Abstract
Background
Computerized navigation improves the accuracy of minimally invasive pedicle screw placement during spine surgery. Such navigation, however, exposes both the patient and the staff to radiation during surgery. To avoid intraoperative exposure to radiation, tracked ultrasound snapshots—ultrasound image frames coupled with corresponding spatial positions—could be used to map preoperatively defined screw plans into the intraoperative coordinate frame. The feasibility of such an approach, however, has not yet been investigated.
Questions/purposes
Are there vertebral landmarks that can be identified using tracked ultrasound snapshots? Can tracked ultrasound snapshots allow preoperative pedicle screw plans to be accurately mapped—compared with CT-derived pedicle screw plans—into the intraoperative coordinate frame in a simulated setting?
Methods
Ultrasound visibility of registration landmarks was checked on volunteers and phantoms. An ultrasound machine with integrated electromagnetic tracking was used for tracked ultrasound acquisition. Registration was performed using 3D Slicer open-source software (www.slicer.org). Two artificial lumbar spine phantoms were used to evaluate registration accuracy of pedicle screw plans using tracked ultrasound snapshots. Registration accuracy was determined by comparing the ultrasound-derived plans with the CT-derived plans.
Results
The four articular processes proved to be identifiable using tracked ultrasound snapshots. Pedicle screw plans were registered to the intraoperative coordinate system using landmarks. The registrations were sufficiently accurate in that none of the registered screw plans intersected the pedicle walls. Registered screw plan positions had an error less than 1.28 ± 1.37 mm (average ± SD) in each direction and an angle difference less than 1.92° ± 1.95° around each axis relative to the CT-derived positions.
Conclusions
Registration landmarks could be located using tracked ultrasound snapshots and permitted accurate mapping of pedicle screw plans to the intraoperative coordinate frame in a simulated setting.
Clinical Relevance
Tracked ultrasound may allow accurate computer-navigated pedicle screw placement while avoiding ionizing radiation in the operating room; however, further studies that compare this approach with other navigation techniques are needed to confirm the practical use of this new approach.
Introduction
Few surgical procedures motivate computerized navigation technologies more than pedicle screw placement. Additionally, because pedicle screw placement is the standard of care in many spinal deformation diseases, improvements to the procedure impact a large patient population. Although pedicle screw placement is considered a low-risk procedure [4], intraoperative three-dimensional (3-D) navigation through continuous instrument tracking can prevent adverse outcomes and decrease intraoperative ionizing radiation. In particular, real-time 3-D navigation is associated with significantly less operation time and blood loss compared with fluoroscopic guidance [21] and also reduces the radiation dosage to operating staff [1, 3]. Furthermore, having access to 3-D guidance during navigation results in fewer screw removals and reduces the number of potentially unsafe screws [18]. In fact, a recent meta-analysis of published literature revealed that the risk of pedicle perforation drops from 15% to 6% when computer navigation is used for screw placement [16]. The favorable effects of real-time 3-D navigation during pedicle screw placement motivate research into an optimal navigation technology: one that is simple, low-cost, accurate, and safe for patients and the surgical team.
One downside of 3-D navigation through intraoperative CT is that the patient and staff are exposed to ionizing radiation during the procedure. This problem can be overcome by registering a preoperative CT scan with an intraoperative stereotactic guidance system, thereby completely eliminating intraoperative ionizing radiation [8]. One method to align the preoperative CT coordinate frame with the intraoperative coordinate frame is landmark registration. Landmark registration, however, requires accurate localization of landmarks in the intraoperative coordinate frame. To accurately localize landmarks in the intraoperative coordinate frame without exposing the patient and staff to ionizing radiation, we propose the use of tracked ultrasound snapshots (TUSS) [19]. Each tracked ultrasound snapshot is comprised of two pieces of information: an ultrasound image and the position and orientation of that ultrasound image. The feasibility of using TUSS for minimally invasive pedicle screw placement is yet to be determined.
In light of the potential benefits that TUSS technology may bring to minimally invasive spinal surgery, this study aims to determine (1) whether vertebral landmarks can be identified with the use of TUSS; and (2) whether the vertebral landmarks on TUSS can be used to accurately map—compared with CT-derived pedicle screw plans—preoperatively defined pedicle screw plans into the intraoperative coordinate frame in a simulated setting. The presented method is open-source and conveniently available for the research community, an extension of the 3D Slicer application (www.slicer.org).
