Abstract
Diabetic nephropathy is one of the serious complications in patients with either type 1 or 2 diabetes mellitus but current treatments remain unsatisfactory. Results of clinical research studies demonstrate that Panax ginseng can help adjust blood pressure and reduce blood sugar and may be advantageous in the treatment of tuberculosis and kidney damage in people with diabetes. The heat-processing method to strengthen the efficacy of P. ginseng has been well-defined based on a long history of ethnopharmacological evidence. The protective effects of P. ginseng on pathological conditions and renal damage associated with diabetic nephropathy in the animal models were markedly improved by heat-processing. The concentrations of less-polar ginsenosides (20(S)-Rg3, 20(R)-Rg3, Rg5, and Rk1) and maltol in P. ginseng were significantly increased in a heat-processing temperature-dependent manner. Based on researches in animal models of diabetes, ginsenoside 20(S)-Rg3 and maltol were evaluated to have therapeutic potential against diabetic renal damage. These effects were achieved through the inhibition of inflammatory pathway activated by oxidative stress and advanced glycation endproducts. These findings indicate that ginsenoside 20(S)-Rg3 and maltol are important bioactive constituents of heat-processed ginseng in the control of pathological conditions associated with diabetic nephropathy.
Keywords: Panax ginseng, Heat-processing, 20(S)-Rg3, Advanced glycation endproducts, Free radical
INTRODUCTION
The kidney is an excretory organ that plays a vital role in excretion of waste through urine in the human body. Diabetic nephropathy is an important cause of end-stage renal disease and is clinically defined as progressively increasing proteinuria accompanied by hypertension and impairment of glomerular filtration [1,2]. The onset of renal disease is multifactorial, involving oxidative stress, hypertension, hyperglycemia and hyperlipidemia [3-6]. The pathophysiological mechanisms that lead to nephropathy and the morphological features of diabetic renal damage are similar in type 1 and type 2 diabetes mellitus [2,7].
Under hyperglycemic condition, glucose and other reactive carbonyl compounds react non-enzymatically with proteins, lipids, or nucleic acids to form Schiff bases and Amadori products. Additional rearrangement and modification lead to the generation of diverse advanced glycation endproducts (AGEs), which can alter the structure and function of intra- and extracellular molecules, increase oxidative stress, and modulate cell activation, signal transduction, and the expression of cytokines and growth factors through receptor-dependent and receptor-independent pathways [8-11]. In people with diabetes and/or chronic renal failure, AGEs that accumulate in the kidney are responsible for the pathological changes, including increased kidney weight, glomerular hypertrophy, glomerular basement membrane thickening, and progressive albuminuria [12].
Moreover, AGEs stimulate free radical mechanisms and induce membrane peroxidation, which in turn increase membrane permeability [11]. Therefore, AGE accumulation in the kidney has been regarded as an index of progressive renal damage in diabetic nephropathy. Reciprocally, oxidative stress is known to induce AGEs. Protein kinase C and mitogen-activated protein kinase-extracellular signal regulated kinases 1, 2 can be activated by reactive oxygen species (ROS) and signal profibrotic responses in kidney cells [13-15]. Several researchers have demonstrated that ROS generation induced by nicotinamide adenine dinucleotide phosphate oxidase and the mitochondrial electron-transport chain is an early event in the development of diabetic renal disease [1,16,17]. Therefore, a variety of synthetic antioxidants for reducing oxidative damage from generating free radicals and improving oxidative stress via improvement of the antioxidant activating system in the body in order to prevent or treat renal diseases have been proposed [10,13].
Clinical evidence has suggested that an appropriate use of traditional medicines in combination with modern Western medicine, or mainstream anti-diabetic drugs, can prevent or ameliorate the development of diabetic complications. Many diabetic patients choose alternative therapeutic approaches, such as herbal or traditional Chinese medicine along with the mainstream anti-diabetic drugs; thus, making alternative therapy for diabetes a popular remedy option [18]. Based on a large number of chemical and pharmacological research work, numerous bioactive compounds have been found in medicinal plants for diabetes [19]. Among the frequently mentioned herbal medicines that help manage blood glucose, ginseng extracts made from the root, rootlet, berry and leaf of Panax ginseng (Korean ginseng) and P. quinquefolius (American ginseng), have been proven effective for anti-hyperglycemia, insulin sensitization, islet protection, anti-obesity and anti-oxidation in many model systems [20].
EFFICACY OF CONVENTIONAL GINSENG AND GINSENOSIDES ON RENAL DAMAGE
P. ginseng is a perennial plant that belongs to the Panax species, Araliaceae family. Examples of Panax species plants having similar efficacy to P. ginseng include P. quinquefolius, P. notoginseng, P. japonica, P. trifolia, P. pseudoginseng, and P. vietnamensis. These Panax species plants contain dammarane-based saponin in common with 1 to 4 saccharide(s) combined with a dammarane backbone, unlike the other plants [21-23]. In particular, ginseng that contains high concentration of saponins includes ginsenosides Rb1, Rb2, Rc, Rd, Rg1, and Re. These saponins have a variety of pharmaceutical effects that greatly differ in types and intensities, depending on the structures [21].
Alcoholic extract of P. quinquefolius, which shows presence of the major ginsenoside Rg1, Re, Rb1, Rc, Rb2, and Rd with predominance of the ginsenoside Rb1 and Re, was effective in the prevention of diabetic nephropathy through a combination of mechanisms, such as anti-hyperglycemic and antioxidant effects [24].
