Abstract
Compound K is a major metabolite of ginsenoside Rb1, which has various pharmacological activities in vivo and in vitro. However, previous studies have focused on the pharmacokinetics of a single metabolite or the parent compound and have not described the pharmacokinetics of both compounds in humans. To investigate the pharmacokinetics of ginsenoside Rb1 and compound K, we performed an open-label, single-oral dose pharmacokinetic study using Korean Red Ginseng extract. We enrolled 10 healthy Korean male volunteers in this study. Serial blood samples were collected during 36 h after Korean Red Ginseng extract administration to determine plasma concentrations of ginsenoside Rb1 and compound K. The mean maximum plasma concentration of compound K was 8.35±3.19 ng/mL, which was significantly higher than that of ginsenoside Rb1 (3.94±1.97 ng/mL). The half-life of compound K was 7 times shorter than that of ginsenoside Rb1. These results suggest that the pharmacokinetics, especially absorption, of compound K are not influenced by the pharmacokinetics of its parent compound, except the time to reach the maximum plasma concentration The delayed absorption of compound K support the evidence that the intestinal microflora play an important role in the transformation of ginsenoside Rb1 to compound K.
Keywords: Panax ginseng, Pharmacokinetics, Ginsenoside Rb1, Compound K
INTRODUCTION
Ginseng (the root of Panax ginseng Meyer) has been used as a traditional medicine for a long time in Asian countries. Over the last two decades, ginseng has also been used as a dietary supplement or a therapeutic agent in the United States and European countries. Ginseng has a wide range of pharmacological activities such as antiinflammatory, antidiabetic, and anticancer activities [1-4]. These effects of ginseng are attributed to its pharmacologically active components known as ginsenosides. Ginsenosides are a class of dammarane-type triterpene saponins, which can be further classified into 20(S)- protopanaxadiol (ginsenoside Rb1, Rb2, Rb3, Rc, and Rd) and 20(S)-protopanaxatriol (ginsenoside Re, Rg1, Rg2, and Rh1) groups according to their aglycone moieties [5-7]. Ginsenoside compound K, also referred to as 20-O-β-Ɗ-glucopyranosyl-20(S)-protopanaxadiol, is an active metabolite found in the blood stream of humans after oral administration of protopanaxadiol ginsenosides Rb1, Rb2, Rc, and Rd. Protopanaxadiol ginsenosides are metabolized to ginsenoside compound K by the intestinal microflora in humans [8-10] and rats [11-14]. Compound K possesses various pharmacological activities including chemopreventive and antiallergic activities [8,12,15-18]. Red ginseng is produced by steaming raw white ginseng. This process results in the formation of active constituents such as Rh4 and Rf2 in red ginseng, which have higher pharmacological activity than those in white ginseng [19,20]. Compared to the extensive in vivo and in vitro pharmacological studies on compound K, pharmacokinetic studies of this compound in humans have received less attention, and studies with a focus on the parent compounds of ginseng have been performed. Understanding the pharmacokinetics of ginsenoside and its metabolites is very important in designing an optimal dosage regimen and reducing the potential of interaction between ginseng and prescription drugs in patients using both agents [21].
The aim of our study was to investigate the pharmacokinetics of ginsenoside Rb1 and its metabolite compound K after oral administration of extracts of Korean Red Ginseng in healthy Korean subjects.
MATERIALS AND METHODS
Subjects and study design
This study had an open-label, single-oral-dose design. The study was performed in accordance with the Declaration of Helsinki, and informed written consent was obtained from each subject. Study protocol was reviewed and approved by the institutional review board of Soonchunhyang University Cheonan Hospital (reference: SCHCH-2010-68). All study procedures were conducted in accordance with the principles of the Declaration of Helsinki and the Korean Good Clinical Practice guidelines. We enrolled 10 healthy male Korean volunteers in this study. Demographic characteristics of the subjects are shown in Table 1.
Table 1.
