Abstract
Interactions between the enteric pathogen Salmonella typhimurium and the luminal surface of the intestine provoke an acute inflammatory response, mediated in part by epithelial cell secretion of the chemokine IL-8 and other proinflammatory molecules. This study investigated the mechanism by which this pathogen induces IL-8 secretion in physiologically polarized model intestinal epithelia. IL-8 secretion induced by both the prototypical proinflammatory cytokine TNF-α and S. typhimurium was NF-κB dependent. However, NF-κB activation and IL-8 secretion induced by S. typhimurium, but not by TNF-α, was preceded by and required an increase in intracellular [Ca2+]. Additionally, agonists that increased intracellular [Ca2+] by receptor-dependent (carbachol) or independent (thapsigargin, ionomycin) means also induced IL-8 secretion. Furthermore, the ability of S. typhimurium mutants to induce IκB-α degradation, NF-κB translocation, and IL-8 transcription and secretion correlated precisely with their ability to induce an intracellular [Ca2+] increase in model intestinal epithelia, but not with their ability to invade these cells. Finally, S. typhimurium, but not TNF-α, induced a Ca2+-dependent phosphorylation of IκB-α. These results indicate that S. typhimurium–induced activation of NF-κB–dependent epithelial inflammatory responses proceeds by a Ca2+-mediated activation of an IκB-α kinase. These observations raise the possibility that pharmacologic intervention of the acute inflammatory response can be selectively matched to the specific class of initiating event.
Introduction
The mammalian intestinal epithelium is a highly specialized tissue that must maintain complex selective secretory and absorptive functions while interfacing with the external environment of the intestinal lumen (1). Particularly in the colon, the epithelium exists within a diverse ecology of microflora, which, under normal conditions, contribute to the physiology of the lower gut. The intestinal epithelial cells, or enterocytes, have evolved highly selective physical, chemical, and immunological barriers to permit this mutually beneficial co-existence. Despite such adaptations a subset of enteric organisms have evolved a variety of mechanisms to surmount these barriers, disturb the host-prokaryotic equilibrium, and presumably acquire a selective advantage. Luminal pathogens, such as Vibrio cholerae, secrete enterotoxins that result in physiologic alterations of the gut mucosa via receptor-mediated events (2). Other organisms are termed enteroinvasive, as they are able to adhere, invade, and disseminate systemically or, alternatively, proliferate within the enterocyte (e.g., Salmonella, Shigella, and Yersinia spp.). An important representative of this latter group is Salmonella typhimurium, a causative agent of acute enteritis (3). Acute enteritis caused by food- or water-borne S. typhimurium is manifested clinically by severe cramping and secretory diarrhea and is one of the most common causes of infectious gastroenteritis in the United States (2). The resulting dehydration can be fatal, especially in the absence of medical treatment.
Infection of human epithelia by S. typhimurium leads to recruitment and subsequent transepithelial migration of polymorphonuclear leukocytes (PMNs) (reviewed in refs. 4 and 5), resulting in a luminal acute inflammatory infiltrate that is the pathologic hallmark of this infection. Recent work with model cultured epithelial systems has begun to elucidate the molecular mechanisms by which salmonellae induce inflammatory colitis. The epithelial cells do not merely comprise a passive barrier but play an active role in orchestrating this process, particularly by secreting inflammatory mediators that direct PMN movement. One key mediator is the C-X-C chemokine IL-8 (6–9), an 8.5-kDa peptide that has well-studied properties as a neutrophil-specific chemotactic agent. IL-8 is rapidly induced at the transcriptional level, as is the case for most chemokines. In response to inflammatory stimuli, intestinal epithelial cells secrete IL-8 into the basolateral (serosal) matrix. We and others have demonstrated that S. typhimurium interactions with model intestinal epithelia result in de novo induction of IL-8 mRNA (10) and subsequent distribution of IL-8 within the underlying matrix, forming a long-lasting haptotactic gradient that guides PMNs to subepithelial space (11).
Although epithelial IL-8 induction in response to bacterial stimuli has been observed in a number of laboratories, the mechanism by which this activation occurs is not well understood. The goal of this study was thus to elucidate the signaling events by which S. typhimurium induces transcriptional activation of the IL-8 gene. Past analysis of the determinants of inducibility of the IL-8 promoter revealed a critical role for the transcription factor NF-κB, with modifying roles variously attributed to the AP-1 and C/EBPβ transcription factors (12–16). Many cell types, including epithelial cells, utilize NF-κB as a rapidly inducible transcriptional activator in response to immune and proinflammatory signals, and NF-κB has been implicated in the upregulation of numerous immunomodulatory genes (reviewed in refs. 17–19). NF-κB activation has been observed in response to S. typhimurium in nonpolarized cells, and its binding sites have been shown to be essential for expression of IL-8 promoter reporter constructs (20). However, the mechanism by which this pathogen activates NF-κB is not yet characterized. In response to proinflammatory cytokines, NF-κB is activated by a well-characterized post-translational pathway. In quiescent cells, the dimeric NF-κB is sequestered in the cytoplasm by a class of specific inhibitor proteins, IκBs. In most cases, activating stimuli results in phosphorylation of IκB, which targets this protein for polyubiquitination and proteasomal degradation, thus allowing release and nuclear translocation of the active DNA-binding dimer. We first asked whether such mechanisms also mediated NF-κB activation in model intestinal epithelia in response to S. typhimurium and then investigated what early bacterial-induced signals could lead to this activation. Increased intracellular [Ca2+] has been associated with S. typhimurium invasion of cultured eukaryotic cells (21, 22). Because calcium mobilization is a widely used signaling mechanism and has been reported to influence NF-κB activation (23, 24), we asked whether epithelial cell Ca2+ mobilization in response to S. typhimurium might play a role in NF-κB activation and subsequent IL-8 secretion. We report that the ability of S. typhimurium strains to elicit changes in intracellular [Ca2+] strictly correlates with their ability to induce proinflammatory signals from, but not to invade, model intestinal epithelia. These results indicate that the critical proinflammatory signals from an important pathogen are the result of this novel NF-κB activating pathway.
Methods
Cell and bacterial culture.
Confluent monolayers of T84 cells, referred to as model intestinal epithelia, were grown as described previously (25). HT-29 cl/19A epithelial cells were also maintained as described previously (25). S. typhimurium strains (χ3306-wild-type), HilΔ, and S. typhimurium PhoPc (invasion defective strains) were maintained and prepared for use (via overnight growth under nonagitated microaerophilic conditions) as described previously (11). Escherichia coli F-18 was a gift of E. McCormick (Harvard Medical School, Boston, Massachusetts, USA).
Drug treatments.
To chelate intracellular [Ca2+], model intestinal epithelia were treated with 15 μM BAPTA-AM (Molecular Probes Inc., Eugene, Oregon, USA) for 45 minutes before the addition of a stimulating agonist. This treatment was in HBSS with reduced [Ca2+] (0.25 mM CaCl2 instead of 1.25 mM CaCl2). This reduced [Ca2+] allowed maintenance of epithelial tight junctions, yet permitted effective Ca2+ chelation while avoiding the very high BAPTA concentrations, which can acidify cells (26). MG-132 (Calbiochem-Novabiochem Corp., San Diego, California, USA) was used at 50 μM in DMSO, and cells were pretreated for 1 hour. Recombinant human TNF-α (R&D Systems Inc., Minneapolis, Minnesota, USA), carbachol, forskolin, cycloheximide, and thapsigargin (Sigma Chemical Co., St. Louis, Missouri, USA), ionomycin (Calbiochem-Novabiochem), were added as described in the figure legends.
IL-8 secretion.
IL-8 secretion from model intestinal epithelia was measured as described previously (8, 10, 27). Briefly, model intestinal epithelia (resistance > 800 Ω.cm2) were washed 3 times with HBSS and placed into 300 μL of HBSS. Thirty minutes later, agonists were added. Recombinant human TNF-α, carbachol, thapsigargin, ionomycin, and forskolin were added basolaterally. S. typhimurium was added apically by placing model epithelia in empty wells and adding 25 μL of S. typhimurium-containing HBSS (1.6 × 1010 bacteria/mL) to the apical surface. This inoculum has been previously shown to correspond to 30 associated bacteria per T84 cell (8). Thirty minutes later, the model epithelia were returned to the same HBSS in which they had incubated before the addition of S. typhimurium. Five hours after the addition of agonist, T84 cell supernatants were removed and assayed for IL-8 via a sandwich ELISA as described previously (10).
Transient transfection.
Transient transfections and assay of chloramphenicol acetyl transferase (CAT) in HT-29 cl/19A cells were carried out by a modified CaPO4 method as described previously (28). The reporter constructs span +23 to –578 of the human IL-8 promoter as described in ref. 13. Cell lysates were prepared 6 hours after addition of agonist.
Determination of intracellular calcium.
