Abstract
Objective
To describe a new computer-controlled research apparatus for measuring in vivo uterine ligament force-displacement behavior and stiffness and to present pilot data in women with and without prolapse.
Study Design
Seventeen women with varying uterine support underwent testing in the operating room (OR) after anesthetic induction. A tripod-mounted computer-controlled linear servoactuator was used to quantify force-displacement behavior of the cervix and supporting ligaments. The servoactuator applied a caudally-directed force to a tenaculum at 4 mm/s velocity until the traction force reached 17.8N (4 lbs.). Cervix location on POP-Q in clinic, in the OR at rest, and with minimal force (<1.1N), and maximum force (17.8N) was recorded. Ligament “stiffness” between minimum and maximum force was calculated.
Results
The mean (SD) subject age was 54.5 (12.7) years, parity 2.9 (1.1), BMI 29.0 (4.3) kg/m2, and POP-Q point C −3.1 (3.9) cm. POP-Q point C was most strongly correlated with cervix location at maximum force (r=+0.68, p=.003) and at rest (r=+0.62, p=.009). Associations between cervix location at minimum force (r=+0.46, p=.059) and ligament stiffness (r= −0.44,p=.079) were not statistically significant. Cervix location in the OR with minimal traction lay below the lowest point found on POP-Q for 13 women.
Conclusions
POP-Q point C was strongly correlated with cervix location at rest and at maximum traction force; however only 19% of the variation in POP-Q point C location was explained by ligament stiffness. The cervix location in the OR at minimal traction lay below POP-Q point C value in ¾ of women.
Keywords: Pelvic organ prolapse, Uterine ligament stiffness
INTRODUCTION
Pelvic organ prolapse is caused by a complex disease process. Among the many pelvic floor structural elements involved, recent data highlight two major contributing factors: 1) the loss of apical supports of the uterus by the cardinal / uterosacral ligament complex1,2 and 2) birth-related levator ani muscle injury.3,4 While there is information about ex vivo ligament properties including cellular and molecular changes in the connective tissue,5,6,7–9 there is a striking lack of data concerning the in vivo ligament properties and prolapse. Specifically, it is not clear whether abnormal ligaments cause apical descent or if apical descent in some women is the effect of abnormal forces placed on normal ligaments by pressure imbalances due to levator ani muscle damage. The lack of a scientific strategy to measure the in vivo biomechanical aspects of apical support and a structural paradigm based on observed data limits progress in this field. This is especially important because findings in clinic during POP-Q exam and data measured in the operating room (OR) are frequently at variance with one-another yet used in clinical decision making.10
To study this, we developed a system to measure in vivo biomechanical properties of the ligament complex. Our objective is to 1) describe a technique for measurement and display of apical support properties for the cardinal uterosacral ligament complexes, and 2) examine early findings in a pilot sample of women with varying degrees of apical support. In addition, we considered the relationship between uterine location seen during POP-Q examination and these observations.
METHODS
Seventeen women, representing a full spectrum of uterine support as defined by POP-Q point C, from normal to prolapse, were recruited and consented preoperatively to participate in this University of Michigan IRB approved study. To obtain this convenience sample for pilot testing, the study team identified potential participants by examining the upcoming Gynecology surgery schedule. Inclusion criteria included age greater than 18 years and the plan for an operation during which a tenaculum would be placed. Women were excluded if they were pregnant, had uterine fibroids greater than twelve weeks in size, had known history of pelvic inflammatory disease, used steroids chronically, had prior pelvic radiation, were undergoing treatment for a malignancy, or had any other factor for which extra time under anesthesia may place them at increased risk of adverse effects.
The study procedure involved the repetition of one step of the routine surgical procedure (tenaculum placement on the cervix and downward uterine traction) for the purpose of recording the research data. Concomitant operations performed included robotic-assisted supracervical hysterectomies and cervico-sacrocolpopexies, vaginal hysterectomies with and without sacrospinous and uterosacral ligament suspensions, hysteroscopies and mid-urethral slings. Subjects were compensated for their participation.
We developed a tripod mounted computer-controlled linear servoactuator (Model # FAPO-150-12-8”, Firgelli Automation, Inc., Vancouver, Canada) to quantify the force-displacement behavior of the uterine cervix (Figure 1). The servoactuator was a computer controlled motor that allows displacement to be controlled by information provided by the controlling computer. A Transducer Techniques™ load cell (Temecula, CA, USA, Model # TLL-500, capacity 500 lbs., nonlinearity 0.25% of Rated Output) was connected to the end of the servoactuator arm and used to measure the traction force. Location of the cervix before and after tenaculum placement was measured by the study team using a ruler with millimeter markings of the lateral cervix in relation to the hymeneal ring. The lateral margin of the cervix was chosen rather than the anterior lip as is often used during POP-Q examination because the anterior lip may stretch under traction and the lateral margin that is closest to the ligament attachment is less affected by this phenomenon. Displacement during traction was tracked to the nearest 0.1 mm by the servoactuator device. During the first nine trials, the accuracy of these measures was confirmed by videotaping the traction session with a ruler in view.
