Abstract
Astrocytes play a crucial role in regulating and maintaining the extracellular chemical milieu of the central nervous system under physiological conditions. Moreover, proliferation of phenotypically altered astrocytes (a.k.a. reactive astrogliosis) has been associated with many neurologic and psychiatric disorders, including mesial temporal lobe epilepsy (MTLE). Glutamine synthetase (GS), which is found in astrocytes, is the only enzyme known to date that is capable of converting glutamate and ammonia to glutamine in the mammalian brain. This reaction is important, because a continuous supply of glutamine is necessary for the synthesis of glutamate and GABA in neurons. The known stoichiometry of glutamate transport across the astrocyte plasma membrane also suggests that rapid metabolism of intracellular glutamate via GS is a prerequisite for efficient glutamate clearance from the extracellular space. Several studies have indicated that the activity of GS in astrocytes is diminished in several brain disorders, including MTLE. It has been hypothesized that the loss of GS activity in MTLE leads to increased extracellular glutamate concentrations and epileptic seizures. Understanding the mechanisms by which GS is regulated may lead to novel therapeutic approaches to MTLE, which is frequently refractory to antiepileptic drugs. This review discusses several known mechanisms by which GS expression and function are influenced, from transcriptional control to enzyme modification.
Keywords: Gliosis, glutamate, hippocampal sclerosis, limbic system, metabolism, seizures
1. INTRODUCTION
Astrocytes, the most abundant cell type in the brain, were long regarded as elements of mere structural support, with minor contributions to brain physiology. However, this view has dramatically changed, and astrocytes are now considered pivotal for a multitude of brain functions, such as axonal growth (Katz et al., 1983; Lemke, 2001), energy metabolism (Hertz, 2011; Magistretti and Pellerin, 1999; Pellerin et al., 2002), neurotransmitter homeostasis (Danbolt, 2001; Schousboe et al., 2013), water/electrolyte balance (Benfenati et al., 2007; Orkand et al., 1966; Verkhratsky and Steinhauser, 2000), and the immune response (Boddeke et al., 2013). Moreover, astrocytes may be critically involved in the pathophysiology of neurological and psychiatric disorders such as epilepsy (Binder and Steinhauser, 2006; Boison, 2008, 2013; Coulter and Eid, 2012; Haydon and Carmignoto, 2006; Steinhauser and Boison, 2012; Tian et al., 2005), stroke (Nedergaard and Dirnagl, 2005), Alzheimer’s disease (Games et al., 1995; Robinson, 2000), multiple sclerosis (Sharma et al., 2010), schizophrenia (Rajkowska et al., 2002; Takano et al., 2010), and suicide/depression (Hasler et al., 2007; Sanacora et al., 2012).
The importance of astrocytes for brain function is underscored by several key features of these cells. Firstly, astrocytes are highly branched cells with cytoplasmic processes surrounding and interacting with critical functional-anatomical domains such as the perivascular and perisynaptic regions (Peters et al., 1991). Astrocyte processes are also juxtaposed to the soma, dendrites and axons of neurons as well as to the plasma membranes of microglial cells, oligodendrocytes, and other astrocytes (Peters et al., 1991). Thus, astrocytes can potentially influence and be influenced by a large number of cells, synapses, and vascular structures. Secondly, astrocytes harbor virtually all of the constitutive metabolic enzymes, as well as several specialized enzymes, most notably glutamine synthetase (GS) (Martinez-Hernandez et al., 1977) and pyruvate carboxylase (Shank et al., 1985). These cells are therefore crucial for the metabolism and homeostasis of glucose, ammonia and glutamate in the brain. Thirdly, the astrocyte plasma membrane is richly endowed with receptors for neurotransmitters (Bowman and Kimelberg, 1984; Kettenmann et al., 1984), growth factors, cytokines, and chemokines (Boddeke et al., 2013). The astrocyte plasma membrane also contains transporters for numerous molecules, such as potassium (Orkand et al., 1966; Verkhratsky and Steinhauser, 2000), water (Nagelhus et al., 1998), glutamate (Danbolt, 2001), glutamine (Chaudhry et al., 2002), glucose (Morgello et al., 1995; Yu and Ding, 1998), ketone bodies and lactate (Bergersen, 2007; Broer et al., 1997; Debernardi et al., 2003). Thus, astrocytes recognize a variety of extracellular stimuli (e.g., neurotransmitters, cytokines, growth factors) and are able to respond to these and other signals by uptake or release of ions, water, amino acids and energy substrates. Finally, astrocytes can change (transform) their phenotype and proliferate in response to certain (usually damaging) conditions. Proliferation of such transformed (“reactive”) astrocytes in brain tissue is called reactive astrogliosis (Robel et al., 2011).
Reactive astrogliosis is present in a variety of pathological conditions, including intracranial infections (Garden, 2013), hypoxia/ischemia (Nedergaard and Dirnagl, 2005), Alzheimer’s disease (Games et al., 1995), and epilepsy (Cohen-Gadol et al., 2004; Gloor, 1991). Reactive astrogliosis was historically thought to be a secondary “scar tissue” response to loss of neurons; however, an increasing number of studies now suggest that reactive astrocytes are critically involved in the causation of several brain disorders. One such disorder is mesial temporal lobe epilepsy (MTLE), which is among the most common types of medication refractory epilepsies (Hauser et al., 1993). This review discusses the involvement of reactive astrocytes in the pathophysiology of MTLE. The main emphasis will be on the lack of GS in these cells (Eid et al., 2004b; van der Hel et al., 2005), and the mechanisms by which the expression and activity of this enzyme are regulated under normal and pathological conditions. An understanding of these regulatory mechanisms is important, as such information is likely to facilitate the development of novel and more efficacious therapies for MTLE.
2. GS IS DEFICIENT IN ASTROCYTES IN MTLE
MTLE is characterized by recurrent complex partial seizures that originate from a network of brain areas, including the hippocampus, entorhinal cortex, amygdala, lateral temporal cortex, medial thalamus, and inferior frontal lobe (Spencer, 2002). Some of these areas are characterized by pathological changes, most notably patterned loss of neurons (Gloor, 1991; Sommer, 1880), reorganization of synapses with aberrant expression of glutamate receptors (de Lanerolle et al., 1998; Eid et al., 2002), altered properties of microvessels (Eid et al., 2004a; Eid et al., 2005; Heinemann et al., 2012; Lauritzen et al., 2011; Lauritzen et al., 2012a; Liwnicz et al., 1990), and marked proliferation of reactive astrocytes (Cohen-Gadol et al., 2004; Gloor, 1991). The reactive astrocytes in MTLE are characterized by changes in their expression of inwardly rectifying potassium channels (Kir4.1) (Bordey and Sontheimer, 1998; Heuser et al., 2012), water channels (Amiry-Moghaddam et al., 2003; Binder et al., 2012; Eid et al., 2005), monocarboxylate transporters (MCTs) (Lauritzen et al., 2011; Lauritzen et al., 2012a; Lauritzen et al., 2012b), GS (Eid et al., 2004b; van der Hel et al., 2005), and possibly also glutamate transporters (Bjornsen et al., 2007; Tessler et al., 1999). Several additional changes in the phenotype and function of reactive astrocytes in MTLE have been previously described (reviewed in (Boison, 2013)). Based on these findings it has been suggested that reactive astrocytes in the temporal lobe in MTLE contribute to the triggering of seizures via impaired buffering of extracellular potassium (Binder et al., 2006; Bordey and Sontheimer, 1998; Heuser et al., 2012), decreased uptake and metabolism of extracellular glutamate (Cavus et al., 2005; During and Spencer, 1993; Eid et al., 2004b; Petroff et al., 2004; Petroff et al., 2002), release of glutamate from astrocytes (Bezzi et al., 1998; Bezzi et al., 2001; Perez et al., 2012; Santello et al., 2011; Tian et al., 2005), or a combination of the three.
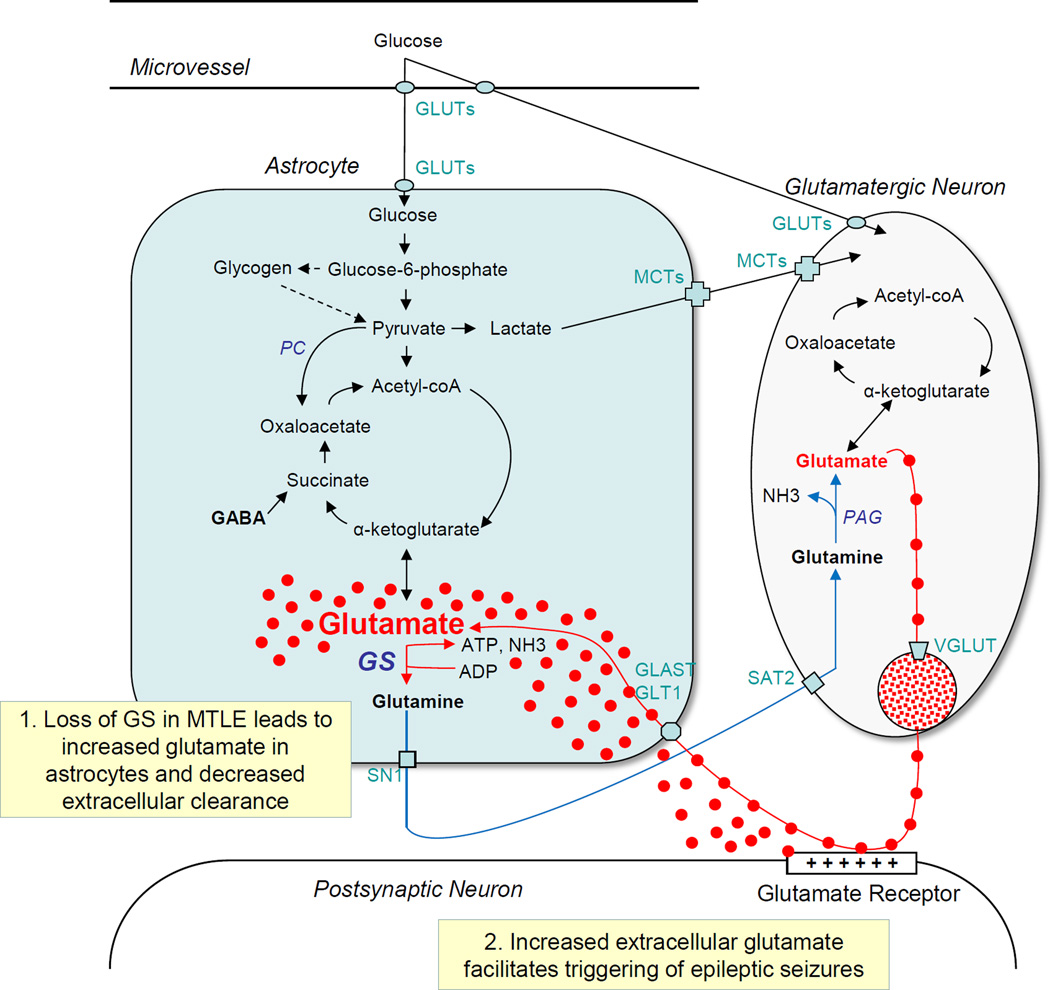
Reactive astrocytes are likely to perturb the glutamate homeostasis in MTLE. Glutamate is a major excitatory and potentially neurotoxic transmitter in the brain, and elevated concentrations of extracellular glutamate and glutamate analogues in the brain can lead to hyperexcitability, seizures and neuronal death (Ben-Ari, 1985; Nadler et al., 1978; Olney et al., 1972). Thus, rapid removal of glutamate from the synaptic cleft and extracellular space is important for termination and fine tuning of excitatory synaptic responses, for prevention of seizures, and for protection of neurons from excitotoxic injury and death. Astrocytes play a crucial role in removal of synaptic and extracellular glutamate because of three key features of these cells (Fig. 1). Firstly, cytoplasmic processes of astrocytes are juxtaposed to excitatory synapses, forming what is often referred to as the tripartite synapse (Halassa et al., 2007; Perea et al., 2009; Peters et al., 1991). Secondly, astrocyte processes take up synaptic glutamate with high efficiency because of the abundance of high-affinity excitatory amino acid transporters on the plasma membrane of the processes (Danbolt, 2001). Thirdly, GS, which is preferentially localized to the cytoplasm of astrocytes (Martinez-Hernandez et al., 1977), converts the glutamate taken up by astrocytes to glutamine according to the following synthetic reaction:
The known stoichiometry of glutamate transport across the astrocyte plasma membrane suggests that rapid metabolism of intracellular glutamate is a prerequisite for efficient glutamate clearance from the extracellular space (Otis and Jahr, 1998).
Figure 1.
Simplified diagram of the brain glutamate metabolism illustrating the involvement of astrocytes, neurons, transporter molecules, and key enzymes. Note that extracellular glutamate is taken up by the astrocyte via glutamate transporters (GLAST, GLT1) and converted to glutamine by glutamine synthetase (GS) in the astrocyte cytoplasm. Loss of GS is hypothesized to increase glutamate in astrocytes and impair the clearance of extracellular glutamate (Box 1). Increased extracellular glutamate is likely to facilitate the triggering of epileptic seizures (Box 2). Abbreviations: GLUTs, glucose transporters; PAG, phosphate activated glutaminase; MCTs, monocarboxylate transporters; MTLE, mesial temporal lobe epilepsy; PC, pyruvate carboxylase; SAT2, system A transporter 2; SN1, system N transporter 1; VGLUT, vesicular glutamate transporter.
