Abstract
Carboxypeptidase E (CPE), a prohormone processing enzyme is highly expressed and secreted from (neuro)endocrine tumors and gliomas, and has been implicated in cancer progression by promoting tumor growth. Our study demonstrates that secreted or exogenously applied CPE promotes survival of pheochromocytoma (PC12) and hepatocellular carcinoma (MHCC97H) cells under nutrient starvation and hypoxic conditions, but had no effect on their proliferation. CPE also reduced migration and invasion of fibrosarcoma (HT1080) cells. We show that CPE treatment mediates survival of MHCC97H cells during metabolic stress by up-regulating the expression of anti-apoptotic protein BCL-2, and other pro-survival genes, via activation of the ERK1/2 pathway.
Keywords: Pheochromocytoma, Hepatocellular carcinoma, Fibrosarcoma, cell survival, cell invasion
1. Introduction
Cancer is the second leading cause of death worldwide, yet despite extensive research, the mechanisms involved in tumor growth, survival and metastatic potential are still not fully understood [1]. Neuroendocrine cancers such as pheochromocytoma, are some of the least understood forms of the disease [2]. It is well established that various types of (neuro)endocrine tumors, including pheochromocytomas, lung small cell carcinomas [3], insulinomas [4], breast adenocarcinoma [5] and glioblastomas [6] secrete large numbers of hormones and growth factors, as well as their processing enzymes, such as carboxypeptidase E (CPE) and peptidylglycine alpha-amidating mono-oxygenase [7]. Among the prohormone processing enzymes, CPE has been the most studied with respect to tumorigenesis. CPE is a multifunctional protein that in addition to its enzymatic function, sub-serves many essential non-enzymatic roles in the endocrine and nervous system, besides a role in cancer (for a review, see ref [8]) and it has been recently shown to act as a trophic factor [9].
High throughput microarray studies have correlated elevated CPE mRNA levels with metastasis in a number of non-endocrine cancers [10], although the form of CPE protein translated from these mRNAs was not analyzed. Recently, an N-terminally truncated splice isoform of CPE, known as CPE- N was identified and shown to be a molecule that induces tumor metastasis [11]. It is elevated in highly metastatic hepatocellular carcinoma (HCC), breast, colon and head and neck cancer cell lines compared to their isogenic low metastatic counterparts. CPE- N is also a powerful biomarker in diagnosing and predicting future metastasis in several cancers including HCC, pheochromocytoma/paraganglioma (PHEO/PGL) and colorectal cancer [11; 12]. CPE- N, up-regulates the expression of Nedd9, a metastatic gene in a HDAC1/2–dependent manner [11]. In addition to the CPE-DN splice variant just described, the primary gene product of the CPE gene, full length wild-type CPE (referred to as CPE) is normally localized in regulated secretory granules of (neuro)endocrine cells. Within these granules it processes peptide hormone and neuropeptide intermediates to their mature bioactive peptides. CPE is secreted from (neuro)endocrine tumor and glioblastoma (GBM) cell lines., It was found that CPE acts as a pro-growth, but anti-metastatic factor [6]. Another recent study has shown that CPE acts extracellularly as a negative regulator of the canonical Wnt signaling pathway [13] and as a neurotrophic factor to protect neurons against oxidative stress induced cell death [9] or during chronic stress [14]. However, the mechanism of action of CPE in these functions is not fully understood.
Neuroendocrine tumors such as PHEO/PGL express both CPE and CPE- N. However, we found that only high levels of the splice isoform, but not CPE, was correlated with enhanced PHEO/PGL tumor metastasis and poor prognosis [11]. In this paper, we investigated whether secreted CPE from these tumors could serve a different role, perhaps as a survival/proliferative factor, similar to that reported for glioblastoma [6]. To determine this possibility, we added neutralizing CPE antibodies to the culture medium of rat pheochromocytoma-derived PC12 cells [15] and found that the cells showed significantly decreased survival in serum free media. We then investigated the mechanism of action of CPE as a survival factor, using a highly metastatic HCC cell line (MHCC97H) lacking background endogenous CPE [11], as a model. We examined the survival effect of exogenously added CPE during metabolic stress on these cells, as well as the signaling pathway and expression of downstream genes. We also used the highly invasive and aggressive fibrosarcoma cell line HT1080, which does not express wild type CPE (see suppl.fig. S1), but only CPE- N, to assay the effects of CPE on invasion and migration.
2. Materials and Methods
2.1. Cell Lines
Rat pheochromocytoma PC12 cells were obtained from ATCC (Manassas, VA). The human HCC cell line, MHCC97H, was obtained from the Liver Cancer Institute, Fudan University (Shanghai, China). The HT1080 fibrosarcoma cells were obtained from TRE-VIGEN Inc. (Gaithersburg, MD).
2.2. Secretion studies for PC12 cells
PC12 cells were seeded on 10 cm dishes and allowed to grow to 70 – 85% confluency in DMEM medium (ATCC, Manassas, VA) supplemented with 10% FBS and 5% horse serum. Cells were later washed with 1× PBS and 1.5 ml of serum free DMEM medium was added to the cells. After 24 h the medium was collected and briefly centrifuged to remove debris. The supernatant containing secreted proteins was collected and stored at −80° C for later use.
2.3. Treatment of PC12 cells with CPE antibodies and cell survival assay
PC12 cells were seeded on a 96 well plate and allowed to grow to 50 – 75% confluency in DMEM supplemented with 10% FBS and 5% horse serum. Cells were washed with 1X PBS and then low glucose (1g/L D-glucose) serum free (LGSF) DMEM medium was added to induce stress. To neutralize the action of any CPE secreted from the cells, 0.25 g of rabbit polyclonal anti-CPE IgG (Anti-CPE #6135, generated in our Lab, was custom made against an 18 aa synthetic peptide corresponding to aa #362-379 of mouse CPE, NCBI accession# NP_038522 and coupled to KLH at the N-terminus ), was added to each of 6 experimental wells. Another six wells had 0.25 g of IgGs from a nonspecific rabbit antibody added to the medium to serve as an antibody control. Six wells were left completely untreated in serum free DMEM medium. This plate containing the three groups of were immediately placed inside the Modular Incubator Chamber (MIC-101) (Billups-Rothenburg), sealed, and flushed with a low oxygen mixture comprising of 1% oxygen, 5% carbon dioxide, and 94% nitrogen for 60 seconds at a rate of 40 li-ters/min after which it was placed into the 37°C incubator. The chamber was subsequently flushed every 12 h as described above, to ensure that a uniform hypoxic environment was maintained. Six wells in a separate plate were untreated in DMEM complete media and placed in the incubator for 24 h to serve as a normal cell control. After the incubation for 24 h under the various conditions all cells were harvested to evaluate the cytotoxic effects of nutrient deprivation and hypoxia on the cells using the a LDH assay (Promega, Madison, WI) according to manufacturer’s instructions.
2.4. Cell viability and proliferation analysis
Cell viability or cytotoxicity was evaluated by determining the levels of LDH released into the culture media after various treatments [16]. LDH was assayed with a Cy-toTox 96 Non-Radioactive Cytotoxicity Assay kit according to the manufacturer’s instructions (Promega, Madison, WI). Proliferation was evaluated by quantification of the DNA binding dye, DRAQ5 that fluoresces at 680 nm upon binding to DNA and is a direct measure of cell density. HCC cells were plated at a density of 40-50% and allowed to grow to ~75% confluency overnight at 37°C, 5% CO2 in DMEM medium supplemented with 10 % fetal calf serum, sodium pyruvate (0.11 mg/ml), penicillin (100 U/ml) and streptomycin (100 mg/ml). The medium was then removed and after rinsing with 1X PBS, the cells were incubated with medium with or without (control) purified recombinant CPE protein (200 nM). The cells were stained with DRAQ5 (BioStatus Ltd., UK) according to the manufacturer’s instructions at the following time points: 0, 8, 24, 34, 48, 56, and 72 h. Cells stained with DRAQ5 were analyzed on the Odyssey infrared imager at 680 nm. Integrated intensity values were obtained and used to generate a growth curve.
