Abstract
Methamphetamine use disorders remain a significant public health concern. Methamphetamine produces its behavioral effects by facilitating release of monoamines like dopamine (DA) and serotonin (5-HT). Results from animal studies show that acute pretreatment with DA and 5-HT antagonists attenuates the effects of methamphetamine, but this area remains largely unexplored in humans. This study sought to assess whether aripiprazole, a partial agonist at D2/5-HT1A receptors and an antagonist at 5-HT2A receptors, would attenuate the reinforcing and subject-rated effects of oral methamphetamine. Seven subjects with histories of recreational stimulant use completed a placebo-controlled, crossover, double-blind protocol in which they first sampled doses of oral methamphetamine (0, 4, 8 or 16 mg) following acute pretreatment with aripiprazole (0 and 15 mg). During each Sampling Session, subjects also completed a battery of subject-rated, cardiovascular, and other performance measures. In subsequent Self-Administration Sessions, subjects were provided the opportunity to earn the previously sampled methamphetamine dose on a progressive-ratio procedure. Methamphetamine functioned as a reinforcer, produced prototypical stimulant-like subject-rated and cardiovascular effects (e.g., increased ratings of Stimulated; elevated blood pressure). Aripiprazole reduced methamphetamine self-administration and attenuated some of the positive subject-rated effects of methamphetamine (e.g., ratings of Like Drug). These results indicate that acute aripiprazole pretreatment attenuates the abuse-related effects of methamphetamine.
Keywords: Methamphetamine, Aripiprazole, Monoamines, Humans
1. Introduction
Methamphetamine use remains a persistent public health concern. Data from the National Survey on Drug Use and Health (NSDUH) suggest that 439,000 Americans reported past-month methamphetamine use and 133,000 individuals indicated past-year initiation of methamphetamine use in 2011 (Substance Abuse and Mental Health Services Administration [SAMHSA], 2012). Methamphetamine use is commonly associated with comorbid psychiatric problems and disorders, as well as needle sharing and risky sexual behaviors, which can lead to increased risk of contracting HIV (see Semple et al., 2004; Shoptaw et al., 2006; Shoptaw et al., 2005; Zweben et al., 2004). These risks highlight the need for a better understanding of methamphetamine abuse in humans.
Several in vitro and in vivo studies have demonstrated that dopamine (DA) and serotonin (5-HT) contribute to the behavioral effects of amphetamine in animals. A seminal preclinical study showed that dose-dependent enhancements in synaptic levels of DA and 5-HT were related to locomotor, sniffing, and stereotyped behavioral responses to amphetamine in rats (Kuczenski and Segal, 1989). Additionally, Wee and colleagues (2007) found that acute pretreatment with aripiprazole, a partial agonist at D2/5-HT1A receptors and an antagonist at 5-HT2A receptors, reduced methamphetamine self-administration in rats. A number of preclinical drug-discrimination studies have implicated both central DA and 5-HT systems in mediating the behavioral effects of methamphetamine (Bergman, 2008; Czoty et al., 2004; Munzar et al., 1999; Munzar and Goldberg, 2000; Tidey and Bergman, 1998). For example, in one of these previous studies, 10 squirrel monkeys were trained to discriminate methamphetamine (0.3 mg/kg) from saline (Tidey and Bergman, 1998). A D2 receptor agonist, (+)-PHNO, dose-dependently increased methamphetamine-appropriate responding, whereas pretreatment with remoxipride, a D2 antagonist, attenuated the discriminative-stimulus effect of methamphetamine. The results of two other studies suggest that 5-HT receptors also contribute to the discriminative-stimulus effects of methamphetamine in animals (Munzar et al., 1999; Sasaki et al., 1995).
These preclinical data indicate that DA and 5-HT antagonists might be viable pharmacotherapies for managing methamphetamine use disorders through extinction processes. Clinical data testing the effects of chronic DA and 5-HT antagonists has not revealed promising results, however. For example, in one study, maintenance on 15 mg aripiprazole increased ratings of methamphetamine-induced euphoria while reducing negative subject-rated effects relative to placebo maintenance (Newton et al., 2008). Clinical trials have either demonstrated that aripiprazole either does not change amphetamine use (Coffin et al., 2013; Sulaiman et al., 2012) or increases it (Tiihonen et al., 2007). Most clinical research has examined the effects of chronic DA/5-HT antagonist dosing on the effects of methamphetamine or methamphetamine use, but no studies have translated preclinical research to determine how acute administration of a DA/5-HT antagonist changes methamphetamine self-administration.
Thus, the present study sought to examine the effects of acute aripiprazole administration on the reinforcing effects of methamphetamine in humans. A progressive-ratio procedure was used, as this procedure has consistently proven to be a sensitive measure of drug reinforcement (e.g., Comer et al., 1997; Comer et al., 1998; Rush et al., 2001; Stoops et al., 2004). A battery of subject-rated, performance, and cardiovascular measures was included to complement the self-administration data. We hypothesized that, when administered concurrently with methamphetamine, 15 mg aripiprazole would act as an antagonist and reduce methamphetamine self-administration as evidenced by a decrease in break points. In addition, we hypothesized that aripiprazole would reduce the stimulant-like subject-rated effects of methamphetamine.
2. Method
2.1 Subjects
Seven non-treatment-seeking adult subjects (5 males, 2 females; 6 White [1 Hispanic], 1 Black) completed the protocol. All subjects reported recreational stimulant use in the past year (i.e., mixed salt amphetamine [Adderall], 3,4-methylededioxymethamphetamine [MDMA; ecstasy], methylphenidate or cocaine). On average (±SEM), subjects were 23 (±2) years of age and weighed 74 (±4) kg. One of the seven subjects reported daily use of cigarettes (15 cigarettes/day) and all reported weekly alcohol use (12±3 drinks/week). In addition to daily cigarette and weekly alcohol use, subjects reported recent recreational use of other drugs. In the month prior to screening, two subjects used marijuana, one subject used opioids, and one subject used benzodiazepines. One subject met Diagnostic and Statistical Manual of Mental Disorders IV (DSM-IV) criteria for alcohol abuse, but a study physician determined that this diagnosis would not interfere with his ability to complete the study.