In vivo brain microdialysis studies of patients with MTLE have shown that the interictal extracellular glutamate concentration is chronically increased in the epileptogenic and astrogliotic hippocampus vs. the contralateral (nonepileptogenic) and nonastrogliotic hippocampus (Cavus et al., 2005; Cavus et al., 2008). As expected, the concentration of extracellular glutamate increases during a seizure; however, it increases more and is cleared more slowly in the epileptogenic than in the nonepileptogenic hippocampus (During and Spencer, 1993).
A deficiency in GS in reactive astrocytes may explain the extracellular glutamate excess in MTLE. Patients with medication-refractory MTLE exhibit markedly reduced concentration and functional activity of GS in reactive astrocytes in the epileptogenic hippocampus [Fig. 2, (Eid et al., 2004b; van der Hel et al., 2005)]. We have hypothesized that the loss of GS in MTLE slows the metabolism of glutamate to glutamine in astrocytes and leads to accumulation of intracellular (astrocytic) and extracellular glutamate (Eid et al., 2004b). We have further postulated that the perturbed glutamate homeostasis caused by the GS deficiency contributes to the triggering of seizures in MTLE. Thus far, in vivo experiments have supported the hypothesis that GS inhibition in the hippocampus leads to accumulation of glutamate in astrocytes (Perez et al., 2012) and to recurrent seizures that resemble those of human MTLE (Eid et al., 2008; Wang et al., 2009). Moreover, humans with mutations in the GS gene also exhibit epileptic seizures (Haberle et al., 2005; Haberle et al., 2006). Hence, deficiency of GS in astrocytes appears to be critically implicated in the causation of seizure disorders, particularly MTLE. Two important questions therefore arise: “What causes the loss of GS in MTLE? And can restoration of the enzyme in astrocytes represent a novel and highly efficacious therapy for this disorder?” The remainder of this discussion will review how GS is regulated in astrocytes under normal and pathological conditions.
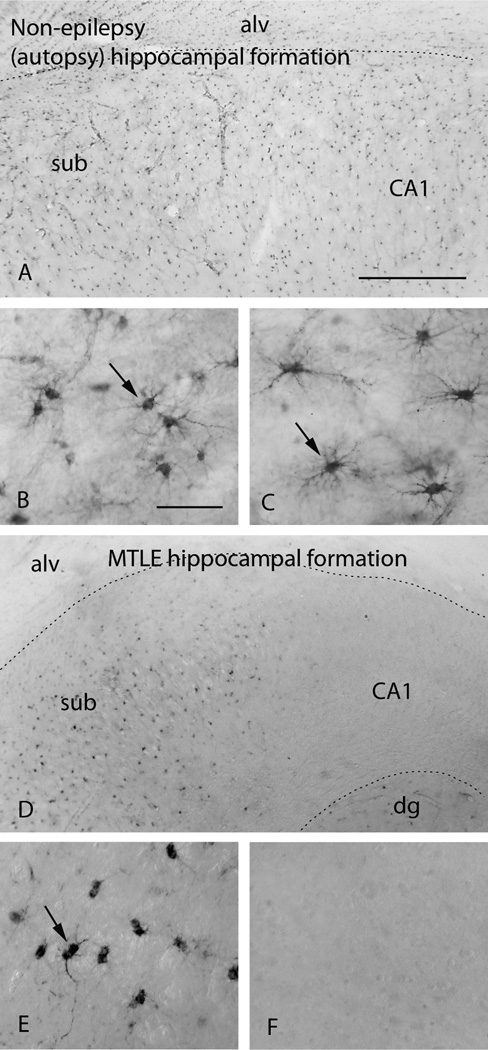
Figure 2.
Immunohistochemistry for GS reveals abundant labeling of astrocytes (arrows) in the subiculum (sub, A, B) and CA1 (A, C) of the hippocampal formation in a human autopsy subject without epilepsy. In contrast, GS is severely deficient in astrocytes in the hippocampal formation in a patient with mesial temporal lobe epilepsy [MTLE, (D–E)]. The deficiency is particularly pronounced in CA1 (D, F). Note that astrocytes in the subiculum of the MTLE hippocampus lack staining for GS in the most distal astrocyte processes (arrow in E) vs. the normal (autopsy) hippocampus (arrow in B). B and C are high power fields of the subiculum and CA1 respectively in A. E and F are high power fields of the subiculum and CA1 respectively in D. Abbreviations: alv, alveus; CA1, Cornu Ammonis subfield 1; dg, dentate gyrus; MTLE, mesial temporal lobe epilepsy. Scale bars: A=0.5 mm (same magnification as D); B=0.1 mm (same magnification as C, E, F). Reproduced from (Eid et al., 2004b), with permission from The Lancet/Elsevier.
3. REGULATION OF GS IN ASTROCYTES
3.1 General concepts
GS is widely distributed among eukaryotes and prokaryotes, and two major isoforms of the enzyme are known to exist. GSI is present exclusively in prokaryotes, while GSII (or GS, as used here) is found in eukaryotes and prokaryotes (Meister, 1985). The active enzyme in humans comprises two ring-like pentamers that form an active decamer that uses manganese (Mn++) as the cofactor (Krajewski et al., 2008). The mammalian enzyme is preferentially localized to hepatocytes surrounding the terminal hepatic veins (Gebhardt and Mecke, 1983), in skeletal muscle cells, and in most glial fibrillary acidic protein (GFAP)-positive astrocytes in the brain (Blondet et al., 1995; Norenberg, 1979; Riepe and Norenberg, 1978).
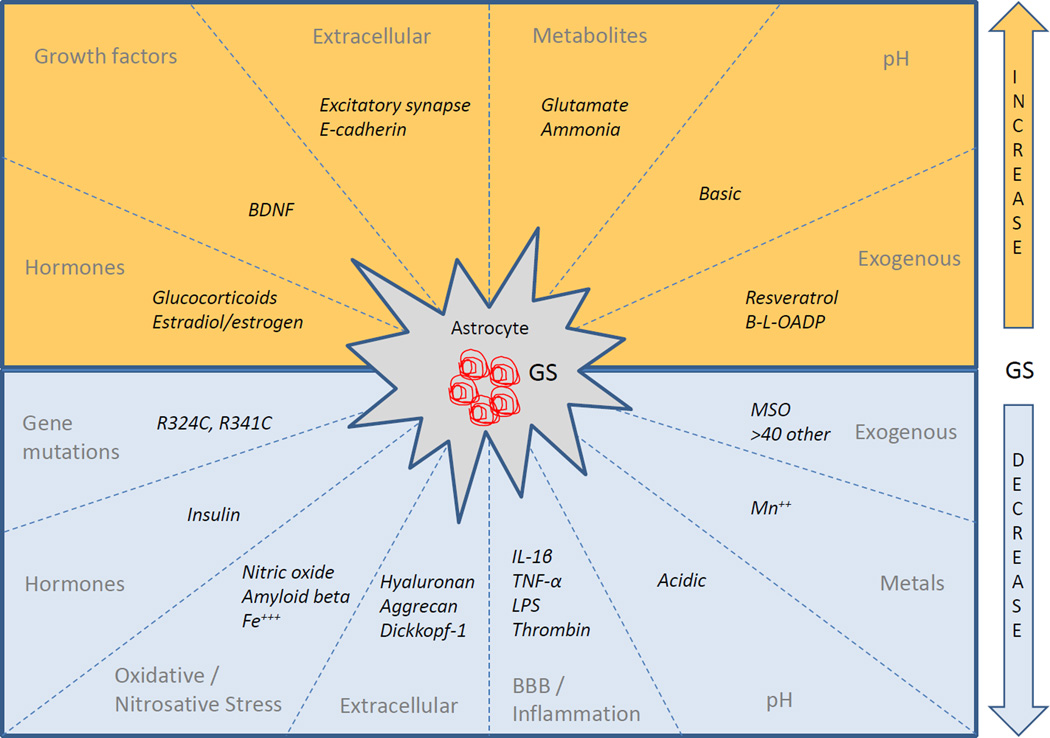
Human GS is encoded by the glutamate-ammonia ligase (GLUL) gene, which is localized to chromosome 1q31. A GS pseudogene (GLULP) is present on chromosome 9p13, and three GS-like genes are localized on 5q33 (GLULL1), on 11p15 (GLULL2), and on 11q24 (GLULL3) (Wang et al., 1996). The GLUL gene, which is likely the main source of GS in mammals, has three regulatory regions that control the transcription of mRNA: (1) the far 2.5-kB upstream region, which contains the canonical TATA, CCAATT and GC boxes, as well as sites for binding transcription factor Sp1 (Fahrner et al., 1993; Lie-Venema et al., 1998); (2) the first intron, which is of particular importance for mRNA expression in Hep G2 hepatoma cells; and (3) the 3’ untranslated region, which is thought to influence localization, stability, and translocation of mRNA (Lie-Venema et al., 1998). Thus, GS may be subject to regulation by a variety of mechanisms targeting the GLUL gene and its mRNA, the expression and stability of the protein, and the kinetic properties of the enzyme. Some of these mechanisms will be discussed below (see also Fig. 3 and Table 1 for an overview).
Figure 3.
Summary of factors that increase (top half) or decrease (bottom half) the expression or activity of GS. For further details and explanation of abbreviations, see Table 1
Table 1.
Regulation of GS
| Factors | Net effect on GS |
Mechanism of Action | References |
|---|---|---|---|
| Gene mutations | |||
| R324C, R341C | ↓ | Disrupts synthesis of GS | (Haberle, et al. 2005) |
| Hormones | |||
| Glucocorticoids | ↑ | Induces gene via GRE and GS silencer element 5’ to GRE |
(Patejunas & Young 1990, Vardimon, et al . 1999, Vardimon, et al. 1988) |
| Estradiol/estrogen | ↑ | ↑ transcription of mRNA | (Blutstein, et al. 2006) |
| Insulin | ↓ | ↓ translation and activity of GS | (Khelil, et al. 1990) |
| Growth factors | |||
| BDNF | ↑ | Unknown | (Dai, et al. 2012) |
| Extracellular interactions | |||
| Excitatory synapses | ↑ | Glutamate or other factor | (Derouiche & Frotscher 1991) |
| Hyaluronan | ↓ | ↓ activity | (Moreno, et al. 2005) |
| Aggrecan | ↓ | ↓ differentiation of astrocytes | (Domowicz, et al. 2008) |
| E-cadherin (pancreas) | ↑ | ↑ Beta-catenin/Wnt-signaling | (Audard, et al. 2008) |
| Dickkopf-1 | ↓ | ↓ Beta-catenin/Wnt-signaling | (Busceti, et al. 2007) |
| Redox and beta amyloid | |||
| Oxidative stress | ↑ | GS oxidation → loss of function | (Butterfield, et al. 2006, Hensley, et al. 1995) |
| Nitrosative stress | ↓ | ↑NOS activity-> GS nitrosylation/nitration -> loss of function |
(Bidmon, et al. 2008, Kosenko, et al . 2003, Swamy, et al. 2011) |
| Fe3+ | ↓ | GS oxidation -> loss of function | (Fernandes, et al. 2011) |
| Beta amyloid | ↓ | GS oxidation -> loss of function | (Boyd-Kimball, et al. 2005) |
|
Inflammation/BBB disruption |
|||
| IL-1 beta + TNF-alpha | ↓ | ↑NO -> GS nitrosylation/ nitration -> loss of function; ↓, induction by glucocorticoids |
(Chao, et al. 1995, Huang & O’Banion 1998) |
| LPS | ↓ | ↑ IL-1 beta | (Letournel-Boulland, et al. 1994) |
| Thrombin | ↓ | ↓ differentiation of astrocytes | (Nelson & Siman 1990) |
|
Metabolites, cofactors metal ions and pH |
|||
| Glutamate | ↑ | Unknown | (Suarez, et al. 2002) |
| Ammonia | ↑ | Unknown | (Suarez, et al. 2002) |
| Increased pH | ↑ | Unknown | (Nissim 1999) |
| Decreased pH | ↓ | Unknown | (Nissim 1999) |
| Mn | ↓ | Unknown | (Deng, et al. 2012) |
| Exogenous compounds | |||
| Resveratrol | ↑ | ↑ GS activity | (dos Santos, et al. 2006) |
| Beta-L-ODAP | ↑ | ↑ GS activity via protein translation | (Miller, et al. 1993) |
| Methionine sulfoximine | ↓ | Irreversible inhibition of GS | (Ronzio & Meister 1968) |
| >40 other compounds, e.g. : –Tabtoxinine-beta-lactam –Alanosine |
↓ | V arious | Reviewed in (Eisenberg, et al. 2000) |
Abbreviations: GRE, glucocorticoid response element; NO, nitric oxide; NOS, nitric oxide synthetase; Beta-L-ODAP, beta-N-oxalyl-alpha, beta-diaminopropionic acid; Wnt, wingless/integrated
3.2 Mutations in the GLUL gene
Mutations in the human GLUL gene were first described by Haberle and colleagues (Haberle et al., 2005), who reported two unrelated newborns who had homozygous mutations in the GLUL gene (R324C and R341C). These mutations were associated with reduced GS activity, severe brain malformations, seizures, multiorgan failure, and early death. A third case of homozygous GLUL mutation was reported later in a 2-year-old infant, who presented with seizures at 13 days of age and developed chronic encephalopathy (Haberle et al., 2011). The considerable morbidity of GLUL mutations is further highlighted by studies of D. melanogaster and transgenic mice. Homozygous mutations in the GLUL gene in D. melanogaster lead to embryo-lethal female sterility (halted embryonic development with failure of eggs to hatch) (Caggese et al., 1992). Mice with prenatal excisions of the GLUL gene in all cell types die during early embryonic development (He et al., 2007), while mice with selective gene deletions in GFAP-positive astrocytes live until postnatal day 3 (He et al., 2010).
