10.1056/NEJM199403103301005. [DOI] [PubMed] [Google Scholar]
- 6.Kraus F. Ueber die wirkung des kalziums auf den kreislauf. Dtsch Med Wochenschr. 1920;46:201–203. [Google Scholar]
- 7.Sridharan MR, Horan LG. Electrocardiographic J wave of hypercalcemia. Am J Cardiol. 1984;54:672–673. doi: 10.1016/0002-9149(84)90273-x. [DOI] [PubMed] [Google Scholar]
- 8.Antzelevitch C, Yan GX. J wave syndromes. Heart Rhythm. 2010;7:549–558. doi: 10.1016/j.hrthm.2009.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shipley RA, Hallaran WR. The four lead electrocardiogram in 200 normal men and women. Am Heart J. 1936;11:325–345. [Google Scholar]
- 10.Grant RP, ESTES EH, Jr., DOYLE JT. Spatial vector electrocardiography; the clinical characteristics of S-T and T vectors. Circulation. 1951;3:182–197. doi: 10.1161/01.cir.3.2.182. [DOI] [PubMed] [Google Scholar]
- 11.Yan GX, Antzelevitch C. Cellular basis for the electrocardiographic J wave. Circulation. 1996;93:372–379. doi: 10.1161/01.cir.93.2.372. [DOI] [PubMed] [Google Scholar]
- 12.Wasserburger RH, Alt WJ. The normal RS-T segment elevation variant. Am J Cardiol. 1961;8:184–192. doi: 10.1016/0002-9149(61)90204-1. [DOI] [PubMed] [Google Scholar]
- 13.Mehta MC, Jain AC. Early repolarization on scalar electrocardiogram. Am J Med Sci. 1995;309:305–311. doi: 10.1097/00000441-199506000-00001. [DOI] [PubMed] [Google Scholar]
- 14.Gussak I, Antzelevitch C. Early repolarization syndrome: clinical characteristics and possible cellular and ionic mechanisms. J Electrocardiol. 2000;33:299–309. doi: 10.1054/jelc.2000.18106. [DOI] [PubMed] [Google Scholar]
- 15.Yan GX, Antzelevitch C. Cellular basis for the Brugada syndrome and other mechanisms of arrhythmogenesis associated with ST segment elevation. Circulation. 1999;100:1660–1666. doi: 10.1161/01.cir.100.15.1660. [DOI] [PubMed] [Google Scholar]
- 16.Haissaguerre M, Derval N, Sacher F, Jesel L, Deisenhofer I, De Roy L, Pasquie JL, Nogami A, Babuty D, Yli-Mayry S, De Chillou C, Scanu P, Mabo P, Matsuo S, Probst V, Le Scouarnec S, Defaye P, Schlaepfer J, Rostock T, Lacroix D, Lamaison D, Lavergne T, Aizawa Y, Englund A, Anselme F, O’Neill M, Hocini M, Lim KT, Knecht S, Veenhuyzen GD, Bordachar P, Chauvin M, Jais P, Coureau G, Chene G, Klein GJ, Clementy J. Sudden cardiac arrest associated with early repolarization. N Engl J Med. 2008;358:2016–2023. doi: 10.1056/NEJMoa071968. [DOI] [PubMed] [Google Scholar]
- 17.Nam GB, Kim YH, Antzelevitch C. Augmentation of J waves and electrical storms in patients with early repolarization. N Engl J Med. 2008;358:2078–2079. doi: 10.1056/NEJMc0708182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Antzelevitch C. Genetic, molecular and cellular mechanisms underlying the J wave syndromes. Circ J. 2012;76:1054–1065. doi: 10.1253/circj.cj-12-0284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Junttila MJ, Sager SJ, Tikkanen JT, Anttonen O, Huikuri HV, Myerburg RJ. Clinical significance of variants of J-points and J-waves: early repolarization patterns and risk. Eur Heart J. 2012;33:2639–2643. doi: 10.1093/eurheartj/ehs110. [DOI] [PubMed] [Google Scholar]
