Abstract
The Schaffer collaterals are among the major glutamatergic inputs to CA1 pyramidal neurons, the primary output of the hippocampus, which also receive sparse recurrent inputs from pyramidal neurons in the CA1 field. Although tonically active α7 nicotinic acetylcholine receptors (nAChRs) have been shown to sustain spontaneous glutamate transmission to CA1 pyramidal neurons in hippocampal slices under resting conditions, it remains to be determined whether these receptors are those expressed by CA3 or CA1 pyramidal neurons. This study was designed to test the hypothesis that the CA3 field of the hippocampus is a significant source of α7 nAChR-sustained glutamatergic transmission to CA1 pyramidal neurons. To this end, spontaneous excitatory postsynaptic currents (EPSCs) were recorded from CA1 and CA3 pyramidal neurons in intact rat hippocampal slices as well as from CA1 pyramidal neurons in CA3-ablated slices under various experimental conditions. Surgical removal of the CA3 region from the slices reduced by 20% the frequency of spontaneous EPSCs recorded from CA1 pyramidal neurons. This finding is in agreement with the concept that the CA3 field contributes significantly to the maintenance of spontaneous glutamatergic synaptic activity in CA1 pyramidal neurons. In addition, the α7 nAChR antagonist methyllycaconitine (MLA, 10 nM) reduced the frequency of spontaneous EPSCs recorded from CA1 pyramidal neurons by 30% in intact slices and 12% in CA3-ablated slices. Taken together, these results demonstrate that tonically active α7 nAChRs in CA3 pyramidal neurons and/or in the Mossy fibers that innervate the CA3 pyramidal neurons do in fact contribute to the maintenance of glutamatergic synaptic activity in CA1 pyramidal neurons of hippocampal slices under resting conditions.
Keywords: hippocampus, EPSC, methyllycaconitine, tetrodotoxin, α7 nAChR, pyramidal neuron
1. Introduction
CA1 pyramidal neurons are the major output neurons of the hippocampus and have been extensively studied with respect to glutamate synaptic transmission, long-term potentiation and depression, learning and memory. The major excitatory drive of CA1 pyramidal neurons originates primarily from CA3 pyramidal neurons and other extrinsic sources, though sparse recurrent glutamatergic inputs also originate from other pyramidal neurons in the CA1 field of the hippocampus [3,7,15]. Glutamate excitatory postsynaptic potentials modify synaptic plasticity by serving as the coincidence detector during back-propagating action potentials [8]. Therefore, understanding the mechanisms that regulate spontaneous glutamatergic transmission in CA1 pyramidal neurons provides valuable information about the neurophysiology of the hippocampal neurocircuitry.
Nicotinic acetylcholine receptors (nAChRs), particularly those containing the α7 subunit, play an important role in induction of long-term potentiation in the hippocampus [14] through modulation of glutamate transmission [9]. Although evidence exists that tonically active α7 nAChRs regulate excitatory and inhibitory synaptic transmission in rat hippocampal slices [2,4,5], the location of α7 nAChRs that control the activity of glutamatergic inputs to CA1 pyramidal neurons remains obscure.
A study carried out in acute hippocampal slices demonstrated that functional α7 nAChRs are present on CA3 pyramidal neurons [11] and that their activation by synaptically released neurotransmitter leads to activation of synaptic nicotinic currents [10]. In vivo studies have also revealed that the excitability of CA3 pyramidal neurons is partially regulated by the activation of α7 nAChRs [13]. However, it is unclear whether α7 nAChR-mediated glutamate release from CA3 pyramidal neurons contributes to the maintenance of spontaneous glutamatergic transmission in CA1 pyramidal neurons under resting conditions. Pyramidal neurons in the CA1 field also express α7 nAChRs [12], but it is hitherto unknown whether activation of these receptors by basal levels of cholinergic transmitter in the hippocampus contributes to the maintenance of spontaneous glutamate synaptic activity in other CA1 pyramidal neurons.
In the present study we assessed the origin of α7 nAChR-dependent spontaneous glutamatergic transmission in CA1 pyramidal neurons. To this end, the frequency and amplitude of spontaneous excitatory postsynaptic currents (EPSCs) recorded from CA1 pyramidal neurons in intact hippocampal slices were compared to those recorded from CA1 pyramidal neurons in CA3-ablated hippocampal slices under specific experimental conditions. Results presented here provide the first direct evidence that under resting conditions α7 nAChR-dependent glutamatergic input to CA1 pyramidal neurons is largely dependent on the structural integrity of the CA3 field.
