Abstract
INTRODUCTION
We experienced a case with long relapse-free survival after successful treatment of chemotherapy and surgery to advanced gastric cancer.
PRESENTATION OF CASE
A 56-year-old man was examined because of rapid weight loss and was diagnosed as having far-advanced gastric cancer with portal vein tumor thrombus (PVTT) and liver, lymph node and peritoneal metastases. Immediately after beginning chemotherapy, gastric obstruction due to gastric cancer was discovered. Therefore gastrojejunostomy, a bypass operation, was performed, and this was followed by the first course chemotherapy with S-1 and cisplatin. After 4 courses of this regimen were completed, PVTT and the peritoneal metastasis could no longer be confirmed, and new lesion had not appeared; therefore, the patient underwent a radical operation with distal gastrectomy, lymph node dissection and partial hepatectomy. After the operation, he received second-line chemotherapy with S-1 and paclitaxel for 1 year. He has been in good health without any signs of recurrence for 3 years and 8 months after the radical operation.
DISCUSSION AND CONCLUSION
Although complete recovery from far-advanced gastric cancer is rarely expected, this case demonstrates that long-term survival is achievable with carefully considered treatment plans.
Keywords: Gastric cancer, Portal vein tumor thrombus, Liver metastasis, Peritoneal dissemination, Gastrojejunostomy
1. Introduction
Gastric cancer with portal vein thrombosis is relative rare. However, this malignancy has a poor prognosis, and long-term survival is often not expected in cases with peritoneal metastases.
Herein, we describe the case of a patient who was clinically cancer free for a long period after chemotherapy. Prior to chemotherapy, the patient had undergone a bypass operation, and chemotherapy was followed by radical gastrectomy and partial hepatectomy.
2. Presentation of case
A 56-year-old man visited his family physician with complaints of dizziness and rapid weight loss. A palpable mass was detected in his upper abdomen, and severe anemia was confirmed by blood tests. Therefore, he was referred to the gastroenterological department of our hospital.
Upper gastrointestinal endoscopy revealed a large type 3 tumor in the gastric antrum, expanding to the duodenal bulb (Fig. 1a). Biopsy of the tumor resulted in a diagnosis of well-differentiated adenocarcinoma.
Fig. 1.
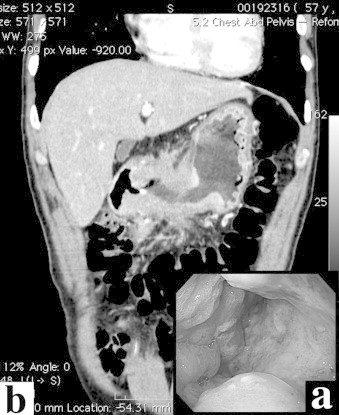
Gastric cancer before anticancer treatment. (a) Findings of upper gastrointestinal endoscopy. The antrum of the stomach is occupied by Bormann 3-type cancer. (b) Computed tomography of the coronal section shows remarkable thickening of the wall at the distal half of the stomach.
Computed tomography (CT) showed a thickening of the entire gastric wall from the antrum to the pylorus (Fig. 1b), with multiple lymph node metastases. In addition, a tumor thrombus progressing to the superior mesenteric vein through the gastroepiploic vein was observed (Fig. 2a). In addition, a liver tumor, 15 mm in size, was noted (Fig. 3). Peritoneal disseminated metastasis was also suspected because of the presence of ascites in the rectovesicular fossa and a rise in the concentration of omental adipose tissue (Fig. 4a).
Fig. 2.
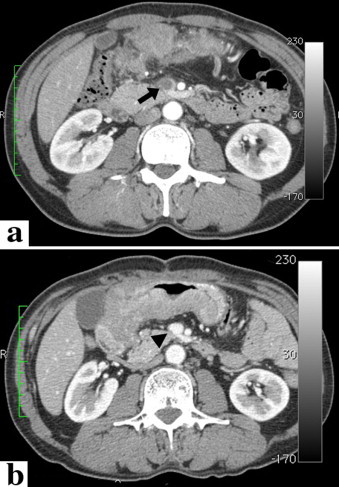
Change in the tumor thrombus in the portal vein after chemotherapy. (a) Computed tomography at the first visit to our hospital. The black arrow indicates the tumor thrombus in the superior mesenteric vein. (b) After 4 courses of chemotherapy, the tumor thrombus disappeared completely (the black arrow head).
Fig. 3.