- 20.Nam GB, Ko KH, Kim J, Park KM, Rhee KS, Choi KJ, Kim YH, Antzelevitch C. Mode of onset of ventricular fibrillation in patients with early repolarization pattern vs. Brugada syndrome. Eur Heart J. 2010;31:330–339. doi: 10.1093/eurheartj/ehp423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ueda K, Nakamura K, Hayashi T, Inagaki N, Takahashi M, Arimura T, Morita H, Higashiuesato Y, Hirano Y, Yasunami M, Takishita S, Yamashina A, Ohe T, Sunamori M, Hiraoka M, Kimura A. Functional characterization of a trafficking-defective HCN4 mutation, D553N, associated with cardiac arrhythmia. J Biol Chem. 2004;279:27194–27198. doi: 10.1074/jbc.M311953200. [DOI] [PubMed] [Google Scholar]
- 22.Noseworthy PA, Tikkanen JT, Porthan K, Oikarinen L, Pietila A, Harald K, Peloso GM, Merchant FM, Jula A, Vaananen H, Hwang SJ, O’Donnell CJ, Salomaa V, Newton-Cheh C, Huikuri HV. The early repolarization pattern in the general population clinical correlates and heritability. J Am Coll Cardiol. 2011;57:2284–2289. doi: 10.1016/j.jacc.2011.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reinhard W, Kaess BM, Debiec R, Nelson CP, Stark K, Tobin MD, Macfarlane PW, Tomaszewski M, Samani NJ, Hengstenberg C. Heritability of early repolarization: a population-based study. Circ Cardiovasc Genet. 2011;4:134–138. doi: 10.1161/CIRCGENETICS.110.958298. [DOI] [PubMed] [Google Scholar]
- 24.Nunn LM, Bhar-Amato J, Lowe MD, Macfarlane PW, Rogers P, McKenna WJ, Elliott PM, Lambiase PD. Prevalence of J-point elevation in sudden arrhythmic death syndrome families. J Am Coll Cardiol. 2011;58:286–290. doi: 10.1016/j.jacc.2011.03.028. [DOI] [PubMed] [Google Scholar]
- 25.Haissaguerre M, Chatel S, Sacher F, Weerasooriya R, Probst V, Loussouarn G, Horlitz M, Liersch R, Schulze-Bahr E, Wilde A, Kaab S, Koster J, Rudy Y, Le MH, Schott JJ. Ventricular fibrillation with prominent early repolarization associated with a rare variant of KCNJ8/KATP channel. J Cardiovasc Electrophysiol. 2009;20:93–98. doi: 10.1111/j.1540-8167.2008.01326.x. [DOI] [PubMed] [Google Scholar]
- 26.Medeiros-Domingo A, Tan BH, Crotti L, Tester DJ, Eckhardt L, Cuoretti A, Kroboth SL, Song C, Zhou Q, Kopp D, Schwartz PJ, Makielski JC, Ackerman MJ. Gain-of-function mutation S422L in the KCNJ8-encoded cardiac K(ATP) channel Kir6.1 as a pathogenic substrate for J-wave syndromes. Heart Rhythm. 2010;7:1466–1471. doi: 10.1016/j.hrthm.2010.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Barajas-Martinez H, Hu D, Ferrer T, Onetti CG, Wu Y, Burashnikov E, Boyle M, Surman T, Urrutia J, Veltmann C, Schimpf R, Borggrefe M, Wolpert C, Ibrahim BB, Sanchez-Chapula JA, Winters S, Haissaguerre M, Antzelevitch C. Molecular genetic and functional association of Bugada and early repolarization syndromes with S422L missense mutation in KCNJ8. Heart Rhythm. 2012;9:548–555. doi: 10.1016/j.hrthm.2011.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Burashnikov E, Pfeiffer R, Barajas-Martinez H, Delpon E, Hu D, Desai M, Borggrefe M, Haissaguerre M, Kanter R, Pollevick GD, Guerchicoff A, Laino R, Marieb M, Nademanee K, Nam GB, Robles R, Schimpf R, Stapleton DH, Viskin S, Winters S, Wolpert C, Zimmern S, Veltmann C, Antzelevitch C. Mutations in the cardiac L-type calcium channel associated J wave sydnrome and sudden cardiac death. Heart Rhythm. 2010;7:1872–1882. doi: 10.1016/j.hrthm.2010.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Watanabe H, Nogami A, Ohkubo K, Kawata H, Hayashi Y, Ishikawa T, Makiyama T, Nagao S, Yagihara N, Takehara N, Kawamura Y, Sato A, Okamura K, Hosaka Y, Sato M, Fukae S, Chinushi M, Oda H, Okabe M, Kimura A, Maemura K, Watanabe I, Kamakura S, Horie M, Aizawa Y, Shimizu W, Makita N. Electrocardiographic characteristics and SCN5A mutations in idiopathic ventricular fibrillation associated with early repolarization. Circ Arrhythm Electrophysiol. 2011;4:874–881. doi: 10.1161/CIRCEP.111.963983. [DOI] [PubMed] [Google Scholar]
