Abstract
S6K (ribosomal S6 kinase p70, p70S6K) activation requires phosphorylation at two stages. The first phosphorylation is independent of insulin stimulation and mediated by an unknown kinase. The second phosphorylation is mediated by mTOR in insulin dependent manner. In this study, we identified JNK1 (c-Jun N-terminal kinase 1) as a kinase in the first phosphorylation. S6K protein was phosphorylated by JNK1 at S411 and S424 in the carboxyl terminal autoinhibitory domain. The phosphorylation was observed in kinase assay with purified S6K as a substrate, and in cells after JNK1 activation by TNF-α or MEKK1 expression. The phosphorylation was detected in JNK2 null cells, but not in JNK1 null cells after TNF-α treatment. When JNK1 activation was inhibited by MKK7 knockdown, the phosphorylation was blocked in cells. The phosphorylation led to S6K protein degradation in NF-κB deficient cells. The degradation was blocked by inhibition of proteasome activity with MG132. In wide type cells, the phosphorylation did not promote S6K degradation when IKK2 (IKKβ, IkB kinase beta) was activated. Instead, the phosphorylation allowed S6K activation by mTOR, which stabilizes S6K protein. In IKK2 null cells or cells treated by IKK2 inhibitor, the phosphorylation led to S6K degradation. These data suggest that S6K is phosphorylated by JNK1 and the phosphorylation makes S6K protein unstable in the absence of IKK2 activation. This study provides a mechanism for regulation of S6K protein stability.
Keywords: Insulin resistance, inflammation, obesity, liver, TNF-α
1. Introduction
Ribosomal S6 kinase p70 (p70S6K, S6K) is a member of the AGC family of serine/threonine protein kinases. It mediates signals of insulin, growth factors, mitogens, nutrients and stresses in the regulation of cell growth and energy metabolism [1]. S6K is required for protein synthesis in cells and is involved in the negative feedback regulation of insulin signaling pathway. In obesity, S6K is activated by several risk factors for type 2 diabetes, such as hyperinsulinemia, inflammation, free fatty acid and branch chain amino acids [1, 2]. The activation contributes to the pathogenesis of type 2 diabetes by induction of insulin resistance. Recent studies suggest that S6K phosphorylates insulin receptor substrate 1 (IRS-1) at multiple serine residues [3-7], by which S6K inhibits insulin signaling at the post receptor level [7]. In vivo, S6K knockout protects mice from obesity-induced insulin resistance [8]. In an early study, we reported that S6K was activated by pro-inflammatory cytokine TNF-α in adipocytes, and the activation was dependent on IKK2 (IKKβ) activity [7]. In the model, S6K mediates IKK2 activity in the inhibition of insulin action. IKK2 regulates S6K through activation of mTOR, an upstream kinase of S6K in the classical insulin signaling pathway [9]. In another study, we observed that in NF-κB p50-KO (knockout) mice, TNF-α was elevated in the blood circulation without induction of insulin resistance [10]. In mechanism, a low level of S6K protein in the liver of p50-KO mice was responsible for the disassociation of TNF-α and insulin resistance. The study suggests that S6K activity is required for inflammation-induced insulin resistance. However, the mechanism of S6K reduction was not known in p50-KO mice. We addressed this issue by investigating the mechanism of S6K protein stability in the current study.
The activity and subcellular location of S6K are controlled by phosphorylation and dephosphorylation [11-13]. Several serine kinases have been reported to phosphorylate S6K in the process of S6K activation. The first phosphorylation occurs at the C-terminal domain (S411, S418, T421, S424 and S429) [14]. The phosphorylation releases the catalytic domain from the inhibition of C-terminal domain in S6K protein. Those kinases of the first phosphorylation step include ERK (T421/S424) [15], P38 [2], and CDC2 (S411) [16]. It is not known if JNK1 plays a role in the first step phosphorylation. JNK1 is a member of MAPK (mitogen activated protein kinase) pathway, which include ERK and p38. The second phosphorylation occurs on Thr389, which is catalyzed by mammalian target of rapamycin (mTOR) [17]. Thr389 phosphorylation promotes docking of phosphatidyl-inositide dependent kinase 1 (PDK1), which phosphorylates S6K at T229. Phosphorylation of T389 and T229 results in full activation of S6K [18]. S6K enzyme activity is inactivated by dephosphorylation that involves in phosphatases such as PP2A and PP1 [19]. In addition to the phosphorylation, S6K activity is regulated by ubiquitination that is catalyzed by E3 ligase ROC1 [20, 21]. The mechanism and biological significance of ubiquitination-mediated regulation of S6K remain to be established in the pathogenesis of type 2 diabetes. In this study, we found that the ubiquitination contributes to S6K protein degradation in p50-KO mice, which protects p50-KO mice from insulin resistance.
We investigated the mechanism of S6K degradation in response to TNF-α. Our data suggests that JNK1 directly phosphorylates S6K1 at the c-terminus autoinhibitory domain to facilitate S6K activation. IKK2 contributes to S6K activation by induction of mTOR activation. In the liver of p50-KO mice, IKK2 activity was reduced and JNK1 activity was elevated. This unique condition led to S6K hyperphosphorylation and protein degradation after ubiquitination.
2. Material and methods
2.1. Animals
Male p50-KO mice (Stock Number: 002849) and the control mice (Stock Number: 100903) were purchased from the Jackson laboratory (Bar Harbor, ME). All of the mice were housed in the animal facility at the Pennington Biomedical Research Center with a 12:12-h light-dark cycle and constant temperature (22–24°C). The mice had free access to water and chow or high fat diet. All procedures were performed in accordance with the National Institutes of Health guidelines for the care and use of animals and were approved by the Institutional Animal Care and Use Committee (IACUC) at the institute. The mice were ear-punched for identification and were group housed at four per cage.
2.2. Cells and reagents
Human embryonic kidney (HEK) 293 cells (CRL-1573) were purchased from the American Type Culture Collection (ATCC). Mouse embryonic fibroblast (MEF) cells including IKK2-KO (IKK2−/−), p65-KO, p50-KO were described elsewhere [10, 22]. p65-KO-siMKK7 stable cell line was established by transfecting cells with a siMKK7 expression plasmid. All cells were maintained in Dulbecco’s Modified Eagle’s culture medium supplemented with 10% fetal calf serum from Sigma (St. Louis, MO 63103). Antibodies specific to p70S6K phosphor-S424 (#9204) and phosphor-Thr389 (#9205), phosphor-IKK (Ser180/Ser181, #2681) were obtained from Cell Signaling (Beverly, MA). Antibodies to JNK (sc-7345), p50 (sc-33022), p38 (sc-728), HA (sc-7392), IκBα (sc-371), phosphor-JNK (sc-6254) and pc-JUN (sc-822) were obtained from Santa Cruz Biotechnology (Santa Cruz, CA). Antibodies to S6K (P70S6K) (ab9366), β-Actin (ab6276) were from Abcam (Cambridge, UK). An antibody to IKKβ (05-535) was from Upstate Biotechnology (Lake Placid, NY 12946). Rapamycin (A-275), and SP600125 (EI-305) were acquired from Biomol (Plymouth Meeting, PA). 15-Deoxyprostaglandin J2 (15dPGJ2, 538927), PD98059 (513000), and SB203580 (203580) were purchased from Calbiochem (San Diego, CA). TNF-α (T6674), MG132, anisomycin was obtained from Sigma. Recombinant IKK2 (IKKβ), JNK and p38 were obtained from Upstate Biotechnology.
