Abstract
Background
Healing and functional recovery have been reported using an extensively porous-coated stem in Vancouver B2 and B3 periprosthetic fractures; however, loss of cortical bone has been observed when using these stems in revision surgery for aseptic loosening. However, it is unclear whether this bone loss influences subsequent loosening.
Question/purposes
We analyze the healing fracture rate and whether the radiographic changes observed around and extensively porous-coated stem used for periprosthetic fractures affect function or loosening.
Methods
We retrospectively reviewed 35 patients with periprosthetic fractures (20 Vancouver B2 and 15 Vancouver B3). Patients’ mean age at surgery was 80 years (range, 51–86 years). No cortical struts were used in this series. We evaluated radiographs for signs of loosening or subsidence. The cortical index and the femoral cortical width were measured at different levels on the immediate pre- and postoperative radiographs and at different periods of followup. The minimum followup was 3 years (mean, 8.3 years; range, 3–17 years).
Results
All fractures had healed, and all stems were clinically and radiographically stable at the end of followup. Nineteen hips showed nonprogressive radiographic subsidence during the first 3 postoperative months without clinical consequences. The cortical index and the lateral and medial cortical thickness increased over time. Increase of femoral cortex thicknesses was greater in cases with moderate preoperative osteoporosis and in cases with stems less than 16 mm in thickness.
Conclusions
Our data suggest an extensively porous-coated stem for Vancouver B2 and B3 periprosthetic fractures leads to a high rate of union and stable fixation. Cortical index and lateral cortex thickness increased in these patients with periprosthetic fractures. Patients with moderate osteoporosis and those using thin stems showed a major increase in femoral cortex thickness over time.
Level of Evidence
Level II, prognostic study. See Guidelines for Authors for a complete description of levels of evidence.
Introduction
Management of Vancouver B2 and B3 femoral periprosthetic fractures [6] is a challenge for the surgeon because these fractures typically occur in elderly patients, around unstable stems, and are often associated with severe bone loss [17]. Cementless long-stem prostheses fixed in the intact diaphysis have been widely used in these patients to obtain fracture healing and stable fixation of the stem in the femoral diaphysis distal to deficient bone present in the proximal femur [13, 15, 17, 22, 23, 32]. Although long stems are a frequent option resulting from their high survival rate regarding rerevision, a loss of cortical bone has been documented in these hips subsequently revised for aseptic loosening [10, 18, 19, 25, 29, 30, 34]. However, it is unclear whether loss of cortical bone influences the risk of revision.
We therefore asked whether (1) the use of an extensively porous-coated stem would reliably allow healing of Vancouver B2 and B3 periprosthetic fractures; (2) the appearance of signs of radiographic loosening and subsidence would affect patient function; and (3) the appearance of radiographic cortical changes observed previously with these implants were related to different factors related to either the patients or the stems. We also assessed the rate of complications, thigh pain, and leg length.
Patients and Methods
We reviewed 40 all periprosthetic fractures (40 patients) operated on between 1992 and 2006. The criterion for inclusion in this study was revision with an extensively porous-coated stem (Solution System; DePuy, Johnson & Johnson, Warsaw, IN, USA) resulting from any B2 or B3 periprosthetic fracture of a cemented or cementless stem. The main indication for this stem was a femoral canal of 18 mm so adequate distal fixation could be obtained. There were no absolute contraindications other than the patient’s preoperative medical condition. Twenty-three fractures were Vancouver B2 and 17 Vancouver B3 type. We excluded five hips because of death from causes unrelated to the operation within the minimum followup. The remaining 35 formed the basis of the followup study. There were 22 women and 18 men (Table 1). Their mean age at the time of surgery was 80.4 years (range, 51–86 years). Five patients were younger than 70 years of age. The original diagnosis was primary osteoarthrosis in 26 patients, femoral neck fracture in eight, posttraumatic arthritis in three, rheumatoid arthritis in two, and developmental dysplasia of the hip in one. We graded preoperative bone osteoporosis according to Moreland and Moreno [30] as minimal (four hips), moderate (six hips), and severe (30 hips). Femoral bone loss was classified according to the Paprosky et al. criteria [34]: Grade 2 (six hips), Grade 3A (11 hips), and Grade 3B (23 hips) (Table 1). The average time between the initial THA and the femoral fracture was 7.7 years (range, 1–20 years). No patients were lost to followup. The minimum followup was 3 years (mean, 8.3 years; range, 3–17 years). No patients were recalled specifically for this study; all data were obtained from medical records and radiographs. Verbal and written informed consent was always obtained from all patients and they were informed preoperatively that they might receive an extensively porous-coated stem.
Table 1.
Patient data (N = 40)
| Type of fracture [6] | B2 fracture | B3 fracture | Total | p values |
|---|---|---|---|---|
| Sex | ||||
| Male | 12 | 6 | 18 | 0.348 |
| Female | 11 | 11 | 22 | |
| Mean age (years [range]) | 79.2 (56–80) | 82.2 (51–86) | 80.4 (51–86) | 0.071 |
| Preoperative bone osteoporosis [28] | ||||
| Minimal and moderate | 8 | 2 | 10 | 0.146 |
| Severe | 15 | 15 | 30 | |
| Bone defect [32] | ||||
| Grade 2 | 4 | 2 | 6 | 0.345 |
| Grade 3A | 8 | 3 | 11 | |
| Grade 3B | 11 | 12 | 23 | |
All hips were templated preoperatively to determine the appropriate stem width. For planning the operation, the surgeons preferred the stems that were long enough to achieve a fixation depth of at least 5 to 7 cm in intact distal diaphyseal bone [18, 25, 30, 34]. All surgeries were performed with a posterolateral approach. The surgeon then confirmed the bone defect by visualization and palpation; diaphyseal bone stock was determined by manually introducing different sizes of flexible reamers to ensure that the diameter was 18 mm or less and at least 5 cm in intact distal bone; after this procedure, we started to ream. Femoral implant selection was based on the femoral bone status that was ascertained during the operation (Table 2). The diameter of the stems ranged from 13.5 to 18 mm (median, 15 mm), and their length ranged from 20.3 to 25.4 cm (median, 25.4 cm), ie, 8 to 10 inches, as specified by the manufacturer (Table 3). Longer stems with more porous coatings were used as needed in femurs with greater degrees of bone stock deficiency. Femora were underreamed by 0.5 mm, although when the component did not progress with impaction, we used line-to-line reaming or even 0.5 mm more for bowed components [25]. To prevent fractures, we placed a prophylactic wire around the distal femur before impaction [26]. The proximal bone stock, despite being excessively poor in quality, was always retained and reapproximated onto the prosthesis with cerclage fixation [35]. No strut grafts were used in any femur.