The Institutional Review Board of the University of Kentucky Medical Center approved this experiment and all subjects gave their written informed consent prior to participating. Subjects were paid $40 per session and earned an additional $40 per session completion bonus if they finished the study. Subjects underwent extensive screening prior to enrollment (e.g., Sevak et al., 2010). To meet inclusion criteria, subjects had to (1) report past-year recreational use of stimulant drugs (e.g., amphetamine, ecstasy, methylphenidate, cocaine) and (2) be in good health with no contraindications to stimulant or antipsychotic medications.
2.2 General Procedures
Subjects reported to the University of Kentucky Laboratory of Human Behavioral Pharmacology (LHBP) at the University of Kentucky Chandler Medical Center for a total of 18 sessions (2 Practice and 16 Experimental). Subjects were informed that during their participation they would receive aripiprazole, methamphetamine and placebo. Other than receiving this general information, subjects were blind to the doses of aripiprazole and methamphetamine to be administered during each session. Subjects were told that the purpose of the study was to determine (1) how different drugs affect mood and behavior, (2) the effects of drugs on physiology (cardiovascular measures), and (3) whether subjects like the drug and are willing to take it again. Other than this general explanation of purpose, subjects were not given any information regarding what outcomes might be expected.
2.2.1 Practice Sessions
Subjects completed two Practice Sessions to familiarize them with the subject-rated questionnaires, the performance task, and the progressive-ratio procedure. The first Practice Session followed the Sampling Session timeline and the second Practice Session followed the Self-Administration Session timeline, as described below, with the exception that no drug was administered. Subject-rated questionnaires, the performance task, and the progressive-ratio procedure were administered on a Macintosh iMac computer (Apple Computer Inc., Cupertino, CA).
2.2.2 Sampling Sessions
A total of eight Sampling Sessions (i.e., one for each aripiprazole and methamphetamine dose combination) were conducted to familiarize subjects with the doses of drug they could choose to work for in subsequent sessions. Sampling Sessions for each dose combination always preceded a Self-Administration Session in which that same dose combination was available, similar to previous studies conducted in our laboratory (e.g., Rush et al., 2001; Stoops et al., 2005; Stoops et al., 2007).
For all sessions, subjects arrived daily at approximately 0800 to the LHBP. Sessions lasted 7 hr. immediately after arriving; urine and expired breath samples were collected to confirm drug and alcohol abstinence, respectively. Female subjects also received urine pregnancy tests prior to each session, which were negative throughout their participation. If subjects tested positive for alcohol or other drugs they were sent home and their session rescheduled. Exceptions included use of THC, due to the long elimination time, and methamphetamine positive results that corresponded to experimental administration. To ensure that subjects were not acutely intoxicated, subjects had to pass a field sobriety test prior to beginning each session. To further enhance safety, neither aripiprazole nor methamphetamine was administered until at least 1.5 and 2 hr, respectively, after subjects arrived at the laboratory. Vital signs were recorded at 30 min intervals between 0830 and 0930 and subjects were provided a standard breakfast (i.e., a juice box, and 2 Nutri-grain® bars or 1 standard single-serving cereal with skimmed milk).
At 0830, subject-rated and performance measures were completed. At 0930, subjects received a single red capsule containing aripiprazole or placebo. At 1000, subjects received eight blue and white capsules (each containing 1/8th of the total methamphetamine dose [0, 4, 8, or 16 mg]) to acquaint them with the effects of the drug dose that could be earned during the following Self-Administration Sessions. Subjects were instructed to pay attention to and make notes about the effects of the drug, as they would later be given the opportunity to work for the capsules again.
For all sessions, subject vitals were recorded and subject-rated measures and performance measures were administered at 1 hr intervals after the second drug (i.e., methamphetamine or placebo) administration (i.e., from 1100 to 1500). Between these measures, subjects were allowed to engage in sedentary, quiet recreational activities (e.g., read newspapers or magazines, complete puzzles, watch television). At 1300, subjects were allowed to eat lunch, which was provided by the LHBP. If no drug effects (cardiovascular or behavioral) were detected at 5 hr post-administration, subjects were released from the laboratory.
2.2.3 Self-Administration Sessions
A single Self-Administration Session followed each Sampling Session. This session differed from Sampling Sessions in that subjects received only the methamphetamine- or placebo-containing capsules they had worked for on the progressive-ratio procedure, which was completed at 0830. The procedure began with a computer screen that asked if the subjects would like to work for one of the capsules administered during the Sampling Session. Subjects responded to this question by clicking on one of two buttons on the computer screen labeled YES or NO. If the subject responded YES, they were then required to click the computer mouse a predetermined number of times to earn a single capsule. To earn the first capsule, subjects had to click the mouse 400 times. The number of mouse clicks required to earn each additional capsule increased by 100 (i.e., 500, 600, 700, 800, 900, 1000, and 1100 responses). To receive all eight capsules, subjects were required to click the mouse a total of 6000 times. If a subject responded NO at any time when they were asked if they would like to work for one of the capsules, the task was terminated. While completing each component of the progressive-ratio procedure, subjects were also able to terminate the task by clicking a button labeled STOP. The dependent measure on this task was the break point (i.e., the last ratio completed). Dosing procedures during Self-Administration Sessions were the same as those described above (i.e., aripiprazole or placebo dose at 0930 after completing the progressive-ratio procedure regardless of the methamphetamine or placebo dose earned; earned methamphetamine or placebo dose at 1000). Subjects also completed all subject-rated measures and performance measures at the times described above for Sampling Sessions.
2.3 Subject-Rated Measures
Computerized subject-rated questionnaires were administered periodically throughout each session, as described above, in fixed order.
