Abstract
Background
Substantial new information has emerged recently about the prognostic value for a variety of new ECG variables. The objective of the present study was to establish reference standards for these novel risk predictors in a large, ethnically diverse cohort of healthy women from the Women's Health Initiative (WHI) study.
Methods and results
The study population consisted of 36,299 healthy racially diverse women. Racial differences in rate-adjusted QT end (QTea) and QT peak (QTpa) intervals as linear functions of RR were small, leading to the conclusion that 450 ms and 390 ms are applicable as thresholds for prolonged and shortened QTea and similarly, 365 ms and 295 for prolonged and shortened QTpa, respectively. As a threshold for increased dispersion of global repolarization (TpeakTend interval), 110 ms was established for white and Hispanic women and 120 ms for African-American and Asian women. Normal standards were derived using lead transformation matrix computed from 116 lead body surface potential maps to derive normal standards for ST monitoring with limb electrodes in Mason-Likar positions and chest leads V3-V6 at the level of V1-V2 and for bipolar vessel-specific left anterior descending (LAD), left circumflex (LCX) and right coronary artery (RCA) leads. The results support the choice 150 μV as a tentative threshold for abnormal ST onset elevation for all monitoring leads. Body mass index (BMI) had a profound effect on Cornell voltage and Sokolow-Lyon voltage in all racial groups and their utility for left ventricular hypertrophy classification remains open.
Conclusions
Common thresholds for all racial groups are applicable for QTea, and QTpa intervals and ST elevation. Race-specific normal standards are required for many other ECG parameters.
Keywords: electrocardiogram, normal standards, QT, TpTe, ST, monitoring
Introduction
Substantial new information has emerged recently about the prognostic value of a variety of ECG variables including QRS non-dipolar voltage (RNDPV) (1,2), QRS duration in the absence of bundle branch blocks (3-5), widened QRS/T angle (6-10), T wave axis deviation (11,12) , prolonged Tpeak-Tend interval (TpTe) (13-17), and T wave complexity (18). Normal standards for these potentially important ECG predictors of future risk of adverse cardiovascular events have not been established. There are abundant electrocardiographic (ECG) data on QT rate adjustment formulas, including a previous report from the Women's Health Initiative (WHI) study containing normal standards for rate-adjusted QT (19). Prognostic value for rate-adjusted QT has been well established for a variety of clinical and general populations (20). However, QT prolongation as a risk marker is known to have many limitations (21-25). Some drugs may prolong QT without inducing malignant arrhythmic events while some other cardioactive agents associated with adverse outcomes may not prolong QT.
The objective of the present study was to establish normative reference standards for an extensive set of ECG variables in a large, ethnically diverse cohort of healthy women from the Women's Health Initiative (WHI) study.
Methods
Study population The WHI is a 40-center, national United States study of risk factors and the prevention of common causes of mortality, morbidity, and impaired quality of life in women. Women aged 50 to 79 years representing an ethnically diverse population of postmenopausal women were recruited from 1994 to 1998. Details of the study design, protocol sampling procedures, and selection and exclusion criteria have been published elsewhere (26). A total of 68,132 women were enrolled in the clinical trial component of the study. Excluded from the present study were women with one or more of the following conditions at the baseline: (1) prior coronary heart disease (CHD) (history or clinical diagnosis of myocardial infarction (MI), angina pectoris, coronary artery bypass surgery or coronary angioplasty); (2) prior (silent) MI by Minnesota Code (MC) criteria (MC 1.1 or MC 1.2 or MC1.3 with MC 4.1-.2 and/or MC 5.1-5.2); (3) history of stroke; (4) diabetes (self report of medication use); (5) congestive heart failure; and (6) use of antihypertensives, calcium channel blockers, antipsychotics or antidepressants; and (7) significant arrhythmias at baseline ECG (MC 8.1.2 through 8.6.4), heart rate <40/min or ≥ 100/min or QRS duration >115 ms, poor ECG quality or lead reversals.
Additional exclusions on the basis of the follow-up through September 2010 were the following conditions: (1) incident MI (WHI definition); (2) incident coronary artery bypass graft or coronary angioplasty; and (3) incident congestive heart failure. A majority of the women excluded had more than one condition meeting the exclusion criteria above. Finally, 133 women with American-Indian ethnicity were excluded because of small sample size and 463 with ethnicity coded as “other”, leaving a total of 36,299 women for the present study for the establishment of normal reference values for ECG parameters.
Electrocardiographic method Standard 12-lead ECGs were recorded in all women in the supine position using MAC PC electrocardiographs (GE Marquette, Inc., Milwaukee, Wisconsin). ECG technicians in all participating centers were trained to use carefully standardized procedures for ECG acquisition including locating the chest electrodes in precise positions using a special device (27) whereby V4 electrode was placed on the breast at 45° angle between midsternal line and the left midaxillary line, V5 halfway between V4 and V6 and V3 halfway between V2 and V4 (27). All electrocardiograms received at the Central ECG Laboratory (EPICARE Center, Dalhousie University, Halifax, Canada and later at Wake Forest University, Winston-Salem, North Carolina) were inspected visually to detect technical errors, missing leads, and inadequate quality, and such records were rejected from ECG data files. The ECGs were processed by the Marquette 12SL program (GE Marquette, Inc., Milwaukee, Wisconsin).
Because of the special importance of QT peak (QTp) and QT end (QTe) for the parameters used in the repolarization model, special algorithms were developed to detect QTp and QTe outlier measurements. Gender-specific predicted values were first computed for QTe, QTp and QT onset interval (QTo). Measured interval values above the 99th percentile of the absolute difference (QT-predicted QT) were replaced by the predicted value. The replacement occurred for QTe >488 ms, QTp >396 ms and QTo >282 ms These outlier measurements were observed mostly with signal quality problems and for QTe often with the uncertainty of locating T wave end when low-amplitude U wave was overlapping the end portion of the T wave. Subsequently, linear rate-adjustment formulas listed in the middle section of Table 1 were applied to obtain rate adjusted QTp and QTe (QTpa and QTea, respectively).
Table 1. Rate-adjustment Formulas for QTpeak and QTend by Ethnicity.
| Ethnic Group | Linear Functions of RR† | R-Square | |
|---|---|---|---|
|
|
|
||
| ‡ QTp | White | 1. QTpa =QTp +132*(1-RR) | 0.508 |
| African-American | 2. QTpa =QTp +131*(1-RR) | 0.461 | |
| Hispanic | 3. QTpa =QTp +138*(1-RR) | 0.480 | |
| Asian/Pacific | 4. QTpa =QTp +142*(1-RR) | 0.496 | |
| § QTe | White | 5. QTea =QTe +182*(1-RR) | 0.735 |
| African-American | 6. QTea =QTe +184*(1-RR) | 0.701 | |
| Hispanic | 7. QTea =QTe +187*(1-RR) | 0.701 | |
| Asian/Pacific | 8. QTea =QTe +179*(1-RR) | 0.650 | |
|
| |||
| All Women | Power and Linear Functions | R-Square | |
|
|
|||
| QTp | Power Function of RR | 9. QTpa=QTp+319*(1-RRˆ0.40-1) | 0.505 |
| QTe | Power Function of RR | 10. QTea =QTe+319*(1-RRˆ0.40) | 0.728 |
| QTp | Linear Function of RR | 11. QTpa = QTp + 132*(1-RR) | 0.502 |
| QTe | Linear Function of RR | 12. QTea=QTe+182*(1-RR) | 0.731 |
| QTe | Linear Function of HR | 13. QTea = QTe + 2.48*(HR-60) | 0.705 |
|
| |||
| ‖ QTpa from QTea and TpTe intervals | 14. QTpa = QTea - (TpTe) + 0.5* (HR-60) | ||
|
| |||
| ‡‡ Other Formulas for QTe | Residual HR-dependence | r | |
|
|
|||
| QTbz | 15. QTbz = QT/RR1/2 | QTbz = 382 + 0.53*HR | 0.31 |
| QTfr | 16. QTfr = QTe/RR1/3 | QTfr = 446-0.53*HR | 0.31 |
| QTfrm | 17. QTfrm = QTe+154*(1-RR) | QTfrm = 436 - 0.38*HR | 0.24 |
| QThg | 18. QThg = QTe + 1.75*(HR-60) | QThg = 459 - 0.73*HR | 0.42 |
RR interval (=60/heart rate (HR) is in seconds, all other intervals in milliseconds in this and in the other tables.
