Abstract
Recent advances in the management of prostate cancer have shown considerable development with time and many novel therapeutic agents have been approved over the past years. For patients with metastatic castration-resistant prostate cancer (mCRPC), initially docetaxel was the standard chemotherapy but once they became refractory to docetaxel, no treatment improved survival. This scenario changed in June 2010 when the US Food and Drug Administration (FDA) approved Cabazitaxel as a new therapeutic option for patients with mCRPC resistant to docetaxel. Cabazitaxel, being a novel tubulin-binding taxane with poor affinity for P-glycoprotein, decreases the chances of resistance. It has shown antitumor activity in preclinical, phase I, II and III clinical studies in docetaxel-resistant tumors. This article summarises the background, pharmacodynamic, kinetics and clinical development of cabazitaxel for the treatment of castration-resistant prostate cancer. Future development and rational use of this drug in other tumors is under therapeutic investigation.
Keywords: Cabazitaxel, castration-resistant prostate cancer, tropic trial
INTRODUCTION
Prostate cancer has become a major public health problem in developed countries in the recent decade. Prostate cancer develops mainly in elderly males more than 50 years. It is the ninth most-common cancer in the world, but wide variations are seen globally. In India, the incidence of prostate cancer was less but is increasing gradually now due to increase in life expectancy, decreasing in mortality of older age group, and absence of regular screening procedures. Most of the patients are diagnosed in late stages making it resistant to treatment, worsening the prognosis, and decreasing the survival rate.
Several factors are associated with increased risk for prostate cancer. Genetics, increasing age, and environmental and geographical factors play a major role. But, dietary factors such as high consumption of fats – fatty acids, alpha linolenic acid found in red meat, etc., deficiency of trace element like selenium and low levels of vitamin D and E have also been implicated in increased risk of development of prostate cancer in some individuals.
Histologically, most of the prostate cancers are adenocarcinomas, the other types are rarely found. Treatment options available for men with localized prostate cancer are conservative management strategies of “watchful waiting” and “active surveillance” to reduce the risk of over-treatment in this group of patients. But, if still the cancer progresses, they will be candidates for hormonal therapy.
The surgical treatment of prostate cancer includes radical prostatectomy along with bilateral pelvic lymphadenectomy.[1] Neo-adjuvant hormonal therapy may be given before definitive local curative treatment to decrease the size of the prostate.
According to the National Institutes of Health guidelines, external beam radiation therapy offers the same long-term survival results as surgery. Patients with low-risk prostate cancer are suitable candidates for low-dose transperineal brachytherapy which is a safe and effective technique.[2]
Besides the above mentioned treatments, several alternative therapeutic options such as cryosurgical ablation of the prostate and high-intensity focused ultrasound have emerged recently as minimally invasive procedures, with the same therapeutic efficacy and low morbidity as compared to established surgical and non-surgical procedures.[3]
Castration is another method to decrease the testicular source of androgens. Surgical castration is considered the “gold standard” method for androgen deprivation which includes bilateral orchiectomy. Medical castration can be achieved by anti-androgen drugs. These include steroidal and non-steroidal anti-androgens which compete with testosterone and di-hydro testosterone at the receptor level in the prostate cell, thus promoting apoptosis and inhibiting the growth of prostate carcinoma.[4] Steroidal anti-androgens includes cyproterone acetate, megestrol acetate, and medroxyprogesterone acetate. Non-steroidal anti-androgens nilutamide, flutamide, and bicalutamide have shown improved quality of life and compliance as compared to surgical castration. Long-acting LHRH (Luteinising hormone-releasing hormone) agonists – busereline, gosereline, leuproreline, triptoreline – have also been used in advanced prostate carcinoma. The US FDA has recently approved the clinical use of LHRH antagonists abarelix and degarelix in metastatic and symptomatic prostate carcinoma, for which no other treatment option is available.[5] Oestrogens, Diesthylstilboesterol can also be used in prostate cancer. They act by downregulating LHRH secretion, androgen inactivation and directly suppressing leydig cell function. Drugs like 5-alpha-reductase inhibitors finasteride and dutasteride have also shown some promise.
For metastatic prostate cancer that has spread beyond the prostate, chemotherapy is started. Earlier, oral chemotherapeutic drug temozolomide was used, but now novel therapeutic agents are available.
Most hormone-dependent cancers become resistant after one to three years and continue to grow despite hormonal therapy. These were previously known as “hormone-refractory prostate cancer” or “androgen-independent prostate cancer,” but now the term “castration-resistant” has replaced “hormone refractory” because they are no longer responsive to even castration treatment. Before 2004, all treatments for castration-resistant prostate cancer (CRPC) were palliative and no treatment prolonged survival rate in such patients. However, now there are several options available for treating CRPC that improve survival.