2.3.1 Adjective-Rating Scale
This questionnaire consisted of 32 items and contained two subscales: Sedative and Stimulant (Oliveto et al., 1992), with each item rated on a 5-point (0–4) Likert-type scale.
2.3.2 Drug-Effect Questionnaire
This questionnaire consisted of 20 items that were presented one at a time (see Rush et al., 2003 for the items) on a 0–100 unit Visual Analog Scale.
2.4 Performance Measure
A computerized version of the DSST, which has been described previously (McLeod et al., 1982), was used in this experiment. This measure is sensitive to the effects of orally administered sedative and stimulant drugs (Rush et al., 2003). Briefly, subjects used a numeric keypad to enter a geometric pattern associated with one of 9 digits displayed on a video screen. Subjects had 90 seconds to enter as many geometric patterns as possible. The dependent measure was the percent of patterns entered correctly (i.e., Percent Trials Correct).
2.5 Cardiovascular Measures
Heart rate and blood pressure were recorded using an automated blood pressure monitor (GE Dinamap® Pro Series 400V2, GE Medical System Information Technologies, Tampa, FL) immediately prior to drug administration and at hourly intervals thereafter until the subject met release criteria at the end of each session.
2.6 Drug Administration
Aripiprazole and methamphetamine were administered in a double-blind fashion. Doses of methamphetamine were prepared in opaque blue and white capsules by weighing methamphetamine HCL powder (supplied by the National Institute on Drug Abuse/Research Triangle Institute, Research Triangle Park, NC) and admixing the appropriate dose with cornstarch. Each methamphetamine capsule contained either 0.5 (4 mg dose), 1 mg (8 mg dose) or 2 mg (16 mg dose). Aripiprazole doses (0 and 15 mg) were prepared by over-encapsulating commercially available drug (Bristol–Myers Squibb, Princeton, NJ) in opaque red capsules with cornstarch. Placebo capsules contained only cornstarch. The order of dose administration was random.
During each Sampling Session, subjects first ingested one red capsule containing the entire dose of aripiprazole for that condition and then ingested eight blue and white capsules, each containing 1/8th of the total dose of methamphetamine for that condition. During each Self-Administration Session, subjects first ingested one red capsule containing the entire dose of aripiprazole for that condition after completing the progressive-ratio procedure and then ingested up to eight blue and white capsules, each containing 1/8th of the total dose of methamphetamine, depending upon progressive-ratio procedure performance.
2.7 Data Analysis
Effects were considered significant for p ≤ 0.05. Effect sizes (eta squared) were also calculated from ANOVA outcomes for which a statistically significant effect was observed (Olejnik and Algina, 2000). Data from the progressive-ratio procedure were analyzed as break point (i.e., the final completed ratio). Progressive-ratio data were analyzed with a two-factor repeated-measures analysis of variance (ANOVA) with Methamphetamine (i.e., 0, 4, 8, and 16 mg) and Aripiprazole (i.e., 0 and 15 mg) as factors (StatView, Cary, NC). F statistics were used to interpret the ANOVA outcomes. One subject did not self-administer methamphetamine and was therefore excluded from all break point analyses. This subject did, however, show changes in response to methamphetamine on other data (e.g., subject ratings) and was therefore included in other analyses.
Because amount of drug ingested during Self-Administration Sessions varied as a function of performance on the progressive-ratio procedure, subject-rated measures, performance measures, and cardiovascular data from these sessions were not analyzed statistically. Thus, peak (i.e., the maximum effect observed following methamphetamine or placebo administration) subject-rated, performance, and cardiovascular data from Sampling Sessions were analyzed. Subject-rated and cardiovascular data were analyzed in a fashion identical to that described for break point data. Area under the curve was also calculated for data from Sampling Sessions and the outcomes were nearly identical to those for peak, so for brevity, only peak data are included here.
3. Results
3.1 Effect Sizes
Table 1 provides effect sizes (eta squared) for measures for which a statistically significant effect was observed in the ANOVA.
Table 1.
Eta Squared values for outcomes with a statistically significant effect in the ANOVA.
| Outcome | Effects | Eta Squared |
|---|---|---|
| Break Point, Progressive-Ratio Procedure | Main Effect of Methamphetamine | 0.24 |
| Main Effect of Aripiprazole | 0.15 | |
| Sedative Subscale, Adjective Rating Scale | Main Effect of Methamphetamine | 0.1 |