QTp and QTpa refer to QTpeak and rate-adjusted QTpeak, respectively.
QTe and QTea refer to QTend and to rate-adjusted QTend, respectively.
A practical formula for QTpa as (QTea - TpTe) with TpTe adjusted for HR.
Formulas 17-20 show the residual dependence on HR of the rate-adjusted QTe by Bazett's (QTbz), Fridricia's (QTfr), Framingham (QTfrm) and Hodge's (QThg) formulas.
Because the application of separate rate adjustment formulas for QTe and QTp rate adjustment is an added complexity in clinical applications, an alternative expression for QTpa was derived as shown in Table 1 using QTea and the TpTe interval. Although in some other population samples TpTe interval has been found to be rate-invariant (15,28), in the present study group of healthy women TpTeinterval was not independent of heart rate (R-square 0.18). Therefore, a simple rate correction factor was derived (Table 1) which adds 5 ms to measured TpTe for each HR increment of 10/min above 60/min and subtracts 5 ms for HR 10/min below 60/min.
Repolarization parameters from the repolarization model The orthogonal Frank XYZ leads were obtained from the 8 independent components (leads I, II, V1-V6) using a transformation matrix from the 116 lead body surface map library of Horáĉek containing recordings for 892 adults aged 16 to 85 years (29) (Supplementary Table 1). Transformation coefficients were also computed specifically for the present study for a set of ST monitoring leads from 8 independent components of the standard 12-lead ECG. These coefficients were used to derive normal standards for ST onset (STo) corresponding to the ST J-point elevation and depression in these monitoring leads with body surface electrode locations at Mason-Likar positions for limb leads (RA and LA electrodes at the right and left subclavicular fossa and LL electrode above left iliac crest), and V3-V6 electrode locations at the level of V1-V2. In addition, three vessel-specific bipolar leads were computed using the coefficients listed in Supplementary Table 1. The vessel specific leads were derived by Horáĉek as the most sensitive bipolar leads for detecting acute ischemic response to coronary obstruction of left anterior descending (LAD), left circumflex (LCX) and right coronary artery (RCA) (30). Bipolar LAD lead is recorded from 1/2 intercostal space below V8 to V3 position, LCX from 1/2 intercostal space above V2 to 1/2 intercostal space above V8, and RCA 1 intercostal space above V2 (at third intercostal space) to left iliac crest.
Repolarization measurements were made utilizing temporal reference points derived from the “global” T wave, the spatial T vector magnitude curve derived from the XYZ leads. RNDPV and a set of 18 repolarization-related ECG variables from our repolarization were chosen for evaluation because of their functional role in generation of normal and abnormal repolarization waveforms or because of their previously shown value as risk predictors (1-18). QRS duration was included as the second depolarization-related parameter in addition to RNDPV because even moderate QRS prolongation has been shown to induce secondary repolarization abnormalities associated with adverse cardiac events (3-5).
The conceptual model used to derive repolarization time (RT) subintervals and other model parameters for the present study has been described in detail in previous publications (6,7,25). RT peak (RTp), the key repolarization model parameter, is considered to represent RT of left ventricular (LV) myocytes at the time of global T wave peak (Tp) when the majority of LV lateral wall myocytes are at some point of phase 3 of their action potential. RTp is computed as a function of QTpa. In normal subjects with normal spatial direction of the repolarization sequence, QTp represents RT of the subepicardial myocyte layers (RTepi). Briefly, RTp = QTpa - (1-Cosθ(Tp|Tref) * (TpTxd)/2, where θ(Tp|Tref) is the spatial angle between the Tp vector and Tref is the reference normal Tp vector with unit xyz components (0.75,0.57,-0.33). TpTxd in turn, is the interval from Tp to Txd, where Txd is the inflexion point (the steepest negative slope) at global T wave downstroke. Thus, RTp is obtained from QTpa by modifying it by the degree of deviation of direction of the initial repolarization from the direction of normal repolarization. LV RT at time point Txd (RTxd) is obtained with an algorithm similar to that for RTepi, whereby RTxd = QTpa - (1+Cosθ(Tp|Tref)* (TpTxd)/2. In normal subjects with normal spatial direction of repolarization RTxd is considered as a representative value for subendocardial myocyte layers (RTendo). In addition to θ(Tp|Tref) noted above, a number of other spatial angles between various QRS and T vectors and other interval and amplitude variables were used in various phases of the study. Their definitions are listed in the footnotes of the corresponding tables.
Statistical methods Frequency distributions of all variables used in various analyses were first inspected to rule out anomalies and outliers. QTe distributions were skewed, but otherwise, no anomalies influencing analyses were observed. QTe and QTp prediction accuracy was evaluated by comparing R-square values of the fit on QT distribution by various QT prediction functions. QTe, QTp and QTo intervals varied substantially with heart rate and race-specific rate adjustment formulas as well as common formulas combining all four racial subgroups were established (Table 1). The stability of the adjusted QT intervals for different QT adjustment functions was compared over various ventricular rates, covering the range of heart rates from 45 to 99/min. Mean values with standard deviations and 96 % normal ranges were established for different racial subgroups for ECG variables considered diagnostically or prognostically important and selected for evaluation. The second and 98th percentile limits rather than the fifth and 95th limits were chosen to define the normal range because this study group was chosen with relatively strict selection criteria to exclude women with CVD at baseline and related outcome events during the subsequent 14 year follow-up. Student's t-test (2 sided) was used to determine the significance of the differences in the mean values between white women and the other racial groups. Data analyses including descriptive statistics and graphics were done using Microsoft Excel 2007version 5.0 (Microsoft Corporation, Redmond, Washington).
Results
Characteristics of the study group Characteristics of the study population are listed in Supplementary Table 2. Of special interest in relation to ECG amplitude is the body mass index (BMI). BMI was 2.9 kg/m2 higher in African-American women than in white women. The impact on BMI on ECG amplitudes will be considered under the subheading “R and S wave amplitudes, Cornell voltage and Sokolow-Lyon voltage”.
Racial differences in rate-adjusted QT and QT subintervals The slope coefficients for rate adjustment as linear function of RR differed relatively little from those of white women in the other 3 ethnic groups (Table 1, top section), and the common linear rate adjustment function was used for rate adjustment when deriving other repolarization-related variables for our repolarization model. Rate adjustment functions for QTp and QTe intervals show that rate adjustment by linear functions of the RR interval gave R-square values as high as the power functions with optimal exponents from log-log regression (middle section in Table 1). Thus, the simpler linear functions were chosen for rate adjustment of QT and QT subintervals.
Formulas 15-18 at the bottom section of Table 1 show the residual dependence on heart rate (HR) of the rate-adjusted QTe by Bazett's (QTbz) (31), Fridricia's (QTfr) (32), Framingham (QTfrm) (33) and Hodge's (QThg) (34) formulas. It is noted that these commonly used formulas left a notable bias with increasing HR in women of our study population, ranging at HR 90 bpm from -22 ms for QThg to +16 ms for QTbz. It appears that the QTe rate sensitivity in this postmenopausal group of healthy women is higher than in the populations where these other formulas were derived. This is noted for instance by comparing the slope coefficient for RR in Formula 12 (QTea=QTe+182*(1-RR)) with the corresponding coefficient in the Framingham formula (QTfrn=QTe+154*(1-RR)) and similarly, the slope coefficients for HR in Formula 13 (QTea=QTe+2.48*(HR-60) and the corresponding coefficient in Hodge's formula (QThg= QTe+1.75*(HR-60).