Table 2.
Prostheses used for primary arthroplasty (N = 40)
| Prosthesis | Number |
|---|---|
| Charnley (DePuy, Warsaw, IN, USA) | 18 |
| Müller (Protek, Bern, Switzerland) | 5 |
| Other cemented stems | 5 |
| RM Isoelastic (Protek) | 1 |
| PCA (Stryker-Howmedica, Rutherford, NJ, USA) | 2 |
| Harris-Galante I (Zimmer, Warsaw, IN, USA) | 6 |
| Mittelmeier (Osteo AG, Selzach, Switzerland) | 2 |
| Omniflex (Stryker, Rutherford, NJ, USA) | 4 |
| Alloclassic (Zimmer, Winthertur, Switzerland) | 3 |
| Other cementless stems | 4 |
Table 3.
Bone defect, stem diameter, and length in the whole series according to the intraoperative bone defect and to the Vancouver fracture type (N = 40 hips)
| Bone defect type [32] | 2 | 3A | 3B | Total |
|---|---|---|---|---|
| Diameter of the stem | ||||
| 13.5 mm | 4 | 1 | 6 | 11 |
| 15 mm | 1 | 6 | 7 | 14 |
| 16.5 mm | 1 | 4 | 9 | 14 |
| 18 mm | – | – | 1 | 1 |
| Total | 6 | 11 | 23 | 40 |
| Length of the stem | ||||
| 8 inches | 4 | 5 | 10 | 19 |
| 10 inches | 2 | 6 | 13 | 21 |
| Total | 6 | 11 | 23 | 40 |
| Type of fracture [6] | B2 | B3 | Total |
|---|---|---|---|
| Diameter of the stem | |||
| 13.5 mm | 7 | 4 | 11 |
| 15 mm | 9 | 5 | 14 |
| 16.5 mm | 5 | 9 | 14 |
| 18 mm | 1 | – | 1 |
| Total | 22 | 18 | 40 |
| Length of the stem | |||
| 8 inches | 12 | 7 | 19 |
| 10 inches | 10 | 11 | 21 |
| Total | 22 | 18 | 40 |
Antibiotic prophylaxis (1 g cefazolin every 6 hours) was discontinued at 48 hours. Subcutaneous heparin as a routine preventive measure for thromboembolic problems was used under the strict protocol of the hospital hematology department until patients were fully mobile. After spending from 3 to 5 days in bed with the leg in abduction using an orthopaedic triangular soft pillow, the patients were allowed to walk with partial weightbearing with two crutches for the younger patients without neurological impairment and minor defects (B2 fractures); older patients with a type B3 fracture were allowed to do nonweightbearing therapy for 3 months until radiographic healing of the fracture was observed.
Clinical evaluation assessed pain, walking ability, and joint motion following the Merle D’Aubigné and Postel scale (range, 1–6) [27]. Clinical failure was considered as no fracture healing, rerevision, or pain (level 4 or less). Moderate pain was considered as level 4 and severe pain as less than level 4. We asked the patient about any type of pain around the hip: groin, lateral, buttock, or thigh pain. We related thigh pain to femoral stem problems when the patients reported rotation of the hip, with the straight leg raising test, and/or weightbearing [8, 24]. Leg length discrepancy was measured by blocks under the operated feet at the first postoperative year examination; any leg length discrepancy over 1 cm was recorded. We considered the fracture healed when the patient was bearing full weight without pain, lacked pain on clinical stressing at the fracture site, and had radiographic evidence of callus bridging the fracture [15]. We also recorded complications after this complex procedure. All data were included except the five patients who died before 3 years after the surgery.
Standard AP and lateral radiographs of the pelvis and the operated femur were made for all patients immediately after the operation, at 3, 6, and 12 months, and annually thereafter. In clinical practice, qualitative and quantitative assessment of cortical index and cortical width from the postoperative radiographs is a somewhat inaccurate and inconsistent method for the evaluation of stress shielding changes [1, 11, 39]. There are other problems inherent to radiographic techniques such as exposure quality and positioning of the prone femur resulting in measurement variability attributable to rotation of the femur when cortical dimensions are involved [1, 7, 11, 39]. To minimize the contributions of each of the potential errors, all postoperative and followup radiographs were made at our institution following the same protocol. The patient was positioned supine with his or her feet together. The x-ray tube was positioned over the symphysis pubis 1 m from and perpendicular to the table. Measurements were made by two experienced readers (EGC, EGR). Variations in magnification were corrected using the known diameter of the femoral head as internal reference. Criteria for healing were the presence of bridging callus on the two views. The femur was divided according to the zones of Gruen et al. [12]. Femoral canal filling was measured as the ratio of stem width to intramedullary canal width and was determined at three levels: level A is 1 cm distal to the lesser trochanter, level B is a point equidistant from the lower edge of the collar and the tip of the prosthesis, and level C is at the distal point of the prosthesis [17]. We compared the radiographs made immediately before and after the operation with those made during the followup evaluations to assess bone remodeling. Femoral bone quality and restoration of the femur were quantitatively assessed on followup AP radiographs by measuring the femoral cortical index [5, 11] as well as the width of the femoral cortex at levels A, B, and C [3, 4]. The cortical index is calculated measuring the quotient of the outside diameter of the shaft to the width of the canal [5]. As a result of the difficulty in evaluating healing [40], and the two observers involved, we also assessed interobserver reliability following the same method at the three levels using an intraclass correlation coefficient. The interobserver reliability was 0.957 (95% confidence interval [CI], 0.821–0.990) at the proximal area, 0.879 (95% CI, 0.557–0.971) at the middle, and 0.969 (95% CI, 0.870–0.993) distally. We did not use the Engh et al. criteria [8] to assess stress shielding because they seem to determine the area rather than the intensity of bone loss [30]. The existence of any residual osteolytic cavities in the femoral cortex was noted and assessed according to Böhm and Bischel [3] as increasing defects, constant defects, or osseous restoration. Migration was assessed by measuring the vertical subsidence of the femoral stem according to the Callaghan et al. method [5]. As a result of the difficulty of assessing subsidence in these hips, given bone loss and different fracture fragments, we only considered a radiographic subsidence greater than 10 mm [17]. Progressive radiographic subsidence was considered after the first 3 postoperative months. Femoral component fixation was graded as radiographic ingrowth, fibrous stable, or unstable according to the criteria for porous prostheses as described by Engh et al. [9].
