Abstract
Background
After neonatal ventral hippocampal lesions (NVHLs), adult rats exhibit evidence of neural processing deficits relevant to schizophrenia, including reduced prepulse inhibition (PPI) of acoustic startle and impaired sensory processing. In intact rats, the regulation of PPI by the ventral hippocampus (VH) is mediated via interactions with medial prefrontal cortex (mPFC) and nucleus accumbens (NAC). We assessed PPI, auditory-evoked responses and expression of 7 schizophrenia-related genes in mPFC and NAC, in adult rats after sham- or real NVHLs.
Methods
Male inbred Buffalo (BUF) rat pups (d7; n=36) received either vehicle or ibotenic acid infusion into the VH. PPI and auditory-evoked dentate gyrus local field potentials (LFPs) were measured on d56 and d66, respectively. Brains were processed for RT-PCR measures of mPFC and NAC Comt, Erbb4, Grid2, Ncam1, Slc1a2, Nrg1 and Reln.
Results
NVHL rats exhibited significant deficits in PPI (p=0.005) and LFPs (p<0.015) proportional to lesion size. Sham vs. NVHL rats did not differ in gene expression levels in mPFC or NAC. As we previously reported, multiple gene expression levels were highly correlated within- (mean r's≈0.5), but not across-brain regions (mean r's≈0). However, for three genes – Comt, Slc1a2 and Ncam1 – after NVHLs, expression levels became significantly correlated, or “coupled,” across the mPFC and NAC (p's < 0.03, 0.002 and 0.05, respectively), and the degree of “coupling” increased with VH lesion size.
Conclusions
After NVHLs that disrupt PPI and auditory processing, specific gene expression levels suggest an abnormal functional coupling of the mPFC and NAC. This model of VH-mPFC-NAC network dysfunction after NVHLs may have implications for understanding the neural basis for PPI- and related sensory processing deficits in schizophrenia patients.
Keywords: catechol-O-methyltransferase, medial prefrontal cortex, nucleus accumbens, prepulse inhibition, schizophrenia, ventral hippocampus
Introduction
Animal models of the pathophysiology of schizophrenia have helped us understand the neurobiological and behavioral consequences of a number of neurodevelopmental insults, even if these insults cannot be definitively linked to schizophrenia per se. One such insult – lesions of the ventral hippocampus in neonatal rats (NVHLs) – has been shown to recreate a number of deficits associated with schizophrenia (Angst et al. 2007; Lipska et al. 1993; Marquis et al. 2006; Marquis et al. 2008; cf. O'Donnell 2012), including reductions in sensorimotor gating as measured by prepulse inhibition of startle (PPI) (Daenen et al. 2003; Le Pen et al. 2003; Le Pen and Moreau 2002; Lipska et al. 1995; Swerdlow et al. 2012a) and sensory registration as measured by auditory evoked potentials (Swerdlow et al. 2012a). In schizophrenia patients, the integrity of the hippocampal-PFC connection is reduced, as assessed by fractional anisotropy, and this deficiency predicts both neurocognitive and functional impairment (Hanlon et al. 2012). To the degree that some forms of schizophrenia are characterized by aberrant ventral hippocampal development and functional connectivity, the NVHL model might help predict some of the expected “neuromaladaptive” consequences of such pathology, and thereby help focus studies of pathophysiology and even therapeutics in this disorder.
The nature of cortical and subcortical disturbances “downstream” from early hippocampal pathology has been a focus of numerous studies, based on the model that hippocampal pathology may be a causal antecedent to circuit perturbations reported in schizophrenia in prefrontal and other cortical regions (O'Donnell 2012; cf. Tseng et al. 2009). Evidence exists for abnormal function in medial prefrontal cortex (mPFC) and ventral forebrain dopamine systems after NVHLs, and for abnormalities in gene expression in frontal and temporal cortex and striatum (e.g. El-Rawas et al. 2009; Flores et al. 2005; Marquis et al. 2006; Marquis et al. 2008; Mitchell et al. 2005; Wong et al. 2005; Yabuki et al. 2013). These findings have come from both “agnostic” large microarray analyses (Wong et al. 2005) and from more focused, hypothesis-driven studies in which genes were selected based on specific biological models of schizophrenia (El-Rawas et al. 2009; Mitchell et al. 2005). Interestingly, the largest empirical survey of gene transcription profiles using over 5000 rat and 25,000 human cDNA's identified no significant effects of NVHLs on the expression of numerous genes that have been associated with schizophrenia risk via genomic analyses in humans (Wong et al. 2005), including catechol-O-methyltransferase (COMT) and neuregulin-1 (NRG1).
We (Swerdlow et al. 2012b) and others (e.g. Flores et al. 2009) have used gene expression patterns across brain regions as a means to understand the role of circuit dynamics in the regulation of behaviors – such as PPI – that are of relevance to schizophrenia; this strategy is based in part on emerging evidence that correlated gene expression levels predict anatomical connectivity between brain regions (Kaufman et al. 2006; Wolf et al. 2011). We reported significant differences in the cortical (mPFC), subcortical (nucleus accumbens; NAC) and ventral hippocampal (VH) expression of Comt, Grid2, Nrg1 and other genes associated with PPI deficits in schizophrenia patients (Greenwood et al. 2011, 2012; Kao et al. 2010; Quednow et al. 2010; Roussos et al. 2008; Sobin et al. 2005; Stefansson et al. 2002) in outbred Sprague Dawley (SD) vs. Long Evans (LE) rat strains that also differed in PPI and PPI-sensitivity to dopamine agonists (Shilling et al. 2008; Swerdlow et al. 2012b). Among other findings, the expression levels of several PPI-associated genes were strongly correlated within brain regions (e.g. NAC comt and NAC Grid2; typical r's ≈ 0.80) but not across brain regions. Because NVHLs are known to cause both structural and gene expression changes in mPFC and NAC, we assessed the patterns of expression of PPI-related genes within and between the mPFC and NAC in adult rats after sham or ibotenic acid NVHLs. Outbred SD and LE rats may have subtle variations in gene sequence, so the present studies utilized inbred albino BUF rats to facilitate future genetic analyses; BUF rats exhibit phenotypes of both basal and DA agonist-disrupted PPI that are indistinguishable from those of SD rats (Swerdlow et al. 2004). Seven genes were selected for expression analyses, based on published reports of single nucleotide polymorphisms associated with PPI (COMT, GRID2, NRG1, NCAM, SLC1A2; Greenwood et al. 2011, 2012) and/or schizophrenia (GRID2, NRG1, RELN, ERBB4; Greenwood et al. 2012).
Methods
All procedures conformed to NIH guidelines and were approved by the UCSD Animal Subjects Committee. Female Buffalo (BUF: BUF/CrCrl) rats (Charles River; Portage, MI) were housed individually in a temperature-controlled room utilizing a reverse 12:12 light/dark cycle. Food and water were offered ad lib. BUF rats were selected for these gene expression studies because they are an inbred strain with a PPI phenotype very comparable to that of Sprague Dawley (SD) rats (Swerdlow et al. 2004) that exhibit consistent and robust PPI deficits after NVHLs (Lipska et al. 1995; Swerdlow et al. 2012a). Females were monitored daily until delivery (litter avg. 6-7 pups, typical of BUF rats; www.harlan.com); the next day all female pups were culled. Surgery was conducted on all male pups on postnatal day (PND) 7, in accordance with several previous reports using this NVHL model (e.g. Angst et al. 2007; Daenen et al. 2003; Le Pen et al. 2003; Le Pen & Moreau 2002; Lipska et al. 1993, 1995; Swerdlow et al. 2012a). On average, pups weighed 13.9 g at surgery. Pups were weaned on PND 28 into groups of 2-3 per cage by condition (sham/lesion) and litter cohort. After surgery, pup weights were taken daily for 1 week to ensure weight gain.
Surgery procedures were previously reported (Swerdlow et al. 2012a). Rat pups (n=36 pups from 10 litters) were brought to the laboratory in their home cages, with their mothers. Pups were weighed and exposed to −3°C for 5-10 min until immobile, then placed on a Kopf stereotaxic surgery station, stabilized on a Styrofoam platform and surrounded by ice chips to maintain hypothermia. A small incision exposed the skull. Bilateral infusions were made into the ventral hippocampus (A: −3.0, L: ± 3.5, D: −5.5) for 84 s with 0.25 μl of 10 μg/μl ibotenic acid (IBO, Sigma Biochemicals, n=21) or phosphate buffered saline (PBS, n=15). Four min later, the injectors were removed, skin was closed with cyanoacrylate glue and each pup was tattooed for identification. After recovery, pups were returned to their home cages with their mothers and were ear tagged at d14. Separate stereotaxic holders were used for IBO vs. PBS infusions, and infusion tubing and injectors were carefully flushed with PBS at the end of each surgical session.