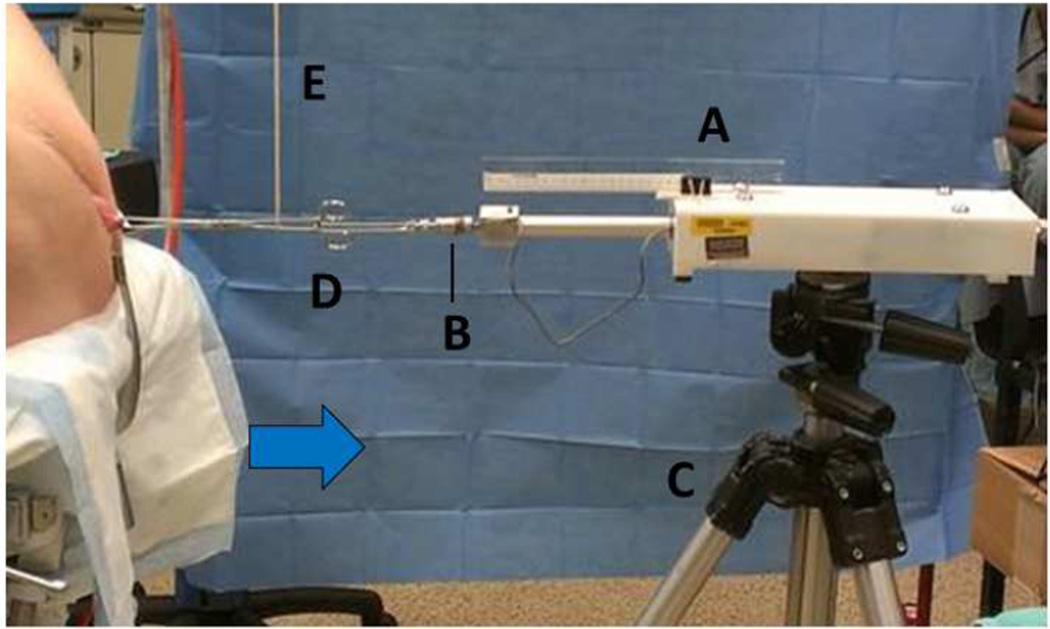
Figure 1. Research apparatus set-up.
A computer-controlled servoactuator (A) with force transducer (B) mounted on a tripod (C) quantifying force-displacement behavior of the uterine cervix while applying caudally-directed tensile force to the handle of a tenaculum (D) supported by a vertical support (E) and attached to the cervix. Blue arrow indicated force vector.
Once the patient was placed in the lithotomy position following anesthesia induction, a short-bladed Sherback posterior-weighted vaginal speculum was placed in the vagina to open the genital hiatus while not interfering with descent of the posterior vaginal fornix (Figure 1). This retracted the levator ani muscles and reducing pelvic floor support interference. A single-tooth tenaculum was placed deep into the cervical stroma incorporating both the anterior and posterior cervical lips, to help prevent soft tissue distortion. The handle of the tenaculum was attached to the load cell and servoactuator by a short bead chain. Prior to any force being applied, the weight of the tenaculum was supported by a long vertically placed string, suspended above from an OR light (Figure 1). Anesthetic data including respiratory rate during testing, use of a paralytic and use of a spinal anesthetic were recorded.
The linear servoactuator was designed to quantify the force-displacement behavior of the uterine cervix and thus calculate the biomechanical properties, including stiffness, of the uterosacral and cardinal ligaments. A typical “ramp and hold” testing technique was used whereby the tenaculum handle was pulled at a constant 4 mm/s velocity in a caudal direction until the traction force reached 17.8 N (4 lbs.) at the end of the “ramp” phase of the test. It was then kept at constant force for 60 seconds (the “hold” phase of the test) to measure how much the ligament tension decreases over time to allow calculation of viscoelastic behavior (these engineering calculations are not reported in this clinical manuscript). A maximal traction force of 17.8 N was chosen based on the only prior study of force displacement that is in the literature.11 Foon et al. evaluated the maximal traction applied by gynecologists during hysterectomy and found that a greater force of 8 lbs (35.6 N) was used.12 Because not all of our patients were undergoing hysterectomy, and because clinically it was felt that 8 lbs might cause cervical lacerations, we chose the more conservative force. In a subset of our subjects, we compared a maximal traction force of 4 versus 6 lbs and found no significant difference in cervix displacement results (data not shown). Because 4 lbs seems well above the physiologic range for women during their normal activities, we chose to use 4 lbs as this force allowed us to demonstrate our study findings while keeping patient safety in mind.
