Abstract
The tachykinin neuropeptide substance P (SP) has an important signaling role in both the nervous and the immune systems. Two naturally occurring variants of the Neurokinin-1 receptor (NK1R) mediate the effects of SP, full-length receptor (NK1R-F) and a truncated form (NK1R-T) that lacks 96 amino acid residues at the C-terminus. We previously reported decreased expression of the NK1R-F in the CNS of HIV-positive individuals in comparison to HIV-negative control subjects. There were no differences in the expression of the NK1R-T in the same groups. In the current study, we quantified the expression of SP precursor mRNA preprotachykinin (TAC1), NK1R (full and truncated forms), viral load (HIV-gag) and several proinflammatory and immune markers (CD4, CCR5, CXCR4, Fractalkine, IL-6, IL-10, CCL2, CCL20, and CD163) in the frontal cortex of autopsied brains from HIV-1 positive individuals with or without HIV associated neuropathology. The expression of SP and, to lesser extent, NK1R-F, were decreased while the expression of CXCR4, CCR5 and CCL2 were increased in CNS of individuals with HIV associated neuropathology. There was no change in HIV loads associated with neuropathology; however, we found a positive correlation between viral loads and the expression of haptoglobin-hemoglobin scavenger receptor CD163. Analysis of CSF from corresponding samples demonstrated an increase in proinflammatory markers (CCL2 MIP-1α and MIP1β) associated with neuropathology. Although our data confirm the overall inflammatory nature of HIV associated neuropathology, we observed a decrease in the expression of SP and NK1R-F, which is also associated with other forms of neuroinflammation.
Keywords: Substance P, Neurokinin-1 receptor, HIV, HIVE, neuropathology, brain
1. Introduction
HIV neuropathology is characterized by changes directly attributed to HIV infection of the central nervous system (CNS) as well as the subsequent changes associated with the inflammatory conditions of the brain, which include the presence of HIV infected macrophages and microglial cells [1–3]. HIV associated neuropathology is the main cause of neurocognitive decline associated with HIV-1 infection [4, 5]. Though prevalence of HIV-associated dementia (HAD) and, to lesser extent, HIV encephalitis (HIVE) has declined in the era of combination antiretroviral therapy (cART) [5–8], HIV replication in CNS during early infection as well as in some individuals treated with cART is likely to contribute to neuropathological and neurocognitive disorders [2]. Neuropathological manifestations of HIV in the CNS include the increased production of multiple pro-inflammatory markers including; the chemokines CCL2 and fractalkine [9–11]; the cytokines TNFα, IL-1, IL-6, GM-CSF [12–16]; the CCR5 and CD163 cellular receptors [17, 18]; free radical nitric oxide [19, 20]; and increased matrix metalloproteinase activity [21, 22].
We previously compared the expression of the two neurokinin-1 receptor (NK1R) isoforms in cingulate cortex of HIV positive individuals and non-infected controls [23]. We demonstrated a decrease in the expression of full-length NK1R (NK1R-F) but not the truncated form of the receptor (NK1R-T) in HIV-positive individuals in comparison to HIV-negative controls. NK1R is a member of the G-protein coupled receptor superfamily and is present in the cells of the nervous system and immune system [24]. A splice variant of the human NK-1R mRNA with a truncated C-terminus has been cloned and identified [24]. The NK1R-F is the predominant form expressed at sites in the human brain, whereas NK1R-T occurs throughout the central nervous system and in peripheral tissues [25]. While it appears that both receptors have equal SP binding properties [26], there are significant differences in the signaling properties between the two isoforms [24, 27–30]. Activation of the SP-NK1R signaling pathway is linked to neurogenic inflammation and neuroinflammation [31–34] with few exceptions [33, 35]. Our previous finding in which NK1R-F expression was decreased in the cingulate cortex of HIV infected individuals was somewhat surprising and led to the hypothesis that the reduced expression of full-length form of NK1R may be significant in deficiencies in cognitive functions associated with neuroAIDS.
In the current study we extend our research on the role of SP-NK1R interactions in neuroAIDS by comparing its expression in CNS of individuals with or without HIV associated neuropathology.
2. Methods
2.1. Subjects and Specimens
Autopsy tissue from the frontal cortex of the brain and cerebrospinal fluid (CSF) specimens from HIV positive individuals were made available through a request to the National NeuroAIDS Tissue Consortium R-187. Histological assessment of brain specimens for diagnoses of HIV-associated neuropathology were made at each of the four NNTC collection sites by board-certified neuropathologists. Standardized protocols and data collection forms were utilized to increase diagnostic reliability between different pathologists. Histological findings for study participants were dichotomized as follows: HIV-NP−: No parenchymal HIV-associated neuropathology. HIV-NP+: Parenchymal HIV-associated neuropathology, including HIV encephalopathy, HIV leukoencephalopathy, and microglial nodular encephalitis. Diagnostic criteria were based upon the 1991 consensus report by Budka et al [1]. Diagnosis of HIVE was based upon histological findings of astrocytosis, multiple disseminated foci of microgliosis, the presence of multinucleated giant cells, and immunohistochemistry for HIV p24 antigen. Diagnosis of HIV leukoencephalopathy included histological findings of microgliosis, astrocytosis, and myelin loss, with or without a presence of multinucleated giant cells. Table 1.