| Main Effect of Aripiprazole | 0.1 | |
| Stimulant Subscale, Adjective Rating Scale | Main Effect of Methamphetamine | 0.25 |
| Active/Alert/Energetic, Drug Effect Questionnaire | Interaction of Methamphetamine and Aripiprazole | 0.07 |
| Euphoric, Drug Effect Questionnaire | Interaction of Methamphetamine and Aripiprazole | 0.06 |
| Good Effects, Drug Effect Questionnaire | Interaction of Methamphetamine and Aripiprazole | 0.09 |
| Like Drug, Drug Effect Questionnaire | Interaction of Methamphetamine and Aripiprazole | 0.09 |
| Rush, Drug Effect Questionnaire | Interaction of Methamphetamine and Aripiprazole | 0.05 |
| Stimulated, Drug Effect Questionnaire | Interaction of Methamphetamine and Aripiprazole | 0.07 |
| Talkative/Friendly, Drug Effect Questionnaire | Main Effect of Methamphetamine | 0.23 |
| Main Effect of Aripiprazole | 0.07 | |
| Any Effect, Drug Effect Questionnaire | Main Effect of Methamphetamine | 0.19 |
| High, Drug Effect Questionnaire | Main Effect of Methamphetamine | 0.34 |
| Performance Improved, Drug Effect Questionnaire | Main Effect of Methamphetamine | 0.23 |
| Restless, Drug Effect Questionnaire | Main Effect of Methamphetamine | 0.12 |
| Shaky/Jittery, Drug Effect Questionnaire | Main Effect of Methamphetamine | 0.12 |
| Willing to Pay For, Drug Effect Questionnaire | Main Effect of Methamphetamine | 0.22 |
| Willing to Take Again, Drug Effect Questionnaire | Main Effect of Methamphetamine | 0.31 |
| Systolic Blood Pressure | Main Effect of Methamphetamine | 0.17 |
| Diastolic Blood Pressure | Main Effect of Methamphetamine | 0.08 |
3.2 Progressive-Ratio Procedure
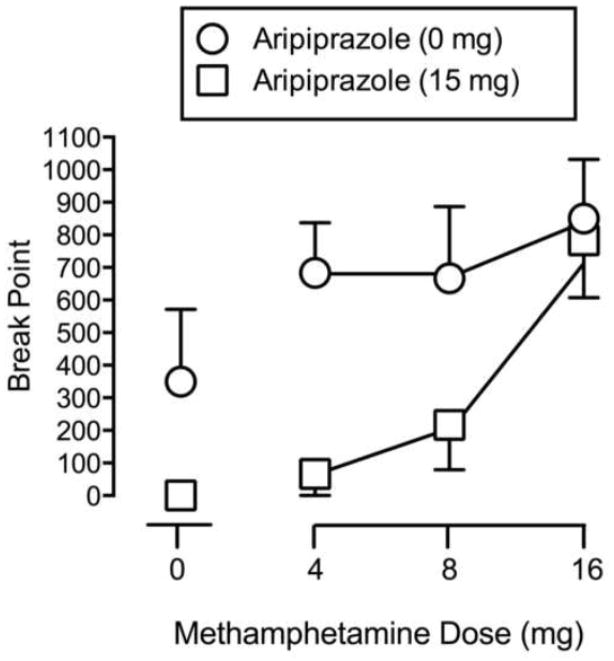
Significant main effects of Methamphetamine (F3,15 = 7.76, p < 0.05) and Aripiprazole (F1, 5 = 6.64, p < 0.05) were found on break point. Each of the doses of methamphetamine increased responding following placebo pretreatment. Aripiprazole pretreatment decreased responding for the low and intermediate dose of methamphetamine (see Figure 1).
Fig. 1.
Dose-response curves for Break Point (Maximum = 1100) on the progressive-ratio procedure as a function of Methamphetamine Dose (mg). Circles indicate 0 mg Aripiprazole. Squares indicate 15 mg Aripiprazole. Error bars indicate 1 SEM.
3.3 Subject-Rated Measures
Significant main effects of Methamphetamine (F3,18 = 4.95, p < 0.05) and Aripiprazole (F1,6 = 7.67, p < 0.05) were observed on the Sedative Subscale from the Adjective-Rating Scale (data not shown). Methamphetamine decreased scores on the Sedative Subscale, which was reversed by aripiprazole. Significant main effects of Methamphetamine (F3,18 = 11.31, p < 0.05) were observed on the Stimulant Subscale from the Adjective-Rating Scale. Methamphetamine dose-dependently increased ratings on these measures regardless of the pretreatment condition (i.e., aripiprazole did not alter responding).
Statistical analysis revealed significant interactions between Methamphetamine and Aripiprazole (F3,18 values ≥ 3.37, p values < 0.05) for six items (Active/Alert/Energetic, Euphoric, Good Effects, Like Drug, Rush, Stimulated) from the Drug-Effect Questionnaire. Figure 2 (top panel) shows outcomes for Like Drug. Methamphetamine produced dose-related increases on this item. At lower doses of methamphetamine (4 and 8 mg), aripiprazole pretreatment decreased subject ratings of Like Drug, whereas it increased these ratings at the highest dose of methamphetamine (16 mg). A similar pattern of effects was observed on the other measures from the Drug-Effect Questionnaire listed above. Additionally, significant main effects of Methamphetamine (F3,18 = 5.44, p < 0.05) and Aripiprazole (F1,6 = 7.53, p < 0.05) were observed on one item (Talkative/Friendly) from the Drug-Effect Questionnaire. Methamphetamine dose-dependently increased ratings on Talkative/Friendly, which was reversed by aripiprazole pretreatment, particularly at the lower doses. Significant main effects of Methamphetamine were observed on seven items (Any Effect, High, Performance Improved, Restless, Shaky/Jittery, Willing to Pay For, Willing to Take Again) from the Drug-Effect Questionnaire (F3,18 values ≥ 3.13, p values ≤ 0.05). Methamphetamine dose-dependently increased ratings on these measures regardless of the pretreatment condition (i.e., there was no main effect of aripiprazole). Figure 2 (middle panel) shows outcomes for Willing to Take Again.
Fig. 2.
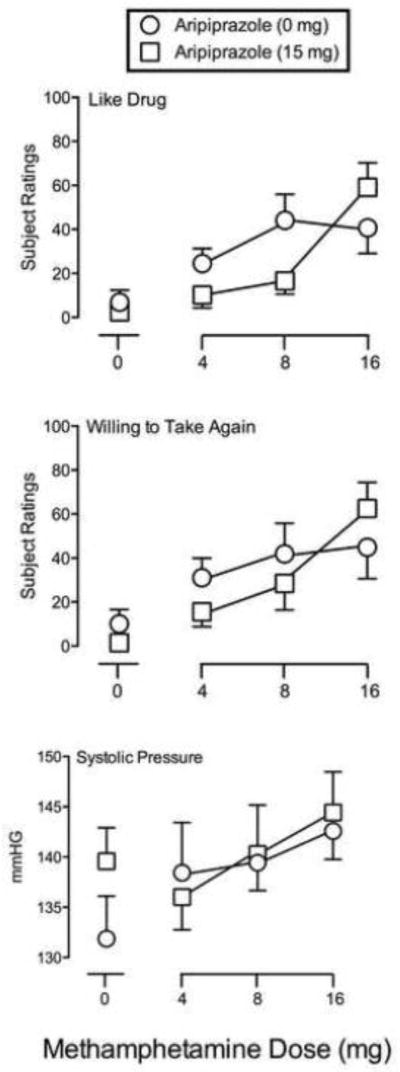
Subject-rated and Cardiovascular measures. Representative measures (Like Drug and Willing to Take Again) from the Drug-Effect Questionnaire (top and middle panels, respectively) and Systolic Pressure (bottom panel) are presented as a function of Methamphetamine Dose (mg). Remaining details are the same as for Figure 1.