Normal standards for ECG Intervals Normal values for PR, QRS and rate-adjusted QT and QT subintervals are listed in Table 2. Most of the mean differences in African-American and Hispanic women from white women are statistically significant although in general these differences can be considered quite small from the clinical point of view. For rate-adjusted QT and QT subintervals, the following values were established as thresholds for interval prolongation: 450 ms for QTea, 365 for QTpa and 270 for QToa. The corresponding thresholds for shortening of these intervals were 390 ms for QTea, 295 for QTpa and 210 ms for QToa. The PR interval was 6 to 9 ms longer in African-American women than in the other racial groups. The value of 220 ms can be recommended as the upper normal limit for African-American women and 210 ms for white, Hispanic and Asian/Pacific women. Also, the upper normal limit for QTpa- QTea was 5 to 8 ms longer in African-American women than in the other 3 racial groups. QTpa- QTea is the rate-adjusted TpTe interval ((TpTe)a), the temporal global RTgrad considered to represent global RT dispersion. The upper normal limit of (TpTe) a interval was 10 ms longer in African-American women than in white women. The value of 110 ms can be recommended for the upper normal limit for (TpTe)a interval for white, Hispanic and Asian/Pacific women and 120 ms for African-American women.
Table 2. Mean values of ECG Intervals with Standard Deviations and the Range of Normal Limits from the 2nd to 98th Percentile by Race.
| All Women | White | African-American | Hispanic | Asian/ Pacific | |
|---|---|---|---|---|---|
|
|
|||||
| Heart Rate (bpm) | 66; 9.2 49 → 88 | 66; 9.2 49 → 87 | 67; 9.8*** 50 → 89 | 66; 8.8NS 50 → 87 | 67; 9.1NS 51→ 88 |
| PR (ms) | 158; 33.9 118 → 212 | 157; 34.6 118 → 212 | 164; 34.9*** 124 → 220 | 155; 22.0** 118 → 204 | 158; 20.9NS 124→ 209 |
| QRS (ms) | 85; 8.1 70 → 104 | 85; 8.0 70 → 104 | 84; 8.5*** 68 → 104 | 85; 8.2NS 70 → 104 | 85; 8.1NS 70 → 104 |
| † QTea (ms) | 413; 14.2 388 → 449 | 413; 14.0 388 → 448 | 414; 14.9NS 388 → 452 | 414; 15.0* 389 → 451 | 418; 16.1*** 393 → 460 |
| † QTpa (ms) | 328; 17.0 294 → 366 | 328; 16.7 295; 365 | 326; 18.9*** 288 → 368 | 328; 17.6NS 294 → 366 | 331; 17.4*** 299 → 372 |
| ‡ QToa (ms) | 236; 15.3 207 → 271 | 237; 15.2 208 → 271 | 233; 16.2*** 202 → 271 | 234; 15.1*** 207 → 269 | 232; 14.4*** 206 → 266 |
| § TpTxd (ms) | 33; 8.5 20 → 56 | 33; 8.2 20 → 54 | 35; 10.3*** 20 → 64 | 34; 9.1*** 20 → 58 | 35; 9. 3*** 22 → 60 |
| # (TpTe)a (ms) | 85; 11.2 63 → 112 | 85; 10.9 64 → 111 | 87; 13.5*** 61 → 119 | 86; 11.8*** 60 → 113 | 87; 14.1NS 54 → 121 |
signifies P<0.0001,
P<0.001,
=P<0.05 and NS nonsignificant for mean difference from white women.
QTea and QTpa are rate-adjusted QTend (QTe) and QTpeak (QRp) intervals by formulas listed in Table 1.
QToa is the rate adjusted QTonset (QTo) interval by formula QToa = QTo +107*(1-RR).
TpTxd signifies crossmural left ventricular repolarization time gradient, equal to the interval from Tp to Txd where Txd is the time point of maximum slope at the global T wave downstroke (see methods).
TpTe computed as the difference (QTea-QTpa) or as TpTe + 0.5*(HR-60).
TpTxd interval is considered to represent regional dispersion of the initial repolarization period dominated by crossmural repolarization of the LV lateral wall. The upper normal limit for TpTxd interval was 54 ms in white women, 10 ms longer (64 ms) in African-American women, 58 ms in Hispanic and 60 ms in Asian/Pacific women.
Normal limits for key repolarization-related ECG parameters Normal values for repolarization-related ECG variables from our repolarization listed in Table 3 show several notable racial differences particularly in African-American women compared to white women and to Hispanic and Asian/Pacific women. The differences in upper normal limits were relatively large for instance for RNDPV, θ(Tinit|Tterm) and for vector magnitudes SToV, ToV, TpV and the ratio ToV/TpV.
Table 3. Mean Values, Standard Deviations and Normal 2nd to 98th Percentile Range for Key ECG Parameters from the Repolarization Model by Race.
| All Women | White | African-American | Hispanic | Asian/Pacific | |
|---|---|---|---|---|---|
| † RTepi | 327 ; 17.1 293→364 | 327 ; 16.8 294→364 | 324 ; 19.2** 284→366 | 326; 17.7NS 292→365 | 330 ; 17.4*** 298→369 |
| † RTxd | 296; 20.2 254→339 | 297 ; 19.7 256→339 | 292 ; 23.7*** 240→343 | 294 ; 21.1*** 249→338 | 297; 21.7NS 252→345 |
| ‡ APDepi | 288; 17.1 253→325 | 288; 16.8 254→324 | 286; 19.1NS 246→326 | 288; 17.8** 253→327 | 293; 17.7*** 258→332 |
| ‡ APDxd | 286 ; 20.2 244→329 | 287 ; 19.7 246→329 | 282 ; 23.7*** 230→333 | 284 ; 21.1*** 239→328 | 287 ; 21.7NS 242→335 |
| § QRp | 39 ; 4.8 30→50 | 39 ; 4.8 30→50 | 38 ; 4.8*** 30→50 | 38 ; 4.8*** 30→50 | 39 ; 4.9 * 30→50 |
| ‖ RNDPV | 41 ; 15.7 19→82 | 41 ; 15.3 22→70 | 46 ; 17.6*** 21→93 | 43 ; 15.7*** 20→85 | 47 ; 18.0*** 20→96 |
| # θ(Rm|Tm) | 43 ; 21.6 9→97 | 43 ; 21.4 13→82 | 43 ; 24.0NS 8→108 | 40 ; 21.7*** 8→96 | 39; 22.0*** 9→99 |
| †† θ(Rp|Tp) | 33 ; 22.1 5→103 | 33 ; 21.7 8→72 | 36 ; 25.3*** 6→116 | 30 ; 22.4*** 5→105 | 30 ; 24.0*** 5→118 |
| ‡‡ θ(Tp|Tref) | 17 ; 13.3 2→53 | 17 ; 12.8 4→38 | 21 ; 17.4*** 3→74 | 18 ; 13.2NS 3→53 | 17 ; 13.4NS 2→51 |
| §§ θ(Tinit|Term) | 31.2 ; 11.5 10→58 | 31.4 ; 11.3 15→50 | 30.9 ; 13.1* 8→61 | 30.6 ; 11.8** 10→59 | 27.8 ; 10.4NS 8→52 |
| ‖‖ θ(Tinit|Tref) | 18 ; 13.4 3→53 | 18 ; 13.0 4→40 | 22 ; 16.8*** 3→72 | 18 ; 12.7NS 3→52 | 17 ; 13.1NS 2→49 |
| ## θ(Tterm|Tref) | 28 ; 9.0 11→39 | 27 ; 8.5 16→36 | 30 ; 13.7*** 11→51 | 29 ; 8.5*** 14→39 | 28 ; 8.9** 13→40 |
| ††† SToV | 32 ; 17.1 6→74 | 31 ; 16.3 9→62 | 42 ; 20.2*** 10→93 | 35 ; 17.4*** 6→76 | 40 ; 19.5*** 9→85 |
| ††† ToV | 103 ; 39.6 38→198 | 102 ; 38.6 46→171 | 114 ; 44.1*** 42→221 | 110 ; 41.6*** 39→205 | 122; 45.7*** 48→235 |
| ††† TpV | 346 ; 123.8 122→629 | 349 ; 122.0 163→565 | 314 ; 131.8*** 95→648 | 340; 128.4** 111→624 | 366 ; 136.7*** 118→628 |
| ‡‡‡ ToV/TpV | 0.31 ; 0.079 0.18→0.52 | 0.30 ; 0.073 0.20→0.42 | 0.38* ; 0.097 0.22→0.64 | 0.33 ; 0.083*** 0.20→0.55 | 0.35 ; 0.084*** 0.21→0.55 |
Signifies P<0.0001,
P<0.001,
=P<0.05 and NS nonsignificant for mean differences from white women.