Table 1.
Sample information
| Number of samples (brain) | Number of samples (CSF) | Age at death, y, mean (SD) | Gender M/F | Duration of HIV since diagnosis, mean years (SD) | Nadir CD4 mean cells/μl(SD) | |
|---|---|---|---|---|---|---|
| HIV-NP− | 16 | 9 | 48(9) | 14/2 | 10(6) | 146(153) |
| HIV-NP+ | 19 | 10 | 42(8) | 16/3 | 12(5) | 108(118) |
2.2. RNA extraction and real-time RT PCR
Total RNA was extracted from brain frontal cortex tissues using Tri-Reagent (Molecular Research Center, Cincinnati, OH), treated with RNAse free DNAse (Ambion/Life Technologies, Grand Island, NY) and reverse transcribed (1μg) using an AffinityScript qPCR cDNA synthesis kit (Agilent, Santa Clara, CA) with random primer, all as instructed by the manufacturer. One-tenth of the resulting cDNA was used as a template for real-time PCR amplification using MyiQ iCycler system (Bio-Rad Laboratories, Inc., Hercules, CA). The sequences of the primers and probes used in this study are listed in Table 2.
Table 2.
Primers used for real-time PCR Assays
| Gene | Primers (forward/reverse, 5′-3′) | Detection |
|---|---|---|
| NK1R-F | TCTTCTTCCTCCTGCCCTACATC GCCCAGACGGAACCTGTCAT |
56-FAM-CCAGATCTCTACCTGAAGAAGTTTATCCAGCA-3BHQ1 |
| NK1R-T | TCTTCTTCCTCCTGCCCTACATC TGG AGAGCTCATGGGGTTGGGATCCT |
5′ FAM-CCAGATCTCTACCTGAAGAAGTTTATCCAGCA-3′ BHQ1 |
| TAC-1 (SP) | CGGACCAGTAATTCAGATCATCA GAGGAACCAGAGAAACTCAGC |
56-FAM-CATGTTGGA/ZEN/TTTTGCGACGGACAGT/3IABkFQ |
| CCR5 | CAAAAAGAAGGTCTTCATTACACC CCTGTGCCTCTTCTTCTCATTTCG |
SYBR |
| CD4 | GGAAAGAAAGTGGTGCTGGGCAAA TCCTTGGTCCCAAAGGCTTCTTCT |
SYBR |
| CXCR4 | TGGCCTTATCCTGCCTGGTATTGT AGGAGTCGATGCTGATCCCAATGT |
SYBR |
| Fractalkine | GAGCCGACTCCTTCTTCC CCTCCATCCTGAGCCTTT |
SYBR |
| IL-6 | AGTGAGGAACAAGCCAGAGC GTCAGGGGTGGTTATTGCAT |
SYBR |
| CCL2 (MCP-1) | CTCGCTCAGCCAGATGCAATCAAT TGGAATCCTGAACCCACTTCTGCT |
SYBR |
| CCL20 (MIP3A) | AGTTTGCTCCTGGCTGCTTTGATG CTGCCGTGTGAAGCCCACAATAAA |
SYBR |
| IL-10 | GTGATGCCCCAAGCTGAGA CACGGCCTTGCTCTTGTTTT |
56-FAM-CCAAGACCCAGACATCAAGGCGCA-3BHQ1 |
| CD163 | AAAAGCGAAGACAGAGACAGC ATCATCTGCATTCAGGCAAG |
SYBR |
| HIV-gag [37] | CATGTTTTCAGCATTATCAGAAGGA TGCTTGATGTCCCCCCACT |
56-FAM-CACCCCACAAGATTTAAACACCATGCTAA-3BHQ1 |
| GAPDH | GTGGTCTCCTCTGACTTCAACA TGCTGTAGCCAAATTCGTTG |
56-FAM-CTGGCATTGCCCTCAACGACC-3BHQ1 |
All primers and probes were synthesized by Integrated DNA Technologies, Inc. (Coralville, IA). PCR fragments were cloned into pGEM-T vector (Promega, Madison, WI) and used to generate standard curves for corresponding genes.
The expression of glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used for normalization of gene expression. All amplification reactions were performed in duplicate and an average mRNA quantity is expressed as copy number per 1,000 copies of GAPDH. The specificity of the amplification with SYBR was confirmed by dye dissociation curve.
2.3. Cytometric Bead Array Assay
A flow cytometry-based Cytometric Bead Array (CBA; BD Biosciences, San Diego, CA) assay was used to measure MIP-1α, MIP-1β, MCP-1, IL-6, CCL5 (RANTES), TNFα, γIFN and GM-CSF in CSF. Aliquots of 70μl of CSF were frozen at −70°C until evaluated by Cytometric Bead Array assay according to the manufacturer’s instructions.
