Abstract
Background
Although pseudotumors have been reported in 32% of asymptomatic metal-on-metal hips, the natural history of asymptomatic pseudotumors is unknown.
Questions/purposes
The purpose of this study was to assess changes over time in asymptomatic pseudotumors and the effect of revision on pseudotumor mass.
Methods
Followup ultrasound was performed a mean of 25.8 months (range, 21–31 months) after the detection of 15 pseudotumors and five isolated fluid collections in a cohort of 20 asymptomatic patients (13 metal-on-metal, three metal-on-polyethylene, and four hip resurfacings) [42]. Changes in pseudotumors and fluid collections size and nature, and serum ion levels were determined.
Results
Among the 15 nonrevised patients, pseudotumors increased in size in six (four solid and two cystic) of 10 patients, three of which had clinically important increases (13–148 cm3; 28–74 cm3; 47–104 cm3). Three pseudotumors (one solid and two cystic) disappeared completely (the largest measured 31 cm3). One solid pseudotumor decreased in size (24 to 18 cm3). In five revised patients, pseudotumors completely disappeared in four patients. The fifth patient had two masses that decreased from 437 cm3 to 262 cm3 and 43 cm3 to 25 cm3. All revision patients had a reduction of chromium (40.42 μ/L to 2.69 μ/L) and cobalt ions (54.19 μ/L to 0.64 μ/L). Of five isolated fluid collections, four completely disappeared (two metal-on-metal and two metal-on-polyethylene) and one (metal-on-metal) increased from 26 cm3 to 136 cm3.
Conclusions
Our observations suggest pseudotumors frequently increase in size in asymptomatic patients with occasional remission of small masses. Revision resulted in remission of pseudotumors.
Level of Evidence
Level IV, therapeutic study. See the Guidelines for Authors for a complete description of levels of evidence.
Introduction
Metal-on-metal bearing surfaces in THA and resurfacing arthroplasty had gained wide interest in the past decade. This interest was increased by the advantage of using large-diameter femoral heads leading to improvement in stability [12, 36, 37] and potential improvement in function [13, 28].
Superior wear characteristics of metal-on-metal bearing surfaces [16, 19] and encouraging early outcomes of various metal-on-metal implant designs were reported by different authors [1, 6, 24, 31]. As metal-on-metal bearing surfaces technology evolved, their shortcomings started to appear. Authors raised concerns regarding elevated serum metal ions and their systemic side effects [4, 17, 19, 26, 30, 38, 40]. Local reactive soft tissue masses termed pseudotumors or lymphocyte-dominated vasculitis-associated lesions started to appear in the literature [7, 10, 11, 14, 22, 32, 34, 39]. It was proposed by some that these lesions represented a form of hypersensitivity reaction to local metal debris [8, 20, 35].
Several joint registries have reported revision rates of 12.48% (confidence interval [CI], 11.04%–14.1%) at 8 years and 14.1% (CI, 13.1%–15.3%) at 11 years with the metal-on-metal total hip prostheses [3, 33]. This eventually led to the recalls of specific metal-on-metal implants [15, 43]. Several authors have reported on the prevalence of these pseudotumor masses in both symptomatic and asymptomatic patients after either large-diameter femoral head THA or hip resurfacing arthroplasty [23, 27, 39, 42]. Hart et al. [23] compared the prevalence of MRI-detected pseudotumors in a group of 30 patients with painful large head metal-on-metal hip arthroplasty and a control group of 28 well-functioning patients and found a similar prevalence of pseudotumor of 57% and 61% in the respective groups. In another study, van der Weegen et al. [39] reported a pseudotumor incidence of 27% in a series of asymptomatic patients with hip resurfacing as detected by MRI. They concluded “that clinical outcomes and plain radiographs…underestimate the presence of pseudotumours in asymptomatic patients.” Kwon et al. reported a prevalence of 4% in asymptomatic patients undergoing hip resurfacing arthroplasty [27]. In a recent report from our institution [42] at a mean of 25.8 months (range, 21–31 months), the prevalence of pseudotumor masses as detected by ultrasound examination in a cohort of asymptomatic patients was 32% and 25% with metal-on-metal large head THA and hip resurfacing arthroplasty, respectively. However, the natural history of pseudotumors in asymptomatic patients after metal-on-metal hip arthroplasty is unclear.
We therefore asked (1) what happens to the size and nature of these pseudotumors when left untreated a minimum of 2 years after their initial detection; (2) what happens to these pseudotumors in patients who undergo revision THA; and (3) what happens to the serum ions levels in patients with metal-on-metal hip arthroplasty and resurfacing who undergo revision for asymptomatic pseudotumors?