Demographic characteristics of 10 healthy male volunteers who participated in this study
| Median±SD | Range | |
|---|---|---|
|
| ||
| Age (yr) | 22±1.3 | 21-25 |
| Body weight (kg) | 71.5±5.7 | 67-84 |
| Height (cm) | 178.5±4.6 | 173-188 |
| Body mass index (kg/m2) | 22.4±1.5 | 20.8-25.8 |
Because of the metabolic nature of compound K and irregular absorption of ginsenosides, we expected large inter-individual variabilities in the pharmacokinetic parameters of ginsenoside Rb1 and compound K. To reduce the inter-individual variability caused by sex on the pharmacokinetics of ginsenoside Rb1 and compound K, we only recruited male volunteers. The exclusion criteria included any significant clinical illness within 2 wk before the study, history of high blood pressure, diabetes, cardiovascular, hepatic, renal, hematological, gastrointestinal, neurologic, psychiatric disease, blood donation within 8 wk before the study, and use of any medicine, including prescription medicines and over-the-counter drugs within 2 wk before the study. In addition, subjects who previously experienced an adverse reaction to ginseng were excluded. All subjects were screened by an investigator on the basis of results of clinical laboratory tests (hematology, blood chemistry, and urinalysis), physical examination, and measurement of vital signs before initiation of the study. Any clinically important abnormalities in the clinical laboratory tests, physical examinations, or vital signs during the pre-study screening were also considered as criteria for exclusion. During the study period, all volunteers were instructed to refrain from smoking and drinking caffeine-containing beverages and instructed not to perform strenuous exercises. In addition, they were continuously observed and were served the same type of food and water. All volunteers fasted from the night before the study day to 1 PM on the study day. At 8 AM on the study day, 9 g of Korean Red Ginseng extract was orally administered to each subject with 100 mL of water. Blood samples were collected before oral administration of Korean Red Ginseng extract and 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 24, and 36 h after administration. We collected 5 mL of blood from an indwelling catheter at each sampling point and transferred the samples to heparin-coated Vacutainer tubes (Becton & Dickinson, Franklin Lakes, NJ, USA). The plasma was separated after centrifugation, and the samples were stored at -70℃ until assay.
Materials and sample preparation
The Korean Red Ginseng, ginsenoside Rb1, and compound K were generously supplied by Korea Ginseng Corporation (Daejeon, Korea). Digoxin as an internal standard was purchased from Sigma-Aldrich Corporation (St. Louis, MO, USA). HPLC-grade methanol and acetonitrile were obtained from Burdick & Jackson (Muskegon, MI, USA). Water was purified using a Milli-Q system (Millipore, Bedford, MA, USA) and used throughout the analytical procedures.
Stock solutions for ginsenoside Rb1 were prepared with methanol as the solvent and were diluted with methanol to use as working solutions. Plasma standards were prepared using drug-free human plasma. Standard samples were prepared at concentrations of 2, 5, 10, 20, 50, 100 ng/mL for ginsenoside Rb1 and compound K. Calibration curves were obtained by plotting the peak area ratios of drug/internal standard versus drug concentrations in the standard samples. To assess the intra- and inter-day precision and accuracy of the assay for both ginsenosides, we analyzed 5 replicates of the plasma standard samples at 4 concentrations. Calibration curves for both compounds were linear throughout the concentration range used in the study with correlation coefficients greater than 0.995. To determine the level of ginsenoside Rb1 administered, an aliquot of the Korean Red Ginseng extract was also prepared and analyzed using an assay method same as that used for ginsenoside Rb1.
Determination of plasma ginsenoside
Quantification of ginsenoside Rb1 and compound K was performed using a Finnigan TSQ Quantum Discovery MAX MS/MS system (Thermo Electron, San Jose, CA, USA). Chromatographic conditions were established using those reported in a previous study with some modifications [22]. Briefly, a Cadenza ODS column (3 μm, 2.0×100 mm; Imtakt, Kyoto, Japan) was used in the separation procedure. The mobile phase was methanol:water run under gradient conditions at a flow rate of 0.25 mL/min. The Finnigan Xcalibur program works on the Windows XP operating system was used for data processing. The injection volume was 5 μL.