During testing, we collected data on cervix location for each subject under the following conditions (Figure 2): 1) point C on POP-Q exam in the clinic, 2) at rest in the OR in lithotomy position with a posterior speculum in place and tenaculum on the cervix but no force applied, 3) with minimal force (1.11 N) in the OR, 4) and with maximum force (17.9 N) in the OR. From this, the ligament “stiffness” (i.e., Δ traction force / Δ cervical displacement between minimal and maximum force) was calculated. After testing was complete, the location of the cervix was also measured while the operating surgeon was asked to pull on the tenaculum with the maximal force he would customarily use during a vaginal hysterectomy (“clinical pull”). This “clinical pull” was purposely not standardized as it was intended to provide additional information about the relationship between our standardized study displacement measurements and the typical amount of force used by clinicians to make surgical decisions. Demographic data including age, parity and BMI were recorded and descriptive statistics performed. All data were checked for the assumption of normality and appropriate bivariate correlation used including Pearson Correlation for normally distributed variables and Spearman Correlation for others. Data analyses were performed using SPSS 19 statistical software. Statistical significance for all analysis was defined at the 5% significance level.
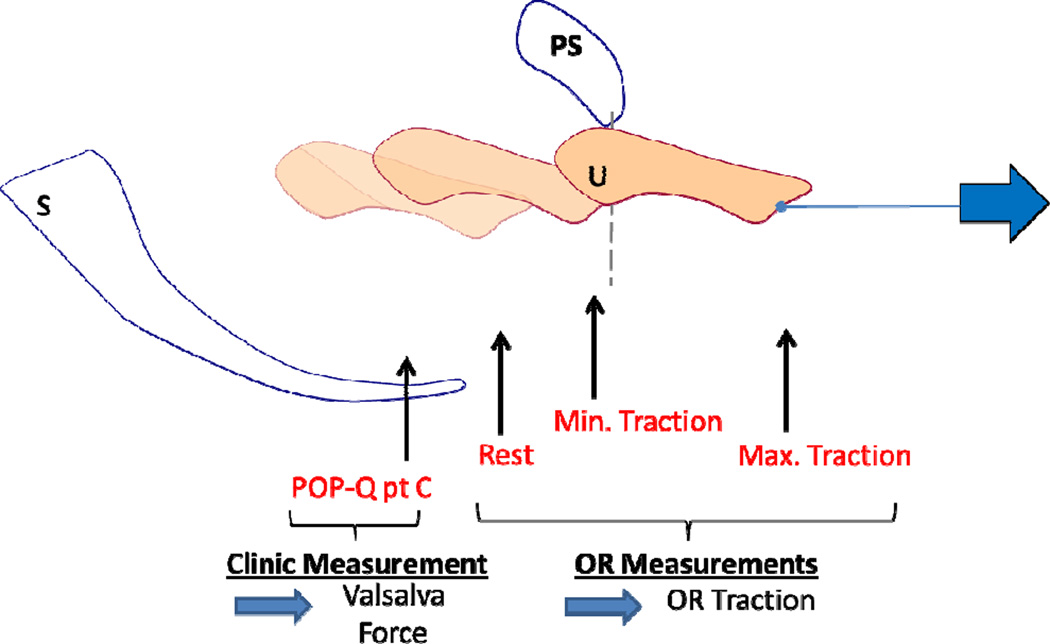
Figure 2. Cervix location measurements.
A schematic drawing of the measurements obtained during testing showing the uterus in four different locations in one subject (uterus (U), pubic symphysis (PS) and sacrum (S)). “POP-Q point C” indicates the location of the cervix measured in clinic at maximal Valsalva. Measurements “Rest”, “Min. Traction”, and “Max. Traction” indicate the cervix location at 0 force, minimal force (1.11N) and maximal force (17.8N) respectively. The latter measurements were taken in the operating room using the servoactuator.
RESULTS
Seventeen women with mean (SD) age of 54.5 (12.7) years, parity of 2.9 (1.1) and BMI 29.0 (4.3) kg/m2 were recruited to include a full range of uterine support from normal support to significant uterine prolapse based on their in office POP-Q point C value made by a Urogynecology faculty or fellow. Point C ranged from −10 cm to +7 cm with a mean of −3.1 (3.9) cm (Table 1). All received a general anesthetic. Five of the women did not receive a muscle paralytic agent and none received spinal/epidural anesthesia.
Table 1.
Subject characteristics.