Qualitative data are expressed as counts and percentages and quantitative data by mean ± SD or range. B2 and B3 fractures were compared using the Student’s t-test for independent data for age, Fisher’s exact test for sex and osteoporosis grade, and the chi-square test for bone defect. Fisher’s exact test was also used to compare the percentage of hips with subsidence between B2 and B3 fractures. The Mann-Whitney U test was used to compare the postoperative clinical score between hips with or without subsidence. The Student’s t-test was used to compare postoperative femoral canal filling at levels A, B, and C between hips with and without subsidence. The evolution of the mean cortical index as well as the width of the cortical bone of the femoral shaft at different levels was assessed using an one-way analysis of variance with repeated measures (Greenhouse-Greisser correction); a two-way analysis of variance for repeated measurements (Greenhouse-Greisser correction) was performed to compare patient sex, preoperative osteoporosis grade, type of fracture, and hips with 2 to 3A or 3B intraoperative bone defects as well as the different stem lengths and diameters using the preoperative and immediate postoperative, 6 months postsurgery, 12 months, and latest followup evaluation radiographs. We studied the main effect and interaction between factors (a substantial interaction indicated that changes are different).
Results
All fractures healed. The mean Merle D’Aubigné and Postel scores at the latest followup study were 5.8 for pain, 5.2 for function, and 4.9 for ROM. The mean knee flexion was 120° (range, 120°–150°) at the 3-month review. There was a leg length discrepancy greater than 1 cm in six patients (15%); of these, two hips had a length discrepancy of 2 cm. Nine patients required the use of one crutch for limp (Table 4). No patient reported thigh pain during walking or during clinical examination.
Table 4.
Postoperative clinical results at latest followup and radiological femoral canal filling at different levels
| Clinical parameter | Value |
|---|---|
| Healing time in months (mean [range]) | 5 (3–8) |
| Pain [27] (SD) | 5.8 (± 0.6) |
| Mobility [27] (SD) | 5.2 (± 0.7) |
| ROM [27] (SD) | 4.9 (± 0.7) |
| Leg length discrepancy > 1 cm (number of cases) | 6 |
| Limp (number of cases) | 9 |
| Mean femoral canal filling (%) (SD) | |
| Level A | 86.8% (± 4.9) |
| Level B | 91.2% (± 7.3) |
| Level C | 94.3% (± 3.6%) |
All hips showed radiographic ingrowth fixation of the stem. Radiolucent lines were seen in four hips in one or two zones of Gruen et al. In most patients residual osteolytic cavities in the femoral cortex filled in but it was slower in the lateral cortex and never completed at the latest followup evaluation. The AP radiograph showed the position of the tip of the stem to be centered in all hips. Seventeen hips had a subsidence of between 10 and 20 mm and two hips greater than that; all stems subsided between the sixth and 12th postoperative week and did not progress thereafter. The mean postoperative clinical score was similar in hips with or without subsidence. The mean canal filling at different levels in the whole series was 86.8% ± 4.9% at level A, 91.2% ± 7.3% at level B, and 94.3% ± 3.6% at level C. Although canal filling was greater in hips without subsidence than in hips with stem subsidence, these differences were statistically significant only at proximal level A (Table 5). Stem subsidence was not related (p = 0.637) with preoperative osteoporosis.
Table 5.
Mean canal filling (% ± SD) at different levels in hips with stem subsidence and no stem subsidence in the immediate postoperative radiograph
| No stem subsidence | Stems with subsidence | ||||||
|---|---|---|---|---|---|---|---|
| Type of fracture [1] | B2 | B3 | Total | B2 | B3 | Total | p values |
| Number of cases | 11 | 10 | 21 | 12 | 7 | 19 | 0.538 |
| Pain [27] | 5.9 | 5.7 | 0.101 | ||||
| Function [27] | 5.2 | 5.1 | 0.952 | ||||
| ROM [27] | 5 | 4.9 | 0.796 | ||||
| Mean canal filling | |||||||
| Level A | 89.4 ± 5.6 | 88.6 ± 4.3 | 89.0 ± 4.9 | 84.9 ± 3.7 | 83.7 ± 3.7 | 84.5 ± 3.7 | 0.002 |
| Level B | 94.3 ± 5.3 | 91.6 ± 9.2 | 93.0 ± 7.4 | 90.1 ± 7.4 | 87.7 ± 6.5 | 89.2 ± 6.9 | 0.104 |
| Level C | 94.4 ± 5.2 | 95.2 ± 8.2 | 94.8 ± 4.5 | 94.2 ± 1.6 | 92.9 ± | 93.7 ± 2.2 | 0.330 |
The cortical index and the lateral and medial cortex thicknesses increased at different levels over time (Fig. 1; Table 6). We found no changes of either the cortical index or the evolution of the femoral cortex regarding sex, bone defect, or fracture type. The femoral cortex thickness over time was higher in cases using stems thinner than 16 mm (p = 0.043) and in cases with a minimum or moderate preoperative osteoporosis (p = 0.008) (Table 7). The cortical index and the femoral cortical showed similar values in Vancouver B2 fractures and minor defects.