Polycarbonate sheets (1.7-mm thick) were machined to precisely fit diagonally across standard fluorescence cuvettes (Sarstedt, Newton, North Carolina, USA) and included a 1.0 × 1.5 cm window cut out of the polycarbonate to incorporate the area targeted by the excitation beam of a Hitachi (Sunnyvale, California, USA) F-4500 fluorescence spectrometer. Transparent polyester filters (Corning Materials, Corning, New York, USA) (0.4 μM pore size) were mounted over the window and coated with 2 mg collagen (added in 200 μL ethanol). T84 cells were plated on these filters at high density (105/cm2) and allowed 7–10 days to become a confluent polarized model intestinal epithelia. These cells and filter apparatus were then washed 3× in HBSS, and placed into HBSS containing 5 μM Fura-2-acetoxymethylester (AM; Molecular Probes) for 60 minutes at 37°C, followed by 3 more washes. The bottom and side edges of the polycarbonate piece were then coated with vacuum grease, and the assembly was placed into a fluorescence cuvette. One milliliter of HBSS was subsequently placed into the basolateral reservoir of the model intestinal epithelia. After verifying the integrity of the grease seal by demonstrating that the HBSS did not visibly leak over to the apical aspect, 1 mL of HBSS was added to the apical reservoir of this model epithelia. This Fura-2–loaded model epithelia was then incubated for an additional 10 minutes to permit diffusional washout of Fura-2 sequestered in the extracellular spaces. The HBSS (both apical and basolateral) was then aspirated out and fresh HBSS added (1 mL to each aspect) without disturbing the grease seal.
Fura-2 loaded epithelia were placed into a spectrofluorometer (thermostated to 37°C) such that the apical surface faced (at a 45° angle) the excitation beam. A 4-mm stir bar (8-mm stirbar cut in half; Fisher Scientific Co., Pittsburgh, Pennsylvania, USA) was then placed in the aspect (apical or basolateral) that was to receive addition of an agonist. Fluorescence emission was read at 505 nm while the excitation wavelength was changed between 340 nm and 380 nm 4 times per second via Intracellular Cation software (Hitachi). After reading fluorescence for 3–5 minutes, S. typhimurium or other agonists were added as indicated in the figure legends. For additions of bacteria, 50 μL of a culture containing 1.6 × 1010 CFU/mL was added.
Values of intracellular [Ca2+], were calculated via the Grynciewitz equation (R–Rmin)/(Rmax–R)*Kd which was taken to be 2.54 × 10–7 M. Rmax and Rmin were measured by adding digitonin (10 μM) and then EGTA (20 mM), respectively, to Fura-2–loaded model epithelia. Fura-2 leakage that occurred over the course of the [Ca2+] measurement was assessed and corrected for by adding EGTA (20 mM) at the end of the measurement and subtracting the changes in fluorescence that immediately (within 2 seconds) resulted. The leakage was assumed to have occurred at a uniform rate during the measurement. We did not observe a significant difference in leakage between epithelia that were treated with bacteria or with only buffer. The autofluorescence of the bacteria as well as changes in autofluorescence of the model epithelia that were induced by the bacteria were measured in non–Fura-2–loaded epithelia and subsequently corrected for by subtraction. To increase the signal/noise ratio of our measurement, the fluorescence values were averaged to produce 1 ratio value and thus 1 value for intracellular [Ca2+] every 2 seconds.
Northern hybridization.
Model intestinal epithelia were treated with cytokine or bacteria as already described here. Total RNA was extracted with TRIzol reagent (GIBCO BRL, Gaithersburg, Maryland, USA) and was analyzed by Northern hybridization analysis as described elsewhere (28). Equal loading of mRNA was demonstrated by ethidium bromide staining or probing for GAPDH. Blots were hybridized with 32P-labeled cDNA probes encoding IL-8.
Western blots.
After experimental treatment, model epithelia (3 × 107 to 5 × 107 cells) were washed in cold HBSS and harvested by scraping. Whole cell extracts from T84 cells were prepared by rapid lysis in SDS loading buffer. Western blots were performed as described previously (28). Anti–IκB-α antisera (Santa Cruz Biotechnology Inc., Santa Cruz, California, USA), anti-p65 (Rockland Immunochemicals, Gilbertsville, Pennsylvania, USA), and anti–phospho-IκB-α (New England Biolabs Inc., Beverly, Massachusetts, USA) were used at a 1:1,000 dilution.
Immunoreactive proteins were detected according to the enhanced chemiluminescent protocol (Amersham Life Sciences Inc., Arlington Heights, Illinois, USA) using 1:1,000 horseradish peroxidase–linked goat anti-rabbit secondary antiserum. Blots were exposed to film for 1–5 minutes.
Electrophoretic mobility shift assay.
After experimental treatment, model epithelia (3 × 107 to 5 × 107 cells) were washed in cold HBSS and harvested by scraping. Cytoplasmic and nuclear extract preparation and binding reactions were performed as described elsewhere (28), with additional wash steps of isolated nuclei to remove potential contamination with cytoplasmic proteins. Competition studies were performed by adding unlabeled double-stranded oligonucleotides to the binding reaction for 10 minutes before the addition of labeled oligonucleotide. For supershift analysis, nuclear extracts from the S. typhimurium–treated epithelial cells were incubated with 1 μL of antisera for 15 minutes at room temperature before addition of binding buffer containing labeled oligonucleotide. Oligonucleotides utilized are as follows: IL8 RE 5′-TTCATCAGTTGCAAATCGTGGAATTTCCTTTCCTCTGGCTA; VCAM-IRF 5′-GGAGTGAAATAGAAAGTCTGTG. Reverse complement strands were designed to leave GATC overhangs on both ends. All oligonucleotides were polyacrylamide gel purified prior to annealing and labeling.
S. typhimurium adherence assay.
S. typhimurium adherence to the apical aspect of model epithelia was measured as described previously (8) except that the current experiments used inverted epithelia to prevent bacteria from contacting and subsequently adhering to the plastic portion of the cell culture inserts.
Results
Both TNF-α and S. typhimurium induce IL-8 secretion by an NF-κB–mediated mechanism.
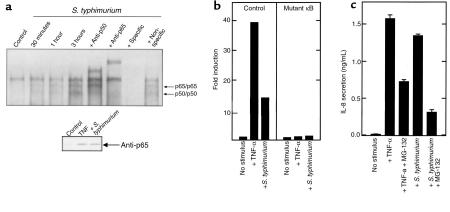
Extensive literature exists implicating the transcriptional activator NF-κB with the induced transcription of the IL-8 gene in response to inflammatory cytokines. Thus, we sought to define the role of NF-κB in regulating S. typhimurium–induced IL-8 secretion in model intestinal epithelia. We used electrophoretic mobility shift assays (EMSAs) to determine whether interactions of S. typhimurium with the physiologic apical surface of model intestinal epithelial could induce NF-κB translocation. For these experiments, a double-stranded oligonucleotide spanning the IL-8 NF-κB motif was used to probe nuclear extracts derived from model intestinal epithelia treated with S. typhimurium or TNF-α (Figure 1a). The probe used in these studies corresponded to the sequence spanning from –67 to –97 on the human IL-8 promoter, the site of a distinctive motif divergent from typical κB motifs (TGGAATTTCC versus the canonical GGGANNTTCC), which has been demonstrated to interact with a specific NF-κB form (homodimeric p65) (12). Resting cells showed no NF-κB binding activity, whereas model epithelia exposed to S. typhimurium showed induction of 2 nucleoprotein complexes within 30 minutes, with maximum induction seen within 3 hours. TNF-α applied basolaterally resulted in the appearance of identical nucleoprotein complexes that were seen within 15 minutes, were maximal at 1 hour, and which declined slightly by 3 hours (data not shown). In all experiments, a low mobility, constitutively present band was seen.
Figure 1.
S. typhimurium and TNF-α induce IL-8 expression by an NF-κB–mediated mechanism. (a) EMSAs with an IL-8 NF-κB motif probe. Nuclear extracts were derived from model intestinal epithelia maintained under control conditions or coincubated with wild-type S. typhimurium as described in Methods for the indicated times. Addition of unlabeled competitor probe or antisera when indicated. Inset: nuclear extracts were Western blotted with anti-p65 as described in Methods. (b) Transient transfection assay with IL-8 reporter constructs. Transfected HT-29 cl19A cells were treated as indicated. Data are reported as fold induction of CAT activity over basal. (c) IL-8 ELISA from model epithelia. Cells were incubated with basolateral TNF-α (10 ng/mL) or apical wild-type S. typhimurium, either with or without 1 hour pretreatment with 50 μM MG-132. Basolateral IL-8 secretion was measured 5 hours later.
To identify the nucleoprotein complexes, supershift analysis was performed on the nucleoprotein complexes formed on the IL-8 NF-κB site in response to stimulation with S. typhimurium for 3 hours. Addition of antisera to the NF-κB component p50 completely supershifted the higher mobility complex, whereas addition of anti-p65 antibody resulted in supershift of both inducible complexes. Furthermore, the presence of 100-fold molar excess of specific probe resulted in the abolition of the inducible bands (as well as a constitutive lower mobility complex), whereas 100-fold molar excess of an unrelated sequence had no effect. We were able to demonstrate the same 3 specific nucleoprotein complexes in other intestinal epithelial cell lines (CACO-2-BBE and HT-29 cl/19A) and generate equivalent supershift patterns with several independent anti–NF-κB antisera (data not shown). EMSA experiments with probes containing consensus NF-κB motifs verified binding of the predicted heterodimeric p50/p65 and homodimeric p50/p50 (data not shown). Immunoblots with nuclear and cytoplasmic extracts confirms the inducible appearance of NF-κB p65 in the nucleus of epithelial cells treated with TNF-α or S. typhimurium (Figure 1a, inset). From these experiments, we conclude that S. typhimurium induces specific NF-κB DNA binding activity of both p50/p65 heterodimers and p65/p65 homodimers.
