Abstract
Dentin sialophosphoprotein (DSPP) plays a vital role in dentinogenesis. Previously, we showed that, in addition to dentin, DSPP is also highly expressed in the alveolar bone and cellular cementum, and plays a crucial role in maintaining periodontal integrity; Dspp deficient mice demonstrate severe periodontal defects including alveolar bone loss, decreased cementum deposition, abnormal osteocyte morphology in the alveolar bone and apical migration of periodontal ligament. DSPP in dentin and bone is cleaved into NH2-terminal and COOH-terminal fragments. While our previous study showed that the proteolytic processing of DSPP is critical for dentinogenesis, it is unclear whether the posttranslational cleavage of DSPP also plays an essential role in maintaining a healthy periodontium. In this study, we analyzed the periodontal tissues in transgenic mice expressing the uncleavable full-length DSPP in the Dspp knockout (Dspp-KO) background (named “Dspp-KO/D452A-Tg mice”), in comparison with those in the wild-type mice, Dspp-KO mice, and mice expressing the normal DSPP transgene in the Dspp-KO background (designated “Dspp-KO/normal-Tg mice”). We found that transgenic expression of the normal DSPP fully rescued the periodontal defects of the Dspp-KO mice, whereas expressing Dspp-KO/D452A-Tg failed to do so. These results indicate that proteolytic processing of DSPP is essential to periodontal integrity.
Keywords: Alveolar bone, cementoblast, dentin, periodontal ligament, periodontal disease
Dentin sialophosphoprotein (DSPP), first discovered by cDNA cloning in 1997, plays a crucial role in dentinogenesis (1). It undergoes post-translational modification and is cleaved into the NH2-terminal fragments (existing in two forms: dentin sialoprotein (DSP) and the proteoglycan form termed DSP-PG) and COOH-terminal fragment (dentin phosphoprotein (DPP)) (1-3). Genetic studies have shown the association of DSPP mutations with dentinogenesis imperfecta (DGI) in humans (4-10). In animal models, Dspp-knock out (Dspp-KO) mice exhibit severe dentin hypomineralization defects with widened predentin zone resembling the dentin defects observed in human DGI (11). These findings strongly suggest the importance of DSPP in the formation and mineralization of dentin.
Besides its established role in tooth formation, DSPP was also discovered in bone, cellular cementum and several non-mineralized tissues (12-20). Its expression in alveolar bone and cellular cementum is remarkably higher than in the long bone (14,19). Recent work from our laboratory showed that DSPP plays an essential role in these periodontal tissues (21). In Dspp deficient mice we observed severe alveolar bone loss with reduced cementum deposition and altered osteocyte morphology (21). The reduction in interdental alveolar bone caused apical migration of periodontal ligaments (PDL) leading to periodontal pockets (21). In vivo studies done by our group showed that proteolytic processing of DSPP is vital for its function in dentinogenesis (22). However, whether the same mechanism applies in periodontal tissues still remains to be tested. For this purpose, we systematically characterized the alveolar bone and cementum of the following groups of mice: 1) mice expressing the uncleavable D452A-DSPP in the Dspp-KO background (named as Dspp-KO/D452A-Tg mice); 2) Dspp-KO mice; 3) Wild-type (WT) mice and; 4) mice expressing the normal DSPP transgene in the Dspp–KO background (referred as Dspp-KO/normal-Tg mice). The results from this study showed that expression of normal DSPP was successful in rescuing the periodontal defects of Dspp-KO mice. However the transgenic expression of D452A-DSPP was unable to rescue the Dspp deficient defects. The findings from from this study provide in vivo evidence that the proteolytic processing of DSPP is necessary for maintaining the health of periodontal tissues.