3.3 Hormones and growth factors
Steroid hormones, insulin, and growth factors modulate the expression of GS. The glucocorticoids dexamethasone, hydrocortisone, and cortisol induce transcription of GS mRNA (Hallermayer et al., 1981; Labow et al., 2001), with resultant increases in GS protein and activity (Khelil et al., 1990). The inductive action of corticosteroids on the GLUL gene is partly controlled by a glucocorticoid response element (Patejunas and Young, 1990; Vardimon et al., 1999; Vardimon et al., 1988) and partly by a GS silencer element 5' to the glucocorticoid response element (Avisar et al., 1999). However, the effect of glucocorticoids is not universal, as cortisol fails to induce the astrocyte GLUL gene in certain experimental paradigms such as the rat adrenalectomy model (Tombaugh and Sapolsky, 1990). The sex hormones estradiol and estrogen enhance the transcription of GS mRNA in astrocytes in the hypothalamus and hippocampus (Blutstein et al., 2006) and in C6 glioma cells (Haghighat, 2005), whereas insulin decreases the translation and functional activity of GS (Khelil et al., 1990). Finally, brain-derived neurotrophic factor (BDNF) enhances the expression of GS and the glutamate transporter GLAST in astrocytes (Dai et al., 2012). Because stress (Friis and Lund, 1974), estrous cycle hormones (Pennell, 2009), glucose metabolism (Garriga-Canut et al., 2006), and neuronal reorganization (Ben-Ari, 2008) have been implicated in the pathophysiology of seizures and epilepsy, it is possible that perturbations in these factors influence the expression of GS in MTLE. Whether and exactly how these factors regulate GS in MTLE remain to be established.
3.4 Extracellular interactions
Brain GS is preferentially localized to the cytoplasm of astrocytes (Martinez-Hernandez et al., 1977), and the protein is particularly enriched in astrocyte processes in specific regions of the neuropil. For example, GS is strongly expressed in astrocyte processes surrounding glutamatergic nerve terminals in the hippocampal formation (Derouiche and Frotscher, 1991). GS is also particularly abundant in a laminar pattern in the molecular layer of the dentate gyrus, corresponding to glutamate binding sites (Derouiche and Ohm, 1994). These patterns of GS enrichment likely reflect an increased demand for extracellular glutamate clearance at regions of high excitatory activity.
The intracellular localization of GS in astrocytes is altered in MTLE and also in Alzheimer’s disease. While GS is completely absent from large numbers of GFAP-positive astrocytes in the epileptogenic hippocampus in human MTLE, most astrocytes in the subiculum of these patients express GS (Eid et al., 2004b). However, the GS protein is markedly deficient in the most distal astrocyte processes in the subiculum in MTLE when compared with the subiculum in non-epileptic subjects (Eid et al., 2004b). Similarly, in the rat pilocarpine model of MTLE, more GS is found in proximal than in distal astrocyte branches (Papageorgiou et al., 2011). The GS-expressing astrocyte cell bodies are located closer to brain microvessels, whereas the density and volume of GS-immunopositive astrocytes are not altered (Papageorgiou et al., 2011). GS is also diminished in distal astrocyte processes near perisynaptic regions of the neuropil in patients with Alzheimer’s disease, despite a 1.4-fold increase in the density of astrocytes (Robinson, 2001).
Although the mechanisms underlying the spatial enrichment of GS are poorly understood, interactions between the astrocyte and the extracellular milieu are likely to be involved. Firstly, loss of excitatory afferents in vivo reduces the expression of GS in astrocyte processes (Derouiche et al., 1993), and primary astrocytes in culture do not express GS unless cocultured with neurons (Linser and Moscona, 1983). Loss of neurons is often observed in MTLE, and a reduction in astrocyte-neuron interactions may therefore underlie the loss of GS in this disorder. Secondly, the extracellular matrix component hyaluronan decreases GS activity in the retina in a model of glaucoma (Moreno et al., 2005). Hyaluronan and its receptor, CD44, are overexpressed in the astrogliotic hippocampal formation in MTLE (Bausch, 2006; Perosa et al., 2002). Thirdly, loss of the extracellular matrix component aggrecan leads to differentiation of astrocytes with induction of GS, GFAP and the glutamate transporter GLAST (Domowicz et al., 2008). Aggrecan accumulates in the brain in response to early-life seizures and therefore may prevent astrocytes from differentiating into a GS-expressing phenotype (McRae et al., 2010). Finally, studies in peripheral organs have shown that the GLUL gene is induced by beta-catenin, a cytoplasmic protein involved in the Wnt pathway (Audard et al., 2008; Cadoret et al., 2002; Kruithof-de Julio et al., 2005). Translocation of the extracellular adhesion molecule E-cadherin from the plasma membrane to the cytoplasm of pseudopapillary tumor cells of the pancreas leads to increased beta-catenin/Wnt signaling and enhanced expression of the GS protein (Audard et al., 2008). In contrast, Wnt signaling is decreased by the secreted glycoprotein, Dickkopf-1. Increased levels of Dickkopf-1 are present in the astrogliotic hippocampus in patients with MTLE, suggesting that the beta-catenin/Wnt pathway may be implicated in the loss of GS in this disorder (Busceti et al., 2007).
3.5 Redox reactions and beta amyloid
The GS protein is exquisitely sensitive to oxidative and nitrosative stresses. Studies of brain tissue from patients with Alzheimer’s disease demonstrate increased markers of oxidative stress in areas of senile plaques and beta amyloid deposits, as evidenced by a decreased W/S (weakly/strongly) ratio of immobilized 2,2,6,6-tetramethyl-4-maleimidopiperdin-oxyl (MAL-6) spin-labeled synaptosomes, and increased phenylhydrazine-reactive protein carbonyl content (Hensley et al., 1995). These indicators of oxidative stress are associated with decreased GS activity (Hensley et al., 1995). Investigations using redox proteomics on temporal lobe specimens from patients with mild cognitive impairment have shown that GS and other proteins are oxidatively modified (Butterfield et al., 2006). Moreover, intracerebral injection of beta amyloid peptide (1–42) in normal rats leads to oxidation of GS (Boyd-Kimball et al., 2005), suggesting that beta amyloid deposits may be implicated in the loss of GS activity in Alzheimer’s disease. It is notable that excessive deposition of beta amyloid has been reported in the temporal lobe in patients with MTLE, suggesting that beta amyloid and oxidative stress may be implicated in GS deficiency in this disorder (Mackenzie and Miller, 1994).
Further evidence supports the involvement of nitrosative/oxidative stress in the GS deficiency in MTLE. Rats treated with kainic acid to induce seizures exhibit increased nitric oxide synthetase activity, increased formation of nitric oxide, and reduced activity of GS in the brain (Swamy et al., 2011). Nitric oxide inhibits the activity of GS by covalent modifications (nitrosylation or nitration) of the enzyme (Kosenko et al., 2003). Induction of seizures in rats using pentylenetetrazole also results in nitration of brain GS, with subsequent reduction in enzyme activity (Bidmon et al., 2008). The reduction in GS activity in this model is not associated with a concurrent decrease in GS protein concentration (Bidmon et al., 2008). Thus, measurements of GS protein by immunological methods (such as Western blots, ELISA, and immunohistochemistry) should be supplemented with measurements of GS enzyme activity, when indicated. Finally, administration of ferric ions (Fe+++) to the brains of rats causes seizures and oxidative damage (Ueda and Willmore, 2000). Ferric ions also inhibit GS and leads to reactive astrogliosis; thus, it is possible that deposition of iron in the brain may contribute to the loss of GS in MTLE (Fernandes et al., 2011; Fernandez et al., 2011). Ferric deposits can occur in pathological conditions associated with brain tissue hemorrhage, such as hypoxia/ischemia, tumors, arteriovenous malformations, and head trauma. These conditions are often linked to development of epilepsy.
3.6 Inflammation and the blood-brain barrier
Neuroinflammation, which is present in epilepsy, can alter the activity and expression of GS. The epileptic brain in patients with MTLE and in animal models is characterized by excessive infiltration of microglial cells and upregulation of interleukin-1 beta (IL-1 beta), tumor necrosis factor alpha (TNF-alpha), IL-6, and NF-kappaB (Vezzani and Granata, 2005). Notably, IL-1 beta triggers seizures when administered to the brains of laboratory animals (Vezzani et al., 1999; Vezzani et al., 2000), and TNF-alpha induces glutamate release from astrocytes in vitro (Santello et al., 2011). Coadministration of IL-1 beta TNF-alpha to human fetal brain cell cultures generates nitric oxide and inhibits GS (Chao et al., 1995). IL-1 beta and TNF-alpha also suppress the induction of GS by dexamethasone in primary mouse astrocytes (Huang and O'Banion, 1998). Finally, treatment of primary astrocyte cultures with lipopolysaccharide increases IL1-beta and downregulates GFAP and GS (Letournel-Boulland et al., 1994). It has been hypothesized that the inhibitory action of cytokines on GS is mediated by an increase in transcription factor c-Jun (Raivich, 2008), which suppresses the expression of GS (Vardimon et al., 1999; Vardimon et al., 2006). Thus, neuroinflammation not only lowers the threshold for seizures, but also reduces the activity of GS, thereby providing a possible causative link between inflammation and excessive glutamatergic neurotransmission.
It is well-known that disruptions of the blood-brain barrier (BBB) can lead to neuroinflammation, and vice versa, that neuroinflammation can result in opening of the BBB (Ransohoff and Brown, 2012; Ransohoff et al., 2003). Several tissue components control the integrity of the BBB, including endothelial cells, vascular basement membrane, pericytes, and perivascular astroglial endfeet. All of these components are altered in patients with MTLE as evidenced by changes in erythropoietin receptors (Eid et al., 2004a), MCTs (Lauritzen et al., 2011), and tight junctions (Rigau et al., 2007) on brain endothelial cells. Furthermore, the BBB in MTLE is characterized by thickening of the basement membrane (Liwnicz et al., 1990); degeneration of pericytes (Liwnicz et al., 1990); and perturbed expression of proteins on perivascular astroglial endfeet, including potassium transporter Kir4.1 (Heuser et al., 2012), dystrophin (Eid et al., 2005), aquaporin 4 (Eid et al., 2005), and MCTs (Lauritzen et al., 2012a). Alterations in the BBB can result in diapedesis of leukocytes and extravasation of plasma components into the brain tissue, with neuroinflammation and seizures as possible consequences (Ballabh et al., 2004; Ransohoff et al., 2003; Salazar et al., 1985; Seiffert et al., 2004). Thus, astrocytes may be affected not only by local tissue inflammation, but also by peripheral blood components leaking into the brain, such as albumin, immunoglobulins, complement, matrix metalloproteinases, and coagulation factors. Serum components, particularly thrombin, have been shown to dedifferentiate astrocytes, resulting in loss of GS (Nelson and Siman, 1990); thus, leakage of thrombin (or possibly prothrombin) across a damaged BBB may be involved in the downregulation of GS in MTLE.
3.7 Metabolites, cofactors, metal ions, and pH
Because GS is intimately involved with regulation of neurotransmitter cycling (in particular, glutamate-glutamine and glutamate-GABA-glutamine cycling) (Chowdhury et al., 2007; Jain et al., 2011; Sibson et al., 2001), it is not surprising that several metabolites associated with neurotransmitter cycling, including the substrates and products of the synthetic reaction catalyzed by GS, play key roles in regulating this enzyme. To better appreciate how the catalytic activity of and flux of substrates through GS can be regulated, it is important to briefly discuss the mechanistic outcome of the kinetic investigations of the purified brain GS (Cooper et al., 1983; Meister, 1985).
The overall mechanism of the GS reaction is complex, and in addition to the synthetic reaction already alluded to above, GS also catalyzes eight other reactions or partial reactions (Cooper et al., 1983; Meister, 1985). However, a detailed discussion of the eight reactions is beyond the scope of this review. It is sufficient to emphasize that a key mechanism of the GS reaction appears to be the formation of an acyl phosphate intermediate followed by a nucleophilic displacement of Pi by ammonia, leading to glutamine formation (Cooper et al., 1983; Meister, 1985). Consistent with this mechanistic construct is the observation that L-methionine-SR-sulfoximine (MSO), an analog of the tetrahedral transition state intermediate, is phosphorylated to MSO-phosphate, which binds tightly to the GS active site and renders GS inactive. Glutamate provides some protection against MSO inactivation, but ammonia does not. Nevertheless, in the presence of ammonia, glutamate provides protection of GS against MSO inactivation (Cooper et al., 1983; Meister, 1985). Because of their implications in the regulation of this enzyme, other catalytic characteristics of GS can be highlighted as follows (Cooper et al., 1983; Meister, 1985): (i) GS possesses separate binding sites for glutamate, ATP, and ammonia, but the substrates do not bind to these sites covalently (Cooper et al., 1983; Meister, 1985). (ii) Ammonia binding to GS requires prior glutamate binding (Cooper et al., 1983; Meister, 1985). Consequently, astrocytic glutamate is important in the role of GS in ammonia detoxification in the brain. (iii) MSO binds to both the glutamate and ammonia binding sites (Cooper et al., 1983; Meister, 1985). Consequently, the convulsant effect of elevated brain MSO can be explained, in part, by elevation of excitatory glutamate and ammonia, neither of which can be detoxified by the action of GS. (iv) Formation of an acyl phosphate intermediate is the key. The slow formation of 2-pyrrolidone-5-carboxylate in the presence of glutamate, ATP, and divalent metal ions (in the absence of ammonia) suggests that GS stabilizes the acyl phosphate intermediate (Cooper et al., 1983; Meister, 1985). Consequently, together with the Km values of its substrates (see below), these kinetic characteristics of GS can help explain how GS can be regulated by its substrates and the inhibitor MSO. On the other hand, L-aspartate, another excitatory neurotransmitter, is neither a substrate nor an effective inhibitor of GS (Cooper et al., 1983).