2.5. Metabolic stress paradigm for HCC cells
HCC cells were plated at a density of 40-50% and allowed to grow to ~75% confluency overnight in as mentioned above. The following day the medium was changed to LGSF medium and the plates were immediately placed inside the Modular Incubator Chamber (MIC-101) (Billups-Rothenburg), sealed, and flushed with a low oxygen mixture comprising of 1% oxygen (as described above for PC12 cells). The chamber was placed into the 37°C incubator and subsequently flushed every 12 h as described above to ensure that a uniform hypoxic environment was maintained.
2.6. Treatment of HCC cells with exogenous CPE
HCC cells were treated with different concentrations (50-400 nM) of recombinant mouse full length CPE (custom expressed in HEK293 cells and purified by Creative Biolabs, Shirley, NY) by adding it to the cell medium at zero time point of each experiment. A concentration of 200 nM was established as the most effective dose for eliciting an increase in the phosphorylation of ERK 1/2. Since the CPE is very stable in the media, no additional CPE was added throughout the experiment. It was also found that human CPE lacking the C-terminal cytoplasmic tail (purchased from Sino Biological Inc., Beijing, China) gave the same results as full length mCPE. For inhibition of CPE activity, the HCC cells were treated with 5 μM guanidino-ethyl-mercapto-succinic acid (GEMSA), a competitive inhibitor of CPE with a Ki ~8 nM [17] along with 200 nM CPE. For inhibition of ERK 1/2, cells were treated with 10 μM U0126 for 30 mins with/without 200 nM CPE. After 30 min the cells were harvested and proteins were analyzed by Western blotting.
2.7. Western Blot for CPE, BCL-2, ERK 1/2, GSK3p/a, AKT, p-catenin andPKC
Proteins from cell lines were prepared using cell lysis buffer (Cell Signaling Technology) supplemented with Complete Inhibitor Cocktail (Roche) to prevent protein degradation. The cell lysate was collected and centrifuged at 15,000 × g for 10 minutes at 4°C and the protein concentration in the supernatant determined using the Bio-Rad Protein Assay. Twenty g of protein was denatured at 95°C for 3 minutes, ran on 4%–12% SDS-PAGE gels, and then transferred onto nitrocellulose membrane (Invitrogen), according to standard protocols. After blocking with 5% nonfat milk at 4°C for three hours, BCL-2 was detected using a monoclonal antibody from Cell Signaling (Cell Signaling Technology, Inc., Danvers, MA) at a 1:1,000 dilution. Phospho-ERK was detected using a monoclonal antibody which crossreacts with p-ERK 1/2 at 1:1000 dilution. CPE was detected using a mouse anti-CPE monoclonal antibody against the 49-200 amino acid sequence (BD Biosciences, San Jose, CA) at 1:4000 dilution. Anti-phospho-AKT monoclonal antibody, anti-AKT polyclonal antibody, anti-phospho-PKC (pan) monoclonal antibody, anti-β-catenin polyclonal antibody, phospho-GSK3β(Ser9), phospho-GSK3β(Tyr216), total-GSK3βpolyclonal antibodies (Cell Signaling), anti-active-β-catenin monoclonal antibody (Santa Cruz) at 1:3000, 1:3000, 1: 2000, 1:3000, 1:3000, 1:1000 dilutions respectively. After incubating with fluorophore-conjugated anti-mouse secondary antibodies (Amer-sham), the bands were visualized by the Odyssey infrared imaging system version 2.1 (LI-COR Biotechnology, Lincoln, NE) and the direct integrated pixel intensity of acquired light from the bands were measured and analyzed by Odyssey Image Studio Ver 2.0 software (LI-COR Biotechnology, Lincoln, NE).
2.8. Quantitative real-time RT-PCR
RNA was extracted from MHCC97H cells using the RNeasy Mini Kit (Qiagen), and first-strand cDNA was synthesized with 1 μg of total RNA from these cells using Transcriptor First Strand cDNA Synthesis Kit (Roche Applied Science). Quantitative PCR was performed using 1 first strand cDNA under the conditions of 95°C for 15 seconds, annealing at 60°C for 60 seconds, extension at 72°C for 30 seconds for 40 cycles, and a final extension at 72°C for 10 minutes using SYBR Green Master Mix (Applied Biosciences). Primer sequences for analyzing 18S RNA were used for normalization. Primer sequences were: for amplifying 18S RNA, fwd: 5′-CTCTTAGCTGAGTGTCCCGC-3′, rev: 5′ CTGATCGTCTTCGAACCTCC-3′; TNF fwd 5′-CAGAGGGCCTGTACCTCATC-3′ rev 5′-GGAAGACCCCTCCCAGATAG-3′; IL-8 fwd: 5′-GTGCAGTTTTGCCAAGGAGT-3′ rev: 5′-CTCTGCACCCAGTTTTCCTT-3′; FOXC2 fwd: 5′-AGTTCATCATGGACCGCTTC-3′ rev: 5′-TCTCCTTGGACACGTCCTTC-3′; NF-kB fwd: 5′-CCTGGATGACTCTTGGGAAA-3′ rev: TCAGCCAGCTGTTTCATGTC-3−; IKBα fwd: 5′-GCAAAATCCTGACCTGGTGT-3′ rev: GCTCGTCCTCTGTGAACTCC-3′. BCL-2 fwd: 5′ TTCTTTGAGTTCGGTGGGGTC-3′ rev_5′-TGCATATTTGTTTGGGGCAGG-3′.
2.9. PCR microarray
The Human Cancer Pathway Finder Superarray (PAHS-033Z; Qiagen) was used to analyze mRNA levels of 84 genes related to cell proliferation, apoptosis, cell cycle, angiogenesis, invasion, and metastasis. Quality control of RNA samples, synthesis of cDNA, and real-time RT-PCR arrays were performed as described previously (4). All genes represented by the array showed a single peak on the melting curve characteristic of the specific products. Data analysis of gene expression was performed using Excel-based PCR Array Data Analysis Software provided by the manufacturer (Qiagen). Fold-changes in gene expression were calculated using the ΔΔ Ct method, and five stably expressed housekeeping genes (β2-Microglobulin (β2M), hypoxanthine phosphoribosyl-transferase 1, ribosomal protein L13a, GAPDH, and β-actin) were used for normalization of the results.
2.10. Wound Healing Assays
Approximately 5 × 104 HCC cells were plated on a 30μ-Dish 35mm high culture-chamber (Ibidi, Martinsried, Germany) and were allowed to form a monolayer. A wound of ~500 μm was created using the manufacturer’s protocol. The cells were then treated with 200 nM CPE in complete DMEM (or left untreated in complete DMEM to serve as a control) and placed on a live cell imaging incubated microscope. Using Metamorph image processing software, a picture was taken of the wound every minute for 12 h. The pictures were then evaluated to determine the time it took for the wound to completely heal with and without CPE treatment. The wound healing data was analyzed by ImageJ software (NIH, Bethesda, MD, USA) (http://imagej.nih.gov/ij/).
2.11. Invasion assays
Cell invasion assays were carried out using Trevigen Cultrex 24 well cell invasion assay kits. Invasion chambers were coated with a 1× solution of Basement Membrane Extract (BME) per the manufacturer’s protocol and allowed to gel overnight. The following day, 5 × 104 serum starved HT1080 cells were added to the top of each chamber in 100 l of serum free media. Five hundred l of complete DMEM, which serves as the chemo-attractant, was added to the bottom of each well. Three chambers were treated with 200 nM CPE and three wells were left as untreated controls. After 19 h of incubation at 37°C, the chambers were removed and the assay was developed using a calcein marker included with the kit, and read on a fluorescent plate reader.
2.12. SiRNA treatment
HCC cells were plated at a density of 40-50% and allowed to grow to ~75% confluency overnight as mentioned above. The following day cells were treat with/out 20 pmole BCL-2 siRNA (Ambion, Carlsbad, CA) with anti-sense seq 5′ AAUGGAUGUACUUCAUCACTA 3′ using Lipofectamine RNAiMAX transfection reagent (Invitrogen, Carlsbad, CA) according to manufacturers’ protocol and incubated under metabolic stress paradigm as mentioned above. After 12 h, subsets of HCC cells were treated with/out BCL-2 siRNA and with/out 200 nM recombinant CPE, and control cells were treated with mock transfection. Forty eight hours after CPE treatment, cell viability or cytotoxicity was evaluated by LDH assay (Promega, Madison, WI). Cells were harvested and total RNA and protein lysates were prepared. Expression of BCL-2 mRNA and protein were determined by qRTPCR and Western blotting respectively as mentioned above.