Materials and Methods
Tracked Ultrasound and Navigation Systems
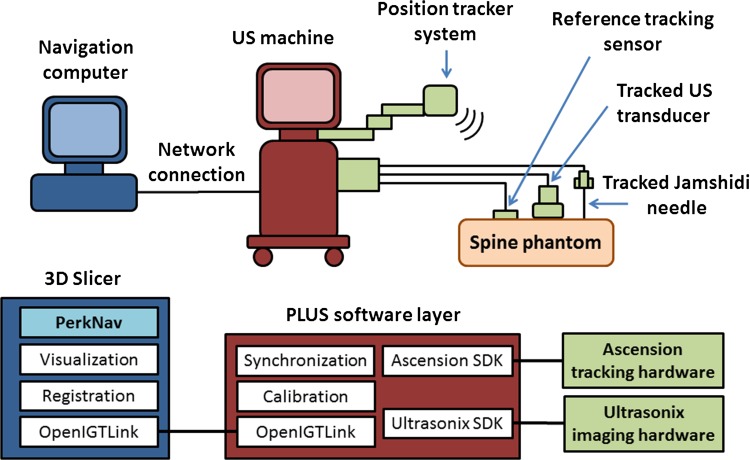
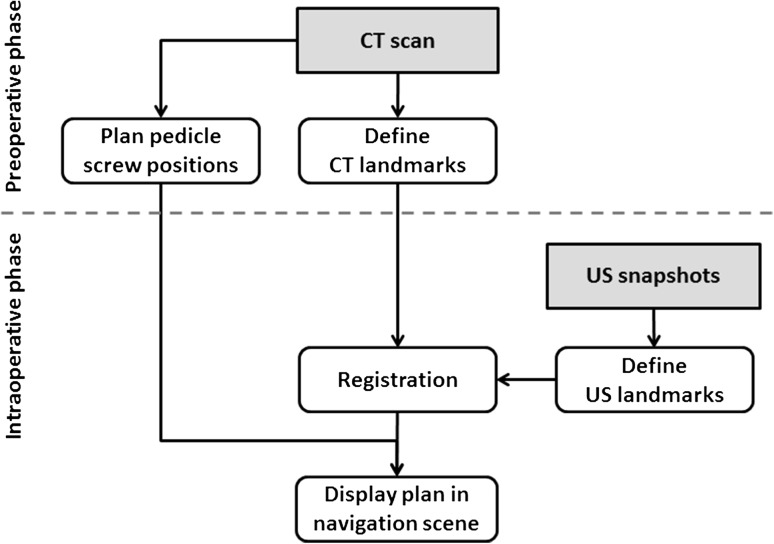
Our intraoperative navigation system is comprised of a navigation computer, an ultrasound machine, and open-source software (Fig. 1). We used a Sonix Tablet (Ultrasonix, Richmond, BC, Canada) ultrasound machine with an integrated GPS extension that allows for electromagnetic tracking of position and orientation. The GPS extension is comprised of a DriveBay electromagnetic tracker (Ascension, Burlington, VT, USA) and an adjustable arm that holds the electromagnetic transmitter. It is the GPS extension that enables TUSS to locate the registration landmarks. The 3-D navigation software is implemented as an extension (SlicerIGT) of the 3D Slicer (www.slicerigt.org) application [19]. The navigation software runs on a dedicated computer and gets real-time tracking and ultrasound image data through a network connection from the ultrasound machine. Communication between the computer and ultrasound machine is facilitated by the OpenIGTLink data communication protocol [17].
Fig. 1.
A schematic overview of the intraoperative navigation hardware and software system is shown. US = ultrasound.
Localizing Vertebral Landmarks in Ultrasound Images
The first question of our study is whether vertebral landmarks can be identified using TUSS.
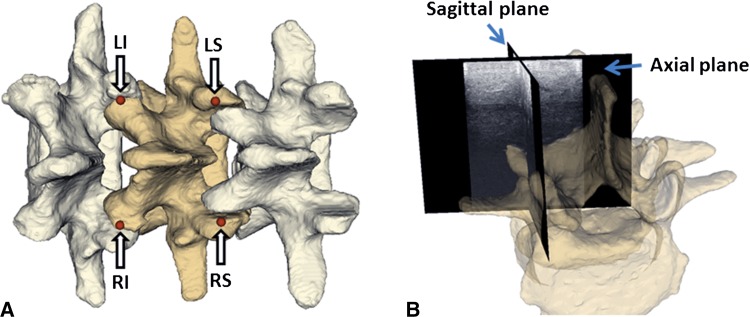
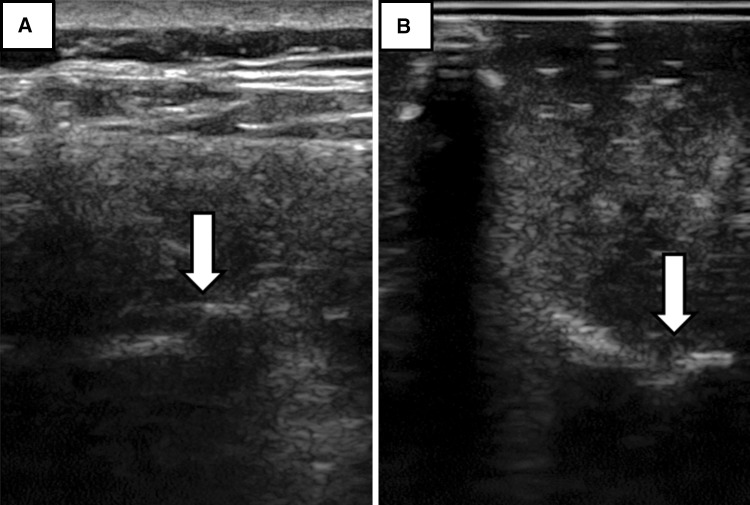
Motivated by the results of Greher et al. [6], which suggest that the articular processes are consistently identifiable in ultrasound images, we chose the posterior-most portions of the articular processes as our registration landmarks. To verify the visibility of these landmarks using TUSS, 10 human subjects were examined (Table 1). The study protocol was approved by the Health Sciences Research Ethics Board at Queen’s University and written informed consent was obtained from subjects before participation in the study. The tracked ultrasound machine was FDA-approved for use in humans. Because finding the articular processes with ultrasound imaging can be a difficult task, we used an axial tracked ultrasound snapshot to help locate the intersecting sagittal ultrasound plane corresponding to the facet joint region (Fig. 2). The ultrasound landmark points were then confirmed using the sagittal ultrasound slices. Ultrasound images of facet joints in the human subjects and phantom models were sonographically similar (Fig. 3).