| Demographic | Mean | SD | Range |
|---|---|---|---|
| Age (yrs) | 54.5 | 12.7 | 36 to 74 |
| Parity | 2.9 | 1.1 | 1 to 6 |
| BMI (kg/m2) | 29.0 | 4.3 | 22.1 to 36.1 |
| POP-Q Ba | 1.35 | 2.3 | −2 to 6 |
| POP-Q C | −3.1 | 3.9 | −10 to +7 |
| POP-Q Bp | −1.6 | 1.2 | −3 to 1 |
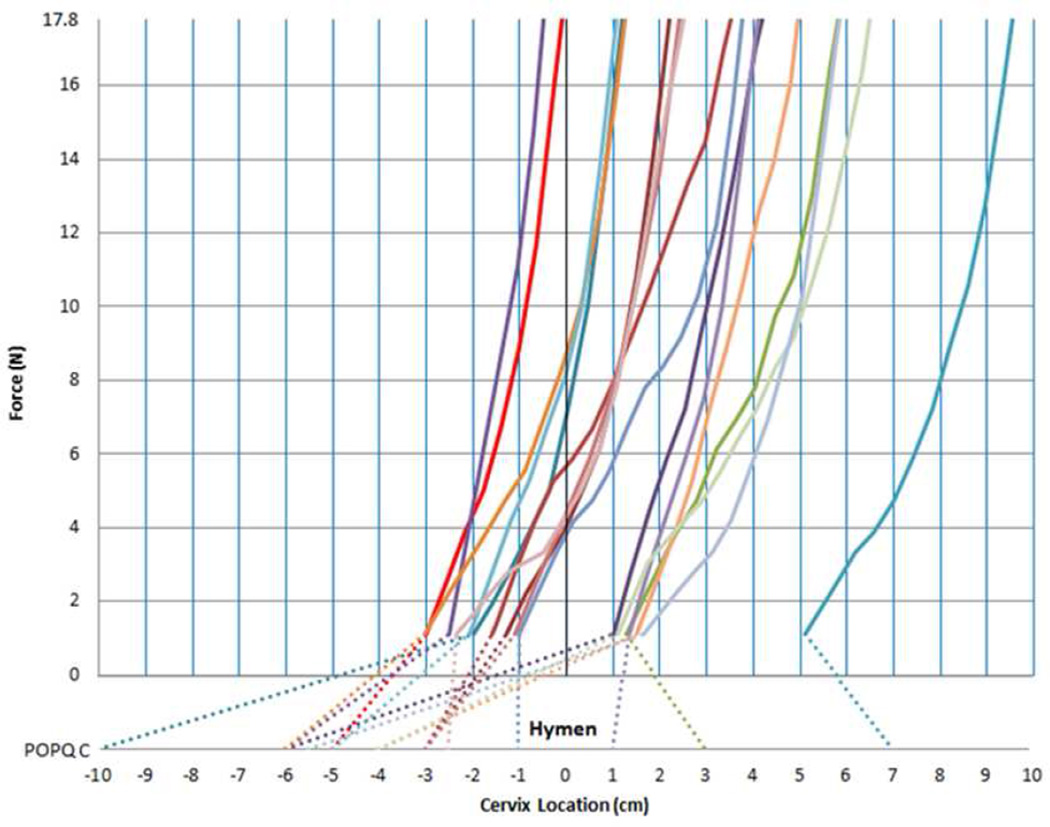
The POP-Q point C location determined during pre-operative POP-Q examination in clinic is displayed in the lower portion of Figure 3. In the upper portion of the graph the traction portion of each subject’s force-displacement curve is shown. Under minimal force (1.1 N) the cervix location varies greatly. Under the maximum force (17.9 N) the location relative to the hymenal ring (upper graph) depends on both the starting position at minimum force as well as the stiffness, represented in Figure 3 as the slope of the force – displacement curve. The latter demonstrates a hyperelastic characteristic wherein the suspensory ligament stiffness increases with increasing cervical displacement. Note the similar shape of each subject’s curve.
Figure 3. Force-displacement graph.
Force-displacement graph demonstrating hyperelastic ramp curves (solid lines) for the 17 individual subjects. X-axis showing Cervix Location in mm based around the Hymen. Y-axis showing Force in N. Dotted line indicating POP-Q point C for each subject.
The cervix location in the OR (solid line) even with minimal force is lower than that seen in the clinic with maximal Valsalva and the prolapse protruding to its maximal clinical extent in 13 individuals (cervix location (cm) at minimal force versus POPQ-point C mean (SD) [range]= −0.42 (2.2) [−3.0 to 5.1] vs. −3.12 (3.9) [−10 to 7]). In two individuals there is a higher position seen in the OR and a similar location in another two subjects. The former is explained by the significant elongation of the anterior cervical lip in these women with large prolapses that led to a much lower measurement of the anterior lip in the POP-Q and higher measure of the lateral cervix. Average cervix locations at various forces are presented in Table 2.
Table 2.
Average cervix locations at various forces.
| Subjects | Mean cervix location for POP-Q pt C (cm) |
Mean cervix location at rest in OR (cm) |
Mean cervix location under minimal force (cm) |
Mean cervix location under maximum force (cm) |
Mean “Stiffness” (N/cm) |
|---|---|---|---|---|---|
| N=17* | −3.1 (3.9) (−10 to 7) |
−1.9 (2.2) (−5.0 to 3.5) |
−0.42 (2.2) (−3.0 to 5.1) |
3.4 (2.6) (−5.0 to 9.5) |
4.9 (1.4) (3.1 to 8.9) |
Data presented are mean (standard deviation) and range.
The correlation of the different parameters is shown in Table 3. POP-Q point C in clinic was most strongly positively correlated with cervix location at maximum force (r=+0.68, r2=0.46, p=.003) and cervix location at rest (r=+0.62, r2=0.38 p=.009). Associations between cervix location at minimum force (r=+0.46, r2=0.21, p=.059) and ligament stiffness (r= −0.44, r2=0.19, p=.079) were not statistically significant in this pilot sample. In other words, only 19% of the variation in POP-Q point C location was explained by ligament stiffness and this was not statistically significant.
