Abstract
Objective:
To evaluate the anti-inflammatory effect of the third-generation CCB, lercanidipine.
Materials and Methods:
The ability of lercanidipine to reduce inflammation in carrageenan-induced paw edema in rats was evaluated, using different doses and its anti-inflammatory effect was compared with that of diclofenac sodium. Mast cell degranulation effect of lercanidipine was also carried out.
Results:
Lercanidipine produced a significant reduction in the inflammatory paw edema at all doses and at all intervals (P < 0.001). The percentage of reduction in edema was found to be proportionate to the doses of lercanidipine. Lercanidipine also significantly reduced the percentage of propranolol-induced mast cell degranulation.
Conclusions:
It was found that lercanidipine exerts a positive anti-inflammatory effect in a dose-dependent manner and was superior to diclofenac sodium.
Keywords: Carrageenan, diclofenac sodium, lercanidipine, mast cells
INTRODUCTION
The second half of the 20th century has seen a constant decrease in cardiovascular mortality in the western industrialized nations.[1] This decrease has occurred concomitantly with an improved control in the incidence of hypertension.
Elevated blood pressure has been related to the risks of major coronary events, such as myocardial infarction and coronary death.[2] Blood pressure levels have also been positively and continuously related to the risk of stroke,[2,3] cerebral hemorrhage, cerebral infarction, and dementia.[3] It is also noteworthy that people are now confronted currently with the rapid development of a ‘second wave’ epidemic of cardiovascular disease that is hitting the developing countries.
Basically, there are six major drug classes used in the treatment of blood pressure. There include diuretics, beta-blockers, calcium-antagonists (CA), angiotensin-converting-enzyme inhibitor (ACE-inhibitors), angiotensin II-antagonists, and alpha-adrenergic blockers. All subgroups of CA are effective and well tolerated in lowering blood pressure. While several studies have cast an unfavorable light on the use of calcium channel blockers (CCBs) as first-line antihypertensive agents,[4,5,6,7] morbidity and mortality results of other trials have evidenced that CA are definitely useful in the treatment of hypertension.
The search for new designs of CA with extended properties has superseded the earlier search for new clinical indications for these drugs.[8,9] Such improved qualities of new dihydropyridines (DHP) would be prolonged action, less side effects, single dosing (improved compliance), and less negative inotropic and chronotropic actions. A second generation of CA has addressed this problem with variable success. While drugs like nitrendipine,[10] or amlodipine,[11] have done reasonably well, others such as mibefradil,[12] or isradipine in the Multicenter Isradipine Diuretic Atherosclerosis Study (MIDAS) trial,[13] have not shown such a favorable outcome.
The third generation of DHP has added an additional property to this class of drugs: High lipophilicity. Currently, one of these drugs commercially available is lercanidipine. As a result of the lipophilic character, this compound is relatively quickly cleared from plasma building up within the phospholipid bilayer of cell membranes. The DHP thus accumulated can interact with its target, the site of the target, and the L-type calcium channel that lies within the double layer of the cell membrane as well. This phenomenon explains on one hand the slow onset, while on the other hand, the long duration of action.
Several agents with CA activity, DHP-derivatives, emerged on the market as early as 1998. The first of these drugs was amlodipine, a DHP with 30-50 hours clinical half-life and the most recent ones are lacidipine and lercanidipine, the latter being well known more for its anti-hypertensive property.[14,15]
The beneficial effects of lercanidipine include myocardial protection during ischemia, antagonism to the vasopressor activity of endothelin-1,[16] improving all indices of mitochondrial function,[17] decreasing fasting blood-glucose, glycosylate-hemoglobin, and improved oral glucose tolerance.
While third-generation DHP-CA Manidipine has been investigated for its pro-inflammatory cytokines secretion reduction property[18] and cardiometabolic properties,[19] drugs such as amlodipine, lacidipine, and nicardipine were evaluated for anti-inflammatory activity on the paw edema produced by carrageenan and compared with the activity of indomethacin,[9] that of the third-generation drug lercanidipine on carrageenan-induced rat hind paw edema has not so far been demonstrated, although a few other aspects of this drug have been investigated.[20,21,22]
Hence, the current study aims to evaluate the anti-inflammatory potential of the drug lercanidipine.
MATERIALS AND METHODS
Animals
Adult male Wistar rats (150-200 g) were used. The rats were housed in a temperature-controlled room in a 12 h/12 h dark/light cycle, and a standard dry diet (pellets) and water were made available ad libitum. All experiments were performed according to the guidelines of the Institutional Ethics Committee as well as those of CPCSEA. All efforts were made to minimize pain and suffering and to reduce the number of animals used.
Methods
Two methods were employed to verify the anti-inflammatory effects of lercanidipine.