2.4. Substance P assay
A modified commercially available antigen competition enzyme immunoassay (EIA) from Cayman Chemical Company (Ann Arbor, MI) was used to quantitate SP, as previously described [36].
2.5. Statistical analysis
The results are expressed as Scatter dot plot and Mean of log10-adjusted values for each individual. Shapiro-Wilkes tests were performed to assess normality of distribution. Evaluation of significant differences between parameters was performed with independent sample t-tests or Mann-Whitney, as appropriate. In all cases, p<0.05 was considered significant. Correlation was determined using Pearson correlation test.
3. Results
The mRNA expression of both SP and NK1R-F was decreased in the frontal cortex of individuals with HIV associated neuropathology, though only the decrease in SP was significant. We also observed an increase in the mRNA expression of the HIV co-receptors CXCR4 and CCR5 as well as the chemokine CCL2. HIV viral load, determined by quantitating HIV-1-gag RNA [37], was unchanged between two groups (Fig. 1). In addition, the mRNA expression levels for NK1R-T, CD4, fractalkine, IL-6, IL-10, CCL20 and CD163 were unchanged (not shown).
Figure 1. RNA levels of SP, NK1R, HIV and immune markers in frontal cortex of subjects from HIV-NP− and HIV-NP+ groups.
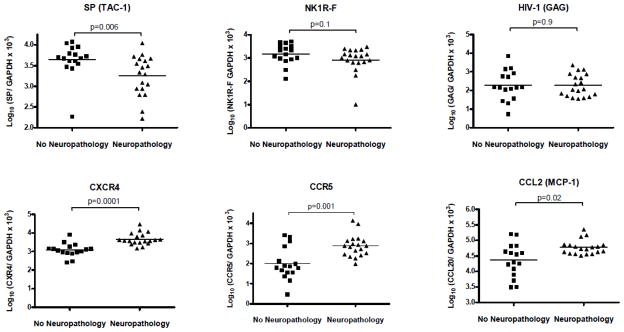
Total RNA was extracted from frontal cortex of HIV-1 infected subjects with (n=19) or without (n=16) HIV-associated neuropathology and assayed for specific RNA levels by real-time RT PCR. RNA levels are expressed as log10 copies number relative to GAPDH mRNA. Results are presented as data for individual samples and mean. Differences in levels of CXCR4 and CCR5 were compared by independent sample t-tests between the two groups. Mann-Whitney tests were performed to compare differences between SP, NK1R-Full, CCL2 and HIV-1 GAG in the two groups.
Analysis of CSF available for selected donors revealed increased levels of MIP-1α, MIP-1β and CCL2 in HIV-NP+ group (Fig. 2). While there was an increasing trend in IL-6 expression, this change was not significant. The levels of SP, TNFα (Fig. 2), RANTES, IFNγ and GM-CSF were unchanged (not shown).
Figure 2. Protein levels of SP and immune markers in CSF of subjects from HIV-NP− and HIV-NP+ groups.
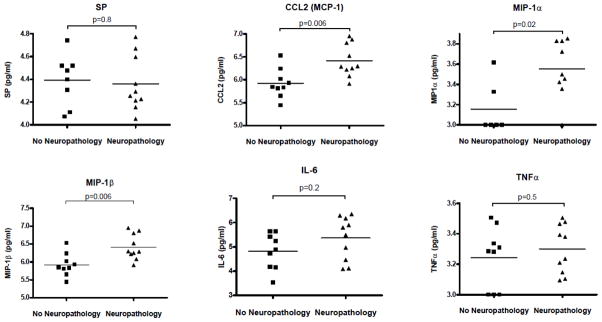
Levels of immune markers were analyzed in CSF of HIV-1 infected subjects with (n=10) or without (n=9) HIV-associated neuropathology using flow cytometry-based Cytometric Bead Array or antigen competition enzyme immunoassay for SP. Data are expressed as log10 pg/ml. Differences between the two groups were assessed for all markers using Mann-Whitney tests.