Having characterized the NF-κB activation that occurs in model intestinal epithelia in response to S. typhimurium, we next investigated whether this activation was necessary for induction of IL-8 secretion. Using transient transfection assays we found that both S. typhimurium and TNF-α induced expression of an IL-8 promoter-CAT reporter gene construct; however, an identical construct bearing a mutation in the NF-κB motif at position –72 could not be induced by either agonist (Figure 1b). This indicates that an intact NF-κB binding motif is necessary for IL-8 activation by either agonist. To investigate further the requirement of NF-κB activation for IL-8 secretion, we used the cell permeable peptide aldehyde MG-132, which blocks the catalytic activity of the 26 S proteasome subunit, abrogating the cells ability to degrade IκB isoforms and thus activate NF-κB (29, 30). Pretreatment of model intestinal epithelia with this inhibitor reduced IL-8 secretion induced by either S. typhimurium or TNF-α, implying that proteasomal catalysis, known to be required for NF-κB activation, was necessary for the IL-8 secretory activity induced by both stimuli (Figure 1c). Taken together, these observations indicate that NF-κB is necessary for IL-8 secretion in response to S. typhimurium, as well as TNF-α, in polarized intestinal epithelial cells.
S. typhimurium–mediated, but not TNF-α –mediated, NF-κB activation and IL-8 secretion requires an increase in intracellular [Ca2+].
We next sought to identify the signals induced by apically applied S. typhimurium that were responsible for NF-κB activation and subsequent IL-8 secretion. Because S. typhimurium induces a [Ca2+] influx in nonpolarized cells (21), we tested whether this occurred in response to bacterial interaction with the apical membrane of polarized cells and, if so, whether such [Ca2+] signals might mediate NF-κB activation and subsequent IL-8 secretion in response to S. typhimurium. We thus developed a system (see Methods) of measuring intracellular [Ca2+] in model intestinal epithelia in which S. typhimurium access could be restricted to the apical surface. To verify that this system provided limited polarized access, we measured changes in intracellular [Ca2+] in response to addition of carbachol (100 μM) added either apically or basolaterally. When added basolaterally, carbachol induced an intracellular [Ca2+] increase of more than 500 nM, whereas carbachol added apically was without effect. As the receptor used by this agonist (the acetylcholine receptor) is only found on the basolateral aspect of intestinal epithelial cells (31), this result indicates our model system provided the limited access these studies required.
We next performed a control experiment that had important effects on the calculations of [Ca2+] changes. Specifically, we measured whether S. typhimurium induced Fura-2 leakage from Fura-2–loaded model epithelia and whether the autofluorescence of model epithelia not loaded with this agent exhibited changes in response to S. typhimurium that would significantly affect the Fura-2 ratio (λex, 340 nm/380 nm; λem, 505 nm). Although there was detectable Fura-2 leakage from model epithelia (which, when occurring into a Ca2+-containing buffer, causes an increase in the Fura-2 340/380 ratio), neither the rate nor amount of leakage was different from control and S. typhimurium–treated epithelia. Therefore, we simply subtracted the fluorescence changes due to this leakage as described in Methods). However, in the absence of Fura-2, we did observe significant fluorescence changes in the autofluorescence of S. typhimurium and model epithelia. These changes occurred about 1 minute after the addition of the bacteria to the epithelia and, somewhat surprisingly, occurred as a quick step rather than a slow drift. This increase in autofluorescence was observed at excitation λ = 340 nm but not λ = 380 nm, thus increasing the Ca2+-sensitive ratio, thereby potentially enabling investigators to misconstrue this change in autofluorescence for an increase in intracellular [Ca2+]. We hypothesize this change in autofluorescence is due to shape changes in the epithelial cells and/or the bacteria. In calculating values of intracellular [Ca2+], we corrected for such artifacts by subtraction of the autofluorescence of non–Fura-2–loaded model epithelia. That our reported changes in intracellular [Ca2+] include such corrections may be one possible reason some of our results regarding stimulus-induced changes in intracellular [Ca2+] differ somewhat from those reported by Pace et al. (21).
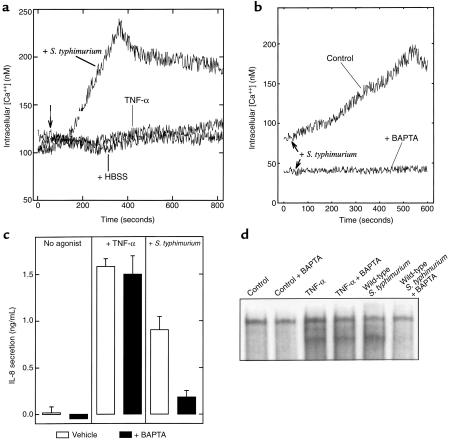
Having defined our ability to measure intracellular [Ca2+] in polarized model epithelia, we used this system to measure intracellular [Ca2+] in response to S. typhimurium. Apically applied S. typhimurium induced an increase in intracellular [Ca2+] within 1 minute that peaked at about 4 minutes and declined slowly thereafter but did not return to baseline during the remainder of our measurements (Figure 2a). In contrast, no changes in intracellular [Ca2+] were observed in response to TNF-α even though this cytokine elicits similar (or greater) levels of NF-κB activation and IL-8 secretion as S. typhimurium (Figure 1c). TNF-α did not prevent Ca2+ mobilization as TNF-α–treated epithelia that were subsequently exposed to S. typhimurium exhibited changes in intracellular [Ca2+] that were indistinguishable from model epithelia treated with S. typhimurium alone (data not shown). To determine whether the observed increase in intracellular [Ca2+] played a role in mediating S. typhimurium elicitation of an inflammatory response, experiments were performed with the Ca2+ chelator BAPTA. Preloading model intestinal epithelia with the chelator prevented any detectable increase in free intracellular [Ca2+] in response to S. typhimurium (Figure 2b) and resulted in abolition of the IL-8 secretory response to S. typhimurium (Figure 2c). In contrast, BAPTA loading had no effect on IL-8 secretion induced by TNF-α. In addition, EMSAs demonstrated that calcium chelation specifically abolished S. typhimurium -induced NF-κB activation, while having no effect on NF-κB activation induced by TNF-α (Figure 2d). This marked differential effect of Ca2+ chelation on TNF-α and S. typhimurium induced activation of this proinflammatory pathway was observed at several time points of stimulation and over a range of concentrations of BAPTA (data not shown). These 2 pathways may be able to act additively to induce inflammation, as S. typhimurium and TNF-α together induced more IL-8 secretion than either agonist alone (S. typhimurium, 10 ng/mL TNF-α, and S. typhimurium + 10 ng/mL TNF-α induced 1.55 ± 0.20, 1.25 ± 0.25, and 2.56 ± 0.6 ng/mL IL-8, respectively). Further, TNF-α–treated epithelia (20 ng/mL, 15 minutes) that were subsequently treated with S. typhimurium exhibited [Ca2+] increases indistinguishable from those exhibited by epithelia treated with S. typhimurium only. Together, these results indicate that an increase in intracellular [Ca2+] is part of the signaling pathway by which S. typhimurium, but not TNF-α, activates proinflammatory events in model intestinal epithelia.
Figure 2.
S. typhimurium, but not TNF-α, induce Ca2+ mobilization that is required for NF-κB activation and IL-8 secretion. (a, b) Fluorometric assay of intracellular calcium. Model intestinal epithelia were loaded with the Ca2+ indicator Fura-2 and placed in a thermostated spectrofluorometer and intracellular Ca2+ was measured as described in Methods. TNF-α was added basolaterally or S. typhimurium was added apically as indicated. In b, intracellular [Ca2+] was measured in response to S. typhimurium in the absence or presence of intracellular BAPTA, which was simultaneously loaded with Fura-2. (c) IL-8 ELISA. Model intestinal epithelia were stimulated basolaterally with TNF-α or infected apically with S. typhimurium in the presence (filled bars) or absence (open bars) of 15 μM BAPTA. Basolateral IL-8 secretion was measured 5 hours later. (d) EMSA with IL-8 probe. Model intestinal epithelia were treated as in c. After stimulation (30 minutes for TNF-α; 1 hour for S. typhimurium), nuclear extracts were isolated and NF-κB translocation assessed as described in Methods.
Next, we sought to better characterize the increase in intracellular [Ca2+] observed in response to S. typhimurium. We did not observe any increase in intracellular [Ca2+] when model intestinal epithelia were treated with either S. typhimurium supernatants (at concentrations of at least 5× those in our live cultures) or heat-killed S. typhimurium (by incubation at 70°C for 30 minutes; data not shown). These experiments indicated that elicitation of this signaling event requires epithelial contact with intact S. typhimurium. We next sought to temporally resolve Ca2+ mobilization and S. typhimurium adherence. At 30 seconds after addition of the bacteria, there were on average 3.96 ± 0.5 specifically adhered bacteria per epithelial cell. Bacterial adherence continued to increase slowly for as long as we measured, with 12.12 ± 0.5 bacteria adhered per epithelial cell at 45 minutes. However, there was no significant difference in IL-8 secretion (5 hours after exposure to bacteria) from epithelia exposed to S. typhimurium for 30 seconds or 45 minutes (105 ± 8% for 30 seconds compared with 45 minutes). Therefore, by the time the intracellular [Ca2+] had significantly increased, S. typhimurium adherence sufficient for maximal epithelial IL-8 secretion had taken place.