Table 1.
Clinical parameters of human subjects
| Parameter | Value |
|---|---|
| Height (m) ± SD | 171.2 ± 8.1 |
| Weight (kg) ± SD | 75.9 ± 20.0 |
| Body mass index (kg/m2) ± SD | 25.7 ± 6.2 |
| Age (years) ± SD | 29.1 ± 8.2 |
| Sex (male/female) | 5/5 |
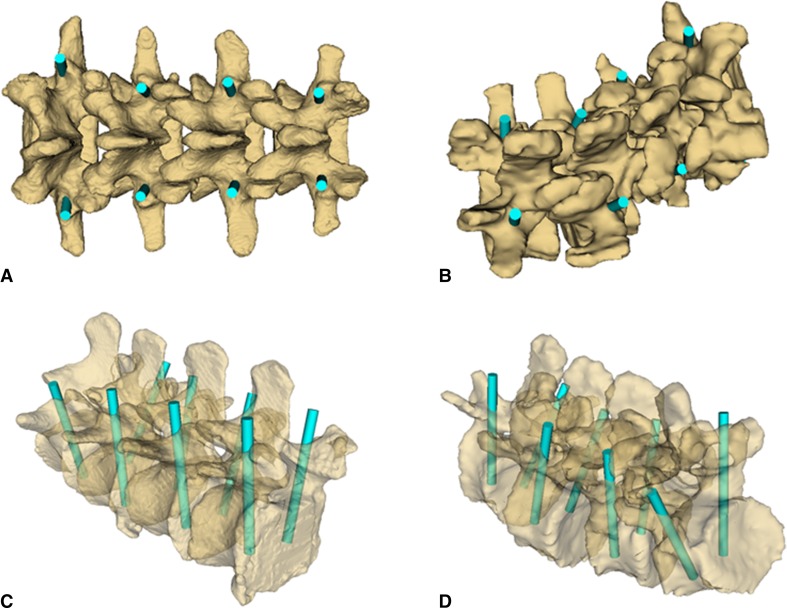
Fig. 2A–B.
(A) Arrows point to the four selected landmarks for vertebra registration. LI = left inferior; LS = left superior; RI = right inferior; RS = right superior. (B) The ultrasound snapshot image planes illustrate how to guide the sagittal plane to the facet joint area. The semitransparent vertebra overlaid on ultrasound snapshots is only for illustration and is not visible during actual landmark definition.
Fig. 3A–B.
(A) Example ultrasound images of the facet joint regions in the transverse plane in a human subject are shown. Arrows point to the facet joints. (B) Example ultrasound images of the facet joint regions in the transverse plane in a phantom model are shown. Arrows point to the facet joints.
Mapping Preoperative Pedicle Screw Plans to the Intraoperative Coordinate Frame
The second question of our study is whether tracked ultrasound snapshots allow preoperative pedicle screw plans to be accurately mapped into the intraoperative coordinate frame. To answer this question, we manufactured two rapid prototyped spine segments: one spine model was generated by manually contouring a CT scan of a healthy spine and the other by contouring a CT scan of a degenerative spine. All CT scans were acquired by a GE Lightspeed 16 (GE, Waukesha, WI, USA) machine. The two models are comprised of a plastic model of the vertebral column suspended in an opaque tissue-like polyvinyl chloride-based gel. Each of the models covers the L2–L5 region of the spine.