Table 1 shows potential approaches of conventional ginsenosides to prevent chemical, surgical and/or genetic-induced renal damage. Ginsenoside Rg1 (20 to 50 mg/kg) attenuated kidney damage with improvement on glomerular structure in spontaneously hypertensive rats and inhibited renal interstitial fibrosis in rats with unilateral ureteral obstruction, via suppressing oxidative stress [25,26]. Treatment of ginsenoside Re (20 mg/kg) restored the levels of both glutathione and malondialdehyde in the kidney of streptozotocin (STZ)-induced diabetic rats [27]. Ginsenoside Rb1 (25 to 60 mg/kg) ameliorated renal dysfunction in glycerol-induced acute renal failure in rats [28] and attenuated acute renal injury induced by ischemia reperfusion by activating the Nrf2/ARE pathway [29]. Ginsenoside Rb1 (12.5 to 50 mg/kg) also inhibited renal interstitial fibrosis in rats with unilateral ureteral obstruction by modulating thrombospondin-1 and vascular endothelial growth factor expression [30]. Ginsenoside Rd (5 mg/kg) attenuated renal dysfunction by preventing oxidative stress in cisplatin or cephaloridine-induced acute renal failure and ischemic-reperfused rats [31-33]. Although the efficacy on nephropathy are poorly understood, total saponins of P. notoginseng has been tested on chronic renal failure (non-uremic) patients and showed good therapeutic results as improving the renal function and lowering urinary protein [34]. In addition, P. ginseng supplementation maintained good glycemic control and improved plasma glucose and insulin regulation safely beyond usual therapy in well-controlled type 2 diabetes [35].
Table 1.
Potential approaches of conventional ginsenosides to prevent chemical, surgical and/or genetic-induced renal damage
| Ginsenoside | Animal model | Mechanism | Reference |
|---|---|---|---|
|
| |||
| Rg1 | Spontaneously hypertensive rat | Repair of glomerular structure | 26 |
| UUO | Renal interstitial fibrosis↓ | 25 | |
| Repair of peritubular capillary | |||
| Thrombospondin-1↓, VEGF↑ | |||
| Re | STZ-induced diabetic rat | Oxidative stress↓ | 27 |
| Rb1 | Glycerol-induced acute renal failure | Renal function↑ | 28 |
| Oxidative stress↓ | |||
| Repair of renal morphology | |||
| UUO | Oxidative damage↓ | 30 | |
| Renal TGF-β1↓ | |||
| Renal interstitial fibrosis↓ | |||
| Intestinal ischemia reperfusion-induced renal injury | Renal function↑ | 29 | |
| Oxidative stress↓ | |||
| Nrf2/ARE pathway↑ | |||
| Rd | Cisplatin-induced acute renal failure | Renal function↑ | 31-33 |
| Cephaloridine-induced renal failure | Oxidative stress↓ | ||
| Renal ischemia-reperfusion | |||
UUO, unilateral ureteral obstruction; VEGF, vascular endothelial growth factor, STZ, streptozotocin; Nrf2, nucuear factor erythroid 2-related factor 2; ARE, antioxidant response element.
EFFICACY OF LESS-POLAR GINSENOSIDES ON FREE RADICAL AND RENAL DAMAGE
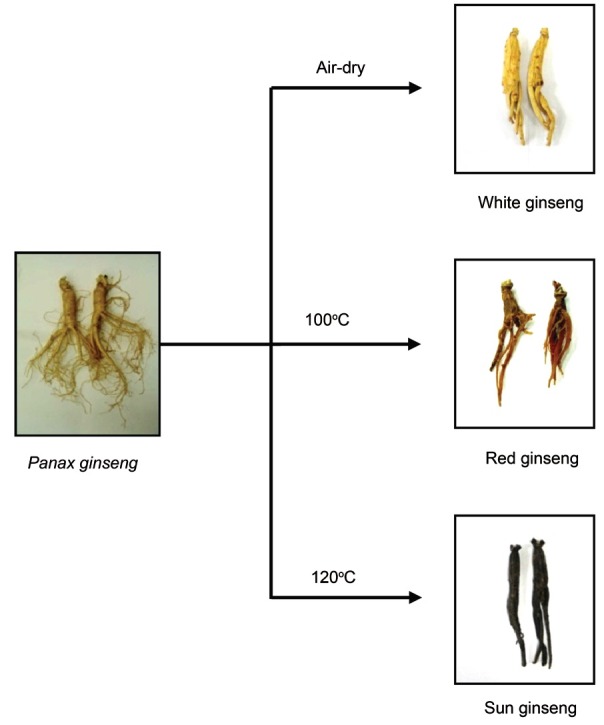
Research in efforts to develop methods for increasing the pharmaceutical effect of ginseng by conversion of the dammarane-based saponin by high temperature and high pressure thermal processing has been conducted. The heat-processing method to strengthen the efficacy of ginseng has been well defined in Korea, based on the long history of ethnopharmacological evidence [21,23]. P. ginseng cultivated in Korea is harvested after 4 to 6 years of cultivation, and it is classified into three types depending on how it is processed. Fresh ginseng can be consumed in an unprocessed state. White ginseng (harvested when 4 to 6 years old) is dried ginseng root. Red ginseng is ginseng root steamed at 98℃ to 100℃ after peeling (Fig. 1) [22]. Red ginseng is more widely used than white ginseng in Asian countries, because steaming induces changes in the chemical constituents and enhances the biological activities of ginseng [22,36,37]. A novel heat-processing method of autoclaving ginseng at a higher temperature than red ginseng was recently developed to achieve an even stronger activity than that of red ginseng; this ginseng product was termed sun ginseng (SG) (Fig. 1) [38].
Fig. 1. Classification of Panax ginseng products by heat-processing methods.