The chemotherapeutic drug docetaxel was approved by FDA in 2004 as treatment for metastatic CRPC with a median survival benefit of 2 to 3 months.[6] A combination of docetaxel, bevacizumab, thalidomide, and prednisone has also proved to be effective in the treatment of CRPC.[7]
But, some patients who received docetaxel-based chemotherapy for CRPC still showed progression of the cancer due to development of resistance; therefore, a novel taxane cabazitaxel was developed as a second-line chemotherapy treatment which was approved by the FDA in 2010.[8]
Furthermore, in 2010, the FDA also approved the first prostate cancer vaccine. This vaccine, sipuleucel-T (Provenge®, manufactured by Dendreon), is effective in the treatment of CRPC with a median survival benefit of 4.1 months.[9] Another treatment emerged in the same year, as a second line hormonal therapy abiraterone (Zytiga) passed a phase III trial for CRPC patients who had failed chemotherapy. Results were positive with overall survival increased to 4.6 months when compared to placebo and was thus approved by US FDA to be used in combination with prednisone to treat patients with metastatic castration-resistant prostate cancer who have received previous docetaxel chemotherapy.[10]
CHEMICAL STRUCTURE
Taxanes are extracted from the bark of western yew tree. Cabazitaxel (previously known as XRP-6258) is a novel semi-synthetic antineoplastic agent belonging to the taxane class extracted from the needles of various species of yew trees (Taxus species). It is a 7,10 dimethyloxy derivative of docetaxel.[11]
The chemical name of cabazitaxel is (2α,5β;,7β;,10β;,13α)-4-acetoxy-13-({(2R,3S)-3[(tertbutoxycarbonyl) amino]- 2-hydroxy-3-phenylpropanoyl} oxy)-1-hydroxy-7,10-dimethoxy-9oxo-5,20- epoxytax-11-en-2-yl benzoate-propan-2-one (1:1). The structural formula is given in Figure 1.
Figure 1.
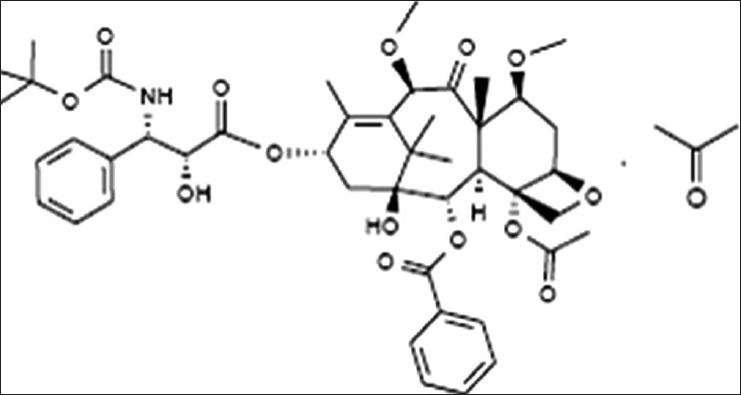
The structural formula of cabazitaxel
PHYSICAL PROPERTIES
It is a white to off-white powder with a molecular formula of C45H57NO14•3H6O and a molecular weight of 835.93 g/mol (for the solvent-free compound). Its melting point is 157°C. It is lipophilic, practically insoluble in water, and soluble in alcohol. It is incompatible to direct light and heat.[12]
MECHANISM OF ACTION
The first taxane developed was paclitaxel in 1970s but was approved by FDA only in 1992 for treatment of refractory ovarian cancer. Docetaxel is a semisynthetic and more potent congener of paclitaxel used in refractory ovarian and breast cancer.
These taxanes exhibit a unique pharmacological action by binding to different sites on intracellular β-tubulin subunit of microtubule and promote the assembly of tubulin into microtubules. These microtubule bundles impair the natural dynamics of microtubules and appear in the mitotic phase of the cell cycle leading to mitotic block and apoptosis of the cancer cell.
Resistance to taxanes is associated mainly with increased expression of multidrug resistance (MDR) 1 gene that encodes P-glycoprotein, an ATP-dependent drug efflux pump which decreases the intracellular concentration of these drugs. Cabazitaxel was superior to paclitaxel and docetaxel because of its poor affinity to P-glycoprotein due to presence of the extra methyl groups. This enables it to be effective in docetaxel-resistant tumors.[13]
The extra methyl groups also impart cabazitaxel with a unique ability to cross blood-brain barrier, the clinical advantages of which are yet to be explored.