Carrageenan-induced paw edema
Lercanidipine was evaluated for anti-inflammatory activity by carrageenan-induced rat paw edema method.[23] Rats were divided into five groups of six animals each. Group 1 served as the negative Control, which received dimethyl sulfoxide (DMSO) (solvent for lercanidipine) 0.1 ml intraperitoneally as well as carrageenan. Group 2 was the positive control and received diclofenac sodium, i.p., at the dose of 2.5 mg/kg BW as well as carrageenan. Groups 3, 4, and 5 were experimental and received carrageenan and lercanidipine hydrochloride dissolved in DMSO, in different doses at the rate of 300, 600, and 900 μg/kg BW. Lercanidipine i.p. was administered 1 hour before the carrageenan injection, and the latter was procured from Sigma-Aldrich. Lercanidipine was bought from Tocris Bioscience, UK.
Induction and measurement of the inflammation
Inflammatory edema was induced as recommended by Winter et al.,[23] by injection of 0.1 ml of freshly prepared suspension of carrageenan (0.1 ml l% w/v) solution in the sub-plantar region of right hind paw of rats.
The hind paw volumes were measured by a plethysmometer 1, 2, and 3 h after carrageenan injection and the difference between the treated and untreated hind paw volumes in the same rat was taken as the edema volume.
The percentage inhibition of edema (anti-inflammatory activity) of the drugs was calculated by the following equation: % inhibition of edema = (1−D/C) × 100. D represents the paw volume in drug-treated animals and C represents the paw volume of control group. The results are expressed as means ± S.E.M. Differences in mean values between groups were analyzed by a one-way analysis of variance (ANOVA) followed by a post hoc Tukey–Kramer Multiple Comparisons Test.
Mast cell degranulation
In vitro test for mast cell degranulation was carried out by the method of Kelley and Weiner,[24] using the following formula: % of degranulation = (1−T/C) × 100, where T = mast cell count in the experimental tissues and C = mast cell count in the control.
Male albino rats were sacrificed by cervical dislocation, the mesentery carefully removed and cut into small bits of about 1 sq cm. These bits of tissues, belonging to the experimental group, were incubated for about 5 min in Tyrode solution containing various doses of lercanidipine hydrochloride (5, 10, 20 mg/ml) and propranolol, a degranulating agent, (50 μg/ml) while the control tissue was incubated with propranolol only. The tissues were removed after 10 minutes and carefully spread over glass slides and stained with 1% toluidine blue. Mast cells are counted in five random fields under high power (X400). The percentage of degranulated mast cells is calculated in each treatment group. Sodium cromoglycate (10 μg/ml), a mast cell stabilizer, was used as standard.
Statistical analysis
Data were expressed as mean ± SEM. The results were analyzed by ANOVA followed by Tukey-Kramer multiple comparisons test. P < 0.05 was considered as a significant difference.
RESULTS
Carrageenan-induced paw edema
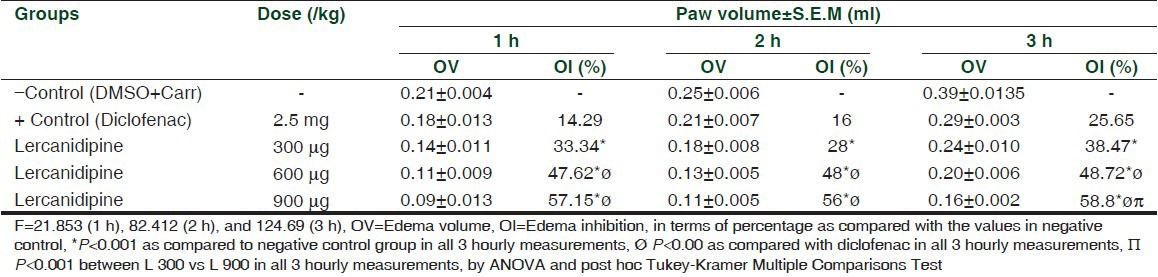
Injection of carrageenan produced an obvious paw edema as seen from the paw volume data at 1, 2, and 3 h after carrageenan injection [Table 1]. Administration of lercanidipine produced a clear and significant reduction in the edema at all doses and intervals (P < 0.001). The higher the dose, the higher was the percentage of reduction of edema. Diclofenac sodium, administered i.p., also reduced the edema but when compared with the efficacy of lercanidipine, the difference in the level of percentage of inhibition of the latter was much greater than that of diclofenac at doses of 600 and 900 mg at all time intervals. At the dose of 300 mg, the difference was not significant.
Table 1.