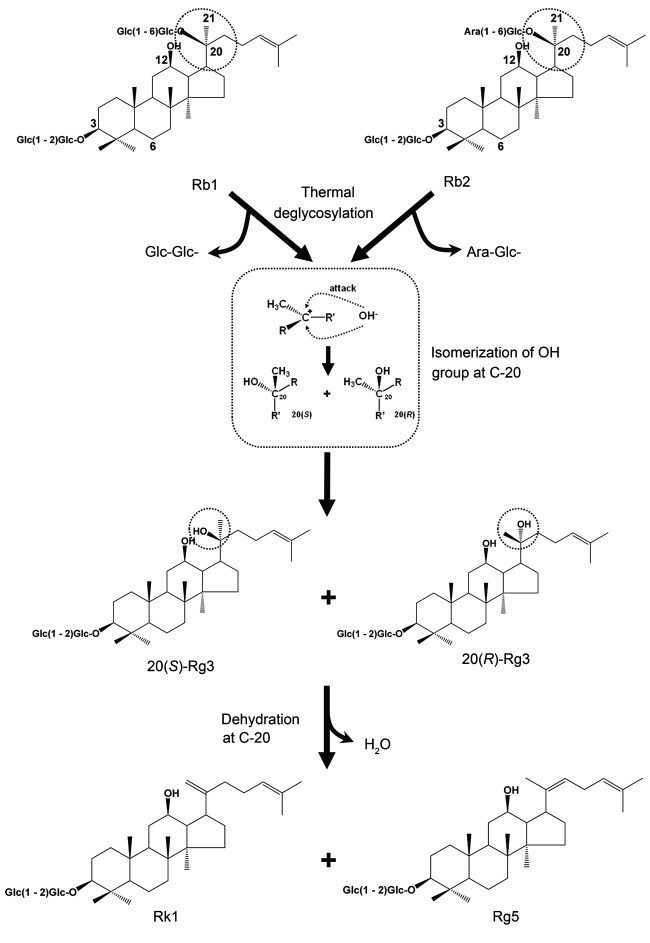
In vivo evidence for the roles of free radicals and AGEs in diabetic kidney disease comes mainly from studies in STZ-induced type 1 diabetic rats [11]. We have previously showed that the protective effects of both P. ginseng and P. quinquefolius on renal damages in STZ-induced diabetic rats were significantly improved by heat-processing [39,40]. The kidney protecting active components of heat-processed P. ginseng were identified by investigating further the changes in the constituents of P. ginseng by heat-processing and its antioxidant activity [23,41-53]. As a representative example, protopanaxadiol-type ginsenoside Rb1 and Rb2 are known to produce stereoisomers 20(S)-Rg3 and 20(R)-Rg3 by dissociation of a glycosyl residue located at carbon 20 by thermal processing, as illustrated in Fig. 2; followed by a dehydration reaction at carbon 20 to produce ginsenoside Rg5 and Rk1 [45,52]. Among them, 20(S)-Rg3 showed the strongest hydroxyl radical (•OH)-scavenging activity, and the following were shown in decreasing order; Rg5, 20(R)-Rg3, and Rk1 at a concentration of 0.5% [45].
Fig. 2. Structural changes of ginsenoside Rb1 and Rb2 brought about by heat-processing.
According to the studies in STZ diabetic rats, the elevated serum glucose, glycosylated protein, and thiobarbituric acid-reactive substance levels in diabetic rats were significantly reduced by the 20(S)-Rg3 administrations (20 mg/kg) in STZ-induced diabetic rats [49]. In addition, the renal dysfunction of diabetic rats was significantly ameliorated by the 20(S)-Rg3 administrations in a dose-dependent manner. A growing body of evidence supports the important roles of renal N-methyl-d-aspartate (NMDA) receptors, originally identified in the central nervous system, in renal blood flow and nephrotoxicity. Moreover, the nephrotoxicity and renal vasoconstriction were attenuated by treatment with an NMDA receptor antagonist [54,55]. The elevated NMDA-NR1 levels of diabetic rats were significantly decreased in the groups administered 20(S)-Rg3 or aminoguanidine (20 mg/kg body weight/d). These beneficial effects on diabetic renal damage were related to the inhibitory effect of 20(S)-Rg3 against NMDA receptor-mediated nephrotoxicity [49].
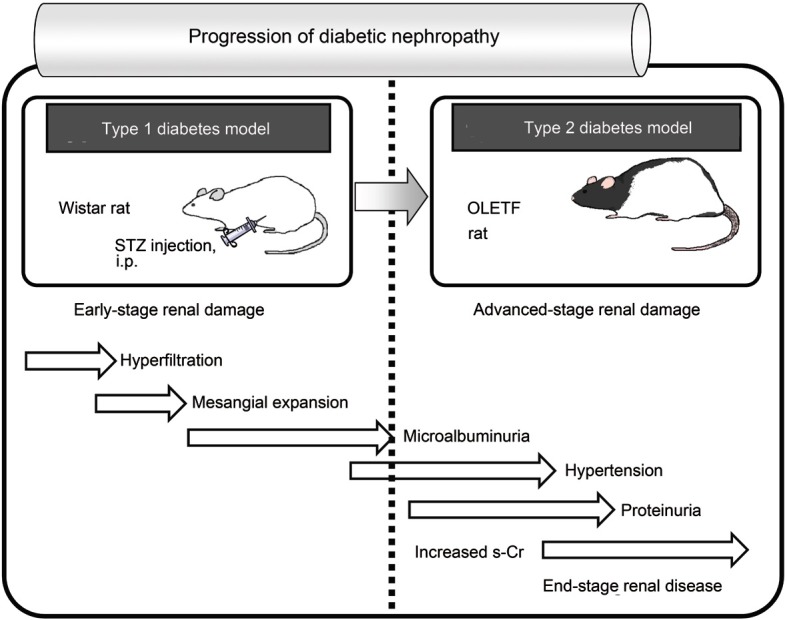
The sign of early diabetic nephropathy is an increased urinary albumin level, and advanced diabetic nephropathy is characterized by proteinuria and decreasing CCr levels [11]. The Otsuka Long-Evans Tokushima Fatty (OLETF) rat is an animal model of spontaneously obese type 2 diabetes characterized by hyperglycemia, insulin resistance, hyperinsulinemia, hypertriglyceridemia, and hypercholesterolemia, and with complications such as nonalcoholic fatty liver and renal disorders; in addition, these typical characteristics of OLETF rats are known to be useful for analyzing the complex forms of human diabetes (Fig. 3) [56,57].
Fig. 3. Schematic description for the progression of diabetic nephropathy in the type 1 and 2 diabetes animal models. STZ, streptozotocin; i.p., intraperitoneal; OLETF, Otsuka Long-Evans Tokushima Fatty.
In our previous study to identify the effect of 20(S)-Rg3 at advanced-stage of diabetic renal damage in OLETF rats, the elevation of pro-inflammatory protein expressions, such as inducible nitric oxide synthase and 3-nitrotyrosine were significantly reduced by the administrations of 20(S)-Rg3. Nε-(-(carboxymethyl)lysine (CML) is known as a marker of cumulative oxidative stress and is involved in the development of diabetic nephropathy [14,58,59]. In addition, the activation of receptor for AGEs by CML results in an activation of NF-κB and production of proinflammatory cytokines [60,61]. The elevation of CML levels in diabetic control rats were prevented by the 20(S)-Rg3 administration. These findings imply that the 20(S)-Rg3 prevents the progression of renal damage and dysfunction in type 2 diabetic rats via inhibiting oxidative stress and AGE formation [62]. Based upon chemical and biological activity tests, 20(S)-Rg3 was found to prevent the progression of renal damage and dysfunction in type 1 and 2 diabetic rats, via inhibiting oxidative stress and inflammation [23].
