Abstract
Objective
To investigate any additional effect of dual transcranial direct current stimulation (tDCS) compared with single tDCS in chronic stroke patients with aphasia.
Methods
Eleven chronic stroke patients (aged 52.6±13.4 years, nine men) with aphasia were enrolled. Single anodal tDCS was applied over the left inferior frontal gyrus (IFG) and a cathodal electrode was placed over the left buccinator muscle. Dual tDCS was applied as follows: 1) anodal tDCS over the left IFG and cathodal tDCS over the left buccinator muscle and 2) cathodal tDCS over the right IFG and anodal tDCS over the right buccinator muscle. Each tDCS was delivered for 30 minutes at a 2-mA intensity. Speech therapy was provided during the last 15 minutes of the tDCS. Before and after the stimulation, the Korean-Boston Naming Test and a verbal fluency test were performed.
Results
The dual tDCS produced a significant improvement in the response time for the Korean-Boston Naming Test compared with the baseline assessment, with a significant interaction between the time and type of interventions. Both single and dual tDCS produced a significant improvement in the number of correct responses after stimulation with no significant interaction. No significant changes in the verbal fluency test were observed after single or dual tDCS.
Conclusion
The results conveyed that dual tDCS using anodal tDCS over the left IFG and cathodal tDCS over the right IFG may be more effective than a single anodal tDCS over the left IFG.
Keywords: Stroke, Aphasia, Transcranial direct current stimulation, Electrical stimulation therapy
INTRODUCTION
Aphasia is a common consequence of stroke and is caused by brain injury to the left cerebral hemisphere [1]. Although patients with aphasia tend to recover to a considerable extent after a stroke, most of the recovery occurs within the first 6 months and is incomplete [2]. Majority of aphasic patients continue to suffer from chronic deficits, for which conventional rehabilitative treatment is less effective [3,4]. Therefore, there is an increasing need to develop new interventions that enhance the recovery from chronic aphasia after a stroke.
Transcranial direct current stimulation (tDCS) is a non-invasive technique that has been found to modulate the excitability of neurons in the cerebral cortex [5,6]. The polarity of the current applied to the scalp determines the effect of tDCS on the underlying cortex. Anodal tDCS has been found to increase cortical excitability, whereas cathodal tDCS decreases it. Numerous reports have demonstrated the modulating effect of tDCS on various cortical functions, including motor learning, working memory, and language [7-9]. In recent studies, the application of anodal tDCS to the left frontal hemisphere was shown to increase the naming accuracy and response time in aphasic patients and healthy individuals [10-12]. These results suggest that stimulation of the perilesional area of the left hemisphere could result in improved recovery from aphasia.
In contrast, the role of the right hemisphere in the recovery from aphasia is still controversial. Previous studies hypothesized that the homologous language region in the right hemisphere has a capacity for language processing that is normally inhibited through transcallosal connections [13]. Although recruitment of the homologous language area in the right hemisphere is thought to be a crucial recovery pathway for relatively large stroke lesions in the left hemisphere, increased activity in the right hemisphere is not always beneficial for all patients. Increased activation of the right hemisphere may result in increased inter-hemispheric inhibition to the detriment of the left hemisphere and could interfere with the recovery of language function [14,15]. Consistent with the above hypothesis, some studies found that suppression of the right hemispheric homologous language region through the use of inhibitory techniques, such as repetitive transcranial magnetic stimulation (rTMS) or tDCS, resulted in an improvement in language [16,17]. Kang et al. [16] applied cathodal tDCS on the right Broca's homologue area for 5 consecutive days and reported improved picture naming in aphasic patients. Furthermore, You et al. [17] reported that cathodal tDCS to the right superior temporal gyrus produced a greater improvement in comprehension compared to the anodal tDCS to the left superior temporal gyrus or sham tDCS.