2.3. Plasmids and Transfection
Plasmid for HA-S6K1 (8984) was obtained from Addgene (Cambridge, MA) [23]. Expression vector for flag-tagged ubiquitin was constructed using pcDNA3 expression vector. Expression vectors for HA-S6KΔC and HA-S6K (S5A) were constructed using vector pRK7. The wild type (WT) and mutant S6K proteins were expressed in HEK293 or MEF cells by transient transfection using Lipofectamine. The bacterial expression vectors for GST-S6K WT, GST-S6K (S411A), GST-S6K (S418A), GST-S6K (S421A), GST-S6K (S424A), GST-S6K (S429A), GST-S6K (S411A+424A), GST-S6KΔC and GST-S6K(S5A) were constructed in PGEX-4T-2 vector. Point mutation and GST-S6K protein preparation were made using protocols as described elsewhere [22].
2.4. Western blot
The whole cell lysate was prepared and the Western blot was conducted according to methods described elsewhere [7].
2.5. Kinase Assay
The kinase assay was conducted according to methods reported earlier [7]. Briefly, purified GST-S6K protein was diluted in kinase assay buffer (20 mM HEPES, pH 7.6, 20 mM MgCl2, 20 mM glycerophosphate, 1 mM dithiothreitol, 10 μM ATP, 1 mM EDTA, 1 mM sodium orthovanadate, 0.4 mM phenylmethylsulfonyl fluoride, 20 mM creatine phosphate). The kinase assay was conducted at 37°C for 30 min in 20 μl of kinase assay buffer containing 5 μCi of γ-32P ATP and 2 μl of kinase, such as JNK, IKK2 and p38. The recombinant kinases were purchased from Upstate Technology. The phosphorylated S6K was resolved in SDS-PAGE and visualized by autoradiography. In non-radioactive kinase assays, the results were determined in immunoblot with phosphor-S6K antibodies.
2.6. Immunoprecipitation (IP)
Immunoprecipitation was carried out using whole cell lysates (400 μg protein) with 2-4 μg of antibody, and 20 μl of protein A- or protein G-Sepharose beads (Amersham Biosciences). The cell lysate was prepared by sonication in the cell lysis buffer. IP was conducted by incubating the whole cell lysate with antibody for 3-4 h at 4°C. The immune complex was washed five times in cell lysis buffer before analysis by immunoblotting.
2.7. Serum TNF-α
Serum TNF-α was measured using a multiplex kit (Cat.# MADPK-71k-03, Linco Research, Inc. St. Charles, Missouri, USA).
2.8. Quantitative Real Time RT-PCR
The TaqMan RT-PCR reaction was used to quantify S6k (Rps6kb1) mRNA in the total RNA extract that was prepared with the Trizol reagent. Primers for Rps6kb1 (Mm00659517_m1) and Tnf-α (Mm00443258_m1) were obtained from Applied Biosystems (Foster City, CA). Mouse ribosome 18S rRNA_s1 (without intron-exon junction) was used as an internal control to normalize mRNA expression. Reaction was conducted with 7900 HT Fast real time PCR System (Applied Biosystems, Foster City, CA).
2.9. Statistical analysis
All experiments were repeated independently at least three times with consistent results. Student’s t-test or one-way ANOVA was used as appropriate in statistical analysis of the data. p < 0.05 was considered statistically significant.
3. Results
3.1. S6K protein level is decreased in p50-KO mice
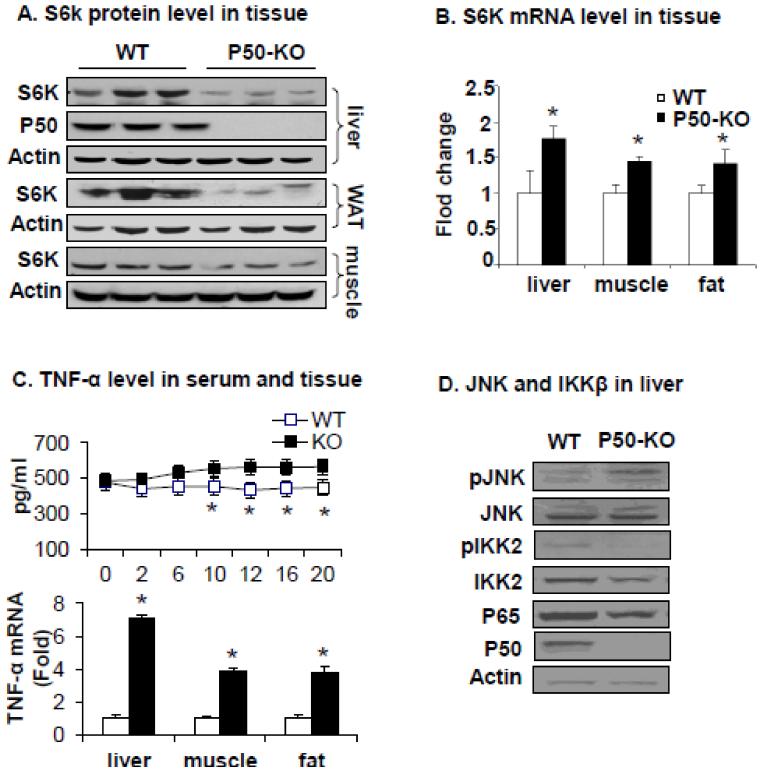
In our previous study, a reduction in S6K protein was observed in the liver of p50-KO mice, and the reduction contributed to the disassociation of inflammation and insulin resistance in p50-KO mice [10]. The study suggests that S6K protein is a key to understand the link between inflammation and insulin resistance. In this study, we explored the mechanism of S6K protein reduction. First, we examined S6K protein in all of insulin sensitive tissues including liver, adipose tissue, and skeletal muscle of p50-KO mice. S6K protein level was dramatically decreased in liver and fat, but modestly reduced in the muscle of p50-KO mice (Fig. 1A). Then, S6K mRNA was determined in these tissues to understand the mechanism of S6K reduction (Fig. 1B). The mRNA was increased in all three tissues, suggesting an increase in S6K mRNA expression.
Fig. 1.
S6K and TNF-α level in p50-KO mice. (A) S6K protein level in tissues. S6K protein was examined in the white adipose tissue (WAT), liver, and muscle of WT and p50-KO mice. (B) S6K mRNA level was determined by qRT-PCR in the liver, muscle and WAT. (C) TNF-α in p50-KO mice. TNF-α protein was determined in the serum by ELISA. TNF-α mRNA was determined in the liver, muscle and WAT with qRT-PCR. (D) JNK and IKKβ in liver. Liver tissues of p50-KO mice were examined for JNK, IKK2 and NF-kB p65 and p50. The experiments were conducted 3 times with consistent results and the representative blots are presented. In the bar figure, each data point represents mean ± SEM (n=6). * P<0.05, ** P<0.001.