Startle chambers (SR-LAB; San Diego Instruments) consisted of Plexiglas cylinders (8.7 cm internal diameter) resting on Plexiglas stands in a sound-attenuated room (60 dB ambient noise). Stimuli were delivered by a mounted speaker located 24 cm above the cylinder. Startle magnitude was detected and recorded by a piezoelectric device located beneath the cylinder.
Sham and NVHL group rats had comparable weights at weaning (d28; mean g (SEM) sham=72.93 (12.27); NVHL=69.95 (7.11)). PPI testing took place on PND 56, when deficits in NVHL rats have been reported previously (Lipska et al. 1995; Swerdlow et al. 2012a); weights at this time were also comparable across groups (mean g (SEM) sham vs. NVHL=224.33 (24.51) vs. 218.62 (21.52)). Testing consisted of a 5 min acclimation period of 70 dB(A) background followed by 4 blocks: blocks 1 and 4 had 4 and 3 120 dB(A) 40 ms noise PULSEs, respectively; block 2 and 3 had 5 trial types (1) PULSE; (2) 3 prepulse+PULSE trials (20 ms noise 3, 5, or 10 dB over background followed 100 ms by a PULSE); (3) NOSTIMs. Mean inter-trial interval was 15 s, (range 6-24 s), and total session length was 19 min; the session had a total of 106 trials, divided as follows: Blocks 1 & 4: 7 120 dB(A) pulses and 7 NOSTIM trials; Block 2-3: 16 PULSE trials, 30 PPI trials and 46 NOSTIMs.
On PND 59, rats were surgically implanted with stainless steel EEG electrodes in the dentate gyrus. Rats received 0.1 ml atropine sulfate subcutaneously (Vedco, 0.054 mg/ml) 15–30 min before full anesthesia with sodium pentobarbital (Abbott, 60 mg/kg i.p.), and then were secured in a Kopf stereotaxic instrument in a flat skull position (tooth bar 3.3 mm below interaural line). An uncut tripole electrode (Plastics One, Roanoke, Va., USA, 0.010) was modified with Jam Nut fastener (Plastics One, 37761) placed on the head plug for precise placement of the connector cable (Plastics One, 100 cm TT2, 335-000). A 2-cm incision exposed the skull, and the skull surface was cleaned. Two holes were drilled for ground and reference wire placement on dura (AP: +2.0, L: ± 1.0). Two holes were drilled for anchor screws (Plastics One, 0-80×3/32), and another for the recording electrode, positioned with its ventral tip in the dentate gyrus (DG: AP: −4.1, L: + 1.0, DV: −3.2). Acrylic dental cement (A-M Systems, Carlsborg, Wash., USA; Dental Cement Powder, 525000 and Solvent, 526000) firmly attached the electrode and anchor screws to the skull, and the incision was closed around the head plug. Rats recovered on a heating pad before being placed in their home cages.
EEG testing began 7d post-surgery. A single startle chamber (SR-LAB, San Diego Instruments, San Diego, Calif., USA) consisted of a Plexiglas cylinder 8.2 cm in diameter resting on a 12.5 × 25.5-cm Plexiglas frame within a ventilated enclosure (background noise: 65 dB(A)). The cylinder was modified with an elevated roof that allowed animals to move freely despite the presence of an EEG headpiece. The test chamber was also modified with electrical insulation, and an electrical interface cable that was fastened to the EEG headpiece. Otherwise, stimulus delivery methods were identical to those described above for startle testing. EEG signals were recorded via a preamplifier cable connection from the rat to an A-M Systems 2 Channel Microelectrode AC Amplifier (Carlsborg, Wash., USA, Model 1800). The recording cable contained three male pins at the proximal end, which connected to the preamplifier, and three male amphenol pins at the distal end of the cable, to connect with the female pins in the head plug. The filter settings on the amplifier were 1.0 Hz low cutoff and 500 Hz high cutoff. The notch filter was in the ‘out’ position; the mode was set for record, and gain was set at 10 K. The amplified signals were recorded on the SR-LAB microcomputer. Auditory stimuli (53 ms duration) consisted of a frequent standard tone (8 KHz, 81.6 dB(A), 87.76% of trials), and two rare “oddball” tones (7.5 KHz or 8.5 KHz, 87.0 dB(A), 12.24% of trials) in an attempt to measure mismatch negativity (MMN), presented in pseudo-randomized sequences. After a 5 min acclimation period, each of 50 test blocks included 172 standard tones and 12 of oddball tone presentations, with a constant inter-stimulus interval of 300 ms; total session length was 59 minutes. After inspection of early data revealed inadequate signal fidelity, adjustments were made to the equipment; as a result, complete data sets were obtained from only 24 out of the 36 rats, and analyses focused on LFPs elicited in response to the standard tones.
EEG data files were imported into Brain Vision Analyzer (v2.0.2) and processed offline and blind to condition using an automated processing script. EEG responses to standard tones were centered across the 50 ms prestimulus baseline period and screened for artifacts (activity exceeding ±300 digital units). LFP Averaging for each rat was performed using remaining artifact-free segments (mean number of accepted sweeps=5746.13, SD=1242.01, with no significant sham vs. NVHL group differences). LFP peaks (screening window) were identified for P30 (20-40 ms) and N40 (30-60 msec). P90 was calculated as the mean voltage across the 65-105 ms range relative to the 50 ms prestimulus baseline.
Rats then remained housed for 7-14 d to minimize possible effects of startle and EEG testing on gene expression. Rats were then sacrificed, their brains removed, weighed and placed in ice-cold saline for 30 sec. Coronal tissue slabs were cut with a wire tissue slicer and the NAC and mPFC were removed bilaterally by free-hand razor dissection; one NAC could not be recovered during this process. Each bilateral tissue sample was placed onto dry ice and transferred to an RNase free tube, then stored at −80°C until analyzed for gene expression. Tissue caudal to the thalamus was stored in a 10% formalin solution for histological assessment of hippocampal lesion damage and EEG electrode placement. In this process, all instruments were cleaned between each rat brain dissection with RNAlater (QIAGEN, Inc, Valencia, CA).
For Reverse Transcription Polymerase Chain Reaction (RT - PCR), total RNA was isolated from brain tissue using an RNeasy Mini kit (QIAGEN, Valencia, CA 91355) and protocols followed as per manufacturer (QIAGEN). Samples were spot checked for quality using the Agilent 2100 Bioanalyzer (Agilent Technologies, Santa Clara, CA), which provides a RNA Integrity Number (RIN). An RIN > 7.0 indicates good quality RNA; the RIN for all samples analyzed was > 8.50. To measure RNA concentration, the optical density of 1.5 μL of total RNA at 260 nm was measured in a spectrophotometer (NanoDrop, ND-1000, NanoDrop Technologies, Wilmington, DE). Equal amounts of RNA /sample were used to make cDNA after DNase treatment. First strand cDNA was synthesized using qScript™ cDNA SuperMix as per manufacturer (Quanta Biosciences, Gaithersburg, MD). Real time RT-PCR was performed using Applied Biosystems’ TaqMan Gene Expression Assays in an Applied Biosystems 7900HT Fast Real-Time PCR System (Applied Biosystems, Foster City, CA). Each 20 μL RT-PCR reaction contained 10 μL of 2X Universal PCR Master Mix, 1 μL of primer/probe mix (900 nM/250 nM final concentration), 4 μL of nuclease-free water, and 5 μL of cDNA template (40 ng). Reactions were performed in MicroAmp Optical 384 well reaction plates (Applied Biosystems) as per manufacturer. Genes and assay ID numbers (Applied Biosystems) included: comt rn00561037_m1; nrg1 rn01482165_m1; grid2 rn00515053_m1; erbb4 rn00572447_m1; reln rn00589609_m1; slc1a2 rn00691548_m1; ncam1 rn00580526_m1 and glyceraldehyde 3-phosphate dehydrogenase (rgapdh) rn01775763_g1. Assays were performed in duplicate. Data were analyzed using SDS 2.3 software from Applied Biosystems. Amplification efficiencies were validated and relative expression values calculated after normalization to the rat GAPDH reference gene.
For histological assessment, 40 μm thick brain sections were mounted on microscope slides and Nissl stained. Tissue from all sham and NVHL rats was inspected by an individual (NRS) who was blind to behavioral, electrophysiological and gene expression results; all lesions or structural abnormalities were hand-drawn onto pages copied from a rat stereotaxic atlas (7 AP levels, coordinates: AP (−3.3) – (−6.3)) [32], and characterized in one of four ways: 1. None (no visible lesion or structural abnormalities); 2. Small (e.g. detectable prominence of the lateral ventricles or minimal thinning of the VH across the medio-lateral (ML) dimension); 3. Medium (e.g. obvious thinning of the VH at most anterior-posterior (AP) levels or identifiable lesion covering up to 50% of the VH at most AP levels); 4. Large (e.g. obvious lesion producing complete loss of VH at most AP levels). The ventral extent of all electrode tips were visualized within the dentate gyrus and plotted free-hand, blind to EEG results, onto a corresponding atlas page (Paxinos & Watson 1998).