Dual-site stimulation using tDCS has received much attention for the purpose of developing more effective methods of stimulation. Vines et al. [18] applied a simultaneous dual-hemispheric tDCS to healthy subjects, using anodal tDCS over the non-dominant and cathodal tDCS over the dominant motor cortices. They confirmed an additive effect on motor function of the non-dominant hand compared with single-site stimulation. Furthermore, dual tDCS using a cathode over the right and an anode over the left parietal cortices produced a stronger neglect-like effect in healthy subjects compared with the single cathodal tDCS over the right parietal cortex [19].
To our knowledge, no study has reported the effect of simultaneous dual tDCS applied over the bihemispheric language-related regions in aphasic patients after suffering from a stroke. Therefore, in this study, we investigated the effects of dual tDCS on the language function of patients with aphasia and compared them with those of single tDCS in order to determine the most effective tDCS method for aphasic patients. We hypothesized that the simultaneous application of anodal tDCS over the left inferior frontal gyrus (IFG) and cathodal tDCS over the right IFG would have a greater effect than a single anodal tDCS over the left IFG.
MATERIALS AND METHODS
Subjects
Eleven chronic stroke patients (aged 52.6±13.4 years, nine men) with aphasia were enrolled in the study. Six of the eleven patients had non-fluent aphasia, while the remaining five had fluent aphasia. The demographic and clinical characteristics of the participants are shown in Table 1. Subjects were enrolled at least 6 months after the onset of aphasia. The inclusion criteria were as follows: native Korean speaker, right handedness before the stroke, single first-ever left hemispheric stroke, and clinically diagnosed with aphasia. Prior to the enrollment into the study, all patients were evaluated by the same professional speech and language pathologist using the Korean-Western Aphasia Battery to determine the type and severity of aphasia. The exclusion criteria included history of seizure and implanted metal object, as these are contraindications relevant to tDCS [20]. We also excluded patients who had severely impaired auditory-verbal comprehension or other neurological diseases, and those who were uncooperative with speech therapy. All participants gave their informed consent.
Table 1.
Demographic and clinical characteristics of subjects
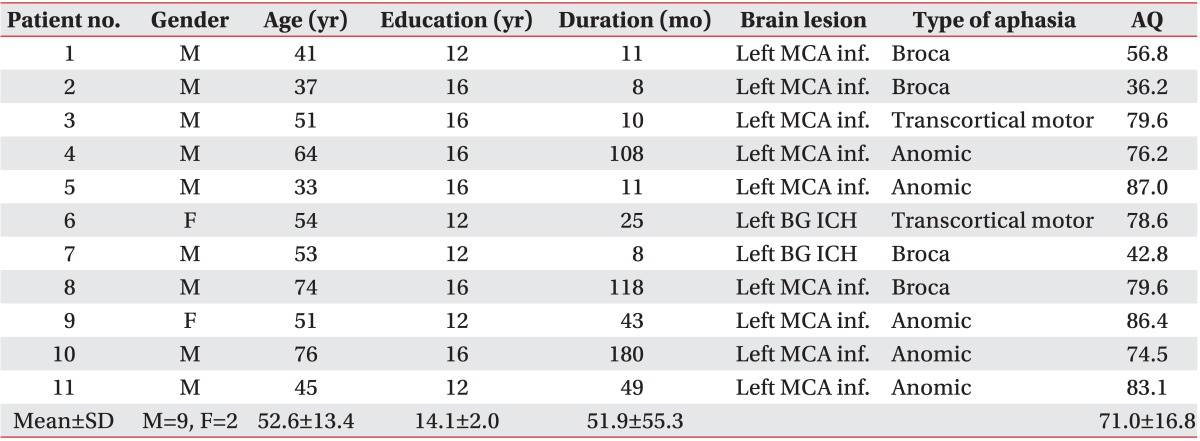
MCA inf., middle cerebral artery infarction; BG, basal ganglia; ICH, intracerebral hemorrhage; AQ, aphasia quotient of Korean-Western Aphasia Battery; SD, standard deviation.