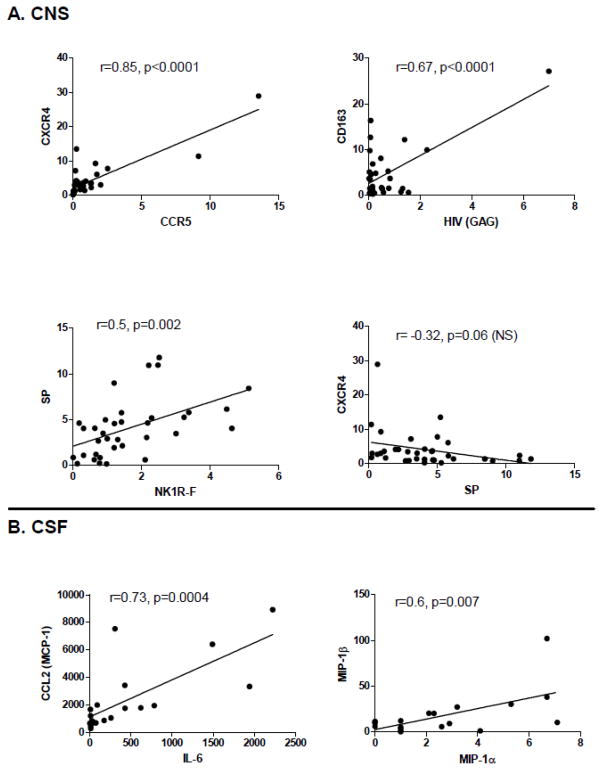
Correlation analysis was performed between markers of inflammation and viral loads in CNS and CSF samples (Fig. 3). A significant correlation in the expression levels was detected between the HIV coreceptors CCR5 and CXCR4 as well as between SP and NK1R-F in CNS. The only marker showing significant correlation with viral load was the haptoglobin-hemoglobin scavenger receptor CD163. A weak negative correlation was detected between the expression of SP and either CXCR4 orCCR5 (only CXCR4 is shown) in CNS (Fig. 3A). A significant correlation was also detected between CCL2 and IL-6 and between MIP-1α and MIP-1β in CSF. (Fig. 3B).
Figure 3. Correlations of gene expression in CNS and CSF of HIV-1 infected subjects.
Correlation analysis between markers in CNS (n=35) and CSF (n=19) of HIV-1 infected subjects was performed using Pearson correlation test. Correlation coefficient (r) and p value are shown, along with a linear regression line.
4. Discussion
We found evidence of inflammation in the frontal cortex and the CSF of individuals with HIV-associated neuropathology which is consistent with previous reports (reviewed [38]). In our cohort, we detected an increased level of CCL2 in both the CNS and the CSF of individuals with HIV-associated neuropathology. The CCL2 chemokine is most commonly associated with HIV infection, CNS viral invasion and viral neuropathology [9, 11, 39]. We also detected a neuropathology-associated increase in the expression of the HIV-1 co-receptors CCR5 and CXCR4 but not CD4. There was a significant correlation between an increase in CCR5 and an increase CXCR4 in the CNS suggesting a common mechanism driving the expression of both HIV co-receptors in individual samples.
Previous studies suggest that macrophage infiltration and viral replication correlate with brain dysfunction in HIV infected individuals [2, 40]. We were unable to detect an increase in either the viral load or CD163 receptor expression, a cell surface marker expressed exclusively on cells of monocyte-macrophage lineage, associated with HIV neuropathology. We did, however, observe a correlation between viral RNA and the expression of CD163 in brain samples. CD163 plays a significant role in HIV infection. Elevated expression of CD163 is associated with HIV infection of monocytes and macrophages in vivo and in vitro and the plasma levels of soluble CD163 may serve as a marker of chronic HIV infection and NeuroAIDS [41–44]. This finding supports previous observations that HIV replicates in the CNS in cells predominantly of monocytes-macrophage origin.
Contrary to proinflammatory markers, the levels of SP and, to the lesser extent, NK1R-F were reduced in brain tissue from donors with HIV associated neuropathology. A significant correlation between the expressions of those two molecules suggests a coordinated decrease in their expression in individual samples. Although there is no direct proof that SP-NK1R interactions are involved in neuroAIDS several lines of evidence indicate that disruption of SP-NK1R signaling may contribute to HIV infection of the CNS. The present finding is consistent with our previous results which demonstrate a decrease in the expression of the NK1R-F but not in NK1R-T in HIV positive individuals in comparison to HIV negative controls [23]. It is also consistent with a report that the expression of the SP precursor, TAC1, is decreased in frontal cortex of patients with HIVE in comparison to HIV negative controls (B. B. Gelman, personal communication CROI, Poster E167, Boston, MA, 2-28-2011). In another study involving an SIV model, we demonstrated an increased expression of SP and NK1-R in SIVE lesions, with macrophages positive for CD163 and SIV being the principal cells expressing NK1-R [45]. The apparent discrepancy between the Rhesus model and human patients may be explained by the fact that in the Rhesus model, we assayed tissue from animals with acute HIVE while the human samples reflect a broader, chronic neuropathology. We were unable to detect any increase in either HIV RNA or CD163 in samples with HIV associated neuropathology in comparison to HIV positive individuals without neuropathology consistent with absence of acute infection/inflammation in HIV-NP+ group. The accumulation of damage to neurons and other CNS resident cells may result in the loss of both NK1R and SP expression, which negates any increases associated with chronic CNS inflammation.
Recently, we demonstrated that SP enhances HIV-1 infection in human fetal brain cell cultures expressing full-length neurokinin-1 receptor [46]. Previously, we also reported an increase in blood SP levels in men and women associated with HIV infection [47, 48]. This increase may be related to elevated SP production by both peripheral neurons and cells of non-neuronal origin in response to the chronic inflammation associated with HIV infection. It was also suggested that substance P plays a major pathogenetic role in HIV infection of the CNS by increasing permeability of brain endothelium exposed to HIV-1 gp120 [49, 50].