Statistical Analyses
Prepulse inhibition was defined as 100-[(startle amplitude on prepulse+pulse trials / startle amplitude on PULSE trials) × 100], and was analyzed by mixed-design analyses of variance (ANOVAs), with lesion (sham vs. ibotenic acid) as a between factor and prepulse intensity and trial block as within factors. For post-hoc analyses, lesion size (categories 1 – 4, above) was used as a grouping factor. Similar analyses were used to assess NVHL effects on startle magnitude on PULSE trials, NOSTIM trials, and startle habituation (startle magnitude on PULSE trials in blocks 1 vs. 4). LFP amplitude for P30, N40 and P90 were treated as independent variables and subjected to one-way ANOVAs with lesion as a grouping factor; post-hoc analyses using lesion size were also conducted as above. For all comparisons, alpha was 0.05.
For gene expression data, fold change (FC) values for all genes were calculated relative to levels within a single sham group rat mPFC, to which FC values of 1.0 were assigned. Values were treated as continuous variables, and ANOVAs were conducted using lesion (sham vs. ibotenic acid) as a between factor and region and gene as within factors. Post-hoc comparisons examined expression separately within each region. Correlations of gene expression both within and across regions were assessed using simple regression analyses and reported together with alpha values corrected for multiple comparisons; regression values were compared using the Fisher r-to-z transformation.
Results
Lesion Histology
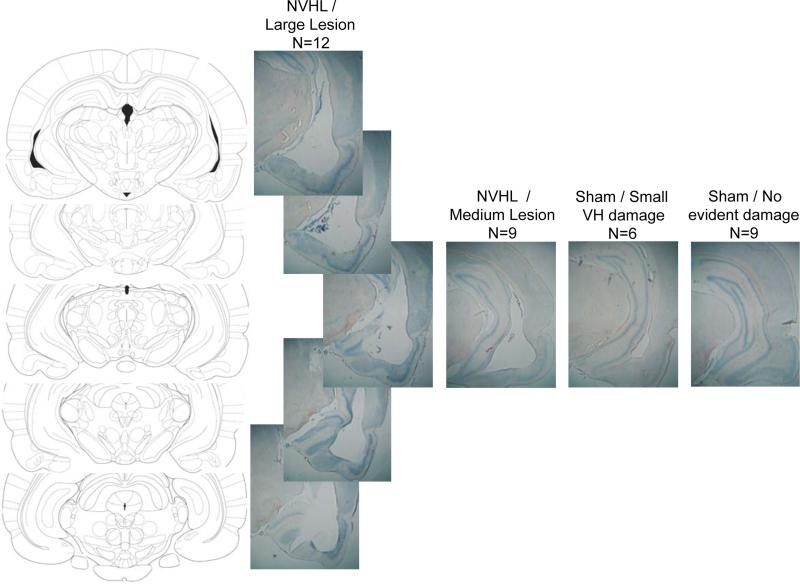
Examples of histological changes in typical brains with small, medium and large NVHL lesions are seen in Figure 1. Interestingly, while most sham group rats had no detectable damage (n=9), histological inspection revealed small abnormalities in 6 of the sham group rats, suggesting that the procedure of lowering a 30 ga needle and infusing PBS at d7 can produce some lasting damage to the VH; in most cases, this damage was manifested by prominence of the lateral ventricle or thinning of the medio-lateral extent of the VH. By contrast, all NVHL group rats had lesions characterized as either medium (n=9) or large (n=12) size, and often involved the loss of substantial amounts of tissue in the full AP extent of the VH complex.
Figure 1.
Coronal sections through the ventral hippocampus from Paxinos and Watson (1998), and 5 vertically positioned photomicrographs of a typical “large” lesion in an NVHL group rat (n=12). Adjacent photomicrographs show typical damage at a roughly comparable AP plane (Bregma – 5.20 – 5.30 mm) in NVHL rats with a “medium” sized lesion (n=9), and in Sham group rats with little (n=6) or no damage noted to the NVHL (n=9).
Behavior
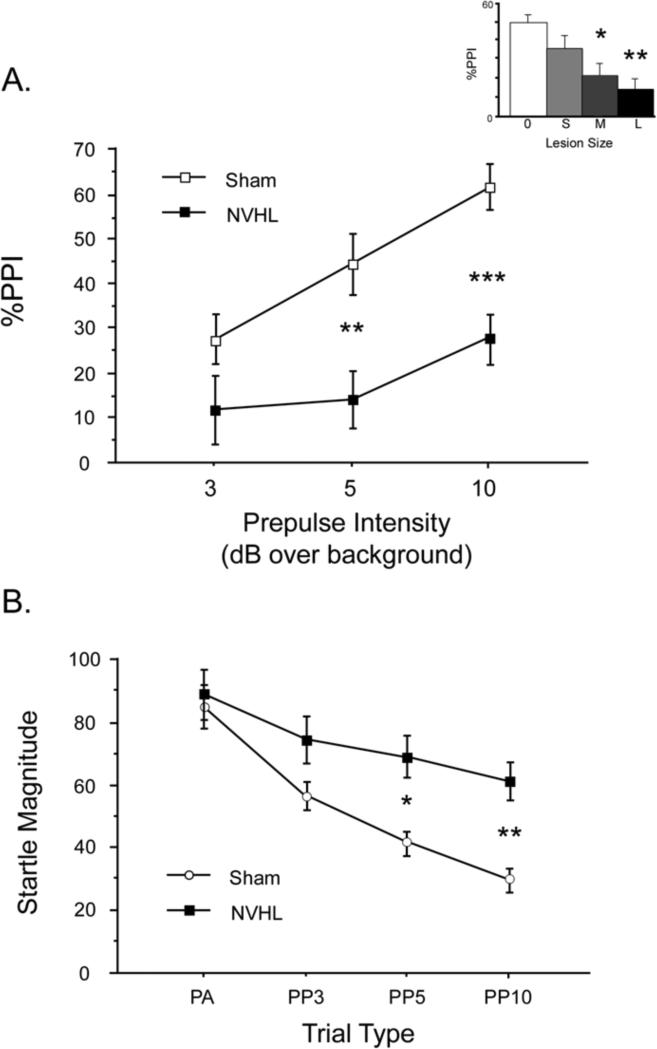
No gross behavioral disturbances were noted in BUF NVHL rats. PPI data are displayed in Figure 2. NVHL rats exhibited profound PPI deficits, and analysis based on lesion size revealed that PPI levels were inversely related to the extent of VH damage across all rats. In the dichotomous comparison of sham vs. NVHL rats, ANOVA revealed a significant effect of lesion group (F=8.93, df 1,34, p<0.006) and prepulse intensity (F=14.93, df 2,68, p<0.0001), but no significant interaction of group × intensity (F=2.15, df 2,68, ns; Figure 2A). When lesion size (none, small, medium, large) was used as a grouping factor, ANOVA revealed the same pattern (main effect of lesion group: p <0.035), with a graded degree of deficits (Figure 2A, inset). Inspection of the data revealed reduced PPI in NVHL vs. sham rats in each of the 6 litters in which rats from both groups were represented.
Figure 2.
NVHLs selectively disrupt sensorimotor gating of startle, and do not significantly alter levels of gene expression in the NAC or mPFC. A. PPI was significantly reduced in NVHL vs. Sham group rats. Main figure shows comparison of NVHL vs. Sham groups across 3 prepulse intensities; inset shows data collapsed across all prepulse intensities, but divided into 4 groups based on histological evidence of no VH damage (0) or small VH damage in Sham group rats (S), or medium (M) or large (L) lesions in NVHL group rats. * p < 0.035; ** p < 0.006; *** p < 0.001. B. Startle magnitude on pulse alone (PA) trials or on trials in which a pulse was preceded by prepulses of 3, 5 or 10 dB over background (PP3, PP5 or PP10, respectively). Elevated startle magnitude on prepulse trials in NVHL rats provides clear evidence of a reduced inhibitory impact of these weak sensory events on the motor reflex, i.e. reduced sensorimotor inhibition. * p <0.025; ** p < 0.006.
These PPI deficits were selective in NVHL rats: no lesion effects on startle magnitude or habituation were detected. Analysis of startle magnitude on PULSE trials for Blocks 1 vs. 4 revealed no significant effect of group (F<1), a significant effect of trial block (F=66.33, df 1,34, p<0.0001), and no significant interaction of group × block (F<1). ANOVA of startle magnitude on PULSE trials during PPI testing in trial blocks 2 and 3 confirmed no group differences (group: F<1; block: F=2.86, df 1,34, ns; group × block: F<1). Analyses using lesion size as a grouping factor generated comparable results (data not shown). NOSTIM levels were also not significantly impacted by lesion status (F=3.09, df 1,34, ns). Importantly, analysis of startle magnitude on PULSE and prepulse+PULSE trials confirmed that the loss of %PPI in NVHL group rats reflected a true loss of sensorimotor gating, i.e. a reduction in the ability of the prepulse to inhibit the magnitude of the response to the PULSE (Figure 2B). ANOVA of startle magnitude across all trial types revealed a significant interaction of lesion group × trial type (F=5.42, df 3, 102, p<0.002), and post-hoc comparisons confirmed comparable startle magnitude on PULSE trials across groups (F<1), but significantly elevated startle magnitude on prepulse+PULSE trials in NVHL rats (F=5.18, df 1,34, p<0.03). Analyses using lesion size as a grouping factor confirmed a graded impact of lesion size on this pattern (data not shown).