Study design and application of tDCS
The study was designed as a randomized, double-blind cross-over study. tDCS was delivered for 30 minutes at an intensity of 2 mA using saline-soaked 5×5-cm sponge electrodes and a constant-current electrical stimulator, Phoresor II Auto PM850 (IOMED, Salt Lake City, UT, USA). Active stimulation electrodes were placed over the left IFG or both the left and right IFG. We used an international 10-20 EEG system to locate the IFG. According to the 10-20 EEG system, the left and right IFG areas were defined as F7 and F8, respectively [21]. Reference electrodes were applied to the buccinator muscle of the same side in order to avoid any unintentional influence of the electrical current on other areas of the brain [22].
All participants received treatments with both single and dual tDCS with a washout period of more than 24 hours between the two experiments. The order of the stimulation experiments was counterbalanced across subjects. For the single stimulation experiment, an anodal tDCS electrode was placed over the left IFG and a cathodal tDCS electrode was positioned over the left buccinator muscle. In the dual stimulation experiment, 1) an anodal tDCS electrode was placed over the left IFG and a counter electric cathodal tDCS electrode was positioned over the left buccinator muscle, and 2) a cathodal tDCS electrode was placed over the right IFG and a counter electric anodal tDCS electrode was positioned over the right buccinator muscle. All electrodes used in the single and dual stimulation experiments were the same; however, the right tDCS electrode was only switched on for 5 seconds.
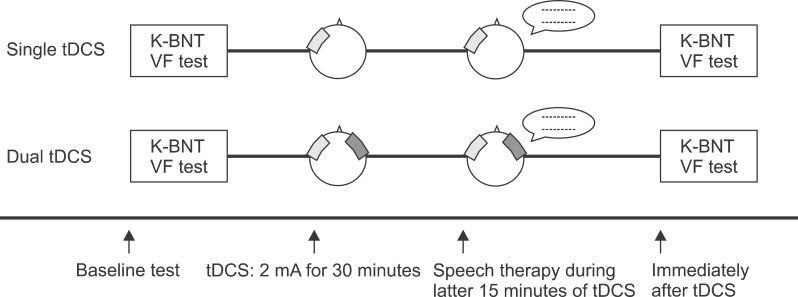
The same therapist administered the speech and language therapy, which was comprised of picture naming and reading short paragraphs, during the last 15 minutes of the tDCS application (Fig. 1).
Fig. 1.
Study design for the single and dual transcranial direct current stimulation (tDCS). The single tDCS applied anodal tDCS over the left inferior frontal gyrus (IFG) and reference electrode over the left buccinators muscle. The dual tDCS was conducted with 1) anodal tDCS over the left IFG and cathodal tDCS over the left buccinators muscle and 2) cathodal tDCS over the right IFG and anodal tDCS over the right buccinators muscle. All subjects were assessed with the Korean-Boston Naming Test (K-BNT) and the verbal fluency (VF) test before and immediately after stimulation.
Evaluation of language function
Before and after each tDCS session, changes in the language functions of the subjects were assessed by a picture naming test and a verbal fluency test. We constructed four sets of the modified, short version of the Korean-Boston Naming Test, which includes 15 frequency-balanced items in each group [23]. Subjects were asked to pronounce the correct names of the pictures that were presented on a personal computer screen. The response time and the number of correct responses were recorded. The verbal fluency test utilized two stimulating pictures (cookie theft and beach) from the Korean-Western Aphasia Battery [24]. A different picture was shown before and after tDCS, and the subjects were requested to describe the picture within 60 seconds. The total number of syllables used was counted. Both the picture naming test and the verbal fluency test were video recorded and reviewed by a single speech-language therapist, who was blinded to the type of stimulation.