The relationship of protein and mRNA suggests that the S6K protein reduction may occur at the post-translational level, not at the transcriptional level. This possibility led us to focus on S6K protein degradation. In search for factors that may contribute to S6K degradation, we focused on TNF-α that is able to induce S6K activation [7]. TNF-α was examined in the plasma and an elevation was found in p50-KO mice (Fig. 1C). Expression of TNF-α mRNA was elevated in all tissues examined (Fig. 1C). The data suggests that TNF-α elevation is associated with S6K reduction in p50-KO mice. TNF-α signal is mediated by JNK1 and IKK2. Phosphorylation of JNK1 and IKK2 was examined in the liver tissues in a Western blot. The data showed that JNK1 activity was enhanced as indicated by its phosphorylation status (Fig. 1D). IKK2 activity was reduced from the low phosphorylation status (Fig. 1D). In addition, proteins for IKK2 (IKKβ) and NF-κB p65 subunit were both reduced in p50-KO tissue (Fig. 1D), suggesting a defect in the IKK2/NF-kB signaling pathway. In wild type cells, JNK1 and IKK2 are both activated by TNF-α. The enhanced JNK1 activity and reduced IKK2 activity suggest that the two pathways were dis-balanced in the liver of p50-KO mice. NF-κB p50 subunit was absent in the tissue confirming the complete knockout of p50 activity in the mice (Fig. 1D).
3.2. Identification of S6K as a new target of JNK1
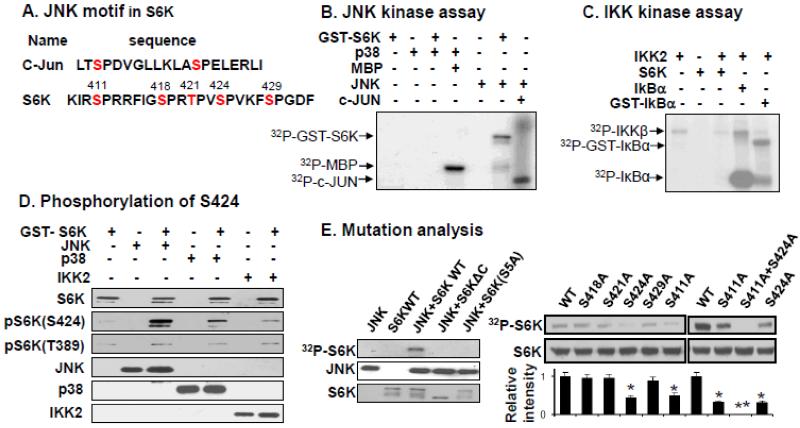
The data suggests that an increase in JNK1 activity is associated with the reduction in S6K protein in p50-KO mice. We searched the literature for their relationship and did not found any information about it. Protein degradation after phosphorylation is a common pathway for clearance of a signaling protein in the cytosol. This pathway may play a role in the S6K reduction. If this is the case, S6K is likely phosphorylated by JNK1. To test this possibility, we examined amino acid sequence of S6K protein for JNK1-sensitive domains. We found five S6K motifs (S411, S418, T421, S424, S429) as potential candidates of JNK targets (Fig. 2A). In the analysis, the authentic JNK1 target in c-JUN protein was used as a template (Fig. 2A). To test this possibility, we prepared a full-length recombinant S6K protein using the bacterial GST-S6K expression system for JNK1 kinase assay. S6K protein was phosphorylated by JNK1 in the assay as indicated by enhanced radiolabeling of S6K protein (Fig. 2B). The phosphorylation was not observed in the presence of p38. In the positive controls, JNK1 phosphorylated the classical substrate c-JUN, and p38 phosphorylated its standard substrate MBP (Fig. 2B). The relationship for IKK2 and S6K was determined too in the kinase assay. We found IKK2 phosphorylated the classical substrate IkBα, but not S6K (Fig.2C). The data suggests that JNK1, but not P38 and IKK2, directly phosphorylates S6K. The data establishes the kinase/substrate relationship for JNK1/S6K.
Fig. 2.
S6K phosphorylation by JNK1 in kinase assay. A. JNK1 target sequences in S6K. Amino acid sequence in c-JUN was used as a consensus sequence in the S6K analysis. Five candidates for JNK-sensitive residues are shown in red font in the c-terminal of S6K. B. JNK1 kinase assay. The kinase assay was conducted with purified JNK1 (kinase) and GST-S6K1 (substrate). The phosphorylation was tracked with radioactivity of 32P. C. IKK2 kinase assay. Purified IKK2 and S6K1 were used with 32P. IkBα was an authentic IKK2 substrate. D. Phosphorylation detected with antibody. S424 phosphorylation in S6K was investigated with phosphor-specific antibody to pS6K(S424) after the kinase assay. In the negative control, IKK2 and p38 were used in the kinase assay. The phosphor-specific antibody to pT389 was used in control. E. Mutation analysis of S6K. Mutant proteins were tested in the JNK1 kinase assay and relative strength of 32P signal was quantified (n=3). The experiments were repeated three times with consistent results and representative blots are presented in this figure. * P<0.05, ** P<0.001 over control.
The phosphor-serine residues in S6K protein were investigated with phosphor-specific antibodies and mutation analysis. S424 is one of five Ser/Thr-Pro sites in S6K, and an antibody to pS6K(S424) is commercially available. S6K phosphorylation was examined in a Western blot using the antibody. The result suggests that S424 was phosphorylated by JNK1 (Fig. 2C). In the test, kinases of IKK2 and p38 were included in the negative control. A weak pS6K(S424) signal was observed in the presence of IKK2 or p38 (Fig. 2C). However, the signal is much weaker relative to that induced by JNK1, suggesting a weak background noise of the phospho-antibody. In the negative control, phosphorylation of Thr389 (mTOR target) was examined and it was not changed by any of the kinases. The data suggest that JNK1 phosphorylates S424 in the S6K c-terminal.
To test JNK1 effect on other S/T-P sites of S6K, we used mutant S6K in the radioactive kinase assay. The mutation was made by c-terminal truncation and point mutation. In S6KΔC, the c-terminal was deleted from S6K protein. In S6KS5A, the five serine/threonine residues in the c-terminal were all replaced by alanine (A). Mutation in either way blocked S6K phosphorylation by JNK1 (Fig. 2E), suggesting that JNK1 targets are exclusively located at the c-terminal (Fig. 2E). To identify the exact target serine residues, we conducted extensive point mutation at the c-terminal by replacing each S/T residue with alanine individually. The mutants were tested in the kinase assay (Fig. 2E). Mutation of either S411 (S411A) or S424 (S424A) individually reduced, but did not abolish the JNK1-mediated phosphorylation (Fig. 2E). Mutation of the two residues together (S411A+S424A) completely blocked the phosphorylation (Fig. 2E). Mutation of other residues (S418, S421 and S429) did not reduce the phosphorylation. These data suggest that in the five residues of c-terminal, both S411 and S424 are JNK targets.