Electrophysiology
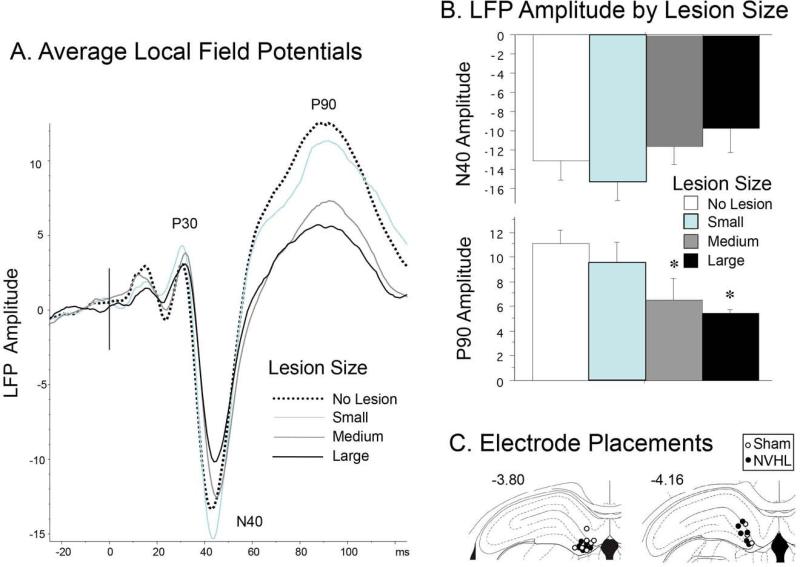
Grand average LFPs are seen in Figure 3A. Separate analyses were conducted on the amplitudes of three evoked field potential components: P30, N40 and P90. One-factor ANOVA's detected no significant effect of lesion on P30 or N40 amplitude (F's=0.17 and 2.32, respectively). Inspection of the N40 data (Figure 3B) suggests that the lack of significant group difference (lesion-induced reduction in N40 amplitude; d=0.65) may reflect deficient power due to sample attrition; this effect size was substantially larger when analyses compared only the subgroups with no VH damage vs. those with largest VH lesions (d=1.20). Analysis of P90 amplitude revealed a significant effect of lesion group (F=7.40, df 1,22, p<0.013), with a graded amplitude-reducing effect of lesion size (Figure 3A), and a substantial contrast between groups with no VH damage vs. those with the largest lesions (F=23.99, df 1,8, p<0.0015). Consistent with this pattern, N40 amplitude correlated significantly with P90 amplitude across all rats (r=−0.48, p<0.019). Histological assessment confirmed electrode placements in the DG for all 24 rats (Figure 3C).
Figure 3.
Local field potentials. A. Grand average LPS across 4 lesion groups, labeled for specific temporal components. B. Amplitude (mean ± SEM) for N40 and P90 waves (* significantly smaller than “No Lesion” group, p<0.05). C. Locations of ventral tips of recording electrodes.
Gene Expression
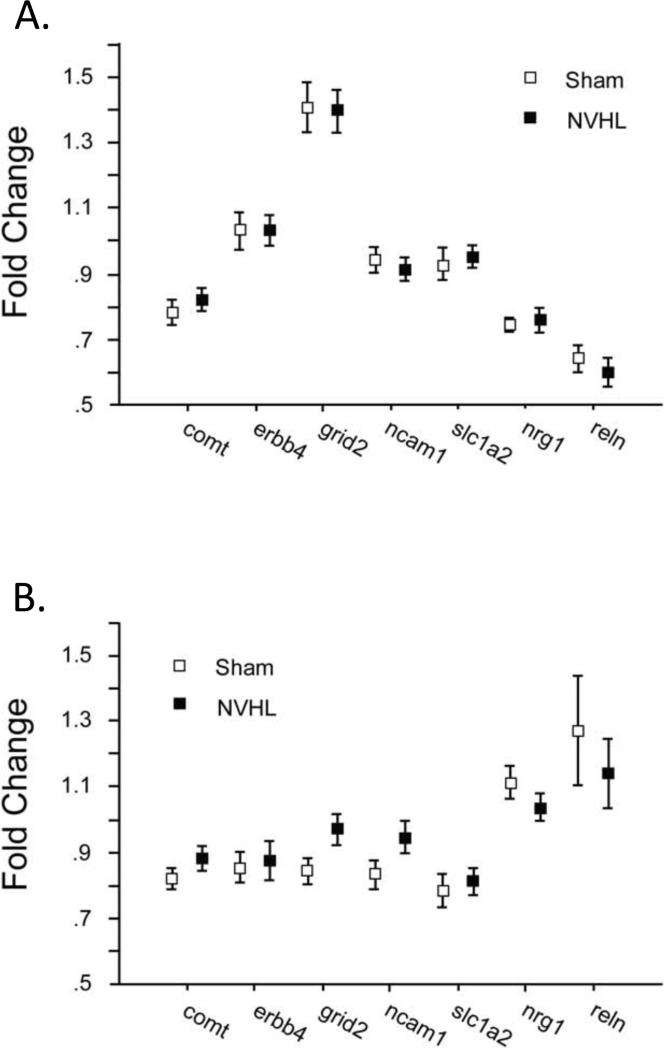
NVHLs had no significant effects on the level of expression of the 7 genes assessed in either the NAC or mPFC (Figure 4). ANOVAs of fold-change values detected no main effect of lesion (NAC: F<1; mPFC: F<1), significant effects of gene (NAC: F=100.04, df 6,198, p<0.0001; mPFC: F=12.14, df 6,204, p<0.0001), and no significant interactions of lesion × gene (NAC: F<1; mPFC: F=1.28, df 6,204, ns). Similar patterns were detected with ANOVAs using lesion size as a grouping factor (not shown).
Figure 4.
A. Expression levels of 7 genes in the NAC in Sham group (n=15) and NVHL rats (n=20). B. Expression levels of 7 genes in the mPFC in Sham group (n=15) and NVHL rats (n=21).
Consistent with our previous report (Swerdlow et al. 2012b), multiple gene expression levels correlated within brain regions (Table 1), but not across brain regions (Table 2). Within the NAC, of the 21 possible pair-wise relationships among the 7 genes in sham group rats (e.g. NAC Comt vs. NAC Erbb4), 18 regression terms were positive, 10 reached uncorrected significance levels (p<0.05), and 7 reached corrected significance levels (0.05/21=0.0024); a similar pattern was detected among NVHL rats (19 positive regression terms, 10 pairs significant uncorrected, 6 significant corrected). For sham group rats, within the mPFC, 15 pairs had positive regression terms, 10 reached uncorrected significance levels, and 5 reached corrected significance levels; a similar pattern was detected among NVHL rats (19 positive regression terms, 11 pairs significant uncorrected, 7 pairs significant corrected).
Table 1.
Simple regression analyses revealed highly correlated expression levels (fold change) of 7 genes within either the mPFC (top 2 grids) or NAC (bottom 2 grids) in both Sham and NVHL group rats.
| mPFC Sham (n=15) | ||||||
|---|---|---|---|---|---|---|
| comt | erbb4 | grid2 | ncam | slc1a2 | Nrg1 | |
| erbb4 | 0.53* | |||||
| grid2 | 0.67** | 0.67** | ||||
| ncam | 0.68** | 0.71** | 0.76*** | |||
| slc1a2 | 0.59* | −0.05 | 0.20 | −0.07 | ||
| nrg1 | −0.20 | 0.60* | 0.36 | 0.42 | −0.54* | |
| reln | −0.19 | 0.62* | 0.07 | 0.29 | −0.59* | 0.79**** |
| mPFC NVHL (n=21) | ||||||
|---|---|---|---|---|---|---|
| comt | erbb4 | grid2 | ncam | slc1a2 | nrg1 | |
| erbb4 | 0.60*** | |||||
| grid2 | 0.72**** | 0.78**** | ||||
| ncam | 0.78**** | 0.70**** | 0.88**** | |||
| slc1a2 | 0.66*** | 0.06 | 0.28 | 0.26 | ||
| nrg1 | 0.07 | 0.56** | 0.38 | 0.46 | −0.46* | |
| reln | 0.02 | 0.58** | 0.25 | 0.40 | −0.52* | 0.75**** |
| NAC Sham (n=15) | ||||||
|---|---|---|---|---|---|---|
| comt | erbb4 | grid2 | ncam | slc1a2 | nrg1 | |
| erbb4 | 0.85**** | |||||
| grid2 | 0.54* | 0.72*** | ||||
| ncam | 0.78*** | 0.84**** | 0.89**** | |||
| slc1a2 | 0.65** | 0.57* | 0.43 | 0.57* | ||
| nrg1 | 0.45 | 0.42 | −0.24 | 0.03 | 0.20 | |
| reln | 0.44 | 0.21 | −0.31 | −0.03 | 0.28 | 0.80*** |
| NAC NVHL (n=20) | ||||||
|---|---|---|---|---|---|---|
| comt | erbb4 | grid2 | ncam | slc1a2 | nrg1 | |
| erbb4 | 0.71*** | |||||
| grid2 | 0.39 | 0.49* | ||||
| ncam | 0.80**** | 0.82**** | 0.66*** | |||
| slc1a2 | 0.61** | 0.32 | 0.44 | 0.49* | ||
| nrg1 | 0.26 | 0.53* | 0.44 | 0.54* | 0.09 | |
| reln | 0.24 | 0.41 | 0.10 | 0.27 | −0.01 | 0.77**** |
p <0.05
p < 0.01
p < 0.005
p < 0.0005
Table 2.