For purified sheep brain GS, its pH optimum is in the range of 7.0 to 7.4 (Meister, 1985). However, its pH optimum can vary between 4.8 and 8.5, depending on the divalent cations present (Meister, 1985). At pH 7.2, Mg++ is more effective than Co++ or Mn++ at their respective pH optima (Meister, 1985). Equivalent activities are observed with these two divalent cations. In the presence of Mg++, the apparent Km values for ATP, L-glutamate, and ammonium ion are, respectively, 2.3, 2.5, and 0.18 mM for sheep brain GS (Meister, 1985). Unlike the liver enzyme, brain GS does not respond to physiological levels of feedback modifiers or end-product metabolites derived from L-glutamine (Wedler and Toms, 1986).
There is evidence that brain GS is a Mn++ metalloprotein (Denman and Wedler, 1984; Wedler et al., 1982; Wedler and Ley, 1994; Wedler et al., 1994). Consistent with this conclusion are the observations that, regardless of whether the transferase activities or the biosynthetic activities of sheep brain GS were measured, the apparent Km for Mn++ (∼20 µM) was approximately two orders of magnitude lower than the corresponding apparent Km for Mg(II) (1.7–3.5 mM) (Wedler and Toms, 1986). In conjunction with the differential roles of divalent cations in activating brain GS (Meister, 1985), the finding that brain GS is a Mn metalloprotein, having a high affinity for Mn++, strongly suggests that intracellular levels of Mn++ in astrocytes constitute one critical factor in regulating brain GS activity and flux (Wedler et al., 1994), because brain GS is largely astroglial in localization (Martinez-Hernandez et al., 1977). Brain levels of manganese become elevated in several neurodegenerative (e.g., Alzheimer’s disease and Parkinson’s disease) and metabolic (e.g., hepatic encephalopathy) diseases and in manganese toxicity (Lai et al., 1999; Lai et al., 2000). Of relevance to the regulation of brain GS is the finding that significant levels of the elevated Mn are likely detected in astrocytes, because these cells are known to accumulate Mn avidly (Lai et al., 1999; Lai et al., 2000; Wedler et al., 1994). Consequently, the elevation of astrocytic Mn levels may exert modulatory effects on GS activity and flux and ultimately on glutamate-glutamine and glutamate-GABA-glutamine cycling in those disease states (Chowdhury et al., 2007; Jain et al., 2011; Lai et al., 1999; Lai et al., 2000; Sibson et al., 2001; Wedler et al., 1994). A case in point is hepatic encephalopathy (Lai et al., 1999; Lai et al., 2000) (see below). Furthermore, the mechanistic interrelations between elevated Mn levels, GS regulation, and neurotransmitter cycling may have important pathophysiological implications in seizure disorders.
When their brain levels are elevated, two substrates of GS, namely ammonia and glutamate, are excitotoxic and exert convulsant effects (Cooper and Lai, 1987; Lai and Cooper, 1986, 1991; Lai et al., 1989). Indeed, GS is the only brain enzyme efficient at removing excess brain ammonia (Cooper and Lai, 1987; Cooper et al., 1983). Thus, when GS is functionally compromised, it can no longer convert ammonia into the comparatively harmless glutamine. One result of the functional compromise of GS is that brain ammonia will rise and ultimately become proconvulsant. The pathophysiological mechanisms of hepatic encephalopathy provide some insight into how brain GS can be functionally compromised. Hepatic encephalopathy is associated with increases in brain levels of ammonia and Mn (Cooper and Lai, 1987; Lai and Cooper, 1986, 1991; Lai et al., 1999; Lai et al., 2000; Lai et al., 1989), the increase in Mn being especially notable in the basal ganglia.
In hepatic encephalopathy, astrocytes typically show Alzheimer Type II histopathological changes (Norenberg, 1988). Two neurotoxins (namely, ammonia and Mn) implicated in this disease are likely to be mechanistically responsible for these pathological changes in astrocytes. Ammonia treatment of primary cultures of astrocytes also induces them to swell and exhibit pathological morphologies very similar, if not identical to Alzheimer Type II histopathological changes (Norenberg, 1988). Ammonia and Mn++ act synergistically in inhibiting glutamate uptake by astrocytes in culture (Hazell and Norenberg, 1997). Similarly, Mn++ treatment of astrocytes in primary culture induces them to swell (Rama Rao et al., 2007) and the effects of ammonia and Mn++ on astroglial swelling are at least additive, if not synergistic (Hazell and Norenberg, 1997; Jayakumar et al., 2004; Rama Rao et al., 2007).
Treatment of astrocytes in primary culture with Mn++ induces decreased expression of glutamate transporters and GS in these cells (Deng et al., 2012). This effect of Mn++ can lower the capacity of Mn-treated astrocytes to detoxify brain ammonia (Deng et al., 2012) by (i) lowering the catalytic capacity of astroglial GS (through decreasing GS expression) and (ii) decreasing the availability of externally supplied glutamate, a key GS substrate (Hazell and Norenberg, 1997). Moreover, in chronic Mn toxicity, GS expression is decreased, and swelling of astrocytes [e.g., resulting from combined treatment with ammonia and Mn++] (Norenberg, 1988; Rama Rao et al., 2007) leads to decreased GS activity, even though astrocytes are accumulating Mn++, which, upon entering the cytoplasmic space of astrocytes, may bind to GS and thereby activates it (Wedler and Toms, 1986). Taken together, these observations prompted us to conclude that when the brain, and consequently the extracellular, levels of ammonia and Mn++ are elevated, as is the case in hepatic encephalopathy (Cooper and Lai, 1987; Hazell and Norenberg, 1997; Jayakumar et al., 2004; Lai and Cooper, 1986; Lai et al., 1999; Lai et al., 2000; Norenberg, 1988; Rama Rao et al., 2007), the astroglial capacity to detoxify ammonia via the action of GS is decreased because of lower availability of glutamate, a substrate of GS, and decreased expression of GS (Deng et al., 2012; Morello et al., 2007). The net result of these pathophysiological mechanisms derived from elevation of extracellular glutamate and ammonia increases the likelihood of seizures.
Another metabolic and signaling mechanism known to regulate astroglial GS is pH (Nissim, 1999). Elevation of intracellular pH markedly stimulates astroglial glutamine synthesis (Nissim, 1999). A few mechanisms may account for this pH effect (Nissim, 1999). Potassium ion-induced alkalinization of astrocytes is associated with enhanced glutamate uptake and glutamine synthesis by astrocytes (Nissim, 1999). On the other hand, even though this enhanced glutamate uptake provides more substrate for GS, the increased astroglial accumulation of glutamate may decrease intracellular pH in astrocytes because of transporter-mediated inward H+ and outward K+ and OH- transport (Nissim, 1999). Consequently, this acidosis induced by astroglial glutamate accumulation may exert an inhibitory effect on GS (Nissim, 1999). Nevertheless, the quantitative dynamics of the regulation of astroglial glutamine synthesis by elevation of intracellular pH remains incompletely understood and is an important area that merits further investigation.
3.8 Exogenous compounds
Several exogenous compounds can influence the expression and activity of GS. Perhaps the most extensively studied such compound is MSO, which competes for binding with glutamate in the active site of GS (Ronzio and Meister, 1968). MSO is phosphorylated by GS in the presence of ATP and results in an irreversible, noncovalent inhibition of the enzyme (Eisenberg et al., 2000; Ronzio and Meister, 1968). The effects of MSO on brain function were first discovered when animals were fed nitrogen chloride-treated (agenized) zein (a maize protein) and subsequently developed hysteria and seizures. The neuropsychiatric signs were caused by MSO, which developed as a byproduct during the agenization process (Bentley et al., 1949). Later studies showed that administration of MSO to mammals inhibited GS and resulted in slowing of the glutamine-glutamate-GABA metabolic pathway (Liang et al., 2006), with increased glutamate in astrocytes (Perez et al., 2012), and epileptic seizures as some of the consequences (Eid et al., 2008; Folbergrova et al., 1969).
Over forty additional exogenous inhibitors of GS have been identified [see Eisenberg et al. for an extensive review (Eisenberg et al., 2000)]. While most of the inhibitors are synthetic, some are found in nature, e.g. tabtoxinine-beta-lactam produced by Pseudomonas syringae pv. tabaci (Langston-Unkefer et al., 1984), alanosine produced by Streptomyces alanosinicus (Anandaraj et al., 1980), and a 110-kD protein identified in tomato roots (Gallardo and Canovas, 1992).
Considerably fewer exogenous compounds have been shown to increase the activity or expression of GS. The phytoalexin resveratrol enhances glutamate uptake and GS activity in C6 glioma cells (dos Santos et al., 2006). Beta-N-oxalyl-L-alpha,beta-diaminopropionic acid (beta-L-ODAP), which is a toxin derived from the seeds of Lathyrus sativus, increases GS activity by 155 percent in astrocytes in the rat cerebral cortex. The effect is dose-dependent and requires protein translation (Miller et al., 1993). Finally, treatment of mice in utero with 2,2',4,4' tetrabromo diphenyl ether (BDE47) results in significant upregulation of GS mRNA in the brain (Haave et al., 2011).
4. CONCLUSIONS
GS, which is found in astrocytes, is a critical enzyme for metabolism of ammonia, glutamate, and GABA in the central nervous system, and perturbations in the homeostasis of these metabolites are likely to profoundly affect brain function. An increasing number of studies have revealed a spectrum of deficiencies and aberrant expression patterns of brain GS in patients with epilepsy, stroke, Alzheimer’s disease, multiple sclerosis, schizophrenia, and suicide/depression. While the exact causes of these alterations are not fully understood, several mechanisms are possible, such as: (a) perturbations in glucocorticoids, sex hormones, and growth factors; (b) oxidative and nitrosative stresses; (c) loss or gain of extracellular interactions; (d) neuroinflammation/BBB dysfunction; and (e) perturbations of metabolites, cofactors, metal ions, and pH. Knowledge of the mechanisms by which GS is altered in brain pathology is likely to guide the development of novel therapeutic interventions targeting the expression and activity of this important enzyme.
Research highlights.