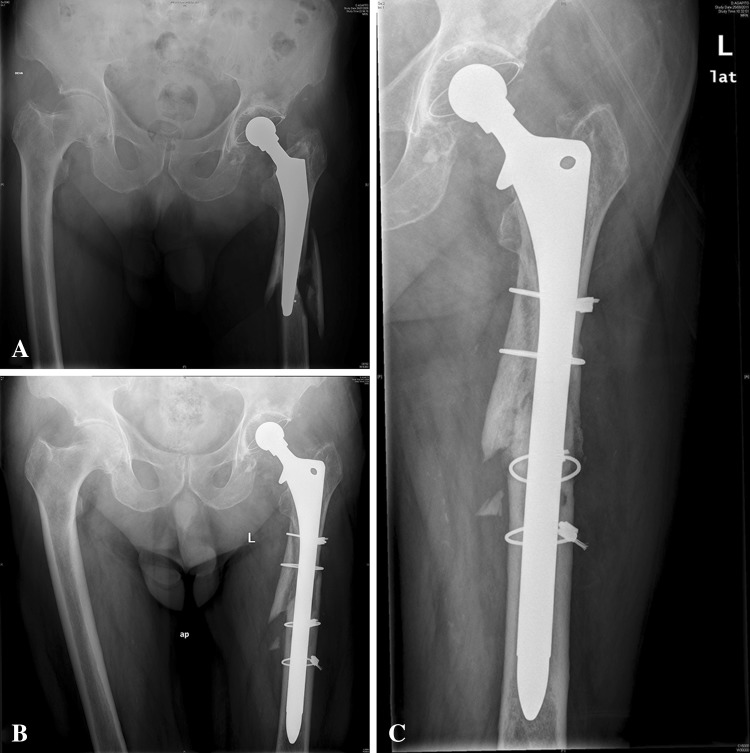
Fig. 1A–C.
(A) Radiographs of a 72-year-old man who presented a loosened cemented total hip prosthesis. (B) AP radiograph of the same hip made 3 months after revision hip surgery placed an extensively porous-coated stem. (C) AP radiograph of the same hip made 10 years postoperatively showing a stable stem and an increase of the medial cortex and a decrease of the proximal lateral cortex.
Table 6.
Variations in the cortical index and femoral cortex at levels A, B, and C in the hips included in the followup study (N = 35)
| Different levels | Presurgery | Postsurgery | At 6 months | At 12 months | At latest followup | p values* |
|---|---|---|---|---|---|---|
| Level A | ||||||
| Cortical index | 1.49 ± 0.15 | 1.55 ± 0.15 | 1.56 ± 0.13 | 1.57 ± 0.17 | 1.59 ± 0.18 | 0.022 |
| Lateral cortex | 4.05 ± 1.60 | 4.92 ± 2.44 | 5.19 ± 2.29 | 5.48 ± 2.53 | 5.24 ± 2.54 | 0.001 |
| Medial cortex | 4.24 ± 1.82 | 5.64 ± 2.25 | 5.84 ± 2.41 | 6.54 ± 3.08 | 7.06 ± 4.25 | < 0.001 |
| Level B | ||||||
| Cortical index | 1.50 ± 0.18 | 1.60 ± 0.16 | 1.55 ± 0.18 | 1.61 ± 0.17 | 1.61 ± 0.17 | < 0.001 |
| Lateral cortex | 4.11 ± 1.45 | 5.16 ± 1.87 | 4.69 ± 2.09 | 5.35 ± 1.69 | 5.18 ± 2.15 | 0.009 |
| Medial cortex | 4.30 ± 1.72 | 5.83 ± 2.09 | 5.83 ± 1.93 | 6.27 ± 2.09 | 6.46 ± 2.54 | < 0.001 |
| Level C | ||||||
| Cortical index | 1.52 ± 0.18 | 1.59 ± 0.18 | 1.58 ± 0.16 | 1.60 ± 0.17 | 1.61 ± 0.18 | 0.009 |
| Lateral cortex | 3.83 ± 1.44 | 4.86 ± 1.42 | 4.46 ± 1.72 | 5.09 ± 1.21 | 4.94 ± 1.59 | < 0.001 |
| Medial cortex | 4.56 ± 1.65 | 5.37 ± 1.52 | 5.61 ± 1.98 | 5.89 ± 2.34 | 6.03 ± 2.86 | 0.002 |
* Greenhouser-Geisser correction test.
Table 7.