Pharmacokinetic and statistical analysis
The plasma concentration-time data for ginsenoside Rb1 and compound K were analyzed using a non-compartmental method with the Phoenix WinNonlin software ver. 6.1 (Pharsight Corporation, Mountain View, CA, USA). The area under the plasma concentration-time curves, which were extrapolated to infinity (AUCinf), and the area under the plasma concentration-time curve between 0 and 36 h (AUCt) were calculated using the trapezoidal rule. Further, we examined the pharmacokinetic parameters such as the terminal half-life (t1/2), maximum concentration (Cmax), and the time to reach Cmax (Tmax). The values of the pharmacokinetic parameters are presented as the mean±SD. To determine the correlation between pharmacokinetic parameters of ginsenoside Rb1 and compound K, correlation analysis was performed by calculating the Spearman’s rank correlation coefficient. Statistical analyses were performed using IBM-SPSS Statistics ver. 20.0 (IBM Corporation, Armonk, NY, USA). A p-value less than 0.05 was considered to be statistically significant.
Tolerability assessment
Throughout the study period, adverse events were recorded on the basis of spontaneous reports from volunteers, clinical examination, and questionnaires distributed by the investigator. At the end of study period, the investigator assessed all the adverse events reported in terms of intensity, duration, and relationship to the administered study drug.
RESULTS AND DISCUSSION
Pharmacokinetics of ginsenoside Rb1 and compound K
An LC-MS/MS method for quantification of ginsenoside Rb1 and compound K was developed for pharmacokinetic study. Representative chromatograms of ginsenoside Rb1, compound K, and internal standard are shown in Fig. 1.
Fig. 1. Representative analytical chromatogram of ginsenoside Rb1 (A), compound K (B), and the internal standard (C).
The amount of ginsenoside Rb1 administered to each subject was determined to be 45.81 mg/9 g of the Korean Red Ginseng extract. The plasma concentration-time curves of ginsenoside Rb1 and compound K are shown in Fig. 2.
Fig. 2. Mean plasma concentration-time curve of ginsenoside Rb1 and compound K after oral administration of Korean Red Ginseng extract in 10 healthy male Korean subjects. ● Plasma ginsenoside Rb1 (Rb1) concentration, ▲ plasma compound K (C-K) concentration.
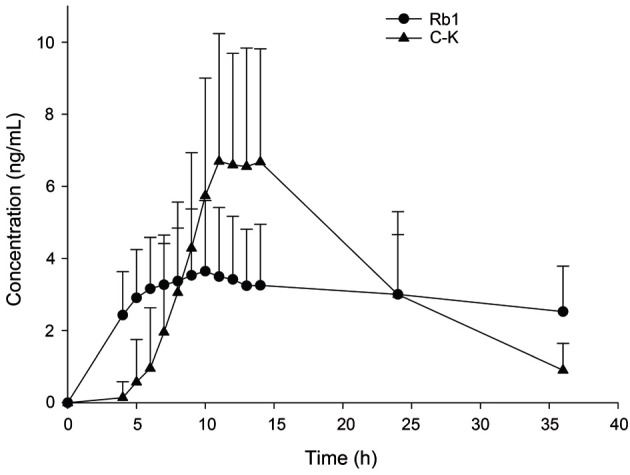
The Cmax of plasma ginsenoside Rb1 and compound K were 3.94±1.97 and 8.35±3.19 ng/mL, respectively (Table 2). Compound K was detected in the plasma of all subjects. Compound K could be detected in the plasma between 4 and 9 h after oral administration of drug. The Tmax of compound K was 12.20±1.81 h.
Table 2.
Pharmacokinetic parameters of ginsenoside Rb1 and compound K after single oral administration of Korean Red Ginseng extract
| Parameters | Ginsenoside Rb1 | Compound K |
|---|---|---|
|
| ||
| AUCt (ng/mL·h) | 102.3±51.0 | 110.7±51.2 |
| AUCinf (ng/mL·h) | 307.7±145.6 | 123.9±57.5 |
| Cmax (ng/mL) | 3.94±1.97 | 8.35±3.19 |
| Tmax (h) | 8.70±2.63 | 12.20±1.81 |
| t1/2 (h) | 58.47±14.28 | 7.82±1.69 |
Values are presented as mean±SD.
AUCt, area under the plasma concentration-time curve between 0 and 36 h; AUCinf, area under the plasma concentration-time curve extrapolated to infinity; Cmax, maximum plasma concentration; Tmax, time to reach Cmax; t1/2, elimination half-life.