Material and methods
Mouse generation
Detailed description for the generation of transgenic mice used in this study has been described in our recent reports (22,23). Briefly, the D452A-DSPP and the normal DSPP constructs are downstream to the 3.6-kb rat Col 1a1 promoter. The mouse lines that were crossbred with Dspp-KO mice (strain name: B6; 129-Dspptm1Kul/Mmnc; MMRRC, UNC, Chapel Hill, NC, USA) and characterized in our recent study by Zhu et al. (22) were used in this investigation. The mice expressing the D452A-DSPP transgene in the Dspp-KO background are referred to as Dspp-KO/D452A-Tg mice while those expressing the normal DSPP transgene in the Dspp-KO background are called Dspp-KO/normal-Tg mice. Primers used for genotyping the transgenic and Dspp knockout mice have been described previously (22). The animal protocols used in this study were approved by the Animal Welfare Committee of Texas A&M University, Baylor College of Dentistry (Dallas, TX, USA). In this study we analyzed the alveolar bone and cementum of the following four groups of mice: Dspp-KO/D452A-Tg mice, WT (C57/BL6J) mice, Dspp-KO mice and Dspp-KO/normal-Tg mice.
Histology
Under anesthesia, 3- and 6-months-old Dspp-KO/ D452A-Tg, WT, Dspp-KO and Dspp-KO/normal-Tg mice were perfused from the ascending aorta with 4% paraformaldehyde in 0.1 M phosphate buffer. The dissected mandibles were then fixed in the same fixative for 24 h, and then decalcified for approximately 2 wk using the protocols as previously described (22). The tissues were then embedded in paraffin, and serial sections of 5 μm were prepared and stained with hematoxylin & eosin (H&E).
Micro-computed tomography
Samples from the above mentioned four groups of mice at 3- and 6-months of age were analyzed by micro-computed tomography (μ-CT) using a μ-CT35 imaging system (Scanco Medical, Bassersdorf, Switzerland). A high-resolution scan (3.5 μm slice increment) of the alveolar bone in the furcation of the first mandibular molar with a fixed dimension for all samples was performed. The data acquired from five samples per group were used for quantitative analyses using the Student's t-test. p < 0.05 was considered statistically significant, and the data are presented as mean ± SD.
Backscattered and resin-cast scanning electron microscopy
The mandibles from the four groups of mice at 3-months of age were dissected and prepared as previously described (21). For backscattered scanning electron microscopy (SEM), the specimens were coated with carbon and examined with a FEI/Philips XL30 Field emission environmental SEM (Philips, Hillsboro, OR, USA). Following this, the surface was acid etched (21), coated with gold and palladium, and examined with SEM.
Results
Failure to rescue the alveolar bone defects by D452A-DSPP transgene
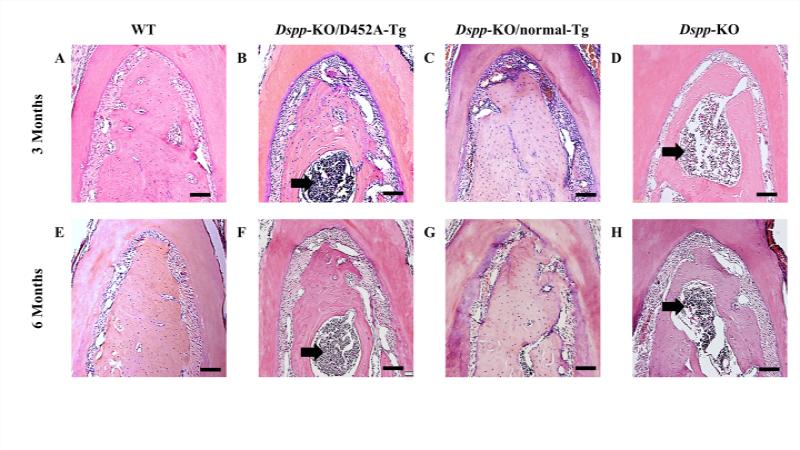
Results from the histological analyses showed severe alveolar bone loss with marked increase in inflammatory cells in the furcation region of the first mandibular molar in Dspp-KO mice at 3- and 6-months of age (Fig. 1D, H), when compared to the WT samples of the same ages (Fig. 1A, E). The transgenic expression of D452A-DSPP was unable to rescue these alveolar bone defects (Fig. 1B, F) and resembled those of the Dspp-KO mice (Fig. 1D, H). However, the alveolar bone of Dspp-KO/ normal-Tg mice at both ages (Fig. 1C, G) appeared similar to the WT samples (Fig. 1A, E) and hence, the transgenic expression of the normal DSPP was able to reverse the Dspp deficient alveolar bone defects to normal.