2. Materials and Methods
2.1 Slice preparation
Hippocampal slices were prepared from 30–35-day-old male Sprague-Dawley rats (from Charles River Laboratories, Wilmington, MA). Animal care and handling were done strictly in accordance with the guidelines set forth by the Institutional Animal Care and Use Committee of the University of Maryland. Animals were euthanized by asphyxiation in a CO2 atmosphere followed by decapitation. Their brains were removed and placed in ice-cold artificial cerebrospinal fluid (ACSF), which was composed of (in mM): NaCl, 125; NaHCO3, 25; KCl, 2.5; NaH2PO4, 1.25; CaCl2, 2, MgCl2, 1; and dextrose, 25. The ACSF was bubbled with 95% O2 and 5% CO2. The hippocampi were dissected out and sectioned in the transverse plane into 300–350-μm thick slices with a vibratome (Leica VT1000S, Leica Microsystems Inc., Bannockburn). In some cases, the CA3 field of the hippocampus was surgically removed immediately after sectioning. Slices were stored at room temperature for at least 45 min in an immersion chamber containing ACSF continuously bubbled with 95% O2 and 5% CO2 before recordings. For incubation experiments some of the slices were transferred to a chamber containing ACSF with test compounds that was continuously bubbled with 95% O2 and 5% CO2.
2.2. Electrophysiological recordings
Spontaneous EPSCs and inhibitory postsynaptic currents (IPSCs) were recorded at −70 mV and 0 mV, respectively, from the soma of CA1 and CA3 pyramidal neurons according to the standard whole-cell mode of the patch-clamp technique in the presence of the muscarinic receptor antagonist atropine (0.5 μM). The internal pipette solution contained (in mM): ethylene-glycol bis(β-amino-ethyl ether)-N-N′-tetraacetic acid, 10; HEPES, 10; Cs-methane sulfonate, 130; CsCl, 10; MgCl2, 2; and lidocaine N-ethyl bromide (QX-314), 5 (pH adjusted to 7.3 with CsOH). All recordings were done at room temperature (22–24°C). Only a single neuron was studied per slice. Therefore, the number of neurons represents the number of hippocampal slices analyzed. The number of animals studied in each set of experiments is stated in the figure legend. EPSCs and their kinetics were analyzed in 5-min recordings using the Clampfit module of the pCLAMP 9.0 software (Molecular Devices, Sunnyvale, USA).
2.3. Drugs
Atropine sulfate, (−)bicuculline methiodide, 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX), N-(2,6-dimethylphenylcarbamoylmethyl) triethylammonium bromide (QX-314), 2-amino-5-phosphonovaleric acid (APV), and tetrodotoxin (TTX) were purchased from Sigma Chemical Co. (St. Louis, MO). Methyllycaconitine citrate (MLA) was a gift from Professor M. H. Benn (Dept. Chemistry, Univ. Calgary, Alberta, Canada).