EFFICACY OF MALTOL ON AGES, FREE RADICLAS AND RENAL DAMAGE
Although the main pharmacologically active constituents of ginseng are believed to be ginsenosides, researchers also have paid attention to the other components. It is well known that Maillard reaction products (MRPs) produced in both heat-treated food systems and in sugar-amino acid model systems have antioxidant activity [63-65]. MRPs in ginseng were reported to increase by heat-processing; these compounds are arginyl-fructosyl-glucose, arginyl-fructose, maltol, maltol-3-O-β-D-glucoside, and so on [66,67]. Maltol is formed by sucrose pyrolysis or thermal degradation of starch [68,69], and is extensively used in food, beverage, tobacco, brewery, cosmetics and pharmaceuticals industries [70]. When ginseng extracts were analyzed with GC-MS, content of maltol in red ginseng and SG was about 4 and 36 times higher, respectively, than in white ginseng [41,50]. There are several lines of evidence regarding the antioxidant activities of maltol, and antioxidants are known to protect against glycation-derived free radicals and may have a therapeutic potential [8,61]. Maltol with hydroxypyrone structure acts as a potent metal-chelating agent [71], and complexes of maltol with metals are now applied to the treatment for some diseases [72-74].
Numerous AGE inhibitors have been investigated by in vitro AGE-inhibitory activity tests, but some classes of AGEs inhibition is primarily mediated by their transition metal-chelating or antioxidant activities [75,76]. In our previous in vitro study, maltol exhibited a stronger inhibitory effect against glucose-induced AGE generation than aminoguanidine, a well-known AGE inhibitor. In addition, the •OH scavenging activity of maltol was slightly stronger than that of aminoguanidine, and this effect was interpreted to be important because •OH scavenging activity in electron spin resonance spectrometer is mediated by the transition metal-chelating and free radical scavenging activities of the compounds [77].
In STZ-diabetic rats, maltol (50 mg/kg body weight/d) significantly decreased the renal fluorescent AGE level, suggesting that it would inhibit oxidative damage and irreversible renal damage caused by protein glycation reaction under diabetes [78]. In addition, the elevated CML expression, a major AGE in human tissues, and receptor for AGE levels in diabetic control rats were significantly reduced by the 50 mg/kg body weight/d of maltol administration. These findings imply that the beneficial effect of maltol in type 1 diabetic rats was mainly mediated by the inhibition of AGE generation (Table 2) [78].
Table 2.
Effect and mechanism action of heat-processed components in Panax ginseng on renal damage
| Ginsenoside | Animal model | Mechanism | Reference |
|---|---|---|---|
|
| |||
| 20(S)-Rg3 | STZ-induced diabetic rat | Renal function↑ | 49 |
| NMDA-mediated nephrotoxicity↓ | |||
| Otsuka Long-Evans Tokushima Fatty rat | Renal function↑ | 62 | |
| Oxidative stress↓ | |||
| Advanced glycation endproduct↓ | |||
| Lipopolysaccharide-induced renal injury | Renal function↑ | 48 | |
| Oxidative stress↓ | |||
| Renal inflammation↓ | |||
| Maltol | STZ-induced diabetic rat | Advanced glycation endproduct↓ | 78 |
| Oxidative stress↓ | |||
STZ, streptozotocin; NMDA, N-methyl-d-aspartate receptor.
CONCLUSION AND PERSPECTIVES
Diabetic nephropathy has been the major cause of patients needing chronic haemodialysis since 1998 [79,80]. Prevention of the occurrence and progression of diabetic nephropathy has become a very important issue. Results of clinical research studies demonstrate that P. ginseng can help adjust blood pressure and reduce blood sugar and may be advantageous in the treatment of tuberculosis and kidney damage in people with type 2 diabetes [34,35]. In this mini-review, we have summarized that the contents of free radical-scavenging active components, such as 20(S)-Rg3 and maltol in P. ginseng were significantly increased, depending on the temperature of heat-processing. Based on the observations on the roles of 20(S)-Rg3 and maltol in AGEs and free radicals in vitro and in vivo studies (type 1 and/or 2 diabetes models), 20(S)-Rg3 and maltol were evaluated to have therapeutic potential against diabetic renal damage in the early-stage. The identification and management of diabetic kidney disease in the early-stage is important because the majority of people have no symptoms until the disease is very advanced [81]. Therefore, the beneficial effects of ginsenoside 20(S)-Rg3 and maltol in the early-stage have important implication by preventing the advancement of diabetic renal damage to advanced-stages. Considering the relevant use and individual daily consumption of ginseng, it is clear that 20(S)-Rg3 and maltol are important bioactive constituents of heat-processed ginseng, especially in the control of diabetic renal complication. This investigation of bioactive constituents of heat-processed P. ginseng is important for the scientific elucidation of improved efficacies of ginseng by traditional and modern heat-processing methods, and may contribute to the development of ginseng-derived novel drugs.
Acknowledgments
This paper was studied with the support of the Ministry of Education Science and Technology and The Korean Federation of Science and Technology Societies. This work was also supported by the Korea Institute of Science and Technology institutional program (2Z03840).