Decreased SP levels in the CNS may be related to neuronal damage and dysfunction possibly contributing to the cognitive deficiencies associated with neuroAIDS. SP-NK1R interactions may play an important role in stress, depression and drug-induced cognitive dysfunction (reviewed [51]. One possible mechanism by which the expression of SP could be regulated involves the transcription factor ΔFosB [52–54]. Additional evidence suggesting that tachykinins may be involved in the control of psychiatric disorders was provided by Bardelli et al. [55]. This study demonstrated that the protein levels of NK1R were significantly decreased while the levels of NK2R were increased compared to healthy subjects in monocytes from patients with major depressive disorder under stable antidepressant therapy.
Overall this study demonstrates that, despite clear evidence that inflammation is present in CNS of patients with HIV associated neuropathology, the expression of SP and, to lesser extent, NK1R-F are decreased. Our results justify further research of interaction between HIV, neuropeptides and proinflammatory factors which may lead to future therapeutic strategies targeting neurokinin receptors in neuroAIDS.
Acknowledgments
We thank Richard Tustin, Eric Reidel, Nguyen Ngoc and Angela Winters for technical help. We thank Christa Heyward for editorial assistance.
This work was supported by National Institutes of Health grants R01-MH049981, P01-MH076388, and 1UO1 MH-090325 to S.D.D. Brain tissue specimens, data, and support were provided by the National NeuroAIDS Tissue Consortium (request R187). This publication was made possible from NIH funding through the NIMH and NINDS Institutes by the following grants:
Manhattan HIV Brain Bank: U01MH083501, R24MH59724
Texas NeuroAIDS Research Center U01MH083507, R24 NS45491
National Neurological AIDS Bank 5U01MH083500, NS 38841
California NeuroAIDS Tissue Network U01MH083506, R24MH59745
Statistics and Data Coordinating Center U01MH083545, N01MH32002
Its contents are solely the responsibility of the authors and do not necessarily represent the official view of the NNTC or NIH.
Footnotes
Conflict of interest
The authors declare that they have no competing interests
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Budka H, Wiley CA, Kleihues P, Artigas J, Asbury AK, Cho ES, et al. HIV-associated disease of the nervous system: review of nomenclature and proposal for neuropathology-based terminology. Brain Pathol. 1991;1(3):143–52. doi: 10.1111/j.1750-3639.1991.tb00653.x. [DOI] [PubMed] [Google Scholar]
- 2.Gelman BB, Lisinicchia JG, Morgello S, Masliah E, Commins D, Achim CL, et al. Neurovirological correlation with HIV-associated neurocognitive disorders and encephalitis in a HAART-era cohort. J Acquir Immune Defic Syndr. 2012 Dec 13; doi: 10.1097/QAI.0b013e31827f1bdb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Everall I, Vaida F, Khanlou N, Lazzaretto D, Achim C, Letendre S, et al. Cliniconeuropathologic correlates of human immunodeficiency virus in the era of antiretroviral therapy. J Neurovirol. 2009;15(5–6):360–70. doi: 10.3109/13550280903131915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cherner M, Masliah E, Ellis RJ, Marcotte TD, Moore DJ, Grant I, et al. Neurocognitive dysfunction predicts postmortem findings of HIV encephalitis. Neurology. 2002;59(10):1563–7. doi: 10.1212/01.wnl.0000034175.11956.79. [DOI] [PubMed] [Google Scholar]
- 5.Morgello S, Mahboob R, Yakoushina T, Khan S, Hague K. Autopsy findings in a human immunodeficiency virus-infected population over 2 decades: influences of gender, ethnicity, risk factors, and time. Arch Pathol Lab Med. 2002;126(2):182–90. doi: 10.5858/2002-126-0182-AFIAHI. [DOI] [PubMed] [Google Scholar]
- 6.Sacktor N, Lyles RH, Skolasky R, Kleeberger C, Selnes OA, Miller EN, et al. HIV-associated neurologic disease incidence changes:: Multicenter AIDS Cohort Study, 1990–1998. Neurology. 2001;56(2):257–60. doi: 10.1212/wnl.56.2.257. [DOI] [PubMed] [Google Scholar]
- 7.Jellinger KA, Setinek U, Drlicek M, Bohm G, Steurer A, Lintner F. Neuropathology and general autopsy findings in AIDS during the last 15 years. Acta Neuropathol. 2000;100(2):213–20. doi: 10.1007/s004010000245. [DOI] [PubMed] [Google Scholar]
- 8.Gray F, Chretien F, Vallat-Decouvelaere AV, Scaravilli F. The changing pattern of HIV neuropathology in the HAART era. J Neuropathol Exp Neurol. 2003 May;62(5):429–40. doi: 10.1093/jnen/62.5.429. [DOI] [PubMed] [Google Scholar]