3. Results
3.1. Surgical ablation of the CA3 field suppresses spontaneous EPSCs in CA1 pyramidal neurons
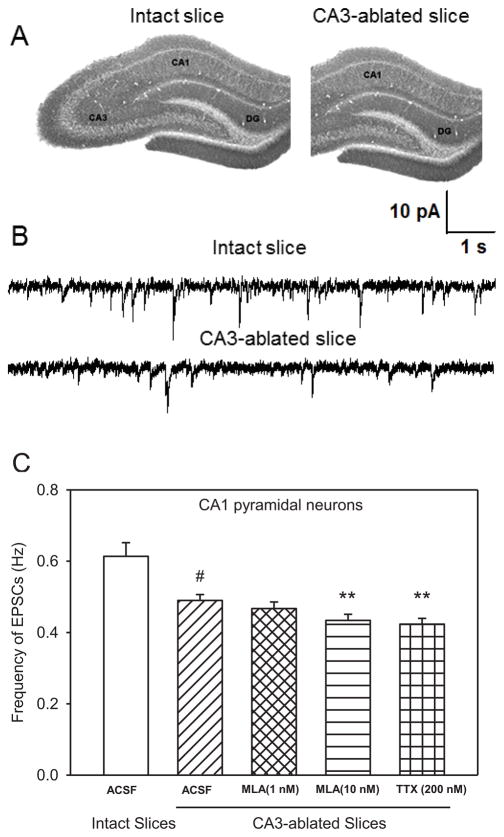
To determine the extent to which the CA3 region contributes to spontaneous glutamatergic transmission in CA1 pyramidal neurons in hippocampal slices under resting conditions, the frequency and amplitude of spontaneous EPSCs recorded from CA1 pyramidal neurons in CA3-ablated slices were compared to those recorded from the same type of neurons in intact slices (Figure 1A, B). The mean frequency of EPSCs recorded from CA1 pyramidal neurons in CA3-ablated slices was 0.49 ± 0.02 Hz, which is 19.7 % lower than that recorded from CA1 pyramidal neurons in intact slices (Figure 1C). The mean peak amplitude of EPSCs recorded from CA1 pyramidal neurons in CA3-ablated slices was also significantly smaller than that recorded from CA1 pyramidal neurons in the intact slices (Table 1).
Figure 1. Effect of CA3 ablation on the effects of MLA and TTX on the frequency of spontaneous EPSCs in CA1 pyramidal neurons.
A. Images of hippocampal slices representing the intact and CA3-ablated preparation. B. Sample traces of spontaneous EPSCs recorded from CA1 pyramidal neurons of 30-day-old rats in intact and CA3-ablated slices. C. Comparison of spontaneous EPSCs recorded from CA1 pyramidal neurons under various conditions. Graph shows mean ± S.E.M frequency of EPSCs from intact slices (ACSF) and CA3-ablated slices under control (ACSF), 15-min superfusion with MLA (1 nM), 15-min superfusion with MLA (10 nM), and 1-h incubation followed by superfusion with 200 nM TTX. Data were obtained from ten neurons from seven rats for intact slices, twelve neurons from seven rats in CA3-ablated slices (control and MLA), and eight neurons from five rats for TTX. # p < 0.001 compared to intact slices by one-way ANOVA followed by Dunnett post-hoc test; ** p < 0.01 compared to ACSF in CA3-ablated slices by one-way ANOVA followed by Dunnett post-hoc test.
Table 1.
Characteristics of spontaneous EPSCs recorded from CA1 pyramidal neurons in intact hippocampal slices and CA3-ablated slices after superfusion with ACSF containing α7 nAChR antagonist MLA (10 nM) for 15 min and incubation for 1 hr or more with ACSF containing TTX (200 nM).
| Slice Preparation | Intact Slices | CA3-ablated Slices | ||||
|---|---|---|---|---|---|---|
| Treatment | ACSF | MLA | TTX | ACSF | MLA | TTX |
| Frequency of EPSCs (Hz) | 0.61 ± 0.02 | 0.43 ± 0.02*** | 0.46 ± 0.03*** | 0.49 ± 0.02*** | 0.43 ± 0.02## | 0.42 ± 0.02## |
| Amplitude (pA) | 12.8 ± 1.76 | 10.9 ± 1.84* | 7.06 ± 0.92*** | 9.94 ± 0.92** | 9.05 ± 1.62# | 7.38 ± 0.81## |
| Rise time 10 to 90% (ms) | 2.49 ± 0.41 | 2.29 ± 0.33 | 2.11 ± 0.25 | 2.18 ± 0.54 | 2.05 ± 0.63 | 2.14 ± 0.71 |
| τd (ms) | 14.9 ± 1.12 | 13.9 ± 1.20 | 12.7 ± 0.94 | 12.6 ± 1.52 | 12.1 ± 1.83 | 13.67 ± 2.18 |
Data are presented as mean ± S.E.M.
p < 0.05;
p < 0.01;
p < 0.001 compared to control (ACSF) value in intact hippocampal slice preparation according to one way ANOVA followed by Dunnett post-hoc test.
p <0.05;
p < 0.01 compared to control (ACSF) value in CA3-ablated slice preparation according to one way ANOVA followed by Dunnett post-hoc test.