Statistical analysis
All analyses were performed using the software package SPSS ver. 20.0 (SPSS Inc., Chicago, IL, USA). The Kolmogorov-Smirnov test revealed that the data were not normally distributed in our study. Therefore, Wilcoxon signed-rank tests were used to evaluate the differences between language function before and after tDCS for each stimulation experiment. Baseline values of both types of stimulation were analyzed using the Mann-Whitney U test. Interactions between the time and type of intervention were determined using the Friedman test. Statistical significance was considered to be at a level of p<0.05.
RESULTS
None of the subjects reported severe adverse effects during or after the experiments, and they all tolerated the single and dual tDCS without interrupting the experiment.
Picture naming test
Response time
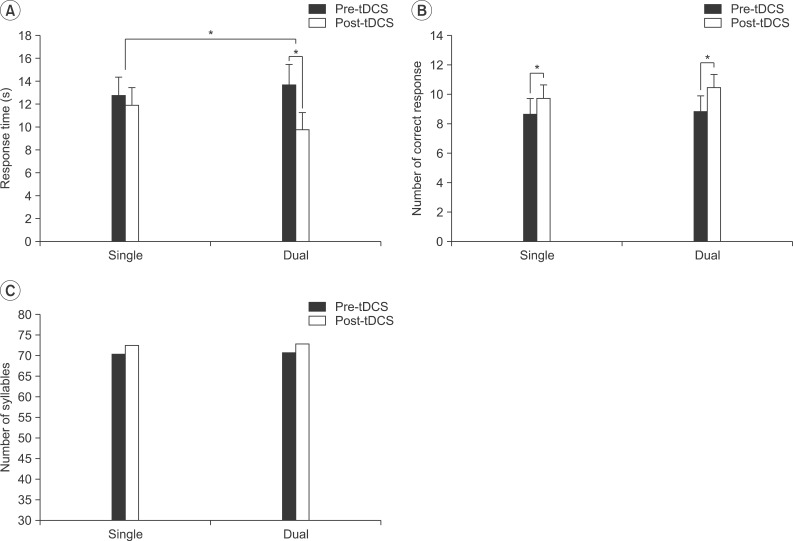
Baseline values for the response time did not differ significantly (single tDCS, 12.76±1.61 seconds; dual tDCS, 13.68±1.81 seconds; p>0.05). The mean response time for the picture naming task was decreased from 12.76±1.61 to 11.90±1.58 seconds, which was not statistically significant (p>0.05) in the single tDCS, but was significantly decreased from 13.68±1.81 to 9.76±1.51 seconds (p<0.05) in the dual tDCS (Fig. 2A). The Friedman test demonstrated a significant interaction between the time (pre-stimulation vs. post-stimulation) and type (single vs. dual) of interventions (χ2=4.455, p=0.035). The dual tDCS induced a greater effect on the response time in the picture naming test than in the single tDCS.
Fig. 2.
Changes in the response time (A) and the number of correct response (B) for the picture naming test, and the number of syllables in the verbal fluency test (C) were demonstrated after application of the single and dual transcranial direct current stimulation (tDCS). *p<0.05.
Correct response
No statistical significant difference in the baseline values of the numbers of correct responses was observed between the two types of intervention (single tDCS, 8.64±1.08; dual tDCS, 8.82±1.08; p>0.05). The number of correct responses significantly increased from 8.64±1.08 to 9.73±0.92 (p<0.05) in the single tDCS (Fig. 2B) and from 8.82±1.08 to 10.45±0.93 (p<0.05) in the dual tDCS. No significant interaction between the time (pre-stimulation vs. post-stimulation) and type (single vs. dual) of intervention was observed in the number of correct responses in the picture naming test (χ2=0.4, p=0.53).
Verbal fluency test
Under conditions of both the single and dual tDCS, no significant difference was found between the number of syllables used in the pre- and post-stimulation (Fig. 2C).