References
- 1.Li JM, Shah AM. ROS generation by nonphagocytic NADPH oxidase: potential relevance in diabetic nephropathy. J Am Soc Nephrol. 2003;14(8 Suppl 3):S221–S226. doi: 10.1097/01.ASN.0000077406.67663.E7. [DOI] [PubMed] [Google Scholar]
- 2.Alsaad KO, Herzenberg AM. Distinguishing diabetic nephropathy from other causes of glomerulosclerosis: an update. J Clin Pathol. 2007;60:18–26. doi: 10.1136/jcp.2005.035592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sarafidis PA, Ruilope LM. Insulin resistance, hyperinsulinemia, and renal injury: mechanisms and implications. Am J Nephrol. 2006;26:232–244. doi: 10.1159/000093632. [DOI] [PubMed] [Google Scholar]
- 4.Njoroge FG, Monnier VM. The chemistry of the Maillard reaction under physiological conditions: a review. Prog Clin Biol Res. 1989;304:85–107. [PubMed] [Google Scholar]
- 5.The Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329:977–986. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 6.Kasuga M. Insulin resistance and pancreatic beta cell failure. J Clin Invest. 2006;116:1756–1760. doi: 10.1172/JCI29189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Parving HH. Diabetic nephropathy: prevention and treatment. Kidney Int. 2001;60:2041–2055. doi: 10.1046/j.1523-1755.2001.00020.x. [DOI] [PubMed] [Google Scholar]
- 8.Baynes JW. Role of oxidative stress in development of complications in diabetes. Diabetes. 1991;40:405–412. doi: 10.2337/diab.40.4.405. [DOI] [PubMed] [Google Scholar]
- 9.Wendt T, Tanji N, Guo J, Hudson BI, Bierhaus A, Ramasamy R, Arnold B, Nawroth PP, Yan SF, D’Agati V, et al. Glucose, glycation, and RAGE: implications for amplification of cellular dysfunction in diabetic nephropathy. J Am Soc Nephrol. 2003;14:1383–1395. doi: 10.1097/01.ASN.0000065100.17349.CA. [DOI] [PubMed] [Google Scholar]
- 10.Williams ME. New therapies for advanced glycation end product nephrotoxicity: current challenges. Am J Kidney Dis. 2003;41(3 Suppl 1):S42–S47. doi: 10.1053/ajkd.2003.50083. [DOI] [PubMed] [Google Scholar]
- 11.Schrijvers BF, De Vriese AS, Flyvbjerg A. From hyperglycemia to diabetic kidney disease: the role of metabolic, hemodynamic, intracellular factors and growth factors/cytokines. Endocr Rev. 2004;25:971–1010. doi: 10.1210/er.2003-0018. [DOI] [PubMed] [Google Scholar]
- 12.Vlassara H, Striker LJ, Teichberg S, Fuh H, Li YM, Steffes M. Advanced glycation end products induce glomerular sclerosis and albuminuria in normal rats. Proc Natl Acad Sci U S A. 1994;91:11704–11708. doi: 10.1073/pnas.91.24.11704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nishikawa T, Edelstein D, Du XL, Yamagishi S, Matsumura T, Kaneda Y, Yorek MA, Beebe D, Oates PJ, Hammes HP, et al. Normalizing mitochondrial superoxide production blocks three pathways of hyperglycaemic damage. Nature. 2000;404:787–790. doi: 10.1038/35008121. [DOI] [PubMed] [Google Scholar]
- 14.Cooper ME. Interaction of metabolic and haemodynamic factors in mediating experimental diabetic nephropathy. Diabetologia. 2001;44:1957–1972. doi: 10.1007/s001250100000. [DOI] [PubMed] [Google Scholar]
- 15.Tuttle KR, Johnson EC, Cooney SK, Anderberg RJ, Johnson EK, Clifton GD, Meek RL. Amino acids injure mesangial cells by advanced glycation end products, oxidative stress, and protein kinase C. Kidney Int. 2005;67:953–968. doi: 10.1111/j.1523-1755.2005.00159.x. [DOI] [PubMed] [Google Scholar]
- 16.Rahbar S, Figarola JL. Novel inhibitors of advanced glycation endproducts. Arch Biochem Biophys. 2003;419:63–79. doi: 10.1016/j.abb.2003.08.009. [DOI] [PubMed] [Google Scholar]
- 17.Newsholme P, Haber EP, Hirabara SM, Rebelato EL, Procopio J, Morgan D, Oliveira-Emilio HC, Carpinelli AR, Curi R. Diabetes associated cell stress and dysfunction: role of mitochondrial and non-mitochondrial ROS production and activity. J Physiol. 2007;583(Pt 1):9–24. doi: 10.1113/jphysiol.2007.135871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ceylan-Isik AF, Fliethman RM, Wold LE, Ren J. Herbal and traditional Chinese medicine for the treatment of cardiovascular complications in diabetes mellitus. Curr Diabetes Rev. 2008;4:320–328. doi: 10.2174/157339908786241142. [DOI] [PubMed] [Google Scholar]
- 19.Li WL, Zheng HC, Bukuru J, De Kimpe N. Natural medicines used in the traditional Chinese medical system for therapy of diabetes mellitus. J Ethnopharmacol. 2004;92:1–21. doi: 10.1016/j.jep.2003.12.031. [DOI] [PubMed] [Google Scholar]
- 20.Yin J, Zhang H, Ye J. Traditional chinese medicine in treatment of metabolic syndrome. Endocr Metab Immune Disord Drug Targets. 2008;8:99–111. doi: 10.2174/187153008784534330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Park JH, Kim JM, Han SB, Kim NY, Surh YJ, Lee SK, Kim ND, Park MK. A new processed ginseng with fortified activity. In: Hur H, Choi KJ, Kim YC, eds. Advances in ginseng research. Korean Society of Ginseng; Seoul: 1998. pp. 146–159. [Google Scholar]