- 9.Dhillon NK, Williams R, Callen S, Zien C, Narayan O, Buch S. Roles of MCP-1 in development of HIV-dementia. Front Biosci. 2008;13:3913–8. doi: 10.2741/2979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tong N, Perry SW, Zhang Q, James HJ, Guo H, Brooks A, et al. Neuronal fractalkine expression in HIV-1 encephalitis: roles for macrophage recruitment and neuroprotection in the central nervous system. J Immunol. 2000;164(3):1333–9. doi: 10.4049/jimmunol.164.3.1333. [DOI] [PubMed] [Google Scholar]
- 11.Eugenin EA, Osiecki K, Lopez L, Goldstein H, Calderon TM, Berman JW. CCL2/monocyte chemoattractant protein-1 mediates enhanced transmigration of human immunodeficiency virus (HIV)-infected leukocytes across the blood-brain barrier: a potential mechanism of HIV-CNS invasion and NeuroAIDS. J Neurosci. 2006;26(4):1098–106. doi: 10.1523/JNEUROSCI.3863-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wesselingh SL, Takahashi K, Glass JD, McArthur JC, Griffin JW, Griffin DE. Cellular localization of tumor necrosis factor mRNA in neurological tissue from HIV-infected patients by combined reverse transcriptase/polymerase chain reaction in situ hybridization and immunohistochemistry. J Neuroimmunol. 1997;74(1–2):1–8. doi: 10.1016/s0165-5728(96)00160-9. [DOI] [PubMed] [Google Scholar]
- 13.Brabers NA, Nottet HS. Role of the pro-inflammatory cytokines TNF-alpha and IL-1beta in HIV-associated dementia. Eur J Clin Invest. 2006;36(7):447–58. doi: 10.1111/j.1365-2362.2006.01657.x. [DOI] [PubMed] [Google Scholar]
- 14.Benveniste EN. Cytokine circuits in brain. Implications for AIDS dementia complex. Res Publ Assoc Res Nerv Ment Dis. 1994;72:71–88. [PubMed] [Google Scholar]
- 15.Yadav A, Collman RG. CNS inflammation and macrophage/microglial biology associated with HIV-1 infection. J Neuroimmune Pharmacol. 2009;4(4):430–47. doi: 10.1007/s11481-009-9174-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kraft-Terry SD, Stothert AR, Buch S, Gendelman HE. HIV-1 neuroimmunity in the era of antiretroviral therapy. Neurobiol Dis. 2010;37(3):542–8. doi: 10.1016/j.nbd.2009.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gelman BB, Chen T, Lisinicchia JG, Soukup VM, Carmical JR, Starkey JM, et al. The National NeuroAIDS Tissue Consortium brain gene array: two types of HIV-associated neurocognitive impairment. PLoS One. 2012;7(9):e46178. doi: 10.1371/journal.pone.0046178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yuferov V, Ho A, Morgello S, Yang Y, Ott J, Kreek MJ. Expression of Ephrin Receptors and Ligands in Postmortem Brains of HIV-Infected Subjects With and Without Cognitive Impairment. J Neuroimmune Pharmacol. 2013;8(1):333–44. doi: 10.1007/s11481-012-9429-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu X, Jana M, Dasgupta S, Koka S, He J, Wood C, et al. Human immunodeficiency virus type 1 (HIV-1) tat induces nitric-oxide synthase in human astroglia. J Biol Chem. 2002;277(42):39312–9. doi: 10.1074/jbc.M205107200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Polazzi E, Levi G, Minghetti L. Human immunodeficiency virus type 1 Tat protein stimulates inducible nitric oxide synthase expression and nitric oxide production in microglial cultures. J Neuropathol Exp Neurol. 1999;58(8):825–31. doi: 10.1097/00005072-199908000-00005. [DOI] [PubMed] [Google Scholar]
- 21.Ragin AB, Wu Y, Ochs R, Du H, Epstein LG, Conant K, et al. Marked relationship between matrix metalloproteinase 7 and brain atrophy in HIV infection. J Neurovirol. 2011;17(2):153–8. doi: 10.1007/s13365-011-0018-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ragin AB, Wu Y, Ochs R, Scheidegger R, Cohen BA, McArthur JC, et al. Serum matrix metalloproteinase levels correlate with brain injury in human immunodeficiency virus infection. J Neurovirol. 2009;15(3):275–81. doi: 10.1080/13550280902913271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Douglas SD, Lynch KG, Lai JP. Neurokinin-1 receptor mRNA expression differences in brains of HIV-infected individuals. J Neurol Sci. 2008;272(1–2):174–7. doi: 10.1016/j.jns.2008.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fong TM, Anderson SA, Yu H, Huang RR, Strader CD. Differential activation of intracellular effector by two isoforms of human neurokinin-1 receptor. Mol Pharmacol. 1992;41(1):24–30. [PubMed] [Google Scholar]