Patients and Methods
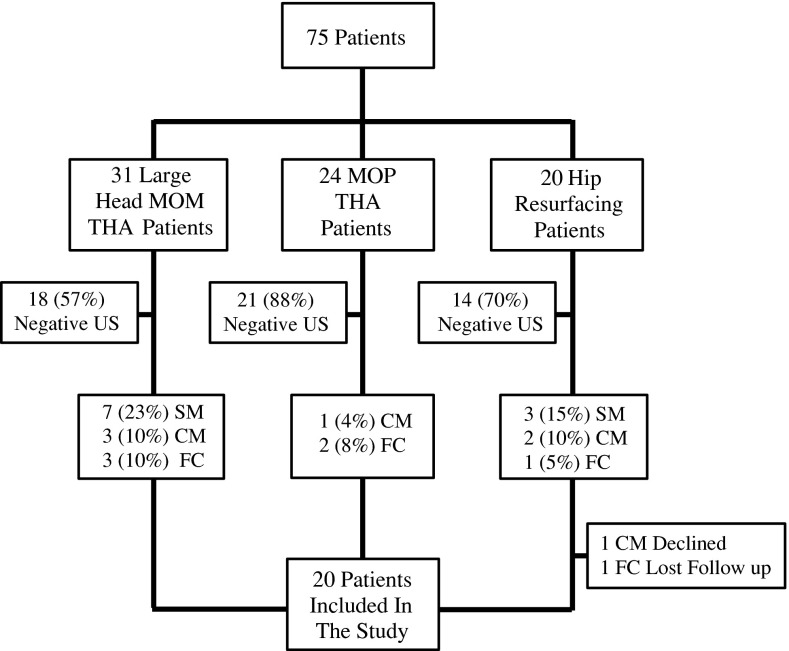
We previously reported a cohort of 75 patients who underwent a primary hip arthroplasty procedure [42] between September 1, 2004, and June 30, 2007. In that study, asymptomatic patients (patients with a WOMAC [5] of > 80 indicating the absence of symptoms) were recruited prospectively during routine followup. Thirty-one patients underwent metal-on-metal THA (M/L Taper®/Durom® THA; Zimmer Inc, Warsaw, IN, USA), 24 underwent metal-on-polyethylene THA (M/L Taper®/Trilogy® hip arthroplasty; Zimmer Inc), and 20 underwent metal-on-metal hip resurfacing arthroplasty (Durom® hip resurfacing; Zimmer Inc). All patients were evaluated with an ultrasound at a mean of 26 months (range, 21–31 months). Twenty-two of the 75 (29%) patients had positive ultrasound findings as follows: solid masses were detected in seven patients (23%) in the metal-on-metal THA group and three patients (15%) in the hip resurfacing arthroplasty group; cystic masses were detected in three patients (10%) in the metal-on-metal THA group (one patient had an associated fluid collection), two patients (10%) in the hip resurfacing arthroplasty group, and one patient (4%) in the metal-on-polyethylene THA group; and isolated fluid collections were detected in three patients (10%) in the metal-on-metal THA group, in two patients (8%) in the metal-on-polyethylene THA group, and in one patient (5%) in the hip resurfacing arthroplasty group [42]. An isolated fluid collection is defined as a fluid collection not associated with a solid or cystic pseudotumor mass.
The 22 patients with the positive ultrasound findings [42] were selected for this study (Fig. 1). Institutional review board approval was obtained. Consent to participate in the study was prospectively obtained from the targeted patients. One patient from the hip resurfacing arthroplasty group declined to participate in the study and was excluded. Contact was lost with another patient from the same group leaving 20 patients. Charts of the participating patients were reviewed. Data extracted included age, sex, and type of implant used. The followup visit notes were reviewed for development of hip pain, discomfort, and instability. The mean age of patients at the time of the ultrasound followup was 61 years (range, 50–72 years). Twelve patients were female and eight were male. Thirteen patients had a large-diameter femoral head THA (M/L Taper/Durom; Zimmer Inc), three a metal-on-polyethylene THA (M/L Taper/Trilogy; Zimmer Inc), and six a metal-on-metal hip resurfacing arthroplasty (Durom; Zimmer Inc) (Table 1). The mean component inclination angle as measured on a single digitally recorded AP pelvic radiograph with use of an electronic protractor, was 42° (range, 35°–52°). The mean arc of cover was 16 mm (range, 12–21 mm). Two patients in the resurfacing arthroplasty group were excluded (one declined and one was lost to followup).
Fig. 1.
The study group was selected from a previous cohort of 75 patients reported by Williams et al. [42]. Twenty-two patients with positive ultrasound findings (SM, CM, FC) were eligible for this study. MOM = metal on metal; MOP = metal on polyethylene; US = ultrasound; SM = solid mass; CM = cystic mass; FC = fluid collection.
Table 1.
Demographics at followup (n = 20 patients)
| Variable | Metal-on-metal THA | Metal-on-polyethylene THA | Resurfacing hip arthroplasty |
|---|---|---|---|
| Number of patients | 13 | 3 | 4 |
| Age (years)* | 62 (53–72) | 58 (52–66) | 61 (50–72) |
| Sex (female:male) | 8:5 | 2:1 | 2:2 |
* The values are given as the mean with the range in parentheses.
The results of the ultrasound and the unknown natural history of pseudotumors were discussed with all participants. All patients were offered the option of a revision THA, but only five of 20 patients chose this option, one of whom was male and four who were female. Four patients were from the large-diameter femoral head metal-on-metal THA group and one patient was from the resurfacing hip arthroplasty group. Three patients were completely asymptomatic. One patient (large-diameter metal-on-metal THA) had no pain symptoms but experienced a sensation of fullness around the hip. The last patient (resurfacing hip arthroplasty) had occasional groin pain. Revision surgery was performed at a mean of 55 months (range, 40–72 months) after the primary procedure. Infection was preoperatively ruled out in all patients using serological markers and aspiration if the serological markers were elevated. Revision surgery was performed by three of the participating surgeons (NVG, CPD, DSG). All revisions were performed through a posterolateral approach. Intraoperatively, patients had a variable amount of gray-tinged joint fluid in association with an identifiable pseudotumor mass. Trunion corrosion was observed in all patients from the large-head THA group. Pseudotumors were debulked when encountered through the posterolateral approach. No attempt was made to debulk pseudotumors that were not accessible through this approach. The pseudotumor mass was debulked in only two patients (both in the large-head THA group) and no debulking was attempted in the remaining three patients. No substantial bone loss was observed. In two patients, the revision bearing surface used was a ceramic head (BIOLOX® Delta ceramic femoral head; Zimmer Inc) with a titanium adaptor sleeve on highly crosslinked polyethylene (Longevity® Highly Crosslinked Polyethylene; Zimmer Inc) and in three patients, a metal head (VerSys® femoral head; Zimmer Inc) on highly crosslinked polyethylene (Longevity® Highly Crosslinked Polyethylene; Zimmer Inc). The decision to use either bearing surface was dictated mainly by the surgeon’s preference. Tissue samples were sent for pathological evaluation and for microbiology. Pathological assessment revealed necrotizing lymphocytic granulomatous reactions consistent with the diagnosis of pseudotumor in every case. All patients received prophylactic antibiotics and anticoagulation in accordance with our center’s protocol. Postoperatively, two female patients had recurrent instability requiring a second revision in one patient who also had a femoral nerve palsy that slowly resolved.