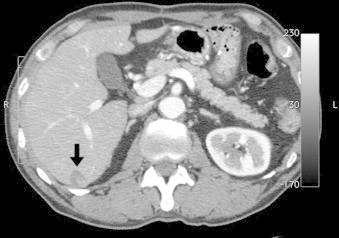
The solitary metastatic tumor of the liver (the black arrow) did not change, despite the administration of chemotherapy before the radical operation.
Fig. 4.
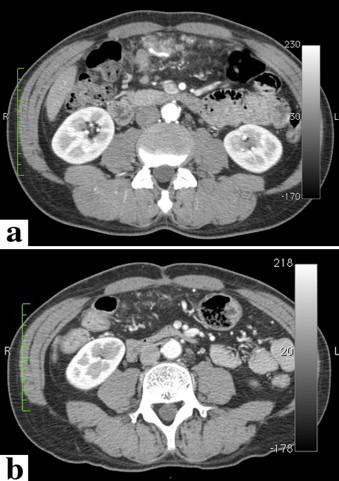
Change in the peritoneal disseminated metastases around the greater omentum. (a) Before chemotherapeutic treatment, many nodules of various sizes and an increase in the concentration of adipose tissue were observed. (b) After treatment, many nodules had almost disappeared.
Laboratory results confirmed severe anemia (red blood cell count, 3.27 × 106/mm3; hemoglobin level, 6.4 g/dl; hematocrit, 23%) and the carcinoembryonic antigen level was significantly high at 770 ng/dl. Liver and renal function test results were within normal limits, and no abnormal findings were observed.
On the basis of these findings, the patient was diagnosed as having far-advanced, unresectable gastric cancer. Therefore, we started a chemotherapy regimen of S-1 (120 mg/body) and cisplatin (60 mg/m2) 2 weeks after his first visit to our hospital.
During the first course of the chemotherapy, the patient complained of frequent nausea and vomiting. Abdominal radiography showed severe dilatation of the proximal stomach, confirming that stenosis of the stomach was the cause of his complaints. Five weeks after beginning chemotherapy, gastrojejunostomy (a bypass operation) was performed to improve and maintain nutritional status and facilitate continuation of anticancer chemotherapy. Chemotherapy was resumed 2 weeks after the operation, and therapy could be administered as scheduled.
After completing 4 courses of the chemotherapy regimen, planned CT examination showed that the tumor thrombus of the portal venous system (Fig. 2b) and disseminated peritoneal metastases had disappeared (Fig. 4b). In addition, the number of liver metastases had not increased and stable disease was achieved in the gastric lesions and metastatic lymph nodes. The radical resection was considered possible, and this was performed 6 months after the beginning of chemotherapy. Peritoneal disseminated metastases, confirmed during the first operation, were not confirmed macroscopically, and cancer cells were not detected in a cytological specimen from peritoneal lavage. On the basis of the above findings, we performed radical surgery, i.e., distal gastrectomy (Fig. 5) (Billroth II reconstruction) with regional lymph node dissection and partial hepatectomy. Five weeks after the operation, the second regimen of chemotherapy with S-1 (120 mg/body) and paclitaxel (50 mg/m2) was administered in 14 courses over a period of 1 year. The patient has been followed-up on a regular basis at our hospital, and there have been no signs of recurrence 4.5 years after radical surgery.
Fig. 5.
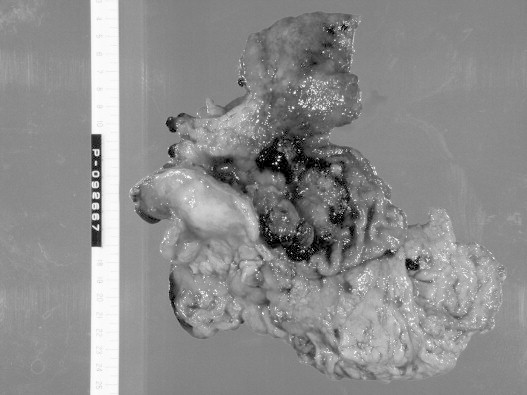
Macroscopic findings of the resected stomach. Type 3 cancer involving the entire circumference of the stomach was confirmed.