- 25.Caberlotto L, Hurd YL, Murdock P, Wahlin JP, Melotto S, Corsi M, et al. Neurokinin 1 receptor and relative abundance of the short and long isoforms in the human brain. European Journal of Neuroscience. 2003;17(9):1736–46. doi: 10.1046/j.1460-9568.2003.02600.x. [DOI] [PubMed] [Google Scholar]
- 26.Tuluc F, Lai JP, Kilpatrick LE, Evans DL, Douglas SD. Neurokinin 1 receptor isoforms and the control of innate immunity. Trends Immunol. 2009;30(6):271–6. doi: 10.1016/j.it.2009.03.006. [DOI] [PubMed] [Google Scholar]
- 27.Bohm SK, Khitin LM, Smeekens SP, Grady EF, Payan DG, Bunnett NW. Identification of potential tyrosine-containing endocytic motifs in the carboxyl-tail and seventh transmembrane domain of the neurokinin 1 receptor. J Biol Chem. 1997;272(4):2363–72. doi: 10.1074/jbc.272.4.2363. [DOI] [PubMed] [Google Scholar]
- 28.Li H, Leeman SE, Slack BE, Hauser G, Saltsman WS, Krause JE, et al. A substance P (neurokinin-1) receptor mutant carboxyl-terminally truncated to resemble a naturally occurring receptor isoform displays enhanced responsiveness and resistance to desensitization. Proc Natl Acad Sci U S A. 1997;94(17):9475–80. doi: 10.1073/pnas.94.17.9475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sasakawa N, Sharif M, Hanley MR. Attenuation of agonist-induced desensitization of the rat substance P receptor by progressive truncation of the C-terminus. FEBS Lett. 1994;347(2–3):181–4. doi: 10.1016/0014-5793(94)00532-x. [DOI] [PubMed] [Google Scholar]
- 30.Lai JP, Lai S, Tuluc F, Tansky MF, Kilpatrick LE, Leeman SE, et al. Differences in the length of the carboxyl terminus mediate functional properties of neurokinin-1 receptor. Proc Natl Acad Sci U S A. 2008;105(34):12605–10. doi: 10.1073/pnas.0806632105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thornton E, Vink R. Treatment with a substance P receptor antagonist is neuroprotective in the intrastriatal 6-hydroxydopamine model of early Parkinson’s disease. PLoS One. 2012;7(4):e34138. doi: 10.1371/journal.pone.0034138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chauhan VS, Kluttz JM, Bost KL, Marriott I. Prophylactic and therapeutic targeting of the neurokinin-1 receptor limits neuroinflammation in a murine model of pneumococcal meningitis. J Immunol. 2011;186(12):7255–63. doi: 10.4049/jimmunol.1100721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kennedy PG, Rodgers J, Bradley B, Hunt SP, Gettinby G, Leeman SE, et al. Clinical and neuroinflammatory responses to meningoencephalitis in substance P receptor knockout mice. Brain. 2003;126(Pt 7):1683–90. doi: 10.1093/brain/awg160. [DOI] [PubMed] [Google Scholar]
- 34.Salisbury E, Rodenberg E, Sonnet C, Hipp J, Gannon FH, Vadakkan TJ, et al. Sensory nerve induced inflammation contributes to heterotopic ossification. J Cell Biochem. 2011;112(10):2748–58. doi: 10.1002/jcb.23225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jiang MH, Chung E, Chi GF, Ahn W, Lim JE, Hong HS, et al. Substance P induces M2-type macrophages after spinal cord injury. Neuroreport. 2012;23(13):786–92. doi: 10.1097/WNR.0b013e3283572206. [DOI] [PubMed] [Google Scholar]
- 36.Campbell DE, Bruckner P, Tustin NB, Tustin R, 3rd, Douglas SD. Novel method for determination of substance P levels in unextracted human plasma by using acidification. Clin Vaccine Immunol. 2009;16(4):594–6. doi: 10.1128/CVI.00406-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Palmer S, Wiegand AP, Maldarelli F, Bazmi H, Mican JM, Polis M, et al. New real-time reverse transcriptase-initiated PCR assay with single-copy sensitivity for human immunodeficiency virus type 1 RNA in plasma. J Clin Microbiol. 2003;41(10):4531–6. doi: 10.1128/JCM.41.10.4531-4536.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gelman BB, Moore DJ. HIV-1 Neuropathology. In: Gendelman HE, Grant I, Everall IP, Fox HS, Gelbard HA, Lipton SA, Swindells S, editors. The Neurology of AIDS. Oxford: 2012. pp. 518–35. [Google Scholar]
- 39.Ansari AW, Heiken H, Meyer-Olson D, Schmidt RE. CCL2: a potential prognostic marker and target of anti-inflammatory strategy in HIV/AIDS pathogenesis. Eur J Immunol. 2011;41(12):3412–8. doi: 10.1002/eji.201141676. [DOI] [PubMed] [Google Scholar]
