Abstract
Background
Patient-specific CT-based instrumentation may reduce implant malpositioning and improve alignment in TKA. However, it is not known whether this innovation is an advance that benefits patients.
Questions/purposes
We evaluated (1) the precision of patient-specific TKA by comparing the incidence of outliers in postoperative alignment between TKAs using patient-specific instruments and TKAs using conventional instruments, and (2) the reliability of patient-specific instruments by intraoperatively investigating whether the surgery could be completed with patient-specific instruments alone.
Methods
In this randomized controlled trial, we compared patient-specific TKA instruments from one manufacturer (n = 50) with conventional TKA instruments (n = 50). Postoperative hip-knee-ankle angles, femoral component rotation, and coronal and sagittal alignments of each component were measured. The validity of the patient-specific instrument was examined using cross-checking procedures with conventional instruments during the surgeries. When the procedure could not be completed accurately with patient-specific instruments, the procedure was converted to TKA using conventional instruments, and the frequency of this occurrence was tallied.
Results
Outliers in the hip-knee-ankle angle were comparable between groups (12% in the patient-specific instrument group and 10% in the conventional instrument group). Other parameters such as sagittal alignment and femoral component rotation did not differ in terms of outliers. Patient-specific guides were abandoned in eight knees (16%) during the surgery because of malrotation of the femoral components and decreased slope of the tibia.
Conclusions
Accuracy was comparable between TKAs done with patient-specific instruments and those done with conventional instruments. However, the patient-specific instrument procedures had to be aborted frequently, incurring expenses that did not benefit patients.
Level of Evidence
Level II, therapeutic study. See Instructions for Authors for a complete description of levels of evidence.
Introduction
Implant alignment is an important factor affecting the long-term outcome of TKA [26]. Malalignment in the coronal plane exceeding 3º may result in an increased risk of component loosening [14, 20, 25]. Computer navigation-assisted TKA was introduced to minimize these outliers in component positioning and alignment [7], and has been reported to be effective [2–4, 19, 22]. In addition, the risk of fat embolism and the amount of bleeding may be less than is observed with conventional methods because some of these approaches do not place an alignment rod in the intramedullary canal of the femur [15]. Osteoarthritic knees with extraarticular deformities of the femur or retained hardware in the femur also can be addressed effectively with navigated TKA without breaching the femoral canal. However, navigated TKAs usually take longer than conventional methods, and there may be an increased risk of infection and pin site loosening or fracture [5, 22].
Patient-specific instrumentation was introduced as another subcategory of computer-assisted orthopaedic surgery characterized by three-dimensional (3-D) preoperative planning with the use of a CT or MRI scan [11]; this approach seeks to minimize the above-mentioned drawbacks of navigated TKA. Several studies have reported that patient-specific instruments improved the accuracy of implant positioning compared with conventional instruments [18, 21, 27]. However, these studies were not randomized trials, and so the question of selection bias (among other kinds of bias) may have affected their results. Moreover, currently available patient-specific instruments determine femoral component rotation based on the principle of the measured resection technique without taking individual soft tissue balance into account. This is a potential limitation, as this approach may be more prone to incorrect femoral component rotation and coronal instability compared with the gap-balancing technique [8, 10].
Accordingly, we sought to evaluate (1) the precision of patient-specific TKA by comparing the incidence of outliers in postoperative alignment between TKAs using patient-specific instruments and TKAs using conventional instruments and (2) the reliability of patient-specific instruments by intraoperatively investigating whether the surgery could be completed with patient-specific instruments alone. As a secondary question, we sought to determine whether the intraoperative decision to abandon the use of patient-specific instrumentation (where it was deemed necessary to do so) would result in an increased likelihood of inaccurate alignment.
Patients and Methods
In this randomized controlled trial, we studied 100 consecutive patients scheduled to undergo TKA. The patients were recruited and enrolled from November 2011 to July 2012. We included only patients with primary osteoarthritis. Patients with any history of previous surgery or trauma to the affected knee were excluded, as were patients who declined to participate in the trial. The internal review board of our hospital (Seoul National University Hospital Institutional Review Board Protocol Number H-1011-011-338) approved the study and informed consent was obtained from all participants. This study was registered in advance at the Clinical Research information Service, which is one of the primary registration systems listed with the WHO International Clinical Trials Registry Platform (Protocol Number KCT0000110).