3. Discussion
Portal vein tumor thrombus (PVTT) originating from gastric cancer is a rare condition. According to the Annual Report of the pathological autopsy cases in Japan (vol. 40, 1998), the incidence of portal vein metastasis in gastric cancer was 1.2% (29/2330).1 Takayasu et al. also reported only 1 case of gastric cancer combined with PVTT in 3176 cases of gastric resection.2
The prognosis of gastric cancer patients who develop PVTT is poor. The median survival of gastric cancer patients with PVTT is 5.4 months, and the 5-year survival rate is less than 10%.3 Sugawara et al. analyzed the clinical data of 11 cases reported in the literature, with or without liver metastasis.4 Of 6 patients with liver metastasis, 5 died, and the longest survival time was 7 months. Five of the 11 patients did not have associated liver metastasis. Surgical resection was performed in 4 of these 5 patients: 3 patients were alive for 2 months, 14 months, and 24 months postoperatively. The remaining patient died 21 months after surgery. Although patients with PVTT but without liver metastasis seem likely to achieve long-term prognosis, the precise prognostic superiority of patients without liver metastasis is difficult to determine. PVTT is considered to be an early step in liver metastasis because metastasis is established when cancer cells released from the primary focus enter the portal bloodstream and are transported to the liver.
The standard therapeutic regimen for advanced unresectable gastric cancer in Japan is described in the Japanese gastric cancer treatment guidelines.5 Based on results from the SPIRIT trial6 and the JCOG 9912 trial,7 the combination of S-1 and cisplatin is recommended as the first-line regimen in the guideline. Although the complete response plus partial response rate is 54%, the median and progression-free survival times are only 13.0 months and 6.0 months, respectively. Therefore, the effectiveness of this regimen has not been satisfactory. Recently, the effectiveness of combination therapy with trastuzumab to treat human epidermal growth factor receptor type 2 (Her2)-positive advanced gastric cancer was reported in the ToGA trial,8 and this regimen of capecitabine, cisplatin, and trastuzumab is recommended in the Japanese guidelines.
Previous case studies have reported on the effectiveness of chemotherapy for PVTT originating from gastric cancer; however, the regimens used differ across studies and the basis for the use of these regimens has not been described.9–11 Accordingly, their use does not seem to be justified.
Peritoneal metastasis originating from gastric cancer is a disease with a very poor prognosis, and treatment is difficult in almost all cases. Many reports on the effectiveness of paclitaxel for peritoneal metastasis have recently been published, and combination treatment with S-1 was administered in almost all cases.12,13 Furthermore, the validity of intraperitoneal paclitaxel injection, in addition to systemic administration, was reported.14 The efficacy and tolerability of combined intravenous and intraperitoneal paclitaxel injection and oral administration of S-1 were also proved in another phase 2 study.15
As described in the Japanese guidelines,5 S-1 is a key drug for gastric cancer; however, since it is administered orally, it cannot be used in cases of gastrointestinal stenosis. In our case, in order to continue oral administration of S-1, gastrojejunostomy was performed as soon as possible upon appearance of gastric pyloric stenosis. Bypass operations, such as gastrojejunostomy,16,17 should be performed in order to not only continue optimal chemotherapy, but also to maintain a satisfactory nutritional status.
4. Conclusion
We encountered a case of successful, consecutive anticancer treatment for far-advanced gastric cancer with PVTT and liver and peritoneal metastases. Treatment included a gastrojejunal bypass operation, chemotherapy, and a radical operation, followed by adjuvant chemotherapy for 1 year. Although complete recovery from this malignancy is rarely expected, this case demonstrates that long-term survival is achievable with carefully considered treatment plans.
Conflict of interest statement
None.
Funding
None.
Ethical approval statement
Written informed consent was obtained from the patient for publication of this case report and case series and accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request.
Author contribution
All the contributions to the paper attribute to the first author, Takashi Orii.
Footnotes
This is an open-access article distributed under the terms of the Creative Commons Attribution-NonCommercial-No Derivative Works License, which permits non-commercial use, distribution, and reproduction in any medium, provided the original author and source are credited.