Figure 1. Failure to process DSPP into fragments leads to alveolar bone defects.
H&E staining of alveolar bone at both 3- and 6-months of age, showed that the Dspp-KO (D and H) and Dspp-KO/D452A-Tg mice (B and F) had significant alveolar bone loss in the furcation region of the first mandibular molar with inflammation (black arrows), compared to the WT mice (A and E). The transgenic expression of normal DSPP (C and G, Dspp-KO/normal-Tg mice) completely rescued the alveolar bone defects of Dspp-KO mice; the alveolar bone Dspp-KO/normal-Tg mice (C and G) appeared similar to those of the WT mice (A and E). Bar: 100 μm
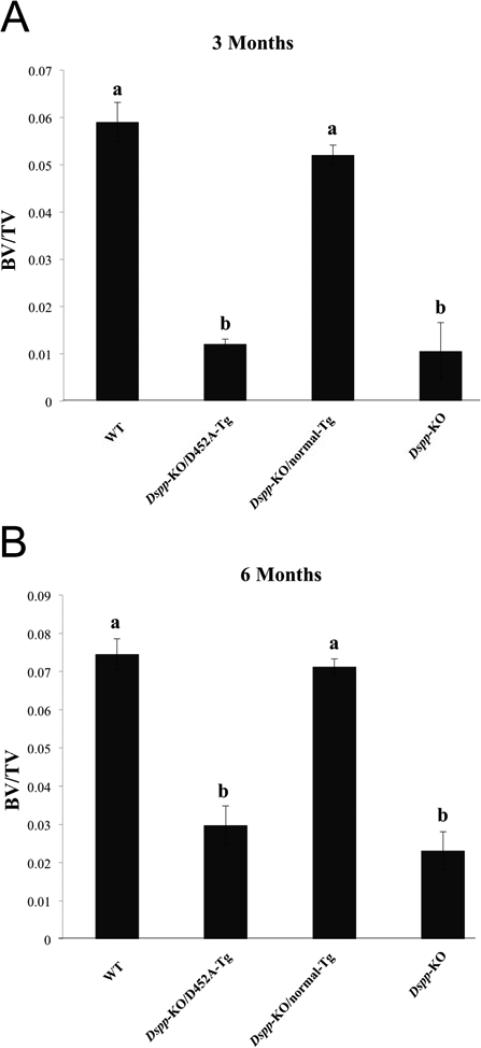
Results from the μ-CT analyses (Fig. 2A, B) also showed that the transgenic expression of normal DSPP was able to revert the bone volume fraction of Dspp-KO mice to a level comparable to the WT mice at both 3-months (Fig. 2A) and 6-months (Fig. 2B) of age, whereas, the expression of D452A-DSPP was unable to achieve the same result.
Figure 2. Quantitative μ-CT analyses.
The quantitative μ-CT analyses of inter-radicular alveolar bone in the 3-month-old (A) and 6-month-old (B) mice showed that transgenic expression of normal DSPP transgene was able to reverse the bone volume fraction of Dspp-KO mice at both ages to a level comparable to WT mice. There was no statistically significant difference between the bone volume fraction of the WT (indicated by “a”) and Dspp-KO/normal-Tg mice (indicated by “a”). The Dspp-KO/D452A-Tg mice (marked as “b”) showed no significant improvement over Dspp-KO mice (marked as “b”) at either age. P < 0.05 (Student's t-test) was considered statistically significant. The differences between a and b were statistically significant (p < 0.05). The differences between a and a, or between b and b, were not statistically significant (p >0.05). The data represent mean ± SD (n = 5).