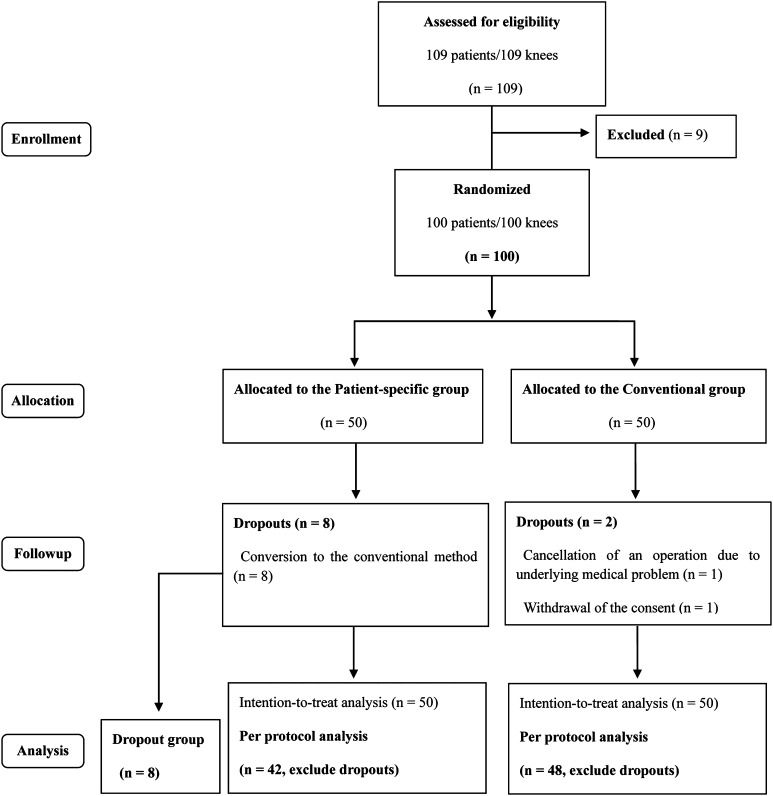
Knees were allocated according to a permuted block randomization program and the patient and surgeon were notified a few days before the surgery. Eligible knees were assigned to the group of TKAs using patient-specific instruments (n = 50) or to the group of TKAs using conventional instruments (n = 50) (Fig. 1). There were no differences between groups in preoperative demographics and clinical and radiographic data (Table 1). A CT-based Signature™ Personalized Patient Care System (Biomet Inc, Warsaw, IN, USA) was used in the patients in the patient-specific instruments group. CT scans were obtained according to the protocol provided by the manufacturer encompassing hip, knee, and ankle centers 4 to 6 weeks before each surgery. Raw images were sent to Biomet Inc for validation and uploaded to the 3-D surgical planning software operated by Materialise (Leuven, Belgium). The surgeon was notified of the preliminary surgical plan on templating and alignment of components, which he then could modify and confirm. Patient-specific guides were produced to fit on the distal femur and proximal tibia of the patient based on the calculated resection levels and proper positioning and size of the prosthesis components. Corresponding bone models also were ordered in all cases. All models were made from polyamide using rapid prototyping. Thereafter, they were delivered to our hospital and sterilized.
Fig. 1.
A flow diagram of the study is shown, which is based on a Consolidated Standards of Reporting Trials (CONSORT) flow diagram for a randomized controlled study. A subgroup (dropout group) was added during the study.
Table 1.
Comparison of preoperative demographics and clinical status between the two groups
| Demographic | Patient-specific instrument group | Conventional group | p value |
|---|---|---|---|
| Age (years)* | 70 ± 7.2 | 70 ± 5.1 | 0.891 |
| Gender (F/M) | 39 / 3 | 43 / 5 | 0.719 |
| BMI (kg/m2)* | 27 ± 4.2 | 27 ± 2.7 | 0.332 |
| Laterality (right/left) | 18 / 24 | 29 / 19 | 0.138 |
| Mechanical tibiofemoral angle (degrees)* | Varus 9.2 ± 4.1 | Varus 8.6 ± 5.2 | 0.573 |
| ROM (degrees)* | 9–131 ± 13.4 | 8–134 ± 13.8 | 0.113 |
| Patella resurfacing (resurface/preserve) | 31/11 | 26/22 | 0.079 |
* Presented as mean and standard deviation.
In the patient-specific instrument group, the initial target position of the implants was set to restore the mechanical alignment of the lower extremity in the coronal plane at 3° flexion from the mechanical axis of the femur and at 3° posterior slope to the mechanical axis of the tibia in the sagittal plane. Femoral component rotation was set at a position parallel to the clinical transepicondylar axis.