DISCUSSION
We investigated the effect of the simultaneous application of anodal tDCS over the left IFG and cathodal tDCS over the right IFG on the language function of aphasic patients and compared the results with those of a single anodal tDCS on the left IFG. Our results demonstrated that the mean response time in the picture naming test was significantly shortened only after the dual tDCS, with an interaction between the two conditions, suggesting that dual tDCS may be more effective than single tDCS. In contrast, both single and dual tDCS were effective in enhancing the accuracy of picture naming, and the number of correct responses was significantly increased after both stimulation conditions.
The current consensus is that two important mechanisms are involved in the recovery from aphasia [25,26]. First, in patients with relatively small lesions in the left hemisphere, the recruitment of perilesional cortical neuronal elements plays an important role in the recovery from aphasia. A number of functional magnetic resonance imaging studies have demonstrated that greater activation in the left hemisphere is associated with a better outcome for language recovery [13,27,28]. Thus, the enhancement of the excitability of the left language-related cortical regions by non-invasive brain stimulation may improve recovery from aphasia [10,12].
Second, the recruitment of the homologous language area and speech-motor region in the right hemisphere may be a crucial pathway in patients with relatively large left hemispheric lesions. The right hemispheric perisylvian area is known to be involved to some extent in language recovery, but is less effective than the structural reorganization of the left hemispheric language areas [26]. The recruitment of the right hemispheric neuronal elements may be facilitated by a decrease in transcallosal inhibition resulting from the left hemispheric lesion. Although activation of the right hemisphere during the subacute phase of aphasia may be beneficial in severely affected patients, prolonged right hemispheric activity is considered to be deleterious as it may exert an increased inhibitory influence on the left perilesional activity [29,30]. Therefore, the inhibition of right hemispheric activity using cathodal tDCS may have a positive effect on language recovery, particularly in chronic aphasic patients [31].
Bihemispheric brain stimulation is based on the inter-hemispheric rivalry theory, which hypothesizes that unilateral lesions in the brain cause an imbalance between dominant and non-dominant hemispheres [32,33], and this may interfere with the natural recovery process [14]. Theoretically, increasing the excitability of the ipsilesional cortical region and decreasing the excitability of the contralesional, unaffected cortical region may help to restore the balance between both hemispheres and thus promote the appropriate recovery process [34]. For bihemispheric stimulation, tDCS has an advantage over rTMS because the dual stimulation can be applied over two hemispheres simultaneously using a single device. In addition, tDCS is cheaper, portable and less noisy compared to rTMS [35]. In the present study, both single and dual tDCS methods produced an improvement in accuracy in the picture naming test with no adverse effects, which suggests that both methods can be used as effective tools to enhance language function in patients with chronic aphasia after stroke. However, the dual tDCS additionally produced a greater improvement in response time in the picture naming test than the single tDCS. Therefore, it can be proposed that the dual tDCS contributed to an additive effect on the results obtained with the single tDCS in the improvement of the language function of chronic aphasic patients.
The patients included in our study varied greatly with respect to the subtypes of aphasia, lesion location, and the extent of brain damage. Regardless of the type of aphasia, the role of the left frontal cortex in its recovery has been demonstrated. A recent functional magnetic resonance imaging study revealed that the activation of the left frontal cortex was correlated with naming accuracy in stroke patients with aphasia [27]. Furthermore, increasing the excitability of the left frontal cortex using tDCS improved naming ability, irrespective of the subtype of the aphasia, and the extent of the stroke lesion [10]. The results from our study are consistent with previous reports and confirm that the activation of the left frontal cortex, particularly the IFG, improves naming ability in various types of aphasia.
However, the results did not demonstrate any significant change in the verbal fluency test after either type of tDCS. In the study by Cattaneo et al. [36], the application of tDCS to Broca's area improved the phonemic and semantic fluency of healthy individuals; however, their study design, outcome measurements and subjects differed from those in our study. Moreover, the Korean-language version of the verbal fluency test has not yet been validated as a quantitative method, especially in stroke patients and may need further investigation in order to define the different effects of tDCS on specific language functions.