To answer our first question, we obtained an ultrasound scan as per the subsequent protocol on all 15 patients who did not undergo hip revision surgery at a mean of 66 months (range, 46–83 months) from the index THA. The measurements of the mass on the repeat ultrasound were compared with the ones on the initial scan. To answer the second question, for those five patients who underwent hip revision surgery, the date of the revision, indication for the revision, intraoperative findings, implants used, pathology reports, and development of postoperative complications were recorded. Also, a postrevision ultrasound examination as per the subsequent protocol was obtained at a mean of 17 months (range, 10–27 months) after the revision surgery and the measurements of any residual mass were compared with the initial scan. To answer the third research question, for the patients who underwent revision surgery, their serum metal ions levels were measured as per the subsequent protocol and were compared with their preoperative serum metal ion levels. All patients completed the WOMAC [5], SF-12 [41], and UCLA activity level [2] questionnaires at the time of the followup. At last followup, the mean WOMAC global score was 91 (range, 58–100; SD = 11.5), the mean SF-12 physical component was 47 (range, 15–62; SD = 12.7), and the mean UCLA activity level was and 7 (range, 3–10; SD = 1.0), respectively. Compared with the previous assessment [42], there were no differences in quality-of-life scores (Table 2).
Table 2.
Quality-of-life scores for patients with pseudotumor or isolated fluid collection: comparison with our previous study*,†
| Quality-of-life measure | Scores from Williams et al. [42] (minimum 2-year followup) (n = 22) | Scores from the current study (minimum 4-year followup) (n = 19) |
|---|---|---|
| WOMAC (points)† | ||
| Global | 95.3 ± 6.0 (80–100) | 91.3 ± 11.5 (58–100) |
| Pain | 94.8 ± 7.6 (75–100) | 92.4 ± 11.9 (65–100) |
| Stiffness | 93.8 ± 11.4 (63–100) | 87.5 ± 15.6 (50–100) |
| Function | 95.7 ± 6.2 (78–100) | 91.5 ± 11.8 (56–100) |
| SF-12 (mental component) | 53.8 ± 8.1 (33–61) | 53.9 ± 6.8 (41–65) |
| SF-12 (physical component) | 49.7 ± 12.3 (12–63) | 47.3 ± 12.7 (15–62) |
| UCLA activity level | 8 ± 1.8 (4–10) | 7 ± 1.9 (3–10) |
* The values are given as the mean and the SD with the range in parentheses; †WOMAC scores are normalized to a range of 0 to 100 with 0 being the worst and 100 being the best.
Ultrasound protocol as published by Williams et al. [42] was used. The ACUSON Antares Ultrasound System (Siemens Medical Solutions USA, Mountain View, CA, USA) was used. A standardized template was used to conduct the scans. One sonographer (DK), who participated in the first study, performed the ultrasound examinations. The Siemens VFX9-4 linear transducer and/or the Siemens CH6-2 curvilinear transducer were used for anterior, posterior, and/or lateral views, depending on each patient’s specific body habitus. The presence, size, and location of any fluid, cystic mass, or solid mass were recorded as well as any involvement of neurovascular structures and any muscle damage. Measurement of the wall thickness of fluid or cystic masses was not attempted. A fluid collection was defined as any collection that is anechoic, has round smooth borders, good through transmission, with posterior enhancement and an intact posterior wall. A cystic mass was defined as a complex cyst that has both cystic and solid components, a cyst with internal septation with or without central hyperechoic debris. A solid mass was defined as a mass that has a clear border, central homogenous echo, posterior shadowing, no associated cystic component, and no central color Doppler flow. A minimum size of 10 mm in any dimension was defined as an abnormality. The volume of any fluid or mass was calculated by multiplying the maximum recorded dimension in millimeters in each of three planes and dividing by 1000 to convert to volume in cubic centimeters (cm3) [42].