Changes in carrageenan-induced mean paw volume after administration of diclofenac and lercanidipine in different doses
Mast cell degranulation
Comparison was made in the number of mast cells degranulating after incubation with propranolol only and along with lercanidipine. The results are shown in Table 2. Figure 1 shows the appearance of mast cells in a normal piece of rat mesentery simply incubated in Tyrode solution without addition of any agents. Figures 2a and b demonstrate the appearance of degranulation. Microscopic examination of bits of rat mesentery incubated at 37°C with propranolol alone showed a mean of 65.9% of mast cells degranulating, while the percentage of degranulation in the mesentery of rats incubated with lercanidipine at a concentration of 5, 10, and 20 mg/ml showed values of 38.6%, 21.5%, and 14.2%, respectively [Table 2].
Table 2.
Mesentery cell count data, showing number of cells per field in control (normal), and experimental rat mesenteries exhibiting degranulating mast cells
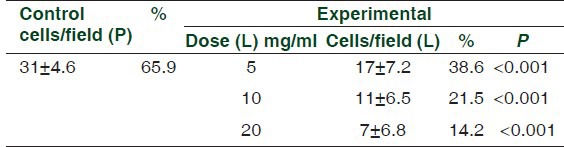
Figure 1.
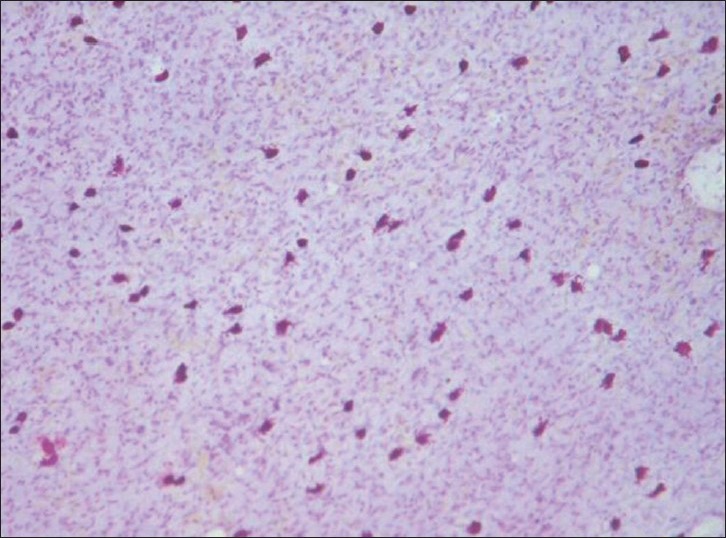
Normal mast cells in the mesentery of control rats. Note the intact mast cells. Toluidine Blue stain, ×100
Figure 2.
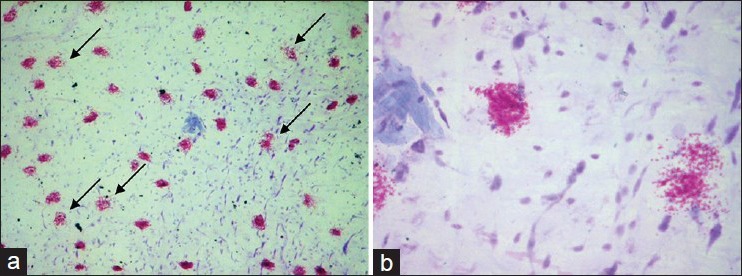
(a and b) Degranulating mast cells in the rat mesentery after incubation with propranolol. Note the damage to the cell membrane and spilling out of granules from the mast cells. a = ×400, b = ×1000
The dose of propranolol (P) remained the same (50 μg/ml). The dose (L) refers to the concentration of lercanidipine in the Tyrode solution. All the values are highly significant. Counting was done at a magnification of ×400.
DISCUSSION
Inflammation and increased capillary permeability are important factors in the pathogenesis of many diseases including atherosclerosis, while oxidative stress (OS), inflammation, and insulin resistance are among the mechanisms that have been recently implicated in pathogenesis of essential hypertension (EH). Peripheral polymorphonuclear leukocytes (PMNLs) are primed in EH patients, releasing uncontrolled superoxide anion contributing to OS and chronic low-grade inflammation in these patients. PMNL priming correlates with insulin resistance and PMNL intracellular calcium ((Ca2+) i). Japanese Society of Hypertension Committee published Guidelines for the Management of Hypertension in 2009 (JSH2009),[25] which recommend CCBs as one of the first choice of antihypertensive drugs among angiotensin II receptor blockers, angiotensin-converting enzyme inhibitors, diuretics, and beta-adrenergic blockers. Because DHP-CCBs have the greatest hypotensive efficacy without affecting organ blood flow, they are indicated in the patients with complications and the elderly.
Recent studies have attributed additional anti-oxidative characteristics to the anti-hypertensive drug lercanidipine (Vasodip), a third-generation CCB. It has been shown that the low-grade systemic inflammation and insulin resistance detected in EH patients may be attenuated by the use of lercanidipine, adding new unknown anti-inflammatory properties to this CCB.