PHARMACOKINETICS
Peak drug concentration following an i.v. dose given every 3 weeks is achieved at the end of the 1-hour drug infusion (Cmax). A triphasic pharmacokinetic model is used to determine drug half-life. Rapid initial phase (α phase) lasts 4 minutes; Intermediate phase (β phase) is of 2 hours, and prolonged terminal phase (γ phase) lasts 95 hours.
Plasma proteins binding is 89 to 92%, mainly to serum albumin and lipoproteins but is equally distributed between plasma and blood.
The drug is extensively metabolized in the liver via the CYP3A4/5 isoenzymes and to a lesser extent by CYP2C8 (10%-20%). No formal studies have been done for evaluating drug-drug interactions with CYP3A4 inhibitors or inducers; hence, concomitant administration should be avoided. But, this field requires further investigations and trials.
Excretion is mainly by enterohepatic circulation (76%) and in urine (3.7%) as unchanged drug or metabolites.[14]
Special populations
Patients with mild to moderate renal impairment do not require dose alteration but should be used cautiously in patients with hepatic impairment as its concentration increases in such patients. Cabazitaxel is reported to be teratogenic as it belongs to category D and is recommended to be discontinued during lactation. Safety and efficacy is not established in pediatric patients. In geriatric population, there are no overall differences in efficacy or pharmacokinetics in individuals ≥65 years of age compared with younger adults; however, such patients had higher incidence of febrile neutropenias and other toxicities.
ADVERSE EFFECTS
The main dose-limiting toxicity is fatal febrile neutropenia. It is contraindicated in patients with neutrophil counts ≤ 1500/mm3. Complete blood counts should be monitored weekly during the first cycle of therapy and prior to each treatment cycle thereafter. If febrile neutropenia occurs, immediate treatment should be started with G-CSF (filgrastim, pegfilgrastim). If it is still not corrected, cabazitaxel therapy must be interrupted until resolution occurs and neutrophil count is >1500/mm3 and upon resumption of therapy, dosage be reduced to 20 mg/m2 every 3 weeks.[12]
Severe hypersensitivity reactions like hypotension, bronchospasm, and generalized rash/erythema has also been reported. If these reactions occur, infusion should be immediately discontinued and appropriate supportive treatment should be administered. Hence, it is also contraindicated in patients with known hypersensitivity reactions to cabazitaxel and other taxanes.
Other toxicities include mild to moderate nausea and vomiting, severe diarrhea, neurotoxicity fatigue, and aloplecia. Grade 1 neurotoxicity was observed including paresthesias, diminished deep tendon reflexes, and impaired vibratory sensations. Renal failure has been reported generally in association with sepsis, dehydration, or obstructive uropathy. It is also embryotoxic and may cause fetal harm.
Special precautions for use of cabazitaxel
Adequate patient evaluation and monitoring is required; hence, it should be administered under the supervision of a qualified clinician.[15] Primary prophylaxis with G-CSF is essential in the following high-risk groups to reduce the risks of neutropenic complications:
Age > 65 years
Extensive prior radiation
Poor nutrition
Previous febrile neutropenia
Poor performance status
Other serious medical comorbidities.
Diarrheal deaths have been reported with cabazitaxel due to dehydration and electrolyte imbalances. Hydration, antiemetics, and antidiarrheals should be used for symptomatic treatment but for grade >3 diarrheas, dose reduction should be considered.
Severe hypersensitivity reactions have been reported with cabazitaxel; thus, premedication with H2-antagonists and corticosteroids is recommended.
Cabazitaxel should not be used in patient suffering from hepatic impairment.
PRECLINICAL DATA
The preclinical data of cabazitaxel suggest the ability to overcome first-generation taxane resistance. The first in vitro assessment of cabazitaxel was reported by Bissery et al. Four cell lines were assessed, including P388 (lymphoblastic leukemia), HL60 (promyelocytic leukemia), KB (cervical adenocarcinoma), and Calc18 (breast carcinoma), all showing antitumor activity at nanomolar concentrations.[16]
In subsequent in vivo models-colon C38 and pancreas P03 murine tumor xenografts, cabazitaxel showed nearly complete tumor regression. The activity of the agent was clearly present in cell lines resistant to standard cytotoxic agents, including anthracyclines, vinca alkaloids, and the older taxanes docetaxel and paclitaxel, probably due to lower affinity for the P-glycoprotein efflux pump.