3.2. Suppression by the α7 nAChR antagonist MLA of spontaneous EPSCs in CA1 pyramidal neurons of CA3-ablated and intact hippocampal slices
Superfusion of CA3-ablated hippocampal slices with ACSF containing MLA (1 nM) had no significant effect on the frequency of EPSCs recorded from CA1 pyramidal neurons (Figure 1B). In contrast, superfusion of intact hippocampal slices with ACSF containing 1 nM MLA reduced by 18.5 ± 1.4% the frequency of EPSCs. At 10 nM, MLA reduced by 12.2 ± 1.9% and 29.5 ± 2.6% the frequency of EPSCs recorded from CA1 pyramidal neurons in CA3-ablated slices (Figure 1B) and intact slices (Table 1), respectively. MLA did not affect the peak amplitude, rise time, or decay-time constant (τd) of the spontaneous EPSCs recorded in CA3-ablated slices (Table 1).
3.3. The Na+-channel blocker tetrodotoxin (TTX) suppresses the glutamatergic synaptic activity in CA1 pyramidal neurons in CA3-ablated slices
After surgical removal of the CA3 region, slices were incubated for 1 h in TTX (200 nM)-containing ACSF and subsequently superfused with the same solution. TTX reduced the frequency and peak amplitude of EPSCs recorded from CA1 pyramidal neurons in CA3-ablated slices by 14.3 ± 2.2% and 25.8 ± 3.4%, respectively (Table 1; Figure 1B). In intact hippocampal slices, TTX (200 nM) reduced the frequency and amplitude of EPSCs recorded from CA1 pyramidal neurons by 24.6 ± 2.1% and 44.8 ± 3.9 %, respectively (Table 1). Neither the rise time nor τd of the EPSCs was affected by TTX (Table 1).
3.4. Spontaneous IPSCs in CA1 pyramidal neurons were not affected by CA3 removal
Bicuculline-sensitive spontaneous IPSCs were recorded from CA1 pyramidal neurons at 0 mV in CA3-ablated slices. The frequency and peak amplitude of IPSCs recorded from the neurons in CA3-ablated slices (1.02 ± 0.07 Hz and 22.8 ± 0.82 pA, respectively; data from five neurons from four rats) were comparable to the values obtained from CA1 pyramidal neurons in intact slices (1.12 ± 0.09 Hz and 25.2 ± 0.73 pA; data from four neurons from three rats). Also, CA3 ablation had no significant effect on the τd or rise time of GABAergic IPSCs (data not shown).
3.5. MLA suppress spontaneous glutamatergic synaptic activity in CA3 pyramidal neurons
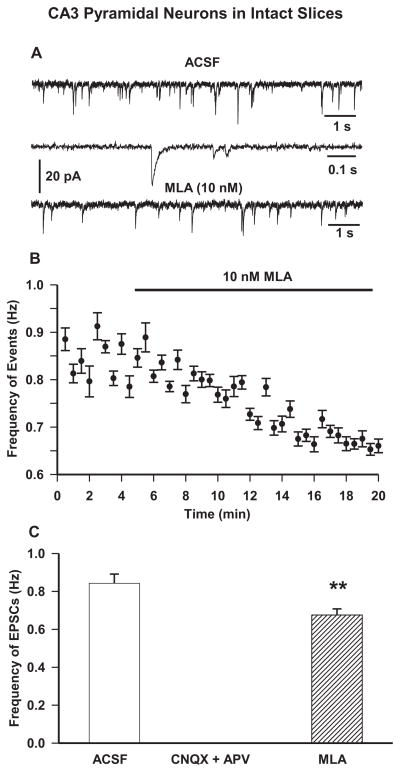
EPSCs recorded from CA3 pyramidal neurons at −70 mV appeared as inward events (Figure 2A) with a peak amplitude of 15.7 ± 1.6 pA, rise time of 3.3 ± 0.6 ms, and τd of 20.6 ± 1.8 ms. These spontaneous events were blocked following 10 min bath application of ACSF containing CNQX (10 μM)-plus-APV (50 μM). In order to assess the contribution of tonically active α7 nAChRs to glutamatergic activity in CA3 pyramidal neurons, EPSCs were recorded in slices superfused with ACSF containing MLA (10 nM). The frequency of EPSCs was reduced by the continuous superfusion of the slices with MLA-containing ACSF, with an onset for inhibition of about 8–10 min (Figure 2B). The maximal effect of MLA was observed at approximately 15 min after the beginning of the superfusion, at which time MLA (10 nM) caused a significant 22.4 ± 2.3 % reduction in frequency of EPSCs (Figure 2B,C). MLA had no significant effect on the τd, rise time, or mean peak amplitude of EPSCs.