Shibata [23] reported that compound K was detected 8 h after the oral administration of ginseng powder in healthy men with some individual variation. Recently, the pharmacokinetics of compound K was compared in healthy male subjects after oral administration of fermented and non-fermented ginseng [24]. They reported that the Tmax of compound K in the group that received non-fermented ginseng was 12.04±4.96 h. These results are consistent with our results despite the difference in dose administered. The pharmacokinetics of compound K has been reported in another recent study, in which fermented ginseng, which consists of compound K, was administered [25]. Thus, the absorption characteristics of this compound could not be directly compared to our results. However, the half-life of compound K, 9.9±5.5 h, was very similar to that reported in our study. The t1/2 of compound K was more than 7 times shorter than that of ginsenoside Rb1. Although the plasma concentration of compound K was very low at 36 h after taking the extract, the compound could be detected in 8 subjects (0.90±0.74 ng/mL).
Correlation between pharmacokinetic parameters of ginsenoside Rb1 and compound K
Many studies showed that ginsenoside Rb1 is transformed into compound K by intestinal microflora after oral administration in humans [1,2,9,10]. Lee et al. [26] investigated the relationship between compound K transforming activities of human intestinal microflora and pharmacokinetic parameters of compound K. Their result showed large inter-individual variation in compound K transforming activities and no correlation between compound K transforming activity and pharmacokinetic parameters such as Cmax and AUC.
In the present study, correlation analyses were performed to investigate whether a correlation existed between the pharmacokinetic parameters the of parent compound, ginsenoside Rb1, and its metabolite, compound K. There was no correlation between all pharmacokinetic parameters, except Tmax, of ginsenoside Rb1 and compound K (Fig. 3). Despite the large inter-individual variability during the time course of Korean ginseng extract administration, the Tmax of ginsenoside Rb1 was significantly correlated to that of compound K (r=0.697, p<0.05).
Fig. 3. Correlation between the time to reach maximum concentration (Tmax) of plasma ginsenoside Rb1 (Rb1) and compound K (C-K) after oral administration of Korean Red Ginseng extracts in 10 healthy male Korean subjects.
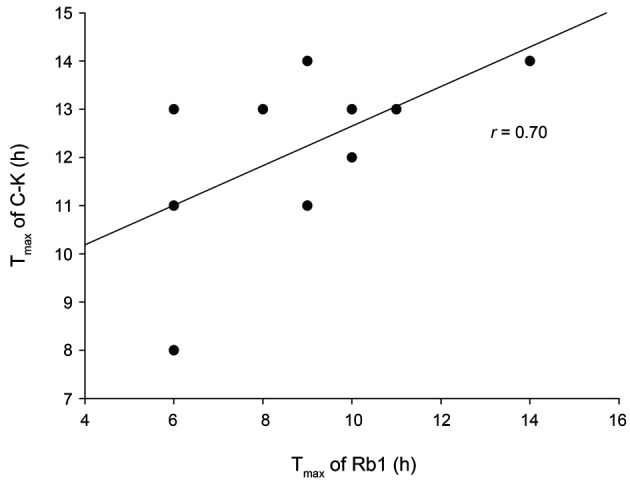
These results suggest that the amount of compound K absorbed is independent of the amount of ginsenoside Rb1 absorbed, and only the Tmax of ginsenoside Rb1 can influence that of compound K.
Tolerability
In the present study, no serious adverse event was reported, and the Korean Red Ginseng extract was well-tolerated at doses up to 9 g in the 10 healthy Korean volunteers.
In this clinical trial, the investigator initially studied the pharmacokinetics of ginsenoside Rb1 and compound K and compared each pharmacokinetic parameter of these agents in the same group of subjects. In summary, the pharmacokinetic characteristics, especially absorption and elimination, showed significant difference between ginsenoside Rb1 and compound K, and no correlation was observed between the pharmacokinetic parameters of the parent compound and the metabolite, except in Tmax. The Tmax of compound K was longer than that of its parent compounds, which suggested that intestinal microflora may play an important role in the transformation of ginsenoside Rb1 to compound K and in the pharmacokinetics of compound K.
Acknowledgments
This study was supported by the 2010 grant from the Korean Society of Ginseng funded by Korea Ginseng Corporation. This study was also partly supported by the Soonchunhyang University research grant.