Furthermore, the activity of cabazitaxel was documented in human tumor xenografts. In three human colorectal cell lines (HCT-116, HCT-8, and HT-29), high antitumor activity was observed.[17]
Long-term tumor-free survival and complete tumor regression were observed in pancreatic xenografts (MIA PaCa-2), head and neck xenografts (SR475), and prostate xenografts (DU145).[18]
CLINICAL TRIALS
Phase I
A phase I clinical trial of cabazitaxel was conducted by Mita et al. in 25 patients with advanced solid tumors to evaluate the pharmacokinetics and safety at increasing doses starting at 10 mg/m2 intravenously every 3 weeks. Twenty-five patients with advanced solid tumor refractory to conventional treatments were enrolled, eight of whom had CRPC. These 25 patients were treated with 102 courses of 3-weekly cabazitaxel at 4 dose levels, ranging from 10 to 25 mg/m2. Pharmacokinetic parameters were dose proportional and half life of cabazitaxel showed a triphasic model.
The main dose-limiting toxicity observed at the dose of 25 mg/m2 every 3 weeks was neutropenia. Common non-hematological toxicities were mild in nature and included low-grade diarrhea (52%), nausea (40%), and vomiting (16%). Grade 1 neurosensory symptoms were also common and manifested as sacral paresthesias, diminished deep tendon reflexes, and impaired vibration and position sensations. Two patients experienced grade 1 hypersensitivity reactions.
In this phase I trial, primary evidence of anticancer activity was observed. Three patients achieved partial responses including two patients with CRPC, one of whom had previously received docetaxel, but 12 patients had stable disease for more than 4 months. Cabazitaxel was well tolerated at dose levels of 25 mg/m2, but due to incidence of grade 4 neutropenia, the recommended phase II dose was 20 mg/m2.[14]
Phase II
No phase II study was conducted in patients with metastatic prostate cancer but was done in breast cancer patients to evaluate the dose of cabazitaxel for phase III trial. Pivot et al. conducted phase II trials in 71 patients with metastatic breast cancer and administered i.v. cabazitaxel 20 mg/m2 every 3 weeks.
After follow up of 20 months, the median survival was 12.3 months and median time to progression was 2.7 months. Objective response rate was 14%, with eight partial and two complete responses seen. Eighteen patients (30%) had stable disease for at least 3 months. Seventy-three percent of patients experienced neutropenia, 55% experienced leucopenia, 35% fatigue, 32% nausea, 30% diarrhea, 18% vomiting, 17% sensory neuropathy, and 6% experienced hypersensitivity reactions.[19]
Phase III
The TROPIC (Treatment of hormone refractory metastatic prostate cancer previously treated with docetaxel containing regimen) trial established the efficacy and safety of cabazitaxel in metastatic castration-resistant prostate cancer. de Bono et al. conducted an open-label randomized phase III trial in men with metastatic castration-resistant prostate cancer who had received previous hormone therapy with docetaxel but whose disease had still progressed during or after treatment. A total of 755 patients were allocated to treatment groups (377 mitoxantrone, 378 cabazitaxel). These men were treated with 10 mg oral prednisone daily, and were randomly assigned to receive either 12 mg/m2 mitoxantrone intravenously or 25 mg/m2 cabazitaxel intravenously every 3 weeks. A maximum of 10 cycles were allowed, due to the risk of mitoxantrone-induced cardiotoxicity.
The primary endpoint was overall survival which was 15.1 months in the cabazitaxel group and 12.7 months in the mitoxantrone group. Secondary endpoints included progression-free survival and safety. Median progression-free survival was 2.8 months in the cabazitaxel arm and 1.4 months in the mitoxantrone arm. There was also significant improvement in tumor response, time to tumor progression, PSA response rate, and median time to PSA progression in cabazitaxel arm. However, pain control and time to pain progression were similar among the two treatment arms.
Similar to the phase I and II trials, the most common toxicity associated with cabazitaxel therapy was neutropenia. Grade 3 neutropenia occurred in 82% of cabazitaxel patients, commonest being febrile neutropenia. The non-hematologic toxicities observed in the cabazitaxel arm included diarrhea, fatigue, asthenia, and peripheral neuropathy. Thus, it was concluded that treatment with cabazitaxel plus prednisone is clinically efficacious and improves overall survival in patients with metastatic castration-resistant prostate cancer whose disease has progressed during or after docetaxel therapy.[8]
On the basis of all the above mentioned trials, the US FDA approved cabazitaxel in June 2010 for metastatic castration-resistant prostate cancer whose disease progresses even after docetaxel treatment.