Reversal of periodontal defects by normal DSPP but not by D452A-DSPP transgene
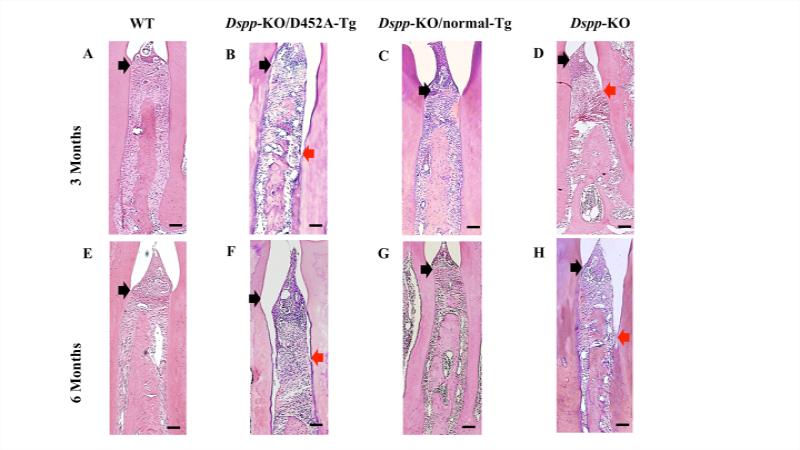
In the interdental regions between the first and second mandibular molars, the WT (Fig. 3A, E) and Dspp-KO/ normal-Tg mice (Fig. 3C, G) showed epithelial attachment at the cemento-enamel junction (CEJ) with healthy interdental alveolar bone deposition at both 3- and 6-months of age. However, in the Dspp-KO (Fig. 3D, H) and Dspp-KO/ D452ATg mice (Fig. 3B, F), the H&E staining showed a remarkable loss of alveolar bone and disruption of periodontal ligaments interdentally, along with the apical migration of the junctional epithelium. The defects in the Dspp-KO (Fig. 3H) and Dspp-KO/ D452A-Tg mice (Fig. 3F) appeared worse at 6-months of age with the epithelial attachment recessing even more apically than at the age of 3 months.
Figure 3. Failure to process DSPP into fragments leads to loss of epithelium attachment in the interdental region.
At 3- and 6-months of age, H & E staining showed that the interdental epithelial attachment between the first and second mandibular molars of the WT (A and E) and Dspp-KO/normal-Tg mice (C and G) was at the cemento-enamel junction (CEJ) (indicated with a black arrow), whereas the Dspp-KO (D and H) and Dspp-KO/D452A-Tg mice (B and F) showed alveolar bone loss with an apically recessed epithelial attachment (black arrows denote the CEJ; red arrows indicate the actual epithelial attachment). Bar: 100 μm.
D452A-DSPP transgene had no effects on the reduced cellular cementum of Dspp-KO mice
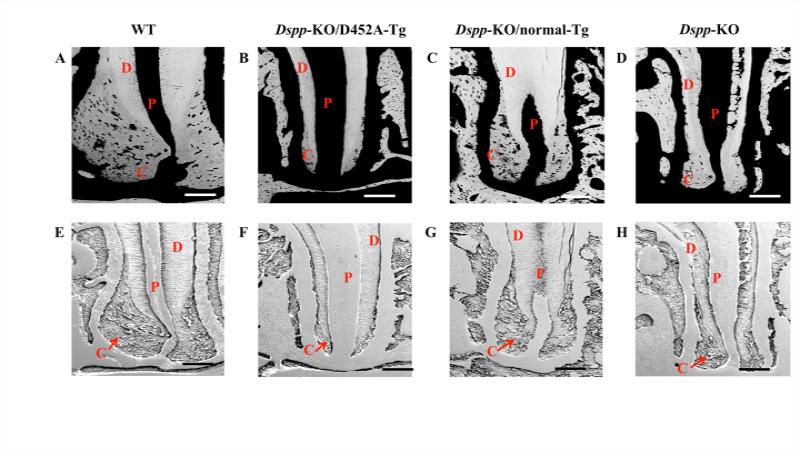
Using backscattered and resin-cast SEM (Fig. 4), we observed an overall loss of cellular cementum in the apical region of mandibular first molar in the 3-month-old Dspp-KO(Fig. 4D, H) and Dspp-KO/ D452A-Tg mice (Fig. 4B, F), when compared with the WT mice of the same ages (Fig. 4A, E). The transgenic expression of normal DSPP in the Dspp-KO/ normal-Tg mice (Fig. 4C, G) was able to rescue the loss of cellular cementum to a great extent. The cellular cementum deposition in the Dspp-KO/ normal-Tg mice (Fig. 4C, G) still showed some differences from the WT sample (Fig. 4A, E), but was markedly improved when compared with the Dspp-KO (Fig. 4D, H) and Dspp-KO/ D452A-Tg mice (Fig. 4B, F).
