Abstract
Background
There are no large database cohorts describing the causes for failure of primary THAs in France. Because implants and causes for revision vary between national registers, it is important to obtain data from all countries.
Questions/purposes
We therefore determined (1) the mechanisms of failure of primary THAs, (2) their order of appearance with time, (3) the types of surgical techniques and implant designs used to perform revision THAs, and (4) 90-day complications after revision THA in France.
Methods
We prospectively collected data on all 2107 first-time revision THAs from 30 tertiary centers from January 1, 2010, to December 31, 2011. A dual-mobility liner had been used in 251 hips. Mean time from primary procedure to revision THA was 11.2 years (range, 1 day to 42 years). Mean age at revision was 70 years (range, 17–104 years).
Results
The causes for revision were mechanical loosening (42%), periprosthetic fracture (12%), infection (11%), wear/osteolysis (11%), dislocation (10%), surgical technique error (6%), and implant fracture (3%). The most common type of revision procedure was all-component revision (49%). A dual-mobility liner was used in 1184 hips (62%). The 90-day dislocation rate was less than 4%, and mortality rate was 1.6%.
Conclusions
Contrary to other reported data, we found dislocation was not the main cause for failure of primary THAs but was still the more frequent early complication after revision. These findings might be related to the use of dual-mobility sockets in more than 10% of primary THAs and more than 60% of revision THAs.
Level of Evidence
Level IV, therapeutic study. See Instructions for Authors for a complete description of levels of evidence.
Introduction
Although THA has been associated with high levels of function and high survival rates, failures do still occur. In 1979, the first THA register was created in Sweden [14], rapidly followed by other similar attempts, mainly in Nordic countries such as Norway [6] or Denmark [13], and then in English-speaking countries such as Great Britain [17], Australia [1], and New Zealand [18]. On the other hand, some developed countries (eg, the United States, Germany, Italy, France) have not yet developed nationwide THA registers, despite existing institution, regional, or multicenter databases. Likely owing to variations in factors such as patient selection, implants, and local hospital situations, various registers have reported differences in reasons for revisions. In the United States, Bozic et al. [2] published in 2009 a multicenter study collecting data on a large scale but not inclusive of all hospitals. Dislocation was the main cause for failure in that study, whereas aseptic loosening was the leading cause in the British registry [17]. A substantial difference in France when compared to other European countries or the United States during the last decade has been the growing use of cementless distal locking femoral components and, above all, the widespread use of dual-mobility implants, which currently represent 1/3 of the cementless sockets implanted in France [10, 16]. In addition, findings change over time. Wolf et al. [24], using a large national administrative database, showed adverse outcomes after revision THA have worsened in the past 20 years in the United States, with 90-day mortality rate and composite adverse outcome rate (death, hemorrhage, infection, pulmonary embolism, sepsis, deep venous thrombosis, and myocardial infarction) reaching 5% and 11%, respectively, in 2006 to 2008. Surprisingly, that report provided no information about the 90-day dislocation rate after revision THA, which ranges from 5% to 30% in the literature [19]. Owing to the differences between countries and over time, it is crucial for countries without national registers to understand the causes of failure and analyze the types of revised THAs performed in their countries.
To compensate for the lack of nationwide databases, the French Society of Orthopaedic and Traumatologic Surgery (Société Française de Chirurgie Orthopédique et Traumatologique [SoFCOT]) initiated in 2009 a large prospective multicenter observational (ie, noninterventional; no modification to indication, surgical technique, or implants usually employed by participating centers) study to evaluate whether the causes for failure of primary THAs differed from those of existing national register data and whether the surgical techniques and implant designs showed different trends when compared to the literature.
We determined (1) the causes of failure of primary THAs, (2) their order of appearance with time, (3) the types of surgical technique and implant designs currently used to perform first revision THAs, and (4) 90-day complications after these first revisions.