2.13. Statistical analysis
Data presented represent the means of a minimum of 3 to 6 separate cultures. Data were analyzed by Student’s t-test or one-way analysis of variance (ANOVA). Error bars indicate standard error of the mean and P values represent the results of Student’s t-test (*p < 0.05, **p < 0.01, ***p < 0.001) unless mentioned otherwise.
3. Results
3.1 Secreted CPE promotes PC12 cell survival under metabolic stress
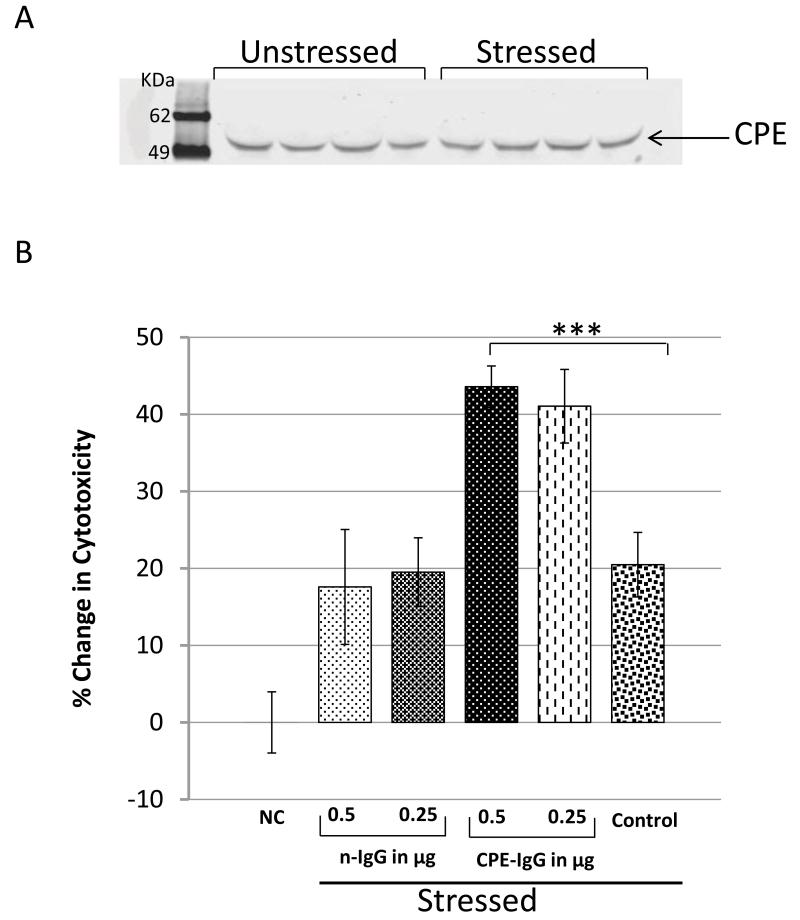
To test our hypothesis that CPE acts extracellularly as a pro-survival factor for neuroendocrine tumor cells, we used rat PC12 cells, an immortalized cell line of pheo-chromocytoma origin, to study the effects of endogenous secreted CPE on cell survival under metabolic stress. First we analyzed the 24 h secretion medium from PC12 cells with and without metabolic stress to determine if CPE is secreted. Fig. 1A shows that CPE is secreted from PC12 cells into the media with and without metabolic stress and therefore could act on the cells in an autocrine/paracrine manner. To study the effect of CPE on PC12 cell survival, cells were grown to 50-75% confluency and then treated under different conditions (see Methods) with and without anti-CPE neutralizing antibodies. All cells were incubated for 24 h and then harvested to evaluate the cytotoxic effects of nutrient deprivation and hypoxia (metabolic stress) on the cells [16]. Fig. 1B shows that cells that had been treated with the polyclonal anti-CPE IgGs exhibited 2-fold more cyto-toxicity than cells that were treated with non-specific IgGs. The cells not treated with IgGs clearly showed resistance to metabolic stress in the presence of secreted CPE (as shown in Fig 1B) similar to cells treated with non-specific IgGs. Control cells that were not metabolically stressed were healthy and showed no cytotoxicity (Fig. 1B, NC). Thus, the survival of neuroendocrine PC12 cells during metabolic stress depends on the presence of CPE.
Figure 1.
Treatment of PC12 cells with CPE antibodies inhibited cell survival. A) Immunoblot of CPE secreted into the media from cells with/without metabolic stress. PC12 cells were subjected to metabolic stress by replacing DMEM supplemented with 10% FBS (complete media) with low glucose (1g/L D-glucose) serum free (LGSF) DMEM media under hypoxic condition. B) Bar graphs show viability of PC 12 cells maintained either in normal complete media indicated as NC (Normal cells), or under metabolic stress conditions (control) with addition of non-specific IgG (nIgG), or with rabbit polyclonal anti-CPE IgGs (CPE-Ab). Cell viability was evaluated by the LDH assay (*p < 0.05, ** p < 0.01, *** p < 0.001, Student’s t-test).
3.2 Exogenous CPE protects HCC cells from cytotoxicity under metabolic stress
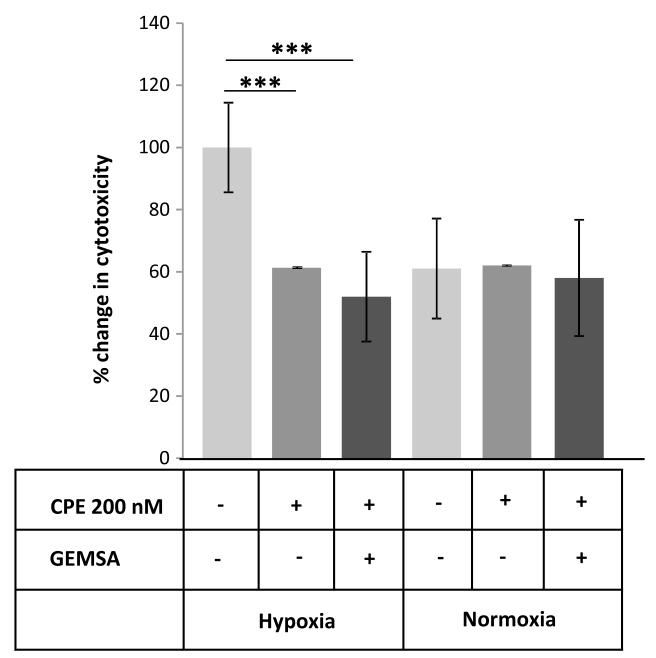
To confirm that CPE is a survival factor indicated in the study on PC12 cells above, where CPE was effectively removed (Fig. 1B), we used MHCC97H (referred to as HCC) cells to test the effect of adding exogenous CPE to a system that does not synthesize or secrete endogenous CPE. HCC cells were maintained in low glucose serum free (LGSF) DMEM or high glucose (4.5g/L D-glucose) supplemented with 10% FBS under hypoxic or normoxic conditions. These cells were treated with or without 200 nM re-combinant mouse CPE for 24 h and the cytotoxicity was measured for the treated and control cells using the LDH assay. Fig. 2 shows that cells treated with recombinant mCPE exhibited significantly less cytotoxicity under metabolic stress than the untreated controls, further supporting our hypothesis that CPE serves as a survival factor. To determine if the survival effect of CPE is dependent on its enzymatic activity, HCC cells were subjected to metabolic stress as described above, in the presence of CPE pre-treated with its inhibitor, GEMSA. Fig. 2 shows that under metabolic stress, cells treated with CPE and GEMSA showed decreased cytotoxicity compared to untreated cells (ANOVA between Control, CPE treated and CPE-GEMSA treated groups; during Hypoxia, F (2, 27) = 93.48, p < 0.001; Normoxia, F (2, 27) = 0.43, p = 0.65). This result indicates that the survival effect of CPE under metabolic stress does not depend on its enzymatic activity.