All surgeries were performed by one experienced surgeon (MCL), with the same type of implant (Vanguard® PS Mobile Bearing Knee; Biomet Inc). In the conventional instrument group, surgeries were performed with conventional manual instruments including an intramedullary femoral guide, an extramedullary tibial guide, and the femoral component sizing device. Femoral component rotations were determined using the gap-balancing technique controlled by the gravity traction method in the conventional instrument group and were predetermined according to the preoperative plan in the patient-specific instrument group (Fig. 2). The patella was resurfaced in all cases except in the case of a thin patella (< 20 mm) or intact cartilage. In the patient-specific instrument group, all bone cutting of the tibia and femur was performed under guidance of patient-specific instruments, except the femoral intercondylar notch. Before applying the patient-specific guides, the soft tissue around the contact points with the instruments was removed to expose the bare bone because all positioning guides were designed based on CT images.
Fig. 2A–B.
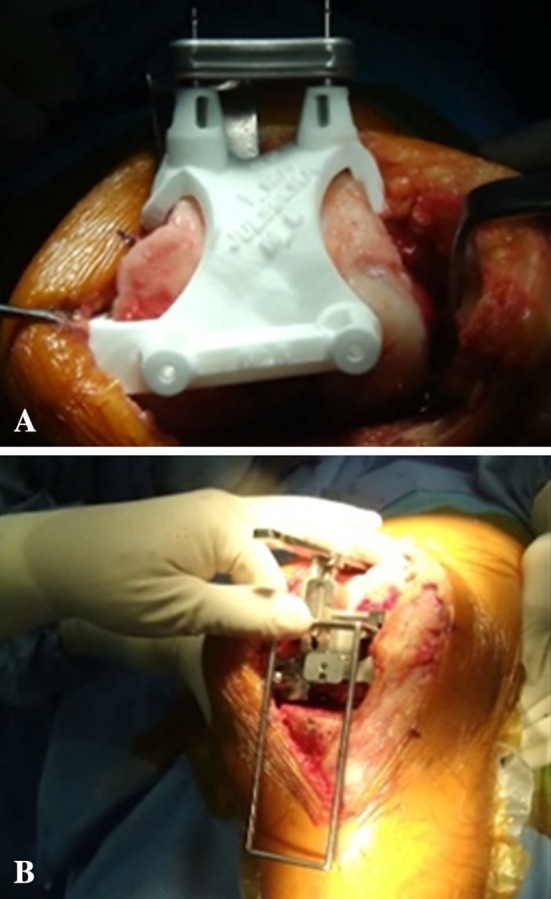
(A) The femoral component rotations in the patient-specific instrument group were determined preoperatively based on anatomic landmarks. (B) Intraoperative cross checks on the femoral component rotations were performed using the gap-balancing technique. The gravity traction method was used for gap balancing, which was used in the same manner as that for the conventional instrument group. The rectangular flexion gap was estimated using a custom-made ladder.
The adequacy of intraoperative alignment and femoral component rotation determined by the patient-specific guide was cross checked at every step with conventional methods before making the bone cuts for each step. Navigation was not used for this purpose. In tibial and distal femoral cutting, conventional extramedullary guides were used for cross-checking procedures. For cross checking femoral component rotation before anterior and posterior cutting of the femur, pinholes made by each method were marked with India ink and compared intraoperatively (Fig. 3). If there was a discrepancy greater than 3° between the two methods, we abandoned the patient-specific instruments and finished the rest of the surgery with conventional instruments on the premise that the traditional gap-balancing technique is the gold standard method. The patients whose surgery was converted intraoperatively to conventional instruments were excluded from the patient-specific instrument group. These patients were not added to the conventional instruments group, but rather they were categorized as a third group (dropout group). We tallied the rate of intraoperative conversion to conventional instruments in the patient-specific instrument group and recorded the specific causes for this conversion.
Fig. 3.