EFFECT OF CABAZITAXEL ON PSA
In patients treated with cabazitaxel, after 3 months of therapy, the PSA reduction rate > 50% was 46.2%, the PSA progression rate was 15.4%, and the disease control rate was 83.3%.[20] In the Phase III TROPIC trial also, the PSA response rate was 39.2% (P = 0.002) and median time to PSA progression was 6.1 months (P = 0.001) with cabazitaxel.[8]
MECHANISM OF TAXANE RESISTANCE AND STRATEGIES TO OVERCOME
Multiple mechanisms of taxane resistance have been observed. Cancer cells which have a MDR1 gene may increase efflux of the drug through a P-glycoprotein pump, thereby minimizing the intracellular concentration of taxanes.[13] Modulation of microtubule characteristics for example increased dynamic activity of the microtubules after drug treatment may alter responsiveness to taxanes.[21] Also, mutations leading to alterations in the microtubule-binding site of taxanes or in microtubule-associated proteins can also decrease the efficacy of taxanes.[22] One of the important mechanisms of resistance is overexpression of the βIII isoforms of tubulin, as taxanes bind to tubulin and prevent the assembly of microtubules. Thus, βIII tubulin over-expression showed decreased efficacy of docetaxel and also resistance to castration.[23] Androgen receptor signaling cascades are associated with prostate cancer cellular proliferation and decreased apoptosis. Alterations in these androgen-signaling pathways may lead to deranged interactions with the tumor microenvironment, via mediators such as VEGF (Vascular endothelial growth factor) and transforming growth factor-β. This can lead to chemotherapy resistance in patients being treated with taxanes.[24]
Though taxanes were the only chemotherapeutic agents that showed a survival advantage in prostate cancer, a high level of taxane resistance decreased its efficacy. Thus, various strategies were needed to overcome this resistance.
One major approach can be intermittent treatment or “drug holidays” with a taxane, which has two advantages. First, with less constant exposure to the drug, there may be a delay in the development of taxane-resistant disease. Second, this “breaks in therapy” may clinically improve the quality of life for the patients and allows them to recover from the cumulative toxicity of chemotherapeutic drugs which may also allow taxane therapy to be prolonged thereby improving the outcome.[25] Several trials have demonstrated that after the initial chemotherapy has stabilized the tumor growth, intermittent treatment holidays would give patients an opportunity to recover from toxicity, increasing the clinical benefit and ultimately prolonging survival.[26]
The other approach is administering chemotherapy combinations which offer synergistic clinical benefit and also helps in overcoming drug resistance. One such combination in prostate cancer could be taxanes with platinum-based chemotherapies, such as carboplatin which has DNA-alkylating properties. Two studies have shown moderate clinical benefits when carboplatin was added to docetaxel in patients whose disease progressed on docetaxel alone.[27] Angiogenesis inhibition can also be a potential target in prostate cancer. A phase II trial was conducted with 2 antiangiogenic agents, bevacizumab and thalidomide, with docetaxel in flexible dosing schedules including drug holidays. The results of this study showed major improvement in PSA responses (90%) and prolonged median overall survival (28.2 months).[7] Recent studies demonstrated the role of androgen receptor targets in castration-resistant prostate cancer. Abiraterone which targets the CYP17 enzyme, decreasing the testosterone production showed a survival advantage in patients who had progressive disease on docetaxel.[28] Similarly, MDV3100, an androgen receptor antagonist, has also shown to be more clinically beneficial than treatment with a taxane alone.[29] A phase I study combined targeted radiation 153Sm-EDTMP (153Sm ethylenediamine tetramethylene phosphonate) with docetaxel in patient with osteoblastic bony metastases also showed promising results.[30] Furthermore, a combination study of radium-223 and docetaxel is currently ongoing.
USES
The current approved indication of cabazitaxel is only in prostate cancer. It is used in combination with prednisone for the treatment of hormone-refractory metastatic prostate cancer in patients whose disease has progressed following prior treatment with docetaxel-based therapy. This regimen improved overall survival compared with mitoxantrone and prednisone. The dose administered is 25 mg/m2 every 3 weeks in combination with oral prednisone 10 mg daily. Primary and secondary prophylaxis should be done with G-CSF, antihistaminics, corticosteroids, and antiemetics.
FUTURE PROSPECTS
Cabazitaxel is one of the drugs approved for metastatic CRPC. Other drugs approved are Abiraterone, a CYP17 inhibitor and Sipuleucel-T, the therapeutic immunotherapy, both offering survival benefits over conventional chemotherapy.
Abiraterone is a selective oral inhibitor of androgen biosynthesis by inhibiting cytochrome P450 17 (17α-hydroxylase-17,20-lyase), the key enzyme in androgen biosynthesis. By targeting CYP17A, abiraterone decreases androgen production in the adrenal glands, prostate, and tumor tissues. On the basis of preclinical, phase I and phase II clinical trials, a phase III trial was planned in which abiraterone (1000 mg/day) was compared to placebo, both combined with prednisone (5 mg twice daily), in 1 196 patients with docetaxel-refractory metastatic CRPC. There was a 35% reduction in the risk of death and a median overall survival of 14.8 months with abiraterone vs 10.9 months with placebo. Secondary endpoints (PSA response) also favored the abiraterone group, and the toxicities of this drug were mainly hypokalemia and fluid retention. Based on the results of this study, the FDA approved abiraterone in combination with prednisone as a treatment for docetaxel-refractory metastatic CPRC in April 2011.