3.4 Performance Measure
Statistical analysis revealed no significant interactions or main effect of either Methamphetamine or Aripiprazole on Percent Trials Correct on the DSST.
3.5 Cardiovascular Measures
A significant main effect of Methamphetamine was found for Systolic (F3,18 = 5.46, p < 0.05) and Diastolic Blood Pressure (F3,18 = 5.15, p < 0.05). Methamphetamine dose-dependently increased blood pressure (see Figure 2 for Systolic Pressure) and aripiprazole did not alter these outcomes (i.e., there was no main effect of aripiprazole). No significant effects were observed for heart rate.
4. Discussion
Methamphetamine functioned as a positive reinforcer (i.e., active doses increased break points, with the high dose producing near-maximal responding) and produced prototypical stimulant-like effects (e.g., increased ratings of Stimulated; elevated blood pressure). When combined with methamphetamine, aripiprazole decreased break points on the progressive-ratio procedure and attenuated some positive subject-rated effects of lower methamphetamine doses. Aripiprazole failed to attenuate the subject-rated effects of the high dose of methamphetamine on some measures (e.g., Like Drug), which could contribute to the lack of effect observed for aripiprazole on self-administration of that dose. Neither drug altered accuracy on the DSST. Finally, aripiprazole alone did not significantly alter cardiovascular measures, despite an apparent increase produced by aripiprazole alone on Systolic Blood Pressure (Figure 2), nor did it increase ratings of negative subjective effects and was therefore considered safe and tolerable when combined with methamphetamine.
Concurrent administration of aripiprazole, a partial agonist at D2/5-HT1A receptors and an antagonist at 5-HT2A receptors, significantly attenuated the reinforcing effects of methamphetamine. These data are consistent with those from preclinical self-administration research (e.g., Wee et al., 2007), as well as drug-discrimination results in humans (Sevak et al., 2011) and animals (e.g., Munzar et al., 1999; Munzar and Goldberg, 2000; Tidey and Bergman, 1998; Bergman, 2008) demonstrating that acute pretreatment with DA or 5-HT receptor antagonists shifts methamphetamine dose-response curves rightward and/or downward.
The present findings support the notion that a partial agonist, such as aripiprazole, acts as an antagonist when there are high levels of neurotransmitter present in the synapse, as would occur after the acute administration of methamphetamine. Thus, in agreement with the previous findings in humans and animals, results from the current study indicate that methamphetamine produces reinforcing effects that are sensitive to a pharmacological modification. These results are also consistent with previous studies from our laboratory which have shown that acute pretreatment with aripiprazole attenuates both the discriminative-stimulus and subject-rated effects of d-amphetamine and methamphetamine (Lile et al., 2005; Stoops et al., 2006, Sevak et al., 2011). Taken together, these data suggest that monoaminergic systems play a critical role in mediating the abuse-related effects of amphetamines in both animals and humans.
The present results are somewhat discordant with the findings of a previous human laboratory study that showed aripiprazole enhanced the subject-rated effects of methamphetamine (Newton et al., 2008), although numeric increases in some subject-rated measures were observed for the highest methamphetamine dose tested here. The data analysis strategy used here cannot demonstrate that the increase observed was statistically significant, however. Enhanced subject-rated effects could contribute to the increased use of amphetamine in aripiprazole treated patients in a clinical trial (Tiihonen et al., 2007). The reason for these discrepancies between these studies is unknown but could be attributed to the use of different methods. For example, Newton and colleagues tested the effects of chronically dosed aripiprazole, included only subject-rated measures to evaluate abuse-related effects, administered methamphetamine intravenously, and recruited methamphetamine-dependent subjects. The other studies (Brauer and de Wit, 1995; 1996; 1997; Wachtel et al., 2002) included only subject-rated measures to evaluate abuse-related effects, assessed monoamine agonists other than aripiprazole and recruited normal adults with little to no history of stimulant use. In the current study, progressive-ratio procedures and subject-rated measures were used to evaluate abuse-related effects and recreational stimulant users were recruited. Results from our laboratory have shown that acute administration of monoamine partial agonists/antagonists attenuate the discriminative-stimulus and positive subject-rated effects of amphetamines (Rush et al., 2003; Lile et al., 2005; Sevak et al., 2011). The current study, which also found attenuated subject-rated and self-administration effects when aripiprazole was administered, extends these findings to another procedure (i.e., self-administration) and provides further evidence supporting the role of monoamines in the abuse-related effects of amphetamines in humans.
These findings should be viewed cautiously in the context of the results from a small clinical trial which found a greater number of amphetamine-positive urine samples in participants receiving aripiprazole maintenance relative to placebo treatment (Tiihonen et al., 2007) and more recent studies that found no significant differences in methamphetamine abstinence in subjects receiving daily aripiprazole treatment relative to placebo (Coffin et al., 2013; Sulaiman et al., 2012). Those clinical studies implemented repeated (i.e., chronic) dosing procedures and this could be the cause for discrepancy (see Lile et al., 2011). The present results, which show reductions in the reinforcing effects of methamphetamine when combined with a D2/5-HT1A receptor partial agonist, still provide useful information on the neuropharmacological mechanisms contributing to the behavioral effects of methamphetamine in humans and support the use of progressive-ratio procedures in elucidating neuropharmacological mechanisms of stimulant drugs of abuse in humans, as well as for screening putative pharmacotherapies. The studies testing chronic aripiprazole doses (Coffin et al., 2013; Sulaiman et al., 2012; Newton et al., 2008; Tiihonen et al., 2007) also provide valuable information about the neuropharmacology of amphetamines, particularly about changes that may occur during long-term monoamine antagonist dosing, but are more clinically relevant from a medications development perspective.