Patients and Methods
Initiated in 2009 by the SoFCOT board and under the direction of two of us (CD, MH), all members of the SoFCOT and French Hip and Knee Society were invited to participate in the study, provided at least 30 revision THAs were performed each year at their institutions. This prospective multicenter cohort study, which started January 1, 2010, was strictly observational for all comers without any modification of the participating centers’ usual practice. The study was restricted to first revisions of conventional THA or resurfacing and included all consecutive such operations performed until December 31, 2011. Revision was defined as change of at least one part of the prosthesis. Rerevisions of THA and revisions of partial hip arthroplasties were excluded. During the 2-year inclusion period, a total of 2153 first revision THAs were performed in 30 tertiary centers (24 public university and general hospitals, two private hospitals, four private clinics) that voluntarily enrolled in the program and prospectively included the patients. During the same period, including first and rerevision THAs, approximately 3800 and 34,000 revision THAs were performed in the 30 participating centers and in France, respectively. Therefore, our data represent 12% of the procedures performed in France. Data were directly collected in a dedicated computerized database (FileMaker® Pro, San Diego, CA, USA). Demographic data of the cohort are summarized (Table 1). A dual-mobility liner was used in 251 hips (12%) during the primary procedure. Postoperative complications after primary THA included dislocation in 106 hips (5%), early infection treated by lavage in 45 hips (2%), hematoma drainage in 23 hips (1%), and sciatic nerve palsy in eight hips (0.4%).
Table 1.
Demographic data of the cohort study (2107 hips in 2107 patients)
| Variable | Value |
|---|---|
| Initial diagnosis (number of patients) | |
| Primary arthritis | 1306 (62%) |
| Hip dysplasia | 264 (13%) |
| Femoral head necrosis | 182 (9%) |
| Posttraumatic arthritis | 104 (5%) |
| Femoral neck fracture | 81 (4%) |
| Inflammatory coxitis | 51 (2%) |
| Rapid destructive arthritis | 23 (1%) |
| Infection | 10 (0.5%) |
| Miscellaneous | 32 (1%) |
| Not specified | 54 (2.5%) |
| Female/male (number of patients) | 1201 (57%)/906 (43%) |
| Age at primary THA (years)* | 59 (60, ± 14, 14–97) |
| Previous nonarthroplasty procedures (number of hips) | |
| No | 1842 (87%) |
| Yes | 265 (13%) |
| Fracture fixation | 83 |
| Shelf procedure | 57 |
| Femoral osteotomy | 50 |
| Surgical reduction of congenital hip dislocation | 18 |
| Acetabular osteotomy | 15 |
| Other | 42 |
| Type of primary THA (number of hips) | |
| Conventional | 2085 (99%) |
| Resurfacing | 13 (0.5%) |
| Not specified | 9 (0.5%) |
| Approach for primary THA (number of hips) | |
| Posterior | 1386 (66%) |
| Lateral | 373 (18%) |
| Anterior | 214 (10%) |
| Trochanteric osteotomy | 115 (5%) |
| Not specified | 19 (1%) |
| Fixation of primary THA (number of hips) | |
| Acetabular side: cemented/cementless/not specified | 792 (38%)/1291 (61%)/24(1%) |
| Femoral side: cemented/cementless/not specified | 1143 (54%)/941 (45%)/23 (1%) |
| Femoral head size (mm)† | 28 (22–56) |
| Age at revision THA (years)* | 70 (73, ± 13.1, 17–104) |
| Mean time from primary THA* | 11 years (11 years, ± 8 years; 1 day to 42 years) |
* Values are expressed as mean, with median, ± SD, and range in parentheses; †values are expressed as median, with range in parentheses.