Figure 2. Effect of pharmacological agents on spontaneous EPSCs in CA3 pyramidal neurons in intact hippocampal slices.
A. Sample recordings of spontaneous EPSCs recorded from CA3 pyramidal neurons of 30-day-old rats at −70 mV under control (first trace) and in presence of α7 nAChR antagonist MLA (10 nM) for 15 min (third trace). The second trace shows EPSCs at an expanded time scale. B. Scatter plot showing effect of superfusion of MLA (10 nM) was derived from averaging the number of events in 30-s intervals. Results are presented as mean ± S.E.M.. Baseline rates were recorded for 5 min before bath application of MLA (thick black bar). Plot represents data from eight neurons from four rats. C. Mean frequency of EPSCs in control condition, 15 min superfusion with ACSF containing glutamate receptor antagonists APV (50 μM) and CNQX (10 μM) and 15 min superfusion with ACSF containing 10 nM MLA. Graph and error bars represent mean and S.E.M., respectively, of data obtained from fourteen neurons from nine rats for control, five neurons from three rats for APV+CNQX, and eight neurons from four rats for MLA. (** p < 0.01 by paired t-test).
4. Discussion
The present results demonstrate that the structural integrity of the CA3 field of the hippocampus is an important determinant of the α7 nAChR-dependent glutamatergic drive to CA1 pyramidal neurons in rat hippocampal slices under resting conditions. As discussed below, these tonically active α7 nAChRs may be present on the CA3 pyramidal neurons/axons and/or on the Mossy fibers that innervate the CA3 pyramidal neurons that in turn innervate the CA1 pyramidal cells. We have previously shown that basal concentrations of ACh and/or choline in hippocampal slices are sufficient to maintain α7 nAChRs tonically active [4,5] and regulate both GABAergic and glutamatergic synaptic transmission to CA1 pyramidal neurons. The finding that the α7 nAChR antagonist MLA suppressed the frequency of spontaneous EPSCs recorded from CA3 pyramidal neurons strongly suggests that, via activation of α7 nAChRs on mossy fiber terminals [17] and/or on CA3 pyramidal neurons [10], basal levels of choline/ACh sustain glutamatergic input to CA3 pyramidal neurons.
Synaptic glutamate release onto hippocampal pyramidal neurons occurs via depolarization of terminals (an action potential-insensitive process) and of presynaptic neurons (an action potential-dependent process). In intact hippocampal slices, action potential block by TTX reduced the EPSC frequency and amplitude recorded from CA1 pyramidal neurons by approximately 25 and 45%, respectively (Table 1), suggesting that a significant portion of glutamatergic input to CA1 pyramidal neurons originate from presynaptic neuron firing. Our current finding that surgical removal of CA3 region caused a reduction of 20% in the frequency and 22% in the peak amplitude of EPSCs (Table 1; Figure 2A) in CA1 pyramidal neurons supports the concept that CA1 pyramidal neurons receive action potential-dependent glutamatergic inputs from the CA3 field. The observation that the frequency of GABAergic IPSCs was unaffected by CA3 ablation indicated that the suppression of glutamate transmission seen in CA1 pyramidal neurons is not due to a nonspecific damage to the slice preparation.