Figure 2.
CPE increased survival of MHCC97H cells in hypoxia conditions. MHCC97H cells were induced with metabolic stress by method described above. Cells were maintained either in normal condition (Normoxia) or under metabolic stress conditions (Hypoxia) with addition of mouse recombinant CPE (mCPE), and with or without CPE inhibitor, GEMSA. Cell viability was evaluated by the LDH assay (* p < 0.05, ** p < 0.01, *** p < 0.001, Student’s t-test).
3.3 CPE up-regulates BCL-2 survival gene expression in HCC cells
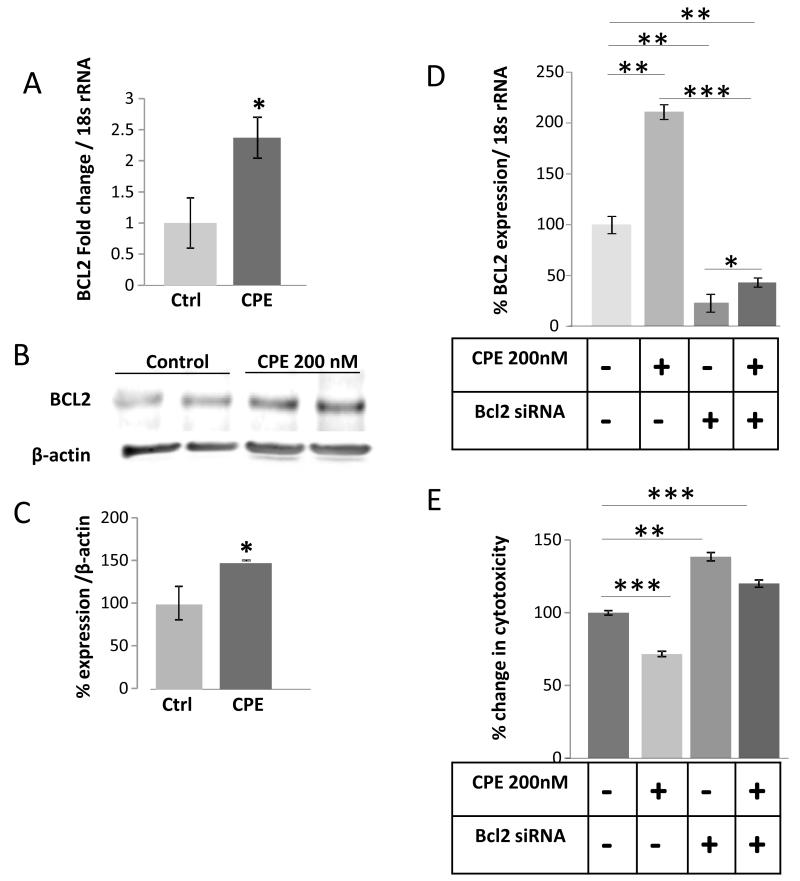
To investigate the mechanism by which CPE increases survival of tumor cells, we again used the CPE-deficient HCC cells. Under the same stressed conditions as were used for cytotoxicity studies, HCC cells were either treated or not treated with 200 nM CPE in LGSF media and then incubated for 24 h in the hypoxia chamber. The cells were then collected and RNA and protein were extracted for evaluation of the expression of the survival gene, BCL-2 [18]. Using qRT-PCR to assay for BCL-2 mRNA, it was shown that HCC cells treated with CPE exhibited a ~2.4-fold increase in BCL-2 mRNA expression (t-test p < 0.05) (Fig. 3A). Western blot analysis also revealed an increase in expression of the pro-survival/anti-apoptotic protein BCL-2 in cells that were treated with CPE (t-test p < 0.05) (Fig. 3B and C). To demonstrate that CPE is directly regulating BCL-2 we silenced BCL-2 by siRNA (Fig. 3D). Suppression of BCL-2 increased cytotoxicity of HCC cells and treatment of CPE did not rescue the cytotoxicity (ANOVA between mock control, CPE treated, BCL-2 siRNA treated and siRNA-CPE treated groups; during Hypoxia, F (3, 20) = 45.44, p < 0.0001). Thus, under metabolic stress, CPE promotes survival of HCC cells by directly increasing the expression of both BCL-2 mRNA and protein.
Figure 3.
CPE up-regulates BCL-2 survival gene expression in HCC cells. Bar graphs show BCL-2 mRNA (A and D), BCL-2 protein (C), Western blots showing BCL-2 (B) in MHCC97H cells after treatment with/without recombinant CPE (200nM) and with/without BCL-2 siRNA. (E) Cell viability was evaluated by the LDH assay in these cells, (*p < 0.05, **p < 0.01, ***p < 0.001, Student’s t-test).
3.4 CPE up-regulates BCL-2 through ERK signaling in HCC cells
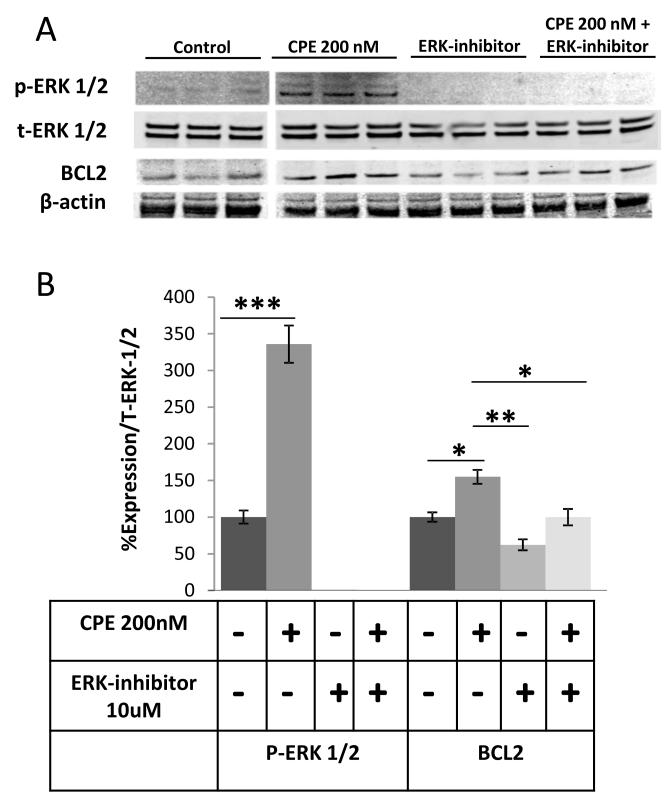
To investigate the signaling pathway by which CPE mediates cell survival, and the increase in BCL-2 expression, we assayed for phosphorylation of ERK, a protein kinase that is known to activate various cell survival cascades and ERK pathway is one of the main pathways that regulate BCL-2 both transcriptionally and post-transcriptionally [19; 20]. Immunoblotting showed that ERK1/2 were phosphorylated as early as 15 min after treatment of HCC cells with CPE (Fig. 4A, B). An increase in BCL-2 protein expression was observed after 30 min treatment with CPE. Furthermore this phosphorylation was nullified and BCL-2 expression was reduced when the cells were treated with CPE in the presence of ERK inhibitor U0126 (10 μM) (Fig. 4A and B) (ANOVA between Control, CPE treated, ERK inhibitor treated and CPE-ERK inhibitor treated groups; for p-ERK 1/2, F (3, 20) = 71.60, p < 0.0001; for BCL-2, F (3, 20) = 18.98, p < 0.001). Thus, the signaling cascade for up-regulation of BCL-2 expression by CPE involves the phosphorylation of ERK1/2.
Figure 4.
CPE up-regulates ERK 1/2 phosphorylation in HCC cells. (A), Western blots and (B) bar graphs showing BCL-2 and phosho-ERK 1/2 and total-ERK ½ (E) expression in MHCC97H cells after treatment with/without recombinant CPE (200nM) and 10μM ERK inhibitor for 30 mins under metabolic stress conditions. β-actin served as a loading control. (*p < 0.05, ** p < 0.01, *** p < 0.001, Student’s t-test).