The following patient information was collected at the time of the revision procedure and transferred to the database: weight, height, BMI, American Society of Anesthesiologists (ASA) score [5], Merle d’Aubigné functional score [15], Charnley categories [3], and Oxford Hip Score adapted for the French language [4]. To compare the surgical technique and implant designs used at revision with those in the literature, the type of procedure, surgical approach, fixation techniques, and implants used were detailed. Finally, medical and surgical complications after the revision procedure were collected during the first 3 months after revision. Surgical complications were defined as intraoperative and postoperative. The latter included those that may jeopardize implant longevity and/or patient function, therefore leading to an additional procedure. Several variables had missing values, including weight, height, and BMI (98 missing, 5%), ASA score (100 missing, 5%), Merle d’Aubigné functional score (34% missing), Oxford Hip Score (638 missing, 30%), surgical approach (49 missing, 2%), type of revision performed (18 missing, 1%), fixation of the acetabular (318 missing, 15%) and femoral component (327 missing, 16%), cup design (197 missing, 9%), and femoral head diameter used at revision (165 missing, 8%). We did not use any specific method to handle missing data as most of the patients had limited missing values and we did not perform a multivariate analysis. Of the 2153 index files included, 46 (2%) were excluded because of inadequate data, leaving 2107 first revision THAs that represent the final data set of this study.
The primary variable was the reason for revision in a closed list of eight choices: aseptic loosening, dislocation, periprosthetic fracture, implant fracture, infection, wear and/or osteolysis, surgical technique error (ie, intraoperative fractures, early migration, obvious component malposition, gross impingement, psoas conflict, and leg length discrepancy), and miscellaneous. We also investigated the occurrence of reasons for revision with time by determining the average time between primary and corresponding revision surgery for each reason for revision.
Results
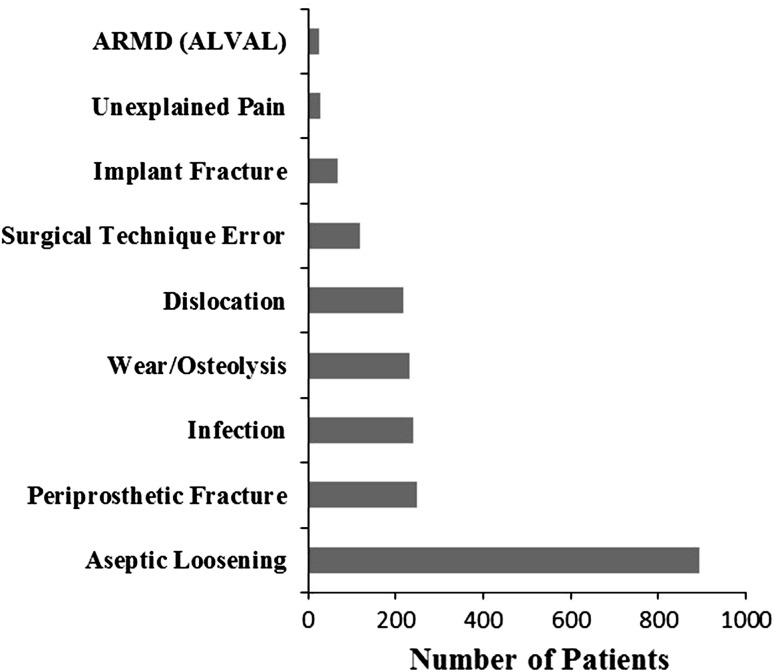
The causes for failure of primary THAs in the 2107 first revision THAs were as follows: mechanical loosening (891 hips, 42%), periprosthetic fracture (249 hips, 12%), infection (240 hips, 11%), wear and/or osteolysis (230 hips, 11%), dislocation (10%), technical error (118 hips, 6%), implant fracture (67 hips, 3%), adverse reaction to metallic debris (21 hips, 1%), and other (72 hips, 4%) (Fig. 1). Revision for dislocation occurred in 207 of 1895 THAs (11%) that had a femoral head diameter of 32 mm or less (range, 22–32 mm) and in 11 of 133 THAs (8%) that had a ball head size of greater than 32 mm (range, 36–56 mm). The mean time from primary procedure to first revision THA was 11 years (median, 11 years; SD, ± 8 years; range, 1 day to 42 years). When causes for revision were examined in order of appearance over time (Table 2), surgical technique errors were the earliest reason for revision at a mean followup of 2.7 years (range, 0–25 years) while wear and/or osteolysis was the latest event at a mean followup of 16 years (range, 3.7–32 years).
Fig. 1.
A graph shows the causes of THA revision in order of frequency. ARMD (ALVAL) = adverse reaction to metallic debris (aseptic lymphocytic vasculitis associated lesion).