The Serum Ion Assay Protocol as published in Williams et al. [42] was used, and serum levels of chromium and cobalt were measured at the Trace Elements Laboratory–London Health Sciences Centre (London, Ontario, Canada) with use of inductively coupled plasma mass spectrometry (Thermo Fisher ELEMENT 2, High Resolution Sector Field Inductively Coupled Plasma Mass Spectrometer; Thermo Fisher Scientific, Waltham, MA, USA). This is considered to be the gold standard for trace metal ion analysis [29].
Results
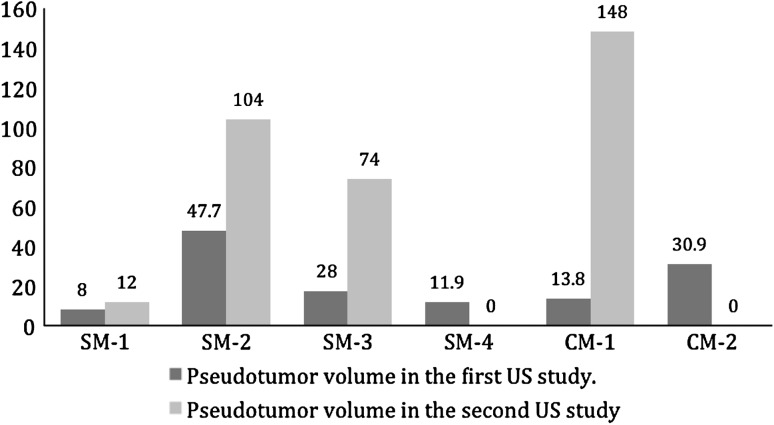
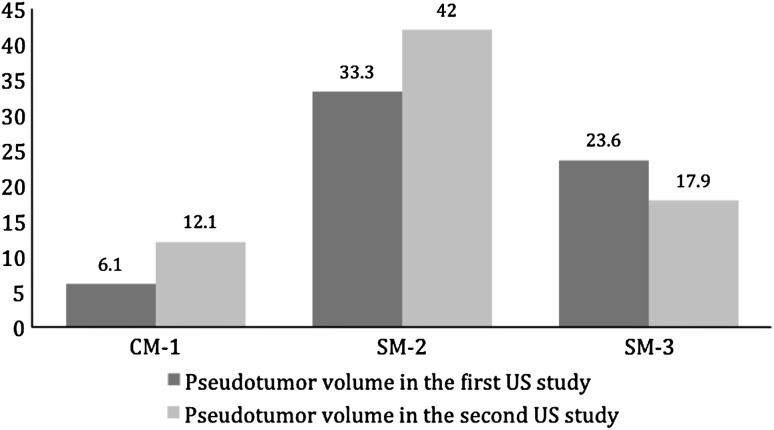
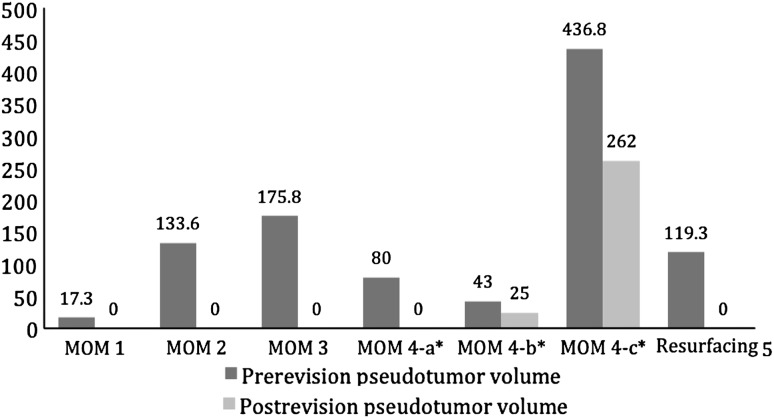
Most of the pseudotumors increased in size when left untreated. Of the 15 nonrevised patients, 10 patients had pseudotumors (six large-head metal-on-metal THA, three hip resurfacing arthroplasty, and one metal-on-polyethylene THA). All patients continued to be asymptomatic. In the large-head metal-on-metal THA group, four pseudotumor masses (three solid and one cystic) increased in size (Fig. 2). Three had what we considered a clinically important increase (14–148 cm3; 28–74 cm3; and 48–104 cm3). The fourth patient had an increase from 8 to 12 cm3. Two pseudotumors (a solid mass measuring 12 cm3 and a cystic mass measuring 31 cm3) completely disappeared with no intervention. Only one patient with a pseudotumor that increased in size had ultrasound evidence of abductor muscle damage in this group. In the hip resurfacing arthroplasty group, two pseudotumors increased in size (Fig. 3). One cystic mass increased from 6 to 12 cm3 and transformed into a solid mass. Another solid mass increased from 33 to 42 cm3 and involved the iliopsoas muscles. Both increases were considered clinically unimportant. A third solid mass decreased in size from 24 to 18 cm3 (Fig. 3). In the metal-on-polyethylene THA group, one small cystic mass measuring 10 cm3 completely disappeared. Pseudotumors completely disappeared in four of five patients who underwent revision surgery. Three patients (two large-head metal-on-metal and one resurfacing arthroplasty) had a single solid pseudotumor measuring 17, 134, and 119 cm3, respectively. One patient (large-head metal-on-metal THA) had a cystic mass measuring 176 cm3 (Fig. 4). The fifth patient (large-head metal-on-metal THA) had three solid masses measuring 80, 43, and 437 cm3. Of these, two masses decreased in size from 437 to 262 cm3 and 43 to 25 cm3 and transformed into cystic masses. The third mass is believed to have completely disappeared (Fig. 4), although postrevision ultrasound did show a fluid collection of 181 cm3. One patient in the revision group had evidence of abductor muscle damage.
Fig. 2.
Pseudotumor sizes (cm3) increased over time in four of six nonrevised large head metal-on-metal THAs. SM = solid mass; US = ultrasound; CM = cystic mass.
Fig. 3.
Pseudotumor sizes (cm3) increased over time in two of three nonrevised hip resurfacing arthroplasties. CM = cystic mass; SM = solid mass; US = ultrasound.
Fig. 4.
Pseudotumors disappeared (* or decreased in size [cm3]) after revision of four large head metal-on-metal THAs and one resurfacing hip arthroplasty. * One large head metal-on-metal patient had three different masses labeled a, b, and c.
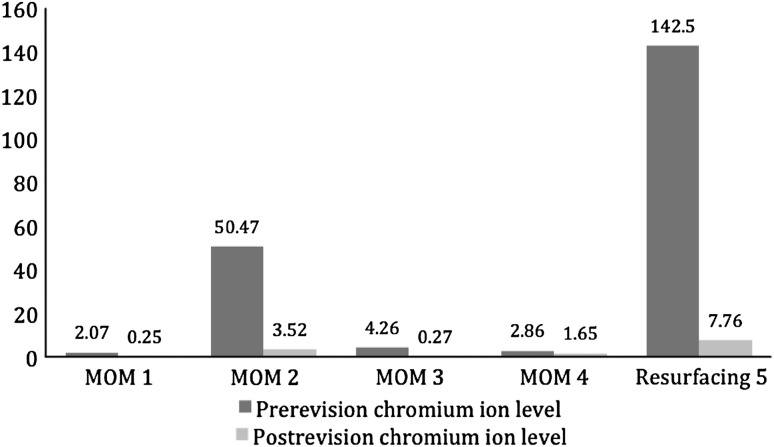
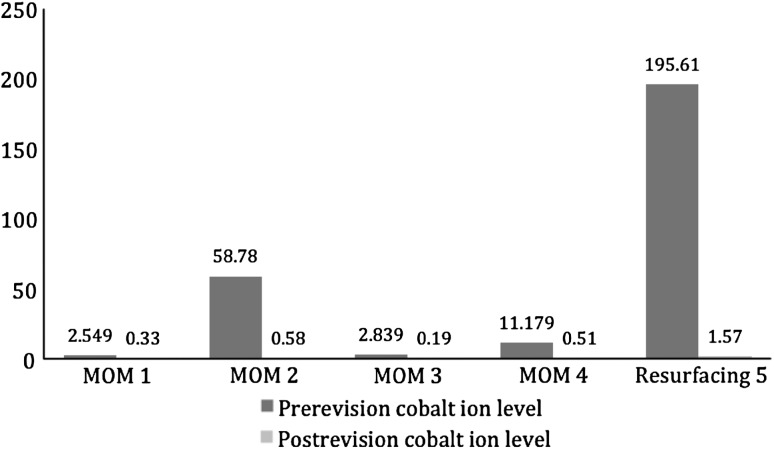
All postrevision patients had reduction of serum chromium (Fig. 5) and cobalt (Fig. 6) ions. Serum chromium ion levels dropped from a mean of 40.42 parts per billion (μg/L) (range, 2.07–142.46 μg/L) to a mean of 2.69 μg/L (range, 0.25–7.76 μg/L). Serum cobalt ion levels dropped from a mean of 54.19 μg/L (range. 2.55–195.61 μg/L) to a mean of 0.64 μg/L (range, 0.19–1.57 μg/L).