- 22.Yun TK. Brief introduction of Panax ginseng C.A. Meyer. J Korean Med Sci. 2001;16 Suppl:S3–S5. doi: 10.3346/jkms.2001.16.S.S3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yokozawa T, Kang KS, Yamabe N, Kim HY. Therapeutic potential of heat-processed Panax ginseng with respect to oxidative tissue damage. Drug Discov Ther. 2007;1:30–44. [PubMed] [Google Scholar]
- 24.Sen S, Chen S, Feng B, Wu Y, Lui E, Chakrabarti S. Preventive effects of North American ginseng (Panax quinquefolium) on diabetic nephropathy. Phytomedicine. 2012;19:494–505. doi: 10.1016/j.phymed.2012.01.001. [DOI] [PubMed] [Google Scholar]
- 25.Xie XS, Liu HC, Wang FP, Zhang CL, Zuo C, Deng Y, Fan JM. Ginsenoside Rg1 modulation on thrombospondin-1 and vascular endothelial growth factor expression in early renal fibrogenesis in unilateral obstruction. Phytother Res. 2010;24:1581–1587. doi: 10.1002/ptr.3190. [DOI] [PubMed] [Google Scholar]
- 26.Chen H, Yin J, Deng Y, Yang M, Xu L, Teng F, Li D, Cheng Y, Liu S, Wang D, et al. The protective effects of ginsenoside Rg1 against hypertension target-organ damage in spontaneously hypertensive rats. BMC Complement Altern Med. 2012;12:53. doi: 10.1186/1472-6882-12-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cho WC, Chung WS, Lee SK, Leung AW, Cheng CH, Yue KK. Ginsenoside Re of Panax ginseng possesses significant antioxidant and antihyperlipidemic efficacies in streptozotocin-induced diabetic rats. Eur J Pharmacol. 2006;550:173–179. doi: 10.1016/j.ejphar.2006.08.056. [DOI] [PubMed] [Google Scholar]
- 28.Zhang HA, Wang M, Zhou J, Yao QY, Ma JM, Jiang CL. Protective effect of ginsenoside against acute renal failure and expression of tyrosine hydroxylase in the locus coeruleus. Physiol Res. 2010;59:61–70. doi: 10.33549/physiolres.931650. [DOI] [PubMed] [Google Scholar]
- 29.Sun Q, Meng QT, Jiang Y, Xia ZY. Ginsenoside Rb1 attenuates intestinal ischemia reperfusion induced renal injury by activating Nrf2/ARE pathway. Molecules. 2012;17:7195–7205. doi: 10.3390/molecules17067195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xie XS, Liu HC, Yang M, Zuo C, Deng Y, Fan JM. Ginsenoside Rb1, a panoxadiol saponin against oxidative damage and renal interstitial fibrosis in rats with unilateral ureteral obstruction. Chin J Integr Med. 2009;15:133–140. doi: 10.1007/s11655-009-0133-9. [DOI] [PubMed] [Google Scholar]
- 31.Yokozawa T, Liu ZW, Dong E. A study of ginsenoside-Rd in a renal ischemia-reperfusion model. Nephron. 1998;78:201–206. doi: 10.1159/000044911. [DOI] [PubMed] [Google Scholar]
- 32.Yokozawa T, Owada S. Effect of ginsenoside-Rd in cephaloridine-induced renal disorder. Nephron. 1999;81:200–207. doi: 10.1159/000045277. [DOI] [PubMed] [Google Scholar]
- 33.Yokozawa T, Liu ZW. The role of ginsenoside-Rd in cisplatin-induced acute renal failure. Ren Fail. 2000;22:115–127. doi: 10.1081/JDI-100100858. [DOI] [PubMed] [Google Scholar]
- 34.Peng SL, Guo ZA. Effect of total saponins of Panax notoginseng on urinary albumin in patients with chronic renal failure. Zhongguo Wei Zhong Bing Ji Jiu Yi Xue. 2010;22:744–746. [PubMed] [Google Scholar]
- 35.Vuksan V, Sung MK, Sievenpiper JL, Stavro PM, Jenkins AL, Di Buono M, Lee KS, Leiter LA, Nam KY, Arnason JT, et al. Korean red ginseng (Panax ginseng) improves glucose and insulin regulation in well-controlled, type 2 diabetes: results of a randomized, double-blind, placebo-controlled study of efficacy and safety. Nutr Metab Cardiovasc Dis. 2008;18:46–56. doi: 10.1016/j.numecd.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 36.Kasai R, Besso H, Tanaka O, Saruwatari YI, Fuwa T. Saponins of red ginseng. Chem Pharm Bull (Tokyo) 1983;31:2120–2125. doi: 10.1248/cpb.31.2120. [DOI] [Google Scholar]
- 37.Matsuura H, Hirao Y, Yoshida S, Kunihiro K, Fuwa T, Kasai R, Tanaka O. Study of red ginseng: new glucosides and a note on the occurrence of maltol. Chem Pharm Bull (Tokyo) 1984;32:4674–4677. doi: 10.1248/cpb.32.4674. [DOI] [Google Scholar]
- 38.Kim WY, Kim JM, Han SB, Lee SK, Kim ND, Park MK, Kim CK, Park JH. Steaming of ginseng at high temperature enhances biological activity. J Nat Prod. 2000;63:1702–1704. doi: 10.1021/np990152b. [DOI] [PubMed] [Google Scholar]
- 39.Kim HY, Kang KS, Yamabe N, Nagai R, Yokozawa T. Protective effect of heat-processed American ginseng against diabetic renal damage in rats. J Agric Food Chem. 2007;55:8491–8497. doi: 10.1021/jf071770y. [DOI] [PubMed] [Google Scholar]
- 40.Kim HY, Kang KS, Yamabe N, Yokozawa T. Comparison of the effects of Korean ginseng and heat-processed Korean ginseng on diabetic oxidative stress. Am J Chin Med. 2008;36:989–1004. doi: 10.1142/S0192415X08006417. [DOI] [PubMed] [Google Scholar]
- 41.Kang KS, Kim HY, Pyo JS, Yokozawa T. Increase in the free radical scavenging activity of ginseng by heat-processing. Biol Pharm Bull. 2006;29:750–754. doi: 10.1248/bpb.29.750. [DOI] [PubMed] [Google Scholar]
- 42.Kang KS, Yokozawa T, Kim HY, Park JH. Study on the nitric oxide scavenging effects of ginseng and its compounds. J Agric Food Chem. 2006;54:2558–2562. doi: 10.1021/jf0529520. [DOI] [PubMed] [Google Scholar]