3.3. JNK1 is involved in TNF-α-mediated S6K activation
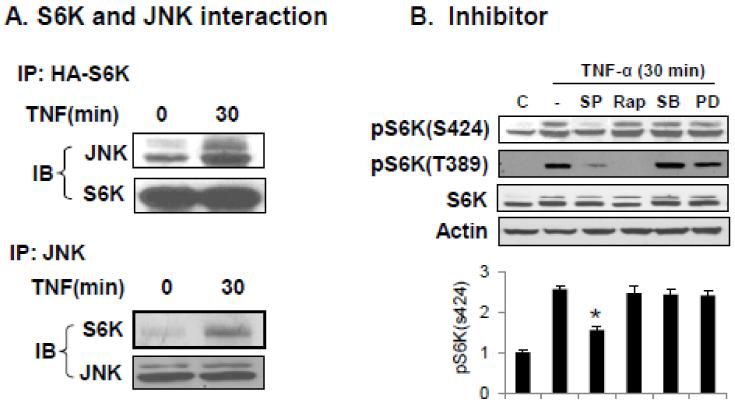
If JNK1 phosphorylates S6K in a physiological condition, the two proteins should interact with each other in cells when JNK1 is activated. To test this possibility, we performed immunoprecipitation (IP) using HA-tagged S6K protein (HA-S6K) that allows isolation of recombinant S6K with HA antibody. HA-S6K was expressed in HEK293 cells, and then purified in IP from the cells, which were treated with TNF-α to activate JNK1. In the IP product of S6K, a strong JNK1 signal was detected (Fig. 3A). In the IP product of JNK1, a strong S6K signal was found (Fig. 3A). The results suggest that S6K was associated with JNK1 in the cells. The status of S6K phosphorylation was examined in the same cell system. An increase in pS6K(S424) signal was observed in the cells after TNF-treatment for 30 minutes (Fig. 3B). The phosphorylation was inhibited by JNK inhibitor (SP), but not by mTOR inhibitor (Rap), p38 inhibitor (SB) or ERK inhibitor (PD) (Fig. 3B). T389 phosphorylation (pS6K389) was increased in the cells by TNF-α, and the signal was dramatically inhibited by JNK inhibitor (SP) and mTOR inhibitor (Rap) (Fig. 3B), suggesting that the JNK-mediated phosphorylation is likely required for Thr389 phosphorylation by mTOR. These data suggests that S424 phosphorylation may be required for S6K activation by TNF-α. S6K phosphorylation in response to mitogen was proposed to be required for subsequent phosphorylation by mTOR in the Avruch’s model [18]. These data suggest that JNK may prime S6K through phosphorylation of S424 and other S/T residues at the c-terminal.
Fig. 3.
S6K-JNK interaction in cells. (A) Association of S6K and JNK in HEK 293 cells. The cells were transfected with HA-S6K and treated with TNF-α. Immunoprecipitation was conducted with HA antibody or JNK antibody. The product was blotted for endogenous JNK or HA-S6K in an immunoblot. (B) Inhibition of S424 phosphorylation by JNK inhibitor. HEK 293 cells were pretreated with JNK inhibitor SP600125 (SP, 25μM), mTOR inhibitor Rapamycin (Rap, 200 nM), p38 inhibitor SB203580 (SB, 5 μM) or ERK inhibitor PD98059 (PD) and then examined for S6K phosphorylation after TNF-α treatment. Upper: pS6K (S424) and pS6K (T389) were examined in a Western blot. The representative blots are presented. Lower: quantification of S424 phosphorylation in three plots. The relative signal intensity at each lane was normalized with the S6K1 protein. Each data points represents mean ± SEM (n=3). The experiments were repeated three times with consistent results and representative blots are presented in this figure. * P<0.05 over control.
3.4. S6K degradation in NF-κB deficient cell lines
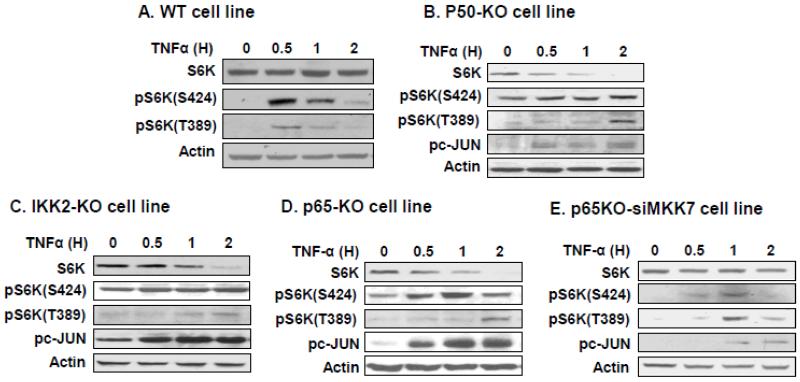
Above data suggests that NF-kB activity may be required for stabilization and activation of S6K. To test this possibility, we examined S6K protein in MEF cells with modified NF-kB activity. In wild type (WT) MEFs, the S6K reduction was not observed in response to TNF-α challenge although S6K (S424) phosphorylation was enhanced (Fig. 4A). In p50-KO MEFs, S6K was reduced at 30 min and S6K disappearance was observed at 2 h after the TNF-treatment (Fig. 4B). The reduction was associated with enhanced JNK1 activity as indicated by persistent c-JUN phosphorylation. To test the role of IKK2/NF-κB further, S6K was examined in MEFs, in which the pathway activity is decreased by knockout of IKK2 or p65 gene. In both cell lines, S6K was reduced by TNF-α treatment (Fig. 4, C and D). The reduction was companied by a strong S6K phosphorylation at S424 and strong c-JUN phosphorylation by JNK1. These results suggest that in the absence of IKK2/NF-κB activity, JNK1 is activated persistently to induce S6K over phosphorylation, which is suggested by the consistent pS6K(S424) signal in the presence of S6K protein reduction (Fig. 4, C and D). To test the possibility, we inhibited JNK1 activity in p65-KO MEFs by knocking down MKK7. MKK7 (also known as JNKK2) is required for TNF-induced JNK activation. The knockdown attenuated JNK1 activity as indicated by reduction in c-JUN phosphorylation (Fig. 4E). The JNK1 inhibition blocked S6K(S424) phosphorylation and S6K protein reduction. The data suggests that persistent JNK1 activation induces S6K degradation in the absence of IKK2/NF-κB activity.
Fig. 4.
TNF-α induce S6K degradation in KO cell lines. (A) S6K in wild type cells. Wild type cell lines were treated with TNF-α (20 ng/ml) for indicated time. S6K and its phosphorylation were determined in the whole cell lysates in an immunoblot for S6K, pS6K(S424), pS6K(T389), and pc-JUN. (B) S6K degradation in p50-KO MEF cells. (C) S6K degradation in IKK2 null cells. (D) S6K degradation in p65 null cells. (E) Inhibition of S6K degradation by MKK7 knockdown in p65-KO cells. MKK7 was knocked down using siRNA in p65-KO cells. The experiments were repeated three times with consistent results and representative blots are presented in this figure.
IKK2/NF-κB pathway may activate S6K protein by inducing mTOR activity. mTOR phosphorylates S6K at T389 to activate S6K. In IKK2/NF-κB deficient MEFs, mTOR activation by TNF-α is impaired. This conclusion is supported by decreased T389 phosphorylation in IKK2/NF-κB deficient MEFs. In WT MEFs, T389 phosphorylation was detected at 30 mins (Fig. 4A). In the IKK2/NF-κB deficient cells, the phosphorylation was not detected at 30 mins, but observed at 2 h after TNF-treatment (Fig. 4, B-D), suggesting that IKK2/NF-κB activity is required for mTOR activation by TNF-α in normal cells. These data suggests that IKK2/NF-κB pathway is required for TNF-α activation of S6K.