- 30.Schwartz PJ, Ackerman MJ, George AL, Jr., Wilde AA. Impact of genetics on the clinical management of channelopathies. J Am Coll Cardiol. 2013;62:169–180. doi: 10.1016/j.jacc.2013.04.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Litovsky SH, Antzelevitch C. Transient outward current prominent in canine ventricular epicardium but not endocardium. Circ Res. 1988;62:116–126. doi: 10.1161/01.res.62.1.116. [DOI] [PubMed] [Google Scholar]
- 32.Antzelevitch C, Sicouri S, Litovsky SH, Lukas A, Krishnan SC, Di Diego JM, Gintant GA, Liu DW. Heterogeneity within the ventricular wall. Electrophysiology and pharmacology of epicardial, endocardial, and M cells. Circ Res. 1991;69:1427–1449. doi: 10.1161/01.res.69.6.1427. [DOI] [PubMed] [Google Scholar]
- 33.Antzelevitch C, Yan GX. Cellular and ionic mechanisms responsible for the Brugada syndrome. J Electrocardiol. 2000;33(Suppl):33–39. doi: 10.1054/jelc.2000.20321. [DOI] [PubMed] [Google Scholar]
- 34.Yan GX, Lankipalli RS, Burke JF, Musco S, Kowey PR. Ventricular repolarization components on the electrocardiogram: cellular basis and clinical significance. J Am Coll Cardiol. 2003;42:401–409. doi: 10.1016/s0735-1097(03)00713-7. [DOI] [PubMed] [Google Scholar]
- 35.Postema PG, van Dessel PFHM, Kors JA, Linnenbank AC, van Harpen G, Ritsema van Eck HJ, van Geloven N, De Bakker JMT, Wilde AAM, Tan HT. Local depolarization abnormalities are the dominant pathophysiologic mechanism for type 1 electrocardiogram in Brugada syndrome: a study of electrocardiograms, vectorcardiograms, and body surface potential maps during ajmaline provocation. J Am Coll Cardiol. 2010;55:789–797. doi: 10.1016/j.jacc.2009.11.033. [DOI] [PubMed] [Google Scholar]
- 36.Nademanee K, Veerakul G, Chandanamattha P, Chaothawee L, Ariyachaipanich A, Jirasirirojanakorn K, Likittanasombat K, Bhuripanyo K, Ngarmukos T. Prevention of ventricular fibrillation episodes in Brugada syndrome by catheter ablation over the anterior right ventricular outflow tract epicardium. Circulation. 2011;123:1270–1279. doi: 10.1161/CIRCULATIONAHA.110.972612. [DOI] [PubMed] [Google Scholar]
- 37.Wilde AA, Postema PG, Di Diego JM, Viskin S, Morita H, Fish JM, Antzelevitch C. The pathophysiological mechanism underlying Brugada syndrome: depolarization versus repolarization. J Mol Cell Cardiol. 2010;49:543–553. doi: 10.1016/j.yjmcc.2010.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Futterman LG, Lemberg L. Brugada. Am J Crit Care. 2001;10:360–364. [PubMed] [Google Scholar]
- 39.Fujiki A, Usui M, Nagasawa H, Mizumaki K, Hayashi H, Inoue H. ST segment elevation in the right precordial leads induced with class IC antiarrhythmic drugs: insight into the mechanism of Brugada syndrome. J Cardiovasc Electrophysiol. 1999;10:214–218. doi: 10.1111/j.1540-8167.1999.tb00662.x. [DOI] [PubMed] [Google Scholar]
- 40.Antzelevitch C. Late potentials and the Brugada syndrome. J Am Coll Cardiol. 2002;39:1996–1999. doi: 10.1016/s0735-1097(02)01887-9. [DOI] [PubMed] [Google Scholar]