RTepi is epicardial repolarization time and RTxd a representative value of subendocardial repolarization time.
APDepi = RTepi - ETepi, and APDendo = RTxd - ETendo, where ETepi and ETendo are epicardial excitation time (=QRp) and endocardial excitation time (taken as 10 ms).
QRp = interval from QRSonset to the peak of QRS vector magnitude curve.
RNDPV = QRS nondipolar voltages (square roots of the total variance of components 4 to 8 from singular value decomposition).
θ(Rm|Tm) = spatial angle between the mean QRS and T vectors.
θ(Rp|Tp) = spatial angle between the peak QRS and T vectors.
θ(Tp|Tref) = spatial angle between the Tp vector and the reference T vector (Tref), signifying spatial deviation angle of repolarization direction from the direction of the normal repolarization sequence.
θ(Tinit|Term) = spatial angle between the mean initial and terminal T vectors from quintiles 1-3 and quintiles 4-5 of global T wave, respectively.
θ(Tinit|Tref) = spatial angles between the mean initial T vector and Tref vector.
θ(Tterm|Tref) = spatial angle between the mean terminal T vector and Tref vector.
Symbol 'V' with SToV, ToV and TpV signifies vector magnitude of STo, To and Tp, respectively.
ToV/TpV is the magnitude ratio of ToV and TpV vectors.
For many of the ECG variable, the upper limits in Hispanic and Asian/Pacific women were similar to those in white women and differed more widely in African-American women for instance for angular ECG variables. It thus appears that race-specific normal limits are necessary for these novel ECG parameters from our repolarization.
Normal limits for Q wave durations The prevalence and the upper normal limits for significant Q waves (≥100 μV followed by R wave ≥100 μV) are shown in Supplementary Figure 1. The prevalence was 15% for lead III and the upper limit was 49 ms. The prevalence of the Q waves considered significant was 11% or less for the other leads shown and 0.6% or less for chest leads V1-V3 (not shown). Importantly, the limits for Q wave duration were <35 ms for aVL and aVF and <30 ms for leads I, II, V4-V6.
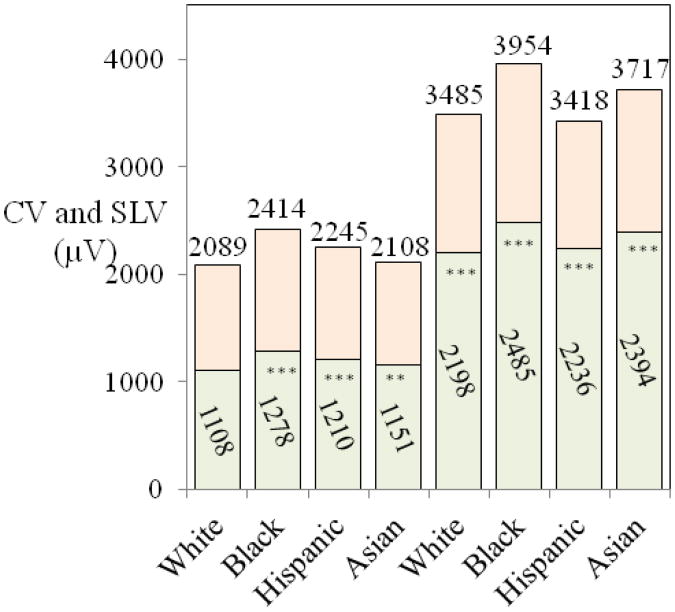
R and S wave amplitudes, Cornell voltage and Sokolow-Lyon voltage Normal limits for R and S wave amplitudes (Supplementary Figure 2) show the expected trends for R and S wave amplitudes, with R wave amplitudes increasing from V1 to V5 and S wave amplitudes decreasing grom V2 to V6. The amplitudes in limb leads were substantially lower than in the chest leads because of lower lead vector strength and QRS vector loop projection differences. Clinically more relevant than the R and S wave amplitudes in different leads are the upper limits for Cornell voltage (CV) and Sokolow-Lyon voltage (SLV) used in clinical criteria for left ventricular hypertrophy (LVH) (Figure 1). The values in Black, Hispanic and Asian women differ significantly from white women, suggesting that rate-specific limits are warranted.
Figure 1.
Mean values (in lower columns) and upper normal limits (98th percentiles, listed on top for Cornell Voltage (CV) (four columns on the left) and Sokolow-Lyon Voltage (SLV) (four columns on the right) by ethnicity. The mean values and the upper limits in black, Hispanic and Asian women differ significantly from white women and race-specific upper normal limits are necessary.
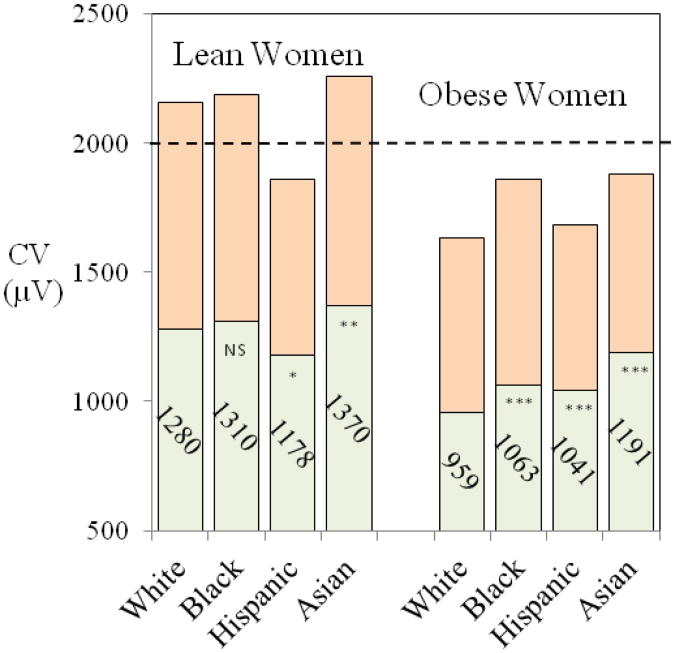
Figure 2 shows the mean values and upper second percentile limits for CV in African-American, Hispanic and Asian/Pacific women for lean and obese women with obesity status matched for body mass index (BMI) in white women (BMI < 23 kg/m2 for lean women and BMI >32 kg/m2 for obese BMI group). The horizontal dashed line represents the commonly used CV (35) threshold for LVH derived in predominantly white women. The limits are above the 2000 μV threshold for LVH in lean white, African-American and Asian/Pacific women but considerably below the threshold in lean Hispanic women and in obese women in all ethnic groups. CV reduction was particularly pronounced in extreme obesity.