3.5. JNK1 activation leads to S6K S424 phosphorylation
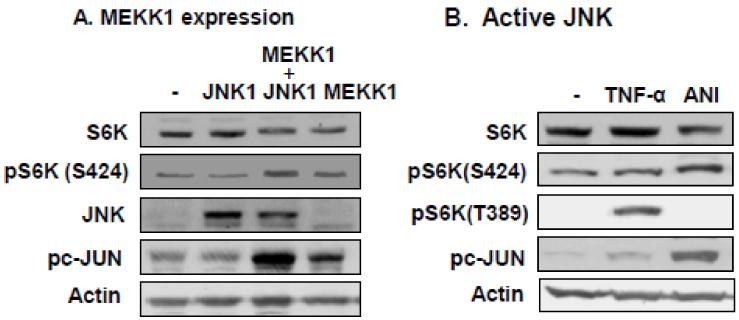
To determine the role of JNK1 in the control of S6K phosphorylation in cells, JNK1 was activated by MEKK1 over-expression or chemical activator (Anisomycin). In transient transfection, over-expression of wild type JNK1 did not enhance JNK1 activity, and had no effect on phosphorylation of the substrate protein c-JUN (Fig. 5A). In this condition, S424 phosphorylation was not changed in S6K. When JNK1 was activated by MEKK1 (a potent activator of JNK) in a cotransfection, the phosphorylation of both c-JUN and S6K was enhanced (Fig. 5A). Expression of MEKK1 alone increased the phosphorylation as well, but at much low activities. Activation of JNK1 by the chemical activator (Anisomycin) led to the same effect in S6K phosphorylation (Fig. 5B). In the control, S6K phosphorylation was induced by TNF-α in the same model, but S6K protein was not reduced dramatically (Fig. 5B). Activation of IKK2/NF-κB by TNF-α is likely responsible for the stabilization of S6K. These data suggest that JNK1 over activation from genetic or chemical modification is able to induce S6K phosphorylation. The data confirms that S424 phosphorylation does not induce a dramatic S6K degradation in WT cells.
Fig. 5.
The effects of JNK1 activation on S6K phosphorylation. A. S6K protein in cells with JNK1 activation. HEK293 cells were transfected with the expression vector for JNK1 alone or in cotransfection with MEKK1 expression vector. S6K protein was determined in the cell lysate together with signals of JNK, pc-JUN, and pS6K (S424). B. S6K phosphorylation in response to anisomycin. HEK293 cells were treated with TNF-α (20 ng/ml) or anisomycin (ANI) for 30 mins to activate JNK. Signals of S6K, pS6K(S424), pS6K(T389) and pc-JUN were determined in the whole cell lysates in an immunoblot. The experiments were repeated three times with consistent results and representative blots are presented in this figure.
3.6. S6K in JNK1 deficient cells
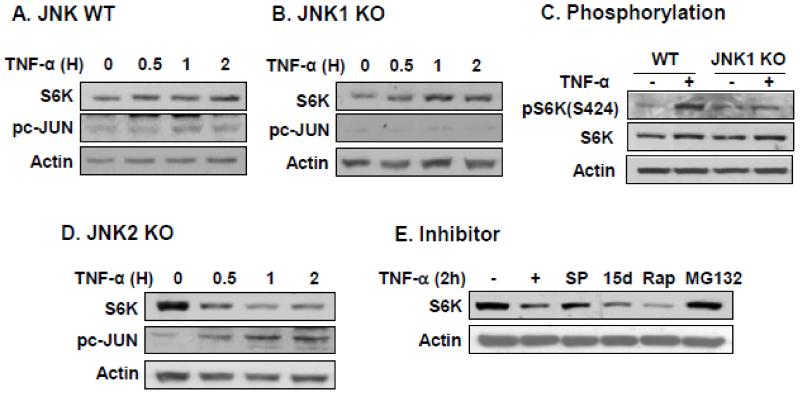
JNK1 and JNK2 are two closely related JNK isoforms, but have different functions. JNK1 is activated by stress responses. JNK2 negatively regulates JNK1 activation in certain conditions. In this study, we differentiated JNK1 and JNK2 activities in the regulation of S6K protein. The study was performed in JNK1−/− and JNK2−/− MEF cells. S6K protein was determined in the cells after TNF-α treatment. In WT MEFs, S6K protein was not reduced in response to TNF-α (Fig. 6A). In JNK1−/− cells, S6K was not reduced by the TNF-treatment, and instead, S6K protein was modestly elevated (Fig. 6B). The increased S6K was associated with a low signal for pS6K(S424) and c-JUN phosphorylation in JNK1−/− cells (Fig. 6, B and C), in which the phosphorylation was not observed at 2 h (Fig. 6, B and C). In contrast, S6K protein was reduced dramatically in JNK2−/− cells in response to TNF-α (Fig. 6D). The reduction was associated with a high JNK activity as shown by c-JUN phosphorylation. The S6K reduction was blocked by JNK inhibitor (SP) and proteasome inhibitor MG132 (Fig. 6E). The JNK inhibitor was not able to block the degradation completely in the test. This might be due to enhanced JNK1 activity in compensation for JNK2 inactivation. In contract, the reduction was enhanced when IKK or mTOR activities were inhibited by IKK inhibitor (15d) or mTOR inhibitor (Rap) (Fig. 6E). Given that IKK2 enhances mTOR activity, the data supports that mTOR may stabilize S6K protein. Taken together, the results suggest that JNK1 (not JNK2) activity is required for S6K degradation. S6K degradation is dependent on proteasome and S6K protein is stabilized by mTOR.
Fig. 6.
S6K was not degraded in JNK1 knockout MEF. The S6K degradation was examined in JNK1−/− and JNK2−/− embryo fibroblast cells. MEF cells were treated with TNF-α after serum-starvation overnight to induce the protein degradation. S6K protein level was determined in an immunoblot with S6K antibody. Phosphorylated c-JUN (pc-JUN) was a positive control for JNK activity. (A) S6K in wild type cells. (B) S6K in JNK1 null cells. (C) S6K phosphorylation in wild type and JNK1−/− cells. pS6K (S424) was determined in wild type and JNK1−/− cells after TNF-treatment for 2 hours. (D) S6K degradation in JNK2 KO cells. (E) Inhibition of S6K degradation by inhibiting JNK or proteasome. Before TNF-treatment, JNK2−/− cells were pretreated with JNK inhibitor SP600125 (SP, 25μM), IKK inhibitor 15dPGJ2 (5μM), mTOR inhibitor Rapamycin (Rap, 200 nM) or proteasomal inhibitor MG132. The experiments were repeated three times with consistent results and representative blots are presented in this figure.