Table 2.
Summary of causes for revision by order of appearance with time
| Cause for revision | Followup (years) | |||
|---|---|---|---|---|
| Mean | SD | Minimum | Maximum | |
| Surgical technique error | 2.8 | 3.1 | 0.005 | 24.8 |
| Infection | 5.6 | 6.6 | 0.025 | 34.5 |
| Dislocation | 7.1 | 7.1 | 0.003 | 33.5 |
| Implant fracture | 9.4 | 7.8 | 0.46 | 20.6 |
| Periprosthetic fracture | 9.8 | 8.3 | 0.022 | 37.1 |
| Mechanical loosening | 14.7 | 7.3 | 0.027 | 42 |
| Wear/osteolysis | 16 | 5.7 | 3.7 | 32 |
Fixation of the components employed at revision was cementless on the acetabular and femoral side in 51% and 54%, respectively. A cementless distal locking stem was used in 179 hips (8%), particularly in 25% of revisions performed for periprosthetic femoral fractures. A dual-mobility liner was used in 1184 hips (62%) during the revision procedure. The patient clinical status, surgical approach, and type of THA revision procedure are summarized (Table 3).
Table 3.
Patient clinical status, surgical approach, and types of procedure at first revision THA
| Variable | Value |
|---|---|
| Patient clinical status at revision | |
| ASA score of 3 and 4 (number of patients) | 602 (30%) |
| Devane score of 1 and 2 (number of patients) | 921 (45%) |
| BMI* | 26.5 (25.7, ± 5.3, 13.8–66) |
| Merle d’Aubigné score (points)* | 11 (12, ± 4.2, 0–18) |
| Oxford Hip Score (points)* | 37.8 (39, ± 12.4, 12–60) |
| Approach for revision THA | |
| Posterior | 1410 (69%) |
| Lateral | 293 (14%) |
| Anterior | 129 (6%) |
| Trochanteric osteotomy | 205 (10%) |
| Rottinger | 21 (1%) |
| Type of revision procedure | |
| Complete | 1018 (49%) |
| Cup-liner-head | 604 (29%) |
| Stem-head | 163 (8%) |
| Cup-liner | 125 (6%) |
| Stem-liner | 69 (3%) |
| Head-liner | 67 (3%) |
| Resection/spacer | 22 (1%) |
| Head/liner only | 14/7 (1%) |
* Values are expressed as mean, with median, ± SD, and range in parentheses; ASA = American Society of Anesthesiologists.
Intraoperative complications were reported in 146 hips (7%), mainly on the femoral side, with greater trochanteric fracture and femoral shaft fracture or perforation in 42 hips (2.1%) and 42 hips (2%), respectively. At 3 months after revision, surgical complications were encountered in 245 hips (12%). The four main complications were dislocation in 77 hips (4%), deep infection in 67 (3%), hematoma drainage in 21 (1%), and sciatic nerve damage in 13 (0.6%). Medical complications were dominated by cardiovascular complications (27%) and pulmonary and neurologic complications (10% each). Thirty-four patients (1.6%) died within 3 months after first revision THA. The mean age of these patients was 80 years (range, 43–97 years) versus 70 years (range, 16–103 years) for the remaining living patients.
Discussion
In 2007, SoFCOT initiated a multicenter THA register in France, but so far, this voluntarily based register reports only approximately 2% of primary and first revision THA procedures, due to very low surgeon compliance and absence of health authority involvement. Therefore, the current SoFCOT initiative was undertaken in 2009 to stimulate the interest of the French orthopaedic community in demographic and register policies. It is crucial to obtain data from all countries, as notable differences have been reported in terms of causes for revision and implants used from one register to another. Using a large prospective multicenter French cohort, we therefore determined (1) the causes of failure of primary THAs, (2) their order of appearance with time, (3) the types of surgical technique and implant designs currently used to perform first revision THAs, and (4) 90-day complications after these first revisions.