Glutamine synthetase (GS) is critical for metabolism of brain glutamate and ammonia
Brain GS is deficient in several brain disorders, including epilepsy
Deficiency in GS may lead to perturbed neurotransmission and epileptic seizures
Here we discuss the mechanisms that regulate GS in the brain
Knowledge of such mechanisms may facilitate new therapies
ACKNOWLEDGEMENTS
TE and JCKL are supported by grants from the National Institutes of Health (NIH): NINDS K08 NS058674 and R01 NS070824. This work was also made possible by grants from the National Center for Advancing Translational Sciences (NCATS; UL1 RR024139) and the National Center for Research Resources (NCRR; TL1 RR024137). NCATS and NCRR are components of the NIH, and NIH roadmap for Medical Research. This work is solely the responsibility of the authors and does not necessarily represent the official view of NIH. The authors would like to
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Amiry-Moghaddam M, Williamson A, Palomba M, Eid T, de Lanerolle NC, Nagelhus EA, Adams ME, Froehner SC, Agre P, Ottersen OP. Delayed K+ clearance associated with aquaporin-4 mislocalization: phenotypic defects in brains of alpha-syntrophin-null mice. Proc Natl Acad Sci U S A. 2003;100:13615–13620. doi: 10.1073/pnas.2336064100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anandaraj SJ, Jayaram HN, Cooney DA, Tyagi AK, Han N, Thomas JH, Chitnis M, Montgomery JA. Interaction of L-alanosine (NSC 153, 353) with enzymes metabolizing L-aspartic acid, L-glutamic acid and their amides. Biochem Pharmacol. 1980;29:227–245. doi: 10.1016/0006-2952(80)90333-0. [DOI] [PubMed] [Google Scholar]
- Audard V, Cavard C, Richa H, Infante M, Couvelard A, Sauvanet A, Terris B, Paye F, Flejou JF. Impaired E-cadherin expression and glutamine synthetase overexpression in solid pseudopapillary neoplasm of the pancreas. Pancreas. 2008;36:80–83. doi: 10.1097/mpa.0b013e318137a9da. [DOI] [PubMed] [Google Scholar]
- Avisar N, Shiftan L, Ben-Dror I, Havazelet N, Vardimon L. A silencer element in the regulatory region of glutamine synthetase controls cell type-specific repression of gene induction by glucocorticoids. J Biol Chem. 1999;274:11399–11407. doi: 10.1074/jbc.274.16.11399. [DOI] [PubMed] [Google Scholar]
- Ballabh P, Braun A, Nedergaard M. The blood-brain barrier: an overview: structure, regulation, and clinical implications. Neurobiol Dis. 2004;16:1–13. doi: 10.1016/j.nbd.2003.12.016. [DOI] [PubMed] [Google Scholar]
- Bausch SB. Potential roles for hyaluronan and CD44 in kainic acid-induced mossy fiber sprouting in organotypic hippocampal slice cultures. Neuroscience. 2006;143:339–350. doi: 10.1016/j.neuroscience.2006.07.037. [DOI] [PubMed] [Google Scholar]
- Ben-Ari Y. Limbic seizure and brain damage produced by kainic acid: mechanisms and relevance to human temporal lobe epilepsy. Neuroscience. 1985;14:375–403. doi: 10.1016/0306-4522(85)90299-4. [DOI] [PubMed] [Google Scholar]
- Ben-Ari Y. Epilepsies and neuronal plasticity: for better or for worse? Dialogues Clin Neurosci. 2008;10:17–27. doi: 10.31887/DCNS.2008.10.1/ybenari. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benfenati V, Amiry-Moghaddam M, Caprini M, Mylonakou MN, Rapisarda C, Ottersen OP, Ferroni S. Expression and functional characterization of transient receptor potential vanilloid-related channel 4 (TRPV4) in rat cortical astrocytes. Neuroscience. 2007;148:876–892. doi: 10.1016/j.neuroscience.2007.06.039. [DOI] [PubMed] [Google Scholar]
- Bentley HR, Mc DE, et al. Action of nitrogen trichloride (agene) on proteins; isolation of crystalline toxic factor. Nature. 1949;164:438. doi: 10.1038/164438a0. [DOI] [PubMed] [Google Scholar]
- Bergersen LH. Is lactate food for neurons? Comparison of monocarboxylate transporter subtypes in brain and muscle. Neuroscience. 2007;145:11–19. doi: 10.1016/j.neuroscience.2006.11.062. [DOI] [PubMed] [Google Scholar]
- Bezzi P, Carmignoto G, Pasti L, Vesce S, Rossi D, Rizzini BL, Pozzan T, Volterra A. Prostaglandins stimulate calcium-dependent glutamate release in astrocytes. Nature. 1998;391:281–285. doi: 10.1038/34651. [DOI] [PubMed] [Google Scholar]
- Bezzi P, Domercq M, Brambilla L, Galli R, Schols D, De Clercq E, Vescovi A, Bagetta G, Kollias G, Meldolesi J, Volterra A. CXCR4-activated astrocyte glutamate release via TNFalpha: amplification by microglia triggers neurotoxicity. Nat Neurosci. 2001;4:702–710. doi: 10.1038/89490. [DOI] [PubMed] [Google Scholar]
- Bidmon HJ, Gorg B, Palomero-Gallagher N, Schleicher A, Haussinger D, Speckmann EJ, Zilles K. Glutamine synthetase becomes nitrated and its activity is reduced during repetitive seizure activity in the pentylentetrazole model of epilepsy. Epilepsia. 2008;49:1733–1748. doi: 10.1111/j.1528-1167.2008.01642.x. [DOI] [PubMed] [Google Scholar]
- Binder DK, Nagelhus EA, Ottersen OP. Aquaporin-4 and epilepsy. Glia. 2012 doi: 10.1002/glia.22317. [DOI] [PubMed] [Google Scholar]
- Binder DK, Steinhauser C. Functional changes in astroglial cells in epilepsy. Glia. 2006;54:358–368. doi: 10.1002/glia.20394. [DOI] [PubMed] [Google Scholar]
- Binder DK, Yao X, Zador Z, Sick TJ, Verkman AS, Manley GT. Increased seizure duration and slowed potassium kinetics in mice lacking aquaporin-4 water channels. Glia. 2006;53:631–636. doi: 10.1002/glia.20318. [DOI] [PubMed] [Google Scholar]
- Bjornsen LP, Eid T, Holmseth S, Danbolt NC, Spencer DD, de Lanerolle NC. Changes in glial glutamate transporters in human epileptogenic hippocampus: inadequate explanation for high extracellular glutamate during seizures. Neurobiol Dis. 2007;25:319–330. doi: 10.1016/j.nbd.2006.09.014. [DOI] [PubMed] [Google Scholar]
- Blondet B, Hantaz-Ambroise D, Ait-Ikhlef A, Cambier D, Murawsky M, Rieger F. Astrocytosis in wobbler mouse spinal cord involves a population of astrocytes which is glutamine synthetase-negative. Neurosci Lett. 1995;183:179–182. doi: 10.1016/0304-3940(94)11145-9. [DOI] [PubMed] [Google Scholar]
- Blutstein T, Devidze N, Choleris E, Jasnow AM, Pfaff DW, Mong JA. Oestradiol up-regulates glutamine synthetase mRNA and protein expression in the hypothalamus and hippocampus: implications for a role of hormonally responsive glia in amino acid neurotransmission. J Neuroendocrinol. 2006;18:692–702. doi: 10.1111/j.1365-2826.2006.01466.x. [DOI] [PubMed] [Google Scholar]
- Boddeke EWGM, Eggen BJL, Biber KPH. Cytokine, chemokine, and growth factor receptors and signaling. In: Kettenmann H, Ransom BR, editors. Neuroglia. 3 ed. New York: Oxford University Press; 2013. pp. 266–280. [Google Scholar]
- Boison D. The adenosine kinase hypothesis of epileptogenesis. Prog Neurobiol. 2008;84:249–262. doi: 10.1016/j.pneurobio.2007.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boison D. Epilepsy. In: Kettenmann H, Ransom BR, editors. Neuroglia. 3 ed. New York: Oxford University Press; 2013. pp. 896–905. [Google Scholar]
- Bordey A, Sontheimer H. Properties of human glial cells associated with epileptic seizure foci. Epilepsy Res. 1998;32:286–303. doi: 10.1016/s0920-1211(98)00059-x. [DOI] [PubMed] [Google Scholar]
- Bowman CL, Kimelberg HK. Excitatory amino acids directly depolarize rat brain astrocytes in primary culture. Nature. 1984;311:656–659. doi: 10.1038/311656a0. [DOI] [PubMed] [Google Scholar]
- Boyd-Kimball D, Sultana R, Poon HF, Lynn BC, Casamenti F, Pepeu G, Klein JB, Butterfield DA. Proteomic identification of proteins specifically oxidized by intracerebral injection of amyloid beta-peptide (1–42) into rat brain: implications for Alzheimer's disease. Neuroscience. 2005;132:313–324. doi: 10.1016/j.neuroscience.2004.12.022. [DOI] [PubMed] [Google Scholar]
- Broer S, Rahman B, Pellegri G, Pellerin L, Martin JL, Verleysdonk S, Hamprecht B, Magistretti PJ. Comparison of lactate transport in astroglial cells and monocarboxylate transporter 1 (MCT 1) expressing Xenopus laevis oocytes. Expression of two different monocarboxylate transporters in astroglial cells and neurons. J Biol Chem. 1997;272:30096–30102. doi: 10.1074/jbc.272.48.30096. [DOI] [PubMed] [Google Scholar]
- Busceti CL, Biagioni F, Aronica E, Riozzi B, Storto M, Battaglia G, Giorgi FS, Gradini R, Fornai F, Caricasole A, Nicoletti F, Bruno V. Induction of the Wnt inhibitor, Dickkopf-1, is associated with neurodegeneration related to temporal lobe epilepsy. Epilepsia. 2007;48:694–705. doi: 10.1111/j.1528-1167.2007.01055.x. [DOI] [PubMed] [Google Scholar]
- Butterfield DA, Poon HF, St Clair D, Keller JN, Pierce WM, Klein JB, Markesbery WR. Redox proteomics identification of oxidatively modified hippocampal proteins in mild cognitive impairment: insights into the development of Alzheimer's disease. Neurobiol Dis. 2006;22:223–232. doi: 10.1016/j.nbd.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Cadoret A, Ovejero C, Terris B, Souil E, Levy L, Lamers WH, Kitajewski J, Kahn A, Perret C. New targets of beta-catenin signaling in the liver are involved in the glutamine metabolism. Oncogene. 2002;21:8293–8301. doi: 10.1038/sj.onc.1206118. [DOI] [PubMed] [Google Scholar]
- Caggese C, Caizzi R, Barsanti P, Bozzetti MP. Mutations in the glutamine synthetase I (gsI) gene produce embryo-lethal female sterility in Drosophila melanogaster. Dev Genet. 1992;13:359–366. doi: 10.1002/dvg.1020130506. [DOI] [PubMed] [Google Scholar]
- Cavus I, Kasoff WS, Cassaday MP, Jacob R, Gueorguieva R, Sherwin RS, Krystal JH, Spencer DD, Abi-Saab WM. Extracellular metabolites in the cortex and hippocampus of epileptic patients. Ann Neurol. 2005;57:226–235. doi: 10.1002/ana.20380. [DOI] [PubMed] [Google Scholar]
- Cavus I, Pan JW, Hetherington HP, Abi-Saab W, Zaveri HP, Vives KP, Krystal JH, Spencer SS, Spencer DD. Decreased hippocampal volume on MRI is associated with increased extracellular glutamate in epilepsy patients. Epilepsia. 2008;49:1358–1366. doi: 10.1111/j.1528-1167.2008.01603.x. [DOI] [PubMed] [Google Scholar]
- Chao CC, Hu S, Ehrlich L, Peterson PK. Interleukin-1 and tumor necrosis factor-alpha synergistically mediate neurotoxicity: involvement of nitric oxide and of N-methyl-D-aspartate receptors. Brain Behav Immun. 1995;9:355–365. doi: 10.1006/brbi.1995.1033. [DOI] [PubMed] [Google Scholar]
- Chaudhry FA, Reimer RJ, Edwards RH. The glutamine commute: take the N line and transfer to the A. J Cell Biol. 2002;157:349–355. doi: 10.1083/jcb.200201070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowdhury GM, Patel AB, Mason GF, Rothman DL, Behar KL. Glutamatergic and GABAergic neurotransmitter cycling and energy metabolism in rat cerebral cortex during postnatal development. J Cereb Blood Flow Metab. 2007;27:1895–1907. doi: 10.1038/sj.jcbfm.9600490. [DOI] [PubMed] [Google Scholar]
- Cohen-Gadol AA, Pan JW, Kim JH, Spencer DD, Hetherington HH. Mesial temporal lobe epilepsy: a proton magnetic resonance spectroscopy study and a histopathological analysis. J Neurosurg. 2004;101:613–620. doi: 10.3171/jns.2004.101.4.0613. [DOI] [PubMed] [Google Scholar]
- Cooper AJ, Lai JC. Cerebral ammonia metabolism in normal and hyperammonemic rats. Neurochem Pathol. 1987;6:67–95. doi: 10.1007/BF02833601. [DOI] [PubMed] [Google Scholar]
- Cooper AJ, Vergara F, Duffy TE. Cerebral glutamine synthetase. In: Hertz L, Kvamme E, McGeer EG, Schousboe A, editors. Glutamine, glutamate, and GABA in the central nervous system. New York: Alan R. Liss Inc; 1983. pp. 77–93. [Google Scholar]
- Coulter DA, Eid T. Astrocytic regulation of glutamate homeostasis in epilepsy. Glia. 2012;60:1215–1226. doi: 10.1002/glia.22341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai M, Xia XB, Xiong SQ. BDNF regulates GLAST and glutamine synthetase in mouse retinal Muller cells. J Cell Physiol. 2012;227:596–603. doi: 10.1002/jcp.22762. [DOI] [PubMed] [Google Scholar]