Figure 4. Failure to process DSPP into fragments leads to decreased cellular cementum deposition.
In these backscattered SEM (A-D) and resin-casted SEM (E-H) images, the dentin is denoted as “D”; pulp as “P” and cementum as “C”. In the backscattered SEM images, the black areas represent unmineralized or hypomineralized areas. Therefore, the pulpal space appears as black (denoted as P); Cementum (C) is less mineralized than Dentin (D) and appears darker than dentin. The Dspp-KO (D) and Dspp-KO/D452A-Tg mice (B) showed increased black areas periapically indicating decreased cellular cementum deposition compared to the WT (A) and Dspp-KO/normal-Tg mice (C). The resin-casted SEM gave a better visualization of the periapical region of cementum.. The WT (E) and Dspp-KO/normal-Tg mice (G) showed a thick layer of cementum deposition at the root apex whereas; Dspp-KO (H) and Dspp-KO/D452A-Tg mice (F) showed little or no cementum in the same region. Bar: 200 μm.
Discussion
Previous in vivo experiments showed that the proteolytically processed NH2-terminal and COOH-terminal fragments (including DSP, DSP-PG, and DPP) of DSPP are the active forms essential for dentinogenesis (22). In the present study, we analyzed the periodontium of the same mouse lines used in our previous study (22) to examine whether transgenic expression of D452A-DSPP (in which Asp452, a key cleavage-site residue, was replaced by Ala452) or normal-DSPP could rescue the phenotypes observed in alveolar bone and cellular cementum of Dspp deficient mice. We found that transgenic expression of normal-DSPP (with no mutation at the cleavage site) was able to rescue the alveolar and cellular cementum phenotypes in Dspp-deficient mice to a level comparable to the WT mice, but the D452A-DSPP transgene failed to do so. These results support that similar to dentinogenesis, the proteolytic processing of DSPP is an activation step, essential for the development and maintenance of the periodontium.
Accumulating evidence suggest that the periodontal defects observed in Dspp deficient mice are due to the intrinsic defects in alveolar bone and cellular cementum caused by loss of DSPP function, but are not secondary to the chronic periodontitis. First, Dspp is highly expressed in the alveolar bone and cellular cementum (14, 21); Second, our current study showed that transgenic expression of normal Dspp rescued the periodontal defects of Dspp deficient mice; Third, detailed analyses showed that the alveolar bone loss occurred independently from and even earlier than chronic periodontitis in these Dspp deficient mice (data not shown). However, inflammation may exacerbate the periodontal defects in Dspp deficient mice as chronic periodontitis itself also could cause alveolar bone loss and periodontal ligament destruction. Therefore, it remains to be determined whether treatment or prevention of the chronic periodontitis would relieve or rescue the periodontal defects seen in Dspp deficient mice.
The present study suggest that the function of DSPP may be mediated by its processed fragments. However, the proteolytic processing of DSPP gives rise to three fragments: DSP, DSP-PG and DPP, each of these may have a specific role in the periodontal tissues. Therefore, it is essential to find out the roles of each of these fragments in the alveolar bone and cellular cementum. We recently found that the NH2-terminal fragment of DSPP (DSP and DSP-PG) have an inhibitory effect on dentinogenesis (24). Presumably, DSP and DSP-PG may have similar inhibitory roles in the alveolar bone and cellular cementum. Considering the tooth and periodontal defects of Dspp deficient mice, it would be reasonable to point out that the DPP fragment may carry the stimulatory effects on dentinogenesis and periodontal development. In the future, it would be necessary to dissect out that the inhibitory effects are mediated by DSP or DSP-PG or both of them, and to determine whether the stimulatory effects are mediated by the DPP.
Acknowledgements
This work was supported by Grant DE005092 (to CQ) from the National Institutes of Health.