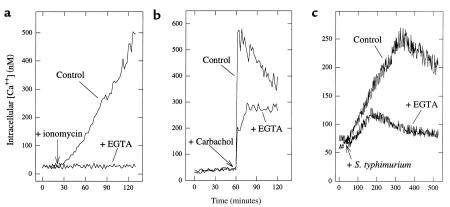
To ascertain whether the increase in intracellular [Ca2+] results from a Ca2+ influx, release from intracellular stores, or both, we measured the effect of adding EGTA to chelate extracellular Ca2+ on agonist-induced intracellular [Ca2+] increases. As expected, the increase in intracellular [Ca2+] induced by ionomycin (resulting from Ca2+ influx; see later here) was abolished by EGTA (Figure 3a). The increase in intracellular [Ca2+] induced by carbachol is thought to result from release of intracellular stores followed by Ca2+ influx (32). Thus, as expected, the carbachol-induced increase in intracellular [Ca2+] was reduced but not eliminated by EGTA (Figure 3b). In response to S. typhimurium, EGTA also reduced but did not eliminate the increase in intracellular [Ca2+] (Figure 3c). The effect of EGTA was likely due to influences on the epithelia rather than the bacteria, as this compound was only added to the basolateral aspect of our model system 10 seconds before the bacteria (kept in Ca2+ containing buffer) were added to the apical aspect. Thus, like carbachol, S. typhimurium appears to induce release of both intracellular Ca2+ stores and Ca2+ influx.
Figure 3.
Chelation of extracellular Ca2+ indicates S. typhimurium–induced [Ca2+] increase results from both intracellular stores and Ca2+ influx. Fluorometric assays of intracellular calcium. Intracellular [Ca2+] was measured in Fura-2–loaded model intestinal epithelia in response to (a) ionomycin (1 μg/mL), (b) carbachol (100 μM), or (c) S. typhimurium in the presence or absence of EGTA (5 mM), which was added 10 seconds before the addition of an agonist.
Agonists that increase intracellular [Ca2+] induce NF-κB activation and IL-8 secretion in model epithelium.
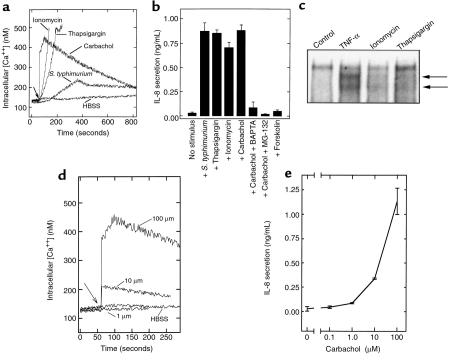
Having demonstrated that increases in intracellular [Ca2+] are required for S. typhimurium–induced activation of NF-κB and subsequent IL-8 secretion, we next measured whether other well-defined agonists that induce Ca2+ mobilization might also elicit these proinflammatory responses. Ionomycin permits extracellular Ca2+ to enter cells by facilitated diffusion down a [Ca2+] gradient (33). Thapsigargin elevates cytosolic [Ca2+] from intracellular stores by inhibiting the endoplasmic reticulum (ER) Ca2+-ATPase, thus allowing efflux to the cytoplasm (34). Carbachol initially increases intracellular [Ca2+] by activating the acetylcholine receptor that, via a G-protein/phosphatidylinositol-specific phospholipase C–mediated mechanism, causes release of ER Ca2+. Each of these agonists was able to elevate intracellular [Ca2+] in model epithelia (Figure 4a) and stimulate IL-8 secretion (Figure 4b). Furthermore, this IL-8 secretion could be ablated by either Ca2+ chelation with BAPTA or proteasomal blockade with MG-132 (Figure 4b and data not shown). These agents also cause NF-κB translocation in model epithelia within 1 hour (Figure 4c) as well as in nonpolarized cells (23). Thus, there is a close correlation between increases in intracellular [Ca2+] and activation of IL-8 secretion by a pathway involving activation of NF-κB.
Figure 4.
Stimuli that induce an increase in intracellular [Ca2+] elicit IL-8 secretion. (a) Fluorometric assays of intracellular [Ca2+]. Intracellular [Ca2+] was measured in Fura-2–loaded model intestinal epithelia in response to S. typhimurium, thapsigargin (10 μM) ionomycin (1 μg/mL), carbachol (100 μM), or forskolin (10 μM). (b) IL-8 ELISA. Five hours after addition of the agonist, basolateral IL-8 secretion was measured by ELISA. BAPTA (added in AM form) and MG-132 were used when indicated as described in Methods. (c) EMSA with IL-8 κB probe. Model epithelia were treated with TNF-α, ionomycin (1 μg/mL) or thapsigargin (10 μM) for 1 hour before extract preparation. NF-κB complexes are indicated with arrows. (d) Intracellular [Ca2+] and (e) IL-8 secretion, in response to the indicated dose of carbachol.
A number of agonists that induce Ca2+ mobilization, including carbachol, activate polarized chloride secretion in model intestinal epithelia. Because such chloride secretion leads to volume (and perhaps other) changes in epithelial cells, we considered the possibility that the activation of IL-8 secretion we observed in response to Ca2+-mobilizing agonists was more directly attributable to chloride secretion than to an increase in intracellular [Ca2+]. However, we found that 10 μM forskolin, which activates chloride secretion to a similar extent as carbachol by a cAMP-mediated Ca2+-independent mechanism (35), did not induce significant levels of IL-8 secretion (Figure 4b), indicating Ca2+-mediated activation of IL-8 secretion is not subsequent to chloride secretion.
We next examined the relationship between receptor-mediated changes in intracellular [Ca2+] and activation of IL-8 secretion. A dose response to carbachol was measured for both events (Figure 4, d and e). At concentrations of less than 1 μM carbachol, we did not observe a detectable increase in intracellular [Ca2+] or IL-8 secretion. At 10 μM carbachol, changes in both intracellular [Ca2+] and IL-8 secretion increased concomitantly to about 30% of the response observed at 100 μM. Higher concentrations of carbachol did not lead to a greater increase in intracellular [Ca2+] or IL-8 secretion (data not shown). Last, like Ca2+ mobilization, carbachol-induced IL-8 mRNA synthesis and secretion were only observed when this agonist was added basolaterally (data not shown). This close correlation between changes in intracellular [Ca2+] and IL-8 secretion induced in model intestinal epithelia by a receptor-mediated agonist supports the notion that the Ca2+ mobilization observed in response to S. typhimurium plays an important role in activating secretion of inflammatory mediators.
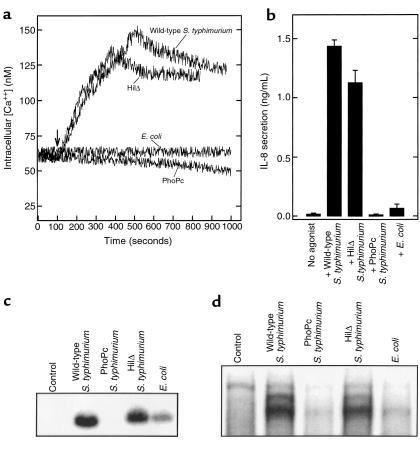
The ability of S. typhimurium mutants to induce an increase in intracellular [Ca2+] correlates with their ability to activate NF-κB and elicit IL-8 secretion. To explore further the relationship between S. typhimurium-induced changes in intracellular [Ca2+], NF-κB activation, and IL-8 secretion we used wild-type S. typhimurium, nonpathogenic noninvasive gut E. coli (F-18, isolated from the feces of a healthy human), and previously characterized invasion-defective S. typhimurium mutants PhoPc and HilΔ. These mutants adhere normally to model epithelia and show similar inability to invade these cells (27, 36) despite their marked phenotypic differences (37–39). We have recently shown that HilΔ but not PhoPc induce IL-8 secretion from model epithelia (27). Here, we investigated whether the ability of these organisms to induce NF-κB activation and subsequently IL-8 synthesis correlated with their ability to elicit changes in intracellular [Ca2+]. Both wild-type S. typhimurium and HilΔ induced an increase in intracellular [Ca2+] and IL-8 secretion, whereas PhoPc and E. coli F-18 elicited very little of either (Figure 5, a and b). This pattern of induction of IL-8 secretion in response to these different organisms correlated very closely with changes in levels of steady-state IL-8 mRNA (Figure 5c) and nuclear translocation of NF-κB as measured by EMSA (Figure 5d). These observations are consistent with the recent demonstration that S. typhimurium invasion is not required for NF-κB activation (40) or IL-8 secretion (27). Thus, among the strains assayed, there is an absolute correlation between a bacterium’s ability to elicit Ca2+ mobilization, translocate NF-κB, and induce IL-8 transcription and IL-8 secretion. Epithelial cell Ca2+ mobilization appears to be an important determinant in whether the intestinal mucosa will initiate an inflammatory response to a particular organism.
Figure 5.
S. typhimurium ability to induce an increase in [Ca2+] correlates with its ability to elicit activation of NF-κB and subsequent IL-8 secretion. Model intestinal epithelia were treated with the indicated strain of S. typhimurium or a nonpathogenic strain of gut E. coli. Bacteria were applied apically. (a) Fluorometric assay of intracellular [Ca2+]. (b) IL-8 secretion measured by ELISA. IL-8 was assayed from the basolateral media after 5 hours. (c) Northern blot probed with IL-8. T84 cell mRNA prepared after infection for 5 hours with the indicated organisms. (d) EMSA with IL-8 probe. Nuclear extracts prepared 3 hours after infection.