- Danbolt NC. Glutamate uptake. Prog. Neurobiol. 2001;65:1–105. doi: 10.1016/s0301-0082(00)00067-8. [DOI] [PubMed] [Google Scholar]
- de Lanerolle NC, Eid T, von Campe G, Kovacs I, Spencer DD, Brines M. Glutamate receptor subunits GluR1 and GluR2/3 distribution shows reorganization in the human epileptogenic hippocampus. Eur J Neurosci. 1998;10:1687–1703. doi: 10.1046/j.1460-9568.1998.00171.x. [DOI] [PubMed] [Google Scholar]
- Debernardi R, Pierre K, Lengacher S, Magistretti PJ, Pellerin L. Cell-specific expression pattern of monocarboxylate transporters in astrocytes and neurons observed in different mouse brain cortical cell cultures. J Neurosci Res. 2003;73:141–155. doi: 10.1002/jnr.10660. [DOI] [PubMed] [Google Scholar]
- Deng Y, Xu Z, Xu B, Xu D, Tian Y, Feng W. The protective effects of riluzole on manganese-induced disruption of glutamate transporters and glutamine synthetase in the cultured astrocytes. Biol Trace Elem Res. 2012;148:242–249. doi: 10.1007/s12011-012-9365-1. [DOI] [PubMed] [Google Scholar]
- Denman RB, Wedler FC. Association-dissociation of mammalian brain glutamine synthetase: effects of metal ions and other ligands. Arch Biochem Biophys. 1984;232:427–440. doi: 10.1016/0003-9861(84)90559-9. [DOI] [PubMed] [Google Scholar]
- Derouiche A, Frotscher M. Astroglial processes around identified glutamatergic synapses contain glutamine synthetase: evidence for transmitter degradation. Brain Res. 1991;552:346–350. doi: 10.1016/0006-8993(91)90103-3. [DOI] [PubMed] [Google Scholar]
- Derouiche A, Heimrich B, Frotscher M. Loss of layer-specific astrocytic glutamine synthetase immunoreactivity in slice cultures of hippocampus. Eur J Neurosci. 1993;5:122–127. doi: 10.1111/j.1460-9568.1993.tb00477.x. [DOI] [PubMed] [Google Scholar]
- Derouiche A, Ohm TG. Glutamine synthetase immunoreactivity in the human hippocampus is lamina-specific. Neurosci Lett. 1994;165:179–182. doi: 10.1016/0304-3940(94)90739-0. [DOI] [PubMed] [Google Scholar]
- Domowicz MS, Sanders TA, Ragsdale CW, Schwartz NB. Aggrecan is expressed by embryonic brain glia and regulates astrocyte development. Dev Biol. 2008 doi: 10.1016/j.ydbio.2007.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- dos Santos AQ, Nardin P, Funchal C, de Almeida LM, Jacques-Silva MC, Wofchuk ST, Goncalves CA, Gottfried C. Resveratrol increases glutamate uptake and glutamine synthetase activity in C6 glioma cells. Arch Biochem Biophys. 2006;453:161–167. doi: 10.1016/j.abb.2006.06.025. [DOI] [PubMed] [Google Scholar]
- During MJ, Spencer DD. Extracellular hippocampal glutamate and spontaneous seizure in the conscious human brain. Lancet. 1993;341:1607–1610. doi: 10.1016/0140-6736(93)90754-5. [DOI] [PubMed] [Google Scholar]
- Eid T, Brines ML, Cerami A, Spencer DD, Kim JH, Schweitzer JS, Ottersen OP, de Lanerolle NC. Increased expression of erythropoietin receptor on blood vessels in the human epileptogenic hippocampus with sclerosis. J Neuropathol Exp Neurol. 2004a;63:73–83. doi: 10.1093/jnen/63.1.73. [DOI] [PubMed] [Google Scholar]
- Eid T, Ghosh A, Wang Y, Beckstrom H, Zaveri HP, Lee TS, Lai JC, Malthankar-Phatak GH, de Lanerolle NC. Recurrent seizures and brain pathology after inhibition of glutamine synthetase in the hippocampus in rats. Brain. 2008;131:2061–2070. doi: 10.1093/brain/awn133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eid T, Kovacs I, Spencer DD, de Lanerolle NC. Novel expression of AMPA-receptor subunit GluR1 on mossy cells and CA3 pyramidal neurons in the human epileptogenic hippocampus. Eur J Neurosci. 2002;15:517–527. doi: 10.1046/j.0953-816x.2001.01887.x. [DOI] [PubMed] [Google Scholar]
- Eid T, Lee TS, Thomas MJ, Amiry-Moghaddam M, Bjornsen LP, Spencer DD, Agre P, Ottersen OP, de Lanerolle NC. Loss of perivascular aquaporin 4 may underlie deficient water and K+ homeostasis in the human epileptogenic hippocampus. Proc Natl Acad Sci U S A. 2005;102:1193–1198. doi: 10.1073/pnas.0409308102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eid T, Thomas MJ, Spencer DD, Runden-Pran E, Lai JC, Malthankar GV, Kim JH, Danbolt NC, Ottersen OP, de Lanerolle NC. Loss of glutamine synthetase in the human epileptogenic hippocampus: possible mechanism for raised extracellular glutamate in mesial temporal lobe epilepsy. Lancet. 2004b;363:28–37. doi: 10.1016/s0140-6736(03)15166-5. [DOI] [PubMed] [Google Scholar]
- Eisenberg D, Gill HS, Pfluegl GM, Rotstein SH. Structure-function relationships of glutamine synthetases. Biochim Biophys Acta. 2000;1477:122–145. doi: 10.1016/s0167-4838(99)00270-8. [DOI] [PubMed] [Google Scholar]
- Fahrner J, Labruyere WT, Gaunitz C, Moorman AF, Gebhardt R, Lamers WH. Identification and functional characterization of regulatory elements of the glutamine synthetase gene from rat liver. Eur J Biochem. 1993;213:1067–1073. doi: 10.1111/j.1432-1033.1993.tb17854.x. [DOI] [PubMed] [Google Scholar]
- Fernandes SP, Dringen R, Lawen A, Robinson SR. Inactivation of astrocytic glutamine synthetase by hydrogen peroxide requires iron. Neurosci Lett. 2011;490:27–30. doi: 10.1016/j.neulet.2010.12.019. [DOI] [PubMed] [Google Scholar]
- Fernandez LL, de Lima MN, Scalco F, Vedana G, Miwa C, Hilbig A, Vianna M, Schroder N. Early post-natal iron administration induces astroglial response in the brain of adult and aged rats. Neurotox Res. 2011;20:193–199. doi: 10.1007/s12640-010-9235-6. [DOI] [PubMed] [Google Scholar]
- Folbergrova J, Passonneau JV, Lowry OH, Schulz DW. Glycogen, ammonia and related metabolities in the brain during seizures evoked by methionine sulphoximine. J Neurochem. 1969;16:191–203. doi: 10.1111/j.1471-4159.1969.tb05937.x. [DOI] [PubMed] [Google Scholar]
- Friis ML, Lund M. Stress convulsions. Arch Neurol. 1974;31:155–159. doi: 10.1001/archneur.1974.00490390037002. [DOI] [PubMed] [Google Scholar]
- Gallardo F, Canovas FM. A macromolecular inhibitor of glutamine synthase activity in tomato root extracts. Phytochemistry. 1992;31:2267–2271. [Google Scholar]
- Games D, Adams D, Alessandrini R, Barbour R, Berthelette P, Blackwell C, Carr T, Clemens J, Donaldson T, Gillespie F, et al. Alzheimer-type neuropathology in transgenic mice overexpressing V717F beta-amyloid precursor protein. Nature. 1995;373:523–527. doi: 10.1038/373523a0. [DOI] [PubMed] [Google Scholar]
- Garden G. Glia in bacterial and viral central nervous system infections. In: Kettenmann H, Ransom BR, editors. Neuroglia. 3 ed. New York: Oxford University Press; 2013. pp. 849–060. [Google Scholar]
- Garriga-Canut M, Schoenike B, Qazi R, Bergendahl K, Daley TJ, Pfender RM, Morrison JF, Ockuly J, Stafstrom C, Sutula T, Roopra A. 2-Deoxy-D-glucose reduces epilepsy progression by NRSF-CtBP-dependent metabolic regulation of chromatin structure. Nat Neurosci. 2006;9:1382–1387. doi: 10.1038/nn1791. [DOI] [PubMed] [Google Scholar]
- Gebhardt R, Mecke D. Heterogeneous distribution of glutamine synthetase among rat liver parenchymal cells in situ and in primary culture. Embo J. 1983;2:567–570. doi: 10.1002/j.1460-2075.1983.tb01464.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gloor P. Mesial temporal sclerosis: Historical background and an overview from a modern perspective. In: Luders H, editor. Epilepsy Surgery. New York: Raven Press; 1991. pp. 689–703. [Google Scholar]
- Haave M, Folven KI, Carroll T, Glover C, Heegaard E, Brattelid T, Hogstrand C, Lundebye AK. Cerebral gene expression and neurobehavioural development after perinatal exposure to an environmentally relevant polybrominated diphenylether (BDE47) Cell Biol Toxicol. 2011;27:343–361. doi: 10.1007/s10565-011-9192-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haberle J, Gorg B, Rutsch F, Schmidt E, Toutain A, Benoist JF, Gelot A, Suc AL, Hohne W, Schliess F, Haussinger D, Koch HG. Congenital glutamine deficiency with glutamine synthetase mutations. N Engl J Med. 2005;353:1926–1933. doi: 10.1056/NEJMoa050456. [DOI] [PubMed] [Google Scholar]
- Haberle J, Gorg B, Toutain A, Rutsch F, Benoist JF, Gelot A, Suc AL, Koch HG, Schliess F, Haussinger D. Inborn error of amino acid synthesis: human glutamine synthetase deficiency. J Inherit Metab Dis. 2006;29:352–358. doi: 10.1007/s10545-006-0256-5. [DOI] [PubMed] [Google Scholar]
- Haberle J, Shahbeck N, Ibrahim K, Hoffmann GF, Ben-Omran T. Natural course of glutamine synthetase deficiency in a 3 year old patient. Mol Genet Metab. 2011;103:89–91. doi: 10.1016/j.ymgme.2011.02.001. [DOI] [PubMed] [Google Scholar]
- Haghighat N. Estrogen (17beta-estradiol) enhances glutamine synthetase activity in C6-glioma cells. Neurochem Res. 2005;30:661–667. doi: 10.1007/s11064-005-2754-5. [DOI] [PubMed] [Google Scholar]
- Halassa MM, Fellin T, Haydon PG. The tripartite synapse: roles for gliotransmission in health and disease. Trends Mol Med. 2007;13:54–63. doi: 10.1016/j.molmed.2006.12.005. [DOI] [PubMed] [Google Scholar]
- Hallermayer K, Harmening C, Hamprecht B. Cellular localization and regulation of glutamine synthetase in primary cultures of brain cells from newborn mice. J Neurochem. 1981;37:43–52. doi: 10.1111/j.1471-4159.1981.tb05289.x. [DOI] [PubMed] [Google Scholar]
- Hasler G, van der Veen JW, Tumonis T, Meyers N, Shen J, Drevets WC. Reduced prefrontal glutamate/glutamine and gamma-aminobutyric acid levels in major depression determined using proton magnetic resonance spectroscopy. Arch Gen Psychiatry. 2007;64:193–200. doi: 10.1001/archpsyc.64.2.193. [DOI] [PubMed] [Google Scholar]
- Hauser WA, Annegers JF, Kurland LT. Incidence of epilepsy and unprovoked seizures in Rochester, Minnesota: 1935–1984. Epilepsia. 1993;34:453–468. doi: 10.1111/j.1528-1157.1993.tb02586.x. [DOI] [PubMed] [Google Scholar]
- Haydon PG, Carmignoto G. Astrocyte control of synaptic transmission and neurovascular coupling. Physiol Rev. 2006;86:1009–1031. doi: 10.1152/physrev.00049.2005. [DOI] [PubMed] [Google Scholar]
- Hazell AS, Norenberg MD. Manganese decreases glutamate uptake in cultured astrocytes. Neurochem Res. 1997;22:1443–1447. doi: 10.1023/a:1021994126329. [DOI] [PubMed] [Google Scholar]
- He Y, Hakvoort TB, Vermeulen JL, Labruyere WT, De Waart DR, Van Der Hel WS, Ruijter JM, Uylings HB, Lamers WH. Glutamine synthetase deficiency in murine astrocytes results in neonatal death. Glia. 2010;58:741–754. doi: 10.1002/glia.20960. [DOI] [PubMed] [Google Scholar]
- He Y, Hakvoort TB, Vermeulen JL, Lamers WH, Van Roon MA. Glutamine synthetase is essential in early mouse embryogenesis. Dev Dyn. 2007;236:1865–1875. doi: 10.1002/dvdy.21185. [DOI] [PubMed] [Google Scholar]
- Heinemann U, Kaufer D, Friedman A. Blood-brain barrier dysfunction, TGFbeta signaling, and astrocyte dysfunction in epilepsy. Glia. 2012;60:1251–1257. doi: 10.1002/glia.22311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hensley K, Hall N, Subramaniam R, Cole P, Harris M, Aksenov M, Aksenova M, Gabbita SP, Wu JF, Carney JM, et al. Brain regional correspondence between Alzheimer's disease histopathology and biomarkers of protein oxidation. J Neurochem. 1995;65:2146–2156. doi: 10.1046/j.1471-4159.1995.65052146.x. [DOI] [PubMed] [Google Scholar]
- Hertz L. Astrocytic energy metabolism and glutamate formation--relevance for 13C-NMR spectroscopy and importance of cytosolic/mitochondrial trafficking. Magn Reson Imaging. 2011;29:1319–1329. doi: 10.1016/j.mri.2011.04.013. [DOI] [PubMed] [Google Scholar]
- Heuser K, Eid T, Lauritzen F, Thoren AE, Vindedal GF, Tauboll E, Gjerstad L, Spencer DD, Ottersen OP, Nagelhus EA, de Lanerolle NC. Loss of Perivascular Kir4.1 Potassium Channels in the Sclerotic Hippocampus of Patients With Mesial Temporal Lobe Epilepsy. J Neuropathol Exp Neurol. 2012;71:814–825. doi: 10.1097/NEN.0b013e318267b5af. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang TL, O'Banion MK. Interleukin-1 beta and tumor necrosis factor-alpha suppress dexamethasone induction of glutamine synthetase in primary mouse astrocytes. J Neurochem. 1998;71:1436–1442. doi: 10.1046/j.1471-4159.1998.71041436.x. [DOI] [PubMed] [Google Scholar]