Simple regression analyses revealed no correlations of gene expression between the mPFC and NAC in Sham group rats, but significantly correlated levels (fold change) in 3 out of 7 genes in NVHL group rats.
| Sham (n=15) | NVHL (n=20) | |
|---|---|---|
| comt | 0.05 | 0.49* |
| erbb4 | −0.34 | 0.28 |
| grid2 | 0.42 | 0.20 |
| ncam | 0.14 | 0.45* |
| slc1a2 | 0.06 | 0.67*** |
| nrgl | 0.09 | 0.14 |
| reln | 0.02 | 0.17 |
p <0.05
p < 0.002 (and “r” significantly greater than Sham group value, p<0.025)
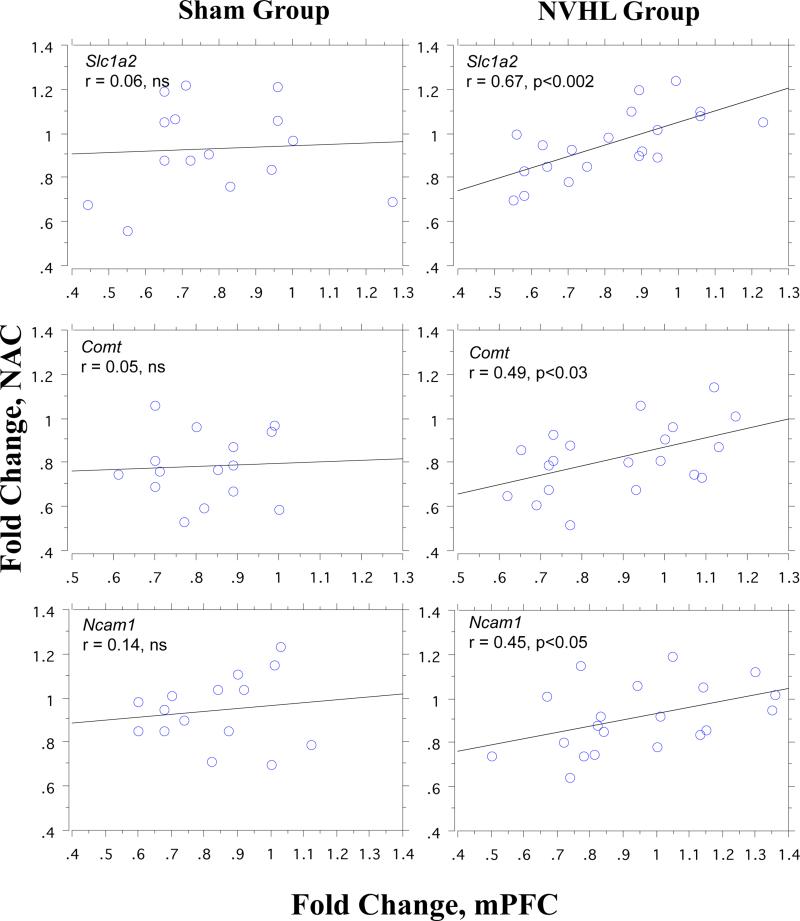
By contrast, correlations of expression levels for each of the 7 genes across regions (e.g. NAC Comt vs. mPFC Comt) in sham group rats failed to reach even uncorrected (p<0.05) significance levels (Table 2). However, among NVHL rats, expression levels across the NAC and mPFC were correlated significantly for Comt (r=0.49, p<0.03), Ncam1 (r=0.45, p<0.05) and Slc1a2 (r=0.67, p<0.002); the latter value reached corrected alpha levels (0.05/7=0.0071), and was significantly greater than sham group values by Fisher's r-to-z transformation (z=−2.01, p<0.025) (Figure 5). Among rats with “large” lesions, r-values were increased to 0.72, 0.59 and 0.73 for Comt, Ncam1 and Slc1a, respectively, and expression levels for Nrg1 were also significantly correlated across structures (r=0.67, p<0.025); correlations for “large” lesion rats also exceeded those for sham group rats for Comt (z=−1.94, p<0.027) and Slc1a2 (z=−1.97, p<0.025). When comparing only rats with no identifiable lesion vs. those with large lesions, group differences in correlated expression levels also reached significance for Ncam1 (z=−1.67, p<0.05).
Figure 5.
Coupling of Comt, Slc1a2 and Ncam1 expression in the NAC and mPFC in NVHL lesion but not sham group rats. Simple regression plots of correlated expression of Comt (top row) and Slc1a2 (middle row) and Ncam1 (bottom row) in NAC (Y-axis) vs. mPFC (X-axis) in Sham group (n=15) and NVHL group rats (n=20). Correlations are significant in NVHL group for each gene and for Slc1a2, this r in NVHL group rats was significantly larger than in Sham group rats (p<0.025 by Fisher's r-to-z transformation). Correlations among NVHL group rats were generally even more robust when only rats with large NVHL lesions were included, and when they were compared to Sham group rats without evidence of VH damage (see text).
In general, levels of gene expression in the NAC and mPFC did not correlate significantly with mean PPI levels; this was true in analyses that included all rats, and in those restricted to either sham group or NVHL rats. Of the 42 possible correlations (7 genes × 2 regions (NAC, mPFC) × 3 groups (all rats, sham group, NVHL)), only three correlations achieved uncorrected significance (PPI vs. NAC Reln, r=−0.36, p<0.04 for all rats; r=−0.60, p<0.02 for sham group rats; and PPI vs. NAC Nrg1, r=−0.62, p<0.014 for sham group rats). Similarly, expression levels did not correlate significantly with P90 LFP amplitudes; of the 42 possible correlations, only two correlations achieved uncorrected significance (P90 vs. mPFC Erbb4, r=0.65, p<0.015, and P90 vs. mPFC Grid2, r=0.55, p<0.045, both for NVHL rats).
Discussion
The present study reproduces the loss of PPI in NVHL rats, first reported by our group in 1995 (Lipska et al. 1995), and subsequently replicated and extended by other groups and in our laboratory (Daenen et al. 2003; Le Pen et al. 2003; Le Pen and Moreau 2002; Swerdlow et al. 2012a). We had previously detected these deficits in outbred SD rats, and the present findings extend these lesion effects to an inbred albino strain, perhaps more appropriate for genetic analyses. The loss of sensorimotor gating was particularly clear in the present NVHL rats, via analyses demonstrating a loss of the inhibitory impact of acoustic prepulses, in the absence of changes in baseline startle reactivity (cf. Swerdlow et al. 2008) (Figure 2B). The selective behavioral effect of these lesions was also demonstrated by their lack of impact on startle habituation and general motor activity, as assessed by cage displacements without stimulus delivery. This pattern of results supports the validity of the NVHL model to study the biology of deficient sensorimotor gating induced by an early developmental brain insult.