Risk factors regarding the cortical index and femoral cortex at levels A in the hips included in the followup study (N = 35)
| Sex | Presurgery | Postsurgery | At latest evaluation | p values* | p values† |
|---|---|---|---|---|---|
| Cortical index | |||||
| Male | 1.48 ± 0.17 | 1.55 ± 0.18 | 1.56 ± 0.23 | 0.574 | 0.915 |
| Female | 1.50 ± 0.12 | 1.55 ± 0.12 | 1.61 ± 0.14 | ||
| Lateral cortex | |||||
| Male | 4.12 ± 1.56 | 5.05 ± 2.43 | 5.33 ± 2.83 | 0.971 | 0.789 |
| Female | 4.00 ± 1.66 | 4.82 ± 2.52 | 5.17 ± 2.38 | ||
| Medial cortex | |||||
| Male | 4.37 ± 2.47 | 5.35 ± 3.16 | 6.13 ± 4.21 | 0.322 | 0.478 |
| Female | 4.14 ± 1.18 | 5.85 ± 1.25 | 7.76 ± 4.24 | ||
| Osteoporosis | |||||
| Cortical index | |||||
| Minimal or Moderate | 1.60 ± 0.11 | 1.66 ± 0.14 | 1.70 ± 0.18 | 0.154 | 0.022 |
| Severe | 1.45 ± 0.13 | 1.51 ± 0.13 | 1.55 ± 1.17 | ||
| Lateral cortex | |||||
| Minimal or Moderate | 4.60 ± 1.05 | 4.90 ± 1.86 | 5.49 ± 1.56 | 0.713 | 0.690 |
| Severe | 3.86 ± 1.72 | 4.92 ± 2.64 | 5.15 ± 2.82 | ||
| Medial cortex | |||||
| Minimal or Moderate | 5.00 ± 1.01 | 6.10 ± 1.17 | 8.33 ± 6.43 | 0.575 | 0.343 |
| Severe | 3.97 ± 1.97 | 5.47 ± 2.51 | 6.62 ± 3.23 | ||
| Paprosky bone defect | |||||
| Cortical index | |||||
| Types 2–3A | 1.51 ± 0.16 | 1.60 ± 0.16 | 1.61 ± 0.12 | 0.472 | 0.088 |
| Types 3B | 1.48 ± 0.13 | 1.51 ± 0.13 | 1.57 ± 0.17 | ||
| Lateral cortex | |||||
| Types 2–3A | 4.17 ± 1.69 | 5.77 ± 1.69 | 5.64 ± 1.80 | 0.236 | 0.227 |
| Types 3B | 3.97 ± 1.56 | 4.28 ± 2.75 | 4.94 ± 2.99 | ||
| Medial cortex | |||||
| Types 2–3A | 4.73 ± 2.08 | 6.24 ± 2.29 | 6.66 ± 3.15 | 0.250 | 0.483 |
| Types 3B | 3.87 ± 1.54 | 5.18 ± 2.16 | 7.36 ± 4.97 | ||
| Fracture type | |||||
| Cortical index | |||||
| Type B2 | 1.54 ± 0.16 | 1.59 ± 0.17 | 1.64 ± 0.15 | 0.737 | 0.046 |
| Type B3 | 1.44 ± 0.11 | 1.51 ± 0.11 | 1.53 ± 0.20 | ||
| Lateral cortex | |||||
| Type B2 | 4.39 ± 1.91 | 5.23 ± 2.83 | 5.86 ± 2.17 | 0.549 | 0.139 |
| Type B3 | 3.70 ± 1.12 | 4.55 ± 1.97 | 4.59 ± 2.80 | ||
| Medial cortex | |||||
| Type B2 | 4.59 ± 1.93 | 5.90 ± 2.27 | 8.07 ± 5.01 | 0.365 | 0.195 |
| Type B3 | 3.67 ± 1.67 | 5.36 ± 2.26 | 5.99 ± 3.05 | ||
| Stem diameter | |||||
| Cortical index | |||||
| 13.5–15 mm | 1.51 ± 0.16 | 1.58 ± 0.16 | 1.64 ± 0.20 | 0.321 | 0.043 |
| 16.5–18 mm | 1.45 ± 0.10 | 1.50 ± 0.10 | 1.49 ± 0.09 | ||
| Lateral cortex | |||||
| 13.5–15 mm | 4.36 ± 1.79 | 5.16 ± 2.83 | 6.01 ± ± .58 | 0.115 | 0.080 |
| 16.5–18 mm | 3.54 ± 1.07 | 4.51 ± 1.60 | 3.94 ± 1.94 | ||
| Medial cortex | |||||
| 13.5–15 mm | 4.54 ± 2.05 | 5.89 ± 2.30 | 8.12 ± 4.83 | 0.137 | 0.101 |
| 16.5–18 mm | 3.74 ± 1.26 | 5.21 ± 2.18 | 5.27 ± 2.17 | ||
| Stem length | |||||
| Cortical index | |||||
| 8 inches | 1.51 ± 0.13 | 1.56 ± 0.13 | 1.63 ± 1.16 | 0.555 | 0.513 |
| 10 inches | 1.48 ± 0.15 | 1.54 ± 0.16 | 1.55 ± 0.20 | ||
| Lateral cortex | |||||
| 8 inches | 3.88 ± 1.53 | 4.51 ± 2.20 | 4.73 ± 2.36 | 0.731 | 0.306 |
| 10 inches | 4.20 ± 1.68 | 5.27 ± 2.63 | 5.67 ± 2.67 | ||
| Medial cortex | |||||
| 6–8 inches | 4.72 ± 2.04 | 6.59 ± 2.14 | 8.41 ± 5.18 | 0.434 | 0.029 |
| 10 inches | 3.83 ± 1.55 | 4.84 ± 2.06 | 5.93 ± 2.96 | ||
*p values for interaction effect between factors (test of within-subjects effects [Greenhouse-Geisser]); †p values for the main risk factors effect of the groups.
There were no dislocations. There were three intraoperative greater trochanter fractures; two had undiagnosed intraoperative fissures at the level of the stem tip; one of these healed spontaneously and the other produced a displaced fracture treated with a plate and screws. One patient had a supracondylar femoral postoperative fracture distal to the stem that was also treated by a plate and screws. Cerclage wires were removed in two hips as a result of superficial infection; additional surgical débridement and antibiotic therapy treatment were required in both cases with normal protein C levels at 6 weeks. Three patients had a hematoma during the first postoperative week; all three had received preoperative anticoagulant therapy. At 1 year after surgery, none of the three had any clinical sign of infection.
Discussion
Cementless long stems have been widely used to obtain fracture healing and stable fixation of the stem in the femoral diaphysis distal after Vancouver B2 and B3 periprosthetic femoral fractures [22, 33]. These stems provide intramedullary fixation of the fracture fragments with distal stability; they bypass the fracture and cortical deficiencies; there is no issue with cement inhibition and there is the potential to achieve biological ingrowth around the porous coating and attain long-term stability [33]. We therefore asked whether (1) the use of an extensively porous-coated stem would reliably allow healing of Vancouver B2 and B3 periprosthetic fractures; (2) the appearance of signs of radiographic loosening and subsidence would affect patient function; and (3) the appearance of radiographic cortical changes observed previously with these implants was related to different factors related to either the patients or the stems.