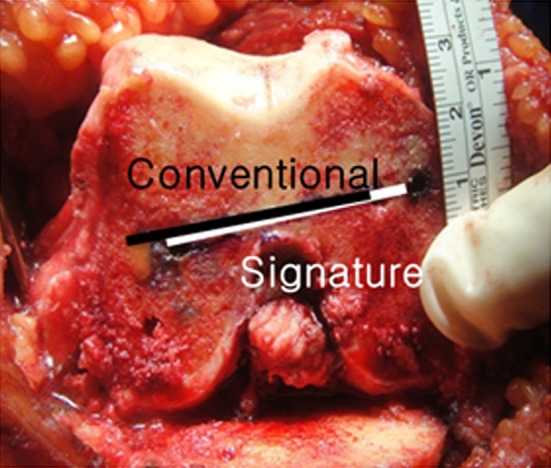
Two pairs of pin holes made by the patient-specific instruments (Signature) and the conventional instruments were compared with a goniometer intraoperatively. If the femoral component rotations suggested by the patient-specific instruments differed by greater than 3° from those suggested by the conventional instruments, the operation was performed with the conventional method thereafter.
We also performed an intention-to-treat analysis, in which results from all patients were analyzed according to the group to which they were assigned. There were no significant differences between this analysis and the one described in the previous paragraph, either in terms of the number of outliers or the mean values for radiographic alignment, thus the results are presented as a per-protocol analysis, with the groups as described in the previous paragraph, because some of the procedures with the patient-specific instruments could not be completed with that approach.
Postoperative mechanical tibiofemoral angles were measured and analyzed with full-weightbearing long-cassette plain radiographs; the coronal-sagittal alignments of each component then were measured with a postoperative CT scan using 3-D reconstruction software (OnDemand3D™; Cybermed Inc, Seoul, Korea). Rotation of the femoral component was assessed with axial CT images by comparing the posterior condylar axis of the implant and clinical transepicondylar axis. The amount of postoperative drainage and operation time were recorded. The outcome on postoperative alignment was evaluated by assessing the frequency of outliers in each parameter. An outlier was defined as greater than 3° from the mechanical axis in the coronal plane and greater than 3° from the initial target angles in the sagittal plane.
An a priori sample size analysis for this study suggested that at least 50 TKAs were required in each group, on an assumption that a 20% difference in the outlier rate is clinically significant between groups (α = 0.05; 1 − β = 0.8). In this calculation, we allowed for a dropout rate of 5% [1]. The postoperative radiographic outcomes were analyzed for all groups including the dropout group. However, the statistical analyses were performed only between the per-protocol population of the patient-specific instrument group (n = 42) and the conventional instrument group (n = 48). The dropout group (n = 8) was analyzed in a different set and the results are shown separately. The differences between groups were examined using a Student’s t-test for continuous variables and Pearson’s chi-square test or Fisher’s exact test for categorical variables. Statistical analyses of outcomes were performed with SPSS® 18.0 statistical software (SPSS Inc, Chicago, IL, USA).
Results
The postoperative hip-knee-ankle angle (mechanical tibiofemoral angle) of the leg did not differ between groups in our per-protocol analysis. Coronal alignments of the femoral and tibial components also were similar between groups, as were sagittal alignments of the femoral and tibial components (Table 2). The percentage of outliers from the mechanical axis of the leg were not different in the two groups (12% in the patient-specific instrument group versus 10% in the conventional instrument group; p = 0.542) (Table 3). The percentages of outliers in terms of coronal and sagittal alignments of each component also were not different between groups (Table 3). Femoral components were placed at an average of 0.5° internally from the clinical transepicondylar axis in the patient-specific instrument group and 1.2° internally from the axis in the conventional instrument group (p = 0.213) (Table 2). Blood loss via postoperative drain did not differ between groups (783.7 mL in the patient-specific instrument group versus 843.8 mL in the conventional instrument group). Surgical time was longer in the patient-specific instrument group than in the conventional instrument group (59.4 versus 46.6 minutes, respectively; p < 0.001).
Table 2.