- 40.Glass JD, Fedor H, Wesselingh SL, McArthur JC. Immunocytochemical quantitation of human immunodeficiency virus in the brain: correlations with dementia. Ann Neurol. 1995;38(5):755–62. doi: 10.1002/ana.410380510. [DOI] [PubMed] [Google Scholar]
- 41.Burdo TH, Lentz MR, Autissier P, Krishnan A, Halpern E, Letendre S, et al. Soluble CD163 made by monocyte/macrophages is a novel marker of HIV activity in early and chronic infection prior to and after anti-retroviral therapy. J Infect Dis. 2011;204(1):154–63. doi: 10.1093/infdis/jir214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tippett E, Cheng WJ, Westhorpe C, Cameron PU, Brew BJ, Lewin SR, et al. Differential expression of CD163 on monocyte subsets in healthy and HIV-1 infected individuals. PLoS One. 2011;6(5):e19968. doi: 10.1371/journal.pone.0019968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Burdo TH, Lo J, Abbara S, Wei J, DeLelys ME, Preffer F, et al. Soluble CD163, a novel marker of activated macrophages, is elevated and associated with noncalcified coronary plaque in HIV-infected patients. J Infect Dis. 2011;204(8):1227–36. doi: 10.1093/infdis/jir520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Subramanian S, Tawakol A, Burdo TH, Abbara S, Wei J, Vijayakumar J, et al. Arterial inflammation in patients with HIV. JAMA. 2012;308(4):379–86. doi: 10.1001/jama.2012.6698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vinet-Oliphant H, Alvarez X, Buza E, Borda JT, Mohan M, Aye PP, et al. Neurokinin-1 receptor (NK1-R) expression in the brains of SIV-infected rhesus macaques: implications for substance P in NK1-R immune cell trafficking into the CNS. Am J Pathol. 2010;177(3):1286–97. doi: 10.2353/ajpath.2010.091109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schwartz L, Spitsin SV, Meshki J, Tuluc F, Douglas SD, Wolfe JH. Substance P enhances HIV-1 infection in human fetal brain cell cultures expressing full-length neurokinin-1 receptor. J Neurovirol. 2013;19(3):219–27. doi: 10.1007/s13365-013-0166-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Douglas SD, Cnaan A, Lynch KG, Benton T, Zhao H, Gettes DR, et al. Elevated substance P levels in HIV-infected women in comparison to HIV-negative women. AIDS Res Hum Retroviruses. 2008;24(3):375–8. doi: 10.1089/aid.2007.0207. [DOI] [PubMed] [Google Scholar]
- 48.Douglas SD, Ho WZ, Gettes DR, Cnaan A, Zhao H, Leserman J, et al. Elevated substance P levels in HIV-infected men. AIDS. 2001;15(15):2043–5. doi: 10.1097/00002030-200110190-00019. [DOI] [PubMed] [Google Scholar]
- 49.Annunziata P, Cioni C, Santonini R, Paccagnini E. Substance P antagonist blocks leakage and reduces activation of cytokine-stimulated rat brain endothelium. J Neuroimmunol. 2002;131(1–2):41–9. doi: 10.1016/s0165-5728(02)00262-x. [DOI] [PubMed] [Google Scholar]
- 50.Annunziata P, Cioni C, Toneatto S, Paccagnini E. HIV-1 gp120 increases the permeability of rat brain endothelium cultures by a mechanism involving substance P. AIDS. 1998;12(18):2377–85. doi: 10.1097/00002030-199818000-00006. [DOI] [PubMed] [Google Scholar]
- 51.Douglas SD, Lai JP, Tuluc F, Schwartz L, Kilpatrick LE. Neurokinin-1 receptor expression and function in human macrophages and brain: perspective on the role in HIV neuropathogenesis. Ann N Y Acad Sci. 2008;1144:90–6. doi: 10.1196/annals.1418.007. [DOI] [PubMed] [Google Scholar]
- 52.Berton O, Covington HE, 3rd, Ebner K, Tsankova NM, Carle TL, Ulery P, et al. Induction of deltaFosB in the periaqueductal gray by stress promotes active coping responses. Neuron. 2007;55(2):289–300. doi: 10.1016/j.neuron.2007.06.033. [DOI] [PubMed] [Google Scholar]
- 53.Vialou V, Robison AJ, Laplant QC, Covington HE, 3rd, Dietz DM, Ohnishi YN, et al. DeltaFosB in brain reward circuits mediates resilience to stress and antidepressant responses. Nat Neurosci. 2010;13(6):745–52. doi: 10.1038/nn.2551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Winstanley CA, LaPlant Q, Theobald DE, Green TA, Bachtell RK, Perrotti LI, et al. DeltaFosB induction in orbitofrontal cortex mediates tolerance to cocaine-induced cognitive dysfunction. J Neurosci. 2007;27(39):10497–507. doi: 10.1523/JNEUROSCI.2566-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bardelli C, Amoruso A, Manzetti E, Fresu LG, Valsesia R, Zeppegno P, et al. Recurrent major depressive disorder: Imbalance of neurokinin (NK)-1 and NK-2 receptor expression in monocytes. Pharmacol Res. 2013;68(1):24–30. doi: 10.1016/j.phrs.2012.10.022. [DOI] [PubMed] [Google Scholar]