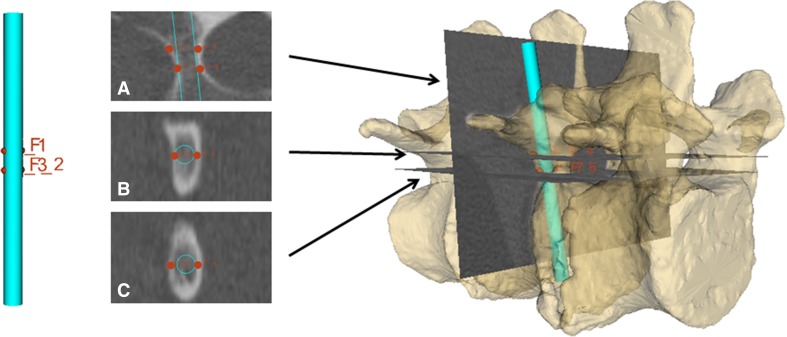
The positions of each pedicle screw are planned using four points on the CT scan (Fig. 4). Optimal positions and orientations of the screws are determined by manually selecting four points; one point is placed on each of the right-anterior, right-posterior, left-anterior, and left-posterior portions of the pedicles using coronal CT slices. Then, the four corresponding points on the 3-D screw models are selected and registered to the CT points. Screw positions on both the healthy and the degenerative models were planned (Fig. 5) and all screws were 4 mm in diameter and 50 mm in length. To intraoperatively map CT-derived pedicle screw plans to the intraoperative coordinate frame using TUSS snapshots, we propose a landmark registration scheme (Fig. 6). In the first step, a preoperative CT scan is used to define pedicle screw plans as well as the registration landmarks (chosen to be the posterior-most portions of the articular processes).
Fig. 4.
The planning of pedicle screws is facilitated using landmark points (red dots) on the CT image and the screw plan.
Fig. 5A–D.
(A) CT-derived screw plans are shown on the healthy spine model in the posterior view. (B) CT-derived screw plans are shown on the degenerative spine model in the posterior view. (C) CT-derived screw plans are shown on the healthy spine model in the oblique view with semitransparent bone models. (D) CT-derived screw plans are shown on the degenerative spine model in the oblique view with semitransparent bone models.
Fig. 6.
The proposed surgical workflow using ultrasound-based registration is shown.
After the pedicle screw plans and registration landmarks have been defined, the corresponding landmarks are localized on the spine model using TUSS in the intraoperative phase. Finally, the CT-derived landmarks and the TUSS-derived landmarks are registered using the Fiducial Registration module, which is available in the 3D Slicer software. This registration allows the preoperative CT-based pedicle screw plans to be mapped to the intraoperative navigation coordinate system.
To evaluate the quality of our mapped pedicle screw plans, we report translation and orientation errors between ultrasound-derived screw plans and the ground truth CT-derived screw plans. Translational error was measured at the center of the screw plan, which was positioned near the center of the pedicles during the planning phase. Orientation errors were decomposed into three Euler angles using the left-right, posterior-anterior, and inferior-superior anatomical axes. Breaches of the pedicle wall or vertebral body were also examined.
Results
The four selected registration landmarks—the posterior-most portions of each vertebra’s four articular processes—were visible under ultrasound imaging in all 10 human subjects as well as in the two synthetic spine models.
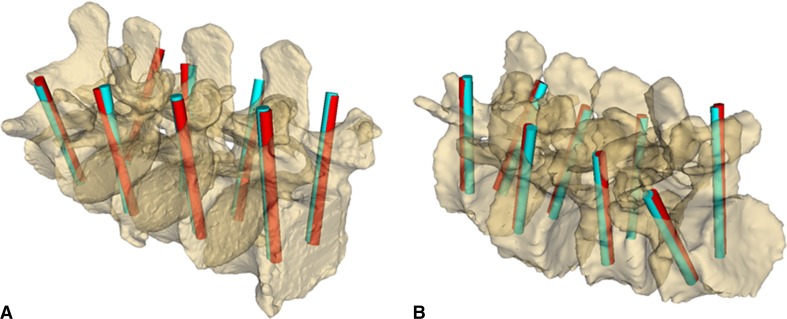
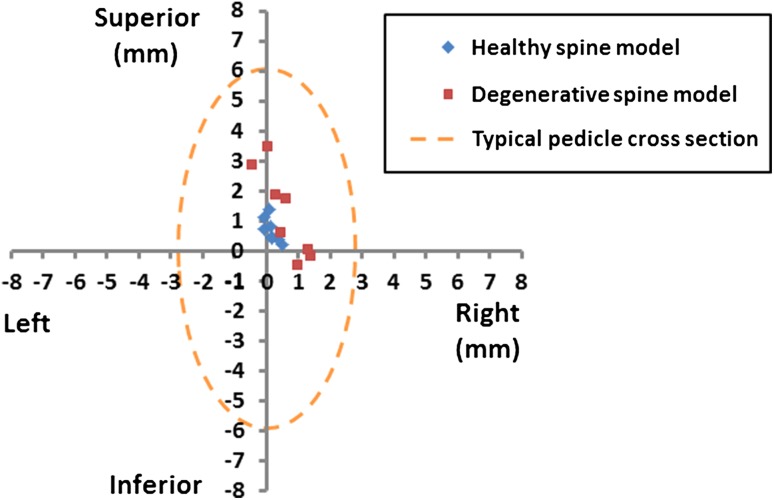
All of the pedicle screw plans were successfully mapped into the intraoperative coordinate frame using TUSS-identified landmarks (Fig. 7). The position and orientation differences between the ground truth CT-based plans and the TUSS-based plans, for all anatomical directions and axes, are summarized in Table 2. Additionally, we have plotted the translational error in the coronal plane of individual screw centers (Fig. 8); the coronal plane was selected because projection of the error data onto this plane is most relevant from the perspective of clinical complications. Note that the maximum translation error (3.51 mm) occurred in the superior direction in the degenerative model. Finally, perforation of the pedicle wall by the TUSS-based screw plans was not detected in any of the pedicles.