The failure of PhoPc to induce Ca2+ mobilization and subsequent NF-κB activation did not result from diminished adherence to our model epithelia, as we found PhoPc adherence at the onset of [Ca2+] increase was equal to that of the other strains (at 30 seconds, adherence of PhoPc and HilΔ was 85% ± 22 and 96% ± 19 of wild-type values, respectively). Nor does adherence of this organism to model intestinal epithelia differ at later times (36 and data not shown).
S. typhimurium–induced Ca2+–mediated events are necessary for the induced phosphorylation and degradation of IκB-α.
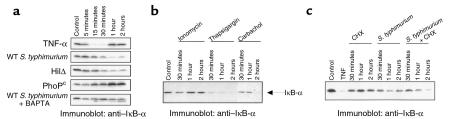
In response to many stimuli, the nuclear translocation of NF-κB is preceded by inducible phosphorylation and proteolytic degradation of an IκB isoform. We thus measured levels of IκB-α by Western blot analysis of whole cell extracts from model intestinal epithelia. Immunoblots from unstimulated cells revealed a single 37-kDa band, corresponding to the observed molecular weight of IκB-α (Figure 6a). Cells treated with TNF-α show the expected degradation of IκB-α within 30 minutes, with resynthesis observed within 1 hour. IκB-α degradation was observed in response to S. typhimurium (the IL-8–inducing wild-type and HilΔ mutant), with maximal degradation seen at 1 hour. The S. typhimurium mutant PhoPc, which does not induce IL-8 secretion or NF-κB translocation, did not cause IκB-α degradation, consistent with our previous observations. Finally, chelating the intracellular Ca2+ of model epithelia with BAPTA abolished IκB-α degradation in response to wild-type S. typhimurium (Figure 6a, last row).
Figure 6.
Proinflammatory S. typhimurium induce Ca2+-dependent degradation of IκB-α. Immunoblots with anti–IκB-α antibodies. (a) Whole cell extracts were prepared at the indicated times from model intestinal epithelia treated with basolateral TNF-α, apical wild-type S. typhimurium (WT), HilΔ and PhoPc mutants, and wild type with BAPTA-pretreated epithelia. (b) Monolayers were treated with basolateral ionomycin (1 μg/mL), thapsigargin (10 μM), or carbachol (100 μM) for the times indicated. (c) Model epithelia were treated with 20 μg/mL cycloheximide, S. typhimurium, or CHX 1 hour before S. typhimurium infection.
Calcium mobilizing agents demonstrated to induce IL-8 synthesis were also capable of inducing IκB-α degradation (Figure 6b). Ionomycin induced partial degradation at 30 minutes with resynthesis by 1 hour, whereas thapsigargin was a much more potent and sustained inducer. In contrast, the receptor-mediated calcium mobilization elicited by carbachol acted in a much more delayed fashion, similar to the kinetics observed with S. typhimurium induction. The slower kinetics of IκB-α degradation induced by carbachol and Salmonella could imply that NF-κB activation occurs via the action of a secondarily synthesized cytokine. Both TNF-α and IL-1 are known to be secreted by T84 cells in response to Salmonella infection. Salmonella-induced IκB-α degradation was therefore assayed from epithelia cultured in the presence of the protein synthesis inhibitor cycloheximide (CHX) (Figure 6c). CHX has no discernible effects on IκB-α levels over 2 hours of treatment, whereas S. typhimurium results in peak IκB-α degradation at 1 hour. Model epithelia pretreated with CHX for 1 hour before Salmonella infection exhibited no reduction of induced IκB-α degradation, ruling out the possibility of a protein synthesis–dependent intermediate involved in the Salmonella-activated pathway. To the contrary, an augmentation of IκB-α degradation was observed at 2 hours. This result is due to the blockade of IκB-α resynthesis, which is induced at the transcriptional level within 2 hours by Salmonella (data not shown). Thus, IκB-α degradation can be elicited by calcium-dependent pathways and, when induced by Salmonella strains, does not require protein synthesis and correlates exactly with the ability to induce IL-8 expression or translocate NF-κB.
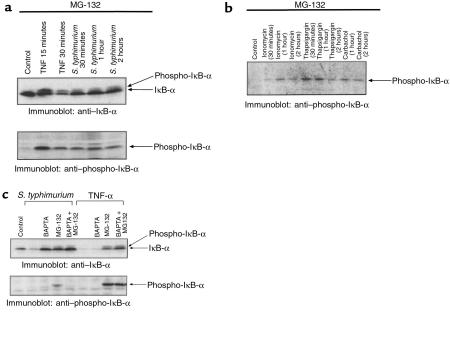
A more proximal regulatory event in the NF-κB activating pathway is the phosphorylation of IκB-α on 2 conserved serine residues (Ser 32 and 36). Phosphorylated IκB-α is highly labile but can be stabilized under conditions of proteasomal blockade with the inhibitor MG-132. Stabilized IκB-α can be detected via its reduced electrophoretic mobility or via phospho-specific IκB-α antibodies. Using MG-132 pretreatment to block the proteolytic activity of the proteasome, the induced degradation of IκB-α in response to TNF-α or S. typhimurium could be inhibited, allowing viewing of a higher molecular weight species consistent with the phosphorylated form (Figure 7a, top row). This finding implicates the proteasome in the activation pathways of both inducers. Immunoblots of the same extracts with an antibody specific to phospho-Ser-IκB-α confirmed the inducible phosphorylation event (Figure 7a, bottom row). Similarly, induction of phospho-IκB-α was observed in an MG-132–treated model epithelium stimulated with calcium mobilizing agonists (Figure 7b). Collectively, these data indicated that all of these agents are capable of inducing an IκB-α kinase activity specific to IκB-α Ser 32.
Figure 7.
Proinflammatory S. typhimurium induces Ca2+-dependent phosphorylation of IκB-α. Immunoblots with anti–IκB-α or anti–phospho-IκB-α antibodies. (a) All model epithelia were pretreated with 50 μM MG-132 for 30 minutes. Whole cell extracts were prepared at the indicated times from cells treated with basolateral TNF-α or apical wild-type S. typhimurium (WT). Extracts were electrophoresed and immunoblotted with anti–IκB-α and anti–phospho-IκB-α antibodies. (b) Cells were pretreated with 50 μM MG-132 for 1 hour before addition of agonists as described in Figure 6b before immunoblot with antiphospho-IκB-α antisera. (c) Epithelia were treated with MG-132 and/or BAPTA as described in Methods, before activation with TNF-α (30 minutes) or Salmonella (1 hour). Extracts were immunoblotted with anti–IκB-α and anti–phospho-IκB-α antibodies as indicated.
Finally, this technique of proteasomal blockade was used to test the effects of intracellular calcium chelation on IκB-α phosphorylation and degradation. BAPTA pretreatment of model epithelium totally blocked the Salmonella-induced degradation of IκB-α, while having no effect on TNF-α–induced degradation (Figure 7c, top row). Pretreatment of cells with MG-132 totally blocked IκB-α degradation in response to both. The combination of BAPTA and MG-132 likewise blocked degradation induced by both activators. The same extracts were then analyzed with antibodies specific to phospho-IκB-α. Under conditions of MG-132 pretreatment, in which phospho-IκB-α would be stabilized, we observed S. typhimurium– and TNF-α–induced phosphorylation similar to that observed in Figure 7a (Figure 7c, bottom row). Strikingly, BAPTA pretreatment had no effect on the inducible phosphorylation of IκB-α in response to TNF-α, but completely abolished phosphorylation in response to S. typhimurium. Taken together, these experiments indicate that Salmonella-induced Ca2+ mobilization leads to phosphorylation and subsequent degradation of IκB-α in a manner distinct from TNF-α–mediated pathways.
Discussion
In this report, we have identified signaling events by which an important enteric pathogen induces the intestinal epithelium to orchestrate a mucosal inflammatory response. Specifically, we find that in response to proinflammatory strains of S. typhimurium, the intestinal epithelium activates the immunomodulatory transcription factor NF-κB (and consequent secretion of the potent PMN chemokine IL-8) by a mechanism involving increases in intracellular [Ca2+]. Mechanistically, the changes in intracellular [Ca2+] result in phosphorylation and degradation of IκB-α. Because the clinical manifestations of salmonellosis result primarily from the inflammatory response to this pathogen (3, 11), these events appear essential to the pathogenesis of this infectious disease. Furthermore, considering that active phases of inflammatory bowel disease are also characterized by an influx of PMN and that levels of IL-8 are elevated in vivo in these disorders (41–44), Ca2+-mediated NF-κB activation may also be involved in initiating the aberrant epithelial chemokine secretion that may be a mediator of these intestinal disorders.
Bacterial-induced calcium mobilization.
Several lines of evidence indicate that S. typhimurium–induced intracellular [Ca2+] increases are dissociable from bacterial entry into model epithelium. The invasion-defective S. typhimurium mutant HilΔ was able to elicit this response (Figure 5a). Pace et al. have demonstrated that blocking S. typhimurium invasion with cytochalasin D, an inhibitor of actin polymerization, does not prevent the observed increase in intracellular [Ca2+] (21). Furthermore, others have recently reported S. typhimurium invasion was not required for NF-κB activation in intestinal epithelial cells, although bacterial contact was necessary (40). Interestingly, noninvasive enteropathogenic E. coli also induces both Ca2+ mobilization (45, 46) and NF-κB activation (47). We recently found that blocking S. typhimurium invasion with cytochalasin D also failed to block epithelial chemokine secretion of either IL-8 or pathogen-elicited epithelial chemoattractant (27 and unpublished data). Thus, although changes in intracellular [Ca2+] may play a role in host cell internalization of S. typhimurium (22), the Ca2+-mediated activation of NF-κB and IL-8 secretion observed in response to S. typhimurium is independent of bacterial invasion.