Comparison of mean values of postoperative radiographic outcomes
| Alignment | Mean values (degrees) | p value | Dropout (n = 8) | |
|---|---|---|---|---|
| Patient-specific (n = 42) | Conventional (n = 48) | |||
| Mechanical tibiofemoral angle | Varus 0.5 ± 2.4 (varus 5.7 to valgus 5.0) | Valgus 0.3 ± 1.7 (varus 2.4 to valgus 4.0) |
0.067 | Valgus 0.7 ± 1.4 (varus 1.2 to valgus 2.7) |
| Coronal alignment (femur) | Varus 1.0 ± 1.4 (varus 3.7 to valgus 2.6) | Varus 0.6 ± 1.4 (varus 2.8 to valgus 3.9) | 0.170 | Varus 0.4 ± 0.9 (varus 2.0 to valgus 0.5) |
| Coronal alignment (tibia) | Valgus 0.2 ± 1.4 (varus 2.4 to valgus 2.9) | Valgus 0.7 ± 1.1 (varus 1.3 to valgus 4.2) | 0.095 | Varus 0.1 ± 0.8 (varus 1.0 to valgus 1.5) |
| Sagittal Alignment (femur) | Flexion 3.2 ± 2.3 (flexion 8.0 to extension 3.2) | Flexion 2.9 ± 2.0 (flexion 7.0 to extension 3.0) | 0.664 | Flexion 2.6 ± 1.2 (flexion 1.0 to 5.0) |
| Sagittal Alignment (tibia) | Posterior slope 3.0 ± 2.0 (neutral to posterior slope 8.0) | Posterior slope 3.6 ± 1.9 (neutral to posterior slope 6.8) | 0.316 | Posterior slope 2.9 ± 1.7 (posterior slope 0.5 to 5.1) |
| Femoral component rotation | IR 0.5 ± 1.8 (IR 4.5 to ER 2.4) | IR 1.2 ± 2.4 (IR 8.2 to ER 3.9) | 0.213 | IR 0.1 ± 0.7 (IR 0.8 to ER 1.2) |
Mean values are presented with standard deviation and range in parentheses; statistical analyses performed only between.
Patient-specific and Conventional groups; IR = internal rotation, ER = external rotation.
Table 3.
Comparison of outliers in postoperative radiographic alignment
| Alignment | Rate of outliers | p value | Dropout (n = 8) | |
|---|---|---|---|---|
| Patient-specific (n = 42) | Conventional (n = 48) | |||
| Mechanical tibiofemoral angle | 12% (5/42) | 10% (5/48) | 0.542 | None |
| Coronal alignment (femur) | 4.8% (2/42) | 2.1% (1/48) | 0.450 | None |
| Coronal alignment (tibia) | 0% (0/42) | 4.2% (2/48) | 0.282 | None |
| Sagittal alignment (femur) | 10% (4/42) | 6% (3/48) | 0.478 | None |
| Sagittal alignment (tibia) | 4.8% (2/42) | 6.3% (3/48) | 0.518 | None |
| Femoral component rotation | 10% (4/42) | 13% (6/48) | 0.458 | None |
Statistical analyses performed only between Patient-specific and Conventional groups.
Use of the patient-specific guides was abandoned intraoperatively in eight knees (16%) during the surgery because there were discrepancies greater than 3° in the femoral component rotation compared with the conventional gap method. External rotation was excessive in six knees and insufficient in two knees (ranging from −4.0° to +7.5°). Among the knees with excessive femoral component rotation, two were accompanied by unacceptably decreased posterior slope of the tibia, and these were corrected by recutting the tibia with an extramedullary guide. Otherwise, coronal and sagittal alignments were generally visually acceptable when cross checked with the conventional femoral and tibial guides.
The postoperative alignment of the dropout group (patients in whom patient-specific guides were abandoned intraoperatively) was acceptable in its mean value in that it was within 3o of the target (Table 2). Patients in this group did not have radiographic outliers in any parameters (Table 3). The intention-to-treat analysis did not change the findings in terms of mean alignment or frequency of outliers.
Discussion
There is an interest in patient-specific guides because they are a relatively new technology and several advantages have been proposed including improved accuracy of component alignment, minimized incidence of outliers, and conciseness of the surgical procedures. Despite much debate on the usefulness of the instruments [18, 21, 23, 29], there are few randomized trials [6, 29] to validate the surgical accuracy of this technology to date. In our study, the postoperative radiographic outcomes of the patient-specific instrument group were not significantly different from those of the conventional instrument group in all parameters. However, the patient-specific instruments were found to be unreliable, resulting in a relatively large number of dropouts (16%, n = 8) owing to malrotation of the femoral component and decreased posterior slope of the tibia.