There were several limitations to our study. First, we had a small cohort available for study and the resulting incompleteness of the clinical outcome data. Second, this is not a comparative analysis with the same implant used for aseptic loosening as in a matched case-control study. Third, healing can be difficult to evaluate on a radiograph. Although most authors agree that the presence of bridging callus on at least two views is a definition for healing of diaphyseal fractures, the number of cortices bridged by callus may actually be more reliable [40]. We performed the interobserver analysis at the three different levels on radiographs, obtaining a high intraclass correlation coefficient; however, some bias may have been introduced in the radiographic assessment. Fourth, qualitative and quantitative assessment of cortical index and cortical width based on the postoperative radiographs can be inaccurate and inconsistent when evaluating stress shielding changes [1, 7, 11]. We are aware that some findings, although statistically significant, showed small differences in cortical thickness that might not be clinically important.
Like in other reports [2, 13, 15, 17, 24, 37], all periprosthetic fractures in this series healed (Table 8). With a firm fixation of the stem in the distal fracture fragment and an approximation of the proximal comminuted fragments, the fracture can heal and at the same time bone mass in the proximal femur can be restored [3, 17]. Springer et al. reported that periprosthetic femoral fractures treated with uncemented extensively porous-coated implants had greater survival rates, stable fixation, and a lower incidence of nonunion than other types of stems [37]. The mean patient age in our series was 80 years, so the patient’s age did not appear to be a negative effect on the patient’s function or loosening [16, 23]. The number of complications was not low, perhaps owing to the removal additional bone in the process of revision.
Table 8.
Results for cementless long-stems in femoral periprosthetic fractures
| Study | Type of stem | Number of cases | Vancouver fracture type | Mean age (years) | Mean followup (years) | Union | Loosening |
|---|---|---|---|---|---|---|---|
| Ko et al. [16] | Wagner | 12 | B2 | 74.5 | 5 | 12 | 0 |
| MacDonald et al. [23] | Solution | 14 | B2, B3 | 63.6 | 8.2 | 14 | 1 |
| Springer et al. [37] | Solution | 30 | B2, B3 | 65.3* | 3.5 | 30 | 3 |
| O’Shea et al. [33] | Solution | 22 | B2, B3 | 75 | 3 | 20 | 2 |
| Current study | Solution | 35 | B2, B3 | 80.4 | 8.3 | 35 | 0 |
*The mean age correspond to the total series, 136 consecutive hips including different cemented and cementless stems.
The rate for early nonprogressive subsidence in our series was relatively high, although this did not affect the function or subsequent loosening. Primary solid fixation in the diaphyseal zone of the femur is not always possible in this complex revision surgery with comminuted and osteopenic bone, which could explain the early subsidence [3, 16]. These clinical results should be interpreted cautiously because factors other than the revised stem such as advanced age, bilaterality, or polyarthritis may be involved. Although extramedullary allograft cortical struts have been used in other series [14, 34], they were not used in this series. We agree with Nadaud et al. [32] that a mismatch in the intramedullary canal diameter of available allografts makes it difficult to achieve a tight press-fit in the host bone in revision surgery. To date, cortical strut grafts have been associated with some devascularization in small underlying bone fragments [2]. In cases in which the fracture and bone loss extend below the level of the femoral isthmus, other options include cementless fixation using a modular fluted femoral stem [2, 21, 31, 36], long-stem cemented fixation coupled with impaction bone grafting [20, 38], distal locking stems [28], or even allograft-prosthesis composite or tumor prostheses have also been used [15, 26, 37, 39].
Contrary to the reported findings in cases with revision surgery for aseptic loosening using this stem [10], the cortical index and the lateral and medial cortex increased at different levels over time in Vancouver B2 and B3 periprosthetic fractures. Patients with stems thinner than 16 mm and patients with a minimum or moderate preoperative osteoporosis showed greater femoral cortex thickness over time; this may be the result of a global increase in bone rather than at these specific sites. Böhm and Bischel [3] emphasize the importance of mechanical stability as well as of the careful removal of cement, scar, and granulation tissue for spontaneous restoration of bone stock [17, 35]. Stable distal anchoring of the stem, which seems to create a new biomechanical balance for the metaphysis, is the best way to heal the fracture [3, 16, 17]. In such injuries, it is important to avoid devascularizing proximal femoral fragments so as to preserve their osteogenic potential, which will promote the healing response [33]. Whether bone remodeling is caused by decreased stress shielding or by changes in the local environment resulting from revision surgery is unclear [3, 16, 17]. As Berry reported [2], our study also shows a healing response can be used to gain fracture healing and preservation and reconstitution of the host femur. Because of the difficulty of densitometric analysis of change in the bone using retrospective comparison of serial radiographs [39], future studies with a dual-energy x-ray absorptiometry scan could be very useful.
In conclusion, an extensive porous-coated stem without allograft can be used to treat difficult Vancouver B2 and B3 periprosthetic femoral fractures. Although the number of complications and stem subsidence is not low, all fractures healed without compromising subsequent function or loosening at a mean of 8 years. In contrast to hips treated with these stems for aseptic loosening, we observed no loss of cortical bone at different levels over time. Patients with thinner stems and those with minimum or moderate preoperative osteoporosis both achieved greater femoral cortex thickness.
Footnotes
Each author certifies that he or she, or a member of his or her immediate family, has no funding or commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research editors and board members are on file with the publication and can be viewed on request.
Clinical Orthopaedics and Related Research neither advocates nor endorses the use of any treatment, drug, or device. Readers are encouraged to always seek additional information, including FDA-approval status, of any drug or device prior to clinical use.
Each author certifies that his or her institution approved the human protocol for this investigation, that all investigations were conducted in conformity with ethical principles of research, and that informed consent for participation in the study was obtained.