Fig. 7A–B.
(A) Overview of pedicle screw plan positions as defined in the CT image (blue rods) and as registered using ultrasound snapshots (red rods) in the healthy spine model is shown. (B) Overview of pedicle screw plan positions as defined in the CT image (blue rods) and as registered using ultrasound snapshots (red rods) in the degenerative spine model is shown.
Table 2.
Translation (position) and orientation error of the ultrasound-based pedicle screw center relative to the CT-based pedicle screw center
| Error type | Healthy model, mean ± SD | Degenerative model, mean ± SD |
|---|---|---|
| Translation R (mm) | 0.16 ± 0.19 | 0.55 ± 0.59 |
| Translation A (mm) | −0.01 ± 1.22 | −0.35 ± 0.40 |
| Translation S (mm) | 0.68 ± 0.38 | 1.28 ± 1.37 |
| Rotation L-R (degrees) | 1.92 ± 1.95 | 1.60 ± 1.56 |
| Rotation P-A (degrees) | −0.05 ± 0.42 | 0.81 ± 1.15 |
| Rotation I-S (degrees) | 0.40 ± 0.99 | −0.79 ± 0.46 |
R = right; A = anterior; S = superior directions; L-R = left-right; P-A = posteroanterior; I-S = inferosuperior rotation axes.
Fig. 8.
A scatterplot of translation errors of individual TUSS-based screw positions relative to the CT-based screw positions in the left-right, inferosuperior anatomical plane is shown.
Discussion
Computerized navigation improves the accuracy of minimally invasive pedicle screw placement during spine surgery. However, navigation using intraoperative radiography or CT exposes both the patient and the operating team to ionizing radiation during surgery. To address the issue of how to eliminate intraoperative radiation from minimally invasive spine surgery, our study aimed to investigate the possibility of using tracked ultrasound for intraoperative navigation. In particular, our study was designed to address two questions. First, can vertebral landmarks be identified using tracked ultrasound snapshots? Second, can these landmarks be used to accurately map preoperative pedicle screw plans into the intraoperative coordinate frame? Affirmative answers to both of these questions are suggested by our experimental outcomes; with respect to our first question, our four chosen landmarks—the posterior-most portions of the four articular processes—were visible in all human subjects as well as the two spine models. With regard to our second question, all of the pedicle screw plans were successfully mapped into the intraoperative coordinate frame, and none of the registrations resulted in screw placement plans that perforated the pedicle walls.
Our study is, however, not without limitations. First, our study suffers from a small sample size; we checked for the visibility of landmarks on only 10 human subjects and two spine models and registered screw placement plans on only two spine models. Additionally, and in part as a consequence of our small sample size, our range of clinical parameters (Table 1) is by no means comprehensive. Although we acknowledge these shortcomings, because this is a proof-of-concept study, we believe that despite our sample size, our results are still sufficient to act as the foundation for more comprehensive studies in the future. In addition, our study also has the limitation that screw placement planning was done only on the lumbar spine and not in other segments of the spine. Placement of pedicle screws in lumbar vertebrae has a higher success rate than placement in other regions of the spine; in particular, as the transverse pedicle diameter falls below 4 mm, the success rate of screw placement decreases [11]. In line with this observation, pedicle screw placement is also less accurate in pediatric patients than in adult patients. Considering that our pedicle screw plans were performed on lumbar vertebrae of adult spine models, the results of our study likely approach a theoretical, or best-case, accuracy. That our study measures screw placement error by comparing screw placement plans—no screws were physically placed in our study—further suggests that our results represent a best-case accuracy. That is, physical placement of screws would likely decrease accuracy and might result in breaches of the pedicle walls not evident in the registered plans. One possible option to improve accuracy—and an option that will likely be used in subsequent trials—is to use additional landmarks should the geometric configuration of the four originally selected TUSS landmarks not adequately match the configuration of the CT landmarks. In particular, differences in geometric configuration between TUSS-selected and CT-selected landmarks could be automatically detected by the registration software and the operator could be warned that additional landmarks are required for accurate registration. Finally, we should note one last limitation, not of the study but of our technique. Although our TUSS identification of landmarks eliminates intraoperative radiation, preoperative and postoperative CT scans are still required to plan screw placements and to determine the accuracy of implanted screws, respectively. This means that the patient is still exposed to radiation. Thus, for cases in which surgery can be performed using intraoperative CT guidance and no preoperative CT scan, our TUSS-based guidance will not reduce the radiation exposure to the patient. In cases in which preoperative scanning is a requirement, however, our method will result in a significant decrease in the radiation dosage to the patient compared with intraoperative CT-based navigation. Furthermore, independent of the requirement for preoperative scanning, using TUSS for intraoperative guidance completely eliminates radiation exposure to the operating team [1].