Figure 2.
Mean values (lower columns) and upper normal limits (98th percentiles, top columns) for Cornell Voltage in women by ethnic group with obesity status matched for body mass index (BMI) in white women (BMI < 23 kg/m2 for lean women and BMI >32 kg/m2 for obese BMI group). The limits are above the 2000 μV threshold for LVH in lean white, African-American and Asian/Pacific women but considerably below the threshold in lean Hispanic women and in obese women in all ethnic groups.
Normal standards for T wave amplitudes The statistics in Supplementary Table 3 show that T wave amplitudes in most ECG leads were lower in African-American and Hispanic women than in white and Asian-Pacific women. Race specific limits may be necessary for T wave amplitudes although combined limits for white and Asian-Pacific women and African-American and Hispanic women could also be considered. TV1 and TV2 can be biphasic, most commonly positive/negative. In V1, the amplitude of the positive initial component is low. Figure 3 summarizes for the combined group of women the normal 96 % range (2nd to 98th percentile) for T wave amplitudes in chest leads including initial T V1 amplitude for biphasic positive/negative (+/-) T wave.
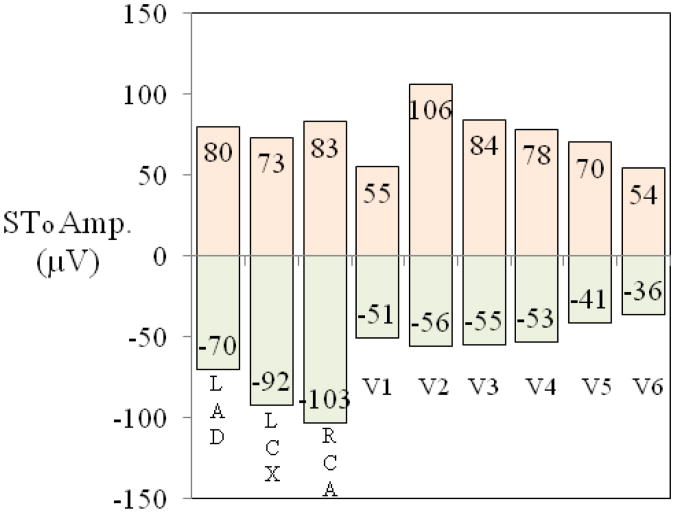
Figure 3.
Normal limits (96% range) for STonset (STo, the J-point amplitude) in vessel-specific bipolar left anterior descending (LAD), left circumflex (LCX) and right coronary artery (RCA) leads and in chest leads V1-V6 for the combined group of women. Bipolar LAD lead is recorded from 1/2 interspace below V8 to V3, LCX from 1/2 interspace above V2 to 1/2 interspace above V8, and RCA 1 interspace above V2 (at third interspace) to left iliac crest. Electrode placements for limb leads are the same as for Mason-Likar leads and V3-V6 positions for monitoring leads are at the level of V1 and V2.
Normal limits for ST onset elevation and depression in standard and monitoring leads Normal ranges (from 2nd to 98th percentile) for ST J-point amplitude (labeled as STofor ST onset) for standard leads I, II, aVL, aVF and V1-V6 for the combined group of women (Supplementary Figure 4) show that the upper normal limits for STo were all well below 100 μV with the exception of lead V2 (112 μV) . The upper limits for STo in monitoring leads were also below 100 μV except 106 μV for V2 (Figure 3). Although not shown, the upper normal limits for monitoring leads M-I and M-II were higher than in standard leads I and II but still below 100 μV. Lower normal limits were approximately 50 μV for monitoring chest leads V1-V6 and approximately 100 μV for vessel specific bipolar leads.
Discussion
The primary goal of the present investigation was to establish normal reference values for an extensive set of ECG variables in post-menopausal women. Previously available normal standards for women have been largely in younger age groups of white women, with limited sample size in older age groups and in other racial groups. Normal values were established for several novel repolarization-related ECG variables such as rate-adjusted QTp and QTe and the TpTe interval which is considered to represent global RT dispersion. Normal standards for these rate-adjusted intervals have not been available before except for QTea. Similarly, normal standards have not been available previously for θ(Rm|Tm) and θ(Tp|Tref), prognostically important spatial angles reflecting deviations of repolarization from normal direction of repolarization sequence.
Rate-adjustment for QTe and QTp In this group of healthy postmenopausal women, formulas for QTea and QTpa as linear functions of the RR interval gave as high R-square values as the power functions with optimal exponents from log-log regression. Racial differences in rate sensitivity (slope coefficients of regression on RR interval) were small in these healthy women and a common linear rate adjustment formula gave an adequate rate adjustment in all racial groups. QT measurements in the present study were made by the Marquette 12SL program. QT measurements made by different algorithms differ slightly due to differing procedures for identification of T wave end (36). Outlier QT and QT subinterval values were replaced in the present study by predicted interval values which can be expected to enhance the stability of the upper normal limits established for rate-adjusted intervals.
Common upper normal limits for all racial groups combined were established as 450 ms for QTea, and 365 ms for QTpa. TpTe interval which reflects global RT dispersion also retained a slight rate dependence and for this reason rate adjusted TpTe was calculated as TpaTea interval, the difference between rate adjusted QTe and QTp. Alternatively, rate adjusted TpTe interval (TpTe)a can be computed by the formula: (TpTe)a = TpTe + 0.5*(HR-60), so that 5 ms is added to TpTe for each heart rate increment by 10 bpm above 60/min. This simpler approach was also used to derive rate adjusted QTp from QTea and TpTe (Formula 14 in Table 1). Considering the upper normal limit for rate-adjusted TpTe interval (last row in Table 2), 110 ms can be recommended as practical limits for white and Hispanic women and 120 ms for African-American women and Asian/Pacific women.
The normal standards established for QT and QT subintervals are primarily intended for use in evaluation of QT prolongation prevalence in epidemiological studies and for prognostic evaluation of their utility in risk prediction. The upper normal limit of 450 ms for QTea established for the healthy women in our study can be compared with the 98th percentile limits rate-adjusted QT of 457 ms by Bazett's formula and 445 ms by Fridericia's formulas documented in a combined group of 79,743 men and women by Mason et al. in an ECG measurement file from pharmaceutical company–sponsored clinical trials (37). That document contained extensive evaluation data with 16 tables on QT and three other ECG variables (PR and QRS interval and frontal plane QRS/T angles). However, potential residual rate-dependent bias for QT adjustment formulas was not evaluated in their study population which may limit the utility of these standards at higher heart rates. Also, the study of Mason et al. did not consider the known gender differences in QT rate sensitivity (38) which leaves open the question of the applicability to women of the formulas from combined gender groups.
Monitoring for ST elevation and depression Limb electrodes placed by clinical ECG monitoring personnel in Mason-Likar positions can be considered already as a de facto standard clinical procedure in ST monitoring. The results from the present study suggest that normal standards for ST elevation and depression in women for standard leads with electrodes placed on the breast are also applicable for monitoring chest leads with chest electrodes placed on the breast at V1-V2 level.
The lower limits for ST change (mean - 2SD) in special vessel-specific monitoring leads as a response to induced acute ischemia in the report of Horáĉek et al. (30) were 114 μV for LAD, 53 μV for LCX and 24 μV for RCA, indicating that approximately 98% of the ischemic changes exceeded these limits. The upper normal limits for STo in Figure 3 for monitoring leads and Supplementary Figure 4 for standard leads support the choice of 150 μV for all monitoring leads as a tentative value for abnormal ST elevation evaluated during stable artifact- free recording periods as ensured by ST monitoring quality control software. The threshold can be modified upwards for instance to 200 μV if less stable periods cause too many alarms. For monitoring of the change in ST elevation or depression, 50 μV increase or decrease from baseline STo value can be suggested as a tentative triggering threshold for alarm.