Readers should be aware of limitations to this study. First, the 30 tertiary hip centers that voluntarily participated in data collection may not be representative of the common practice in France. The majority were teaching university hospitals or private groups experienced in hip arthroplasty. In fact, the total number of THA revisions (first and subsequent) performed annually in France was estimated at 17,000. During the 2-year study period, the 30 centers involved performed about 1900 THA revisions and rerevisions annually, which represent approximately 12% of the nationwide THA revision practice. Second, missing data were encountered but mainly for functional status at revision (Merle d’Aubigné scores, Oxford Hip Scores), which was not within the scope of this study.
Our results for causes of failure of primary THAs were in the range of annual register data, except for dislocation, which, with a 10% rate, was in the fifth position (Table 4). The 2010 annual report of the Swedish Hip Arthroplasty Register confirmed aseptic loosening remained the prominent cause for revision of primary THA (72.6%), far ahead of dislocation (8.7%) and infection (7.8%) over more than 40 years (from 1967 to 2010) [21]. In fact, we believe it more appropriate to compare our data with those collected over the same recent 2-year time period (2010–2011) as provided by the Australian [1], Great Britain and Wales [17] and New Zealand registers [18] (Table 4). In these three registers, aseptic loosening remained the first reason for first revision THA (30%–45%) and dislocation was in second place (15%–31%), followed by infection (13%–17%), periprosthetic fracture (9%–15%), and pain (2%–26%). Our study can also be compared to the US epidemiologic study published in 2009 by Bozic et al. [2] (Table 3). In that paper, the Healthcare Cost and Utilization Project Nationwide Inpatient Sample database was used to analyze clinical, demographic, and economic data from 51,345 revision THAs performed between October 1, 2005, and December 31, 2006, in more than 1000 hospitals and a large, nationally representative Medicare population. The most common causes of revision were instability/dislocation (22%), mechanical loosening (20%), and infection (15%). Of course, our study is different in several ways from the study of Bozic et al. [2]. The US study was retrospective, reported on more than 50,000 procedures, and included all revision THA procedures (as opposed to just first revisions). Complete revision of all components was performed in the minority of procedures in both studies, 41% and 48%, respectively. Partial revision procedures were limited to acetabular components in 13% and 35%, respectively; femoral components in 13% and 11%, respectively; and bearings in 13% and 4%, respectively. Resection arthroplasty was more frequent in the US study (9%) than in the French study (1%). Despite the fact that all THA revision and rerevision procedures were accounted for in the US study, it is surprising that hip instability/dislocation was the primary indication reported for revision THAs (22%). For Bozic et al. [2], this finding emphasized the need for improvement in understanding the current causes of THA instability and additional advances in surgical techniques. Our 10% dislocation rate was lower than those in the US study and the three registers reported above. Interestingly, if dual-mobility cups were excluded from our primary THA cohort, the rate of revision for dislocation would have reached 12% and thus would have become the second most frequent cause of revision, as reported in nearly all registers.
Table 4.
Comparison of the five major causes for annual revision rates among three registers, the US study, and our study
| Register/Study | Year | Percentage of revisions by cause | |||||
|---|---|---|---|---|---|---|---|
| Aseptic loosening | Dislocation (Rank) | Infection | Periprosthetic fracture | Pain | Other* | ||
| Australian register [1] | 2011 | 29.9 | 27.6 (2) | 16.7 | 14.7 | 2 | 9 |
| National Joint Registry of Great Britain and Wales [17]† | 2011 | 42 | 13 (3) | 12 | 8 | 24 | NA |
| New Zealand register [18]† | 2010 | 41 | 30.6 (2) | 13.2 | 9.5 | 10.7 | NA |
| Bozic et al. [2] | 2005–2006 | 19.7 | 22.5 (1) | 14.8 | 6.2 | NA | 36.8 |
| Current study | 2010–2011 | 41.5 | 10.4 (5) | 11.2 | 11.8 | 1.3 | 23.8 |
* Other includes implant failure, implant fracture, wear, osteolysis, mechanical problems, surgical technique errors, and adverse reaction to metallic debris (aseptic lymphocytic vasculitis associated lesion); NA = not applicable; †in the New Zealand and Great Britain and Wales registries, there was often more than one reason listed on the data form and all are entered.