Compared to sham group rats, NVHL rats also exhibited large effect size reductions in N40- and statistically significant reductions in P90-auditory sensory LFPs recorded from dentate gyrus in response to tone stimuli. Deficits in these components were most pronounced in rats with larger lesions, consistent with our previous report of N40 reduction in NVHL rats using surface recordings (Swerdlow et al 2012a). In contrast to the observed N40 and P90 effects, NVHLs had no apparent impact on the earlier P30 LFP component. A similar dissociation of earlier vs. later LFP components has also been observed in rodents after subchronic ketamine exposure (Connolly et al., 2004; Featherstone et al., 2012; Maxwell et al., 2006), a pharmacologic approach to modeling schizophrenia-like sensory and cognitive deficits. Although P30 and N40 LFPs and surface recordings have been the focus of many previous studies of sensory registration and/or gating, relatively less is known about the nature of the P90 component in rats. This P90 response exhibits comparable morphology to P80s observed in mice and P200s observed in humans in response to repetitive auditory stimuli recordings (Amann et al., 2010; Connolly 2004), consistent with other shifts in DG LFP latencies across species (Tamura et al. 2011). While the neural basis for P90 deficits after NVHLs is not known, it is likely that NVHLs damage or destroy perforant pathway inputs to the DG, and substantially alter the intrinsic laminar structure of the DG. In larger lesions, there is also likely some degree of lateral anatomical shift in compensation for the substantial loss of tissue in the ventral hippocampus, centered approximately 5 mm from the DG recording sites. While the distribution of electrode placements in sham and NVHL groups did not differ markedly to visual inspection, it is very likely that the local cellular environments within these sites responsible for LFPs differed substantially between lesion groups. The lack of correlation of any LFP amplitude with either startle or PPI is generally consistent with our observations with N40 amplitude in NVHL rats recorded from surface electrodes (Swerdlow et al. 2012a), and supports the ability to dissociate deficits of sensorimotor gating from those of sensory processing in this NVHL model.
Despite their substantial impact on PPI and dentate gyrus LFPs, NVHLs did not alter levels of brain regional expression of 7 genes associated with PPI and its deficits in schizophrenia patients, consistent with previous reports (Wong et al. 2005). Thus, a lesion that reproduces a developmentally-linked schizophrenia phenotype – deficient PPI – did not change the levels of expression of genes associated with these PPI deficits, in brain regions known to regulate these deficits. Perhaps this should not be surprising, since levels of gene expression (this study in rats) are not necessarily related to the nuclear DNA or particular SNPs for those genes (previous studies in humans). However, higher levels of gene expression are suggestive of higher levels of protein expression and activity, and particular SNPs (e.g., COMT Val158Met) are also associated with higher levels of protein activity. Thus, for some genes, including COMT, one phenotype might result from either of two potentially independent processes: 1) an event (e.g. epigenetic) that changes levels of gene expression, or 2) the presence of a specific SNP. Nonetheless, for the levels of 7 genes selected in this study based on their association with PPI or it deficits in schizophrenia patients, none were altered by NVHLs that did produce a profound loss of PPI.
As in our previous study with an overlapping but non-identical set of genes, expression levels across multiple genes were highly correlated within individual brain regions. Thus, 37 out of 42 of the r-values for expression levels among pairs of different genes within the NAC, and 34 out of 42 within the mPFC, were positive, and many reached robust levels of statistical significance. In total, 41 out of 84 correlations reached significance levels between 0.05 - 0.0024, and another 25 correlations reached significance levels < 0.0024; this is not likely to be a pattern based on chance. We had previously speculated (Swerdlow et al. 2012b) that this intra-region “coupling” of expression levels across different genes reflected a common source for expression “drive”, i.e. the levels of metabolic or cellular activity within the surrounding brain region. It is also conceivable that such strong intra-regional correlations reflect the fact that these genes are related via one or more signaling pathways within neurons or circuits.
A relationship between correlated gene expression and neural connectivity between different brain regions has been reported in both worms (Kaufman et al. 2006) and adult rodents (Wolf et al. 2011), and has been hypothesized to reflect developmental mechanisms such as those underlying axonal guidance to those regions. In the mammalian brain, this link between correlated expression and anatomical interconnectivity is thought to be present in a disproportionate number of genes implicated in the pathogenesis of schizophrenia (Wolf et al. 2011) and other neurodevelopmental disorders, including autism. Among genes associated with reduced PPI in schizophrenia, in outbred male and female Sprague Dawley (SD) and Long Evans adult rats, we reported only modestly correlated expression across 3 brain regions – NAC, mPFC and VH – that are known to share strong neural connections (Swerdlow et al. 2012b). Even to the extent that such correlations achieved uncorrected statistical significance, they appeared to largely reflect an “artifact” of the influence of the admixture of different strains and sexes. For example, among SD male rats – arguably the sample closest to the present male inbred BUF rats – no significant correlations were detected across the 3 brain regions, for Grid2, Nrg1 or Comt. Clearly, using these genes and brain regions, and the limited power afforded by modest sample sizes, “normal” levels of neural or functional connectivity were insufficient to generate correlated gene expression levels between regions.
Using inbred BUF rats, the present study also did not generally show even modest correlations in the levels of gene expression across the known interconnected NAC and mPFC regions in sham group rats. However, patterns suggesting stronger correlated expression between the NAC and mPFC were detected in NVHL rats for 4 out of the 7 genes tested: Comt, Ncam1, Slc1a2 and Nrg1. Clearly, this is only an empirical observation, open for mechanistic interpretation. We speculate that such a “coupling” might reflect greater functional interconnectivity as a result of the absence and/or alteration of normal developmental signaling to these structures by the VH or other brain regions damaged by the NVHLs; such an interpretation would be consistent with evolving models for the broader circuit impact of early development damage to the VH (O'Donnell 2012; Tseng et al. 2009). An important test of the developmental nature of these processes would be to assess correlated expression levels after VH lesions made during adulthood; such lesions do not disrupt PPI, but are associated with enhanced sensitivity to the PPI-disruptive effects of DA agonists (Swerdlow et al. 1995). For both sham and NVHL group brains, each structure clearly was characterized by distinct patterns of the relative levels of expression of the 7 genes in this study (compare Figures 2C and 2D). Nonetheless, for several of these genes, in NVHL rats, levels of expression in one region significantly predicted levels in the other structure. We do not yet fully understand the relationship between inter-regional correlated gene expression and anatomical or functional connectivity, let alone the mechanisms that might account for such a relationship. The present data only suggest that changes in these normal relationships might be triggered by early developmental interventions such as NVHLs, and that these changes might prove useful in understanding the underlying mechanisms for functional alterations in brain circuits and their potential role in developmental brain disorders.
Of the genes whose fronto-striatal expression levels appear to be “coupled” in NVHL rats, all had been pre-selected based on published evidence of associations with PPI or its deficits in schizophrenia. Thus, it is very possible that coupling might also be observed with other genes, including those unrelated to PPI and schizophrenia, and more generally, that this coupling is not related in any special way to either PPI or schizophrenia. Of the 7 genes tested here, a positive effect of NVHLs on coupled NAC and mPFC expression was particularly evident for Slc1a2. Also known as “GLT1” (glutamate transporter) or EAAT2 (excitatory amino acid transporter – 2) – SLC1A2 is the dominant protein regulating glutamate transport in the mammalian central nervous system (Conti and Weinberg, 1999; Danbolt, 2001). PPI in rats is regulated by glutamate and other EAAs within the nucleus accumbens (e.g. Wan and Swerdlow 1996). Pharmacological upregulation of Slc1a2 potently disrupts PPI in SD rats, an effect that appears to be dependent on reduced glutamate activity at metabotropic [(mGluR)2/3] glutamate receptors (Bellesi et al. 2009; Bellesi and Conti 2010). In those studies, PPI levels were found to correlate strongly (r=−0.67) with SLC1A2 levels in frontal cortex (Bellesi et al. 2009), an effect that was not detected in the present study, in either sham or NVHL group rats. Nonetheless, given the apparent role of SLC1A2 in regulating both PPI and glutamate transport, it would seem reasonable to hypothesize that NVHL-induced changes in the fronto-striatal coupling of this protein might have an impact not only on PPI levels, but also on distributed frontal cortico-striato-pallido-thalamic (CSPT) circuit dynamics.
The VH innervates both the mPFC and NAC, and our studies suggest that these projections follow very distinct anatomical pathways (Miller et al. 2010; Saint Marie et al. 2010), that presumably have distinct developmental courses regulated by different genes. Conditions that would foster greater mPFC-NAC functional interconnectivity might be created by NVHLs via reduced competition at a synaptic level – i.e. the formation of reciprocal connections between the NAC and mPFC within dendritic territories normally occupied by VH inputs - or by the loss of a differentiating signal normally provided by VH innervation of either structure. NVHLs result in both restructuring and electrophysiological changes within the mPFC (Ryan et al. 2012). Others have reported aberrant limbic-cortical connectivity associated with both endogenous (Anticevic et al. 2013) and drug-induced psychosis (Driesen et al. 2013) in humans. To the degree that the observed stronger correlations of Comt, Ncam1, Slc1a2 and Nrg1 across regions reflects stronger mPFC-NAC connectivity, this suggests that an abnormal functional coupling of these regions might occur in response to a specific early developmental insult linked to schizophrenia. Such an aberrant coupling of fronto-striatal systems has been described in previous NVHL models (Chambers et al. 2010), and has been demonstrated in other disorders associated with reduced PPI, such as obsessive-compulsive disorder, where therapeutic response to medication or psychotherapy is associated with a relative uncoupling of these regions, as reflected by a reduction in correlated metabolic activity (Schwartz et al. 1996; Schwartz 1998). If the present NVHL / expression coupling model can be replicated and understood, it is conceivable that future studies might explore strategies for uncoupling NVHL-induced fronto-striatal gene expression patterns as avenues for early therapeutic or even preventative interventions in disorders such as schizophrenia.
