Abstract
INTRODUCTION
Ciliated hepatic foregut cysts (CHFC) are rare, typically benign liver lesions. Primary squamous cell carcinoma (SCC) of the liver is also a rare entity with only approximately 25 reported cases in the literature. Recently, there have been four reports of malignant transformation of CHFC into primary squamous cell carcinoma of the liver. Here we report a fifth with unique presentation and review the literature.
PRESENTATION OF CASE
A 34 year-old man, with a history of ulcerative colitis, was incidentally found to have a 10 cm lesion in the right anterior sector plus left medial section of the liver on computerized tomography (CT) scan. The patient was asymptomatic at presentation and neoplastic markers were not elevated. Sequential transarterial chemoembolization (TACE) and portal vein embolization (PVE) allowed for left lateral section plus segment 1 hypertrophy and subsequent resection. Histology later revealed the cyst to be a CHFC and showed its malignant transformation. At 6 month follow-up, the patient has lung and abdominal recurrence.
DISCUSSION
With now the fifth case of malignant transformation of CHFC being reported, approximately 5% of all reported CHFC have undergone malignant transformation. This frequency, taken together with the aggressive disease course and poor prognosis, suggests that CHFC must not be presumed benign and should be regarded with clinical suspicion.
CONCLUSION
Accurate diagnosis of CHFC is mandatory given its potential malignant transformation. Even in asymptomatic CHFC, surgical excision is recommended. In addition, in cases of otherwise unresectable lesions, sequential TACE and PVE may provide optimal hypertrophy of future liver remnant.
Keywords: Ciliated hepatic foregut cyst, Squamous cell carcinoma, Transcatheter arterial chemoembolization (TACE), Portal vein embolization (PVE), Liver
1. Introduction
Ciliated hepatic foregut cysts (CHFC) are rare, typically benign liver lesions of congenital origin. During embryologic development, the primitive foregut gives rise to the tracheobronchial tree, and it is speculated that the common origin of the esophagus and liver accounts for the histological findings in ciliated hepatic foregut cysts.1 Despite first being described in 1857 by Friedreich, a literature review reveals only about 100 reported cases of CHFC.2 While this number may not reflect the prevalence of these cysts due to their benign, asymptomatic nature, it is agreed that CHFC are an uncommon finding.3
While the previous belief was that CHFC were benign lesions with rare morbidity, there have been four recent reports of malignant transformation of CHFC into primary squamous cell carcinoma (SCC) of the liver.1,3,5,6 Primary SCC of the liver is itself a very rare entity with approximately 25 reported cases in the literature.7 Pathogenesis of primary SCC of the liver is not entirely clear but previous cases have been associated with various diseases including non-parasitic cysts, hepatic cirrhosis, hepatolithiasis, hepatic teratoma, and Caroli's disease.7,8 There has been only one reported case of primary SCC without latent hepatic insult.7
Hepatic primary SCC is highly malignant with high rates of recurrence and poor overall prognosis.8 The disease state is typically advanced upon diagnosis and presents clinically as right upper quadrant pain, vague fullness, jaundice, weight loss, nausea, and/or vomiting. Hepatomegaly and tenderness with palpitation may be present.7 The outcomes of cases in the literature suggest a prognosis of less than 1 year survival with only isolated cases achieving 5 year survival.7,8 There is no current treatment standard, but most commonly, TACE, surgical resection, and radiation therapy have been utilized.7,8
Herein, this report identifies a fifth case of primary SCC of the liver arising from a CHFC. This case is unique in that the liver lesion was asymptomatic and was found incidentally on computed tomography (CT). In addition, successful management with sequential transcatheter arterial chemoembolization (TACE) and portal vein embolization (PVE) allowed for a therapeutic surgical intervention.
2. Case presentation
A 34-year-old man with a past medical history remarkable for ulcerative colitis underwent a CT scan due to an elevated C-reactive protein (CRP) and erythrocyte sedimentation rate (ESR), in addition to anemia on his routine blood work. Unexpectedly, the CT revealed a 10 cm hypoenhancing lesion in the right anterior sector plus left medial section of the liver (Fig. 1).
Fig. 1.
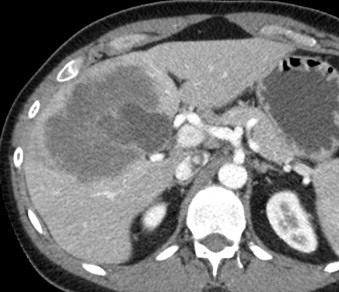
Incidental finding of hypoenhancing hepatic lesion noted on CT conducted in response to elevated ESR and CRP.