We found that our four chosen landmarks—the posterior-most portions of each vertebra’s four articular processes—were visible in all of the human patients as well as in the two synthetic spine models. These results agree with those of Greher et al. [6], in which the articular processes of the L3, L4, and L5 vertebrae were visible in all of the 20 healthy adult volunteers in their study. As mentioned previously, locating the articular processes in ultrasound images can be a challenging task. The need to accurately select a particular portion of the articular process—in our case, the posterior-most portion—for registration purposes further adds to the challenge. Subjectively, we found that the use of tracked ultrasound snapshots—which allows orthogonal ultrasound planes to be simultaneously visualized—facilitated the process compared with using regular ultrasound (Fig. 4); however, we will leave it to future studies to quantitatively evaluate the effectiveness of tracked ultrasound snapshots for vertebral landmark identification compared with (nontracked) ultrasound and other modalities.
Registration with TUSS landmark points allowed all pedicle screw plans to be successfully mapped to the intraoperative coordinate frame with none of the mapped plans perforating the pedicle wall. It should be noted that the translational errors found in our evaluation study were not uniform across different directions. In particular, errors were largest along the inferosuperior anatomical direction, a trend that may be attributed to the elongated shape of the facet joints in the inferosuperior direction. Also noteworthy is that the average of translation errors of the screw plans is biased in the superior direction (Fig. 8) by 0.9 mm, which suggests a systematic error in our method. This is within the error of the electromagnetic tracker claimed by the manufacturer (1 mm) and could likely be compensated by recalibration of the tracking magnetic field preceding the operation. Thus, improved accuracy may follow recalibration. Screw plans are evaluated in differing ways in the literature; Liang et al. [12] compute position and orientation errors by comparing screw placements to a ground truth plan, Kawaguchi et al. [9] determine critical breaches of the screws using postoperative CT, and Zhang et al. [22] use perforation of the pedicle wall and deviation from the lateral pedicle wall. Following these evaluation methods, we reported both the position and orientation accuracy of the TUSS-registered screw plans—with reference to the ground truth plans—and also examined the registered plans for pedicle wall perforations.
We now briefly compare our TUSS-based guidance technique with other guidance strategies. The use of artificial mechanical constraints is a simple and robust method for controlling the movement of surgical tools during pedicle screw placement. Some groups have developed rapid prototyped templates for the lamina based on a preoperative CT scan of the spine [9, 13]. Although this strategy avoids intraoperative radiation—like our TUSS-based method—it requires direct contact with a relatively large bone surface—unlike our TUSS-based method—and is therefore unsuitable for minimally invasive procedures. The most advanced mechanical apparatus designed for pedicle screw placement is the SpineAssist (MAZOR Surgical Technologies, Caesarea, Israel) miniature robot, which is mounted on a T-frame fixed rigidly to the spine [13]. Although this robot provides excellent clinical outcomes [7], its cost and complexity, which are far greater than our TUSS-based system, may impose limitations on its applicability as the standard of care. In other approaches, Liang et al. [12] used the intersection of two laser planes to guide the pedicle probe to position the guidewires for screws and Von Jako et al. [20] used electromagnetic tracking in minimally invasive percutaneous pedicle screw placement. Although these two technologies reduce the use of fluoroscopy during the procedure, they do not eliminate radiation entirely like our TUSS-based system does. Another approach is to use intraoperative CT imaging for guidance [10, 14]; however, little advantage was found using intraoperative CT imaging compared with navigation using preoperative CT alone [2]. Moreover, intraoperative CT guidance requires investment into expensive instrumentation and exposes the patient and operating team to radiation during the operation. In contrast, our TUSS-based navigation system relies on two technologies—electromagnetic tracking and ultrasound imaging—that are low-cost and pose no risk to patients or staff. We would also like to emphasize that as the accuracy of automatic CT to ultrasound registration improves, landmark registration may be replaced in the future by automatic techniques. This replacement would eliminate the need to train operators in ultrasound landmark recognition and would also shorten the procedure time. These automatic registration methods are either based on image-to-image registration [21] or require prior segmentation of the vertebrae [15]. Biomechanical constraints can also be applied in the registration algorithm to account for the characteristic deformation of the spinal column between CT and ultrasound. However, registration still fails in a significant number of trials even under experimental conditions when used in image-based [5] or surface-based algorithms [15]. Because success rates are reportedly below 90%, and surgical cases would probably result in a lower success rate than experimental cases, we have chosen not to use these otherwise promising automatic registration methods. As a final note, because our method is implemented as free and open-source software, we expect it to disseminate easily among researchers and ultimately among clinicians performing pedicle screw placements; furthermore, we expect that the customizable nature of our software will encourage our TUSS-based system to be studied as a guidance option for other minimally invasive spinal interventions such as vertebroplasty.