Only 0.6% of the ECGs were associated with horizontal or downsloping ST segments (MC 4.2 or 4.1) and thus the ST elevations in these normal women were associated with upwards-sloping ST segments. ST elevation associated with J-point “notches” has recently been the focus of investigations on ST segment patterns considered by some investigators to represent early repolarization (39). This topic was not considered in the present investigation.
Effective display formats are available for ST monitoring applications such as introduced by Pelter et al. using a 3-D plot of ST onset elevation values over the monitoring period in all 12 ECG leads (40). The 150 μV threshold introduced here can be applied for acute ischemia. A simple ST Index (STI) can also be considered for monitoring of ST elevation status in patients suspected for acute coronary syndrome using the formula: STI = 100*STo /BLSTo where BLSTo is the baseline STovalue at admission. A threshold value can then be selected for ST elevation change alarm as 10% or 15% change in STI.
Normal standards for QRS/T angle and other repolarization-related ECG parameters Normal standards for θ(Rm|Tm), θ(Tp|Tref) and other angular variables reflecting deviations of repolarization from normal direction of repolarization sequence revealed that race-specific normal limits are necessary to identify prognostically important repolarization abnormalities. These race-specific upper normal limits are listed in Table 3, including normal values for RTp, RTxd and spatial vector magnitudes for STo, To and Tp.
Electrode placement, obesity, breast protuberance and ECG amplitudes Ancillary analyses (Figure 2) indicated that CV and SLV values differed substantially with BMI in all ethnic groups. In particular, both CV and SLV were lower in obese than in lean women, suggesting possible effect of increased distance from cardiac source to the body surface in obese women with large breast. However, a previous investigation in 6814 women in the Atherosclerosis in Communities (ARIC) study concluded that breast protuberance alone had only a relatively small effect on CV and SLV (R-square < 0.01) and n contrast, chest size (ellipsoidal approximation of bony thorax circumference) was a dominant factor influencing CV and SLV than breast protuberance (27). There is a scarcity of data with direct comparison of chest lead amplitudes with electrode placement under the breast and on the breast. Macfarlane et al. compared R wave amplitudes in V3-V6 in 84 women and noted that R amplitude in V4 was 28 μV higher (95% CI -9 to +65μV) and in V6 134 μV lower (95% CI-160 to -108 μV) with electrode placed under the breast vs. in standard positions on the breast (41). Repeatability of measured amplitudes was higher with electrodes placed on the breast than under the breast. The role of constitutional factors as determinants of CV and SLV appears complex and the question of the utility of current ECG criteria for LVH remains open.
The electrode placement issue has also baring on normal standards for STo in monitoring leads, derived in the present investigation with chest electrodes placed on the breast rather than under the breast. Since the chest size rather than breast protuberance is the dominant factor influencing chest lead amplitudes, it appears reasonable to conclude that the normal standards for monitoring leads would not differ substantially whether derived from source data with electrodes on the breast or under the breast. Limited data available for ST J-point amplitude in older women with chest electrodes placed under the breast reveal that the upper normal limit for ST J-point amplitude in women 40 years old and over is 140 μV or less in V2, 80 μV or less in V4 and 60 μV or less in V6 (42). The corresponding upper normal limits in Supplementary Figure 4 for chest leads V2, V4 and V6 with electrodes placed on the breast are 102 μV, 78 μV and 63 μV respectively, and in Figure 3 for monitoring leads V2, V4 and V6 106 μV, 78 μV and 54 μV, respectively. These differences can be considered relatively unimportant considering other sources of variability in the monitoring setup.
Clinical perspectives As noted in the Introduction, substantial new information has emerged recently about prognostic value for a variety of novel depolarization- and repolarization-related ECG variables. Of particular clinical interest among these novel ECG parameters are TpTe interval and spatial angles θ(Rm|Tm) and θ(Tp|Tref). TpTe interval is considered to represent global dispersion of repolarization thought to be a marker of the risk of arrhythmic events and sudden cardiac death (13-17). θ(Rm|Tm) and θ(Tp|Tref) are of clinical interest as measures of abnormal deviation of spatial direction of repolarization sequence associated with acute coronary syndrome and CHD mortality risk (7-10). The present report also introduces guidelines for practical clinical monitoring of ST elevation in women. A recent report from the Practical Use of the Latest Standards for Electrocardiography (PULSE) Trial concluded that there is overutilization particularly of arrhythmia monitoring in the hospital settings but at the same time there is underutilization of ischemia monitoring and QT monitoring when the need of monitoring is clinically indicated (43). Another comprehensive consensus report covering all aspects of hospital cardiac care monitoring commented on the lack of adequate guidelines for QT monitoring and noted that quality control problems cause frequent false alarms in QT and ST monitoring hindering their wider acceptance (44). There is scarcity of normal ECG standards particularly for older women. For instance, the most comprehensive reference document in Electrocardiology (51) contains normal standards derived from a sample of white women but it contains only 79 women in age group 50 years old and older. The extensive set of normal standards introduced in the present communication from racially diverse cohort of postmenopausal women should provide a valuable supplement to available ECG reference data.
Limitations of the study Our study group consisted of healthy postmenopausal women. A separate investigation is required to evaluate gender differences in normal reference values in younger women and to establish normal reference standards for men not yet available for a variety of newer ECG predictor variables. ST elevation associated with J-point “notches” considered by some investigators to represent early repolarization was not considered here and the topic will require a separate investigation.
Supplementary Material
Supplementary Figure 1. Prevalence (%) and 98th percentile normal limits for Q wave duration for Q waves with amplitude ≥100 μV followed by an R wave ≥100 μV in limb leads and in chest leads V4-V6. The prevalence was 0.6% or less in V1-V3.
Supplementary Figure 2. Upper normal limits for R waves (98th percentiles) and lower normal limits for S waves (2nd percentiles) in limb leads I, II, aVL and aVF and in chest leads V1-V6. The expected trend is seen in V1 to V5 with increasing R and S amplitudes (normal limits listed on top). The amplitudes in limb leads are substantially lower than in the chest leads because of lower lead vector strength and QRS vector loop projection differences.
Supplementary Figure 3. Normal 96 % range (2nd to 98th percentile) for T wave amplitudes in chest leads including initial T V1 amplitude for biphasic positive/negative (+/-) T wave. TV1 was biphasic positive/negative in 2278 women (6.3%) and biphasic negative/positive in only 59 women (0.2%) (not shown).
Supplementary Figure 4. Normal 96% range (from second to 98th percentile) for STonset (STo, the J-point amplitude) in standard leads I, II, aVL, aVF and in chest leads V1-V6 for the combined group of women.
Acknowledgments
Short list of WHI investigators. The WHI program is funded by the National Heart, Lung, and Blood Institute, National Institutes of Health, U.S. Department of Health and Human Services through contracts HHSN268201100046C, HHSN268201100001C, HHSN268201100002C, HHSN268201100003C, HHSN268201100004C, and HHSN271201100004C.