Fig. 5.
Chromium ion levels (in parts per billion [μg/L]) decreased in all revised patients (four large head metal-on-metal THAs and one resurfacing hip arthroplasty).
Fig. 6.
Cobalt ion levels (in parts per billion [μg/L]) decreased in all revised patients (four large head metal-on-metal THAs and one resurfacing hip arthroplasty).
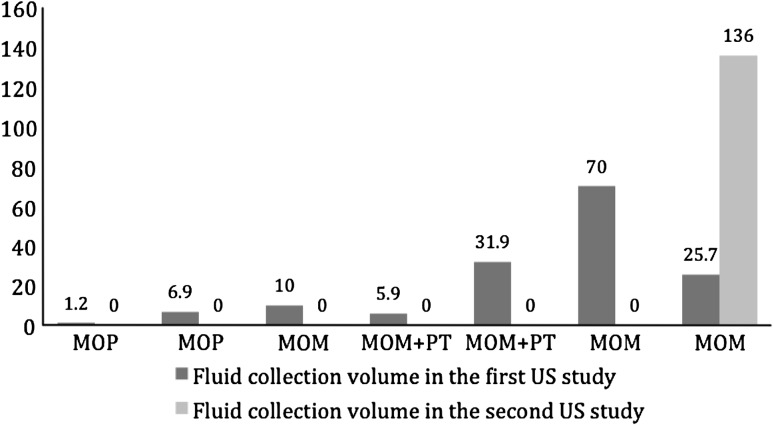
Five patients (three large-head metal-on-metal and two metal-on-polyethylene THA) had isolated fluid collections (1, 7, 10, 26, and 70 cm3) (Fig. 7). Four of the five isolated fluid collections completely disappeared (two metal-on-metal and two metal-on-polyethylene THA). The fifth, an isolated fluid collection, increased in size from 25 to 136 cm3 (Fig. 7). A fluid collection was initially detected in association with a pseudotumor in two large-head metal-on-metal patients (5 cm3 and 32 cm3). One patient had a solid pseudotumor that increased in size (48 to 104 cm3). The other patient underwent revision with complete remission of the pseudotumor (17 cm3). Fluid collections completely disappeared in both patients.
Fig. 7.
Fluid collections disappeared over time in two metal-on-polyethylene and two metal-on-metal THAs and increased in size in one metal-on-metal THA. MOM = metal on metal; MOP = metal on polyethylene; PT = pseudotumor; US = ultrasound.
Discussion
Since pseudotumors were first described [7, 22, 34], several authors have reported prevalences of 0.1% to 61% after metal-on-metal hip arthroplasty even in asymptomatic patients [9, 23, 27, 42]. In a previous report published by our institution [42], pseudotumor prevalence was described for three groups of asymptomatic patients at a minimum of 2 years from the index THA. The prevalence of pseudotumor masses (cystic or solid) was 33% in the metal-on-metal hip arthroplasty group, 25% in the metal-on-metal hip resurfacing group, and 4% in the metal-on-polyethylene group; isolated fluid collection prevalence, respectively, for each group was 10%, 5%, and 8% [42]. As we recognize the high prevalence of image-detected pseudotumors in asymptomatic patients, efforts should be directed toward designing evidence-based followup protocols and establishing clear indications for operative intervention. Guidelines recommending periodic clinical assessment of asymptomatic patients undergoing metal-on-metal hip arthroplasty have already been published [25]; however, the use of routine imaging, metal ion testing, indications for operative revision, and prognostic factors are yet to be established. For those reasons, knowing how pseudotumors behave over time is vital. We therefore assessed the changes over time in asymptomatic patients with previously ultrasound-detected pseudotumors, the effect of revision on the pseudotumor mass and serum ion levels.
Our study was subject to a number of limitations. First, the number of patients studied is small. We followed 22 patients and two patients were lost to followup. Including all previous patients, even those with negative ultrasounds, could have provided more data regarding the incidence of pseudotumor. However, in light of our research question, we only included patients with previously documented ultrasound findings. Second, the patients were diverse, including those with large-head metal-on-metal, metal-on-polyethylene, and hip resurfacing arthroplasty. This resulted in further smaller subgroups and difficulties detecting if any difference in pseudotumor behavior existed between different subgroups. Given the limited number of patients with positive ultrasound findings, we elected not to omit any patient subgroup. Third, ultrasound was used to measure the volume of pseudotumor masses and fluid collection. Being operator-dependent and the lack of data on the clinically meaningful differences in measurement adds another level of uncertainty. Fourth, the diagnosis of pseudotumor was only made by ultrasound and not confirmed by hip biopsy. Despite the previous limitations, we believe that our findings demonstrate the dynamic nature of pseudotumors, stress the importance of close followup, and open the door for future investigations.
We found four of six hips continued to increase in size in the large femoral head THA group but three of six hips if we exclude the one patient who had a clinically unimportant increase (from 8 to 12 cm3). To our knowledge no other study demonstrated the progression in the pseudotumor mass. Knowing a reasonable likelihood of a pseudotumor progressing is important when discussing revision surgery with an asymptomatic patient. It makes a stronger case for more frequent followup or possibly for earlier revision surgery. A complete remission despite no intervention was seen in two small pseudotumor masses (a 12-cm3 solid mass and a 31-cm3 cystic mass) in hips with a large-head metal-on-metal THA. It is difficult to explain why these masses disappeared. At followup, metal ion levels had decreased in both patients compared with their previous ion assessment. This suggests that observing small masses (< 31 cm3) in hope for remission is a reasonable option. These patients should be followed closely. Two of the three patients who progressed started with a similar small mass size. Revision of a metal-on-metal arthroplasty for pseudotumors was observed to have high complication rate [21]. As well, this was the finding in this series despite the limited number of revision patients. We still believe that the benefit of revision in asymptomatic patients should be weighed against the observed high complication rates of the revision surgery.