The patient was asymptomatic and no noticeable orangomegaly or pain was found with palpation. Laboratory studies revealed anemia suggestive of iron deficiency, normal serological tumor markers (CEA, CA 19-9, and AFP), viral hepatitis panel was negative, and CRP/ESR were elevated at 3.0 mg/dL and 74 mm/hr respectively. A dedicated liver magnetic resonance imaging (MRI) was performed and revealed a heterogeneous hepatic lesion in segments 4, 5, and 8. The lesion was noted to have an internal cystic component (Fig. 2). Computer generated volume assessment of the liver was performed at this time and it was found that segments 1, 2, and 3 comprised only 18% of the total liver volume.
Fig. 2.
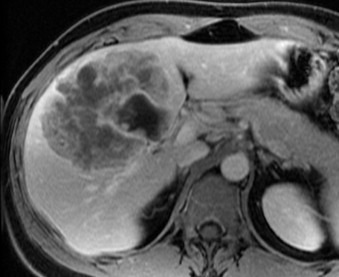
MRI revealing mass in the right anterior sector plus left medial section of the liver with internal cystic component.
Subsequent biopsy of the lesion showed it to be SCC. The patient underwent lipiodol-based transarterial chemoebolization (TACE) of the right hepatic artery and segment 4 branches. This was followed by right portal vein embolization (PVE) two weeks later. Six weeks after PVE, cross sectional imaging demonstrated that segments 1,2, and 3 had hypertrophied from 18% to 33% of total liver volume, providing an adequate liver remnant for right trisectionectomy (Fig. 3). The patient tolerated the procedure well and had an uncomplicated recovery. At 6 months, surveillance imaging demonstrated evidence of extrahepatic recurrence (lung and abdominal). The patient is currently receiving a systemic chemotherapy regimen of carboplatin and paclitaxel.
Fig. 3.
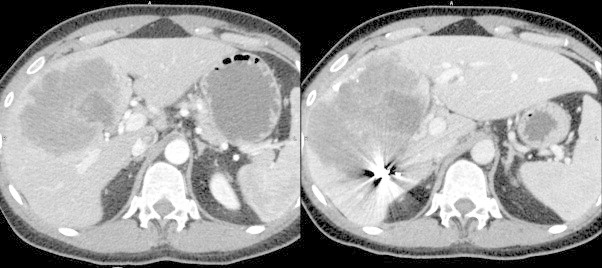
Left lateral segment hypertrophy. The image at left shows the liver before portal vein embolization (PVE), with a small left lateral segment (1067.391 cm3). The figure at right demonstrates scatter from embolization of the right portal vein, as well as significant hypertrophy of the left lateral segment (1801.602 cm3).
3. Histology
The liver specimen demonstrated a smooth mottled capsule with a 13 cm × 7 cm rough white–gray nodular area exhibiting overlying fibrous adhesions. A well-circumscribed yellow–gray mass measuring 14 cm × 12.5 cm × 10.5 cm and a cyst, central within the mass measuring 5.5 cm × 5 cm × 4.5 cm, were revealed by sectioning. The cyst wall exhibited a smooth to focally granular surface and was filled with a soft tan–gray tumor material. Three well-circumscribed nodules adjacent to the large mass were present.
Microscopically, the tumor showed areas of confluent geographic necrosis. Immunohistochemical staining demonstrated tumor cells positive for CK 5/6 and p40, but negative for p16. There were no lymph node metastases in the 3 nodes examined. The cyst was lined with pseudostratified ciliated columnar epithelium and had regions of wall-thickening (Fig. 4). The tumor showed sheets of neoplastic cells with eosinophilic cytoplasm and round-to-oval nuclei. It was determined that the tumor was poorly differentiated squamous cell carcinoma. The resection margins were negative for malignancy.
Fig. 4.
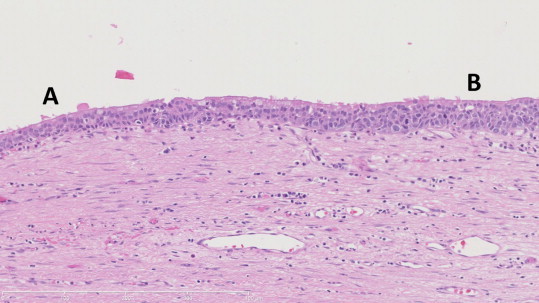
Transition of normal ciliated foregut cyst wall (A) to in situ squamous cell carcinoma (B). On the left (A), the cyst wall resembles that of a bronchogenic cyst. The wall itself is thinner and contains fewer mitoses, which are appropriately located along the basement membrane. On the right (B), the wall is thicker. Here, mitoses are more frequent, and are no longer limited to locations along the basement membrane.