In the present experimental conditions, TTX suppressed by approximately 14% and 25% the frequency of spontaneous EPSCs recorded from CA1 pyramidal neurons in CA3-ablated and intact slices, respectively. One could hypothesize that in the intact slices TTX blocked action potential-dependent glutamatergic inputs to CA1 pyramidal neurons originating from both CA3 and CA1 neurons, whereas in CA3-ablated slices only the CA1 source of action potentital-dependent glutamatergic input was available for the effect of TTX. Based on this hypothesis and the results obtained here, it appears that, under the current experimental condition, 11% (i.e., 25% - 14%) of the action potential-dependent glutamatergic inputs to the CA1 pyramidal originated from the CA3 field and 14% originated from the CA1 field. Anatomical studies have however provided evidence that the contribution of CA1 pyramidal neurons to the glutamatergic synaptic activity in CA1 pyramidal neurons is lower than that of CA3 pyramidal neurons [7, 16]. Thus, our results can be better reconciled by the hypothesis that although CA1 pyramidal neurons in the CA3-ablated slices most likely contribute to the action potential-dependent EPSCs recorded from CA1 pyramidal neurons, the CA3 pyramidal neuron axons that remain in the slices are viable and continue to release glutamate onto the CA1 pyramidal neurons in an action potential-dependent fashion. This notion requires that axon initial segments, the site of initiation of action potentials [6] were retained in the Schaffer collaterals of CA3-ablated slices. Alternatively, it is possible that a few intact CA3 neurons still remained in our CA3-ablated slices that contributed partly to the effect of TTX.
We have previously shown that tonic α7 nAChR activity regulates the action potential-sensitive glutamate activity in CA1 pyramidal neurons, while having no significant influence on action potential-independent glutamatergic transmission in those neurons [5]. Therefore, based on the current demonstration that the structural integrity of the CA3 field of the hippocampus is essential for the α7 nAChR-dependent glutamatergic activity in CA1 pyramidal neurons, it is feasible to propose that under resting conditions tonically active α7 nAChRs on CA3 pyramidal neurons/axons and/or on mossy fibers that synapse onto CA3 pyramidal neurons that in turn synapse onto CA1 pyramidal neurons control the excitatory drive of the latter (see Figure 3). The observation that a small reduction of the frequency of EPSCs in CA1 pyramidal neurons of CA3-ablated slices could still be detected following superfusion of these slices with MLA suggests that in these slices glutamatergic input to CA1 pyramidal neurons is controlled by tonically active α7 nAChRs located on the severed but functional axons of CA3 pyramidal neurons and/or on CA1 pyramidal neurons that synapse onto the neurons under study. In fact, as alluded to earlier, CA1 pyramidal neurons receive glutamatergic input from other CA1 pyramidal neurons [7, 18] and are known to express α7 nAChRs [12].
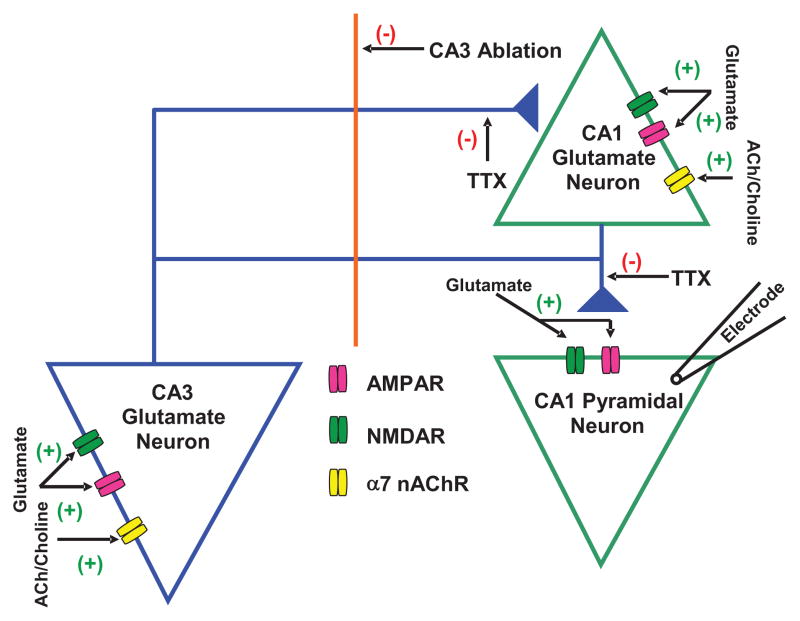
Figure 3. Schematic representation of glutamatergic transmission to CA1 pyramidal neuron.