References
- 1.Bell AL, Brand RA. Roentgenographic changes in the proximal femoral dimensions due to hip rotation. Clin Orthop Relat Res. 1989;240:194–199. [PubMed] [Google Scholar]
- 2.Berry DJ. Treatment of Vancouver B3 periprosthetic femur fractures with a fluted tapered stem. Clin Orthop Relat Res. 2003;417:224–231. doi: 10.1097/01.blo.0000096821.67494.f6. [DOI] [PubMed] [Google Scholar]
- 3.Böhm P, Bischel O. Femoral revision with the Wagner SL revision stem, evaluation of one hundred and twenty-nine revisions followed for a mean of 4.8 years. J Bone Joint Surg Am. 2001;83:1023–1031. doi: 10.1302/0301-620X.83B7.11413. [DOI] [PubMed] [Google Scholar]
- 4.Bosco JA, Lachiewicz PF, DeMasi R. Survivorship analysis of cemented high modulus total hip arthroplasty. Clin Orthop Relat Res. 1993;294:131–139. [PubMed] [Google Scholar]
- 5.Callaghan JJ, Salvati EA, Pellicci PM, Wilson PD, Jr, Ranawat CS. Results of revision for mechanical failure after cemented total hip replacement. 1979 to 1982. A two to five-year follow-up. J Bone Joint Surg Am. 1982;1985(67):1074–1085. [PubMed] [Google Scholar]
- 6.Duncan CP, Masri BA. Fractures of the femur after hip replacement. Instr Course Lect. 1995;44:293–304. [PubMed] [Google Scholar]
- 7.Eckrich SGJ, Noble PC, Tullos HS. Effect of rotation on the radiographic appearance of the femoral canal. J Arthroplasty. 1994;9:419–426. doi: 10.1016/0883-5403(94)90053-1. [DOI] [PubMed] [Google Scholar]
- 8.Engh CA, Bobyn JD, Glassman AH. Porous-coated hip replacement the factors governing bone ingrowth, stress shielding, and clinical results. J Bone Joint Surg Br. 1987;69:45–55. doi: 10.1302/0301-620X.69B1.3818732. [DOI] [PubMed] [Google Scholar]
- 9.Engh CA, Glassman AH, Suthers KE. The case for porous-coated hip implants. The femoral side. Clin Orthop Relat Res. 1990;261:63–81. [PubMed] [Google Scholar]
- 10.Garcia-Cimbrelo E, Garcia-Rey E, Cruz-Pardos A, Madero R. Stress-shielding of the proximal femur using an extensively porous-coated femoral component without allograft in revision surgery. A 5- to 17-year follow-up study. J Bone Joint Surg Br. 2010;92:1363–1369. doi: 10.1302/0301-620X.92B10.24317. [DOI] [PubMed] [Google Scholar]
- 11.Gruen TA. A simple assessment of bone quality prior to hip arthroplasty: cortical index revisited. Acta Orthop Belg. 1997;63(Suppl 1):20–27. [PubMed] [Google Scholar]
- 12.Gruen TA, McNeice GM, Amstutz HC. ‘Modes of failure’ of cemented stem-type femoral components. Clin Orthop Relat Res. 1979;171:17–27. [PubMed] [Google Scholar]
- 13.Gutierrez del Alamo J, Garcia-Cimbrelo E, Castellanos V, Gil-Garay E. Radiographic bone regeneration and clinical outcome with Wagner SL revision stem. A 5-year to 12-year follow-up study. J Arthroplasty. 2007;22:515–524. doi: 10.1016/j.arth.2006.04.029. [DOI] [PubMed] [Google Scholar]
- 14.Kim Y-H, Kim J-S. Revision hip arthroplasty using strut allografts and fully porous-coated stems. J Arthroplasty. 2005;20:454–459. doi: 10.1016/j.arth.2004.09.054. [DOI] [PubMed] [Google Scholar]
- 15.Klein GR, Parvizi J, Rapuri V, Wolf CF, Hozack WJ, Sharkey PF, Purtill JJ. Proximal femoral replacement for the treatment of periprosthetic fractures. J Bone Joint Surg Am. 2005;87:1777–1781. doi: 10.2106/JBJS.D.02420. [DOI] [PubMed] [Google Scholar]
- 16.Ko PS, Lam JJ, Tio MK, Lee OB, Ip FK. Distal fixation with Wagner revision stem in treating Vancouver type B2 periprosthetic femur fracture in geriatric patients. J Arthroplasty. 2003;18:446–452. doi: 10.1016/S0883-5403(03)00148-7. [DOI] [PubMed] [Google Scholar]
- 17.Koldstad K. Revision THR after periprosthetic femoral fractures. An analysis of 23 cases. Acta Orthop Scand. 1994;65:505–508. doi: 10.3109/17453679409000900. [DOI] [PubMed] [Google Scholar]
- 18.Krishnamurthy AB, MacDonald SJ, Paprosky WG. 5- to 13-year follow-up study in cementless femoral components in revision surgery. J Arthroplasty. 1997;12:839–847. doi: 10.1016/S0883-5403(97)90152-2. [DOI] [PubMed] [Google Scholar]
- 19.Lawrence JM, Engh CA, Macalino GE, Lauro GR. Outcome of revision hip arthroplasty done without cement. J Bone Joint Surg Am. 1994;76:965–973. doi: 10.2106/00004623-199407000-00002. [DOI] [PubMed] [Google Scholar]
- 20.Lee G-C, Nelson CL, Virmani S, Manikonda K, Israelite CL, Garino JP. Management of periprosthetic femur fractures with severe bone loss using impaction bone grafting technique. J Arthroplasty. 2010;25:405–409. doi: 10.1016/j.arth.2009.01.024. [DOI] [PubMed] [Google Scholar]
- 21.Levine BR, Della Valle CJ, Lewis P, Berger RA, Sporer SM, Paprosky WJ. Extended trochanteric osteotomy for the treatment of Vancouver B2/B3 periprosthetic fractures of the femur. J Arthroplasty. 2008;23:527–533. doi: 10.1016/j.arth.2007.05.046. [DOI] [PubMed] [Google Scholar]