Sipuleucel-T is a personalized immunotherapy developed for use in mCRPC. This therapy requires isolation of peripheral blood mononuclear cells from patients by leukopheresis, culturing them with a fusion protein of prostatic acid phosphatase and GM-CSF and reinfusion of antigen-presenting cells back into the patient. This process is repeated every two weeks, three times. The initial two small randomized trials showed that sipuleucel-T produced a survival benefit in 225 patients with mCRPC and also had an acceptable toxicity profile consisting mostly of chills, fever, and headache. Thus, a larger phase III study, the IMPACT trial, was subsequently conducted. The median survival in this trial was 25.8 months in sipuleucel-T arm compared with 21.7 months in the placebo arm leading to its FDA approval for the treatment in asymptomatic or minimally symptomatic mCRPC.[9]
These three drugs were approved as salvage chemotherapy in mCRPC showing no response to docetaxel-based therapy.
The toxicities of cabazitaxel at the dose of 25 mg/m2 are one of the concerning issues, mainly febrile neutropenias and neutropenic deaths. Several trials are required by reducing the dose to 20 mg/m2 and comparing it with 25 mg/m2 in metastatic CRPC and calculating the risk benefit ratio. Trials of cabazitaxel addressing the cardiac complications should also be undertaken as several cardiac-related deaths were reported in previous studies.
Various studies concerning drug interactions with CYP 3A4 inhibitors and inducers and pharmacokinetic and safety profiles in patients with hepatic impairment, in pregnancy, lactation, and in pediatric population are required.
Though cabazitaxel has been approved for metastatic CRPC, it still requires stringent phase IV post-marketing surveillances for efficacy and safety before being approved for other tumors.
The financial constraints of cabazitaxel are much more taxing than docetaxel, costing more than double the amount per cycle, because it is still in the development stage and only approved as second line drug after failure with docetaxel in metastatic CRPC. Presently, it is being marketed only by Sanofi-Aventis under brand name Jevtana®. Its market could rise further if it is approved as first line drug in metastatic CRPC or approved in other solid tumors.
At present, there are various ongoing trials of cabazitaxel in combination with other drugs as well as in different population of patients. The important ones are[15] Phase I/II trial of cabazitaxel with cisplatin (NCT00925743) and with gemcitiabine (NCT01001221). Phase I trial of cabazitaxel in patients with varying degrees of hepatic impairment (NCT01140607) and to determine the potential effect on QTcF interval of cabazitaxel in patients with advanced solid tumors (NCT01087021).
Furthermore, investigators are planning more trials combining cabazitaxel with abiraterone and other new investigational agents.
Moreover, no trials have been conducted in India for evaluating the efficacy and safety in Indian population. These areas need further consideration before it can be approved in wider population and for tumors other than prostate cancer.
Alpharadin is another investigational agent which is still not approved for marketing by any health authorities but has completed a successful phase III trial for CRPC patients with bone metastasis and showed improved survival and quality of life. There are also several treatments currently in the pipeline to treat CRPC. These include the 2nd generation hormonal therapies, MDV3100 and Orteronel (TAK-700), the immunotherapy Prostvac, the Clusterin protein inhibitor OGX-011, and the bone metastasis-targeting Cabozantinib (XL-184). Tasquinimod had shown good results in a phase II trial.
But, many highly promising drug candidates have also failed in large-scale clinical trials; Zibotentan, an endothelin-A receptor antagonist, Sunitinib, an antiangiogenic TKI, Ixabepilone, and Satraplatin.