- 41.Nagase S, Kusano KF, Morita H, Fujimoto Y, Kakishita M, Nakamura K, Emori T, Matsubara H, Ohe T. Epicardial electrogram of the right ventricular outflow tract in patients with the Brugada syndrome: using the epicardial lead. J Am Coll Cardiol. 2002;39:1992–1995. doi: 10.1016/s0735-1097(02)01888-0. [DOI] [PubMed] [Google Scholar]
- 42.Eckardt L, Bruns HJ, Paul M, Kirchhof P, Schulze-Bahr E, Wichter T, Breithardt G, Borggrefe M, Haverkamp W. Body surface area of ST elevation and the presence of late potentials correlate to the inducibility of ventricular tachyarrhythmias in Brugada syndrome. J Cardiovasc Electrophysiol. 2002;13:742–749. doi: 10.1046/j.1540-8167.2002.00742.x. [DOI] [PubMed] [Google Scholar]
- 43.Ikeda T, Takami M, Sugi K, Mizusawa Y, Sakurada H, Yoshino H. Noninvasive risk stratification of subjects with a brugada-type electrocardiogram and no history of cardiac arrest. Ann Noninvasive Electrocardiol. 2005;10:396–403. doi: 10.1111/j.1542-474X.2005.00055.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mizobuchi M, Enjoji Y, Nakamura S, Muranishi H, Utsunomiya M, Funatsu A, Kobayashi T. Ventricular late potential in patients with apparently normal electrocardiogram; predictor of Brugada syndrome. Pacing Clin Electrophysiol. 2010;33:266–273. doi: 10.1111/j.1540-8159.2009.02621.x. [DOI] [PubMed] [Google Scholar]
- 45.Antzelevitch C. Brugada syndrome. PACE. 2006;29:1130–1159. doi: 10.1111/j.1540-8159.2006.00507.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Takagi M, Aihara N, Kuribayashi S, Taguchi A, Shimizu W, Kurita T, Suyama K, Kamakura S, Hamada S, Takamiya M. Localized right ventricular morphological abnormalities detected by eletron-beam computed tomography represent arrhythmogenic substrates in patients with the Brugada syndrome. Eur Heart J. 2001;22:1032–1041. doi: 10.1053/euhj.2000.2424. [DOI] [PubMed] [Google Scholar]
- 47.Antzelevitch C. Brugada syndrome: historical perspectives and observations. Eur Heart J. 2002;23:676–678. doi: 10.1053/euhj.2001.3145. [DOI] [PubMed] [Google Scholar]
- 48.Esperer HD, Hoos O, Hottenrott K. Syncope due to Brugada syndrome in a young athlete. Br J Sports Med. 2007;41:180–181. doi: 10.1136/bjsm.2006.030098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Guevara-Valdivia ME, Iturralde Torres P, De Micheli A, Colin Lizalde L, Medeiros Domingo A, Gonzalez-Hermosillo JA. [Electrocardiographic changes during stress test in a patient with “Brugada syndrome”] Arch Cardiol Mex. 2001;71:66–72. [PubMed] [Google Scholar]
- 50.Stix G, Bella PD, Carbucicchio C, Schmidinger H. Spatial and temporal heterogeneity of depolarization and repolarization may complicate implantable cardioverter defibrillator therapy in Brugada syndrome. J Cardiovasc Electrophysiol. 2000;11:516–521. doi: 10.1111/j.1540-8167.2000.tb00004.x. [DOI] [PubMed] [Google Scholar]
- 51.Amin AS, de Groot EAA, Ruijter JM, Wilde AAM, Tan HT. Exercise-induced ECG changes in Brugada syndrome. Circ Arrhythm Electrophysiol. 2009;2:531–539. doi: 10.1161/CIRCEP.109.862441. [DOI] [PubMed] [Google Scholar]
- 52.Das MK, El Masry H. Fragmented QRS and other depolarization abnormalities as a predictor of mortality and sudden cardiac death. Curr Opin Cardiol. 2010;25:59–64. doi: 10.1097/HCO.0b013e328333d35d. [DOI] [PubMed] [Google Scholar]
- 53.Antzelevitch C, Brugada P, Brugada J, Brugada R, Shimizu W, Gussak I, Perez Riera AR. Brugada syndrome: a decade of progress. Circ Res. 2002;91:1114–1119. doi: 10.1161/01.res.0000046046.53721.90. [DOI] [PubMed] [Google Scholar]