- 43.Kang KS, Kim HY, Yamabe N, Yokozawa T. Stereospecificity in hydroxyl radical scavenging activities of four ginsenosides produced by heat processing. Bioorg Med Chem Lett. 2006;16:5028–5031. doi: 10.1016/j.bmcl.2006.07.071. [DOI] [PubMed] [Google Scholar]
- 44.Kang KS, Kim HY, Yamabe N, Nagai R, Yokozawa T. Protective effect of sun ginseng against diabetic renal damage. Biol Pharm Bull. 2006;29:1678–1684. doi: 10.1248/bpb.29.1678. [DOI] [PubMed] [Google Scholar]
- 45.Kang KS, Kim HY, Baek SH, Yoo HH, Park JH, Yokozawa T. Study on the hydroxyl radical scavenging activity changes of ginseng and ginsenoside-Rb2 by heat processing. Biol Pharm Bull. 2007;30:724–728. doi: 10.1248/bpb.30.724. [DOI] [PubMed] [Google Scholar]
- 46.Kang KS, Yokozawa T, Yamabe N, Kim HY, Park JH. ESR study on the structure and hydroxyl radical-scavenging activity relationships of ginsenosides isolated from Panax ginseng C A Meyer. Biol Pharm Bull. 2007;30:917–921. doi: 10.1248/bpb.30.917. [DOI] [PubMed] [Google Scholar]
- 47.Kang KS, Lee YJ, Park JH, Yokozawa T. The effects of glycine and L-arginine on heat stability of ginsenoside Rb1. Biol Pharm Bull. 2007;30:1975–1978. doi: 10.1248/bpb.30.1975. [DOI] [PubMed] [Google Scholar]
- 48.Kang KS, Kim HY, Yamabe N, Park JH, Yokozawa T. Preventive effect of 20(S)-ginsenoside Rg3 against lipopolysaccharide-induced hepatic and renal injury in rats. Free Radic Res. 2007;41:1181–1188. doi: 10.1080/10715760701581740. [DOI] [PubMed] [Google Scholar]
- 49.Kang KS, Yamabe N, Kim HY, Park JH, Yokozawa T. Therapeutic potential of 20(S)-ginsenoside Rg(3) against streptozotocin-induced diabetic renal damage in rats. Eur J Pharmacol. 2008;591:266–272. doi: 10.1016/j.ejphar.2008.06.077. [DOI] [PubMed] [Google Scholar]
- 50.Kang KS, Tanaka T, Cho EJ, Yokozawa T. Evaluation of the peroxynitrite scavenging activity of heat-processed ginseng. J Med Food. 2009;12:124–130. doi: 10.1089/jmf.2007.0646. [DOI] [PubMed] [Google Scholar]
- 51.Yamabe N, Song KI, Lee W, Han IH, Lee JH, Ham J, Kim SN, Park JH, Kang KS. Chemical and free radical-scavenging activity changes of ginsenoside Re by Maillard reaction and its possible use as a renoprotective agent. J Ginseng Res. 2012;36:256–262. doi: 10.5142/jgr.2012.36.2.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lee YJ, Kim HY, Kang KS, Lee JG, Yokozawa T, Park JH. The chemical and hydroxyl radical scavenging activity changes of ginsenoside-Rb1 by heat processing. Bioorg Med Chem Lett. 2008;18:4515–4520. doi: 10.1016/j.bmcl.2008.07.056. [DOI] [PubMed] [Google Scholar]
- 53.Yamabe N, Lee JG, Lee YJ, Park CH, Kim HY, Park JH, Yokozawa T, Kang KS. The chemical and 1,1-diphenyl-2-picrylhydrazyl radical scavenging activity changes of ginsenosides Rb1 and Rg1 by Maillard reaction. J Ginseng Res. 2011;35:60–68. doi: 10.5142/jgr.2011.35.1.060. [DOI] [Google Scholar]
- 54.Deng A, Valdivielso JM, Munger KA, Blantz RC, Thomson SC. Vasodilatory N-methyl-D-aspartate receptors are constitutively expressed in rat kidney. J Am Soc Nephrol. 2002;13:1381–1384. doi: 10.1097/01.ASN.0000013293.11876.4E. [DOI] [PubMed] [Google Scholar]
- 55.Leung JC, Marphis T, Craver RD, Silverstein DM. Altered NMDA receptor expression in renal toxicity: protection with a receptor antagonist. Kidney Int. 2004;66:167–176. doi: 10.1111/j.1523-1755.2004.00718.x. [DOI] [PubMed] [Google Scholar]
- 56.Kawano K, Hirashima T, Mori S, Saitoh Y, Kurosumi M, Natori T. Spontaneous long-term hyperglycemic rat with diabetic complications. Otsuka Long-Evans Tokushima Fatty (OLETF) strain. Diabetes. 1992;41:1422–1428. doi: 10.2337/diab.41.11.1422. [DOI] [PubMed] [Google Scholar]
- 57.Seo YS, Kim JH, Jo NY, Choi KM, Baik SH, Park JJ, Kim JS, Byun KS, Bak YT, Lee CH, et al. PPAR agonists treatment is effective in a nonalcoholic fatty liver disease animal model by modulating fatty-acid metabolic enzymes. J Gastroenterol Hepatol. 2008;23:102–109. doi: 10.1111/j.1440-1746.2006.04819.x. [DOI] [PubMed] [Google Scholar]
- 58.Horie K, Miyata T, Maeda K, Miyata S, Sugiyama S, Sakai H, van Ypersole de Strihou C, Monnier VM, Witztum JL, Kurokawa K. Immunohistochemical colocalization of glycoxidation products and lipid peroxidation products in diabetic renal glomerular lesions. Implication for glycoxidative stress in the pathogenesis of diabetic nephropathy. J Clin Invest. 1997;100:2995–3004. doi: 10.1172/JCI119853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nagai R, Unno Y, Hayashi MC, Masuda S, Hayase F, Kinae N, Horiuchi S. Peroxynitrite induces formation of N(epsilon)-(carboxymethyl) lysine by the cleavage of Amadori product and generation of glucosone and glyoxal from glucose: novel pathways for protein modification by peroxynitrite. Diabetes. 2002;51:2833–2839. doi: 10.2337/diabetes.51.9.2833. [DOI] [PubMed] [Google Scholar]
- 60.Yan SD, Schmidt AM, Anderson GM, Zhang J, Brett J, Zou YS, Pinsky D, Stern D. Enhanced cellular oxidant stress by the interaction of advanced glycation end products with their receptors/binding proteins. J Biol Chem. 1994;269:9889–9897. [PubMed] [Google Scholar]