Five patients underwent revision a mean of 10 months (range, 3–20 months) from the time of pseudotumor diagnosis. The revision of the metal-on-metal bearing surfaces to a metal-on-polyethylene or ceramic-on-polyethylene bearing surface consistently resulted in either the complete remission or marked reduction in the size of the pseudotumor mass. This seems to be independent of the nature of the pseudotumor or surgical debulking of the mass. Complications were seen frequently after revision. Postoperatively, two female patients had recurrent instability requiring a second revision in one patient who also had a femoral nerve palsy that slowly resolved. Both patients had a primary 44-mm femoral head and both were revised to 32-mm femoral head. Only one patient has evidence of abductor muscle damage on ultrasound. The one patient, whose ultrasound still showed a mass after the revision, had the largest pseudotumor mass volume (437 cm3), and her followup ultrasound examination was performed only 10 months after the revision (the group mean was 17 months after revision). We are hopeful that in time, the mass will disappear (similar to the other four revised patients) but we cannot be certain.
Reduction in metal ion levels for all revision patients was observed. Our numbers are limited to establish this rule for certain. However, similar results have been reported in the literature. Ebreo et al. [18] examined a series of 44 patients with large-diameter head THA and hip resurfacing arthroplasty. In the 23 patients who had preoperative metal ions for comparison, median serum cobalt and chromium levels changed from 176.6 nM/L to 5.1 nM/L (p < 0.001) and 117 nM/L to 19 nm/L (p < 0.001), respectively, at a minimum followup of 14 months [18]. In this study, ultrasound detection of isolated fluid collections did not seem to have any substantial clinical sequelae. All fluid collections disappeared except one isolated collection in a female patient who had a metal-on-metal THA; the patient had a higher initial chromium (4.9 μg/L) and cobalt (9.3 μg/L) ion levels compared with the other two patients with a metal-on-metal THA (a mean of 0.88 μg/L and 0.64 μg/L for chromium and cobalt, respectively). She had a normal inclination angle of the cup and her metal ion levels had increased at the followup assessment (chromium to 5.24 μg/L and cobalt to12.07 μg/L). We recommend no surgical intervention for an ultrasound-detected fluid collection. Followup imaging is advised. Persistence or progression of the fluid collection is more likely if the associated metal ion levels are high.
Ultrasound-detected abnormalities are frequently observed in asymptomatic metal-on-metal THA and hip resurfacing arthroplasty. The indications for revision surgery at present are not clear. Our observations suggest that patients with isolated fluid collections can be observed with followup ultrasound anticipating the complete disappearance of the collection. For patients with solid and/or cystic pseudotumor masses, we currently recommend a followup ultrasound and surgical intervention if the mass substantially increases in size.
Acknowledgments
We thank Diana Korlaet CRGS, PDDipAppSci (Medical Ultrasound), for performing the ultrasound scans; and Daphné Savoy BA, for her assistance in the preparation of the manuscript.
Footnotes
This study was funded by a grant from the University of British Columbia Orthopaedics Research Excellence Fund (OREF) using funds from Johnson & Johnson (Canada) Inc (Markham, Ontario, Canada). Each author (BAM, CPD, DSG) certifies that he or she, has or may receive payments or benefits in any one year, an amount of USD 10,000–USD 100,000 (eg, serve as a consultant [BAM, CPD, DSG] or speakers bureaus [CPD]) from a commercial entity (Zimmer, Inc, Warsaw, IN, USA) and an amount of USD 10,000–USD 100,000 (speakers bureaus [CPD]) from a commercial entity (DePuy Synthes Canada Ltd, Markham, Ontario, Canada) related to this work. The remaining authors (SAA, NVG) certify that he or she has no commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article. The institution of the authors (SAA, NVG, BAM, CPD, DSG) has received funding from Johnson & Johnson (Canada) Inc, Zimmer Inc, DePuy Synthes Canada Ltd, Stryker Canada (Hamilton, Ontario, Canada), and Bayer Inc (Toronto, Ontario, Canada). One of the authors’ (SAA) fellowships was supported by the University of Dammam, Saudi Arabia.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research editors and board members are on file with the publication and can be viewed on request.
Clinical Orthopaedics and Related Research neither advocates nor endorses the use of any treatment, drug, or device. Readers are encouraged to always seek additional information, including FDA-approval status, of any drug or device prior to clinical use.
Each author certifies that his or her institution approved the human protocol for this investigation, that all investigations were conducted in conformity with ethical principles of research, and that informed consent for participation in the study was obtained.
References
- 1.Amstutz HC, Su EP, Le Duff MJ. Surface arthroplasty in young patients with hip arthritis secondary to childhood disorders. Orthop Clin North Am. 2005;36:223–230. doi: 10.1016/j.ocl.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 2.Amstutz HC, Thomas BJ, Jinnah R, Kim W, Grogan T, Yale C. Treatment of primary osteoarthritis of the hip. A comparison of total joint and surface replacement arthroplasty. J Bone Joint Surg Am. 1984;66:228–241. [PubMed] [Google Scholar]
- 3.Australian Orthopaedic Association National Joint Replacement Registry (AOANJRR). Available at: https://aoanjrr.dmac.adelaide.edu.au/documents/10180/60142/Annual%20Report%202012?version=1.2&t=1355186837517. Accessed January 5, 2013.