References
- 1.The Japanese Society of Pathology, editor. Vol. 40. Jinshikai; Aichi: 1998 (January–December). (Annual of the pathological autopsy cases in Japan). [in Japanese] [Google Scholar]
- 2.Takayasu K., Tajiri H., Noguchi M., Ishikawa T., Maruyama K. Imaging diagnosis of portal tumor thrombus secondary to gastric cancer. Gastrointest Radiol. 1989;14:161–163. doi: 10.1007/BF01889184. [DOI] [PubMed] [Google Scholar]
- 3.Bang W.E., Jun H.L., Jong S.L. Survival analysis of gastric cancer patients with tumor thrombus in the portal vein. J Surg Oncol. 2012;105:310–315. doi: 10.1002/jso.22083. [DOI] [PubMed] [Google Scholar]
- 4.Sugawara Y., Toshiro K., Hiraishi M., Ishizaki Y., Makuuchi M. Portal tumor thrombi due to gastric cancer. Hepatogastroenterology. 1996;43:1000–1005. [PubMed] [Google Scholar]
- 5.Japanese Gastric Cancer Association Japanese gastric cancer treatment guidelines 2010 (ver. 3) Gastric Cancer. 2011;14:113–123. doi: 10.1007/s10120-011-0042-4. [DOI] [PubMed] [Google Scholar]
- 6.Koizumi W., Narahara H., Hara T. Randomized phase III study of S-1 alone versus S-1 cisplatin in the treatment for advanced gastric cancer (The SPIRITS trial): a phase III trial. Lancet Oncol. 2008;9:215–221. doi: 10.1016/S1470-2045(08)70035-4. [DOI] [PubMed] [Google Scholar]
- 7.Boku N., Yamamoto S., Shirao K. Fluorouracil versus combination of irinotecan plus cisplatin versus S-1 in metastatic gastric cancer: a randomized phase 3 study. Lancet Oncol. 2009;10:1063–1069. doi: 10.1016/S1470-2045(09)70259-1. [DOI] [PubMed] [Google Scholar]
- 8.Bang Y.J., van Cutsem E., Feyereislova A., ToGA Trial Investigators Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet. 2010;376:687–697. doi: 10.1016/S0140-6736(10)61121-X. [DOI] [PubMed] [Google Scholar]
- 9.Asami Y., Miyanaga T., Ito H. A case of advanced gastric cancer with tumor embolus in the portal vein successfully treated with S-1 and CDDP therapy. Gan To Kagaku Ryoho. 2011;38:1175–1178. [PubMed] [Google Scholar]
- 10.Hoshimoto S., Hagiuda J., Igarashi N. A case of advanced gastric cancer with a tumor embolus in the portal vein successfully treated with TS-1 and CDDP. Gan To Kagaku Ryoho. 2004;31:1079–1081. [PubMed] [Google Scholar]
- 11.Yoshida Y., Egami I., Onda M. A case of liver metastasis of gastric cancer with portal vein tumor thrombosis responding to chemotherapy with 5-FU and epirubicin. Gan To Kagaku Ryoho. 1955;22:1245–1248. [PubMed] [Google Scholar]
- 12.Aizaki K., Kohno S., Sasaki K. Response to S-1+ paclitaxel in far-advanced gastric cancer. Gan To Kagaku Ryoho. 2009;36:1877–1880. [PubMed] [Google Scholar]
- 13.Okumura K., Tani S., Shiogai Y., Kodama M., Mekata E., Tan T. Two cases of advanced gastric cancer with peritonitis carcinomatosa that showed disappearance of ascites and obtained a good quality of life by using DIF and paclitaxel. Gan To Kagaku Ryoho. 2012;39:667–670. [PubMed] [Google Scholar]
- 14.Tamura S., Miki H., Nakata K. Intraperitoneal administration of paclitaxel and oral S-1 for a patient with peritoneal dissemination and hydronephrosis due to advanced gastric cancer. Gastric Cancer. 2007;10:251–255. doi: 10.1007/s10120-007-0431-x. [DOI] [PubMed] [Google Scholar]
- 15.Ishigami H., Kitayama J., Kaisaki S. Phase II study of weekly intravenous and intraperitoneal paclitaxel combined with S-1 for advanced gastric cancer with peritoneal metastasis. Ann Oncol. 2010;21:67–70. doi: 10.1093/annonc/mdp260. [DOI] [PubMed] [Google Scholar]
- 16.Mimatsu K., Oida T., Kuboi Y. A long-surviving patient with unresectable advanced gastric cancer responding to S-1 after receiving improved gastrojejunostomy. Int J Clin Oncol. 2004;9:193–196. doi: 10.1007/s10147-004-0387-3. [DOI] [PubMed] [Google Scholar]
- 17.Matsushima Y., Kawabata R., Imamura H. Gastrojejunostomy followed by chemotherapy with S-1 in unresectable gastric cancer with pyloric stenosis. Gan To Kagaku Ryoho. 2012;39:911–914. [PubMed] [Google Scholar]