Appendix and Short list of WHI investigators
Short List of whi Investigators
Program Office
(National Heart, Lung, and Blood Institute, Bethesda, Maryland) Jacques Rossouw, Shari Ludlam, Dale Burwen, Joan McGowan, Leslie Ford, and Nancy Geller
Clinical Coordinating Center: Clinical Coordinating Center
(Fred Hutchinson Cancer Research Center, Seattle, WA) Garnet Anderson, Ross Prentice, Andrea LaCroix, and Charles Kooperberg
Investigators and Academic Centers
(Brigham and Women's Hospital, Harvard Medical School, Boston, MA) JoAnn E. Manson; (MedStar Health Research Institute/Howard University, Washington, DC) Barbara V. Howard; (Stanford Prevention Research Center, Stanford, CA) Marcia L. Stefanick; (The Ohio State University, Columbus, OH) Rebecca Jackson; (University of Arizona, Tucson/Phoenix, AZ) Cynthia A. Thomson; (University at Buffalo, Buffalo, NY) Jean Wactawski-Wende; (University of Florida, Gainesville/Jacksonville, FL) Marian Limacher; (University of Iowa, Iowa City/Davenport, IA) Robert Wallace; (University of Pittsburgh, Pittsburgh, PA) Lewis Kuller; (Wake Forest University School of Medicine, Winston-Salem, NC) Sally Shumaker.
Women's Health Initiative Memory Study
(Wake Forest University School of Medicine, Winston-Salem, NC) Sally Shumaker
Footnotes
A short list of Women's Health Initiative investigators appears in the Appendix. The Women's Health Initiative program is funded by the National Heart, Lung, and Blood Institute, U.S. Department of Health and Human
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Rautaharju PM, Kooperberg C, Larson JC, LaCroix A. Electrocardiographic abnormalities that predict coronary heart disease events and mortality in postmenopausal women: the Women's Health Initiative. Circulation. 2006;113:473. doi: 10.1161/CIRCULATIONAHA.104.496091. [DOI] [PubMed] [Google Scholar]
- 2.Rautaharju PM, Prineas RJ, Wood J, et al. Electrocardiographic predictors of new-onset heart failure in men and in women free of coronary heart disease (from the Atherosclerosis in Communities [ARIC] Study) Am J Cardiol. 2007;100:1437. doi: 10.1016/j.amjcard.2007.06.036. [DOI] [PubMed] [Google Scholar]
- 3.Aro AL, Anttonen O, Tikkanen JT, et al. Intraventricular conduction delay in a standard 12-lead electrocardiogram as a predictor of mortality in the general population. Circulation Arrhythm Electrophysiol. 2011;4:704. doi: 10.1161/CIRCEP.111.963561. [DOI] [PubMed] [Google Scholar]
- 4.Teodorescu C, Reinier K, Uy-Evanado A, et al. Prolonged QRS duration on the resting ECG is associated with SCD risk in coronary disease, independent of prolonged ventricular repolarization. Heart Rhythm. 2011;8:1562. doi: 10.1016/j.hrthm.2011.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kurl S, Mäkikallio TH, Rautaharju P, et al. Duration of QRS complex in resting electrocardiogram is a predictor of sudden cardiac death in men. Circulation. 2012;125:2588. doi: 10.1161/CIRCULATIONAHA.111.025577. [DOI] [PubMed] [Google Scholar]
- 6.Rautaharju PM, Zhou SH, Gregg RE, et al. Electrocardiographic estimates of action potential durations and transmural repolarization time gradients in healthy subjects and in acute coronary syndrome patients-profound differences by sex and by presence vs absence of diagnostic ST elevation. J Electrocardiol. 2011;44:309. doi: 10.1016/j.jelectrocard.2010.11.009. [DOI] [PubMed] [Google Scholar]
- 7.Rautaharju PM, Zhou SH, Gregg RE, et al. Heart rate, gender differences, and presence versus absence of diagnostic ST elevation as determinants of spatial QRS|T angle widening in acute coronary syndrome. Am J Cardiol. 2011;107:744. doi: 10.1016/j.amjcard.2011.02.333. [DOI] [PubMed] [Google Scholar]
- 8.Zhang ZM, Prineas RJ, Case D, et al. The Comparison of the Prognostic Significance of the Electrocardiographic QRS/T Angles in Predicting Incident Coronary Heart Disease and Total Mortality (from the Atherosclerosis Risk In Communities Study) Am J Cardiol. 2007;100:844. doi: 10.1016/j.amjcard.2007.03.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lown MT, Munyombwe T, Harrison, et al. Association of Frontal QRS-T Angle–Age Risk Score on Admission Electrocardiogram With Mortality in Patients Admitted With an Acute Coronary Syndrome. Evaluation of Methods and Management of Acute Coronary Events (EMMACE) Investigators. Am J Cardiol. 2012;109:307. doi: 10.1016/j.amjcard.2011.09.014. [DOI] [PubMed] [Google Scholar]
- 10.Whang W, Shimbo D, Levitan EB, et al. Relations between QRS|T angle, cardiac risk factors, and mortality in the Third National Health and Nutrition Examination Survey (NHANES III) Am J Cardiol. 2012;109:981. doi: 10.1016/j.amjcard.2011.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kors JA, de Bruyne MC, Hoes AW, et al. T axis as an independent indicator of risk of cardiac events in elderly people. Lancet. 1998;352:601. doi: 10.1016/S0140-6736(97)10190-8. [DOI] [PubMed] [Google Scholar]
- 12.Rautaharju PM, Clark-Nelson J, Kronmal RA, et al. Usefulness of T-axis deviation as an independent risk indicator for incident cardiac events in older men and women free from coronary heart disease. The CHS Study. Am J Cardiol. 2001;88:118. doi: 10.1016/s0002-9149(01)01604-6. [DOI] [PubMed] [Google Scholar]
- 13.Panikkath R, Reinier K, Uy-Evanado A, et al. Prolonged Tpeak-to-tend interval on the resting ECG is associated with increased risk of SCD. Circulation Arrhythmia and electrophysiology. 2011;4:441. doi: 10.1161/CIRCEP.110.960658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gupta P, Patel C, Patel H, et al. Tp-e/QT ratio as an index of arrhythmogenesis. J Electrocardiol. 2008;41:567. doi: 10.1016/j.jelectrocard.2008.07.016. [DOI] [PubMed] [Google Scholar]
- 15.Kanters JK, Haarmark C, Vedel-Larsen E, et al. Tpeak Tend interval in long QT syndrome. J Electrocardiol. 2008;41:603. doi: 10.1016/j.jelectrocard.2008.07.024. [DOI] [PubMed] [Google Scholar]
- 16.Castro Hevia J, Antzelevitch C, Tornés Bárzaga F, et al. Tpeak-Tend and Tpeak-Tend dispersion as risk factors for ventricular tachycardia/ventricular fibrillation in patients with the Brugada syndrome. J Am Coll Cardiol. 2006;47:1828. doi: 10.1016/j.jacc.2005.12.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang J, Shan Q, Yang B, et al. Tpeak-Tend interval as a new risk factor for arrhythmic event in patient with Brugada syndrome. J Nanjing Medical University. 2007;21:213. [Google Scholar]
- 18.Al-Zaiti SS, Runco KN, Carey MG. Increased T wave complexity can indicate subclinical myocardial ischemia in asymptomatic adults. J Electrocardiol. 2011;44:684. doi: 10.1016/j.jelectrocard.2011.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rautaharju PM, Prineas RJ, Kadish A, et al. Normal standards for QT and QT subintervals derived from a large ethnically diverse population of women aged 50 to 79 years (The Women's Health Initiative [WHI]) Am J Cardiol. 2006;97:730. doi: 10.1016/j.amjcard.2005.09.108. [DOI] [PubMed] [Google Scholar]
- 20.Zhang Y, Post WS, Blasco-Colmenares E, et al. Electrocardiographic QT interval and mortality: a meta-analysis. Epidemiology. 2011;22:660. doi: 10.1097/EDE.0b013e318225768b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Roden DM. Drug-induced prolongation of the QT interval. N Engl J Med. 2004;350:1013. doi: 10.1056/NEJMra032426. [DOI] [PubMed] [Google Scholar]