The current study had several limitations. First, it did not examine the excitability of the stimulated cortical area directly. Therefore, the behavioral results may indirectly verify our hypothesis. Functional imaging techniques, such as functional magnetic resonance imaging, could be used to clarify this issue in future studies. Second, the total current intensities provided to the whole brain in the single and dual tDCS experiments differed (2.0 mA in the single vs. 4.0 mA in the dual tDCS, respectively). Therefore, a different modulatory effect due to a higher intensity of electric current cannot be ruled out as a cause of the differences in the results. Finally, the population in our study was relatively small and heterogeneous, and thus, we were unavailable to evaluate the effects relative to specific brain lesions or subtypes of aphasia.
Despite the above limitations, our study proposes that dual tDCS can be used as a safe and effective intervention method in addition to conventional speech-language therapy for the treatment of aphasic patients following a stroke.
The results of this study showed that simultaneous, bifrontal dual tDCS is a safe and effective method to improve language function in patients with chronic aphasia after a stroke. Further studies on the cumulative and long-term effects of dual tDCS are required for appropriate clinical application.
ACKNOWLEDGMENTS
This study was supported by the National Research Foundation of Korea grant (No. 2011-0016960) and by a KOSEF grant (M10644000022-06N4400-02210) funded by the Korean government.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Jordan LC, Hillis AE. Disorders of speech and language: aphasia, apraxia and dysarthria. Curr Opin Neurol. 2006;19:580–585. doi: 10.1097/WCO.0b013e3280109260. [DOI] [PubMed] [Google Scholar]
- 2.Nicholas ML, Helm-Estabrooks N, Ward-Lonergan J, Morgan AR. Evolution of severe aphasia in the first two years post onset. Arch Phys Med Rehabil. 1993;74:830–836. doi: 10.1016/0003-9993(93)90009-y. [DOI] [PubMed] [Google Scholar]
- 3.Robey RR. The efficacy of treatment for aphasic persons: a meta-analysis. Brain Lang. 1994;47:582–608. doi: 10.1006/brln.1994.1060. [DOI] [PubMed] [Google Scholar]
- 4.Robey RR. A meta-analysis of clinical outcomes in the treatment of aphasia. J Speech Lang Hear Res. 1998;41:172–187. doi: 10.1044/jslhr.4101.172. [DOI] [PubMed] [Google Scholar]
- 5.Nitsche MA, Paulus W. Excitability changes induced in the human motor cortex by weak transcranial direct current stimulation. J Physiol. 2000;527:633–639. doi: 10.1111/j.1469-7793.2000.t01-1-00633.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Priori A, Berardelli A, Rona S, Accornero N, Manfredi M. Polarization of the human motor cortex through the scalp. Neuroreport. 1998;9:2257–2260. doi: 10.1097/00001756-199807130-00020. [DOI] [PubMed] [Google Scholar]
- 7.Liebetanz D, Nitsche MA, Tergau F, Paulus W. Pharmacological approach to the mechanisms of transcranial DC-stimulation-induced after-effects of human motor cortex excitability. Brain. 2002;125:2238–2247. doi: 10.1093/brain/awf238. [DOI] [PubMed] [Google Scholar]
- 8.Fregni F, Boggio PS, Nitsche M, Bermpohl F, Antal A, Feredoes E, et al. Anodal transcranial direct current stimulation of prefrontal cortex enhances working memory. Exp Brain Res. 2005;166:23–30. doi: 10.1007/s00221-005-2334-6. [DOI] [PubMed] [Google Scholar]