- 61.Ahmed N. Advanced glycation endproducts: role in pathology of diabetic complications. Diabetes Res Clin Pract. 2005;67:3–21. doi: 10.1016/j.diabres.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 62.Kang KS, Yamabe N, Kim HY, Park JH, Yokozawa T. Effects of heat-processed ginseng and its active component ginsenoside 20(S)-Rg3 on the progression of renal damage and dysfunction in type 2 diabetic Otsuka Long-Evans Tokushima Fatty rats. Biol Pharm Bull. 2010;33:1077–1081. doi: 10.1248/bpb.33.1077. [DOI] [PubMed] [Google Scholar]
- 63.Wijewickreme AN, Krejpcio Z, Kitts DD. Hydroxyl scavenging activity of glucose, fructose, and ribose-lysine model Maillard products. J Food Sci. 1999;64:457–461. doi: 10.1111/j.1365-2621.1999.tb15062.x. [DOI] [Google Scholar]
- 64.Bekedam EK, Schols HA, Cammerer B, Kroh LW, van Boekel MA, Smit G. Electron spin resonance (ESR) studies on the formation of roasting-induced antioxidative structures in coffee brews at different degrees of roast. J Agric Food Chem. 2008;56:4597–4604. doi: 10.1021/jf8004004. [DOI] [PubMed] [Google Scholar]
- 65.Chen XM, Kitts DD. Antioxidant activity and chemical properties of crude and fractionated Maillard reaction products derived from four sugar-amino acid Maillard reaction model systems. Ann N Y Acad Sci. 2008;1126:220–224. doi: 10.1196/annals.1433.028. [DOI] [PubMed] [Google Scholar]
- 66.Li X, Zheng Y, Liu M, Zhang L. A study on maillard reaction and its products during processing of red ginseng. Zhongguo Zhong Yao Za Zhi. 1999;24:274–278. [PubMed] [Google Scholar]
- 67.Suzuki Y, Choi KJ, Uchida K, Ko SR, Sohn HJ, Park JD. Arginyl-fructosyl-glucose and arginyl-fructose, compounds related to browning reaction in the model system of steaming and heat-drying processes for the preparation of red ginseng. J Ginseng Res. 2004;28:143–148. doi: 10.5142/JGR.2004.28.3.143. [DOI] [Google Scholar]
- 68.Johnson RR, Alford ED, Kinzer GW. Formation of sucrose pyrolysis products. J Agric Food Chem. 1969;17:22–24. doi: 10.1021/jf60161a013. [DOI] [Google Scholar]
- 69.Ito H. The formation of maltol and isomaltol through degradation of sucrose. Agric Biol Chem. 1977;41:1307–1308. doi: 10.1271/bbb1961.41.1307. [DOI] [Google Scholar]
- 70.Bjeldanes LF, Chew H. Mutagenicity of 1,2-dicarbonyl compounds: maltol, kojic acid, diacetyl and related substances. Mutat Res. 1979;67:367–371. doi: 10.1016/0165-1218(79)90034-X. [DOI] [PubMed] [Google Scholar]
- 71.Yasumoto E, Nakano K, Nakayachi T, Morshed SR, Hashimoto K, Kikuchi H, Nishikawa H, Kawase M, Sakagami H. Cytotoxic activity of deferiprone, maltol and related hydroxyketones against human tumor cell lines. Anticancer Res. 2004;24:755–762. [PubMed] [Google Scholar]
- 72.Harvey RS, Reffitt DM, Doig LA, Meenan J, Ellis RD, Thompson RP, Powell JJ. Ferric trimaltol corrects iron deficiency anaemia in patients intolerant of iron. Aliment Pharmacol Ther. 1998;12:845–848. doi: 10.1046/j.1365-2036.1998.00380.x. [DOI] [PubMed] [Google Scholar]
- 73.Verma S, Cam MC, McNeill JH. Nutritional factors that can favorably influence the glucose/insulin system: vanadium. J Am Coll Nutr. 1998;17:11–18. doi: 10.1080/07315724.1998.10718730. [DOI] [PubMed] [Google Scholar]
- 74.Reffitt DM, Burden TJ, Seed PT, Wood J, Thompson RP, Powell JJ. Assessment of iron absorption from ferric trimaltol. Ann Clin Biochem. 2000;37(Pt 4):457–466. doi: 10.1258/0004563001899645. [DOI] [PubMed] [Google Scholar]
- 75.Jang DS, Kim JM, Lee YM, Kim YS, Kim JH, Kim JS. Puerariafuran, a new inhibitor of advanced glycation end products (AGEs) isolated from the roots of Pueraria lobata. Chem Pharm Bull (Tokyo) 2006;54:1315–1317. doi: 10.1248/cpb.54.1315. [DOI] [PubMed] [Google Scholar]
- 76.Price DL, Rhett PM, Thorpe SR, Baynes JW. Chelating activity of advanced glycation end-product inhibitors. J Biol Chem. 2001;276:48967–48972. doi: 10.1074/jbc.M108196200. [DOI] [PubMed] [Google Scholar]
- 77.Huang D, Ou B, Prior RL. The chemistry behind antioxidant capacity assays. J Agric Food Chem. 2005;53:1841–1856. doi: 10.1021/jf030723c. [DOI] [PubMed] [Google Scholar]
- 78.Kang KS, Yamabe N, Kim HY, Yokozawa T. Role of maltol in advanced glycation end products and free radicals: in-vitro and in-vivo studies. J Pharm Pharmacol. 2008;60:445–452. doi: 10.1211/jpp.60.4.0006. [DOI] [PubMed] [Google Scholar]
- 79.Nakai S, Shinzato T, Nagura Y, Masakane I, Kitaoka T, Shinoda T, Yamazaki C, Sakai R, Ohmori H, Morita O, et al. An overview of regular dialysis treatment in Japan (as of 31 December 2001). Ther Apher Dial. 2004;8:3–32. doi: 10.1111/j.1526-0968.2004.00180.x. [DOI] [PubMed] [Google Scholar]
- 80.Yamabe N, Yokozawa T. Activity of the Chinese prescription Hachimi-jio-gan against renal damage in the Otsuka Long-Evans Tokushima fatty rat: a model of human type 2 diabetes mellitus. J Pharm Pharmacol. 2006;58:535–545. doi: 10.1211/jpp.58.4.0014. [DOI] [PubMed] [Google Scholar]
- 81.Levin A. Identification of patients and risk factors in chronic kidney disease: evaluating risk factors and therapeutic strategies. Nephrol Dial Transplant. 2001;16 Suppl 7:57–60. doi: 10.1093/ndt/16.suppl_7.57. [DOI] [PubMed] [Google Scholar]