- 22.Lindahl H, Garellick G, Regner H, Herberts P, Malchau H. Three hundred and twenty-one periprosthetic femoral fractures. J Bone Joint Surg Am. 2006;88:1215–1222. doi: 10.2106/JBJS.E.00457. [DOI] [PubMed] [Google Scholar]
- 23.MacDonald SJ, Paprosky WG, Jablonsky WS, Magnus RG. Periprosthetic femoral fractures treated with a long-stem cementless component. J Arthoplasty. 2001;16:379–383. doi: 10.1054/arth.2001.20536. [DOI] [PubMed] [Google Scholar]
- 24.McAuley JP, Culpepper WJ, Engh CA. Total hip arthroplasty. Concerns with extensively porous coated femoral components. Clin Orthop Relat Res. 1998;355:182–188. doi: 10.1097/00003086-199810000-00019. [DOI] [PubMed] [Google Scholar]
- 25.McAuley JP, Engh CA., Jr Femoral fixation in the face of considerable bone loss. Cylindrical and extensively coated femoral components. Clin Orthop Relat Res. 2004;429:215–221. doi: 10.1097/01.blo.0000150274.21573.f4. [DOI] [PubMed] [Google Scholar]
- 26.Meek RMD, Garbuz DS, Masri BA, Greidanus NV, Duncan CP. Intraoperative fracture of the femur in revision total hip arthroplasty with a diaphyseal fitting stem. J Bone Joint Surg Am. 2004;86:480–485. doi: 10.2106/00004623-200403000-00004. [DOI] [PubMed] [Google Scholar]
- 27.Merle D’AubignéR. Numerical classification of the function of the hip. Rev Chir Orthop Reparatrice Appar Mot. 1970;56:481–486. [PubMed] [Google Scholar]
- 28.Mertl P, Philippot R, Rosset P, Migaud H, Tabutin J, Van de Velde D. Distal locking stem for revision femoral loosening and peri-prosthetic fractures. Int Orthop. 2011;35:275–282. doi: 10.1007/s00264-010-1182-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moreland JR, Bernstein ML. Femoral revision hip arthroplasty with uncemented, porous-coated stems. Clin Orthop Relat Res. 1995;319:141–150. [PubMed] [Google Scholar]
- 30.Moreland JR, Moreno MA. Cementless femoral revision arthroplasty of the hip. Minimum 5 years follow-up. Clin Orthop Relat Res. 2001;393:194–201. doi: 10.1097/00003086-200112000-00022. [DOI] [PubMed] [Google Scholar]
- 31.Mulay S, Hassan T, Birtwistle S, Power R. Management of types B2 and B3 femoral periprosthetic fractures by a tapered, fluted, and distally fixed stem. J Arthroplasty. 2005;20:751–756. doi: 10.1016/j.arth.2004.11.020. [DOI] [PubMed] [Google Scholar]
- 32.Nadaud MC, Griffin WL, Fehring TK, Mason JB, Tabor OB, Jr, Odum S, Nussman DS. Cementless revision total hip arthroplasty without allograft in severe proximal femoral defects. J Arthroplasty. 2005;20:738–744. doi: 10.1016/j.arth.2004.12.053. [DOI] [PubMed] [Google Scholar]
- 33.O’Shea K, Quinlan JF, Kutty S, Mulcahy D, Brady OH. The use of uncemented extensively porous-coated femoral components in the management of Vancouver B2 and B3 periprosthetic femoral fractures. J Bone Joint Surg Br. 2005;87:1617–1621. doi: 10.1302/0301-620X.87B12.16338. [DOI] [PubMed] [Google Scholar]
- 34.Paprosky WG, Greidanus NV, Antoniou J. Minimum 10-year results of extensively porous-coated stems in revision hip arthroplasty. Clin Orthop Relat Res. 1999;369:230–242. doi: 10.1097/00003086-199912000-00024. [DOI] [PubMed] [Google Scholar]
- 35.Parvizi J, Rapuri VR, Purtill JJ, Sharkey PF, Rothman RH, Hozack WJ. Treatment protocol for proximal femoral periprosthetic fractures. J Bone Joint Surg Am. 2004;86(Suppl 2):8–16. doi: 10.2106/00004623-200412002-00003. [DOI] [PubMed] [Google Scholar]
- 36.Sporer SM, Paprosky WG. Revision total hip arthroplasty. The limits of fully coated stems. Clin Orthop Relat Res. 2003;417:203–209. doi: 10.1097/01.blo.0000096803.78689.0c. [DOI] [PubMed] [Google Scholar]
- 37.Springer BD, Berry DJ, Lewallen DG. Treatment of periprosthetic femoral fractures following total hip arthroplasty with femoral component revision. J Bone Joint Surg Am. 2003;85:2156–2162. doi: 10.2106/00004623-200311000-00015. [DOI] [PubMed] [Google Scholar]
- 38.Tsiridis E, Narvani AA, Haddad FS, Timperley JA, Gie GA. Impaction femoral allografting and cemented revision for periprosthetic femoral fractures. J Bone Joint Surg Br. 2004;86:1124–1132. doi: 10.1302/0301-620X.86B8.14854. [DOI] [PubMed] [Google Scholar]
- 39.West JD, Mayor MB, Collier JP. Potential errors in quantitative densitometric analysis of orthopaedic radiographs. A study after total hip arthroplasty. J Bone Joint Surg Am. 1987;69:58–64. [PubMed] [Google Scholar]
- 40.Whelan DB, Bhandari M, McKee MD, Guyatt GH, Kreder HJ, Stephen D, Schemitsch EH. Interobserver and intraobserver variation in the assessment of the healing of tibial fractures after intramedullary fixation. J Bone Joint Surg Br. 2002;84:15–18. doi: 10.1302/0301-620X.84B1.11347. [DOI] [PubMed] [Google Scholar]