- 54.Kurita T, Shimizu W, Inagaki M, Suyama K, Taguchi A, Satomi K, Aihara N, Kamakura S, Kobayashi J, Kosakai Y. The electrophysiologic mechanism of ST-segment elevation in Brugada syndrome. J Am Coll Cardiol. 2002;40:330–334. doi: 10.1016/s0735-1097(02)01964-2. [DOI] [PubMed] [Google Scholar]
- 55.Belhassen B, Viskin S. Pharmacologic approach to therapy of Brugada syndrome: quinidine as an alternative to ICD therapy? In: Antzelevitch C, Brugada P, Brugada J, Brugada R, editors. The Brugada Syndrome: From Bench to Bedside. Blackwell Futura; Oxford: 2004. pp. 202–211. [Google Scholar]
- 56.Belhassen B, Glick A, Viskin S. Efficacy of quinidine in high-risk patients with Brugada syndrome. Circulation. 2004;110:1731–1737. doi: 10.1161/01.CIR.0000143159.30585.90. [DOI] [PubMed] [Google Scholar]
- 57.Watanabe H, Chinushi M, Osaki A, Okamura K, Izumi D, Komura S, Hosaka Y, Tanabe Y, Furushima H, Washizuka T, Aizawa Y. Elimination of late potentials by quinidine in a patient with Brugada syndrome. J Electrocardiol. 2006;39:63–66. doi: 10.1016/j.jelectrocard.2005.07.009. [DOI] [PubMed] [Google Scholar]
- 58.Di Diego JM, Antzelevitch C. Cellular basis for ST-segment changes observed during ischemia. J Electrocardiol. 2003;36(Suppl):1–5. doi: 10.1016/j.jelectrocard.2003.09.001. [DOI] [PubMed] [Google Scholar]
- 59.Di Diego JM, Fish JM, Antzelevitch C. Brugada syndrome and ischemia-induced ST-segment elevation. Similarities and differences. J Electrocardiol. 2005;38:14–17. doi: 10.1016/j.jelectrocard.2005.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Childers R. R wave amplitude in ischemia, injury, and infarction. J Electrocardiol. 1996;29:171–178. doi: 10.1016/s0022-0736(96)80058-6. [DOI] [PubMed] [Google Scholar]
- 61.Cordeiro JM, Mazza M, Goodrow R, Ulahannan N, Antzelevitch C, Di Diego JM. Functionally distinct sodium channels in ventricular epicardial and endocardial cells contribute to a greater sensitivity of the epicardium to electrical depression. Am J Physiol Heart Circ Physiol. 2008;295:H154–H162. doi: 10.1152/ajpheart.01327.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kandori A, Shimizu W, Yokokawa M, Noda T, Kamakura S, Miyatake K, Murakami M, Miyashita T, Ogata K, Tsukada K. Identifying patterns of spatial current dispersion that characterise and separate the Brugada syndrome and complete right-bundle branch block. Med Biol Eng Comput. 2004;42:236–244. doi: 10.1007/BF02344637. [DOI] [PubMed] [Google Scholar]
- 63.Hoogendijk MG, Potse M, Linnenbank AC, Verkerk AO, Den Ruijter HM, van Amersfoorth SC, Klaver EC, Beekman L, Bezzina CR, Postema PG, Tan HL, Reimer AG, van der Wal AC, Ten Harkel AD, Dalinghaus M, Vinet A, Wilde AA, de Bakker JM, Coronel R. Mechanism of right precordial ST-segment elevation in structural heart disease: Excitation failure by current-to-load mismatch. Heart Rhythm. 2010;7:238–248. doi: 10.1016/j.hrthm.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 64.Marquez MF, Bisteni A, Medrano G, De Micheli A, Guevara M, Iturralde P, Colin L, Hermosillo G, Cardenas M. Dynamic electrocardiographic changes after aborted sudden death in a patient with Brugada syndrome and rate-dependent right bundle branch block. J Electrocardiol. 2005;38:256–259. doi: 10.1016/j.jelectrocard.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 65.Heng SJ, Clark EN, Macfarlane PW. End QRS notching or slurring in the electrocardiogram: influence on the definition of “early repolarization”. J Am Coll Cardiol. 2012;60:947–948. doi: 10.1016/j.jacc.2012.03.061. [DOI] [PubMed] [Google Scholar]