HIGHLIGHTS.
Inbred BUF rats sustained sham or neonatal ventral hippocampal lesions (NVHLs).
As adults, NVHL rats exhibited profound deficits in prepulse inhibition of startle.
NVHL rats also exhibited deficits in auditory-evoked field potentials from dentate gyrus.
Expression levels of 7 PPI-related genes in mPFC and NAC were unchanged by NVHLs.
Levels of 3 genes correlated significantly in mPFC and NAC in NVHL but not sham rats.
Acknowledgements
Supported by MH42228 and MH59803. The authors are grateful for informative correspondence with Dr. Eric Turner, and for assistance in manuscript preparation provided by Ms. Maria Bongiovanni. NRS has received Consulting fees from Neurocrine, Inc. GAL has received Consulting fees from Astellas Pharma, Inc.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Amann LC, Gandal MJ, Halene TB, Ehrlichman RS, White SL, McCarren HS, Siegel SJ. Mouse behavioral endophenotypes for schizophrenia. Brain Res. Bull. 2010;83:147–61. doi: 10.1016/j.brainresbull.2010.04.008. [DOI] [PubMed] [Google Scholar]
- Angst MJ, Macedo CE, Guiberteau T, Sandner G. Alteration of conditioned emotional response and conditioned taste aversion after neonatal ventral hippocampus lesions in rats. Brain Res. 2007;1143:183–192. doi: 10.1016/j.brainres.2007.01.093. [DOI] [PubMed] [Google Scholar]
- Anticevic A, Brumbaugh MS, Winkler AM, Lombardo LE, Barrett J, Corlett PR, Kober H, Gruber J, Repovs G, Cole MW, Krystal JH, Pearlson GD, Glahn DC. Global prefrontal and fronto-amygdala dysconnectivity in bipolar I disorder with psychosis history. Biol. Psychiatry. 2013;73:565–573. doi: 10.1016/j.biopsych.2012.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellesi M, Conti F. The mGluR2/3 agonist LY379268 blocks the effects of GLT-1 upregulation on prepulse inhibition of the startle reflex in adult rats. Neuropsychopharmacology. 2010;35:1253–1260. doi: 10.1038/npp.2009.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellesi M, Melone M, Gubbini A, Battistacci S, Conti F. GLT-1 Upregulation Impairs Prepulse Inhibition of the Startle Reflex in Adult Rats. GLIA. 2009;57:703–713. doi: 10.1002/glia.20798. [DOI] [PubMed] [Google Scholar]
- Chambers RA, Sentir AM, Conroy SK, Truitt WA, Shekhar A. Cortical-striatal iIntegration of cocaine history and prefrontal dysfunction in animal modeling of dual diagnosis. Biol. Psychiatry. 2010;67:788–792. doi: 10.1016/j.biopsych.2009.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connolly PM, Maxwell C, Liang Y, Kahn JB, Kanes SJ, Abel T, Gur RE, Turetsky BI, Siegel SJ. The effects of ketamine vary among inbred mouse strains and mimic schizophrenia for the P80, but not P20 or N40 auditory ERP components. Neurochem. Res. 2004;29:1179–1188. doi: 10.1023/b:nere.0000023605.68408.fb. [DOI] [PubMed] [Google Scholar]
- Conti F, Weinberg RJ. Shaping excitation at glutamatergic synapses. Trends Neurosci. 1999;22:451–458. doi: 10.1016/s0166-2236(99)01445-9. [DOI] [PubMed] [Google Scholar]
- Danbolt NC. Glutamate uptake. Prog. Neurobiol. 2001;65:1–105. doi: 10.1016/s0301-0082(00)00067-8. [DOI] [PubMed] [Google Scholar]
- Daenen EW, Wolterink G, Van Der Heyden JA, Kruse CG, Van Ree JM. Neonatal lesions in the amygdala or ventral hippocampus disrupt prepulse inhibition of the acoustic startle response; implications for an animal model of neurodevelopmental disorders like schizophrenia. Eur. Neuropsychopharmacol. 2003;3:187–197. doi: 10.1016/s0924-977x(03)00007-5. [DOI] [PubMed] [Google Scholar]
- Driesen NR, McCarthy G, Bhagwagar Z, Bloch M, Calhoun V, D'Souza DC, Gueorguieva R, He G, Ramachandran R, Suckow RF, Anticevic A, Morgan PT, Krystal JH. Relationship of resting brain hyperconnectivity and schizophrenia-like symptoms produced by the NMDA receptor antagonist ketamine in humans. Mol. Psychiatry. 2013 doi: 10.1038/mp.2012.194. Epub ahead of print, Jan. 22. doi: 10.1038/mp.2012.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Rawas R, Saadé NE, Thiriet N, Atwehc S, Jaber M, Al-Amin HA. Developmental changes in the mRNA expression of neuropeptides and dopamine and glutamate receptors in neonates and adult rats after ventral hippocampal lesion. Schizophr. Res. 2009;113:298–307. doi: 10.1016/j.schres.2009.05.009. [DOI] [PubMed] [Google Scholar]
- Featherstone RE, Liang Y, Saunders JA, Tatard-Leitman VM, Ehrlichman RS, Siegel SJ. Subchronic ketamine treatment leads to permanent changes in EEG, cognition and the astrocytic glutamate transporter EAAT2 in mice. Neurobiol Dis. 2012;47:338–46. doi: 10.1016/j.nbd.2012.05.003. [DOI] [PubMed] [Google Scholar]
- Flores G, Alquicer G, Silva-Gómez AB, Zaldivar G, Stewart J, Quirion R, Srivastava LK. Alterations in dendritic morphology of prefrontal cortical and nucleus accumbens neurons in post-pubertal rats after neonatal excitotoxic lesions of the ventral hippocampus. Neuroscience. 2005;133:463–470. doi: 10.1016/j.neuroscience.2005.02.021. [DOI] [PubMed] [Google Scholar]
- Flores C, Bhardwaj SK, Labelle-Dumais C, Srivastava LK. Altered netrin-1 receptor expression in dopamine terminal regions following neonatal ventral hippocampal lesions in the rat. Synapse. 2009;63:54–60. doi: 10.1002/syn.20584. [DOI] [PubMed] [Google Scholar]
- Greenwood TA, Lazzeroni LC, Murray SS, Cadenhead KS, Calkins ME, Dobie DJ, et al. Analysis of 94 candidate genes and twelve endophenotypes for schizophrenia from the Consortium on the Genetics of Schizophrenia. Am. J. Psychiatry. 2011;168:930–946. doi: 10.1176/appi.ajp.2011.10050723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwood TA, Light GA, Swerdlow NR, Radant AD, Braff DL. Association analysis of 94 candidate genes and schizophrenia-related endophenotypes. PLoS Genet. 2012;7:e1002134. doi: 10.1371/journal.pone.0029630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanlon FM, Houck JM, Klimaj SD, Caprihan A, Mayer AR, Weisend MP, Bustillo JR, Hamilton DA, Tesche CD. Frontotemporal anatomical connectivity and working-relational memory performance predict everyday functioning in schizophrenia. Psychophysiology. 2012;49:1340–1352. doi: 10.1111/j.1469-8986.2012.01448.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao WT, Wang Y, Kleinman JE, Lipska BK, Hyde TM, Weinberger DR, Law AJ. Common genetic variation in Neuregulin 3 (NRG3) influences risk for schizophrenia and impacts NRG3 expression in human brain. Proc. Natl. Acad. Sci. U. S. A. 2010;107:15619–15624. doi: 10.1073/pnas.1005410107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman A, Dror G, Meilijson I, Ruppin E. Gene expression of Caenorhabditis elegans neurons carries information on their synaptic connectivity. PLoS Comput. Biol. 2006;2:e167. doi: 10.1371/journal.pcbi.0020167. doi:10.1371/journal.pcbi.0020167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Pen G, Kew J, Alberati D, Borroni E, Heitz MP, Moreau JL. Prepulse inhibition deficits of the startle reflex in neonatal ventral hippocampal-lesioned rats: reversal by glycine and a glycine transporter inhibitor. Biol. Psychiatry. 2003;54:1162–1170. doi: 10.1016/s0006-3223(03)00374-3. [DOI] [PubMed] [Google Scholar]
- Le Pen G, Moreau JL. Disruption of prepulse inhibition of startle reflex in a neurodevelopmental model of schizophrenia: reversal by clozapine, olanzapine and risperidone but not by haloperidol. Neuropsychopharmacology. 2002;27:1–11. doi: 10.1016/S0893-133X(01)00383-9. [DOI] [PubMed] [Google Scholar]
- Lipska BK, Jaskiw GE, Weinberger DR. Postpubertal emergence of hyperresponsiveness to stress and to amphetamine after neonatal excitotoxic hippocampal damage: a potential animal model of schizophrenia. Neuropsychopharmacology. 1993;9:67–75. doi: 10.1038/npp.1993.44. [DOI] [PubMed] [Google Scholar]