Table 3.
Correlation between POPQ point C and dependent variables of cervix location at 0 force (Rest), cervix location at minimal force (Min), cervix location at maximal force (Max), ligament stiffness between minimal and maximal force locations (Stiffness), and the clinical pull of the cervix (Clinical).
| POPQ C | Rest | Min | Max | Stiffness | Clinical |
|---|---|---|---|---|---|
| R | .615 | .459 | .678 | −.438 | .703 |
| R2 | .378 | .211 | .460 | .191 | .494 |
| P value | .009 | .059 | .003 | .079 | .003 |
Cervix location with “clinical pull” was most highly correlated with POP-Q point C (r=0.703, r2=.494, p=0.003) but overall only predicted 49% of the clinic location. When comparing the cervix locations between the “clinical pull” and the maximum force during testing, the later was 6 mm lower [mean (SD) maximum= 3.4 (2.6) cm vs. clinical pull= 2.9 (2.2) cm; p< 0.001]. There were no statistically significant differences among cervix location values in patients who did and did not receive muscle paralytics in this pilot sample (data not shown).
COMMENT
This study reports a measurement technique to quantify and display in vivo biomechanical properties of uterine suspensory support and relate them to clinical findings. It integrates findings of several different aspects of pelvic organ support and quantifies the in vivo stiffness of the ligaments. Importantly, it relates these assessments to clinical evaluation obtained during POP-Q examination. Our aim was to describe and test the feasibility of such a technique so that future studies testing clinical hypotheses about uterine support can be carried out. Furthermore, we present early findings in women with varying support which can be used for power calculations in planning research using this technique. The goal of this line of investigation is to eventually test hypotheses such as the following: Uterine position, as measured by POP-Q point C location at maximal Valsalva, is primarily determined by suspensory ligament stiffness.
In this pilot convenience sample, we found that POP-Q point C was positively correlated with cervix location at maximum force and cervix location at rest in the OR. This parameter, however, explained about half of the variation in POP-Q point C. Ligament stiffness explained only 19% of the POP-Q point C location. This did not support our proposed hypothesis that uterine position, as measured by POP-Q point C location at maximal Valsalva, was primarily determined by ligament stiffness.
Perhaps the most striking observation from these pilot data is that the location of the uterine cervix in the operating room, even with the minimal force (1/4 lbs), was well below the lowest point found on the POP-Q examination during maximal Valsalva for three-quarters of the women. This difference would be even greater considering the fact that we measured to a higher point (lateral cervix) in the OR than in the clinic. Similar findings have been reported by others,10 however standardized forces and displacement rates were not used at the time of measurement in the operating room. Anesthesia may play some role but anesthetic differences published in the past have been small.11 This is a clinically relevant question as the location of the cervix with traction in the operating room is often used over clinic POPQ data to determine whether a hysterectomy and apical suspension is surgically indicated. It remains unclear due to a paucity of long-term outcomes data following prolapse repair with and without concomitant hysterectomy and/or apical suspension, as to the preoperative evaluation method that is most appropriate.
These observations raise two important clinical questions. First, to what degree are the ligaments stressed during Valsalva? Second, what is the significance of support measurements made in the operating room? To answer these questions a basic structural paradigm is needed to help guide discussion.
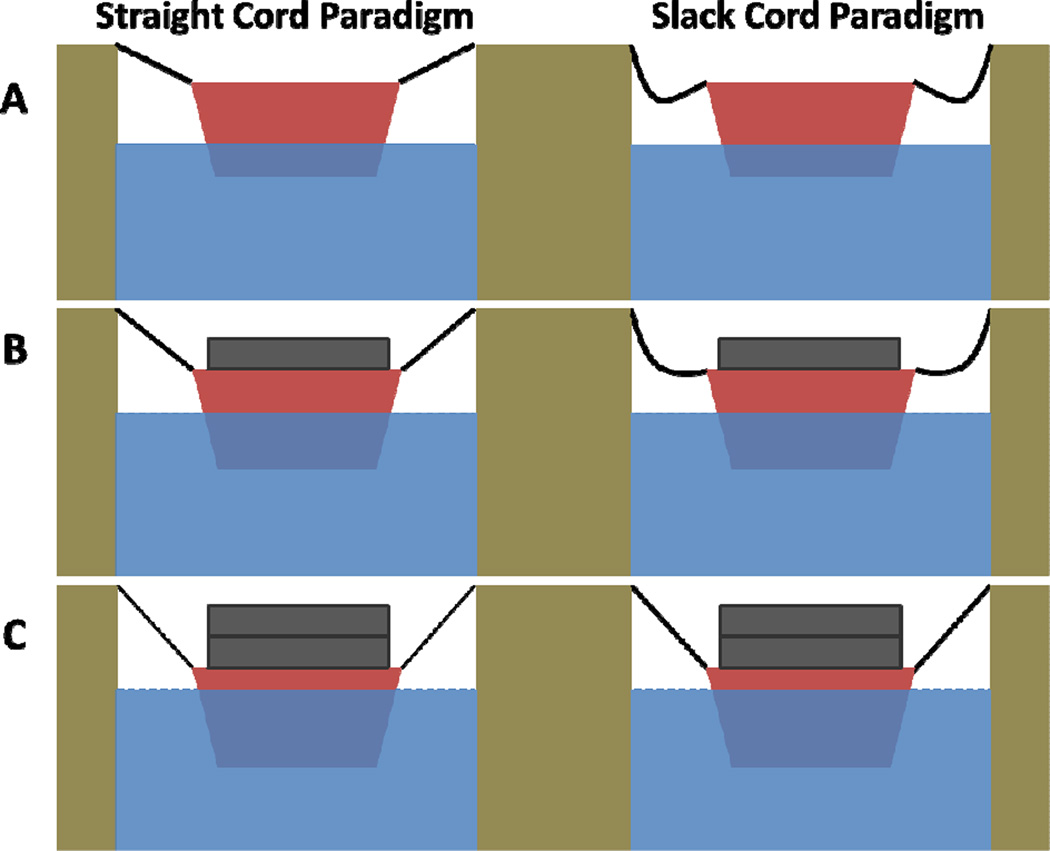