In conclusion, this study suggests that tracked ultrasound technology may be able to make a practical contribution to minimally invasive spinal fusion surgery by eliminating intraoperative ionizing radiation. In particular, we found that tracked ultrasound snapshots of vertebral landmarks can be used to allow preoperative pedicle screw plans to be accurately mapped into the intraoperative coordinate frame. In the immediate future, we hope to determine the effectiveness of TUSS guidance on a larger and more diverse subject sample as well as further test TUSS guidance by placing physical screws in spine models. Looking farther ahead, we would also like to see our TUSS guidance technique be explored in the context of other minimally invasive spinal interventions such as vertebroplasty and believe that the open-source and highly customizable qualities of our software will facilitate such use.
Footnotes
Each author certifies that he or she, or a member of his or her immediate family, has no funding or commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research editors and board members are on file with the publication and can be viewed on request.
Clinical Orthopaedics and Related Research neither advocates nor endorses the use of any treatment, drug, or device. Readers are encouraged to always seek additional information, including FDA-approval status, of any drug or device prior to clinical use.
Each author certifies that his or her institution approved the human protocol for this investigation, that all investigations were conducted in conformity with ethical principles of research, and that informed consent for participation in the study was obtained.
The related experiments were performed at Queen’s University, Kingston, Ontario, Canada.
References
- 1.Bandela JR, Jacob RP, Arreola M, Griglock TM, Bova F, Yang M. Use of CT-based intraoperative spinal navigation: management of radiation exposure to operator, staff, and patients. World Neurosurg. 2011 Nov 7 [Epub ahead of print]. [DOI] [PubMed]
- 2.Costa F, Cardia A, Ortolina A, Fabio G, Zerbi A, Fornari M. Spinal navigation: standard preoperative versus intraoperative computed tomography data set acquisition for computer-guidance system: radiological and clinical study in 100 consecutive patients. Spine (Phila Pa 1976). 2011;36:2094–2098. doi: 10.1097/BRS.0b013e318201129d. [DOI] [PubMed] [Google Scholar]
- 3.Cui G, Wang Y, Kao TH, Zhang Y, Liu Z, Liu B, Li J, Zhang X, Zhu S, Lu N, Mao K, Wang Z, Zhang X, Yuan X, Dong T, Xiao S. Application of intraoperative computed tomography with or without navigation system in surgical correction of spinal deformity: a preliminary result of 59 consecutive human cases. Spine (Phila Pa 1976). 2012;37:891–900. doi: 10.1097/BRS.0b013e31823aff81. [DOI] [PubMed] [Google Scholar]
- 4.Gautschi OP, Schatlo B, Schaller K, Tessitore E. Clinically relevant complications related to pedicle screw placement in thoracolumbar surgery and their management: a literature review of 35,630 pedicle screws. Neurosurg Focus. 2011;31:E8. doi: 10.3171/2011.7.FOCUS11168. [DOI] [PubMed] [Google Scholar]
- 5.Gill S, Abolmaesumi P, Fichtinger G, Boisvert J, Pichora D, Borshneck D, Mousavi P. Biomechanically constrained groupwise ultrasound to CT registration of the lumbar spine. Med Image Anal. 2012;16:662–674. doi: 10.1016/j.media.2010.07.008. [DOI] [PubMed] [Google Scholar]
- 6.Greher M, Scharbert G, Kamolz LP, Beck H, Gustorff B, Kirchmair L, Kapral S. Ultrasound-guided lumbar facet nerve block: a sonoanatomic study of a new methodologic approach. Anesthesiology. 2004;100:1242–1248. doi: 10.1097/00000542-200405000-00028. [DOI] [PubMed] [Google Scholar]
- 7.Hu X, Ohnmeiss DD, Lieberman IH. Robotic-assisted pedicle screw placement: lessons learned from the first 102 patients. Eur Spine J. 2012 Sep 14 [Epub ahead of print]. [DOI] [PMC free article] [PubMed]
- 8.Kalfas IH, Kormos DW, Murphy MA, McKenzie RL, Barnett GH, Bell GR, Steiner CP, Trimble MB, Weisenberger JP. Application of frameless stereotaxy to pedicle screw fixation of the spine. J Neurosurg. 1995;83:641–647. doi: 10.3171/jns.1995.83.4.0641. [DOI] [PubMed] [Google Scholar]