In the present study, reinforcing and subject-rated effects of methamphetamine tended to show the same pattern: methamphetamine increased responding on the progressive-ratio procedure while also producing increases in subject ratings of prototypical stimulant-like effects. Aripiprazole attenuated these effects at lower doses of methamphetamine, while this attenuation was absent at the highest dose administered. The failure of aripiprazole to reduce effects of methamphetamine at the highest tested dose could be due to a surmountable antagonism (i.e., overriding the effect of the antagonist with a higher stimulant dose), which could also contribute to increased amphetamine taking found in a previous clinical trial (Tiihonen et al., 2007). These findings are consistent with an interaction between a partial agonist (i.e. aripiprazole) acting as a competitive antagonist in the presence of an agonist at monoamine receptors (i.e., methamphetamine). Worth noting is that 15 mg of aripiprazole is on the low end of the range of doses indicated for the treatment of schizophrenia (15–30 mg; McGavin and Goa, 2002). Previous studies in our laboratory have assessed different doses of aripiprazole (e.g., 10 and 20 mg) in combination with d-amphetamine and have found further evidence of surmountable antagonism (e.g., Lile et al., 2005; Stoops, 2006).
Finally, there were some limitations to the present study. The doses of methamphetamine tested were low and the subjects in the present study were not methamphetamine-dependent, which would be a more clinically relevant population. Additionally, methamphetamine was administered orally, which is a less common route of abuse (versus intranasal, intravenous, or smoking). Overall, the oral administration of small doses of methamphetamine studied in nondependent individuals limit the generalizability of current findings. A small sample size was enrolled, which may also lead to concerns about generalizability or replicability of results. However, as shown in Table 1, the effect sizes were medium to large using conventional definitions (Olejnik and Algina, 2000), which somewhat obviates these concerns. It is also important to address possible involvement of other receptor systems that could not be assessed by aripiprazole (a partial agonist at D2/5-HT1A receptors and an antagonist at 5-HT2A receptors) administration. Norepinephrine (NE) neurotransmitter system also contributes to the effects of methamphetamine, but this mechanism was not of focus here and its involvement in abuse-related effects of methamphetamine was unable to be assessed. Previous studies have shown that aripiprazole does not increase NE levels, indicating that it does not modulate NE transmission (see Chernoloz et al., 2009; Sevak et al., 2011; Zocchi et al., 2005). Thus, the degree to which NE plays a role in methamphetamine self-administration behavior should be assessed in future studies.
5. Conclusion
In this study, self-administration of low methamphetamine doses and some positive subject-rated effects produced by those doses were reduced by acute aripiprazole pretreatment. These findings indicate that monoamine receptor systems contribute to the abuse-related effects of methamphetamine in humans, which has not consistently been demonstrated. Despite the effects produced by acute aripiprazole administration here, the utility of aripiprazole for managing methamphetamine use disorders appears limited given negative findings from at least three clinical trials, although a fine-grain analysis of treatment responders (as has been done with other antagonist-type methamphetamine treatments) may yield more promising outcomes (Ma et al., 2013).
Highlights.
This study tested how aripiprazole impacts methamphetamine self-administration.
Methamphetamine functioned as a reinforcer.
Aripiprazole attenuated the reinforcing effects methamphetamine.
Acknowledgments
This research was supported by NIDA Grant R01 DA 017711 to CRR and Departmental Startup funds to WWS. These funding agencies had no role other than financial support.
Authors Rush, Stoops, Lile and Sevak designed the study. Authors Stoops, Lile and Rush oversaw the conduct of the research. Authors Stoops and Bennett conducted data analysis. Authors Stoops and Bennett conducted the literature searches, summarized previous related research and wrote the first draft of the manuscript. All authors contributed to and have approved the final manuscript
The authors declare no conflicts of interest relevant to this work.
The authors wish to thank the staff at the University of Kentucky Laboratory of Human Behavioral Pharmacology for expert technical and medical assistance. We appreciate the pharmacy services of Dr. Steve Sitzlar of the University of Kentucky Investigational Drug Service.
Abbreviations
- 5-HT
serotonin
- ANOVA
Analysis of Variance
- DA
dopamine
- DSM-IV
Diagnostic and Statistical Manual of Mental Disorders IV
- DSST
Digit Symbol Substitution Task
- hr
hours
- kg
killigrams
- LHBP
Laboratory of Human Behavioral Pharmacology
- MDMA
3,4-methylenedioxymethamphetamine (ecstasy)
- methamphetamine HCL
methamphetamine hydrochloride
- mg
milligrams
- min
minute
- NSDUH
National Survey on Drug Use and Health
- SEM
Standard Error of the Mean
- SAMHSA
Substance Abuse and Mental Health Services Administration
- THC
tetrahydrocannabinol
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
William W. Stoops, Email: william.stoops@uky.edu.
J. Adam Bennett, Email: abennet8@eastern.edu.
Joshua A. Lile, Email: jalile2@uky.edu.
Rajkumar J. Sevak, Email: rsevak@ucla.edu.
Craig R. Rush, Email: crush2@email.uky.edu.