There are several limitations to our study. The first and most important limitation is that the cross-checking procedures and other intraoperative decision-making procedures were not based on quantified and objective data as in a navigation study. One experienced surgeon (MCL) strictly cross checked the alignment and femoral component rotation with the conventional technique, including manual instruments and anatomic landmarks. There was some room for bias, especially in the traction forces used to determine the femoral component rotation (the manual force applied to distract the tibia distally while an assistant applied countertraction on the thigh to estimate the flexion gap). However, the results of postoperative radiographic measurements including assessment of the femoral component rotation with CT showed that there was no major bias in decisions made during the surgeries. Although a prospective controlled trial using a navigation system would be promising for a future study, cross checking with a navigation system is not completely free from this limitation because the registration steps are still vulnerable to the surgeon’s subjectivity. Second, only one type of patient-specific positioning guide was used in our study. Therefore, it is difficult to generalize our results to other commercially available patient-specific instruments. Third, this study focused on intraoperative validation of patient-specific instruments and comparison of immediate postoperative radiographic outcomes with conventional TKA, without encompassing other important parameters, such as functional improvement, patient satisfaction, longevity, and cost-effectiveness. Finally, the size of the study population was relatively small, although it was adequate based on our a priori sample size analysis. In previous studies with patient-specific instruments, MRI [18, 21, 23, 27, 28] or MRI and CT [13] were used for manufacturing the guides; our study was composed of purely CT-based guides, and this also may affect the degree to which our results can be generalized, as does the fact that we evaluated only one patient-specific system in this study. Results with other systems may differ.
Similar to our findings, Nunley et al. [23] retrospectively compared the patient-specific TKA group (n = 50) and conventional TKA group (n = 50) in their study and concluded that the incidence of outliers was not significantly different between the two groups (32% in the patient-specific TKA group and 40% in the conventional TKA group). However, a study by Ng et al. [21] revealed that the incidence of outliers was significantly higher in the conventional TKA group (9% in the patient-specific TKA group and 22% in the conventional TKA group), and the component angles were more close to neutral in their patient-specific TKA group. However, their study was limited by its restrospective design and because the surgeries were performed by multiple surgeons; by contrast, our study was a randomized controlled trial.
We found that a high proportion of the procedures could not be completed accurately using patient-specific guides. Klatt et al. [16] reported similar results in their small case series with the MRI-based OtisKnee™ system (OtisMed [Stryker], Alameda, CA, USA) and stated the need for more scientific validation of this technology. A navigation system was used for cross-checking procedures in their study, and the recommended coronal alignments by the instrument were 5.5° valgus to 0.5° varus to the mechanical axis in the femur and 3° valgus to 7.5° varus in the tibia. The posterior slope of the tibia also was decreased, ranging from 5.5° anterior slope to 0.5° posterior slope. Another difficulty with patient-specific guides is that they do not take into account the effects of soft tissue balancing. The gap-balancing technique has been advocated over the measured resection technique in some studies, including navigation studies, in terms of less abnormal femoral component rotation and coronal instability and better functional outcome [8, 10, 17, 24]. We used the gravity traction method, which uses gravity as a tension force for reproducing a rectangular flexion gap. The patient-specific instrument group showed the potential problems of the measured resection technique, resulting in excessive or insufficient (> 3°) femoral component rotation compared with the result using the gravity traction method. If cross checking and changes had not been made, there would have been eight cases of malrotation of the femoral components and asymmetric flexion gaps. As an alternative, several studies have proposed a kinematically aligned TKA using patient-specific instruments [9, 12, 23, 27], the intention of which is to improve clinical outcome by restoring prearthritic alignment without the release of soft tissue including collateral ligaments. However, some of these studies [9, 27] on kinematic alignment were limited by small sample size and lack of long-term results; consequently, the time-tested principle of mechanical alignment is used. Our finding that surgical time was longer in the patient-specific instrument group compared with the conventional instrument group also was observed by others who used similar cross-checking procedures [18, 28].
In another case series study by Stronach et al. [28], an average of 2.4 intraoperative changes were made per knee in 66 TKAs using the Signature™ system, and 81% of those surgeon-directed changes improved the implant alignment in the postoperative radiographic assessment. Similarly, TKAs in our patients excluded from the patient-specific instrument group owing to conversions to the conventional method showed no outliers in the postoperative radiographic measurements. We found TKAs using patient-specific instruments to be comparably accurate in terms of implant alignment and positioning to conventional TKAs. However, patient-specific instruments were not sufficiently reliable to complete the surgery independently in a large proportion of patients owing to issues of femoral component rotation and tibial slope. If patient-specific instruments are used, they must be used with caution, and further validation should be performed before they are widely adopted.
Acknowledgments
We thank Sang Min Lee MD for help with acquisition of a certificate of approval from the institutional review board.
Footnotes
Each author certifies that he, or a member of his immediate family, has no commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research editors and board members are on file with the publication and can be viewed on request.