4. Discussion
Malignant transformation of ciliated hepatic foregut cysts is exceedingly rare.6 Since CHFC were first described in 1857, only five such cases, including this one, have been reported (Table 1).1,4–6 Due to their normally asymptomatic nature, CHFC are usually found incidentally during surgery (6%), via imaging studies (40%), or post mortem at autopsy (26%). The remainder present with abdominal symptoms.9 Diagnosis of CHFC preoperatively is difficult because radiographic studies cannot differentiate CHFC from other cysts and thus, biopsy is typically required.4 Malignant transformation of CHFC is even more difficult to diagnose preoperatively due to the sampling error associated with aspiration cytology, and the difficulty associated with distinguishing benign and neoplastic cysts with imaging studies.4 In addition, serological tumor markers such as CA 19-9 seem to lack efficacy for diagnosis as they have been demonstrated to be elevated in benign CHFC and may be normal in malignancy, as in this case.6 Most CHFC are unilocular, and rarely exceed 4 cm in diameter.9 Interestingly, with the exception of the case reported by Zhang et al. which presented with multiple small cysts (3–5 mm), the remaining CHFC that have undergone malignant transformation have been larger than 5 cm in diameter.1
Table 1.
Review of five reported cases of malignant transformation of CHFC in the English Literature.
| Case | Age/gender | Presentation | Pathological features | Outcome | References |
|---|---|---|---|---|---|
| 1 | 51 M | 1-month epigastric pain made worse by eating Mild RUQ pain with palpitation | Cyst occupying the subhepatic space | Uneventful postoperative course, but died 2 months later | [6] |
| 12 cm × 10 cm × 0.1–0.7 cm in size | |||||
| 2 | 21 M | 6-month history of vague fullness | Cyst occupying segments V and VI | Uneventful postoperative course, | [5] |
| Mild RUQ pain, fever, and 13 kg weight loss | Greatest diameter measuring 10 cm | but died 9 months later after widespread intra-abdominal recurrence | |||
| 3 | 40 F | RUQ pain with back and thoracic pain | Cyst occupying segment V | Outcome not provided | [4] |
| 13 cm × 9 cm × 7 cm in size | |||||
| 4 | 60 F | 1-month history of vague fullness | Tumor occupying segment VI | Alive after 6-month follow-up | [1] |
| RUQ pain on palpitation, w/o peritoneal signs | 7 cm × 6 cm × 5 cm in size Mutiple small cystic lesions, 3–5 mm in size | ||||
| 5 | 34 M | Asymptomatic | Tumor occupying segments IV, V, and VIII | Lung and abdominal recurrence at 6-months | *** |
| Central cyst measuring 5.5 cm × 5 cm × 4.5 cm |
Note: *** indicates this paper, RUQ = Right upper quadrant.
It is difficult to definitively say that primary SCC of the liver is a result of malignant transformation of the CHFC wall. However, after establishing no extrahepatic disease, a primary hepatic cancer should be suspected. In addition, given that the cyst was adjacent to and focally connected with the squamous cell carcinoma in this case, it was felt that the cyst wall was the origin of pathogenesis. Squamous cell metaplasia, dysplasia, and malignant transformation of the ciliated columnar epithelium is the proposed mechanism of pathogenesis.
CHFC have historically been considered non-neoplastic lesions. However, with the recent publication of now five cases of squamous cell carcinoma arising from CHFC, approximately 5% of reported CHFC have demonstrated malignant transformation, suggesting the need for serious clinical consideration. Due to the aggressive nature of these malignant lesions, it is mandatory that thorough pathological and radiological studies be conducted in order to differentiate cysts upon discovery. Differential diagnoses of large hepatic cysts include: parasitic cyst, simple hepatic cyst, and biliary cyst, as well as non-cystic lesions if cysts are filled with dense fluid or demonstrate metastatic change.1,5,9 The ciliated epithelium of CHFC is unique and can serve as a differentiating histological marker.
Our case and the existing literature suggest that primary SCC of the liver is an aggressive disease with poor prognosis. Only isolated cases have been reported in which 5 year survival has been achieved and Zhao et al. reports that survival does not usually exceed 1 year.7,8 In part due to the rarity of these lesions, there is no standard recommended treatment. Kaji et al. reported a case of primary hepatic SCC that responded very well to chemotherapeutics (cis-diaminedichloro-platinum (CDDP) and 5-fluorouracil) administered via hepatic arterial injection.10 In addition, recent studies have demonstrated the efficacy of sequential TACE and PVE in management of hepatocellular carcinoma.11 This approach has demonstrated significant increases in the future liver remnant (FLR) and longer recurrence-free and overall survival versus PVE alone.11 In this case, sequential TACE and PVE was well tolerated and was successful in inducing sufficient FLR hypertrophy for surgical intervention.