- 9.Sparing R, Dafotakis M, Meister IG, Thirugnanasambandam N, Fink GR. Enhancing language performance with non-invasive brain stimulation: a transcranial direct current stimulation study in healthy humans. Neuropsychologia. 2008;46:261–268. doi: 10.1016/j.neuropsychologia.2007.07.009. [DOI] [PubMed] [Google Scholar]
- 10.Baker JM, Rorden C, Fridriksson J. Using transcranial direct-current stimulation to treat stroke patients with aphasia. Stroke. 2010;41:1229–1236. doi: 10.1161/STROKEAHA.109.576785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fertonani A, Rosini S, Cotelli M, Rossini PM, Miniussi C. Naming facilitation induced by transcranial direct current stimulation. Behav Brain Res. 2010;208:311–318. doi: 10.1016/j.bbr.2009.10.030. [DOI] [PubMed] [Google Scholar]
- 12.Fridriksson J, Richardson JD, Baker JM, Rorden C. Transcranial direct current stimulation improves naming reaction time in fluent aphasia: a double-blind, sham-controlled study. Stroke. 2011;42:819–821. doi: 10.1161/STROKEAHA.110.600288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Karbe H, Thiel A, Weber-Luxenburger G, Herholz K, Kessler J, Heiss WD. Brain plasticity in poststroke aphasia: what is the contribution of the right hemisphere? Brain Lang. 1998;64:215–230. doi: 10.1006/brln.1998.1961. [DOI] [PubMed] [Google Scholar]
- 14.Lotze M, Markert J, Sauseng P, Hoppe J, Plewnia C, Gerloff C. The role of multiple contralesional motor areas for complex hand movements after internal capsular lesion. J Neurosci. 2006;26:6096–6102. doi: 10.1523/JNEUROSCI.4564-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Loubinoux I, Carel C, Pariente J, Dechaumont S, Albucher JF, Marque P, et al. Correlation between cerebral reorganization and motor recovery after subcortical infarcts. Neuroimage. 2003;20:2166–2180. doi: 10.1016/j.neuroimage.2003.08.017. [DOI] [PubMed] [Google Scholar]
- 16.Kang EK, Kim YK, Sohn HM, Cohen LG, Paik NJ. Improved picture naming in aphasia patients treated with cathodal tDCS to inhibit the right Broca's homologue area. Restor Neurol Neurosci. 2011;29:141–152. doi: 10.3233/RNN-2011-0587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.You DS, Kim DY, Chun MH, Jung SE, Park SJ. Cathodal transcranial direct current stimulation of the right Wernicke's area improves comprehension in subacute stroke patients. Brain Lang. 2011;119:1–5. doi: 10.1016/j.bandl.2011.05.002. [DOI] [PubMed] [Google Scholar]
- 18.Vines BW, Cerruti C, Schlaug G. Dual-hemisphere tDCS facilitates greater improvements for healthy subjects' non-dominant hand compared to uni-hemisphere stimulation. BMC Neurosci. 2008;9:103. doi: 10.1186/1471-2202-9-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Giglia G, Mattaliano P, Puma A, Rizzo S, Fierro B, Brighina F. Neglect-like effects induced by tDCS modulation of posterior parietal cortices in healthy subjects. Brain Stimul. 2011;4:294–299. doi: 10.1016/j.brs.2011.01.003. [DOI] [PubMed] [Google Scholar]
- 20.Nitsche MA, Cohen LG, Wassermann EM, Priori A, Lang N, Antal A, et al. Transcranial direct current stimulation: state of the art 2008. Brain Stimul. 2008;1:206–223. doi: 10.1016/j.brs.2008.06.004. [DOI] [PubMed] [Google Scholar]
- 21.Okamoto M, Dan H, Sakamoto K, Takeo K, Shimizu K, Kohno S, et al. Three-dimensional probabilistic anatomical cranio-cerebral correlation via the international 10-20 system oriented for transcranial functional brain mapping. Neuroimage. 2004;21:99–111. doi: 10.1016/j.neuroimage.2003.08.026. [DOI] [PubMed] [Google Scholar]