When classified by chronologic order of appearance after index surgery (Table 2), surgical technique errors were the earliest reason for revision (mean followup, 2.7 years) and wear and/or osteolysis was the latest reason (mean followup, 16 years). In fact, the two earliest (first 5 years) mechanisms of primary THA failure (surgical technique errors and infection) can be related to surgical expertise and perioperative environment; mid-term (5–10 years) failures (dislocation and implant fracture) mainly relate to implant characteristics (head size, metallurgic considerations, fatigue); and long-term (> 10 years) events (periprosthetic fractures, aseptic mechanical loosening, and wear and/or osteolysis) are linked to patient and implant unavoidable normal aging.
Beside frequent use of dual-mobility cups in THA revision surgery (62% in our cohort), our study showed another French cultural trend in the choice of implants used to perform femoral revision, with prominent use of cementless stems (54%). In particular, distal locking stems, developed in France to address complex femoral revisions secondary to osteolysis or periprosthetic femoral fractures [16], were used in 26% of the cementless femoral implants used for revision.
In our study, dislocation was the most common complication reported during the first 3 months after revision surgery, but our 4% rate is quite low in comparison with the usual range (5%–30%) of dislocation after revision THA reported in the literature [19, 23]. This might be due to the use of dual-mobility cups in 62% of revision, confirming the fact that the dual-mobility concept has become a well-accepted treatment for patients with high risk of instability and/or dislocation after primary and revision THAs in France [7–9, 11, 22]. Nevertheless, the short followup and the risk of intraprosthetic dislocation specific to this type of implant still raise concerns about its long-term efficiency [12, 20].
Finally, our 90-day mortality rate of 1.6% is lower than the 5% reported in the 2006 to 2008 US study by Wolf et al. [24] in the Medicare population and including all revisions, which increased the patient risk profile (aging and comorbidities).
This prospective study highlights some particularities of first revision THA in France: periprosthetic fracture is the second cause for first revision THA and dislocation is the fifth cause, which conflicts with data from the US Nationwide Inpatient Sample database [2]. So far, there is no nationwide THA register in either the United States or France. These differences emphasize the need for registers at a national level and support all current national and international initiatives as endorsed by the International Consortium of Orthopaedic Registers in collaboration with the European Arthroplasty Register and the International Society for Arthroplasty Registries.
Acknowledgments
We thank all the orthopaedic surgeons from the 30 institutions who participated in this project (The SoFCOT Group): CHU d’Amiens, Hôpital Nord (Amiens, France): Jean-François Lardanchet, Patrice Mertl, Jérome Taviaux; Institut Calot (Berck, France): Alain Cazenave; CHU de Caen (Caen, France): Christophe Hulet, Benoit Lebel; CH de Cannes (Cannes, France): Jacques Tabutin; Hôpitaux Civils de Colmar (Colmar, France): Claude Schwartz; Hôpital St-Jacques (Clermond-Ferrand, France): Stéphane Boisgard, Stéphane Descamps, Myriam Galvin, Jean-Paul Levai; CHRU de Grenoble (Grenoble, France): Marc Blaysat, Dominique Saragaglia; CHU Roger Salengro (Lille, France): Henri Migaud, Grégory Kern; Department of Biostatistics, CERIM, Université Lille (Lille, France): Nassima Ramdane-Sebbane; Hôpital E Herriot–Pavillon T (Lyon, France): Jacques Bejui-Hugues, Jean-Paul Carret, Olivier Guyen; Centre Hospitalier Lyon Sud (Lyon, France): Romain Desmarchelier, Michel Fessy, Anthony Viste; Centre ostéo-articulaire des Cèdres (Echirolles, France): Jean-Louis Prudhon, François Steffann; CH Sainte Marguerite (Marseille, France): Jean-Noël Argenson, Xavier Flecher; SINCAL-CHU (Nancy, France): Daniel Molé, Richard Philippe, Olivier Roche; Hôtel