5. Conclusion
CHFC should not be presumed benign and must be regarded with suspicion. The possibility of malignant transformation of CHFC and aggressive nature of primary hepatic SCC necessitates accurate diagnosis of hepatic cysts. Given this review of cases, we recommend surgical excision of CHFC. Also, in cases of otherwise unresectable primary SCC of the liver, sequential TACE and PVE may induce optimal FLR hypertrophy to allow for surgical resection.
Consent
Written informed consent was obtained from the patient for publication of this case report and case series and accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request.
Conflict of interest
None.
Funding
None.
Author contributions
J.M. Wilson and R. Groeschl wrote and edited the manuscript, concept/design development, data collection/analysis. B. George wrote and edited the manuscript, concept/design development. K.K. Turaga wrote and edited the manuscript, concept/design development. P.J. Patel wrote and edited the manuscript, concept/design development, data collection/analysis. K. Saeian wrote and edited the manuscript, concept/design development. T.C. Gamblin wrote and edited the manuscript, concept/design development, data collection/analysis.
Footnotes
This is an open-access article distributed under the terms of the Creative Commons Attribution-NonCommercial-No Derivative Works License, which permits non-commercial use, distribution, and reproduction in any medium, provided the original author and source are credited.
References
- 1.Zhang X., Wang Z., Dong Y. Squamous cell carcinoma arising in a ciliated hepatic foregut cyst: case report and literature review. Pathol Res Pract. 2009;205(7):498–501. doi: 10.1016/j.prp.2008.12.003. [DOI] [PubMed] [Google Scholar]
- 2.Sharma S., Dean A.G., Corn A., Kohli V., Wright H.I., Sebastian A. Ciliated hepatic foregut cyst: an increasingly diagnosed condition. Hepatobiliary Pancreat Dis Int. 2008;7(6):581–589. [PubMed] [Google Scholar]
- 3.Vick D.J., Goodman Z.D., Deavers M.T., Cain J., Ishak K.G. Ciliated hepatic foregut cyst: a study of six cases and review of the literature. Am J Surg Pathol. 1999;23(6):671–677. doi: 10.1097/00000478-199906000-00006. [DOI] [PubMed] [Google Scholar]
- 4.de Lajarte-Thirouard A.S., Rioux-Leclercq N., Boudjema K., Gandon Y., Ramee M.P., Turlin B. Squamous cell carcinoma arising in a hepatic foregut cyst. Pathol Res Pract. 2002;198(10):697–700. doi: 10.1078/0344-0338-00323. [DOI] [PubMed] [Google Scholar]
- 5.Furlanetto A., Dei Tos A.P. Squamous cell carcinoma arising in a ciliated hepatic foregut cyst. Virchows Archiv. 2002;441(3):296–298. doi: 10.1007/s00428-002-0668-z. [DOI] [PubMed] [Google Scholar]
- 6.Vick D.J., Goodman Z.D., Ishak K.G. Squamous cell carcinoma arising in a ciliated hepatic foregut cyst. Arch Pathol Lab Med. 1999;123(11):1115–1117. doi: 10.5858/1999-123-1115-SCCAIA. [DOI] [PubMed] [Google Scholar]
- 7.Naik S., Waris W., Carmosino L., Mehrishi A., Saif M.W. Primary squamous cell carcinoma of the liver. J Gastrointestin Liver Dis. 2009;18(4):487–489. [PubMed] [Google Scholar]
- 8.Zhao R., Zhu K., Wang R., Gao J., Cui K., Yu F. Primary squamous cell carcinoma of the liver: a case report and review of the literature. Oncol Lett. 2012;4(6):1163–1166. doi: 10.3892/ol.2012.894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shaw J.M., Krige J.E., Beningfield S.J., Locketz M.L. Ciliated hepatic foregut cyst: a rare cystic liver lesion. J Gastrointest Surg. 2008;12(7):1304–1306. doi: 10.1007/s11605-007-0364-z. [DOI] [PubMed] [Google Scholar]
- 10.Kaji R., Sasaki N., Tateishi I., Nagata E., Okabe Y., Yoshida T. A case report of primary hepatic squamous cell carcinoma that remarkably responded to low dose arterial injection of anti-cancer drugs. Kurume Med J. 2003;50(1–2):71–75. doi: 10.2739/kurumemedj.50.71. [DOI] [PubMed] [Google Scholar]
- 11.Yoo H., Kim J.H., Ko G.Y., Kim K.W., Gwon D.I., Lee S.G. Sequential transcatheter arterial chemoembolization and portal vein embolization versus portal vein embolization only before major hepatectomy for patients with hepatocellular carcinoma. Ann Surg Oncol. 2011;18(5):1251–1257. doi: 10.1245/s10434-010-1423-3. [DOI] [PubMed] [Google Scholar]