- 4.Beaulé PE, Kim PR, Hamdi A, Fazekas A. A prospective metal ion study of large-head metal-on-metal bearing: a matched-pair analysis of hip resurfacing versus total hip replacement. Orthop Clin North Am. 2011;42:251–257. doi: 10.1016/j.ocl.2011.01.005. [DOI] [PubMed] [Google Scholar]
- 5.Bellamy N, Buchanan WW, Goldsmith CH, Campbell J, Stitt LW. Validation study of WOMAC: a health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. J Rheumatol. 1988;15:1833–1840. [PubMed] [Google Scholar]
- 6.Berton C, Girard J, Krantz N, Migaud H. The Durom large diameter head acetabular component: early results with a large-diameter metal-on-metal bearing. J Bone Joint Surg Br. 2010;92:202–208. doi: 10.1302/0301-620X.92B2.22653. [DOI] [PubMed] [Google Scholar]
- 7.Boardman DR, Middleton FR, Kavanagh TG. A benign psoas mass following metal-on-metal resurfacing of the hip. J Bone Joint Surg Br. 2006;88:402–404. doi: 10.1302/0301-620X.88B3.16748. [DOI] [PubMed] [Google Scholar]
- 8.Campbell P, Ebramzadeh E, Nelson S, Takamura K, De Smet K, Amstutz HC. Histological features of pseudotumor-like tissues from metal-on-metal hips. Clin Orthop Relat Res. 2010;468:2321–2327. doi: 10.1007/s11999-010-1372-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Canadian Hip Resurfacing Study Group A survey on the prevalence of pseudotumors with metal-on-metal hip resurfacing in Canadian academic centers. J Bone Joint Surg Am. 2011;93(Suppl 2):118–121. doi: 10.2106/JBJS.J.01848. [DOI] [PubMed] [Google Scholar]
- 10.Clayton RA, Beggs I, Salter DM, Grant MH, Patton JT, Porter DE. Inflammatory pseudotumor associated with femoral nerve palsy following metal-on-metal resurfacing of the hip. A case report. J Bone Joint Surg Am. 2008;90:1988–1993. doi: 10.2106/JBJS.G.00879. [DOI] [PubMed] [Google Scholar]
- 11.Counsell A, Heasley R, Arumilli B, Paul A. A groin mass caused by metal particle debris after hip resurfacing. Acta Orthop Belg. 2008;74:870–874. [PubMed] [Google Scholar]
- 12.Cuckler JM, Moore KD, Lombardi AVJ, McPherson E, Emerson R. Large versus small femoral heads in metal-on-metal total hip arthroplasty. J Arthroplasty. 2004;19(Suppl 3):41–44. doi: 10.1016/j.arth.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 13.Daniel J, Pynsent PB, McMinn DJ. Metal-on-metal resurfacing of the hip in patients under the age of 55 years with osteoarthritis. J Bone Joint Surg Br. 2004;86:177–184. doi: 10.1302/0301-620X.86B2.14600. [DOI] [PubMed] [Google Scholar]
- 14.Davies AP, Willert HG, Campbell PA, Learmonth ID, Case CP. An unusual lymphocytic perivascular infiltration in tissues around contemporary metal-on-metal joint replacements. J Bone Joint Surg Am. 2005;87:18–27. doi: 10.2106/JBJS.C.00949. [DOI] [PubMed] [Google Scholar]
- 15.DePuy Synthes. Available at: http://www.depuy.com/sites/default/files/DPYUS1%20Recall%20Notice.pdf. Accessed July 2012.
- 16.Dowson D, Hardaker C, Flett M, Isaac GH. A hip joint simulator study of the performance of metal-on-metal joints. Part II: design. J Arthroplasty. 2004;19(Suppl 3):124–130. doi: 10.1016/j.arth.2004.09.016. [DOI] [PubMed] [Google Scholar]
- 17.Dunstan E, Ladon D, Whittingham-Jones P, Carrington R, Briggs TW. Chromosomal aberrations in the peripheral blood of patients with metal-on-metal hip bearings. J Bone Joint Surg Am. 2008;90:517–522. doi: 10.2106/JBJS.F.01435. [DOI] [PubMed] [Google Scholar]
- 18.Ebreo D, Khan A, El-Meligy M, Armstrong C, Peter V. Metal ion levels decrease after revision for metallosis arising from large-diameter metal-on-metal hip arthroplasty. Acta Orthop Belg. 2011;77:777–781. [PubMed] [Google Scholar]
- 19.Garbuz DS, Tanzer M, Greidanus NV, Masri BA, Duncan CP. The John Charnley Award: metal-on-metal hip resurfacing versus large-diameter head metal-on-metal total hip arthroplasty: a randomized clinical trial. Clin Orthop Relat Res. 2010;468:318–325. doi: 10.1007/s11999-009-1029-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Glyn-Jones S, Roques A, Taylor A, Kwon YM, McLardy-Smith P, Gill HS, Walter W, Tuke M, Murray D. The in vivo linear and volumetric wear of hip resurfacing implants revised for pseudotumor. J Bone Joint Surg Am. 2011;93:2180–2188. doi: 10.2106/JBJS.J.01206. [DOI] [PubMed] [Google Scholar]
- 21.Grammatopolous G, Pandit H, Kwon YM, Gundle R, McLardy-Smith P, Beard DJ, Murray DW, Gill HS. Hip resurfacings revised for inflammatory pseudotumour have a poor outcome. J Bone Joint Surg Br. 2009;91:1019–1024. doi: 10.1302/0301-620X.91B8.22562. [DOI] [PubMed] [Google Scholar]
- 22.Gruber FW, Böck A, Trattnig S, Lintner F, Ritschl P. Cystic lesion of the groin due to metallosis: a rare long-term complication of metal-on-metal total hip arthroplasty. J Arthroplasty. 2007;22:923–927. doi: 10.1016/j.arth.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 23.Hart AJ, Satchithananda K, Liddle AD, Sabah SA, McRobbie D, Henckel J, Cobb JP, Skinner JA, Mitchell AW. Pseudotumors in association with well-functioning metal-on-metal hip prostheses: a case-control study using three-dimensional computed tomography and magnetic resonance imaging. J Bone Joint Surg Am. 2012;94:317–325. doi: 10.2106/JBJS.J.01508. [DOI] [PubMed] [Google Scholar]
- 24.Heilpern GN, Shah NN, Fordyce MJ. Birmingham hip resurfacing arthroplasty: a series of 110 consecutive hips with a minimum five-year clinical and radiological follow-up. J Bone Joint Surg Br. 2008;90:1137–1142. doi: 10.1302/0301-620X.90B9.20524. [DOI] [PubMed] [Google Scholar]
- 25.Information for Orthopaedic Surgeons about Metal-on-Metal Hip Implant Surgery. Available at: http://www.fda.gov/MedicalDevices/ProductsandMedicalProcedures/ImplantsandProsthetics/MetalonMetalHipImplants/ucm241667.htm#top. Accessed July 2012.