How S. typhimurium induces an increase in intracellular [Ca2+] is not yet clear, but some insights can be drawn. One possibility that surface determinants present on wild-type or HilΔ mutant S. typhimurium (but not present on the PhoPc mutant) could function as a ligand for host cell membrane receptors. The PhoPc strain (constitutive activation of the PhoP/PhoQ 2 component regulatory system) is thought to represent the phenotypic state characteristic of the intracellular phase of the Salmonella infection process (48). One feature that results is that PhoPc organisms bear structural alterations in the lipid A component of cell wall LPS, which has been shown to induce an attenuated response from infected eukaryotic cells (49). This may imply that defective LPS signaling may play a role in the observed inability of PhoPc to elicit calcium mobilization and subsequent events. Recent reports have demonstrated Ca2+-dependent signaling elicited by LPS in several host cell types (50, 51). We also note that IκB-α degradation or activation of IκB kinase in response to LPS has been reported to occur in monocytic cells (52, 53) with kinetics similar to the Salmonella- (and carbachol-) mediated responses reported herein (in epithelial cells). However, although LPS may play a role, clearly LPS alone is not inducing epithelial cell Ca2+ mobilization or IL-8 secretion, as neither inactive S. typhimurium (heat-killed or gentamicin-treated) or purified S. typhimurium LPS detectably induce these responses.
Signals leading to release of intracellular Ca2+ stores are often initiated via 7-transmembrane spanning α−helix G-protein–coupled receptors (i.e., the classic pathway), but can also be mediated by cell-surface molecules that lack an intracellular domain (as happens, for example via glycophosphoinositide-anchored Fcγ receptors on human neutrophils) presumably by clustering associated signaling molecules (54, 55). It seems reasonable that a receptor-mediated mechanism of either of these types could mediate S. typhimurium–induced Ca2+ mobilization. Receptor-mediated events have been reported in other systems. In airway epithelium, Pseudomona aueriginosa activates NF-κB via interactions with the cellular pilin receptor, and purified bacterial pilin or antibodies to the cellular pilin receptor could mimic this response (56).
Carbachol elicits a well-characterized inositoltriphosphate-mediated (IP3-mediated) Ca2+ release from intracellular stores, followed by a Ca2+ influx. S. typhimurium attachment also induces an increase in cytosolic IP3 in eukaryotic cells (22). In addition, the observation that blocking Ca2+ influx with EGTA had a similar effect on the intracellular [Ca2+] increase induced by either carbachol or S. typhimurium suggests that the S. typhimurium-induced intracellular [Ca2+] increase might occur via a mechanism similar to such classic soluble agonists. Although the slower kinetics of the S. typhimurium–induced response are not typical of such agonists, the kinetics of the response of individual cells may be much faster. However, there is also precedent for slower IP3-independent release of intracellular stores even when an increase in IP3 is observed (57). The release of intracellular Ca2+ stores could lead to the opening of Ca2+ channels, or, alternatively, Ca2+ influx could be the result of a porinlike protein from S. typhimurium similar to the case shown for Neisseria (58).
S. typhimurium may activate epithelial signaling pathways via type III–mediated translocation of an activating factor into epithelial cells. Some examples of this type of mechanism are the injection of Yops by Yersinia spp. (59) and the recently reported observation that enteropathogenic E. coli inserts a tyrosine kinase substrate into host cells (60). There are also examples of S. typhimurium translocating effector proteins such as SopE and SopB into host cells (61–63). Given that SopB has been shown to have phosphoinositol phosphatase activity, it is possible to envisage a specific role for this protein in eliciting Ca2+ mobilization. That HilΔ (which induced Ca2+ mobilization) is deficient in expression of the type III secretion apparatus suggests that normal levels of expression of this complex may not be required for the activation of the NF-κB pathway by this pathogen.
Ca2+-mediated activation of NF-κB.
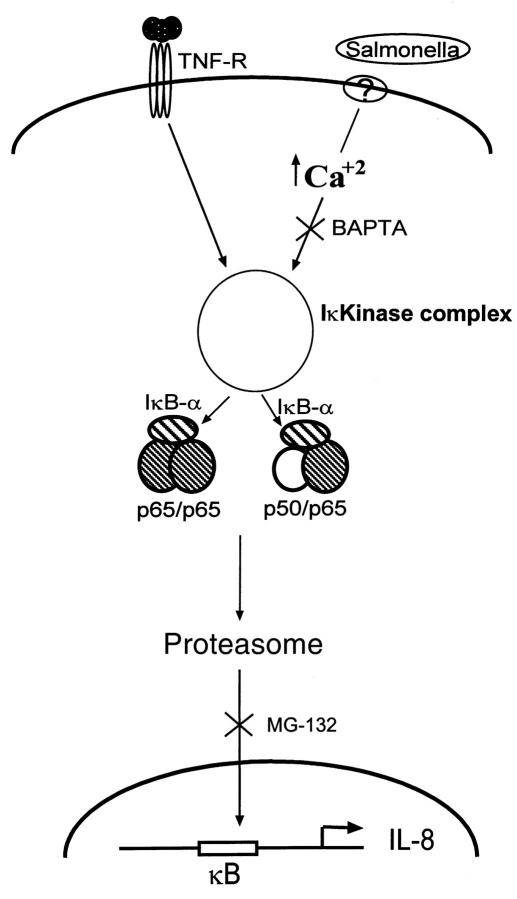
Calcium-dependent NF-κB activation occurs in response to other physiologic stimuli such as acetylcholine receptor ligation (this report), the neuropeptide substance P (24), and endoplasmic reticulum “overload” (23). In addition, our data show that nonreceptor-mediated pharmacologic means of increasing intracellular [Ca2+] (ionomycin, thapsigargin) also induced IL-8 activation. Thus, regardless of their source, increases in intracellular [Ca2+] appear to be able to induce IκB-α degradation and nuclear translocation of NF-κB in certain cell types. Because chelation of intracellular Ca2+ selectively inhibits these S. typhimurium–induced events, we propose the model diagrammed in Figure 7. NF-κB dimers (p50/p65 and p65/p65) exist as cytoplasmic complexes containing IκB-α (as well as, presumably, IκB-β, IκB-ε, p100, and p105). S. typhimurium, but not TNF-α, activates pathways that utilize increases in cytosolic [Ca2+] that, perhaps along with other second messengers, lead to phosphorylation of IκB-α. Experiments with BAPTA indicate that the S. typhimurium–induced calcium signal is occurring proximal to an IκB kinase, whereas our data with the proteasomal inhibitor MG-132 indicate that proteasomal-mediated degradation IκB-α is shared by both pathways.
Work with the prototypical proinflammatory cytokines, TNF-α and IL-1β, has delineated parallel activation pathways involving a direct series of specific protein-protein interactions from the membrane receptors of TNF-α and IL-1β (TNF-αR and Toll-like receptor respectively) to a recently characterized kinase activity thought to be responsible for the specifically phosphorylating IκB-α (reviewed in refs. 18, 64, and 65). The enzymatic activity has been traced to 2 IκB kinases present in a large (800 kDa) multiprotein complex (66, 67). Because of the wide array of NF-κB–inducing stimuli, and the need for tight control over this critical modulator of inflammatory reactions, it has been suggested that the kinase complex functions as a “signalsome,” integrating a variety of second messengers into a final common pathway (67). For example, aside from TNF-α and IL-1, activators of MAP kinase pathways have been shown to result in IKK activation (68). Thus, there is intense interest in characterizing the regulatory components of the “signalsome” and elucidating the multiple pathways that feed into it. A Ca2+-dependent kinase would seem to be a reasonable candidate to provide a link from Ca2+ mobilization to activation of IKK. How such a link occurs and whether it is to the currently conceptualized IKK complex, or other hypothetical kinases, are currently under investigation.
It is possible that numerous host-pathogen interactions that result in inflammatory responses will involve Ca2+-mediated activation of NF-κB, suggesting the existence of an signaling pathway involved in innate immunity. Such a pathway would be particularly important given studies that show that even normal intestinal microflora can induce a similar acute inflammatory response in murine models of inflammatory bowel disease (69, 70). Furthermore, because of the near ubiquitous involvement of the NF-κB systems in immune and inflammatory reactions, the search for safe effective pharmacologic inhibitors of NF-κB has been limited. Our results with the intracellular calcium chelator BAPTA indicate that specific activators of NF-κB, such as bacterial pathogens, may be selectively inhibited without affecting other critical NF-κB activating pathways necessary for normal immune function.
Figure 8.
Model of S. typhimurium–induced NF-κB activation. Both TNF-α and S. typhimurium induce NF-κB nuclear translocation via activation of an IκB kinase and subsequent proteasomal degradation of IκB-α. However, S. typhimurium–mediated, but not TNF-α–mediated, activation of the kinase requires an increase in cytosolic [Ca2+].
Acknowledgments
This work was supported by grants from the National Institutes of Health (DK09800 to A.T. Gewirtz; DK-47662 to J.L. Madara; and HL60033 to A.S. Neish) and Crohn’s and Colitis Foundation of America (to D. Merlin).