Two structural hypotheses have been under consideration for more than a century to understand the role of the uterine suspension ligament. First, the “straight cord” paradigm suggests that the ligaments are straight and their properties are the primary determinants of uterine position and is not supported by our data. For the other, we suggest the term “slack cord” paradigm, which posits that the uterus is “a floating organ” such as a boat, whose position is influenced partially by the ligaments but also by the structures surrounding it, such as water.13 (Figure 4a,b,c) In this model, the ligaments, or cords tethering the boat to the dock, are not the primary factors determining uterine position until it has descended enough to straighten the ligaments lower in the pelvic. This is consistent with the anatomy of the ligaments of living women quantified in 3D that reveals significant curvature to the cardinal ligaments where the upper and lower portions have a 115 degree difference in direction and a 19% longer curved line length than the distance between origin and insertion.14
Figure 4. “Straight Cord Paradigm” versus “Slack Cord Paradigm” boat analogies.
Boat analogy depicting the “Straight Cord Paradigm” versus the “Slack Cord Paradigm” in which the boat represents the uterus, the cords represent the cardinal-uterosacral ligament complex, and the water represents the pelvic floor muscles. Figure 4A shows no load or force in the boat whereas Figures 4B and 4C show increasing loads or force and the subsequent effect on the supporting ligaments.
Our data are consistent with a “slack cord” paradigm where changes in uterine position occur before the ligament stiffness comes into play. Factors other than ligament stiffness are involved in determining the location of the uterus with small degrees of traction. In this situation, overall cervix starting location is highly variable and only marginally determined by its ligament’s properties. Furthermore, the cervix starting location in the OR is quite different than that seen in the clinic. Although it is possible that anesthesia affects this, the magnitude of anesthesia effects is quite small. In our earlier study there was only a 4mm difference between women with and without paralysis.11 In a recent study, a difference in uterine descent between traction with the patient awake and under anesthesia was recorded as 1.6 cm.15 However, traction force was not standardized so the degree to which clinicians pulled harder on anesthetized patients compared to those who were awake is unknown. Even taking these factors into account it seems likely that the biomechanical measurements (including stiffness) obtained during the OR tests possibly lie outside the physiological range; that is, the position of the uterus during a woman’s normal activities, including Valsalva, get to the point where the stiffness of the ligaments is challenged only in a minority of women (Figure 4c). This was most marked for the subjects with relatively normal support of the uterus in the clinic under maximal Valsalva (higher clinical POP-Q).
Our findings extend our knowledge of suspensory ligament functional anatomy. Anatomical studies have characterized the histopathological composition of the uterosacral and cardinal ligaments in women without prolapse showing that they are similar to visceral mesenteries.5,16–18 Altered tissue composition in women with prolapse has been reported.9,19 However, few groups have assessed the biomechanical properties of the uterine suspensory ligaments. Rivaux et al. performed uniaxial tension testing on non-prolapsed fresh cadaveric uterosacral biopsies and demonstrated a non-linear stress–strain relationship and hyperelastic mechanical behavior.20 Reay-Jones et al. performed similar uniaxial tension testing of uterosacral biopsies in a larger cohort of surgical specimens in normal versus prolapsed patients and demonstrated a significant difference among the prolapsed group.21 However a major limitation in the tensile testing of a biopsy specimen is that there is significant anatomic variation from the proximal to distal ligament segments with regard to thickness and smooth muscle composition.22 This limitation is avoided when biomechanical testing is done in vivo, evaluating the entire ligament in situ, with a technique such as that presented in this study.