- 22.Shah R, Hondeghem LM. Refining detection of drug-induced proarrhythmia: QT interval and TRIaD. Heart Rhythm. 2005;2:758. doi: 10.1016/j.hrthm.2005.03.023. [DOI] [PubMed] [Google Scholar]
- 23.Huikuri HV, Castellanos A, Myerburg RJ. SCD due to cardiac arrhythmias. N Engl J Med. 2001;345:1473. doi: 10.1056/NEJMra000650. [DOI] [PubMed] [Google Scholar]
- 24.Hohnloser SH, Klingenheben T, Singh BN. Amiodarone-associated proarrhythmic effects. A review with special reference to torsade de pointes tachycardia. Ann Intern Med. 1994;121:529. doi: 10.7326/0003-4819-121-7-199410010-00009. [DOI] [PubMed] [Google Scholar]
- 25.Rautaharju PM, Zhou SH, Gregg RE, et al. Electrocardiographic estimates of regional action potential durations and repolarization time subintervals reveal ischemia-induced abnormalities in acute coronary syndrome not evident from global QT. J Electrocardiol. 2011;44:718. doi: 10.1016/j.jelectrocard.2011.08.007. [DOI] [PubMed] [Google Scholar]
- 26.The Women's Health Initiative Study Group. Design paper. Design of the Women's Health Initiative Clinical Trial and Observational Study. Control Clin Trials. 1998;19:61. doi: 10.1016/s0197-2456(97)00078-0. [DOI] [PubMed] [Google Scholar]
- 27.Rautaharju PM, Park L, Rautaharju FS, et al. A standardized procedure for locating and documenting ECG chest electrode positions. Consideration of the effect of breast tissue on ECG amplitudes in women. J Electrocardiol. 1998;31:17. doi: 10.1016/s0022-0736(98)90003-6. [DOI] [PubMed] [Google Scholar]
- 28.Andersen MP, Xue JQ, Graff C, et al. New descriptors of T-wave morphology are independent of heart rate. J Electrocardiol. 2008;41:557. doi: 10.1016/j.jelectrocard.2008.07.021. [DOI] [PubMed] [Google Scholar]
- 29.Horáĉek BM, Warren JW, Field DQ, et al. Statistical and deterministic approaches to designing transformations of electrocardiographic leads. J Electrocardiol. 2002;35(suppl):41. doi: 10.1054/jelc.2002.37154. [DOI] [PubMed] [Google Scholar]
- 30.Horácek BM, Warren JW, Penney CJ, MacLeod RS, Title LM, Gardner MJ, Feldman CL. Optimal electrocardiographic leads for detecting acute myocardial ischemia. J Electrocardiol. 2001;34(Suppl):97. doi: 10.1054/jelc.2001.28844. [DOI] [PubMed] [Google Scholar]
- 31.Bazett HC. An analysis of time relationships of electrocardiograms. Heart. 1920;7:353. [Google Scholar]
- 32.Fridericia LS. Duration of systole in the electrocardiogram. Acta Med Scand. 1920;53:469. [Google Scholar]
- 33.Sagie A, Larson MG, Goldberg RJ, Bengtson JR, Levy DA. An improved method for adjusting the QT interval for heart rate (the Framingham Heart Study) Am J Cardiol. 1992;70:797. doi: 10.1016/0002-9149(92)90562-d. [DOI] [PubMed] [Google Scholar]
- 34.Hodges M, Salerno D, Erlien D. Bazett's QT correction reviewed: Evidence that a linear correction for heart rate is better. J Am Coll Cardiol. 1983;1(abstract):694. [Google Scholar]
- 35.Casale PN, Devereux RB, Kligfield P, et al. Electrocardiographic detection of left ventricular hypertrophy: development and prospective validation of improved criteria. J Am Coll Cardiol. 1985;6:572. doi: 10.1016/s0735-1097(85)80115-7. [DOI] [PubMed] [Google Scholar]
- 36.Kligfield P, Hancock EW, Helfenbein ED, et al. Relation of QT interval measurements to evolving automated algorithms from different manufacturers of electrocardiographs. Am J Cardiol. 2006;98:88. doi: 10.1016/j.amjcard.2006.01.060. [DOI] [PubMed] [Google Scholar]
- 37.Mason JW, Ramseth DJ, Chanter DO, Moon TE, Goodman DB, Mendzelevski B. Electrocardiographic reference ranges derived from 79,743 ambulatory subjects. J Electrocardiol. 2007;40:218. doi: 10.1016/j.jelectrocard.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 38.Smetana P, Velislav N, Batchvarova VN, Hnatkova K, Ghuran A, Harries M, Murtagh AM, Malik M. The sex differences in the QT interval duration depend on heart rate. J Am Coll Cardiol. 2002;39:97. [Google Scholar]
- 39.Gussak I, Antzelevitch C, Bjerregaard P. ECG phenomena of the early ventricular repolarization: The Early Repolarization Syndrome. In: Gussak I, Antzelevitch C, editors. Cardiac Repolarization Bridging Basic and Clinical Science. Totowa, NJ: Humana Press, Inc; 2003. pp. 1–548. [Google Scholar]
- 40.Pelter MM, Adams MG, Drew BJ. Transient myocardial ischemia is an independent predictor of adverse in-hospital outcomes in patients with acute coronary syndromes treated in the telemetry unit. Heart Lung. 2003;32:71. doi: 10.1067/mhl.2003.11. [DOI] [PubMed] [Google Scholar]
- 41.Macfarlane PW, Colaco R, Stevens K, Reay P, Beckett C, Aitchison T. Precordial electrode placement in women. Netherlands Heart Journal. 2003;11:118. [PMC free article] [PubMed] [Google Scholar]
- 42.Macfarlane PW, van Oosterom A, Pahlm O, Kligfield P, Janse M, Camm J, editors. Comprehensive Electrocardiography Appendix 1: Adult Normal Limits. London: Springer-Verlag; 2013. pp. 2058–2122. [Google Scholar]
- 43.Funk M, Winkler CG, May JL, et al. Unnecessary arrhythmia monitoring and underutilization of ischemia and QT interval monitoring in current clinical practice: baseline results of the Practical Use of the Latest Standards for Electrocardiography trial. J Electrocardiol. 2010;43:542. doi: 10.1016/j.jelectrocard.2010.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Drew BJ, Califf RM, Funk M, et al. Practice standards for electrocardiographic monitoring in hospital settings: an American Heart Association scientific statement from the Councils on Cardiovascular Nursing, Clinical Cardiology, and Cardiovascular Disease in the Young: endorsed by the International Society of Computerized Electrocardiology and the American Association of Critical-Care Nurses. Circulation. 2004;110:2721. doi: 10.1161/01.CIR.0000145144.56673.59. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. Prevalence (%) and 98th percentile normal limits for Q wave duration for Q waves with amplitude ≥100 μV followed by an R wave ≥100 μV in limb leads and in chest leads V4-V6. The prevalence was 0.6% or less in V1-V3.
Supplementary Figure 2. Upper normal limits for R waves (98th percentiles) and lower normal limits for S waves (2nd percentiles) in limb leads I, II, aVL and aVF and in chest leads V1-V6. The expected trend is seen in V1 to V5 with increasing R and S amplitudes (normal limits listed on top). The amplitudes in limb leads are substantially lower than in the chest leads because of lower lead vector strength and QRS vector loop projection differences.
Supplementary Figure 3. Normal 96 % range (2nd to 98th percentile) for T wave amplitudes in chest leads including initial T V1 amplitude for biphasic positive/negative (+/-) T wave. TV1 was biphasic positive/negative in 2278 women (6.3%) and biphasic negative/positive in only 59 women (0.2%) (not shown).
Supplementary Figure 4. Normal 96% range (from second to 98th percentile) for STonset (STo, the J-point amplitude) in standard leads I, II, aVL, aVF and in chest leads V1-V6 for the combined group of women.