- 9.Kawaguchi Y, Nakano M, Yasuda T, Seki S, Hori T, Kimura T. Development of a new technique for pedicle screw and Magerl screw insertion using a 3-dimensional image guide. Spine (Phila Pa 1976). 2012;37:1983–1988. doi: 10.1097/BRS.0b013e31825ab547. [DOI] [PubMed] [Google Scholar]
- 10.Larson AN, Polly DW, Jr, Guidera KJ, Mielke CH, Santos ER, Ledonio CG, Sembrano JN. The accuracy of navigation and 3D image-guided placement for the placement of pedicle screws in congenital spine deformity. J Pediatr Orthop. 2012;32:e23–29. doi: 10.1097/BPO.0b013e318263a39e. [DOI] [PubMed] [Google Scholar]
- 11.Larson AN, Santos ER, Polly DW, Jr, Ledonio CG, Sembrano JN, Mielke CH, Guidera KJ. Pediatric pedicle screw placement using intraoperative computed tomography and 3-dimensional image-guided navigation. Spine. 2012;37:188–194. doi: 10.1097/BRS.0b013e31822a2e0a. [DOI] [PubMed] [Google Scholar]
- 12.Liang JT, Doke T, Onogi S, Ohashi S, Ohnishi I, Sakuma I, Nakajima Y. A fluorolaser navigation system to guide linear surgical tool insertion. Int J Comput Assist Radiol Surg. 2012;7:931–939. doi: 10.1007/s11548-012-0743-0. [DOI] [PubMed] [Google Scholar]
- 13.Lieberman IH, Togawa D, Kayanja MM, Reinhardt MK, Friedlander A, Knoller N, Benzel EC. Bone-mounted miniature robotic guidance for pedicle screw and translaminar facet screw placement: Part I—Technical development and a test case result. Neurosurgery. 2006;59:641–650. doi: 10.1227/01.NEU.0000229055.00829.5B. [DOI] [PubMed] [Google Scholar]
- 14.Park P, Foley KT, Cowan JA, Marca FL. Minimally invasive pedicle screw fixation utilizing O-arm fluoroscopy with computer-assisted navigation: feasibility, technique, and preliminary results. Surg Neurol Int. 2010;1:44. doi: 10.4103/2152-7806.68705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rasoulian A, Abolmaesumi P, Mousavi P. Feature-based multibody rigid registration of CT and ultrasound images of lumbar spine. Med Phys. 2012;39:3154–3166. doi: 10.1118/1.4711753. [DOI] [PubMed] [Google Scholar]
- 16.Shin BJ, James AR, Njoku IU, Härtl R. Pedicle screw navigation: a systematic review and meta-analysis of perforation risk for computer-navigated versus freehand insertion. J Neurosurg Spine. 2012;17:113–122. doi: 10.3171/2012.5.SPINE11399. [DOI] [PubMed] [Google Scholar]
- 17.Tokuda J, Fischer GS, Papademetris X, Yaniv Z, Ibanez L, Cheng P, Liu H, Blevins J, Arata J, Golby AJ, Kapur T, Pieper S, Burdette EC, Fichtinger G, Tempany CM, Hata N. OpenIGTLink: an open network protocol for image-guided therapy environment. Int J Med Robot. 2009;5:423–434. doi: 10.1002/rcs.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ughwanogho E, Patel NM, Baldwin KD, Sampson NR, Flynn JM. Computed tomography-guided navigation of thoracic pedicle screws for adolescent idiopathic scoliosis results in more accurate placement and less screw removal. Spine (Phila Pa 1976). 2012;37:E473–478. doi: 10.1097/BRS.0b013e318238bbd9. [DOI] [PubMed] [Google Scholar]
- 19.Ungi T, Abolmaesumi P, Jalal R, Welch M, Ayukawa I, Nagpal S, Lasso A, Jaeger M, Borschneck DP, Fichtinger G, Mousavi P. Spinal needle navigation by tracked ultrasound snapshots. IEEE Trans Biomed Eng. 2012;59:2766–2772. doi: 10.1109/TBME.2012.2209881. [DOI] [PubMed] [Google Scholar]
- 20.Von Jako R, Finn MA, Yonemura KS, Araghi A, Khoo LT, Carrino JA, Perez-Cruet M. Minimally invasive percutaneous transpedicular screw fixation: increased accuracy and reduced radiation exposure by means of a novel electromagnetic navigation system. Acta Neurochir (Wien). 2011;153:589–596. doi: 10.1007/s00701-010-0882-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yan CX, Goulet B, Tampieri D, Collins DL. Ultrasound-CT registration of vertebrae without reconstruction. Int J Comput Assist Radiol Surg. 2012;7:901–909. doi: 10.1007/s11548-012-0771-9. [DOI] [PubMed] [Google Scholar]
- 22.Zhang HL, Zhou DS, Jiang ZS. Analysis of accuracy of computer-assisted navigation in cervical pedicle screw installation. Orthop Surg. 2011;3:52–56. doi: 10.1111/j.1757-7861.2010.00110.x. [DOI] [PMC free article] [PubMed] [Google Scholar]