- 22.Galea JM, Jayaram G, Ajagbe L, Celnik P. Modulation of cerebellar excitability by polarity-specific noninvasive direct current stimulation. J Neurosci. 2009;29:9115–9122. doi: 10.1523/JNEUROSCI.2184-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim H, Na DL. Normative data on the Korean version of the Boston Naming Test. J Clin Exp Neuropsychol. 1999;21:127–133. doi: 10.1076/jcen.21.1.127.942. [DOI] [PubMed] [Google Scholar]
- 24.Kim H, Na DL. Normative data on the Korean version of the Western Aphasia Battery. J Clin Exp Neuropsychol. 2004;26:1011–1020. doi: 10.1080/13803390490515397. [DOI] [PubMed] [Google Scholar]
- 25.Heiss WD, Kessler J, Thiel A, Ghaemi M, Karbe H. Differential capacity of left and right hemispheric areas for compensation of poststroke aphasia. Ann Neurol. 1999;45:430–438. doi: 10.1002/1531-8249(199904)45:4<430::aid-ana3>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 26.Heiss WD, Thiel A. A proposed regional hierarchy in recovery of post-stroke aphasia. Brain Lang. 2006;98:118–123. doi: 10.1016/j.bandl.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 27.Fridriksson J, Bonilha L, Baker JM, Moser D, Rorden C. Activity in preserved left hemisphere regions predicts anomia severity in aphasia. Cereb Cortex. 2010;20:1013–1019. doi: 10.1093/cercor/bhp160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Warburton E, Price CJ, Swinburn K, Wise RJ. Mechanisms of recovery from aphasia: evidence from positron emission tomography studies. J Neurol Neurosurg Psychiatry. 1999;66:155–161. doi: 10.1136/jnnp.66.2.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rosen HJ, Petersen SE, Linenweber MR, Snyder AZ, White DA, Chapman L, et al. Neural correlates of recovery from aphasia after damage to left inferior frontal cortex. Neurology. 2000;55:1883–1894. doi: 10.1212/wnl.55.12.1883. [DOI] [PubMed] [Google Scholar]
- 30.Saur D, Lange R, Baumgaertner A, Schraknepper V, Willmes K, Rijntjes M, et al. Dynamics of language reorganization after stroke. Brain. 2006;129:1371–1384. doi: 10.1093/brain/awl090. [DOI] [PubMed] [Google Scholar]
- 31.Floel A, Meinzer M, Kirstein R, Nijhof S, Deppe M, Knecht S, et al. Short-term anomia training and electrical brain stimulation. Stroke. 2011;42:2065–2067. doi: 10.1161/STROKEAHA.110.609032. [DOI] [PubMed] [Google Scholar]
- 32.Murase N, Duque J, Mazzocchio R, Cohen LG. Influence of interhemispheric interactions on motor function in chronic stroke. Ann Neurol. 2004;55:400–409. doi: 10.1002/ana.10848. [DOI] [PubMed] [Google Scholar]
- 33.Shimizu T, Hosaki A, Hino T, Sato M, Komori T, Hirai S, et al. Motor cortical disinhibition in the unaffected hemisphere after unilateral cortical stroke. Brain. 2002;125:1896–1907. doi: 10.1093/brain/awf183. [DOI] [PubMed] [Google Scholar]
- 34.Schlaug G, Renga V, Nair D. Transcranial direct current stimulation in stroke recovery. Arch Neurol. 2008;65:1571–1576. doi: 10.1001/archneur.65.12.1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gandiga PC, Hummel FC, Cohen LG. Transcranial DC stimulation (tDCS): a tool for double-blind sham-controlled clinical studies in brain stimulation. Clin Neurophysiol. 2006;117:845–850. doi: 10.1016/j.clinph.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 36.Cattaneo Z, Pisoni A, Papagno C. Transcranial direct current stimulation over Broca's region improves phonemic and semantic fluency in healthy individuals. Neuroscience. 2011;183:64–70. doi: 10.1016/j.neuroscience.2011.03.058. [DOI] [PubMed] [Google Scholar]