References
- 1.Christensen LP. Ginsenosides chemistry, biosynthesis, analysis, and potential health effects. Adv Food Nutr Res. 2009;55:1–99. doi: 10.1016/S1043-4526(08)00401-4. [DOI] [PubMed] [Google Scholar]
- 2.Kang KS, Kim HY, Yamabe N, Nagai R, Yokozawa T. Protective effect of sun ginseng against diabetic renal damage. Biol Pharm Bull. 2006;29:1678–1684. doi: 10.1248/bpb.29.1678. [DOI] [PubMed] [Google Scholar]
- 3.Yun TK. Panax ginseng: a non-organ-specific cancer preventive? Lancet Oncol. 2001;2:49–55. doi: 10.1016/S1470-2045(00)00196-0. [DOI] [PubMed] [Google Scholar]
- 4.Qi LW, Wang CZ, Yuan CS. American ginseng: potential structure-function relationship in cancer chemoprevention. Biochem Pharmacol. 2010;80:947–954. doi: 10.1016/j.bcp.2010.06.023. [DOI] [PubMed] [Google Scholar]
- 5.Attele AS, Wu JA, Yuan CS. Ginseng pharmacology: multiple constituents and multiple actions. Biochem Pharmacol. 1999;58:1685–1693. doi: 10.1016/S0006-2952(99)00212-9. [DOI] [PubMed] [Google Scholar]
- 6.Gillis CN. Panax ginseng pharmacology: a nitric oxide link? Biochem Pharmacol. 1997;54:1–8. doi: 10.1016/s0006-2952(97)00193-7. [DOI] [PubMed] [Google Scholar]
- 7.Paek IB, Moon Y, Kim J, Ji HY, Kim SA, Sohn DH, Kim JB, Lee HS. Pharmacokinetics of a ginseng saponin metabolite compound K in rats. Biopharm Drug Dispos. 2006;27:39–45. doi: 10.1002/bdd.481. [DOI] [PubMed] [Google Scholar]
- 8.Bae EA, Choo MK, Park EK, Park SY, Shin HY, Kim DH. Metabolism of ginsenoside R(c) by human intestinal bacteria and its related antiallergic activity. Biol Pharm Bull. 2002;25:743–747. doi: 10.1248/bpb.25.743. [DOI] [PubMed] [Google Scholar]
- 9.Hasegawa H, Sung JH, Benno Y. Role of human intestinal Prevotella oris in hydrolyzing ginseng saponins. Planta Med. 1997;63:436–440. doi: 10.1055/s-2006-957729. [DOI] [PubMed] [Google Scholar]
- 10.Tawab MA, Bahr U, Karas M, Wurglics M, Schubert-Zsilavecz M. Degradation of ginsenosides in humans after oral administration. Drug Metab Dispos. 2003;31:1065–1071. doi: 10.1124/dmd.31.8.1065. [DOI] [PubMed] [Google Scholar]
- 11.Karikura M, Miyase T, Tanizawa H, Takino Y, Taniyama T, Hayashi T. Studies on absorption, distribution, excretion and metabolism of ginseng saponins. V. The decomposition products of ginsenoside Rb2 in the large intestine of rats. Chem Pharm Bull (Tokyo) 1990;38:2859–2861. doi: 10.1248/cpb.38.2859. [DOI] [PubMed] [Google Scholar]
- 12.Hasegawa H, Uchiyama M. Antimetastatic efficacy of orally administered ginsenoside Rb1 in dependence on intestinal bacterial hydrolyzing potential and significance of treatment with an active bacterial metabolite. Planta Med. 1998;64:696–700. doi: 10.1055/s-2006-957560. [DOI] [PubMed] [Google Scholar]
- 13.Akao T, Kida H, Kanaoka M, Hattori M, Kobashi K. Intestinal bacterial hydrolysis is required for the appearance of compound K in rat plasma after oral administration of ginsenoside Rb1 from Panax ginseng. J Pharm Pharmacol. 1998;50:1155–1160. doi: 10.1111/j.2042-7158.1998.tb03327.x. [DOI] [PubMed] [Google Scholar]
- 14.Akao T, Kanaoka M, Kobashi K. Appearance of compound K, a major metabolite of ginsenoside Rb1 by intestinal bacteria, in rat plasma after oral administration: measurement of compound K by enzyme immunoassay. Biol Pharm Bull. 1998;21:245–249. doi: 10.1248/bpb.21.245. [DOI] [PubMed] [Google Scholar]