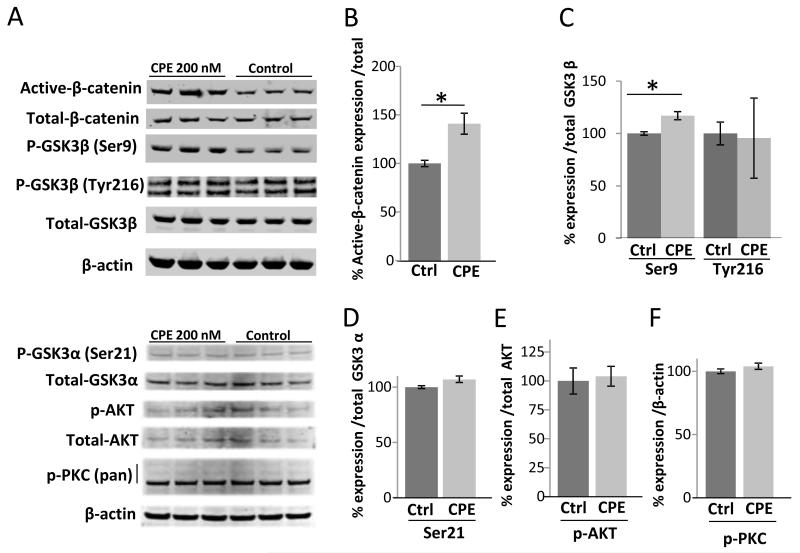
3.5 CPE treatment induces phosphorylation of GSK3P (Ser9) and active P-catenin expression in HCC cells
To determine if CPE plays a role in Wnt/β-catenin signaling in HCC cells, we carried out Western blot analysis for active-β-catenin, phospho-GSK3β (Ser9), (Tyr216), phos-pho-GSK3α (Ser21) and phosphor-AKT and PKC (Fig. 5A-F) for HCC cells treated with CPE under metabolic stress. There was a 40% increase in active-β-catenin (t-test p < 0.03) and 17% increasing in phospho-GSK3β (Ser9) (t-test p < 0.02), however no changes were found in the expression of phospho-GSK3β (Tyr216), phospho -GSK3α (Ser21), phospho-AKT or phospho-PKC. These results showed that CPE could positively regulate active-β-catenin and phospho-GSK3β (Ser9).
Figure 5.
CPE up-regulates active β-catenin and pGSK3β(Ser9) expression in HCC cells. (A) Western blots showing active and total β-catenin, pGSK3β(Ser9), pGSK3β(Tyr216) and total GSK3β, pGSK3α(Ser21) and total GSK3α, pAKT and total AKT and pPKC(pan). Bar graphs show protein expression (B-F) in MHCC97H cells after treatment with/without recombinant CPE (200nM) under metabolic stress conditions. β-actin served as a loading control. (*p < 0.05, Student’s t-test).
3.6. CPE treatment of HCC cells up-regulates expression of other target genes
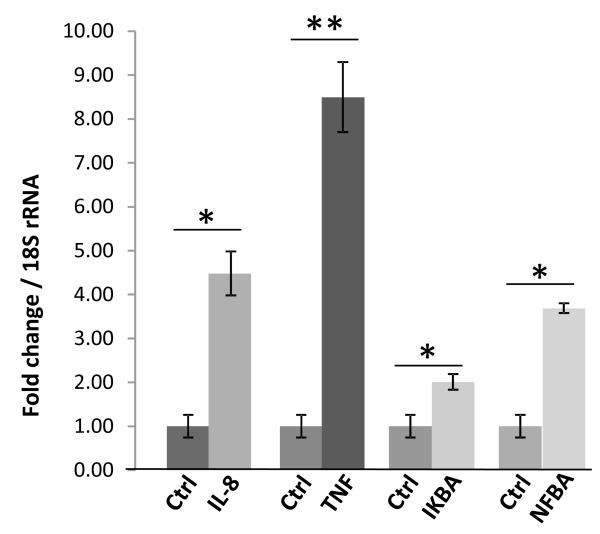
To determine the effect of CPE treatment on up-regulating other target genes in HCC cells under metabolic stress, cells were either treated or not treated with 200 nM CPE in LGSF media, incubated for 24 h in the hypoxia chamber, then removed and RNA extracted from the cells. The samples were run on a PCR array (SA Biosciences-Qiagen) that contained primers for many of the common genes involved in tumorigenesis. From the results we narrowed the list of most likely important genes to a small pool, which we re-tested using custom real time RT-PCR primers designed using the Primer 3 program. Several genes: TNF, NF-κB, I-κB alpha, and IL-8 were consistently upregulated in cells treated with CPE under metabolic stress (Fig. 6). All of these genes have been associated with cell survival in some manner under various types of distress (see Discussion section). We also reconfirmed the expression of BCL-2 gene expression in this PCR array (Supplementary Table 1).
Figure 6.
CPE treatment of MHCC97H cells up-regulated expression of survival genes. Bar graph showing the fold change of mRNA expression of CPE target genes in recom-binant CPE (200nM) MHCC97H cells compared to untreated cells under metabolic stress conditions confirmed by qRT-PCR. Down-stream gene targets of CPE were first analyzed using Human Cancer Pathway Finder PCR Array and up-regulated genes were reconfirmed by qRT-PCR (*p < 0.05, **p < 0.01, Student’s t-test).
3.7. CPE acts to inhibit migration and invasion of cancer cells
To explore the effects of CPE on the metastatic potential of cancer cells, we examined the effect of wound healing with and without CPE treatment of HT1080 cells which are highly invasive. Our results showed that CPE significantly inhibited the migration of these cells. In quantitative terms, the migration capacity decreased by 50% in CPE treated cells compared with control cells (Fig.7 A, B & C). To test CPE’s effects on invasion, we used a chamber assay which allows us to measure the number of cells that migrate through a barrier coated with basement membrane extract (BME) over a specific amount of time. HT1080 cells were seeded in these chambers and allowed to incubate for 19 h with or without CPE (200 nM) and then evaluated for invasion of cells across the membrane using a fluorescent marker substrate. Fig. 7 C shows that ~22% fewer HT1080 cells migrated through the BME barrier when treated with CPE compared to that of untreated cells (control). Any inhibitory effect on HT1080 invasiveness by an outside effector would indicate a strong ability to affect metastasis since these cells are derived from a robust and aggressive cancer[21]. Thus this result suggests that CPE may have an effect on inhibiting tumor cell invasion.
Figure 7.
CPE treatment of HT1080 cells inhibited cell migration and invasion in vitro and has no effect on proliferation of MHCC97H or HT1080 cells. A) Microscopic images of CPE-treated and control HT1080 cells in wound assay, B) graphical representation of wound area covered in 9 hours. The migration of cells was analyzed by a wound-healing assay. Representative photomicrographs were taken at 10× magnification from 3 independent experiments, each repeated in triplicates. C) The invasive properties of the indicated cells were evaluated in an invasion assay using a transwell insert coated with basement membrane extract (BME). Cells that penetrated the BME were counted and analyzed with a histogram. Line graphs showing the staining intensities of MHCC97H (D) and HT1080 (E) cells after DRAQ5 staining at different time points (*** p < 0.001, Student’s t-test).
We also examined the effect of CPE treatment on the proliferation of HCC and HT1080 cells in LGSF media using DRAQ5 staining (Fig. 7 D & E). Our results show that exogenous CPE has no effect on proliferation of these cells.
Discussion
Recent studies on the role of the CPE gene in mediating tumor growth, survival and metastasis have revealed two gene products: full length (wild type) CPE and a N-terminally truncated splice isoform, CPE- N, each of which plays distinctive roles in tumor progression. CPE- N is expressed in many tumors and tumor cell lines which includes HCC, PHEO/PGL, GBM, colon, breast, and head and neck cancers [11]. It functions by entering the nucleus where it is involved in the activation of a metastatic gene, Nedd9, and possibly others [11]. Unlike CPE- N, CPE appears to be expressed only in endocrine and neuroendocrine tumors and in glioblastomas [6; 10]. Until recently, it was presumed that CPE expressed in these tumors function within secretory granules in the cell as an enzyme that processes immature hormone intermediates to mature peptide hormones and neuropeptides which are synthesized in abundance in these tumors [22; 23; 24]. However, a recent report indicated that CPE is secreted in abundance by glioblas-toma cells and has extracellular effects on tumor cell survival, proliferation, migration and invasion [6]. Those findings were provocative, since no mechanism for the effects was provided. In that study, conditioned media from glioblastoma cell lines were used as a source of CPE, leaving open the possibility that the effects could be due to other factors as well, working in concert with CPE.