Dieu (Nantes, France): Alexandre Bocéno, François Gouin, Norbert Passutti; Groupement cliniques privées GOS (Nice, France): Loys Descamps; Clinique Jouvenet (Paris, France): Fabrice Gaudot, Jean-Baptiste Leymarie, Thierry Siguier, Grégory Sorriaux; Hôpital Cochin–Pavillon Ollier (Paris, France): Jean-Pierre Courpied, Matthieu Karoubi; Institut Mutualiste Montsouris (Paris, France): Emmanuel de Thomasson; Hôpital Bichat (Paris, France): Philippe Loriaut, Philippe Massin; Hôpital Lariboisière (Paris, France): Laurent Sedel, Frédéric Zadegan; CHU Jean Bernard (Poitiers, France): Louis-Etienne Gayet, Simon Teyssédou; CH de Pont-Labbé (Pont-Labbé, France): François Gaucher; CHP St Grégoire (Rennes, France): Philippe Triclot; CHU Sud (Rennes, France): Denis Huten, Jean-Christophe Lambotte, Jean-Louis Polard; Clinique Chirurgicale Orthopédique (Rouen, France): Franck Dujardin; CHU de Saint Etienne (Saint Etienne, France): Bertrand Boyer, Frédéric Farizon; Centre de Chirurgie Orthopédique et Traumatologique de Strasbourg (Strasbourg, France): Jean-Yves Jenny; CHU Hautepierre (Strasbourg, France): François Bonnomet, Matthieu Ehlinger, Frédéric Leiber-Wackenheim, Jean-François Kempf; CHU Rangueil (Toulouse, France): Philippe Chiron, Nicolas Reina; and Hôpital du Chesnay (Versailles, France): Philippe Beaufils, Philippe Oger.
Footnotes
The institution of one or more of the authors (AD) has received, during the study period, funding from Société Française de Chirurgie Orthopédique et Traumatologique (SoFCOT, Paris, France).
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research editors and board members are on file with the publication and can be viewed on request.
This work was performed at 30 French orthopaedic tertiary centers (see Acknowledgments).
References
- 1.Australian Orthopaedic Association. National Joint Replacement Report 2011. Available at: http://www.dmac.adelaide.edu.au/aoanjirr/publications. Accessed October 25, 2012.
- 2.Bozic KJ, Kurtz SM, Lau E, Ong K, Vail TP, Berry DJ. The epidemiology of revision total hip arthroplasty in the United States. J Bone Joint Surg Am. 2009;91:128–133. doi: 10.2106/JBJS.H.00155. [DOI] [PubMed] [Google Scholar]
- 3.Charnley J. Low Friction Arthroplasty of the Hip: Theory and Practice. New York, NY: Springer; 1979. [Google Scholar]
- 4.Delaunay C, Epinette JA, Dawson J, Murray D, Jolles BM. Cross cultural adaptations of the Oxford-12 Hip Score to the French speaking population. Orthop Traumatol Surg Res. 2009;95:89–99. doi: 10.1016/j.otsr.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 5.Dripps RD. New classification of physical status. Anesthesiology. 1963;24:111. [Google Scholar]
- 6.Furnes O, Lie SA, Espehaug B, Vollset SE, Engesaeter LB, Havelin LI. Hip disease and the prognosis of total hip replacements: a review of 53,698 primary total hip replacements reported to the Norwegian Arthroplasty Register 1987–99. J Bone Joint Surg Br. 2001;83:579–586. doi: 10.1302/0301-620X.83B4.11223. [DOI] [PubMed] [Google Scholar]
- 7.Guyen O, Pibarot V, Vaz G, Chevillotte C, Caret JP, Bejui-Hugues J. Unconstrained tripolar implants for primary total hip arthroplasty in patients at risk for dislocation. J Arthroplasty. 2007;22:849–858. doi: 10.1016/j.arth.2006.11.014. [DOI] [PubMed] [Google Scholar]
- 8.Hamadouche M, Arnould H, Bouxin B. Is a cementless dual mobility socket in primary THA a reasonable option? Clin Orthop Relat Res. 2012;470:3048–3053. doi: 10.1007/s11999-012-2395-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hamadouche M, Biau DH, Huten D, Musset T, Gaucher F. The use of a cemented dual mobility socket to treat recurrent dislocation. Clin Orthop Relat Res. 2010;468:3248–3254. doi: 10.1007/s11999-010-1404-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.[Health Authority. Evaluation of total hip prostheses: generic descriptions revising the list of reimbursable products and services of hip joint implants] [in French]. September 2007. Available at: http://www.has.sante.fr/portail/upload/docs/application/pdf/rapport_evaluation_des_prothesesde_hanche.pdf. Accessed November 25, 2012.