3.7. Regulation of S6K stability by ubiquitination
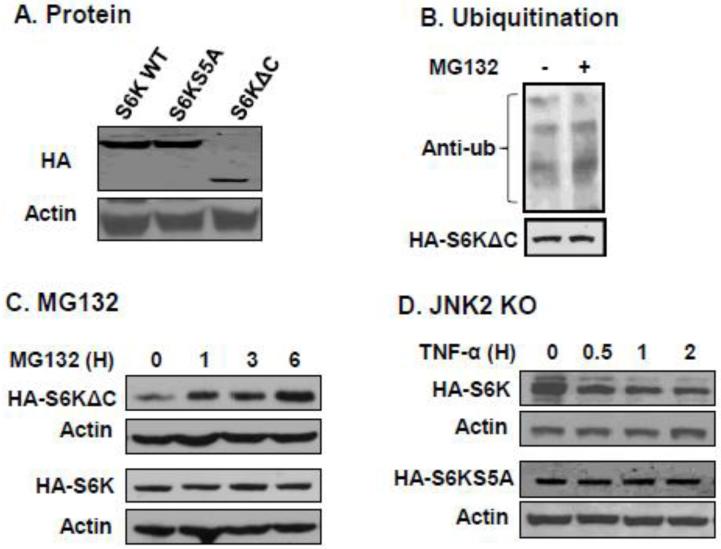
It is believed that the c-terminal keeps S6K from activation. The phosphorylation releases S6K from the c-terminal inhibition [2]. However, it is not known how the c-terminal controls S6K protein stability. To address this issue, we compared WT S6K and S6K mutants in protein stability. S6KΔC lacks 104 amino acids at the c-terminal. In S6KS5A, the five serine/threonine residues (S411, S418, T421, S424, and S429) were replaced by alanine. The WT and mutant S6K were HA-tagged, and their protein stability was determined in 293 cells after a transient transfection. After 48 h in transfection, WT and S6KS5A exhibited same protein abundance in the transfected cells (Fig. 7A). However, S6KΔC protein was dramatically decreased in the system (Fig. 7A).
Fig. 7.
Deletion of the c-terminal regulatory domains enhances the ubiquitination of S6K. (A) Expression levels of S6K WT, S6KS5A and S6KΔC were determined in HEK293 in 48 hours after transfection. (B) Ubiquitination of S6K. HA-S6KΔC was expressed in 293 cells in cotransfection with flag-tagged ubiquitin. MG132 (10 μM) was added at 48 h after transfection. HA-S6KΔC was collected by immunoprecipitation with HA antibody 6 h later and then detected for ubiquitination with flag antibody in an immunoblot. (C) Degradation of HA-S6KΔC in HEK293 cells. HEK293 cells were transiently transfected with HA-S6K and HA-S6KΔC. The cells were treated with vehicle (DMSO) or MG132 (10 μM) for different time as indicated. The protein amount was determined with the anti-HA antibody. (D) Blocking S6K degradation by mutation of c-terminal serine/threonine residues. HA-S6K and HA-S6KS5A were stably expressed in JNK2−/− cells. S6K degradation was induced TNF-α (20 ng/ml) for different time. The protein was quantified in the whole cell lysates with anti-HA antibody. The experiments were repeated three times with consistent results and representative blots are presented in this figure.
Protein degradation is often a result of protein modification by ubiquitination. If S6KΔC is degraded, it may be ubiquitinated. To test this possibility, we examined modification of S6KΔC protein by ubiquitination in this study. Flag-tagged ubiquitin was expressed with HA-S6KΔC in 293 cells in a cotransfection assay. Protein degradation was blocked using the proteasomal inhibitor MG132. The HA-S6KΔC protein was collected in IP with HA antibody. Ubiquitin was detected in the isolated HA-S6KΔC protein using the anti-flag antibody in an immunoblot (Fig. 7B). The ubiquitination was observed in the basal condition, and the signal was enhanced by MG132 treatment. The data suggest that without the c-terminal, S6K protein is subject to ubiquitination that promotes S6K degradation.
A time course study was conducted to examine S6KΔC degradation at 1, 3 and 6 h after addition of proteasomal inhibitor MG132 (Fig. 7C). The study was done in the absence of JNK1 activation to exclude the effect of phosphorylation. In the test, S6KΔC protein was increased gradually in a time-dependent manner. In the same condition, WT S6K protein did not exhibit the increase. The data suggests that loss of c-terminal makes S6K protein unstable, suggesting that a change in the c-terminal structure is important in the control of S6K protein stability.
In the full length protein, the c-terminal is regulated by phosphorylation status. If the phosphorylation is absent, the c-terminal will not have a structural change and this condition should keep S6K from activation. Our data suggests that the condition also prevents S6K from degradation. To test this effect of phosphorylation, we compared S6K and S6KS5A in protein stability in JNK2 null cells, which have an increased JNK1 activity. The two proteins were stably expressed in JNK2−/− MEFs, respectively, through stable transfection to avoid the influence of transfection efficiency. The protein abundance was examined in cells after TNF-α treatment to activate JNK1. A reduction was observed in WT S6K protein, but not in the mutant S6K (S6KS5A) (Fig. 7D). The data suggests that the c-terminal phosphorylation is required for the S6K protein degradation.
4. Discussion
S6K protein degradation is a new mechanism in the regulation of S6K activity. In the classical insulin signaling pathway, S6K is activated by mTOR at the downstream of PI3K/Akt. The activation involves in S6K phosphorylation at T389 by mTOR, which enhances protein synthesis through phosphorylation of ribosomal protein S6 by S6K. Recently, S6K has been reported to mediate nutrient signals in the regulation of insulin sensitivity [1, 2]. S6K is activated by branch chain amino acids through mTOR [24, 25], and by TNF-α through IKK2 (IKKβ) [7, 9]. In those responses, S6K is shown to phosphorylate IRS-1 in the inhibition of insulin signaling pathway [6, 7]. S6K activation is controlled by phosphorylation at two stages, one dependent on insulin by mTOR-mediated phosphorylation and one independent of insulin by MAP kinase. There is little information about S6K regulation by degradation [2]. In the current study, we identified JNK1 as a kinase in the insulin-independent phosphorylation of S6K. In addition, we found that the phosphorylation may lead to S6K protein degradation, which represents a new mechanism in the regulation of S6K activity. This mechanism provides an answer to the reduced S6K activity that was observed in our study of p50-KO mice [26].
We found that JNK1 is a kinase to phosphorylate S6K at S411 and S424 in the c-terminal autoinhibitory pseudosubstrate domain in cells. The proline-directed sites of S6K in the c-terminal contain five serine/threonine residues (S411, S418, T421, S424 and S429), which are phosphorylated in response to growth factors and mitogens. The phosphorylation primes S6K for subsequent phosphorylation by mTOR [27, 28]. The modification leads to a conformational change in S6K, which relieves the inhibitory activity of c-terminal domain [14, 29]. Mutation studies suggest that although the modification contributes to S6K1 activation in cells, the phosphorylation is not required for S6K activation in vitro. Mutation of these serine/threonine sites to alanine residues or deletion of 101 amino acids at the c-terminus only modestly reduces S6K1 activity in vitro. Substitution of the Ser/Thr residues with phosphor-mimetic residues (D3E) modestly increases S6K activity [23, 30, 31]. The phosphorylation occurs in response to mitogen-responsive kinases [27], the role of JNK was unclear in the S6K regulation [2]. Our point mutation data suggest that JNK1 phosphorylates S6K at S411 and S424 at the c-terminal in cells. Western blot data suggests that IKK2 and p38 may phosphorylate S6K protein (Fig. 2C). This possibility was tested in the kinase assay using radioactive 32P-labeled ATP. The result suggests that IKK2 does not phosphorylate S6K directly. No P38 activity was observed in the same condition (Fig. 2B). The data suggests that IKK2 and P38 do not directly phosphorylate S6K. The Western blot signal might be non-specific for IKK2 and P38.