In our present study we examined the extracellular role of CPE in tumor cell growth and survival from several cancer types using purified recombinant CPE. First we showed that rat pheochromocytoma cells, a neuroendocrine tumor cell line (PC12) secretes CPE (Fig. 1A) and addition of an anti-CPE neutralizing antibody in the cell medium resulted in increased cytotoxic effects and poor survival of the cells under metabolic stress (nutrient starvation and hypoxia) (Fig. 1B). To confirm that CPE is a survival factor indicated in the study on PC12 cells above, where CPE was effectively removed (Fig. 1 B), we used MHCC97H (referred to as HCC) cells to test the effect of adding exogenous CPE to a system that does not synthesize or secrete endogenous CPE. In our gain of function experiments (Fig. 2), we found that HCC cells, that do not synthesize CPE, showed significantly less cytotoxicity under these metabolic stress conditions when purified recombinant CPE protein was added to the culture medium. This effect was also observed when CPE was treated with 5^M GEMSA, a specific and potent inhibitor of CPE, indicating that the extracellular role of CPE in imparting resistance to the metabolic stress is independent of its enzymatic activity. It is not so surprising that this would be the case as CPE has a pH optimum at ~5-5.5 with no activity above pH 7.0 [25], hence it is not expected to be enzymatically active in the culture media.
Chronic metabolic stress is obviously detrimental to cells; therefore treatment with recombinant CPE, that helps protect the cells, suggests that signaling pathways involved in cell survival are activated by CPE. We found that treatment of HCC cells under metabolic stress, with CPE, resulted in increased phosphorylation of ERK1/2 and an increase in the expression of the survival gene BCL-2 [18], at the mRNA and protein levels (Fig. 3). In addition, CPE treatment caused an increase of phospho-GSK3β (Ser9) and active-β-catenin (Fig. 4), suggesting the involvement of the canonical Wnt signaling pathway (see below). Several other genes (TNF, NF-κB, I-κB alpha, and IL-8) (Fig. 5), which could support tumor cell survival were also up-regulated in the CPE-treated HCC cells under metabolic stress. The ability of tumor cells to migrate and invade is a key property of a metastatic phenotype, hence we assessed whether CPE could affect these properties, similar to that reported for glioblastomas [6], in a highly metastatic cell line, HT1080 cells. We showed that addition of CPE to the media of these cells inhibited their metastatic phenotype as measured by wound healing and invasion assays (Fig. 7), however, contrary to that reported for glioblastoma cells [6], CPE had no effect on proliferation of these cells (Fig. 7).
Our findings indicate that CPE is a tumor pro-survival factor that functions ex-tracellularly through ERK signaling to up-regulate expression of BCL-2, the pro-survival (anti-apoptotic) protein which acts to inhibit mitochondrial leakage during cell stress [26]. ERK1/2 regulate cellular activity by acting on more than 100 substrates in the cytoplasm and nucleus, including indirect inducers of gene expression, transcription factors and cell cycle-related kinases, and is a key signaling pathway involved in pituitary tu-morigenesis [27; 28; 29; 30]. ERK signaling could be activated by GPCRs through PKC [31], however we did not observe any change in phosho-PKC after CPE treatment (Fig. 4), suggesting that CPE signaling may be mediated by other signaling cascades. Interestingly, we also did not see any increase in AKT (protein kinase B), a protein kinase family of genes involved in regulating cell survival and is a key regulator of BCL-2 [32]. Hence, induction of BCL-2 by CPE is primarily through ERK signaling.
A key component of the Wnt signaling pathway, β-catenin plays a major role during initiation of carcinogenesis [33]. β-catenin and pGSK3β(Ser9) (inactive form of GSK3β and is a key regulator of nuclear factor (NF)κB nuclear activity [34], discussed below) were significantly increased after CPE treatment. Even though β-catenin acts as a co-activator during cell proliferation, it also plays an important role in cell survival [35]. Hence in our studies, increased expression of β-catenin and activation of the Wnt pathway with the treatment of CPE may contribute to the survival of the HCC cells under these metabolic stress conditions. At the resting state of the cell no change in pGSK3β(Tyr216), the active form of GSK3β was found. We also found no change in GSK3α in CPE mediated cell survival.
The role of the other genes that are up-regulated in promoting survival of the HCC cells is more complex. TNF mRNA was highly expressed with CPE treatment of HCC cells under stress. However, TNF has both pro-apoptotic effects by triggering NFKB activity, as well as anti-apoptotic effects. NF-κB is a transcription factor that plays a crucial role in many physiological and patho-physiological processes, including survival and differentiation [34; 36]. NF-κB has been shown to be activated by several signaling pathways [37; 38]. GSK3p regulates NF-κB activity either positively or negatively in a cell type-dependent manner [39]. Since the NF-κB inhibitor, I-kB mRNA was also increased, the NF-κB pro-apoptotic effects may be nullified. Thus, the likely role of TNF is in supporting pro-survival in this case, consistent with another study showing the increase of NF-κB after CPE induction [40]. TNF drives expression of IL-8 which in vivo would promote angiogenesis [41]. Hence the increase in IL-8 expression with CPE treatment would also support survival of the tumor cells through increased angiogenesis. Interestingly, unlike for glioblastoma cells [6], there was no effect of CPE on proliferation of HCC or HT1080 cells (Fig. 7). This is consistent with studies showing a negative effect of CPE on proliferation of adult neural stem cells [42], as well as negatively regulating the canonical Wnt signaling pathway [13], which normally promotes cancer cell proliferation[43].
The ability of CPE to inhibit migration and invasion of a very aggressive fibrosar-coma cell line, HT1080, suggests that CPE has powerful anti-metastatic effects as was also demonstrated in glioblastomas. The mechanism underlying the inhibition of migration or invasion by CPE is less clear. Since the Wnt pathway components mediate cancer cell invasion [44], one can speculate that the negative regulation of the Wnt pathway by CPE [13] could be responsible for the inhibition of migration and invasion observed with CPE treatment. It has generally been thought that the same pathways or molecules lead to proliferation, survival and metastasis in cancer progression. However, our studies on CPE have led to the recognition that there are "ying and yang" pathways and molecules; ones that specifically drive tumor cell survival and others that promote proliferation, migration and invasion. These pathways are in competition with each other depending on the tumor environment.
With respect to the CPE gene, epithelial-derived tumors have thus far been shown to express only the splice variant, CPE- N, and its main role seems to be to induce metastasis. On the other hand endocrine, neuroendocrine tumors, glioblastomas and neuro-blastomas synthesize both CPE- N and CPE which have competing opposing effects on metastasis. Hence, depending on their relative levels of expression, each will dictate whether the tumor metastasizes or not.
Identifying the role of CPE as an inhibitor of metastasis has resolved an on-going enigma where a clinical study showed that high CPE levels in small cell carcinoma of the lung, a neuroendocrine tumor, indicated good prognosis [23], presumably because of its anti-metastatic effects. On the other hand, our studies on CPE- N in PHEOs showed that high copy numbers of CPE- N mRNA indicated a metastatic tumor, or future recurrence or metastasis with poor prognosis [11]. Given the current knowledge, to use CPE as a prognostic biomarker for (neuro)endocrine tumors such as PHEOs, specific primers for measuring CPE- N and CPE transcripts must be used to avoid confounding results.
Our findings also suggest that CPE could be a good target for various inhibitory therapeutic agents, with the potential benefits of decreased tumor growth and increased susceptibility to hypoxic and nutrient deprived conditions that arise as a result of anti an-giogenic therapies such as Avastin. However, to accomplish this, more work to determine whether CPE is a ligand with its own receptor, or signals through binding to other receptor complexes, such as Wnt3a/frizzled [13], will be necessary. Since invasion is often considered the marker for a particular molecule’s metastatic potential, we can conclude that although CPE may serve to protect a tumor from external stress, it does not promote the spread of the cancer and therefore may actually be a positive attribute in some cases. This fragile dynamic between the pro-survival and anti-metastatic effects of CPE is one that must be considered carefully and further investigated, but certainly offers the potential to be an extraordinarily important aspect of the way we approach treatment of neuroendocrine, endocrine tumors and glioblastomas that secrete CPE.