- Lipska BK, Swerdlow NR, Geyer MA, Jaskiw GE, Braff DL, Weinberger DR. Neonatal excitotoxic hippocampal damage in rats causes post-pubertal changes in prepulse inhibition of startle and its disruption by apomorphine. Psychopharmacology. 1995;122:35–43. doi: 10.1007/BF02246439. [DOI] [PubMed] [Google Scholar]
- Marquis JP, Goulet S, Doré FY. Neonatal lesions of the ventral hippocampus in rats lead to prefrontal cognitive deficits at two maturational stages. Neuroscience. 2006;140:759–767. doi: 10.1016/j.neuroscience.2006.02.048. [DOI] [PubMed] [Google Scholar]
- Marquis JP, Goulet S, Doré FY. Neonatal ventral hippocampus lesions disrupt extradimensional shift and alter dendritic spine density in the medial prefrontal cortex of juvenile rats. Neurobiol. Learn. Mem. 2008;90:339–346. doi: 10.1016/j.nlm.2008.04.005. [DOI] [PubMed] [Google Scholar]
- Maxwell CR, Ehrlichman RS, Liang Y, Trief D, Kanes SJ, Karp J, Siegel SJ. Ketamine produces lasting disruptions in encoding of sensory stimuli. J. Pharmacol. Exp. Ther. 2006;316:315–324. doi: 10.1124/jpet.105.091199. [DOI] [PubMed] [Google Scholar]
- Miller EJ, Saint Marie RL, Breier MR, Swerdlow NR. Pathways from the ventral hippocampus and caudal amygdala to forebrain regions that regulate sensorimotor gating in the rat. Neuroscience. 2010;165:601–611. doi: 10.1016/j.neuroscience.2009.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell CP, Grayson DR, Goldman MB. Neonatal lesions of the ventral hippocampal formation alter GABA-A receptor subunit mRNA expression in adult rat frontal pole Biol. Psychiatry. 2005;57:49–55. doi: 10.1016/j.biopsych.2004.09.017. [DOI] [PubMed] [Google Scholar]
- O'Donnell P. Cortical disinhibition in the neonatal ventral hippocampal lesion model of schizophrenia: New vistas on possible therapeutic approaches. Pharmacol. Ther. 2012;133:19–25. doi: 10.1016/j.pharmthera.2011.07.005. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates, fourth ed. Academic Press; San Diego, CA: 1998. [Google Scholar]
- Quednow BB, Wagner M, Mössner R, Maier W, Kuhn KU. Sensorimotor gating of schizophrenia patients depends on catechol O-methyltransferase Val158Met polymorphism. Schizophr. Bull. 2010;36:341–346. doi: 10.1093/schbul/sbn088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roussos P, Giakoumaki SG, Rogdaki M, Pavlakis S, Frangou S, Bitsios P. Prepulse inhibition of the startle reflex depends on the catechol O-methyltransferase Val158Met gene polymorphism. Psychol. Med. 2008;38:1651–1658. doi: 10.1017/S0033291708002912. [DOI] [PubMed] [Google Scholar]
- Ryan RT, Bhardwaj SK, Tse YC, Srivastava LK, Wong TP. Opposing Alterations in Excitation and Inhibition of Layer 5 Medial Prefrontal Cortex Pyramidal Neurons Following Neonatal Ventral Hippocampal Lesion. Cereb. 2012 doi: 10.1093/cercor/bhs111. Cortex Epub ahead of print, May 10. [DOI] [PubMed] [Google Scholar]
- Saint Marie RL, Miller EJ, Breier MR, Weber M, Swerdlow NR. Projections from ventral hippocampus to medial prefrontal cortex but not nucleus accumbens remain functional after fornix lesions in rats. Neuroscience. 2010;168:498–504. doi: 10.1016/j.neuroscience.2010.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz JM. Neuroanatomical aspects of cognitive-behavioural therapy response in obsessive-compulsive disorder. An evolving perspective on brain and behaviour. Br. J. Psychiatry Suppl. 1998;35:38–44. [PubMed] [Google Scholar]
- Schwartz JM, Stoessel PW, Baxter LR, Jr., Martin KM, Phelps ME. Systematic changes in cerebral glucose metabolic rate after successful behavior modification treatment of obsessive-compulsive disorder. Arch. Gen. Psychiatry. 1996;53:109–113. doi: 10.1001/archpsyc.1996.01830020023004. [DOI] [PubMed] [Google Scholar]
- Shilling PD, Saint Marie RL, Shoemaker JM, Swerdlow NR. Strain differences in the gating-disruptive effects of apomorphine: relationship to gene expression in nucleus accumbens signaling pathways. Biol. Psychiatry. 2008;63:748–758. doi: 10.1016/j.biopsych.2007.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobin C, Kiley-Brabeck K, Karayiorgou M. Lower prepulse inhibition in children with the 22q11 deletion syndrome. Am. J. Psychiatry. 2005;162:1090–1099. doi: 10.1176/appi.ajp.162.6.1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefansson H, Sigurdsson E, Steinthorsdottir V, Bjornsdottir S, Sigmundsson T, Ghosh S, et al. Neuregulin 1 and susceptibility to schizophrenia. Am. J. Hum. Genet. 2002;71:877–892. doi: 10.1086/342734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swerdlow NR, Light GA, Breier MR, Shoemaker JM, Saint Marie RL, Neary AC, et al. Sensory and sensorimotor gating deficits after neonatal ventral hippocampal lesions in rats. Dev. Neurosci. 2012a;34:240–249. doi: 10.1159/000336841. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Lipska BK, Weinberger DR, Braff DL, Jaskiw GE, Geyer MA. Increased sensitivity to the sensorimotor gating-disruptive effects of apomorphine after lesions of medial prefrontal cortex or ventral hippocampus in adult rats. Psychopharmacology. 1995;122:27–34. doi: 10.1007/BF02246438. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Shilling PD, Breier M, Trim RS, Light GA, Saint Marie R. Fronto-temporal-mesolimbic gene expression and heritable differences in amphetamine-disrupted sensorimotor gating in rats. Psychopharmacology. 2012b;224:349–362. doi: 10.1007/s00213-012-2758-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swerdlow NR, Shoemaker JM, Crain S, Goins J, Onozuka K, Auerbach PP. Sensitivity to drug effects on prepulse inhibition in inbred and outbred rat strains. Pharmacol. Biochem. Behav. 2004;77:291–302. doi: 10.1016/j.pbb.2003.11.008. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Weber M, Qu Y, Light GA, Braff DL. Realistic expectations of prepulse inhibition in translational models for schizophrenia research. Psychopharmacology. 2008;199:331–388. doi: 10.1007/s00213-008-1072-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura R, Nishida H, Eifuku S, Nagao K, Fushiki H, Watanabe Y, Ono T. Short-term synaptic plasticity in the dentate gyrus of monkeys. PLoS ONE. 2011;6:e20006. doi: 10.1371/journal.pone.0020006. doi:10.1371/journal.pone.0020006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng KY, Chambers RA, Lipska BK. The neonatal ventral hippocampal lesion as a heuristic neurodevelopmental model of schizophrenia. Behav. Brain Res. 2009;204:295–305. doi: 10.1016/j.bbr.2008.11.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan FJ, Swerdlow NR. Sensorimotor gating in rats is regulated by different dopamine–glutamate interactions in the nucleus accumbens core and shell subregions. Brain Res. 1996;722:168–176. doi: 10.1016/0006-8993(96)00209-0. [DOI] [PubMed] [Google Scholar]
- Wolf L, Goldberg C, Manor N, Sharan R, Ruppin E. Gene Expression in the Rodent Brain is Associated with Its Regional Connectivity. PLoS Comput. Biol. 2011;7:e1002040. doi: 10.1371/journal.pcbi.1002040. doi:10.1371/journal.pcbi.1002040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong AHC, Lipska BK, Likhodi O, Boffa E, Weinberger DR, Kennedy JL, et al. Cortical gene expression in the neonatal ventral-hippocampal lesion rat model. Schizophr. Res. 2005;77:261–270. doi: 10.1016/j.schres.2005.03.011. [DOI] [PubMed] [Google Scholar]
- Yabuki Y, Nakagawasai O, Moriguchi S, Shioda N, Onogi H, Tan-No K, et al. Decreased CaMKII and PKC activities in specific brain regions are associated with cognitive impairment in neonatal ventral hippocampus-lesioned rats. Neuroscience. 2013 doi: 10.1016/j.neuroscience.2012.12.048. doi: http://dx.doi.org/10.1016/j.neuroscience.2012.12.048. [DOI] [PubMed]