To our knowledge, only one other study has been performed, evaluating the force-displacement behavior of the uterosacral/cardinal ligament complex in vivo. Bartscht et al., using manual traction with a spring scale, manually measured the distance from the ectocervix to the hymen in 73 women with normal support under general anesthesia and varying amounts of downward traction (0–4 lbs.).11 They reported the mean cervix locations at interval forces and found that with each additional pound of traction applied, the cervix descended less and less, indicating a progressive loss of compliance as the ligaments reached their elastic limit. However the use of manual traction lacks precision and did not standardize the rate of loading or compensate for the effect of the weight of the spring scale and tenaculum. Our study extends this research by developing an improved measurement equipment capable of precise and reproducible loading rate and magnitude and making observations in women with prolapse. We believe that the ability to quantify the properties of the ligaments do play an important role in characterizing a woman’s pelvic organ support and may help in providing quantitative data to help guide her surgical management. For example, if a woman with a cystocele has relatively normal ligament properties and behavior, then her surgical management may not need to include a hysterectomy or apical suspension compared to a woman with abnormal properties. Ligament integrity may also shed light on which women would benefit from synthetic mesh augmentation during prolapse repair.
Any new testing strategy requires refinement over time. As with any pilot study, our data have limitations, including a relatively small sample size, non- standardized anesthetic, and the lack of a larger control group with normal support and without any other pelvic floor disorders. It is important to recognize that this technique seeks, to the extent possible, to isolate the suspensory ligament properties. Inherently it measures something different from uterine descent during maximal Valsalva maneuver. Our data relate to downward displacement of the uterus under traction. Anatomically, we believe that the cardinal and uterosacral ligaments provide the primary resistance to this movement. However, some supportive role of lateral vaginal tissue (Level II support) is also possible and other effects such as vaginal wall properties and the effects of adjacent organs can come into play as well. Understanding the relationship between these data and clinical findings will take further work. The present data allow for estimates of magnitude and variation needed for a prospectively enrolled case-control comparison under standardized anesthetic conditions. However we note that sub-analysis comparing mean cervix locations under different forces revealed no significant differences between subjects who did and did not receive a paralytic (data not shown).
This study presents a technique that measures in vivo biomechanical properties of uterine apical support minimizing the interference of other pelvic floor supports. Once fully developed, such a technique will allow future, appropriately powered studies to test clinical hypotheses about significant associations between factors such as prolapse severity and uterine ligament integrity.
Acknowledgements
We thank Hogene Kim, M.S. (University of Michigan, Department of Biomechanics), for technical help in developing an early version of this apparatus.
Funding-
This work has been funded by NIH RO1 HD 38665, P50 HD 44406 and K12 HD004438.
IRB University of Michigan HUM 00056743- Consent obtained from all subjects.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures-
Dr. John O. DeLancey and Dr. James Ashton-Miller do not have any directly related conflicts of interest for this study but they are part of the Pelvic Floor Research Group at the University of Michigan, which does receive funding from Johnson and Johnson, American Medical Systems, Kimberly Clark and Proctor and Gamble.
Dr. James Ashton-Miller also receives unrelated funding from Boston Scientific.
All other authors do not have any related conflicts of interest.
Presentation-
This project was presented at the Society of Gynecologic Surgeons’ 39th Annual meeting, April 8–10, 2013 in Charleston, South Carolina, USA.
REFERENCES
- 1.Summers A, Winkel LA, Hussain HK, DeLancey JO. The relationship between anterior and apical compartment support. Am J Obstet Gynecol. 2006;194:1438–1443. doi: 10.1016/j.ajog.2006.01.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rooney K, Kenton K, Mueller ER, FitzGerald MP, Brubaker L. Advanced anterior vaginal wall prolapse is highly correlated with apical prolapse. Am J Obstet Gynecol. 2006;195:1837–1840. doi: 10.1016/j.ajog.2006.06.065. [DOI] [PubMed] [Google Scholar]