References
- 1.Madara JL. Pathobiology of the intestinal epithelial barrier. Am J Pathol. 1990;137:1273–1281. [PMC free article] [PubMed] [Google Scholar]
- 2.Yamada, T., Alpers, D.H., Owyang, C., Powell, D.W., and Silverstein, F.E. 1995. Textbook of gastroenterology. J.B. Lippincott. Philadelphia, PA. 1606–1629.
- 3.Takeuchi A. Electron microscope studies of experimental Salmonella infection. Am J Pathol. 1967;50:109–119. [PMC free article] [PubMed] [Google Scholar]
- 4.McCormick B, Gewirtz A, Madara JL. Epithelial crosstalk with bacteria and immune cells. Curr Opin Gastro. 1998;14:492–497. [Google Scholar]
- 5.McCormick BA, Miller SI, Madara JL. New insights on molecular pathways utilized by salmonella species in cell binding. Front Biosci. 1996;1:d131–d145. doi: 10.2741/a121. [DOI] [PubMed] [Google Scholar]
- 6.Eckmann L, et al. Differential cytokine expression by human intestinal epithelial cell lines: regulated expression of interleukin-8. Gastroenterology. 1993;105:1689–1697. doi: 10.1016/0016-5085(93)91064-o. [DOI] [PubMed] [Google Scholar]
- 7.Eckmann L, Kagnoff M, Fierer J. Epithelial cells secrete the chemokine interleukin-8 in response to bacterial entry. Infect Immun. 1993;61:4569–4574. doi: 10.1128/iai.61.11.4569-4574.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McCormick BA, Colgan SP, Archer CD, Miller SI, Madara JL. Salmonella typhimurium attachment to human intestinal epithelial monolayers: transcellular signalling to subepithelial neutrophils. J Cell Biol. 1993;123:895–907. doi: 10.1083/jcb.123.4.895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jung HC, et al. A distinct array of proinflammatory cytokines is expressed in human colon epithelial cells in response to bacterial invasion. J Clin Invest. 1995;95:55–65. doi: 10.1172/JCI117676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gewirtz AT, et al. Pathogen-induced chemokine secretion from model intestinal epithelium is inhibited by lipoxin A4 analogs. J Clin Invest. 1998;101:1860–1869. doi: 10.1172/JCI1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McCormick B, et al. Surface attachment of Salmonella typhimurium to intestinal epithelia imprints the subepithelial matrix with gradients chemotactic for neutrophils. J Cell Biol. 1995;131:1599–1608. doi: 10.1083/jcb.131.6.1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kunsch C, Rosen CA. NF-kappa B subunit-specific regulation of the interleukin-8 promoter. Mol Cell Biol. 1993;13:6137–6146. doi: 10.1128/mcb.13.10.6137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kunsch C, Lang RK, Rosen CA, Shannon MF. Synergistic transcriptional activation of the IL-8 gene by NF-kappa B p65 (RelA) and NF-IL-6. J Immunol. 1994;153:153–164. [PubMed] [Google Scholar]
- 14.Mukaida N, Shiroo M, Matsushima K. Genomic structure of the human monocyte-derived neutrophil chemotactic factor IL-8. J Immunol. 1989;143:1366–1371. [PubMed] [Google Scholar]
- 15.Mukaida N, Mahe Y, Matsushima K. Cooperative interaction of nuclear factor-kappa B- and cis-regulatory enhancer binding protein-like factor binding elements in activating the interleukin-8 gene by pro-inflammatory cytokines. J Biol Chem. 1990;265:21128–21133. [PubMed] [Google Scholar]
- 16.Mukaida N, Okamoto S, Ishikawa Y, Matsushima K. Molecular mechanism of interleukin-8 gene expression. J Leukoc Biol. 1994;56:554–558. [PubMed] [Google Scholar]
- 17.Ghosh S, May MJ, Kopp EB. NF-kappa B and Rel proteins: evolutionarily conserved mediators of immune responses. Annu Rev Immunol. 1998;16:225–260. doi: 10.1146/annurev.immunol.16.1.225. [DOI] [PubMed] [Google Scholar]
- 18.Bauerle P. Pro-inflammatory signaling: last pieces in the NF-kB puzzle? Curr Biol. 1998;8:19–22. doi: 10.1016/s0960-9822(98)70010-7. [DOI] [PubMed] [Google Scholar]
- 19.Thanos D, Maniatis T. NF-kappa B: a lesson in family values. Cell. 1995;80:529–532. doi: 10.1016/0092-8674(95)90506-5. [DOI] [PubMed] [Google Scholar]
- 20.Hobbie S, Chen L, Davis R, Galan JE. Involvement of mitogen-activated protein kinase pathways in the nuclear responses and cytokine production induced by Salmonella typhimurium in cultured intestinal epithelial cells. J Immunol. 1997;159:5550–5559. [PubMed] [Google Scholar]
- 21.Pace J, Hayman MJ, Galan JE. Signal transduction and invasion of epithelial cells by S. typhimurium. Cell. 1993;72:505–514. doi: 10.1016/0092-8674(93)90070-7. [DOI] [PubMed] [Google Scholar]
- 22.Ruschkowski S, Rosenshine I, Finlay B. Salmonella typhimurium induces an inositol phosphate flux in infected epithelial cells. FEMS Microbiol Lett. 1992;74:121–126. doi: 10.1016/0378-1097(92)90416-l. [DOI] [PubMed] [Google Scholar]
- 23.Pahl HL, Sester M, Burgert HG, Baeuerle PA. Activation of transcription factor NF-kappaB by the adenovirus E3/19K protein requires its ER retention. J Cell Biol. 1996;132:511–522. doi: 10.1083/jcb.132.4.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lieb K, Fiebich BL, Berger M, Bauer J, Schulze-Osthoff K. The neuropeptide substance P activates transcription factor NF-kappa B and kappa B-dependent gene expression in human astrocytoma cells. J Immunol. 1997;159:4952–4958. [PubMed] [Google Scholar]
- 25.Dharmsathaphorn K, Madara JL. Established intestinal cell lines as model systems for electrolyte transport studies. Methods Enzymol. 1990;192:354–389. doi: 10.1016/0076-6879(90)92082-o. [DOI] [PubMed] [Google Scholar]
- 26.Seetoo KF, et al. A cytosolic calcium transient is not necessary for degranulation or oxidative burst in immune complex-stimulated neutrophils. J Leukoc Biol. 1997;62:329–340. doi: 10.1002/jlb.62.3.329. [DOI] [PubMed] [Google Scholar]
- 27.Gewirtz AT, Siber AM, Madara JL, McCormick BA. Orchestration of neutrophil movement by intestinal epithelial cells in response to salmonella typhimurium can be uncoupled from bacterial internalization. Infect Immun. 1999;67:608–617. doi: 10.1128/iai.67.2.608-617.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Neish AS, et al. Endothelial interferon regulatory factor 1 cooperates with NF-kappa B as a transcriptional activator of vascular cell adhesion molecule 1. Mol Cell Biol. 1995;15:2558–2569. doi: 10.1128/mcb.15.5.2558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fiedler MA, Wernke-Dollries K, Stark JM. Inhibition of TNF-alpha-induced NF-kappaB activation and IL-8 release in A549 cells with the proteasome inhibitor MG-132. Am J Respir Cell Mol Biol. 1998;19:259–268. doi: 10.1165/ajrcmb.19.2.3149. [DOI] [PubMed] [Google Scholar]
- 30.Read MA, et al. The proteasome pathway is required for cytokine-induced Endothelial-leukocyte adhesion molecule expression. Immunity. 1995;2:493–506. doi: 10.1016/1074-7613(95)90030-6. [DOI] [PubMed] [Google Scholar]
- 31.Dickinson KE, Frizzell RA, Sekar MC. Activation of T84 cell chloride channels by carbachol involves a phosphoinositide-coupled muscarinic M3 receptor. Eur J Pharmacol. 1992;225:291–298. doi: 10.1016/0922-4106(92)90102-2. [DOI] [PubMed] [Google Scholar]
- 32.Fischer H, Illek B, Negulescu PA, Clauss W, Machen TE. Carbachol-activated calcium entry into HT-29 cells is regulated by both membrane potential and cell volume. Proc Natl Acad Sci USA. 1992;89:1438–1442. doi: 10.1073/pnas.89.4.1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kuznetsov G, Brostrom MA, Brostrom CO. Demonstration of a calcium requirement for secretory protein processing and export. Differential effects of calcium and dithiothreitol. J Biol Chem. 1992;267:3932–3939. [PubMed] [Google Scholar]
- 34.Takemura H, Hughes AR, Thastrup O, Putney JW., Jr Activation of calcium entry by the tumor promoter thapsigargin in parotid acinar cells. Evidence that an intracellular calcium pool and not an inositol phosphate regulates calcium fluxes at the plasma membrane. J Biol Chem. 1989;264:12266–12271. [PubMed] [Google Scholar]