Proposed model shows tonic regulation of glutamatergic input to CA1 pyramidal neurons by α7 nAChRs located on CA3 and CA1 pyramidal neurons. Spontaneous EPSCs were recorded from CA1 pyramidal neurons that received glutamate inputs from CA3 and CA1 pyramidal neurons. The α7 nAChR is reported to be present on CA3 and CA1 pyramidal neurons. The decrease in EPSC frequency by CA3 ablation suggests that CA3 pyramidal neurons regulate the excitability of CA1 pyramidal neurons. The magnitude of effect of MLA (10 nM) on EPSCs was larger in intact slices compared to that in CA3-ablated slices, which suggests that endogenous ACh or choline activates α7 nAChRs located on both CA3 and CA1 regions and controls glutamate activity in CA1 pyramidal neurons. (+) represents activation and (−) represents inhibition in the neurocircuitry.
Glutamate activity in CA1 pyramidal neurons has been implicated in memory processing in the hippocampus. This mechanistic information regarding regulation of tonic α7 nAChR-dependent glutmatergic transmission to CA1 pyramidal neurons through connections between CA3-CA1 pyramidal neurons and, possibly, CA1-CA1 pyramidal neurons (Figure 3) sheds light on the role of cholinergic signaling in cognitive functions and how this may be affected by endogenous ligands such as kynurenic acid in certain brain disorders. Also, this intrinsic regulatory mechanism can be targeted by α7 nAChR orthosteric and allosteric ligands that are developed for clinical use in neurological conditions such as Alzheimer’s disease and schizophrenia, in which α7 nAChR dysfunctions have been noted.
5. Conclusion
The present results provided data that support the hypothesis that tonic α7 nAChR-dependent glutamate drive to CA1 pyramidal neurons is largely dependent on the structural integrity of the CA3 field of the hippocampus.
Highlights.
CA3 ablation decreases frequency of glutamate EPSCs in CA1 pyramidal neurons
MLA or TTX decrease EPSC frequency in CA1 pyramidal neurons to a lesser extent after CA3 ablation
Tonic α7 nAChR-mediated glutamate drive to CA1 pyramidal neurons originates from both CA3 and CA1 neurons
Acknowledgments
The authors are indebted to Ms. Mabel Zelle and Ms. Bhagavathy Alkondon for technical assistance.
Funding
This work was supported by National Institute of Health Grant NS25296 (Edson X. Albuquerque).
Abbreviations
- ACSF
artificial cerebrospinal fluid
- APV
2-amino-5-phosphonovaleric acid
- CNQX
6-Cyano-7-nitroquinoxaline-2,3-dione
- EPSC
excitatory postsynaptic current
- IPSC
inhibitory postsynaptic current
- MLA
methyllycaconitine
- nAChR
nicotinic acetylcholine receptor
- τd
time constant of decay
- TTX
Tetrodotoxin
Footnotes
Conflict of Interest
The authors report no conflict of interest.
Author Contributions
Participated in Research Design: Banerjee, Alkondon, Pereira, Albuquerque
Conducted Experiments: Banerjee
Performed Data Analysis: Banerjee, Alkondon, Pereira, Albuquerque
Contributed to the writing of the manuscript: Banerjee, Alkondon, Pereira, Albuquerque
Other: Albuquerque acquired the funding for the research
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Jyotirmoy Banerjee, Email: banerjee@umaryland.edu.
Manickavasagom Alkondon, Email: malko001@umaryland.edu.
Edson X. Albuquerque, Email: ealbuquerque@som.umaryland.edu.
Edna F.R. Pereira, Email: EPereira@som.umaryland.edu.