Supplementary Material
Supplementary Table 1: List showing all genes used in the PCR Array and data analysis and their respective fold changes after CPE treatment compared to untreated control in HCC cells.
Supplementary Figure S1: Western blot showing the CPE protein band in positive control (ATt20 cells) and no CPE in HT1080 cells. The absence of full length wild-type CPE (~50kD band) in HT1080 cells was verified by immunostaining with both a mouse monoclonal CPE antibody and a rabbit polyclonal antibody #6135 generated against a CPE peptide (mouse pre-proCPE amino acids 362-379) in our laboratory. Absorption control with this peptide abolished CPE ~50kD band. Actin was used as loading control. HT1080 protein lysate (20 μg), secreted media and ATt20 protein lysate (20 μg) were run on the sample gel in triplicates, transferred on a single nitrocellulose blot and was cut and probed separately with polyclonal antibody 6135 or 6135 plus peptide or monoclonal CPE antibody.
Acknowledgements
This research was supported by the Intramural Research Program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, USA,
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errorsmaybe discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest: The authors declare that no conflict of interests exists.
References
- [1].Chambers AF, Groom AC, MacDonald IC. Dissemination and growth of cancer cells in metastatic sites. Nature reviews. Cancer. 2002;2:563–572. doi: 10.1038/nrc865. [DOI] [PubMed] [Google Scholar]
- [2].Neumann HP, Bausch B, McWhinney SR, Bender BU, Gimm O, Franke G, Schipper J, Klisch J, Altehoefer C, Zerres K, Januszewicz A, Eng C, Smith WM, Munk R, Manz T, Glaesker S, Apel TW, Treier M, Reineke M, Walz MK, Hoang-Vu C, Brauckhoff M, Klein-Franke A, Klose P, Schmidt H, Maier-Woelfle M, Peczkowska M, Szmigielski C. Germ-line mutations in nonsyndromic pheochromocytoma. The New England journal of medicine. 2002;346:1459–1466. doi: 10.1056/NEJMoa020152. [DOI] [PubMed] [Google Scholar]
- [3].North WG, Du J. Key peptide processing enzymes are expressed by a variant form of small-cell carcinoma of the lung. Peptides. 1998;19:1743–1747. doi: 10.1016/s0196-9781(98)00130-2. [DOI] [PubMed] [Google Scholar]
- [4].Azzoni C, D’Adda T, Tamburrano G, Coscelli C, Madsen OD, Scopsi L, Bordi C. Functioning human insulinomas. An immunohistochemical analysis of intracellular insulin processing. Virchows Archiv : an international journal of pathology. 1998;433:495–504. doi: 10.1007/s004280050280. [DOI] [PubMed] [Google Scholar]
- [5].Zhang JH, Zhou D, You J, Tang BS, Li PY, Tang SS. Differential Processing of Neuropeptide Proprotein in Human Breast Adenocarcinoma. Journal of endocrinological investigation. 2013 doi: 10.3275/8935. [DOI] [PubMed] [Google Scholar]
- [6].Horing E, Harter PN, Seznec J, Schittenhelm J, Buhring HJ, Bhattacharyya S, von Hattingen E, Zachskorn C, Mittelbronn M, Naumann U. The "go or grow" potential of gliomas is linked to the neuropeptide processing enzyme carboxypeptidase E and mediated by metabolic stress. Acta neuropathologica. 2012;124:83–97. doi: 10.1007/s00401-011-0940-x. [DOI] [PubMed] [Google Scholar]
- [7].He P, Varticovski L, Bowman ED, Fukuoka J, Welsh JA, Miura K, Jen J, Gabrielson E, Brambilla E, Travis WD, Harris CC. Identification of carboxypeptidase E and gamma-glutamyl hydrolase as biomarkers for pulmonary neuroendocrine tumors by cDNA microarray. Human pathology. 2004;35:1196–1209. doi: 10.1016/j.humpath.2004.06.014. [DOI] [PubMed] [Google Scholar]
- [8].Cawley NX, Wetsel WC, Murthy SR, Park JJ, Pacak K, Loh YP. New roles of carboxypeptidase E in endocrine and neural function and cancer. Endocrine reviews. 2012;33:216–253. doi: 10.1210/er.2011-1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].NX CY,C, YP L. Carboxypeptidase E/NFa1: A new neurotrophic factor against oxidative stress-induced apoptotic cell death mediated by ERK and PI3-K/AKT pathways. PloS one. 2013 doi: 10.1371/journal.pone.0071578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Murthy SR, Pacak K, Loh YP. Carboxypeptidase E: elevated expression correlated with tumor growth and metastasis in pheochromocytomas and other cancers. Cellular and molecular neurobiology. 2010;30:1377–1381. doi: 10.1007/s10571-010-9592-y. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- [11].Lee TK, Murthy SR, Cawley NX, Dhanvantari S, Hewitt SM, Lou H, Lau T, Ma S, Huynh T, Wesley RA, Ng IO, Pacak K, Poon RT, Loh YP. An N-terminal truncated carboxypeptidase E splice isoform induces tumor growth and is a biomarker for predicting future metastasis in human cancers. The Journal of clinical investigation. 2011;121:880–892. doi: 10.1172/JCI40433. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- [12].Zhou K, Liang H, Liu Y, Yang C, Liu P, Jiang X. Overexpression of CPE-DeltaN predicts poor prognosis in colorectal cancer patients. Tumour biology : the journal of the International Society for Oncodevelopmental Biology and Medicine. 2013 doi: 10.1007/s13277-013-0952-3. [DOI] [PubMed] [Google Scholar]
- [13].Skalka N, Caspi M, Caspi E, Loh YP, Rosin-Arbesfeld R. Carboxypeptidase E: a negative regulator of the canonical Wnt signaling pathway. Oncogene. 2012 doi: 10.1038/onc.2012.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Murthy SR, Thouennon E, Li WS, Cheng Y, Bhupatkar J, Cawley NX, Lane M, Merchenthaler I, Loh YP. Carboxypeptidase E Protects Hippocampal Neurons During Stress in Male Mice by Up-Regulating Pro-Survival BCL2 Protein Expression. Endocrinology. 2013 doi: 10.1210/en.2013-1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Greene LA, Tischler AS. Establishment of a noradrenergic clonal line of rat adrenal pheochromocytoma cells which respond to nerve growth factor. Proceedings of the National Academy of Sciences of the United States of America. 1976;73:2424–2428. doi: 10.1073/pnas.73.7.2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Korzeniewski C, Callewaert DM. An enzyme-release assay for natural cytotoxicity. J Immunol Methods. 1983;64:313–320. doi: 10.1016/0022-1759(83)90438-6. [DOI] [PubMed] [Google Scholar]
- [17].Fricker LD, Plummer TH, Jr., Snyder SH. Enkephalin convertase: potent, selective, and irreversible inhibitors. Biochemical and biophysical research communications. 1983;111:994–1000. doi: 10.1016/0006-291x(83)91398-0. [DOI] [PubMed] [Google Scholar]
- [18].Jacobson MD, Raff MC. Programmed cell death and Bcl-2 protection in very low oxygen. Nature. 1995;374:814–816. doi: 10.1038/374814a0. [DOI] [PubMed] [Google Scholar]
- [19].Balmanno K, Cook SJ. Tumour cell survival signalling by the ERK1/2 pathway. Cell death and differentiation. 2009;16:368–377. doi: 10.1038/cdd.2008.148. [DOI] [PubMed] [Google Scholar]
- [20].Ballif BA, Blenis J. Molecular mechanisms mediating mammalian mitogenactivated protein kinase (MAPK) kinase (MEK)-MAPK cell survival signals. Cell growth & differentiation : the molecular biology journal of the American Association for Cancer Research. 2001;12:397–408. [PubMed] [Google Scholar]