Our data suggests that S6K integrates signals of JNK1 and IKK2 in the TNF-α signaling pathway. Serine kinases JNK1 and IKK2 are signaling molecules in the TNF-α pathway. In this study, we find that they regulate S6K protein stability and activation. S6K c-terminus was phosphorylated by JNK1. In an early study, we reported that TNF-α activated S6K through IKK2 [7], which induced mTOR activation by inhibition of TCS1 (tuberous sclerosis 1) [9]. In the current study, our data suggest that in the presence of IKK2 activity, JNK1-mediated phosphorylation promotes S6K activation through mTOR activation. In the absence of IKK2 activity, the phosphorylation leads to S6K degradation in cells. This conclusion is supported by S6K degradation in IKK2 null cells and S6K degradation after c-terminus deletion (S6KΔC). These data suggest that JNK1 and IKK2 have different effects in the regulation of S6K activity and stability. The mTOR-mediated phosphorylation acts to stabilize the S6K protein in response to IKK2 activation in the physiological condition.
S6K1 degradation is mediated by ubiquitination and proteasome. We observed that S6K was ubiquitinated in cells. This observation is consistent with those in other reports on S6K [20, 21]. Our data suggests that the modification may happen in the physiological conditions when JNK1 is over activated, which induces S6K over phosphorylation at the c-terminal. The ubiquitination is likely accelerated in the absence of the c-terminus as indicated by S6K mutant (S6KΔC) that lacks 104 amino acids at the C-terminal of S6K. S6KΔC exhibited a low protein level in cells from quick degradation. The protein was ubiquitinated in the basal condition. The ubiquitination was enhanced in cells in response to proteasome inhibitor MG132. These data suggest that ubiquitination leads to S6K degradation.
Our finding explains the S6K reduction in NF-κB p50-KO mice. In p50-KO mice, TNF-α is expressed at a high level from enhanced NF-kB activity in the absence of p50 [26]. The enhanced NF-κB may induce a negative feedback to reduce IKK2 activity [26]. Lack of IKK2 activity leads to persistent JNK1 activation in response to TNF-α. IKK2 is known to inhibit JNK1 activity in general. Current study suggests that in p50-KO hepatocytes, lack of IKK2 activity leads to S6K degradation due to JNK1 over activation. Without IKK2 activity, mTOR cannot be activated to protect S6K in cell treated with TNF-α. The data suggests that S6K reduction is a result of disbalance between IKK2 and JNK1 in p50-KO cells.
In summary, our study provides a new model for regulation of S6K activity. In the physiological condition, S6K activation is induced by TNF-α through activation of JNK1 and IKK2. JNK1 phosphorylates S6K at the c-terminal (S411 and S424) to induce a conformation change in the protein, which is independent of insulin activity. In the presence of IKK2 signal, the structural change promotes S6K1 activation by mTOR-mediated phosphorylation. In the presence of IKK2 inhibition, S6K is subject to ubiquitination and degradation after the JNK1-medaited modification. There are two effects by which IKK2 inhibits S6K degradation: (a) To prevent JNK1 over activation by inhibition of JNK1. (b) To induce mTOR activation that leads to activation and stabilization of S6K protein. In conclusion, JNK1 is a serine kinase that phosphorylates S6K1 at c-terminal that leads to S6K either degradation or activation dependent on mTOR activity.
Highlight.
JNK1 is a serine kinase to phosphorylate S6K in insulin independent manner.
JNK1 phosphorylates S6K at serine 424 in the c-terminal.
The phosphorylation may induce S6K protein degradation in the absence of mTOR activation.
The phosphorylation induces S6K activation when mTOR is activated.
The study explains why inflammation is disassociated with insulin resistance in p50-KO mice.
Acknowledgments
This work was supported by NIH grant (DK068036 and DK085495) to Ye J. qRT-PCR was conducted in the Genetic Core that was supported in part by NORC (NIH 2P30DK072476) center grants from the National Institutes of Health.
Footnotes
Conflict of interest:
We confirm that there are no conflicts of interest associated with this publication that could inappropriately influence its outcome.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author contribution
Jin Zhang conducted the experiment, collected data, and drafted the manuscript, Zhanguo Gao involved in experiment design and data interpretation, and Jianping Ye involved in study design and manuscript preparation.
REFERENCES
- [1].Um SH, D’Alessio D, Thomas G. Nutrient overload, insulin resistance, and ribosomal protein S6 kinase 1, S6K1. Cell Metab. 2006;3:393–402. doi: 10.1016/j.cmet.2006.05.003. [DOI] [PubMed] [Google Scholar]
- [2].Magnuson B, Ekim B, Fingar DC. Regulation and function of ribosomal protein S6 kinase (S6K) within mTOR signalling networks. Biochem J. 2012;441:1–21. doi: 10.1042/BJ20110892. [DOI] [PubMed] [Google Scholar]
- [3].Carlson CJ, White MF, Rondinone CM. Mammalian target of rapamycin regulates IRS-1 serine 307 phosphorylation. Biochem Biophys Res Commun. 2004;316:533–539. doi: 10.1016/j.bbrc.2004.02.082. [DOI] [PubMed] [Google Scholar]
- [4].Harrington LS, Findlay GM, Gray A, Tolkacheva T, Wigfield S, Rebholz H, Barnett J, Leslie NR, Cheng S, Shepherd PR, Gout I, Downes CP, Lamb RF. The TSC1-2 tumor suppressor controls insulin-PI3K signaling via regulation of IRS proteins. J Cell Biol. 2004;166:213–223. doi: 10.1083/jcb.200403069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Ozes ON, Akca H, Mayo LD, Gustin JA, Maehama T, Dixon JE, Donner DB. A phosphatidylinositol 3-kinase/Akt/mTOR pathway mediates and PTEN antagonizes tumor necrosis factor inhibition of insulin signaling through insulin receptor substrate-1. Proc Natl Acad Sci U S A. 2001;98:4640–4645. doi: 10.1073/pnas.051042298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Tremblay F, Brule S, Hee Um S, Li Y, Masuda K, Roden M, Sun XJ, Krebs M, Polakiewicz RD, Thomas G, Marette A. Identification of IRS-1 Ser-1101 as a target of S6K1 in nutrient- and obesity-induced insulin resistance. PNAS. 2007;104:14056–14061. doi: 10.1073/pnas.0706517104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Zhang J, Gao Z, Yin J, Quon MJ, Ye J. S6K Directly Phosphorylates IRS-1 on Ser-270 to Promote Insulin Resistance in Response to TNF-α Signaling Through IKK2. J Biol Chem. 2008;283:35375–35382. doi: 10.1074/jbc.M806480200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Um SH, Frigerio F, Watanabe M, Picard F, Joaquin M, Sticker M, Fumagalli S, Allegrini PR, Kozma SC, Auwerx J, Thomas G. Absence of S6K1 protects against age- and diet-induced obesity while enhancing insulin sensitivity. Nature. 2004;431:200–205. doi: 10.1038/nature02866. [DOI] [PubMed] [Google Scholar]