- 11.Langlais F, Ropars M, Gaucher F, Musset T, Chaix O. Dual mobility cemented cups have low dislocation rates in THA revisions. Clin Orthop Relat Res. 2008;466:389–395. doi: 10.1007/s11999-007-0047-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lecuire F, Benareau J, Rubini J, Basso M. [Intra-prosthetic dislocation of the Bousquet dual mobility socket] [in French] Rev Chir Orthop Reparatrice Appar Mot. 2004;90:249–255. doi: 10.1016/S0035-1040(04)70101-4. [DOI] [PubMed] [Google Scholar]
- 13.Lucht U. The Danish Hip Arthroplasty Register. Acta Orthop Scand. 2000;71:433–439. doi: 10.1080/000164700317381081. [DOI] [PubMed] [Google Scholar]
- 14.Malchau H, Herberts P, Eisler T, Garellick G, Soderman P. The Swedish Total Hip Replacement Register. J Bone Joint Surg Am. 2002;84(suppl 2):2–20. doi: 10.2106/00004623-200200002-00002. [DOI] [PubMed] [Google Scholar]
- 15.Merle d’Aubigné R. [Numerical classification of the function of the hip] [in French] Rev Chir Orthop Reparatrice Appar Mot. 1970;1990(76):371–374. [PubMed] [Google Scholar]
- 16.Mertl P, Philippot R, Rosset P, Migaud H, Tabutin J, Van de Velde D. Distal locking stem for revision femoral loosening and peri-prosthetic fractures. Int Orthop. 2011;35:275–282. doi: 10.1007/s00264-010-1182-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.National Joint Registry for England and Wales. 9th Annual Report 2012. Available at: http://www.njrcentre.org.uk. Accessed January 27, 2013.
- 18.New Zealand Orthopaedic Association. The New Zealand Joint Registry: thirteen year report. Available at: www.cdhb.govt.nz/njr/. Accessed January 27, 2013.
- 19.Philippot R, Adam P, Reckhaus M, Delangle F, Verdot FX, Curvale G, Farizon F. Prevention of dislocation in total hip revision surgery using a dual mobility design. Orthop Traumatol Surg Res. 2009;95:407–413. doi: 10.1016/j.otsr.2009.04.016. [DOI] [PubMed] [Google Scholar]
- 20.Philippot R, Boyer B, Farizon F. Intraprosthetic dislocation: a specific complication of the dual-mobility system. Clin Orthop Relat Res. 2013;471:965–970. doi: 10.1007/s11999-012-2639-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Swedish Hip Arthroplasty Register. Annual Report 2010. Available at: http://www.shpr.se/Libraries/Documents/AnnualReport-2010-2-eng.sflb.ashx. Accessed March 8, 2012.
- 22.Vielpeau C, Lebel B, Ardouin L, Burdin G, Lautridou C. The dual mobility socket concept: experience with 668 cases. Int Orthop. 2011;35:225–230. doi: 10.1007/s00264-010-1156-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wetters NG, Murray TG, Moric M, Sporer SM, Paprosky WG, Della Valle CJ. Risk factors for dislocation after revision total hip arthroplasty. Clin Orthop Relat Res. 2013;471:410–416. doi: 10.1007/s11999-012-2561-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wolf BR, Lu X, Li Y, Callaghan JJ, Cram P. Adverse outcomes in hip arthroplasty: long-term trends. J Bone Joint Surg Am. 2012;94:e103. doi: 10.2106/JBJS.J.00764. [DOI] [PMC free article] [PubMed] [Google Scholar]