Clinical Orthopaedics and Related Research neither advocates nor endorses the use of any treatment, drug, or device. Readers are encouraged to always seek additional information, including FDA approval status, of any drug or device before clinical use.
Each author certifies that his or her institution approved the human protocol for this investigation, that all investigations were conducted in conformity with ethical principles of research, and that informed consent for participation in the study was obtained.
This study was performed at Seoul National University Hospital, Seoul, Korea.
References
- 1.Bathis H, Perlick L, Tingart M, Luring C, Grifka J. CT-free computer-assisted total knee arthroplasty versus the conventional technique: radiographic results of 100 cases. Orthopedics. 2004;27:476–480. doi: 10.3928/0147-7447-20040501-13. [DOI] [PubMed] [Google Scholar]
- 2.Bathis H, Perlick L, Tingart M, Luring C, Zurakowski D, Grifka J. Alignment in total knee arthroplasty: a comparison of computer-assisted surgery with the conventional technique. J Bone Joint Surg Br. 2004;86:682–687. doi: 10.1302/0301-620X.86B5.14927. [DOI] [PubMed] [Google Scholar]
- 3.Bauwens K, Matthes G, Wich M, Gebhard F, Hanson B, Ekkernkamp A, Stengel D. Navigated total knee replacement: a meta-analysis. J Bone Joint Surg Am. 2007;89:261–269. doi: 10.2106/JBJS.F.00601. [DOI] [PubMed] [Google Scholar]
- 4.Blakeney WG, Khan RJ, Wall SJ. Computer-assisted techniques versus conventional guides for component alignment in total knee arthroplasty: a randomized controlled trial. J Bone Joint Surg Am. 2011;93:1377–1384. doi: 10.2106/JBJS.I.01321. [DOI] [PubMed] [Google Scholar]
- 5.Bonutti P, Dethmers D, Stiehl JB. Case report: femoral shaft fracture resulting from femoral tracker placement in navigated TKA. Clin Orthop Relat Res. 2008;466:1499–1502. doi: 10.1007/s11999-008-0150-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chareancholvanich K, Narkbunnam R, Pornrattanamaneewong C. A prospective randomised controlled study of patient-specific cutting guides compared with conventional instrumentation in total knee replacement. Bone Joint J. 2013;95:354–359. doi: 10.1302/0301-620X.95B3.29903. [DOI] [PubMed] [Google Scholar]
- 7.Delp SL, Stulberg SD, Davies B, Picard F, Leitner F. Computer assisted knee replacement. Clin Orthop Relat Res. 1998;354:49–56. doi: 10.1097/00003086-199809000-00007. [DOI] [PubMed] [Google Scholar]
- 8.Dennis DA, Komistek RD, Kim RH, Sharma A. Gap balancing versus measured resection technique for total knee arthroplasty. Clin Orthop Relat Res. 2010;468:102–107. doi: 10.1007/s11999-009-1112-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dossett HG, Swartz GJ, Estrada NA, LeFevre GW, Kwasman BG. Kinematically versus mechanically aligned total knee arthroplasty. Orthopedics. 2012;35:e160–e169. doi: 10.3928/01477447-20120123-04. [DOI] [PubMed] [Google Scholar]
- 10.Fehring TK. Rotational malalignment of the femoral component in total knee arthroplasty. Clin Orthop Relat Res. 2000;380:72–79. doi: 10.1097/00003086-200011000-00010. [DOI] [PubMed] [Google Scholar]
- 11.Hafez MA, Chelule KL, Seedhom BB, Sherman KP. Computer-assisted total knee arthroplasty using patient-specific templating. Clin Orthop Relat Res. 2006;444:184–192. doi: 10.1097/01.blo.0000201148.06454.ef. [DOI] [PubMed] [Google Scholar]
- 12.Howell SM, Howell SJ, Kuznik KT, Cohen J, Hull ML. Does a kinematically aligned total knee arthroplasty restore function without failure regardless of alignment category? Clin Orthop Relat Res. 2013;471:1000–1007. doi: 10.1007/s11999-012-2613-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Howell SM, Kuznik K, Hull ML, Siston RA. Results of an initial experience with custom-fit positioning total knee arthroplasty in a series of 48 patients. Orthopedics. 2008;31:857–863. doi: 10.3928/01477447-20080901-15. [DOI] [PubMed] [Google Scholar]