Footnotes
Gibson MP, Jani P, Liu Y, Wang X, Lu Y, Feng JQ, Qin C. Failure to process dentin sialophosphoprotein (DSPP) into fragments leads to periodontal defects in mice. Eur J Oral Sci
Conflicts of interest
The authors declare no conflicts of interest.
References
- 1.MacDougall M, Simmons D, Luan X, Gu TT, Dupont BR. Assignment of dentin sialophosphoprotein (DSPP) to the critical DGI2 locus on human chromosome 4 band q21.3 by in situ hybridization. Cytogenet Cell Genet. 1997;79:121–122. doi: 10.1159/000134697. [DOI] [PubMed] [Google Scholar]
- 2.Ritchie HH, Wang LH, Knudtson K. A novel rat 523 amino acid phosphophoryn: nucleotide sequence and genomic organization. Biochim Biophys Acta. 2001;1520:212–222. doi: 10.1016/s0167-4781(01)00274-3. [DOI] [PubMed] [Google Scholar]
- 3.Gu K, Chang S, Ritchie HH, Clarkson BH, Rutherford RB. Molecular cloning of a human dentin sialophosphoprotein gene. Eur J Oral Sci. 2000;108:35–42. doi: 10.1034/j.1600-0722.2000.00765.x. [DOI] [PubMed] [Google Scholar]
- 4.Xiao S, Yu C, Chou X, Yuan W, Wang Y, Bu L, Fu G, Qian M, Yang J, Shi Y, Hu L, Han B, Wang Z, Huang W, Liu J, Chen Z, Zhao G, Kong X. Dentinogenesis imperfecta 1 with or without progressive hearing loss is associated with distinct mutations in DSPP. Nat Genet. 2001;27:201–204. doi: 10.1038/84848. [DOI] [PubMed] [Google Scholar]
- 5.Zhang X, Zhao J, Li C, Gao S, Qiu C, Liu P, Wu G, Qiang B, Lo WH, Shen Y. DSPP mutation in dentinogenesis imperfecta Shields type II. Nat Genet. 2001;27:151–152. doi: 10.1038/84765. [DOI] [PubMed] [Google Scholar]
- 6.Kim JW, Simmer JP. Hereditary dentin defects. J Dent Res. 2007;86:392–399. doi: 10.1177/154405910708600502. [DOI] [PubMed] [Google Scholar]
- 7.Holappa H, Nieminen P, Tolva L, Lukinmaa PL, Alaluusua S. Splicing site mutations in dentin sialophosphoprotein causing dentinogenesis imperfecta type II. Eur J Oral Sci. 2006;114:381–384. doi: 10.1111/j.1600-0722.2006.00391.x. [DOI] [PubMed] [Google Scholar]
- 8.Rajpar MH, Koch MJ, Davies RM, Mellody KT, Kielty CM, Dixon MJ. Mutation of the signal peptide region of the bicistronic gene DSPP affects translocation to the endoplasmic reticulum and results in defective dentine biomineralization. Hum Mol Genet. 2002;11:2559–2565. doi: 10.1093/hmg/11.21.2559. [DOI] [PubMed] [Google Scholar]
- 9.Kim JW, Nam SH, Jang KT, Lee SH, Kim CC, Hahn SH, Hu JC, Simmer JP. A novel splice acceptor mutation in the DSPP gene causing dentinogenesis imperfecta type II. Hum Genet. 2004;115:248–254. doi: 10.1007/s00439-004-1143-5. [DOI] [PubMed] [Google Scholar]
- 10.Dong J, Gu T, Jeffords L, Macdougall M. Dentin phosphoprotein compound mutation in dentin sialophosphoprotein causes dentinogenesis imperfecta type III. Am J Med Genet A. 2005;132A:305–309. doi: 10.1002/ajmg.a.30460. [DOI] [PubMed] [Google Scholar]
- 11.Sreenath T, Thyagarajan T, Hall B, Longenecker G, D'Souza R, Hong S, Wright JT, Macdougall M, Sauk J, Kulkarni AB. Dentin sialophosphoprotein knockout mouse teeth display widened predentin zone and develop defective dentin mineralization similar to human dentinogenesis imperfecta type III. J Biol Chem. 2003;278:24874–24880. doi: 10.1074/jbc.M303908200. [DOI] [PubMed] [Google Scholar]