References
- Bergman J. Medications for stimulant abuse: Agonist-based strategies and preclinical evaluations of the mixed-action D-sub-2 partial agonist aripiprazole (Abilify) Exp Clin Psychopharm. 2008;16:475–483. doi: 10.1037/a0014398. [DOI] [PubMed] [Google Scholar]
- Brauer LH, de Wit H. Role of dopamine in d-amphetamine-induced euphoria in normal, healthy volunteers. Exp Clin Psychopharm. 1995;3:371–381. [Google Scholar]
- Brauer LH, de Wit H. Subjective responses to d-amphetamine alone and after pimozide pretreatment in normal, healthy volunteers. Biol Psychiatry. 1996;39:26–32. doi: 10.1016/0006-3223(95)00110-7. [DOI] [PubMed] [Google Scholar]
- Brauer LH, de Wit H. High dose pimozide does not block amphetamine-induced euphoria in normal volunteers. Pharmacol Biochem Be. 1997;56:265–272. doi: 10.1016/s0091-3057(96)00240-7. [DOI] [PubMed] [Google Scholar]
- Chernoloz O, El Mansari M, Blier P. Electrophysiological studies in the rat brain on the basis for aripiprazole augmentation of antidepressants in major depressive disorder. Psychopharmacology. 2009;206:335–344. doi: 10.1007/s00213-009-1611-7. [DOI] [PubMed] [Google Scholar]
- Coffin PO, Santos GM, Das M, Santos DM, Huffaker S, Matheson T, Gasper J, Vittinghoff E, Colfax GN. Aripiprazole for the treatment of methamphetamine dependence: A randomized, double-blind, placebo-controlled trial. Addiction. 2013;108:751–761. doi: 10.1111/add.12073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comer SD, Collins ED, Fischman MW. Choice between money and intranasal heroin in morphine maintained humans. Behav Pharmacol. 1997;8:677–690. doi: 10.1097/00008877-199712000-00002. [DOI] [PubMed] [Google Scholar]
- Comer SD, Collins ED, Wilson ST, Donovan MR, Foltin RW, Fischman MW. Effects of an alternative reinforcer on intravenous heroin self-administration by humans. Eur J Pharmacol. 1998;345:13–26. doi: 10.1016/s0014-2999(97)01572-0. [DOI] [PubMed] [Google Scholar]
- Czoty PW, Ramanathan CR, Mutschler NH, Makriyannis A, Bergman J. Drug discrimination in methamphetamine-trained monkeys: Effects of monoamine transporter inhibitors. J Pharmacol Exp Ther. 2004;311:720–727. doi: 10.1124/jpet.104.071035. [DOI] [PubMed] [Google Scholar]
- Kuczenski R, Segal D. Concomitant characterization of behavioral and striatal neurotransmitter response to amphetamine using in vivo microdialysis. J Neurosci. 1989;9:2051–2065. doi: 10.1523/JNEUROSCI.09-06-02051.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lile JA, Stoops WW, Glaser PEA, Hays LR, Rush CR. Discriminative stimulus, subject-rated and cardiovascular effects of cocaine alone and in combination with aripiprazole in humans. J Psychopharmacol. 2011;25:1469–79. doi: 10.1177/0269881110385597. [DOI] [PubMed] [Google Scholar]
- Lile JA, Stoops WW, Vansickel AR, Glaser PEA, Hays LR, Rush CR. Aripiprazole attenuates the discriminative-stimulus and subject-rated effects of d-amphetamine in humans. Neuropsychopharmacol. 2005;30:2103–2114. doi: 10.1038/sj.npp.1300803. [DOI] [PubMed] [Google Scholar]
- Ma JZ, Johnson BA, Yu E, Weiss D, McSherry F, Saadvandi J, Iturriaga E, Ait-Daoud N, Rawson RA, Mrymoc M, Campbell J, Gorodetzky C, Haning W, Carlton B, Mawhinney J, Weis D, McCann M, Pham T, Stock C, Dickinson R, Elkashef A, Li MD. Fine-grain analysis of treatment effect of topiramate on methamphetamine addiction with latent variable analysis. Drug Alcohol Depend. 2013;130:45–51. doi: 10.1016/j.drugalcdep.2012.10.009. [DOI] [PubMed] [Google Scholar]
- McGavin JK, Goa KL. Aripiprazole. CNS Drugs. 2002;16:779–786. doi: 10.2165/00023210-200216110-00008. [DOI] [PubMed] [Google Scholar]
- McLeod DR, Griffiths RR, Bigelow GE, Yingling J. An automated version of the digit symbol substitution test (DSST) Behav Res Meth Instr. 1982;14:463–466. [Google Scholar]
- Munzar P, Goldberg SR. Dopaminergic involvement in the discriminative-stimulus effects of methamphetamine in rats. Psychopharmacology. 2000;148:209–216. doi: 10.1007/s002130050044. [DOI] [PubMed] [Google Scholar]
- Munzar P, Laufert MD, Kutkat SW, Nováková J, Goldberg SR. Effects of various serotonin agonists, antagonists, and uptake inhibitors on the discriminative stimulus effects of methamphetamine in rats. J Pharmacol Exp Ther. 1999;291:239–250. [PubMed] [Google Scholar]
- Newton TF, Reid MS, De La Garza R, Mahoney JJ, Abad A, Condos R, Palamar J, Halkitis PN, Mojisak J, Anderson A, Li SH, Elkashef A. Evaluation of subjective effects of aripiprazole and methamphetamine in methamphetamine-dependent volunteers. Int J Neuropsychopharmacol. 2008;11:1037–1045. doi: 10.1017/S1461145708009097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olejnik S, Algina J. Measures of effect size for comparative studies: Applications, interpretations and limitations. Contemp Educ Psychol. 2000;25:241–286. doi: 10.1006/ceps.2000.1040. [DOI] [PubMed] [Google Scholar]
- Oliveto AH, Bickel WK, Hughes JR, Shea PJ, Higgins ST, Fenwick JW. Caffeine drug discrimination in humans: Acquisition, specificity and correlation with self-reports. J Pharmacol Exp Ther. 1992;261:885–894. [PubMed] [Google Scholar]
- Rush CR, Essman WD, Simpson CA, Baker RW. Reinforcing and subject-rated effects of methylphenidate and d-amphetamine in non-drug-abusing humans. J Clin Psychopharm. 2001;21:273–286. doi: 10.1097/00004714-200106000-00005. [DOI] [PubMed] [Google Scholar]