References
- 1.Albuquerque EX, Pereira EFR, Alkondon M, Rogers SW. Mammalian nicotinic acetylcholine receptors: from structure to function. Physiol Rev. 2009;89:73–120. doi: 10.1152/physrev.00015.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alkondon M, Pereira EFR, Eisenberg HM, Kajii Y, Schwarcz R, Albuquerque EX. Age dependency of inhibition of α7 nicotinic receptors and tonically active N-methyl-D-aspartate receptors by endogenously produced kynurenic acid in the brain. J Pharmacol Exp Ther. 2011;337:572–582. doi: 10.1124/jpet.110.177386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bekkers JM, Stevens CF. Presynaptic mechanism for long-term potentiation in the hippocampus. Nature. 1990;1990(346):724–729. doi: 10.1038/346724a0. [DOI] [PubMed] [Google Scholar]
- 4.Banerjee J, Alkondon M, Pereira EF, Albuquerque EX. Regulation of GABAergic inputs to CA1 pyramidal neurons by nicotinic receptors and kynurenic acid. J Pharmacol Exp Ther. 2012a;341:500–509. doi: 10.1124/jpet.111.189860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Banerjee J, Alkondon M, Albuquerque EX. Kynurenic acid inhibits glutamatergic transmission to CA1 pyramidal neurons via α7 nAChR-dependent and -independent mechanisms. Biochem Pharmacol. 2012b;84:1078–1087. doi: 10.1016/j.bcp.2012.07.030. [DOI] [PubMed] [Google Scholar]
- 6.Debanne D, Campanac E, Bialowas A, Carlier E, Alcaraz G. Axon Physiology. Physiol Rev. 2011;91:555–602. doi: 10.1152/physrev.00048.2009. [DOI] [PubMed] [Google Scholar]
- 7.Deuchars J, Thomson AM. CA1 pyramid-pyramid connections in rat hippocampus in vitro: Dual intracellular recordings with biocytin filling. Neuroscience. 1996;74:1009–1018. doi: 10.1016/0306-4522(96)00251-5. [DOI] [PubMed] [Google Scholar]
- 8.Frick A, Magee J, Johnston D. LTP is accompanied by an enhanced local excitability of pyramidal neuron dendrites. Nature Neuroscience. 2004;7:126–135. doi: 10.1038/nn1178. [DOI] [PubMed] [Google Scholar]
- 9.Gray R, Rajan AS, Radcliffe KA, Yakehiro M, Dani JA. Hippocampal synaptic transmission enhanced by low concentrations of nicotine. Nature. 1996;383:713–716. doi: 10.1038/383713a0. [DOI] [PubMed] [Google Scholar]
- 10.Grybko MJ, Hahm E, Perrine W, Parnes JA, Chick WS, Sharma G, Finger TE, Vijayaraghavan S. A transgenic mouse model reveals fast nicotinic transmission in hippocampal pyramidal neurons. Eur J Neurosci. 2011;33:1786–1798. doi: 10.1111/j.1460-9568.2011.07671.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grybko MJ, Sharma G, Vijayaraghavan S. Functional distribution of nicotinic receptors in CA3 region of the hippocampus. J Mol Neurosci. 2010;40:114–120. doi: 10.1007/s12031-009-9266-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hefft S, Hulo S, Bertrand D, Muller D. Synaptic transmission at nicotinic acetylcholine receptors in rat hippocampal organotypic cultures and slices. J Physiol. 1999;515:769–776. doi: 10.1111/j.1469-7793.1999.769ab.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huang LT, Sherwood JL, Sun YJ, Lodge D, Wang Y. Activation of presynaptic α7 nicotinic receptors evokes an excitatory response in hippocampal CA3 neurones in anaesthetized rats: an in vivo iontophoretic study. Br J Pharmacol. 2010;159:554–565. doi: 10.1111/j.1476-5381.2009.00529.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ji D, Lape R, Dani JA. Timing and location of nicotinic activity enhances or depresses hippocampal synaptic plasticity. Neuron. 2001;31:131–141. doi: 10.1016/s0896-6273(01)00332-4. [DOI] [PubMed] [Google Scholar]
- 15.Miles R, Wong RK. Excitatory interactions between CA3 neurones in the guinea-pig hippocampus. J Physiol. 1986;373:397–418. doi: 10.1113/jphysiol.1986.sp016055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sayer RJ, Friedlander MJ, Redman SJ. The time course and amplitude of EPSPs evoked at synapses between pairs of CA3/CAI neurones in the hippocampal slice. J Neurosci. 1990;10:826–836. doi: 10.1523/JNEUROSCI.10-03-00826.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sharma G, Grybko MJ, Vijayaraghavan S. Action potential-independent and nicotinic receptor-mediated concerted release of multiple quanta at hippcampal CA3-mossy fiber synapses. J Neurosci. 2008;28:2563–2575. doi: 10.1523/JNEUROSCI.5407-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Somogyi P, Klausberger T. Defined types of cortical interneurone structure space and spike timing in the hippocampus. J Physiol. 2005;562:9–26. doi: 10.1113/jphysiol.2004.078915. [DOI] [PMC free article] [PubMed] [Google Scholar]