- Jain A, Lai JCK, Chowdhury GM, Behar K, Bhushan A. Glioblastoma: current chemotherapeutic status and need for new targets and approaches. In: Abujamra AL, editor. Brain tumors: current and emerging therapeutic strategies. Rijeka: InTech; 2011. pp. 145–176. [Google Scholar]
- Jayakumar AR, Rama Rao KV, Kalaiselvi P, Norenberg MD. Combined effects of ammonia and manganese on astrocytes in culture. Neurochem Res. 2004;29:2051–2056. doi: 10.1007/s11064-004-6878-9. [DOI] [PubMed] [Google Scholar]
- Katz MJ, Lasek RJ, Silver J. Ontophyletics of the nervous system: development of the corpus callosum and evolution of axon tracts. Proc Natl Acad Sci U S A. 1983;80:5936–5940. doi: 10.1073/pnas.80.19.5936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kettenmann H, Backus KH, Schachner M. Aspartate, glutamate and gamma-aminobutyric acid depolarize cultured astrocytes. Neurosci Lett. 1984;52:25–29. doi: 10.1016/0304-3940(84)90345-8. [DOI] [PubMed] [Google Scholar]
- Khelil M, Rolland B, Fages C, Tardy M. Glutamine synthetase modulation in astrocyte cultures of different mouse brain areas. Glia. 1990;3:75–80. doi: 10.1002/glia.440030110. [DOI] [PubMed] [Google Scholar]
- Kosenko E, Llansola M, Montoliu C, Monfort P, Rodrigo R, Hernandez-Viadel M, Erceg S, Sanchez-Perez AM, Felipo V. Glutamine synthetase activity and glutamine content in brain: modulation by NMDA receptors and nitric oxide. Neurochem Int. 2003;43:493–499. doi: 10.1016/s0197-0186(03)00039-1. [DOI] [PubMed] [Google Scholar]
- Krajewski WW, Collins R, Holmberg-Schiavone L, Jones TA, Karlberg T, Mowbray SL. Crystal structures of mammalian glutamine synthetases illustrate substrate-induced conformational changes and provide opportunities for drug and herbicide design. J Mol Biol. 2008;375:217–228. doi: 10.1016/j.jmb.2007.10.029. [DOI] [PubMed] [Google Scholar]
- Kruithof-de Julio M, Labruyere WT, Ruijter JM, Vermeulen JL, Stanulovic V, Stallen JM, Baldysiak-Figiel A, Gebhardt R, Lamers WH, Hakvoort TB. The RL-ET-14 cell line mediates expression of glutamine synthetase through the upstream enhancer/promoter region. J Hepatol. 2005;43:126–131. doi: 10.1016/j.jhep.2005.01.036. [DOI] [PubMed] [Google Scholar]
- Labow BI, Souba WW, Abcouwer SF. Mechanisms governing the expression of the enzymes of glutamine metabolism--glutaminase and glutamine synthetase. J Nutr. 2001;131:2467S–2474S. doi: 10.1093/jn/131.9.2467S. discussion 2486S–2467S. [DOI] [PubMed] [Google Scholar]
- Lai JC, Cooper AJ. Brain alpha-ketoglutarate dehydrogenase complex: kinetic properties, regional distribution, and effects of inhibitors. J Neurochem. 1986;47:1376–1386. doi: 10.1111/j.1471-4159.1986.tb00768.x. [DOI] [PubMed] [Google Scholar]
- Lai JC, Cooper AJ. Neurotoxicity of ammonia and fatty acids: differential inhibition of mitochondrial dehydrogenases by ammonia and fatty acyl coenzyme A derivatives. Neurochem Res. 1991;16:795–803. doi: 10.1007/BF00965689. [DOI] [PubMed] [Google Scholar]
- Lai JC, Minski MJ, Chan AW, Leung TK, Lim L. Manganese mineral interactions in brain. Neurotoxicology. 1999;20:433–444. [PubMed] [Google Scholar]
- Lai JC, Minski MJ, Chan AW, Lim L. Interrelations between manganese and other metal ions in health and disease. Met Ions Biol Syst. 2000;37:123–156. [PubMed] [Google Scholar]
- Lai JC, Murthy CR, Cooper AJ, Hertz E, Hertz L. Differential effects of ammonia and beta-methylene-DL-aspartate on metabolism of glutamate and related amino acids by astrocytes and neurons in primary culture. Neurochem Res. 1989;14:377–389. doi: 10.1007/BF01000042. [DOI] [PubMed] [Google Scholar]
- Langston-Unkefer PL, Macy PA, Durbin RD. Inactivation of Glutamine Synthetase by Tabtoxinine-beta-lactam : Effects of Substrates and pH. Plant Physiol. 1984;76:71–74. doi: 10.1104/pp.76.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauritzen F, de Lanerolle NC, Lee TS, Spencer DD, Kim JH, Bergersen LH, Eid T. Monocarboxylate transporter 1 is deficient on microvessels in the human epileptogenic hippocampus. Neurobiol Dis. 2011;41:577–584. doi: 10.1016/j.nbd.2010.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauritzen F, Heuser K, de Lanerolle NC, Lee TS, Spencer DD, Kim JH, Gjedde A, Eid T, Bergersen LH. Redistribution of monocarboxylate transporter 2 on the surface of astrocytes in the human epileptogenic hippocampus. Glia. 2012a;60:1172–1181. doi: 10.1002/glia.22344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauritzen F, Perez EL, Melillo ER, Roh JM, Zaveri HP, Lee TS, Wang Y, Bergersen LH, Eid T. Altered expression of brain monocarboxylate transporter 1 in models of temporal lobe epilepsy. Neurobiol Dis. 2012b;45:165–176. doi: 10.1016/j.nbd.2011.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemke G. Glial control of neuronal development. Annu Rev Neurosci. 2001;24:87–105. doi: 10.1146/annurev.neuro.24.1.87. [DOI] [PubMed] [Google Scholar]
- Letournel-Boulland ML, Fages C, Rolland B, Tardy M. Lipopolysaccharides (LPS), up-regulate the IL-1-mRNA and down-regulate the glial fibrillary acidic protein (GFAP) and glutamine synthetase (GS)-mRNAs in astroglial primary cultures. Eur Cytokine Netw. 1994;5:51–56. [PubMed] [Google Scholar]
- Liang SL, Carlson GC, Coulter DA. Dynamic regulation of synaptic GABA release by the glutamate-glutamine cycle in hippocampal area CA1. J Neurosci. 2006;26:8537–8548. doi: 10.1523/JNEUROSCI.0329-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lie-Venema H, Hakvoort TB, van Hemert FJ, Moorman AF, Lamers WH. Regulation of the spatiotemporal pattern of expression of the glutamine synthetase gene. Prog Nucleic Acid Res Mol Biol. 1998;61:243–308. doi: 10.1016/s0079-6603(08)60829-6. [DOI] [PubMed] [Google Scholar]
- Linser P, Moscona AA. Hormonal induction of glutamine synthetase in cultures of embryonic retina cells: requirement for neuron-glia contact interactions. Dev Biol. 1983;96:529–534. doi: 10.1016/0012-1606(83)90190-2. [DOI] [PubMed] [Google Scholar]
- Liwnicz BH, Leach JL, Yeh HS, Privitera M. Pericyte degeneration and thickening of basement membranes of cerebral microvessels in complex partial seizures: electron microscopic study of surgically removed tissue. Neurosurg. 1990;26:409–420. doi: 10.1097/00006123-199003000-00006. [DOI] [PubMed] [Google Scholar]
- Mackenzie IR, Miller LA. Senile plaques in temporal lobe epilepsy. Acta Neuropathol. 1994;87:504–510. doi: 10.1007/BF00294177. [DOI] [PubMed] [Google Scholar]
- Magistretti PJ, Pellerin L. Astrocytes Couple Synaptic Activity to Glucose Utilization in the Brain. News Physiol Sci. 1999;14:177–182. doi: 10.1152/physiologyonline.1999.14.5.177. [DOI] [PubMed] [Google Scholar]
- Martinez-Hernandez A, Bell KP, Norenberg MD. Glutamine synthetase: glial localization in brain. Science. 1977;195:1356–1358. doi: 10.1126/science.14400. [DOI] [PubMed] [Google Scholar]
- McRae PA, Baranov E, Sarode S, Brooks-Kayal AR, Porter BE. Aggrecan expression, a component of the inhibitory interneuron perineuronal net, is altered following an early-life seizure. Neurobiol Dis. 2010;39:439–448. doi: 10.1016/j.nbd.2010.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meister A. Glutamine synthetase from mammalian tissues. In: Meister A, editor. Glutamate, Glutamine, Glutathione, and Related Compounds. 1985. pp. 185–199. [DOI] [PubMed] [Google Scholar]
- Miller S, Nunn PB, Bridges RJ. Induction of astrocyte glutamine synthetase activity by the Lathyrus toxin beta-N-oxalyl-L-alpha,beta-diaminopropionic acid (beta-L-ODAP) Glia. 1993;7:329–336. doi: 10.1002/glia.440070408. [DOI] [PubMed] [Google Scholar]
- Morello M, Zatta P, Zambenedetti P, Martorana A, DAngelo V, Melchiorri G, Bernardi G, Sancesario G. Manganese intoxication decreases the expression of manganoproteins in the rat basal ganglia: an immunohistochemical study. Brain Res Bull. 2007;74:406–415. doi: 10.1016/j.brainresbull.2007.07.011. [DOI] [PubMed] [Google Scholar]
- Moreno MC, Sande P, Marcos HA, de Zavalia N, Keller Sarmiento MI, Rosenstein RE. Effect of glaucoma on the retinal glutamate/glutamine cycle activity. Faseb J. 2005;19:1161–1162. doi: 10.1096/fj.04-3313fje. [DOI] [PubMed] [Google Scholar]
- Morgello S, Uson RR, Schwartz EJ, Haber RS. The human blood-brain barrier glucose transporter (GLUT1) is a glucose transporter of gray matter astrocytes. Glia. 1995;14:43–54. doi: 10.1002/glia.440140107. [DOI] [PubMed] [Google Scholar]
- Nadler JV, Perry BW, Cotman CW. Intraventricular kainic acid preferentially destroys hippocampal pyramidal cells. Nature. 1978;271:676–677. doi: 10.1038/271676a0. [DOI] [PubMed] [Google Scholar]
- Nagelhus EA, Veruki ML, Torp R, haug F-M, Laake JH, S N, Agre P, Ottersen OP. Aquaporin-4 water channel protein in the rat retina and optic nerve: polarized expression in Müller cells and fibrous astrocytes. J. Neurosci. 1998;18:2506–2519. doi: 10.1523/JNEUROSCI.18-07-02506.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nedergaard M, Dirnagl U. Role of glial cells in cerebral ischemia. Glia. 2005;50:281–286. doi: 10.1002/glia.20205. [DOI] [PubMed] [Google Scholar]
- Nelson RB, Siman R. Thrombin and its inhibitors regulate morphological and biochemical differentiation of astrocytes in vitro. Brain Res Dev Brain Res. 1990;54:93–104. doi: 10.1016/0165-3806(90)90069-b. [DOI] [PubMed] [Google Scholar]
- Nissim I. Newer aspects of glutamine/glutamate metabolism: the role of acute pH changes. Am J Physiol. 1999;277:F493–F497. doi: 10.1152/ajprenal.1999.277.4.F493. [DOI] [PubMed] [Google Scholar]
- Norenberg MD. Distribution of glutamine synthetase in the rat central nervous system. J. Histochem. Cytochem. 1979;27:756–762. doi: 10.1177/27.3.39099. [DOI] [PubMed] [Google Scholar]
- Norenberg MD. Hepatic encephalopathy: studies with astrocyte cultures. In: Norenberg MD, Hertz L, Schousboe A, editors. The biochemical pathology of astrocytes. New York: Alan R Liss Inc; 1988. pp. 451–464. [Google Scholar]
- Olney JW, Sharpe LG, Feigin RD. Glutamate-induced brain damage in infant primates. J Neuropathol Exp Neurol. 1972;31:464–488. doi: 10.1097/00005072-197207000-00006. [DOI] [PubMed] [Google Scholar]
- Orkand RK, Nicholls JG, Kuffler SW. Effect of nerve impulses on the membrane potential of glial cells in the central nervous system of amphibia. J Neurophysiol. 1966;29:788–806. doi: 10.1152/jn.1966.29.4.788. [DOI] [PubMed] [Google Scholar]
- Otis TS, Jahr CE. Anion currents and predicted glutamate flux through a neuronal glutamate transporter. J Neurosci. 1998;18:7099–7110. doi: 10.1523/JNEUROSCI.18-18-07099.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papageorgiou IE, Gabriel S, Fetani AF, Kann O, Heinemann U. Redistribution of astrocytic glutamine synthetase in the hippocampus of chronic epileptic rats. Glia. 2011;59:1706–1718. doi: 10.1002/glia.21217. [DOI] [PubMed] [Google Scholar]
- Patejunas G, Young AP. Constitutive and glucocorticoid-mediated activation of glutamine synthetase gene expression in the developing chicken retina. J Biol Chem. 1990;265:15280–15285. [PubMed] [Google Scholar]
- Pellerin L, Bonvento G, Chatton JY, Pierre K, Magistretti PJ. Role of neuron-glia interaction in the regulation of brain glucose utilization. Diabetes Nutr Metab. 2002;15:268–273. discussion 273. [PubMed] [Google Scholar]
- Pennell PB. Hormonal aspects of epilepsy. Neurol Clin. 2009;27:941–965. doi: 10.1016/j.ncl.2009.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perea G, Navarrete M, Araque A. Tripartite synapses: astrocytes process and control synaptic information. Trends Neurosci. 2009;32:421–431. doi: 10.1016/j.tins.2009.05.001. [DOI] [PubMed] [Google Scholar]