- 15.Wang CZ, Du GJ, Zhang Z, Wen XD, Calway T, Zhen Z, Musch MW, Bissonnette M, Chang EB, Yuan CS. Ginsenoside compound K, not Rb1, possesses potential chemopreventive activities in human colorectal cancer. Int J Oncol. 2012;40:1970–1976. doi: 10.3892/ijo.2012.1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wakabayashi C, Murakami K, Hasegawa H, Murata J, Saiki I. An intestinal bacterial metabolite of ginseng protopanaxadiol saponins has the ability to induce apoptosis in tumor cells. Biochem Biophys Res Commun. 1998;246:725–730. doi: 10.1006/bbrc.1998.8690. [DOI] [PubMed] [Google Scholar]
- 17.Lee SJ, Sung JH, Lee SJ, Moon CK, Lee BH. Antitumor activity of a novel ginseng saponin metabolite in human pulmonary adenocarcinoma cells resistant to cisplatin. Cancer Lett. 1999;144:39–43. doi: 10.1016/S0304-3835(99)00188-3. [DOI] [PubMed] [Google Scholar]
- 18.Choo MK, Park EK, Han MJ, Kim DH. Antiallergic activity of ginseng and its ginsenosides. Planta Med. 2003;69:518–522. doi: 10.1055/s-2003-40653. [DOI] [PubMed] [Google Scholar]
- 19.Baek NI, Kim DS, Lee YH, Park JD, Lee CB, Kim SI. Ginsenoside Rh4, a genuine dammarane glycoside from Korean red ginseng. Planta Med. 1996;62:86–87. doi: 10.1055/s-2006-957816. [DOI] [PubMed] [Google Scholar]
- 20.Kim NR, Kim JH, Kim CY. Effect of Korean red ginseng supplementation on ocular blood flow in patients with glaucoma. J Ginseng Res. 2010;34:237–245. doi: 10.5142/jgr.2010.34.3.237. [DOI] [Google Scholar]
- 21.Qi LW, Wang CZ, Du GJ, Zhang ZY, Calway T, Yuan CS. Metabolism of ginseng and its interactions with drugs. Curr Drug Metab. 2011;12:818–822. doi: 10.2174/138920011797470128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xie HT, Wang GJ, Sun JG, Tucker I, Zhao XC, Xie YY, Li H, Jiang XL, Wang R, Xu MJ, et al. High performance liquid chromatographic-mass spectrometric determination of ginsenoside Rg3 and its metabolites in rat plasma using solid-phase extraction for pharmacokinetic studies. J Chromatogr B Analyt Technol Biomed Life Sci. 2005;818:167–173. doi: 10.1016/j.jchromb.2004.12.028. [DOI] [PubMed] [Google Scholar]
- 23.Shibata S. Chemistry and cancer preventing activities of ginseng saponins and some related triterpenoid compounds. J Korean Med Sci. 2001;16(Suppl):S28–S37. doi: 10.3346/jkms.2001.16.S.S28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jin H, Seo JH, Uhm YK, Jung CY, Lee SK, Yim SV. Pharmacokinetic comparison of ginsenoside metabolite IH-901 from fermented and non-fermented ginseng in healthy Korean volunteers. J Ethnopharmacol. 2012;139:664–667. doi: 10.1016/j.jep.2011.11.052. [DOI] [PubMed] [Google Scholar]
- 25.Kim JS, Kim Y, Han SH, Jeon JY, Hwang M, Im YJ, Kim JH, Lee SY, Chae SW, Kim MG. Development and validation of an LC-MS/MS method for determination of compound K in human plasma and clinical application. J Ginseng Res. 2013;37:135–141. doi: 10.5142/jgr.2013.37.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee J, Lee E, Kim D, Lee J, Yoo J, Koh B. Studies on absorption, distribution and metabolism of ginseng in humans after oral administration. J Ethnopharmacol. 2009;122:143–148. doi: 10.1016/j.jep.2008.12.012. [DOI] [PubMed] [Google Scholar]