- 35.Henderson RM, Ashford ML, MacVinish LJ, Cuthbert AW. Chloride channels and anion fluxes in a human colonic epithelium (HCA- 7) Br J Pharmacol. 1992;106:109–114. doi: 10.1111/j.1476-5381.1992.tb14301.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McCormick B, Miller S, Carnes D, Madara J. Transepithelial signaling to neutrophils by Salmonellae: a novel virulence mechanism for gastroenteritis. Infect Immun. 1995;63:2302–2309. doi: 10.1128/iai.63.6.2302-2309.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bajaj V, Hwang C, Lee CA. hilA is a novel ompR/toxR family member that activates the expression of Salmonella typhimurium invasion genes. Mol Microbiol. 1995;18:715–727. doi: 10.1111/j.1365-2958.1995.mmi_18040715.x. [DOI] [PubMed] [Google Scholar]
- 38.Garcia Vescovi E, Soncini FC, Groisman EA. Mg2+ as an extracellular signal: environmental regulation of Salmonella virulence. Cell. 1996;84:165–174. doi: 10.1016/s0092-8674(00)81003-x. [DOI] [PubMed] [Google Scholar]
- 39.Miller S, Mekalanos J. Constitutive expression of the phoP regulon attenuates Salmonella virulence and survival within macrophages. J Bacteriol. 1990;172:2485–2490. doi: 10.1128/jb.172.5.2485-2490.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Eaves-Pyles T, Szabo C, Salzman AL. Bacterial invasion is not required for activation of NF-kappaB in enterocytes. Infect Immun. 1999;67:800–804. doi: 10.1128/iai.67.2.800-804.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Daig R, et al. Increased interleukin 8 expression in the colon mucosa of patients with inflammatory bowel disease. Gut. 1996;38:216–222. doi: 10.1136/gut.38.2.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Funakoshi K, et al. Study of cytokines in ulcerative colitis. J Gastroenterol. 1995;30(Suppl. 8):61–63. [PubMed] [Google Scholar]
- 43.Mazzucchelli L, et al. Expression of interleukin-8 gene in inflammatory bowel disease is related to the histological grade of active inflammation. Am J Pathol. 1994;144:997–1007. [PMC free article] [PubMed] [Google Scholar]
- 44.Nielsen OH, Rudiger N, Gaustadnes M, Horn T. Intestinal interleukin-8 concentration and gene expression in inflammatory bowel disease. Scand J Gastroenterol. 1997;32:1028–1034. doi: 10.3109/00365529709011220. [DOI] [PubMed] [Google Scholar]
- 45.Foubister V, Rosenshine I, Finlay BB. A diarrheal pathogen, enteropathogenic Escherichia coli (EPEC), triggers a flux of inositol phosphates in infected epithelial cells. J Exp Med. 1994;179:993–998. doi: 10.1084/jem.179.3.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Baldwin TJ, Ward W, Aitken A, Knutton S, Williams PH. Elevation of intracellular free calcium levels in HEp-2 cells infected with enteropathogenic Escherichia coli. Infect Immun. 1991;59:1599–1604. doi: 10.1128/iai.59.5.1599-1604.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Savkovic SD, Koutsouris A, Hecht G. Activation of NF-kappaB in intestinal epithelial cells by enteropathogenic Escherichia coli. Am J Physiol. 1997;273:C1160–C1167. doi: 10.1152/ajpcell.1997.273.4.C1160. [DOI] [PubMed] [Google Scholar]
- 48.Miller S. PhoP/PhoQ: macrophage-specific modulators of Salmonella virulence? Mol Microbiol. 1991;5:2073–2078. doi: 10.1111/j.1365-2958.1991.tb02135.x. [DOI] [PubMed] [Google Scholar]
- 49.Guo L, et al. Regulation of lipid A modifications by Salmonella typhimurium virulence genes phoP-phoQ. Science. 1997;276:250–253. doi: 10.1126/science.276.5310.250. [DOI] [PubMed] [Google Scholar]
- 50.Woods JS, Ellis ME, Diegez-Acuna FJ, Corral J. Activation of NF-κB in normal rat kidney epithelial (NRK52E) cells is mediated via a redox-insensitive, calcium-dependent pathway. Toxicol Appl Pharmacol. 1999;154:219–227. doi: 10.1006/taap.1998.8583. [DOI] [PubMed] [Google Scholar]
- 51.Hsuan SL, et al. Pasteurella haemolytica leukotoxin and endotoxin induced cytokine gene expression in bovine alveolar macrophages requires NF-kappaB activation and calcium elevation. Microb Pathog. 1999;26:263–273. doi: 10.1006/mpat.1998.0271. [DOI] [PubMed] [Google Scholar]
- 52.Fischer C, et al. Differential effects of lipopolysaccharide and tumor necrosis factor on monocytic IkappaB kinase signalsome activation and IkappaB proteolysis. J Biol Chem. 1999;274:24625–24632. doi: 10.1074/jbc.274.35.24625. [DOI] [PubMed] [Google Scholar]
- 53.Henkel T, et al. Rapid proteolysis of I kappa B-alpha is necessary for activation of transcription factor NF-kappa B. Nature. 1993;365:182–185. doi: 10.1038/365182a0. [DOI] [PubMed] [Google Scholar]
- 54.Brunkhorst BA, et al. Differential roles of FcγRII and FcγRIII in immune complex stimulation of human neutrophils. J Biol Chem. 1992;267:20659–20666. [PubMed] [Google Scholar]
- 55.Strohmeier GR, et al. Role of the Fc gamma R subclasses Fc gamma RII and Fc gamma RIII in the activation of human neutrophils by low and high valency immune complexes. J Leukoc Biol. 1995;58:415–422. doi: 10.1002/jlb.58.4.415. [DOI] [PubMed] [Google Scholar]
- 56.DiMango E, Ratner AJ, Bryan R, Tabibi S, Prince A. Activation of NF-kappaB by adherent Pseudomonas aeruginosa in normal and cystic fibrosis respiratory epithelial cells. J Clin Invest. 1998;101:2598–2605. doi: 10.1172/JCI2865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rosales C, Brown EJ. Signal transduction by neutrophil immunoglobulin G Fc receptors. Dissociation of intracytoplasmic calcium concentration rise from inositol 1,4,5-trisphosphate. J Biol Chem. 1992;267:5265–5271. [PubMed] [Google Scholar]
- 58.Muller A, et al. Neisserial porin (PorB) causes rapid calcium influx in target cells and induces apoptosis by the activation of cysteine proteases. EMBO J. 1999;18:339–352. doi: 10.1093/emboj/18.2.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cornelis GR, Wolf-Watz H. The Yersinia Yop virulon: a bacterial system for subverting eukaryotic cells. Mol Microbiol. 1997;23:861–867. doi: 10.1046/j.1365-2958.1997.2731623.x. [DOI] [PubMed] [Google Scholar]
- 60.Kenny B, et al. Enteropathogenic E. coli (EPEC) transfers its receptor for intimate adherence into mammalian cells. Cell. 1997;91:511–520. doi: 10.1016/s0092-8674(00)80437-7. [DOI] [PubMed] [Google Scholar]
- 61.Wood M, Rosqvist R, Mullan P, Edwards M, Galyov E. SopE, a secreted protein of Salmonella dublin, is translocated into the target eukaryotic cell via a sip-dependent mechanism and promotes bacterial entry. Mol Microbiol. 1996;22:327–338. doi: 10.1046/j.1365-2958.1996.00116.x. [DOI] [PubMed] [Google Scholar]
- 62.Norris FA, Wilson MP, Wallis TS, Galyov EE, Majerus PW. SopB, a protein required for virulence of Salmonella dublin, is an inositol phosphate phosphatase. Proc Natl Acad Sci USA. 1998;95:14057–14059. doi: 10.1073/pnas.95.24.14057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hardt WD, Chen LM, Schuebel KE, Bustelo XR, Galan JE. S. typhimurium encodes an activator of Rho GTPases that induces membrane ruffling and nuclear responses in host cells. Cell. 1998;93:815–826. doi: 10.1016/s0092-8674(00)81442-7. [DOI] [PubMed] [Google Scholar]
- 64.Stancovski I, Baltimore D. NF-kappaB activation: the I kappaB kinase revealed? Cell. 1997;91:299–302. doi: 10.1016/s0092-8674(00)80413-4. [DOI] [PubMed] [Google Scholar]
- 65.Jobin C, et al. TNF receptor-associated factor-2 is involved in both IL-1 beta and TNF- alpha signaling cascades leading to NF-kappa B activation and IL-8 expression in human intestinal epithelial cells. J Immunol. 1999;162:4447–4454. [PubMed] [Google Scholar]
- 66.Chen ZJ, Parent L, Maniatis T. Site-specific phosphorylation of IkappaBalpha by a novel ubiquitination-dependent protein kinase activity. Cell. 1996;84:853–862. doi: 10.1016/s0092-8674(00)81064-8. [DOI] [PubMed] [Google Scholar]
- 67.Maniatis T. Catalysis by a multiprotein IkappaB kinase complex. Science. 1997;278:818–819. doi: 10.1126/science.278.5339.818. [DOI] [PubMed] [Google Scholar]
- 68.Lee FS, Hagler J, Chen ZJ, Maniatis T. Activation of the IkappaB alpha kinase complex by MEKK1, a kinase of the JNK pathway. Cell. 1997;88:213–22. doi: 10.1016/s0092-8674(00)81842-5. [DOI] [PubMed] [Google Scholar]
- 69.Kuhn R, Lohler J, Rennick D, Rajewsky K, Muller W. Interleukin-10-deficient mice develop chronic enterocolitis. Cell. 1993;75:263–274. doi: 10.1016/0092-8674(93)80068-p. [DOI] [PubMed] [Google Scholar]
- 70.Panwala CM, Jones JC, Viney JL. A novel model of inflammatory bowel disease: mice deficient for the multiple drug resistance gene, mdr1a, spontaneously develop colitis. J Immunol. 1998;161:5733–5744. [PubMed] [Google Scholar]