- 14.Jeffery RS, Morris RW, Denham RA. Coronal alignment after total knee replacement. J Bone Joint Surg Br. 1991;73:709–714. doi: 10.1302/0301-620X.73B5.1894655. [DOI] [PubMed] [Google Scholar]
- 15.Kalairajah Y, Simpson D, Cossey AJ, Verrall GM, Spriggins AJ. Blood loss after total knee replacement: effects of computer-assisted surgery. J Bone Joint Surg Br. 2005;87:1480–1482. doi: 10.1302/0301-620X.87B11.16474. [DOI] [PubMed] [Google Scholar]
- 16.Klatt BA, Goyal N, Austin MS, Hozack WJ. Custom-fit total knee arthroplasty (OtisKnee) results in malalignment. J Arthroplasty. 2008;23:26–29. doi: 10.1016/j.arth.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 17.Lee DH, Park JH, Song DI, Padhy D, Jeong WK, Han SB. Accuracy of soft tissue balancing in TKA: comparison between navigation-assisted gap balancing and conventional measured resection. Knee Surg Sports Traumatol Arthrosc. 2010;18:381–387. doi: 10.1007/s00167-009-0983-x. [DOI] [PubMed] [Google Scholar]
- 18.Lombardi AV, Jr, Berend KR, Adams JB. Patient-specific approach in total knee arthroplasty. Orthopedics. 2008;31:927–930. doi: 10.3928/01477447-20080901-21. [DOI] [PubMed] [Google Scholar]
- 19.Mason JB, Fehring TK, Estok R, Banel D, Fahrbach K. Meta-analysis of alignment outcomes in computer-assisted total knee arthroplasty surgery. J Arthroplasty. 2007;22:1097–1106. doi: 10.1016/j.arth.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 20.Moreland JR. Mechanisms of failure in total knee arthroplasty. Clin Orthop Relat Res. 1988;226:49–64. [PubMed] [Google Scholar]
- 21.Ng VY, DeClaire JH, Berend KR, Gulick BC, Lombardi AV., Jr Improved accuracy of alignment with patient-specific positioning guides compared with manual instrumentation in TKA. Clin Orthop Relat Res. 2012;470:99–107. doi: 10.1007/s11999-011-1996-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Novicoff WM, Saleh KJ, Mihalko WM, Wang XQ, Knaebel HP. Primary total knee arthroplasty: a comparison of computer-assisted and manual techniques. Instr Course Lect. 2010;59:109–117. [PubMed] [Google Scholar]
- 23.Nunley RM, Ellison BS, Zhu J, Ruh EL, Howell SM, Barrack RL. Do patient-specific guides improve coronal alignment in total knee arthroplasty? Clin Orthop Relat Res. 2012;470:895–902. doi: 10.1007/s11999-011-2222-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pang HN, Yeo SJ, Chong HC, Chin PL, Ong J, Lo NN. Computer-assisted gap balancing technique improves outcome in total knee arthroplasty, compared with conventional measured resection technique. Knee Surg Sports Traumatol Arthrosc. 2011;19:1496–1503. doi: 10.1007/s00167-011-1483-3. [DOI] [PubMed] [Google Scholar]
- 25.Rand JA, Coventry MB. Ten-year evaluation of geometric total knee arthroplasty. Clin Orthop Relat Res. 1988;232:168–173. [PubMed] [Google Scholar]
- 26.Ritter MA, Herbst SA, Keating EM, Faris PM, Meding JB. Long-term survival analysis of a posterior cruciate-retaining total condylar total knee arthroplasty. Clin Orthop Relat Res. 1994;309:136–145. [PubMed] [Google Scholar]
- 27.Spencer BA, Mont MA, McGrath MS, Boyd B, Mitrick MF. Initial experience with custom-fit total knee replacement: intra-operative events and long-leg coronal alignment. Int Orthop. 2009;33:1571–1575. doi: 10.1007/s00264-008-0693-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stronach BM, Pelt CE, Erickson J, Peters CL. Patient-specific total knee arthroplasty required frequent surgeon-directed changes. Clin Orthop Relat Res. 2013;471:169–174. doi: 10.1007/s11999-012-2573-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Victor J, Dujardin J, Vandenneucker H, Arnout N, Bellemans J. Patient-specific guides do not improve accuracy in total knee arthroplasty: a prospective randomized controlled trial. Clin Orthop Relat Res. 2013 Apr 24. [Epub ahead of print]. [DOI] [PMC free article] [PubMed]