- [21].Marshall CJ, Hall A, Weiss RA. A transforming gene present in human sarcoma cell lines. Nature. 1982;299:171–173. doi: 10.1038/299171a0. [DOI] [PubMed] [Google Scholar]
- [22].Fan X, Olson SJ, Blevins LS, Allen GS, Johnson MD. Immunohistochemical localization of carboxypeptidases D, E, and Z in pituitary adenomas and normal human pituitary. J Histochem Cytochem. 2002;50:1509–1516. doi: 10.1177/002215540205001111. [DOI] [PubMed] [Google Scholar]
- [23].He P, Varticovski L, Bowman ED, Fukuoka J, Welsh JA, Miura K, Jen J, Gabrielson E, Brambilla E, Travis WD, Harris CC. Identification of carboxypeptidase E and gamma-glutamyl hydrolase as biomarkers for pulmonary neuroendocrine tumors by cDNA microarray. Hum Pathol. 2004;35:1196–1209. doi: 10.1016/j.humpath.2004.06.014. [DOI] [PubMed] [Google Scholar]
- [24].Tang SS, Zhang JH, Liu HX, Li HZ. PC2/CPE-mediated pro-protein processing in tumor cells and its differentiated cells or tissues. Mol Cell Endocrinol. 2009;303:43–49. doi: 10.1016/j.mce.2009.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Greene D, Das B, Fricker LD. Regulation of carboxypeptidase E. Effect of pH, temperature and Co2+ on kinetic parameters of substrate hydrolysis. The Biochemical journal. 1992;285(Pt 2):613–618. doi: 10.1042/bj2850613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Boucher MJ, Morisset J, Vachon PH, Reed JC, Laine J, Rivard N. MEK/ERK signaling pathway regulates the expression of Bcl-2, Bcl-X(L), and Mcl-1 and promotes survival of human pancreatic cancer cells. Journal of cellular biochemistry. 2000;79:355–369. [PubMed] [Google Scholar]
- [27].Kolch W. Meaningful relationships: the regulation of the Ras/Raf/MEK/ERK pathway by protein interactions. The Biochemical journal. 2000;351(Pt 2):289–305. [PMC free article] [PubMed] [Google Scholar]
- [28].Schulze A, Lehmann K, Jefferies HB, McMahon M, Downward J. Analysis of the transcriptional program induced by Raf in epithelial cells. Genes & development. 2001;15:981–994. doi: 10.1101/gad.191101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Xaus J, Comalada M, Valledor AF, Cardo M, Herrero C, Soler C, Lloberas J, Celada A. Molecular mechanisms involved in macrophage survival, proliferation, activation or apoptosis. Immunobiology. 2001;204:543–550. doi: 10.1078/0171-2985-00091. [DOI] [PubMed] [Google Scholar]
- [30].Cakir M, Grossman AB. Targeting MAPK (Ras/ERK) and PI3K/Akt pathways in pituitary tumorigenesis. Expert opinion on therapeutic targets. 2009;13:1121–1134. doi: 10.1517/14728220903170675. [DOI] [PubMed] [Google Scholar]
- [31].Naor Z. Signaling by G-protein-coupled receptor (GPCR): studies on the GnRH receptor. Frontiers in neuroendocrinology. 2009;30:10–29. doi: 10.1016/j.yfrne.2008.07.001. [DOI] [PubMed] [Google Scholar]
- [32].Pugazhenthi S, Nesterova A, Sable C, Heidenreich KA, Boxer LM, Heasley LE, Reusch JE. Akt/protein kinase B up-regulates Bcl-2 expression through cAMP-response element-binding protein. The Journal of biological chemistry. 2000;275:10761–10766. doi: 10.1074/jbc.275.15.10761. [DOI] [PubMed] [Google Scholar]
- [33].de La Coste A, Romagnolo B, Billuart P, Renard CA, Buendia MA, Soubrane O, Fabre M, Chelly J, Beldjord C, Kahn A, Perret C. Somatic mutations of the beta-catenin gene are frequent in mouse and human hepatocellular carcinomas. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:8847–8851. doi: 10.1073/pnas.95.15.8847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Billadeau DD. Primers on molecular pathways. The glycogen synthase kinase-3beta. Pancreatology. 2007;7:398–402. doi: 10.1159/000108955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Chung EJ, Hwang SG, Nguyen P, Lee S, Kim JS, Kim JW, Henkart PA, Bottaro DP, Soon L, Bonvini P, Lee SJ, Karp JE, Oh HJ, Rubin JS, Trepel JB. Regulation of leukemic cell adhesion, proliferation, and survival by betacatenin. Blood. 2002;100:982–990. doi: 10.1182/blood.v100.3.982. [DOI] [PubMed] [Google Scholar]
- [36].Perkins ND. NF-kappaB: tumor promoter or suppressor? Trends in cell biology. 2004;14:64–69. doi: 10.1016/j.tcb.2003.12.004. [DOI] [PubMed] [Google Scholar]
- [37].Ghosh S, Karin M. Missing pieces in the NF-kappaB puzzle. Cell. 2002;109(Suppl):S81–96. doi: 10.1016/s0092-8674(02)00703-1. [DOI] [PubMed] [Google Scholar]
- [38].Silverman N, Maniatis T. NF-kappaB signaling pathways in mammalian and insect innate immunity. Genes & development. 2001;15:2321–2342. doi: 10.1101/gad.909001. [DOI] [PubMed] [Google Scholar]
- [39].Grimes CA, Jope RS. The multifaceted roles of glycogen synthase kinase 3beta in cellular signaling. Progress in neurobiology. 2001;65:391–426. doi: 10.1016/s0301-0082(01)00011-9. [DOI] [PubMed] [Google Scholar]
- [40].Meliopoulos VA, Andersen LE, Brooks P, Yan X, Bakre A, Coleman JK, Tompkins SM, Tripp RA. MicroRNA regulation of human protease genes essential or influenza virus replication. PloS one. 2012;7:e37169. doi: 10.1371/journal.pone.0037169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Persad R, Huynh HQ, Hao L, Ha JR, Sergi C, Srivastava R, Persad S. Angiogenic remodeling in pediatric EoE is associated with increased levels of VEGF-A, angiogenin, IL-8, and activation of the TNF-alpha-NFkappaB pathway. Journal of pediatric gastroenterology and nutrition. 2012;55:251–260. doi: 10.1097/MPG.0b013e31824b6391. [DOI] [PubMed] [Google Scholar]
- [42].Lee C, Hu J, Ralls S, Kitamura T, Loh YP, Yang Y, Mukouyama YS, Ahn S. The molecular profiles of neural stem cell niche in the adult subventricular zone. PloS one. 2012;7:e50501. doi: 10.1371/journal.pone.0050501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Clevers H. Wnt/beta-catenin signaling in development and disease. Cell. 2006;127:469–480. doi: 10.1016/j.cell.2006.10.018. [DOI] [PubMed] [Google Scholar]
- [44].Nguyen A, Rosner A, Milovanovic T, Hope C, Planutis K, Saha B, Chaiwun B, Lin F, Imam SA, Marsh JL, Holcombe RF. Wnt pathway component LEF1 mediates tumor cell invasion and is expressed in human and murine breast cancers lacking ErbB2 (her-2/neu) overexpression. Int J Oncol. 2005;27:949–956. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table 1: List showing all genes used in the PCR Array and data analysis and their respective fold changes after CPE treatment compared to untreated control in HCC cells.
Supplementary Figure S1: Western blot showing the CPE protein band in positive control (ATt20 cells) and no CPE in HT1080 cells. The absence of full length wild-type CPE (~50kD band) in HT1080 cells was verified by immunostaining with both a mouse monoclonal CPE antibody and a rabbit polyclonal antibody #6135 generated against a CPE peptide (mouse pre-proCPE amino acids 362-379) in our laboratory. Absorption control with this peptide abolished CPE ~50kD band. Actin was used as loading control. HT1080 protein lysate (20 μg), secreted media and ATt20 protein lysate (20 μg) were run on the sample gel in triplicates, transferred on a single nitrocellulose blot and was cut and probed separately with polyclonal antibody 6135 or 6135 plus peptide or monoclonal CPE antibody.