- 3.DeLancey JO, Morgan DM, Fenner DE, et al. Comparison of levator ani muscle defects and function in women with and without pelvic organ prolapse. Obstet Gynecol. 2007;109:295–302. doi: 10.1097/01.AOG.0000250901.57095.ba. [DOI] [PubMed] [Google Scholar]
- 4.Dietz HP. Quantification of major morphological abnormalities of the levator ani. Ultrasound Obstet Gynecol. 2007;29:329–334. doi: 10.1002/uog.3951. [DOI] [PubMed] [Google Scholar]
- 5.Cole EE, Leu PB, Gomelsky A, et al. Histopathological evaluation of the uterosacral ligament: is this a dependable structure for pelvic reconstruction? BJU Int. 2006;97:345–348. doi: 10.1111/j.1464-410X.2005.05903.x. [DOI] [PubMed] [Google Scholar]
- 6.Rahn DD, Ruff MD, Brown SA, Tibbals HF, Word RA. Biomechanical properties of the vaginal wall: effect of pregnancy, elastic fiber deficiency, and pelvic organ prolapse. Am J Obstet Gynecol. 2008;198:590 e1–590 e6. doi: 10.1016/j.ajog.2008.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jean-Charles C, Rubod C, Brieu M, Boukerrou M, Fasel J, Cosson M. Biomechanical properties of prolapsed or non-prolapsed vaginal tissue: impact on genital prolapse surgery. Int Urogynecol J. 2010;21:1535–1538. doi: 10.1007/s00192-010-1208-z. [DOI] [PubMed] [Google Scholar]
- 8.Moalli PA, Shand SH, Zyczynski HM, Gordy SC, Meyn LA. Remodeling of vaginal connective tissue in patients with prolapse. Obstet Gynecol. 2005;106:953–963. doi: 10.1097/01.AOG.0000182584.15087.dd. [DOI] [PubMed] [Google Scholar]
- 9.Gabriel B, Watermann D, Hancke K, et al. Increased expression of matrix metalloproteinase 2 in uterosacral ligaments is associated with pelvic organ prolapse. Int Urogynecol J Pelvic Floor Dysfunct. 2006;17:478–482. doi: 10.1007/s00192-005-0045-y. [DOI] [PubMed] [Google Scholar]
- 10.Vineyard DD, Kuehl TJ, Coates KW, Shull BL. A comparison of preoperative and intraoperative evaluations for patients who undergo site-specific operation for the correction of pelvic organ prolapse. Am J Obstet Gynecol. 2002;186:1155–1159. doi: 10.1067/mob.2002.122985. [DOI] [PubMed] [Google Scholar]
- 11.Bartscht KD, DeLancey JO. A technique to study the passive supports of the uterus. Obstet Gynecol. 1988;72:940–943. doi: 10.1097/00006250-198812000-00028. [DOI] [PubMed] [Google Scholar]
- 12.Foon R, Agur W, Kingsly A, White P, Smith P. Traction on the cervix in theatre before anterior repair: Does it tell us when to perform a concomitant hysterectomy? Eur J Obstet Gynecol Reprod Biol. 2012;160:205–209. doi: 10.1016/j.ejogrb.2011.11.002. [DOI] [PubMed] [Google Scholar]
- 13.Paramore R. The statics of the female pelvic viscera. London: H. K. Lewis & Co. Ltd.; 1918. [Google Scholar]
- 14.Chen L, Ramanah R, Hsu Y, Ashton-Miller JA, Delancey JO. Cardinal and deep uterosacral ligament lines of action: MRI based 3D technique development and preliminary findings in normal women. Int Urogynecol J. 2013;24:37–45. doi: 10.1007/s00192-012-1801-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chao FL, Rosamilia A, Dwyer PL, Polyakov A, Schierlitz L, Agnew G. Does preoperative traction on the cervix approximate intra-operative uterine prolapse? A randomised controlled trial. Int Urogynecol J. 2012;23:417–422. doi: 10.1007/s00192-011-1656-0. [DOI] [PubMed] [Google Scholar]
- 16.Campbell RM. The anatomy and histology of the sacrouterine ligaments. Am J Obstet Gynecol. 1950;59:1–12. doi: 10.1016/0002-9378(50)90334-6. illust. [DOI] [PubMed] [Google Scholar]
- 17.Range RL, Woodburne RT. The Gross and Microscopic Anatomy of the Transverse Cervical Ligament. Am J Obstet Gynecol. 1964;90:460–467. doi: 10.1016/0002-9378(64)90802-6. [DOI] [PubMed] [Google Scholar]
- 18.Ramanah R, Berger MB, Parratte BM, DeLancey JO. Anatomy and histology of apical support: a literature review concerning cardinal and uterosacral ligaments. Int Urogynecol J. 2012;23:1483–1494. doi: 10.1007/s00192-012-1819-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ewies AA, Al-Azzawi F, Thompson J. Changes in extracellular matrix proteins in the cardinal ligaments of post-menopausal women with or without prolapse: a computerized immunohistomorphometric analysis. Hum Reprod. 2003;18:2189–2195. doi: 10.1093/humrep/deg420. [DOI] [PubMed] [Google Scholar]
- 20.Rivaux G, Rubod C, Dedet B, Brieu M, Gabriel B, Cosson M. Comparative analysis of pelvic ligaments: a biomechanics study. Int Urogynecol J. 2013;24:135–139. doi: 10.1007/s00192-012-1861-5. [DOI] [PubMed] [Google Scholar]
- 21.Reay Jones NH, Healy JC, King LJ, Saini S, Shousha S, Allen-Mersh TG. Pelvic connective tissue resilience decreases with vaginal delivery, menopause and uterine prolapse. Br J Surg. 2003;90:466–472. doi: 10.1002/bjs.4065. [DOI] [PubMed] [Google Scholar]
- 22.Vu D, Haylen BT, Tse K, Farnsworth A. Surgical anatomy of the uterosacral ligament. Int Urogynecol J. 2010;21:1123–1128. doi: 10.1007/s00192-010-1147-8. [DOI] [PubMed] [Google Scholar]