CONCLUSION
Cabazitaxel was approved by the FDA in June 2010 for metastatic castration-resistant prostate cancer whose disease progresses during or after docetaxel treatment. The various phase I and II studies and the TROPIC trial showed that cabazitaxel provided a survival benefit of 2.4 months over mitoxantrone in these patients. However, again this drug has its own toxicities, febrile neutropenia being the major one, hence making its use cautious and judicious. Careful dose selection, proper combination with other therapies, and primary and secondary prophylaxis with G-CSF, antihistaminics, and corticosteroids can further enhance survival and quality of life in metastatic CRPC patients. Its utilization and success in other cancers requires further exploration.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Bianco FJ, Jr, Scardino PT, Eastham JA. Radical prostatectomy: Long-term cancer control and recovery of sexual and urinary function (“trifecta”) Urology. 2005;66(5 Suppl):83–94. doi: 10.1016/j.urology.2005.06.116. [DOI] [PubMed] [Google Scholar]
- 2.Salembier C, Lavagnini P, Nickers P, Mangili P, Rijnders A, Polo A, et al. Tumour and target volumes in permanent prostate brachytherapy: A supplement to the ESTRO/EAU/EORTC recommendations on prostate brachytherapy. Radiother Oncol. 2007;83:3–10. doi: 10.1016/j.radonc.2007.01.014. [DOI] [PubMed] [Google Scholar]
- 3.Beerlage HP, Thüroff S, Madersbacher S, Zlotta AR, Aus G, de Reijke TM, et al. Current status of minimally invasive treatment options for localized prostate carcinoma. Eur Urol. 2000;37:2–13. doi: 10.1159/000020091. [DOI] [PubMed] [Google Scholar]
- 4.Anderson J. The role of antiandrogenmonotherapy in the treatment of prostate cancer. BJU Int. 2003;91:455–61. doi: 10.1046/j.1464-410x.2003.04026.x. [DOI] [PubMed] [Google Scholar]
- 5.Trachtenberg J, Gittleman M, Steidle C, Barzell W, Friedel W, Pessis D, et al. A phase 3, multicentre, open label, randomized study of abarelix versus leuprolide plus daily antiandrogen in men with prostate cancer. J Urol. 2002;167:1670–4. doi: 10.1097/00005392-200204000-00021. [DOI] [PubMed] [Google Scholar]
- 6.Schurko B, Oh WK. Docetaxel chemotherapy remains the standard of care in castration-resistant prostate cancer. Nat Clin Pract Oncol. 2008;5:506–7. doi: 10.1038/ncponc1201. [DOI] [PubMed] [Google Scholar]
- 7.Ning YM, Gulley JL, Arlen PM, Woo S, Steinberg SM, Wright JJ, et al. Phase II trial of bevacizumab, thalidomide, docetaxel, and prednisone in patients with metastatic castration-resistant prostate cancer. J Clin Oncol. 2010;28:2070–6. doi: 10.1200/JCO.2009.25.4524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Bono JS, Oudard S, Ozguroglu M, Hansen S, Machiels JP, Kocak I, et al. Prednisone plus cabazitaxel or mitoxantrone for metastatic castration-resistant prostate cancer progressing after docetaxel treatment: Arandomised open-label trial. Lancet. 2010;376:1147–54. doi: 10.1016/S0140-6736(10)61389-X. [DOI] [PubMed] [Google Scholar]
- 9.Kantoff PW, Higano CS, Shore ND, Berger ER, Small EJ, Penson DF, et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. NEngl J Med. 2010;363:411–22. doi: 10.1056/NEJMoa1001294. [DOI] [PubMed] [Google Scholar]
- 10.Ang JE, Olmos D, Bono JS. CYP17 blockade by abiraterone: Further evidence for frequent continued hormone-dependence in castration-resistant prostate cancer. Br J Cancer. 2009;10:671–5. doi: 10.1038/sj.bjc.6604904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bouchet BP, Galmarini CM. Cabazitaxel. A new taxane with favorable properties. Drugs Today (Barc) 2010;46:735–42. doi: 10.1358/dot.2010.46.10.1519019. [DOI] [PubMed] [Google Scholar]
- 12.Bridgewater, NJ: Sanofi-aventis US; 2010. Jun, Jevtana (cabazitaxel) injection prescribing information. [Google Scholar]
- 13.Kartner N, Riordan JR, Ling V. Cell surface P-glycoprotein associated with multidrug resistance in mammalian cell lines. Science. 1983;221:1285–8. doi: 10.1126/science.6137059. [DOI] [PubMed] [Google Scholar]
- 14.Mita AC, Denis LJ, Rowinsky EK, Debono JS, Goetz AD, Ochoa L, et al. Phase I and pharmacokinetic study of XRP6258 (RPR 116258A), a novel taxane, administered as a 1-hour infusion every 3 weeks in patients with advanced solid tumors. Clin Cancer Res. 2009;15:723–30. doi: 10.1158/1078-0432.CCR-08-0596. [DOI] [PubMed] [Google Scholar]