- Rush CR, Stoops WW, Hays LR, Glaser PEA, Hays LS. Risperidone attenuates the discriminative-stimulus effects of d-amphetamine in humans. J Pharmacol Exp Ther. 2003;306:195–204. doi: 10.1124/jpet.102.048439. [DOI] [PubMed] [Google Scholar]
- Sasaki JE, Tatham TA, Barrett JE. The discriminative stimulus effects of methamphetamine in pigeons. Psychopharmacology. 1995;120:303–310. doi: 10.1007/BF02311178. [DOI] [PubMed] [Google Scholar]
- Semple SJ, Patterson TL, Grant I. The context of sexual risk behavior among heterosexual methamphetamine users. Addict Behav. 2004;29:807–810. doi: 10.1016/j.addbeh.2004.02.013. [DOI] [PubMed] [Google Scholar]
- Sevak RJ, Stoops WW, Glaser PEA, Hays LR, Rush CR. Reinforcing effects of d-amphetamine: Influence of novel ratios on a progressive-ratio schedule. Behav Pharmacol. 2010;21:745–753. doi: 10.1097/FBP.0b013e32833fa7b3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sevak RJ, Vansickel AR, Stoops WW, Glaser PEA, Hays LR, Rush CR. Discriminative-stimulus, subject-rated, and physiological effects of methamphetamine in humans pretreated with aripiprazole. J Clin Psychopharm. 2011;31:470–480. doi: 10.1097/JCP.0b013e318221b2db. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoptaw S, Klausner JD, Reback CJ, Tierney S, Stansell J, Hare CB, Gibson S, Siever M, King WD, Kao U, Dang J. A public health response to the methamphetamine epidemic: The implementation of contingency management to treat methamphetamine dependence. BMC Public Health. 2006;18:214. doi: 10.1186/1471-2458-6-214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoptaw S, Reback CJ, Peck JA, Yang X, Rotheram-Fuller E, Larkins S, Veniegas RC, Freese TE, Hucks-Ortiz C. Behavioral treatment approaches for methamphetamine dependence and HIV-related sexual risk behaviors among urban gay and bisexual men. Drug Alcohol Depen. 2005;78:125–134. doi: 10.1016/j.drugalcdep.2004.10.004. [DOI] [PubMed] [Google Scholar]
- Stoops WW. Aripiprazole as a potential pharmacotherapy for stimulant dependence: Human laboratory studies with d-amphetamine. Exp Clin Psychopharm. 2006;14:413–421. doi: 10.1037/1064-1297.14.4.413. [DOI] [PubMed] [Google Scholar]
- Stoops WW, Glaser PEA, Fillmore MT, Rush CR. Reinforcing, subject-rated, performance and physiological effects of methylphenidate and d-amphetamine in stimulant abusing humans. J Psychopharm. 2004;18:534–543. doi: 10.1177/0269881104047281. [DOI] [PubMed] [Google Scholar]
- Stoops WW, Lile JA, Fillmore MT, Glaser PEA, Rush CR. Reinforcing effects of modafinil: Influence of dose and behavioral demands following drug administration. Psychopharmacology. 2005;182:186–193. doi: 10.1007/s00213-005-0044-1. [DOI] [PubMed] [Google Scholar]
- Stoops WW, Lile JA, Glaser PEA, Rush CR. A low dose of aripiprazole attenuates the subject-rated effects of d-amphetamine. Drug Alcohol Depen. 2006;84:206–209. doi: 10.1016/j.drugalcdep.2006.02.004. [DOI] [PubMed] [Google Scholar]
- Stoops WW, Vansickel AR, Lile JA, Rush CR. Acute d-amphetamine pretreatment does not alter stimulant self-administration in humans. Pharmacol Biochem Be. 2007;87:20–29. doi: 10.1016/j.pbb.2007.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration. Results from the 2011 National Survey on Drug Use and Health: Summary of national findings. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2012. NSDUH Series H-44, HHS Publication No. (SMA) 12-4713. [Google Scholar]
- Sulaiman AH, Gill JS, Said MA, Zainal NZ, Hussein HM, Guan NC. A randomized, placebo-controlled trial of aripiprazole for the treatment of methamphetamine dependence and associated psychosis. Int J of Psychiat Clin Early Online. 2012:1–8. doi: 10.3109/13651501.2012.667116. [DOI] [PubMed] [Google Scholar]
- Tidey JW, Bergman J. Drug discrimination in methamphetamine-trained monkeys: Agonist and antagonist effects of dopaminergic drugs. J Pharmacol Exp Ther. 1998;285:1163–1174. [PubMed] [Google Scholar]
- Tiihonen J, Kuoppasalmi K, Föhr J, Tuomola P, Kuikanmäki O, Vorma H, Sokero P, Haukka J, Meririnne E. A comparison of aripiprazole, methylphenidate, and placebo for amphetamine dependence. Am J Psychiat. 2007;164:160–162. doi: 10.1176/ajp.2007.164.1.160. [DOI] [PubMed] [Google Scholar]
- Wachtel SR, Ortengren A, de Wit H. The effects of acute haloperidol or risperidone on subjective responses to methamphetamine in healthy volunteers. Drug Alcohol Depen. 2002;68:23–33. doi: 10.1016/s0376-8716(02)00104-7. [DOI] [PubMed] [Google Scholar]
- Wee S, Wang Z, Woolverton WL, Pulvirenti L, Koob G. Effect of aripiprazole, a partial dopamine D2 receptor agonist, on increased rate of methamphetamine self-administration in rats with prolonged session duration. Neuropsychopharmacol. 2007;32:2238–2247. doi: 10.1038/sj.npp.1301353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zocchi A, Fabbri D, Heidbreder CA. Aripiprazole increases dopamine but not noradrenaline and serotonin levels in the mouse prefrontal cortex. Neurosci Lett. 2005;387:157–161. doi: 10.1016/j.neulet.2005.06.035. [DOI] [PubMed] [Google Scholar]
- Zweben JE, Cohen JB, Christian D, Galloway GP, Salinardi M, Parent D, Iguchi M Methamphetamine Treatment Project. Psychiatric symptoms in methamphetamine users. Am J Addiction. 2004;13:181–190. doi: 10.1080/10550490490436055. [DOI] [PubMed] [Google Scholar]