- Perez EL, Lauritzen F, Wang Y, Lee TS, Kang D, Zaveri HP, Chaudhry FA, Ottersen OP, Bergersen LH, Eid T. Evidence for astrocytes as a potential source of the glutamate excess in temporal lobe epilepsy. Neurobiol Dis. 2012;47:331–337. doi: 10.1016/j.nbd.2012.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perosa SR, Porcionatto MA, Cukiert A, Martins JR, Passeroti CC, Amado D, Matas SL, Nader HB, Cavalheiro EA, Leite JP, Naffah-Mazzacoratti MG. Glycosaminoglycan levels and proteoglycan expression are altered in the hippocampus of patients with mesial temporal lobe epilepsy. Brain Res Bull. 2002;58:509–516. doi: 10.1016/s0361-9230(02)00822-5. [DOI] [PubMed] [Google Scholar]
- Peters A, Palay SL, Webster Hd. Neurons and their supporting cells. 3rd ed. New York: Oxford University Press; 1991. The fine structure of the nervous system. [Google Scholar]
- Petroff OA, Cavus I, Kim JH, Spencer DD. Interictal extracellular glutamate concentrations are increased in hippocampal sclerosis. Ann Neurol. 2004;56:S43. [Google Scholar]
- Petroff OA, Errante LD, Rothman DL, Kim JH, Spencer DD. Glutamate-glutamine cycling in the epileptic human hippocampus. Epilepsia. 2002;43:703–710. doi: 10.1046/j.1528-1157.2002.38901.x. [DOI] [PubMed] [Google Scholar]
- Raivich G. c-Jun expression, activation and function in neural cell death, inflammation and repair. J Neurochem. 2008;107:898–906. doi: 10.1111/j.1471-4159.2008.05684.x. [DOI] [PubMed] [Google Scholar]
- Rajkowska G, Miguel-Hidalgo JJ, Makkos Z, Meltzer H, Overholser J, Stockmeier C. Layer-specific reductions in GFAP-reactive astroglia in the dorsolateral prefrontal cortex in schizophrenia. Schizophr Res. 2002;57:127–138. doi: 10.1016/s0920-9964(02)00339-0. [DOI] [PubMed] [Google Scholar]
- Rama Rao KV, Reddy PV, Hazell AS, Norenberg MD. Manganese induces cell swelling in cultured astrocytes. Neurotoxicology. 2007;28:807–812. doi: 10.1016/j.neuro.2007.03.001. [DOI] [PubMed] [Google Scholar]
- Ransohoff RM, Brown MA. Innate immunity in the central nervous system. J Clin Invest. 2012;122:1164–1171. doi: 10.1172/JCI58644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ransohoff RM, Kivisakk P, Kidd G. Three or more routes for leukocyte migration into the central nervous system. Nat Rev Immunol. 2003;3:569–581. doi: 10.1038/nri1130. [DOI] [PubMed] [Google Scholar]
- Riepe RE, Norenberg MD. Glutamine synthetase in the developing rat retina: an immunohistochemical study. Exp Eye Res. 1978;27:435–444. doi: 10.1016/0014-4835(78)90022-2. [DOI] [PubMed] [Google Scholar]
- Rigau V, Morin M, Rousset MC, de Bock F, Lebrun A, Coubes P, Picot MC, Baldy-Moulinier M, Bockaert J, Crespel A, Lerner-Natoli M. Angiogenesis is associated with blood-brain barrier permeability in temporal lobe epilepsy. Brain. 2007;130:1942–1956. doi: 10.1093/brain/awm118. [DOI] [PubMed] [Google Scholar]
- Robel S, Berninger B, Gotz M. The stem cell potential of glia: lessons from reactive gliosis. Nat Rev Neurosci. 2011;12:88–104. doi: 10.1038/nrn2978. [DOI] [PubMed] [Google Scholar]
- Robinson SR. Neuronal expression of glutamine synthetase in Alzheimer's disease indicates a profound impairment of metabolic interactions with astrocytes. Neurochem Int. 2000;36:471–482. doi: 10.1016/s0197-0186(99)00150-3. [DOI] [PubMed] [Google Scholar]
- Robinson SR. Changes in the cellular distribution of glutamine synthetase in Alzheimer's disease. J Neurosci Res. 2001;66:972–980. doi: 10.1002/jnr.10057. [DOI] [PubMed] [Google Scholar]
- Ronzio RA, Meister A. Phosphorylation of methionine sulfoximine by glutamine synthetase. Proc Natl Acad Sci U S A. 1968;59:164–170. doi: 10.1073/pnas.59.1.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salazar AM, Jabbari B, Vance SC, Grafman J, Amin D, Dillon JD. Epilepsy after penetrating head injury. I. Clinical correlates: a report of the Vietnam Head Injury Study. Neurology. 1985;35:1406–1414. doi: 10.1212/wnl.35.10.1406. [DOI] [PubMed] [Google Scholar]
- Sanacora G, Treccani G, Popoli M. Towards a glutamate hypothesis of depression: an emerging frontier of neuropsychopharmacology for mood disorders. Neuropharmacology. 2012;62:63–77. doi: 10.1016/j.neuropharm.2011.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santello M, Bezzi P, Volterra A. TNFalpha controls glutamatergic gliotransmission in the hippocampal dentate gyrus. Neuron. 2011;69:988–1001. doi: 10.1016/j.neuron.2011.02.003. [DOI] [PubMed] [Google Scholar]
- Schousboe A, Bak LK, Madsen KK, Waagepetersen HS. Amino acid neurotransmitter synthesis and removal. In: Kettenmann H, Ransom BR, editors. Neuroglial. 3 ed. New York: Oxford University Press; 2013. pp. 443–456. [Google Scholar]
- Seiffert E, Dreier JP, Ivens S, Bechmann I, Tomkins O, Heinemann U, Friedman A. Lasting blood-brain barrier disruption induces epileptic focus in the rat somatosensory cortex. J Neurosci. 2004;24:7829–7836. doi: 10.1523/JNEUROSCI.1751-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shank RP, Bennett GS, Freytag SO, Campbell GL. Pyruvate carboxylase: an astrocyte-specific enzyme implicated in the replenishment of amino acid neurotransmitter pools. Brain Res. 1985;329:364–367. doi: 10.1016/0006-8993(85)90552-9. [DOI] [PubMed] [Google Scholar]
- Sharma R, Fischer MT, Bauer J, Felts PA, Smith KJ, Misu T, Fujihara K, Bradl M, Lassmann H. Inflammation induced by innate immunity in the central nervous system leads to primary astrocyte dysfunction followed by demyelination. Acta Neuropathol. 2010;120:223–236. doi: 10.1007/s00401-010-0704-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sibson NR, Mason GF, Shen J, Cline GW, Herskovits AZ, Wall JE, Behar KL, Rothman DL, Shulman RG. In vivo (13)C NMR measurement of neurotransmitter glutamate cycling, anaplerosis and TCA cycle flux in rat brain during. J Neurochem. 2001;76:975–989. doi: 10.1046/j.1471-4159.2001.00074.x. [DOI] [PubMed] [Google Scholar]
- Sommer W. Erkrankung des Ammonshorns als aetiologisches Moment der Epilepsie. Arch. Psychiatr. Nervenkr. 1880;10:631–675. [Google Scholar]
- Spencer SS. Neural networks in human epilepsy: evidence of and implications for treatment. Epilepsia. 2002;43:219–227. doi: 10.1046/j.1528-1157.2002.26901.x. [DOI] [PubMed] [Google Scholar]
- Steinhauser C, Boison D. Epilepsy: crucial role for astrocytes. Glia. 2012;60:1191. doi: 10.1002/glia.22300. [DOI] [PubMed] [Google Scholar]
- Swamy M, Yusof WR, Sirajudeen KN, Mustapha Z, Govindasamy C. Decreased glutamine synthetase, increased citrulline-nitric oxide cycle activities, and oxidative stress in different regions of brain in epilepsy rat model. J Physiol Biochem. 2011;67:105–113. doi: 10.1007/s13105-010-0054-2. [DOI] [PubMed] [Google Scholar]
- Takano A, Arakawa R, Ito H, Tateno A, Takahashi H, Matsumoto R, Okubo Y, Suhara T. Peripheral benzodiazepine receptors in patients with chronic schizophrenia: a PET study with [11C]DAA1106. Int J Neuropsychopharmacol. 2010;13:943–950. doi: 10.1017/S1461145710000313. [DOI] [PubMed] [Google Scholar]
- Tessler S, Danbolt NC, Faull RL, Storm-Mathisen J, Emson PC. Expression of the glutamate transporters in human temporal lobe epilepsy. Neuroscience. 1999;88:1083–1091. doi: 10.1016/s0306-4522(98)00301-7. [DOI] [PubMed] [Google Scholar]
- Tian GF, Azmi H, Takano T, Xu Q, Peng W, Lin J, Oberheim N, Lou N, Wang X, Zielke HR, Kang J, Nedergaard M. An astrocytic basis of epilepsy. Nat Med. 2005;11:973–981. doi: 10.1038/nm1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tombaugh GC, Sapolsky RM. Hippocampal glutamine synthetase: insensitivity to glucocorticoids and stress. Am J Physiol. 1990;258:E894–E897. doi: 10.1152/ajpendo.1990.258.5.E894. [DOI] [PubMed] [Google Scholar]
- Ueda Y, Willmore LJ. Sequential changes in glutamate transporter protein levels during Fe(3+)-induced epileptogenesis. Epilepsy Res. 2000;39:201–209. doi: 10.1016/s0920-1211(99)00122-9. [DOI] [PubMed] [Google Scholar]
- van der Hel WS, Notenboom RG, Bos IW, van Rijen PC, van Veelen CW, de Graan PN. Reduced glutamine synthetase in hippocampal areas with neuron loss in temporal lobe epilepsy. Neurology. 2005;64:326–333. doi: 10.1212/01.WNL.0000149636.44660.99. [DOI] [PubMed] [Google Scholar]
- Vardimon L, Ben-Dror I, Avisar N, Oren A, Shiftan L. Glucocorticoid control of glial gene expression. J Neurobiol. 1999;40:513–527. doi: 10.1002/(sici)1097-4695(19990915)40:4<513::aid-neu8>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- Vardimon L, Ben-Dror I, Oren A, Polak P. Cytoskeletal and cell contact control of the glucocorticoid pathway. Mol Cell Endocrinol. 2006;252:142–147. doi: 10.1016/j.mce.2006.03.012. [DOI] [PubMed] [Google Scholar]
- Vardimon L, Fox LL, Degenstein L, Moscona AA. Cell contacts are required for induction by cortisol of glutamine synthetase gene transcription in the retina. Proc Natl Acad Sci U S A. 1988;85:5981–5985. doi: 10.1073/pnas.85.16.5981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verkhratsky A, Steinhauser C. Ion channels in glial cells. Brain Res Brain Res Rev. 2000;32:380–412. doi: 10.1016/s0165-0173(99)00093-4. [DOI] [PubMed] [Google Scholar]
- Vezzani A, Conti M, De Luigi A, Ravizza T, Moneta D, Marchesi F, De Simoni MG. Interleukin-1beta immunoreactivity and microglia are enhanced in the rat hippocampus by focal kainate application: functional evidence for enhancement of electrographic seizures. J Neurosci. 1999;19:5054–5065. doi: 10.1523/JNEUROSCI.19-12-05054.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vezzani A, Granata T. Brain inflammation in epilepsy: experimental and clinical evidence. Epilepsia. 2005;46:1724–1743. doi: 10.1111/j.1528-1167.2005.00298.x. [DOI] [PubMed] [Google Scholar]
- Vezzani A, Moneta D, Conti M, Richichi C, Ravizza T, De Luigi A, De Simoni MG, Sperk G, Andell-Jonsson S, Lundkvist J, Iverfeldt K, Bartfai T. Powerful anticonvulsant action of IL-1 receptor antagonist on intracerebral injection and astrocytic overexpression in mice. Proc Natl Acad Sci U S A. 2000;97:11534–11539. doi: 10.1073/pnas.190206797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Kudoh J, Kubota R, Asakawa S, Minoshima S, Shimizu N. Chromosomal mapping of a family of human glutamine synthetase genes: functional gene (GLUL) on 1q25, pseudogene (GLULP) on 9p13, and three related genes (GLULL1, GLULL2, GLULL3) on 5q33, 11p15, and 11q24. Genomics. 1996;37:195–199. doi: 10.1006/geno.1996.0542. [DOI] [PubMed] [Google Scholar]
- Wang Y, Zaveri HP, Lee TS, Eid T. The development of recurrent seizures after continuous intrahippocampal infusion of methionine sulfoximine in rats: a video-intracranial electroencephalographic study. Exp Neurol. 2009;220:293–302. doi: 10.1016/j.expneurol.2009.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wedler FC, Denman RB, Roby WG. Glutamine synthetase from ovine brain is a manganese(II) enzyme. Biochemistry. 1982;21:6389–6396. doi: 10.1021/bi00268a011. [DOI] [PubMed] [Google Scholar]
- Wedler FC, Ley BW. Kinetic, ESR, and trapping evidence for in vivo binding of Mn(II) to glutamine synthetase in brain cells. Neurochem Res. 1994;19:139–144. doi: 10.1007/BF00966808. [DOI] [PubMed] [Google Scholar]
- Wedler FC, Toms R. Interactions of Mn(II) with mammalian glutamine synthetase. In: Schramm VL, Wedler FC, editors. Manganese in metabolism and enzyme function. Orlando: Academic Press; 1986. pp. 221–238. [Google Scholar]
- Wedler FC, Vichnin MC, Ley BW, Tholey G, Ledig M, Copin JC. Effects of Ca(II) ions on Mn(II) dynamics in chick glia and rat astrocytes: potential regulation of glutamine synthetase. Neurochem Res. 1994;19:145–151. doi: 10.1007/BF00966809. [DOI] [PubMed] [Google Scholar]
- Yu S, Ding WG. The 45 kDa form of glucose transporter 1 (GLUT1) is localized in oligodendrocyte and astrocyte but not in microglia in the rat brain. Brain Res. 1998;797:65–72. doi: 10.1016/s0006-8993(98)00372-2. [DOI] [PubMed] [Google Scholar]