- 15.Pal SK, Twardowski P, Sartor O. Critical appraisal of cabazitaxel in the management of advanced prostate cancer. Clin Interv Aging. 2010;5:395–402. doi: 10.2147/CIA.S14570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bissery MC, Bouchard H, Riou J, Vrignaud P, Combeau C, Bourzat JD. Preclinical evaluation of TXD258, a new taxoid. Proceedings of the American Association for Cancer Research. Cancer Research, Incorporated, and American Association for Cancer Research, Incorporated 2000. 2000;41 Abstract 1364. [Google Scholar]
- 17.Vrignaud P, Lejeune P, Chaplin D, Lavelle F, Bissery MC. In vivo efficacy of TXD258, a new taxoid, against human tumor xenografts Proceedings of the American Association for Cancer Research. Cancer Research, Incorporated, and American Association for Cancer Research, Incorporated, 2000. 2000;41 Abstract 1365. [Google Scholar]
- 18.Stone KR, Mickey DD, Wunderli H, Mickey GH, Paulson DF. Isolation of a human prostate carcinoma cell line (DU 145) Int J Cancer. 1978;21:274–81. doi: 10.1002/ijc.2910210305. [DOI] [PubMed] [Google Scholar]
- 19.Pivot X, Koralewski P, Hidalgo JL, Chan A, Gonçalves A, Schwartsmann G, et al. A multicenter phase II study of XRP6258 administered as a 1-h i.v infusion every 3 weeks in taxane-resistant metastatic breast cancer patients. Ann Oncol. 2008;19:1547–52. doi: 10.1093/annonc/mdn171. [DOI] [PubMed] [Google Scholar]
- 20.Heck MM, Höppner M, Horn T, Thalgott M, Gschwend JE, Retz M. Compassionate use of abiraterone and cabazitaxel: First experiences in docetaxel-pretreated castration-resistant prostate cancer patients. Urologe A. 2012;51:390–7. doi: 10.1007/s00120-012-2804-y. [DOI] [PubMed] [Google Scholar]
- 21.Gonçalves A, Braguer D, Kamath K, Martello L, Briand C, Horwitz S, et al. Resistance to Taxol in lung cancer cells associated with increased microtubule dynamics. Proc Natl Acad Sci U S A. 2001;98:11737–42. doi: 10.1073/pnas.191388598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huzil JT, Chen K, Kurgan L, Tuszynski JA. The roles of beta-tubulin mutations and isotype expression in acquired drug resistance. Cancer Inform. 2007;3:159–81. [PMC free article] [PubMed] [Google Scholar]
- 23.Ploussard G, Terry S, Maillé P, Allory Y, Sirab N, Kheuang L, et al. Class III beta-tubulin expression predicts prostate tumor aggressiveness and patient response to docetaxel-based chemotherapy. Cancer Res. 2010;70:9253–64. doi: 10.1158/0008-5472.CAN-10-1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhu ML, Kyprianou N. Androgen receptor and growth factor signaling cross-talk in prostate cancer cells. Endocr Relat Cancer. 2008;15:841–9. doi: 10.1677/ERC-08-0084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Beer TM, Garzotto M, Henner WD, Eilers KM, Wersinger EM. Intermittent chemotherapy in metastatic androgen-independent prostate cancer. Br J Cancer. 2003;89:968–70. doi: 10.1038/sj.bjc.6601232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Madan RA, Pal SK, Sartor O, Dahut WL. Overcoming chemotherapy resistance in prostate cancer. Clin Cancer Res. 2011;17:3892–902. doi: 10.1158/1078-0432.CCR-10-2654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Regan MM, O’Donnell EK, Kelly WK, Halabi S, Berry W, Urakami S, et al. Efficacy of carboplatin-taxane combinations in the management of castration-resistant prostate cancer: A pooled analysis of seven prospective clinical trials. Ann Oncol. 2010;21:312–8. doi: 10.1093/annonc/mdp308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Attard G, Reid AH, A’Hern R, Parker C, Oommen NB, Folkerd E, et al. Selective inhibition of CYP17 with abiraterone acetate is highly active in the treatment of castration-resistant prostate cancer. J Clin Oncol. 2009;27:3742–8. doi: 10.1200/JCO.2008.20.0642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Scher HI, Beer TM, Higano CS, Anand A, Taplin ME, Efstathiou E, et al. Prostate Cancer Foundation/Department of Defense Prostate Cancer Clinical Trials Consortium. Antitumour activity of MDV3100 in castration-resistant prostate cancer: A phase 1-2 study. Lancet. 2010;375:1437–46. doi: 10.1016/S0140-6736(10)60172-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sartor O, Reid RH, Hoskin PJ, Quick DP, Ell PJ, Coleman RE, et al. Quadramet 424Sm10/11 Study Group. Samarium-153-Lexidronam complex for treatment of painful bone metastases in hormone-refractory prostate cancer. Urology. 2004;63:940–5. doi: 10.1016/j.urology.2004.01.034. [DOI] [PubMed] [Google Scholar]