- [9].Lee D-F, Kuo H-P, Chen C-T, Hsu J-M, Chou C-K, Wei Y, Sun H-L, Li L-Y, Ping B, Huang W-C, He X, Hung J-Y, Lai C-C, Ding Q, Su J-L, Yang J-Y, Sahin AA, Hortobagyi GN, Tsai F-J, Tsai C-H, Hung M-C. IKK[beta] Suppression of TSC1 Links Inflammation and Tumor Angiogenesis via the mTOR Pathway. Cell. 2007;130:440–455. doi: 10.1016/j.cell.2007.05.058. [DOI] [PubMed] [Google Scholar]
- [10].Gao Z, Yin J, Zhang J, He Q, McGuinness OP, Ye J. Inactivation of NF-kappaB p50 leads to insulin sensitization in liver through post-translational inhibition of p70S6K. J Biol Chem. 2009;284:18368–18376. doi: 10.1074/jbc.M109.007260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Dennis PB, Pullen N, Pearson RB, Kozma SC, Thomas G. Phosphorylation sites in the autoinhibitory domain participate in p70(s6k) activation loop phosphorylation. J Biol Chem. 1998;273:14845–14852. doi: 10.1074/jbc.273.24.14845. [DOI] [PubMed] [Google Scholar]
- [12].Frodin M, Antal TL, Dummler BA, Jensen CJ, Deak M, Gammeltoft S, Biondi RM. A phosphoserine/threonine-binding pocket in AGC kinases and PDK1 mediates activation by hydrophobic motif phosphorylation. EMBO J. 2002;21:5396–5407. doi: 10.1093/emboj/cdf551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Pullen N, Dennis PB, Andjelkovic M, Dufner A, Kozma SC, Hemmings BA, Thomas G. Phosphorylation and activation of p70s6k by PDK1. Science. 1998;279:707–710. doi: 10.1126/science.279.5351.707. [DOI] [PubMed] [Google Scholar]
- [14].Price DJ, Mukhopadhyay NK, Avruch J. Insulin-activated protein kinases phosphorylate a pseudosubstrate synthetic peptide inhibitor of the p70 S6 kinase. J Biol Chem. 1991;266:16281–16284. [PubMed] [Google Scholar]
- [15].Lehman JA, Calvo V, Gomez-Cambronero J. Mechanism of ribosomal p70S6 kinase activation by granulocyte macrophage colony-stimulating factor in neutrophils: cooperation of a MEK-related, THR421/SER424 kinase and a rapamycin-sensitive, m-TOR-related THR389 kinase. J Biol Chem. 2003;278:28130–28138. doi: 10.1074/jbc.M300376200. [DOI] [PubMed] [Google Scholar]
- [16].Papst PJ, Sugiyama H, Nagasawa M, Lucas JJ, Maller JL, Terada N. Cdc2-cyclin B phosphorylates p70 S6 kinase on Ser411 at mitosis. J Biol Chem. 1998;273:15077–15084. doi: 10.1074/jbc.273.24.15077. [DOI] [PubMed] [Google Scholar]
- [17].Isotani S, Hara K, Tokunaga C, Inoue H, Avruch J, Yonezawa K. Immunopurified mammalian target of rapamycin phosphorylates and activates p70 S6 kinase alpha in vitro. J Biol Chem. 1999;274:34493–34498. doi: 10.1074/jbc.274.48.34493. [DOI] [PubMed] [Google Scholar]
- [18].Alessi DR, Kozlowski MT, Weng QP, Morrice N, Avruch J. 3-Phosphoinositide-dependent protein kinase 1 (PDK1) phosphorylates and activates the p70 S6 kinase in vivo and in vitro. Curr Biol. 1998;8:69–81. doi: 10.1016/s0960-9822(98)70037-5. [DOI] [PubMed] [Google Scholar]
- [19].Thomas G. The S6 kinase signaling pathway in the control of development and growth. Biol Res. 2002;35:305–313. doi: 10.4067/s0716-97602002000200022. [DOI] [PubMed] [Google Scholar]
- [20].Gwalter J, Wang ML, Gout I. The ubiquitination of ribosomal S6 kinases is independent from the mitogen-induced phosphorylation/activation of the kinase. Int J Biochem Cell Biol. 2009;41:828–833. doi: 10.1016/j.biocel.2008.08.018. [DOI] [PubMed] [Google Scholar]
- [21].Wang ML, Panasyuk G, Gwalter J, Nemazanyy I, Fenton T, Filonenko V, Gout I. Regulation of ribosomal protein S6 kinases by ubiquitination. Biochem Biophys Res Commun. 2008;369:382–387. doi: 10.1016/j.bbrc.2008.02.032. [DOI] [PubMed] [Google Scholar]
- [22].Gao Z, Hwang D, Bataille F, Lefevre M, York D, Quon MJ, Ye J. Serine phosphorylation of insulin receptor substrate 1 by inhibitor KappaB kinase complex. J Biol Chem. 2002;277:48115–48121. doi: 10.1074/jbc.M209459200. [DOI] [PubMed] [Google Scholar]
- [23].Schalm SS, Blenis J. Identification of a conserved motif required for mTOR signaling. Curr Biol. 2002;12:632–639. doi: 10.1016/s0960-9822(02)00762-5. [DOI] [PubMed] [Google Scholar]
- [24].Xu G, Marshall CA, Lin TA, Kwon G, Munivenkatappa RB, Hill JR, Lawrence JC, Jr., McDaniel ML. Insulin mediates glucose-stimulated phosphorylation of PHAS-I by pancreatic beta cells. An insulin-receptor mechanism for autoregulation of protein synthesis by translation. J Biol Chem. 1998;273:4485–4491. doi: 10.1074/jbc.273.8.4485. [DOI] [PubMed] [Google Scholar]
- [25].Iiboshi Y, Papst PJ, Kawasome H, Hosoi H, Abraham RT, Houghton PJ, Terada N. Amino acid-dependent control of p70(s6k). Involvement of tRNA aminoacylation in the regulation. J Biol Chem. 1999;274:1092–1099. doi: 10.1074/jbc.274.2.1092. [DOI] [PubMed] [Google Scholar]
- [26].Gao Z, Yin J, He Q, McGuinness OP, Ye J. Inactivation of NF-κB p50 Leads to Insulin Sensitization in Liver through Post-translational Inhibition of p70S6K. J Biol Chem. 2009;284:18368–18376. doi: 10.1074/jbc.M109.007260. Z. J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Mukhopadhyay NK, Price DJ, Kyriakis JM, Pelech S, Sanghera J, Avruch J. An array of insulin-activated, proline-directed serine/threonine protein kinases phosphorylate the p70 S6 kinase. J Biol Chem. 1992;267:3325–3335. [PubMed] [Google Scholar]
- [28].Ferrari S, Bannwarth W, Morley SJ, Totty NF, Thomas G. Activation of p70s6k is associated with phosphorylation of four clustered sites displaying Ser/Thr-Pro motifs. Proc Natl Acad Sci U S A. 1992;89:7282–7286. doi: 10.1073/pnas.89.15.7282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Banerjee P, Ahmad MF, Grove JR, Kozlosky C, Price DJ, Avruch J. Molecular structure of a major insulin/mitogen-activated 70-kDa S6 protein kinase. Proc Natl Acad Sci U S A. 1990;87:8550–8554. doi: 10.1073/pnas.87.21.8550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Han JW, Pearson RB, Dennis PB, Thomas G. Rapamycin, wortmannin, and the methylxanthine SQ20006 inactivate p70s6k by inducing dephosphorylation of the same subset of sites. J Biol Chem. 1995;270:21396–21403. doi: 10.1074/jbc.270.36.21396. [DOI] [PubMed] [Google Scholar]
- [31].Ferrari S, Pearson RB, Siegmann M, Kozma SC, Thomas G. The immunosuppressant rapamycin induces inactivation of p70s6k through dephosphorylation of a novel set of sites. J Biol Chem. 1993;268:16091–16094. [PubMed] [Google Scholar]