- 26.Kim PR, Beaulé PE, Dunbar M, Lee JK, Birkett N, Turner MC, Yenugadhati N, Armstrong V, Krewski D. Cobalt and chromium levels in blood and urine following hip resurfacing arthroplasty with the Conserve Plus implant. J Bone Joint Surg Am. 2011;93(Suppl 2):107–117. doi: 10.2106/JBJS.J.01721. [DOI] [PubMed] [Google Scholar]
- 27.Kwon YM, Ostlere SJ, McLardy-Smith P, Athanasou NA, Gill HS, Murray DW. ‘Asymptomatic’ pseudotumors after metal-on-metal hip resurfacing arthroplasty: prevalence and metal ion study. J Arthroplasty. 2011;26:511–518. doi: 10.1016/j.arth.2010.05.030. [DOI] [PubMed] [Google Scholar]
- 28.Lavigne M, Ganapathi M, Mottard S, Girard J, Vendittoli PA. Range of motion of large head total hip arthroplasty is greater than 28 mm total hip arthroplasty or hip resurfacing. Clin Biomech (Bristol, Avon). 2011;26:267–273. [DOI] [PubMed]
- 29.MacDonald SJ, McCalden RW, Chess DG, Bourne RB, Rorabeck CH, Cleland D, Leung F. Metal-on-metal versus polyethylene in hip arthroplasty: a randomized clinical trial. Clin Orthop Relat Res. 2003;406:282–296. doi: 10.1097/00003086-200301000-00039. [DOI] [PubMed] [Google Scholar]
- 30.Mao X, Wong AA, Crawford RW. Cobalt toxicity—an emerging clinical problem in patients with metal-on-metal hip prostheses? Med J Aust. 2011;194:649–651. doi: 10.5694/j.1326-5377.2011.tb03151.x. [DOI] [PubMed] [Google Scholar]
- 31.McMinn DJ, Daniel J, Ziaee H, Pradhan C. Results of the Birmingham Hip Resurfacing dysplasia component in severe acetabular insufficiency: a six- to 9.6-year follow-up. J Bone Joint Surg Br. 2008;90:715–723. doi: 10.1302/0301-620X.90B6.19875. [DOI] [PubMed] [Google Scholar]
- 32.Murray DW, Grammatopoulos G, Gundle R, Gibbons CL, Whitwell D, Taylor A, Glyn-Jones S, Pandit HG, Ostlere S, Gill HS, Athanasou N, McLardy-Smith P. Hip resurfacing and pseudotumour. Hip Int. 2011;21:279–283. doi: 10.5301/HIP.2011.8405. [DOI] [PubMed] [Google Scholar]
- 33.National Joint Registry (NJR) for England and Wales. Available at: http://www.njrcentre.org.uk/NjrCentre/Portals/0/Documents/England/Reports/9th_annual_report/NJR%209th%20Annual%20Report%202012.pdf. Accessed January 5, 2013.
- 34.Pandit H, Glyn-Jones S, McLardy-Smith P, Gundle R, Whitwell D, Gibbons CL, Ostlere S, Athanasou N, Gill HS, Murray DW. Pseudotumours associated with metal-on-metal hip resurfacings. J Bone Joint Surg Br. 2008;90:847. doi: 10.1302/0301-620X.90B7.20213. [DOI] [PubMed] [Google Scholar]
- 35.Pandit H, Vlychou M, Whitwell D, Crook D, Luqmani R, Ostlere S, Murray DW, Athanasou NA. Necrotic granulomatous pseudotumours in bilateral resurfacing hip arthroplasties: evidence for a type IV immune response. Virchows Arch. 2008;453:529–534. doi: 10.1007/s00428-008-0659-9. [DOI] [PubMed] [Google Scholar]
- 36.Peters CL, McPherson EJ, Jackson JD, Erickson JA. Reduction in early dislocation rate with large-diameter femoral heads in primary total hip arthroplasty. J Arthroplasty. 2007;22:140–144. doi: 10.1016/j.arth.2007.04.019. [DOI] [PubMed] [Google Scholar]
- 37.Stuchin SA. Anatomic diameter femoral heads in total hip arthroplasty: a preliminary report. J Bone Joint Surg Am. 2008;90(Suppl 3):52–56. doi: 10.2106/JBJS.H.00690. [DOI] [PubMed] [Google Scholar]
- 38.Tower SS. Arthroprosthetic cobaltism: neurological and cardiac manifestations in two patients with metal-on-metal arthroplasty: a case report. J Bone Joint Surg Am. 2010;92:2847–2851. doi: 10.2106/JBJS.J.00125. [DOI] [PubMed] [Google Scholar]
- 39.van der Weegen W, Smolders JM, Sijbesma T, Hoekstra HJ, Brakel K, van Susante JL. High incidence of pseudotumours after hip resurfacing even in low risk patients; results from an intensified MRI screening protocol. Hip Int. 2012 Dec 11. doi: 10.5301/hipint.5000004 [Epub ahead of print]. [DOI] [PubMed]
- 40.Vendittoli PA, Amzica T, Roy AG, Lusignan D, Girard J, Lavigne M. Metal ion release with large-diameter metal-on-metal hip arthroplasty. J Arthroplasty. 2011;26:282–288. doi: 10.1016/j.arth.2009.12.013. [DOI] [PubMed] [Google Scholar]
- 41.Ware JE, Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30:473–483. doi: 10.1097/00005650-199206000-00002. [DOI] [PubMed] [Google Scholar]
- 42.Williams DH, Greidanus NV, Masri BA, Duncan CP, Garbuz DS. Prevalence of pseudotumor in asymptomatic patients after metal-on-metal hip arthroplasty. J Bone Joint Surg Am. 2011;93:2164–2171. doi: 10.2106/JBJS.J.01884. [DOI] [PubMed] [Google Scholar]
- 43.Zimmer Inc. Available at: http://www.zimmer.com/web/enUS/pdf/DUROM_SURGEON_LETTER_07-22-08_FINAL1.pdf. Accessed July 2012.