- 12.Qin C, Brunn JC, Cadena E, Ridall A, Tsujigiwa H, Nagatsuka H, Nagai N, Butler WT. The expression of dentin sialophosphoprotein gene in bone. J Dent Res. 2002;81:392–394. doi: 10.1177/154405910208100607. [DOI] [PubMed] [Google Scholar]
- 13.Qin C, Brunn JC, Cadena E, Ridall A, Butler WT. Dentin sialoprotein in bone and dentin sialophosphoprotein gene expressed by osteoblasts. Connect Tissue Res. 2003;44(Suppl 1):179–183. [PubMed] [Google Scholar]
- 14.Baba O, Qin C, Brunn JC, Jones JE, Wygant JN, Mcintyre BW, Butler WT. Detection of dentin sialoprotein in rat periodontium. Eur J Oral Sci. 2004;112:163–170. doi: 10.1111/j.0909-8836.2004.00110.x. [DOI] [PubMed] [Google Scholar]
- 15.Alvares K, Kanwar YS, Veis A. Expression and potential role of dentin phosphophoryn (DPP) in mouse embryonic tissues involved in epithelial-mesenchymal interactions and branching morphogenesis. Dev Dyn. 2006;235:2980–2990. doi: 10.1002/dvdy.20935. [DOI] [PubMed] [Google Scholar]
- 16.Ogbureke KU, Fisher LW. Expression of SIBLINGs and their partner MMPs in salivary glands. J Dent Res. 2004;83:664–670. doi: 10.1177/154405910408300902. [DOI] [PubMed] [Google Scholar]
- 17.Ogbureke KU, Fisher LW. Renal expression of SIBLING proteins and their partner matrix metalloproteinases (MMPs). Kidney Int. 2005;68:155–166. doi: 10.1111/j.1523-1755.2005.00389.x. [DOI] [PubMed] [Google Scholar]
- 18.Ogbureke KU, Fisher LW. SIBLING expression patterns in duct epithelia reflect the degree of metabolic activity. J Histochem Cytochem. 2007;55:403–409. doi: 10.1369/jhc.6A7075.2007. [DOI] [PubMed] [Google Scholar]
- 19.Prasad M, Butler WT, Qin C. Dentin sialophosphoprotein in biomineralization. Connect Tissue Res. 2010;51:404–417. doi: 10.3109/03008200903329789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Prasad M, Zhu Q, Sun Y, Wang X, Kulkarni A, Boskey A, Feng JQ, Qin C. Expression of dentin sialophosphoprotein in non-mineralized tissues. J Histochem Cytochem. 2011;59:1009–1021. doi: 10.1369/0022155411423406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gibson MP, Zhu Q, Liu Q, D'Souza RN, Feng JQ, Qin C. Loss of dentin sialophosphoprotein leads to periodontal diseases in mice. J Periodontal Res. 2013;48:221–227. doi: 10.1111/j.1600-0765.2012.01523.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhu Q, Gibson MP, Liu Q, Liu Y, Lu Y, Wang X, Feng JQ, Qin C. Proteolytic processing of dentin sialophosphoprotein (DSPP) is essential to dentinogenesis. J Biol Chem. 2012;287:30426–30435. doi: 10.1074/jbc.M112.388587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhu Q, Prasad M, Kong H, Lu Y, Sun Y, Wang X, Yamoah A, Feng JQ, Qin C. Partial blocking of mouse DSPP processing by substitution of Gly(451)-Asp(452) bond suggests the presence of secondary cleavage site(s). Connect Tissue Res. 2012;53:307–312. doi: 10.3109/03008207.2011.650301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gibson MP, Liu Q, Zhu Q, Lu Y, Jani P, Wang X, Liu Y, Paine ML, Snead ML, Feng JQ, Qin C. Role of the NH2 -terminal fragment of dentin sialophosphoprotein in dentinogenesis. Eur J Oral Sci. 2013;121:76–85. doi: 10.1111/eos.12020. [DOI] [PMC free article] [PubMed] [Google Scholar]