- 66.Froelicher V. Early repolarization redux: the devil is in the methods. Ann Noninvasive Electrocardiol. 2012;17:63–64. doi: 10.1111/j.1542-474X.2011.00483.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Surawicz B, Macfarlane PW. Inappropriate and confusing electrocardiographic terms: J-wave syndromes and early repolarization. J Am Coll Cardiol. 2011;57:1584–1586. doi: 10.1016/j.jacc.2010.11.040. [DOI] [PubMed] [Google Scholar]
- 68.Kawata H, Noda T, Yamada Y, Okamura H, Satomi K, Aiba T, Takaki H, Aihara N, Isobe M, Kamakura S, Shimizu W. Effect of sodium-channel blockade on early repolarization in inferior/lateral leads in patients with idiopathic ventricular fibrillation and Brugada syndrome. Heart Rhythm. 2012;9:77–83. doi: 10.1016/j.hrthm.2011.08.017. [DOI] [PubMed] [Google Scholar]
- 69.Shinde R, Shinde S, Makhale C, Grant P, Sathe S, Durairaj M, Lokhandwala Y, Di Diego JM, Antzelevitch C. Occurrence of “J waves” in 12-lead ECG as a marker of acute ischemia and their cellular basis. PACE. 2007;30:817–819. doi: 10.1111/j.1540-8159.2007.00760.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hasdai D, Porter A, Birnbaum Y, Strahilevitz J, Sclarovsky S. Predicting postinfarction left ventricular dysfunction based on the configuration of the QRS complex and ST segment in the initial ECG of patients with a first anterior wall myocardial infarction. Cardiology. 1996;87:125–128. doi: 10.1159/000177074. [DOI] [PubMed] [Google Scholar]
- 71.Lerner DJ, Kannel WB. Patterns of coronary heart disease morbidity and mortality in the sexes: a 26-year follow-up of the Framingham population. Am Heart J. 1986;111:383–390. doi: 10.1016/0002-8703(86)90155-9. [DOI] [PubMed] [Google Scholar]
- 72.Every N, Hallstrom A, McDonald KM, Parsons L, Thom D, Weaver D, Hlatky MA. Risk of sudden versus nonsudden cardiac death in patients with coronary artery disease. Am Heart J. 2002;144:390–396. doi: 10.1067/mhj.2002.125495. [DOI] [PubMed] [Google Scholar]
- 73.Kalla H, Yan GX, Marinchak R. Ventricular fibrillation in a patient with prominent J (Osborn) waves and ST segment elevation in the inferior electrocardiographic leads: a Brugada syndrome variant? J Cardiovasc Electrophysiol. 2000;11:95–98. doi: 10.1111/j.1540-8167.2000.tb00743.x. [DOI] [PubMed] [Google Scholar]
- 74.Qi X, Sun F, An X, Yang J. A case of Brugada syndrome with ST segment elevation through entire precordial leads. Chin J Cardiol. 2004;32:272–273. [Google Scholar]
- 75.Johnson P, Lesage A, Floyd WL, Yound WG, Jr., Sealy WC. Prevention of ventricular fibrillation during profound hypothermia by quinidine. Ann Surg. 1960;151:490–495. doi: 10.1097/00000658-196004000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Antzelevitch C. The Brugada syndrome: diagnostic criteria and cellular mechanisms. Eur Heart J. 2001;22:356–363. doi: 10.1053/euhj.2000.2461. [DOI] [PubMed] [Google Scholar]
- 77.Antzelevitch C, Yan GX, Viskin S. Rationale for the use of the terms J-wave syndromes and early repolarization. J Am Coll Cardiol. 2011;57:1587–1590. doi: 10.1016/j.jacc.2010.11.038. [DOI] [PMC free article] [PubMed] [Google Scholar]