Carrageenan-induced rat paw edema is the most suitable and commonly used investigation to look for anti-inflammatory potential of a drug, evaluated by its anti-edematous effect. Occurrence of edema secondary to carrageenan serves as an index of acute inflammatory changes and can be determined from differences in the paw volume measured immediately after carrageenan injection and then every hour for 3-6 hours. Carrageenan-induced edema is said to be biphasic, with the first phase occurring during the first hour after the injection, which is brought about by the release of histamine and serotonin and the second one due to the release of prostaglandins and cyclo-oxygenase products, with kinins providing continuity between the two phases.
In our study, carrageenan was used to induce paw edema and various doses of lercanidipine were used to primarily verify whether lercanidipine exerts any anti-inflammatory action at all like its related compounds, such as nifedipine and verapamil, since such a study has not so far been carried out. Various doses of lercanidipine were also employed in order to find out whether the anti-inflammatory effect, if present, is dose-dependent. Diclofenac sodium was also used in one group of rats as a contrast measure to compare the efficacy of lercanidipine.
Carrageenan has caused inflammation as shown by the values of paw volume data. Statistical analysis of the experimental data suggests a highly significant anti-inflammatory activity by lercanidipine (P < 0.001). The effect is also dose-dependent as seen from the values that after a dose of 900 μg/kg BW, the values of paw volume are comparatively the lowest, much lower than the values after diclofenac sodium injection. Unlike verapamil, the anti-inflammatory potency of which is effective at low dosage, lercanidipine's efficacy is dose-dependent similar to that of nifedipine.[26]
There is significant evidence that mast cells, basophils, and their released mediators are primary effectors of allergic inflammation.[27,28,29] It is believed that many of the deleterious effects that mast cells mediate are due to a variety of proinflammatory molecules exocytosed with exposure to antigen. By inhibiting the degranulation process of mast cells, lercanidipine has proved its anti-inflammatory potential.
CONCLUSIONS
Lercanidipine exerts a dose dependent anti-inflammatory activity and the effect is superior to diclofenac sodium.
ACKNOWLEDGMENT
This study was funded by Indian Council of Medical Research (ICMR), in the form of Short Term Studentship (STS) project to PV. There is no conflict of interest in this study
Footnotes
Source of Support: Study supported by ICMR STS to PV
Conflict of Interest: None declared.
REFERENCES
- 1.Uemura K, Pisa Z. Trends in cardiovascular disease mortality in industrialized countries since 1950. World Health Stat Q. 1988;41:155–68. [PubMed] [Google Scholar]
- 2.MacMahon S, Peto R, Cutler J, Collins R, Sorlie P, Neaton J, et al. Blood pressure, stroke, and coronary disease. Part 1, prolonged differences in blood pressure: Prospective observational studies corrected for the regression dilution bias. Lancet. 1990;335:765–74. doi: 10.1016/0140-6736(90)90878-9. [DOI] [PubMed] [Google Scholar]
- 3.Eastern Stroke and Coronary Heart Disease Collaborative Research Group. Blood pressure, cholesterol and stroke in Eastern Asia. Lancet. 1998;352:1801–7. [PubMed] [Google Scholar]
- 4.Pahor M, Guralnik JM, Furberg CD, Carbonin P, Havlik RJ. Risk of gastrointestinal haemorrhage with calcium antagonists in hypertensive persons over 67 years old. Lancet. 1996;347:1061–5. doi: 10.1016/s0140-6736(96)90276-7. [DOI] [PubMed] [Google Scholar]
- 5.Pahor M, Guralnik JM, Ferrucci L, Corti MC, Salive ME, Cerhan JR, et al. Calcium-channel blockade and incidence of cancer in aged populations. Lancet. 1996;348:493–7. doi: 10.1016/S0140-6736(96)04277-8. [DOI] [PubMed] [Google Scholar]
- 6.Furberg CD, Psaty BM, Meyer JV. Nifedipine. Dose-related increase in mortality in patients with coronary heart disease. Circulation. 1995;92:1326–31. doi: 10.1161/01.cir.92.5.1326. [DOI] [PubMed] [Google Scholar]
- 7.Psaty BM, Heckbert SR, Koepsell TD, Siscovick DS, Raghunathan TE, Weiss NS, et al. The risk of myocardial infarction associated with antihypertensive drug therapies. JAMA. 1995;274:620–5. [PubMed] [Google Scholar]
- 8.Gasser R. Calcium antagonists: Pharmacological agents in search of new clinical indications. Angiology. 1990;41:36–43. doi: 10.1177/000331979004100106. [DOI] [PubMed] [Google Scholar]
- 9.Halici Z, Suleyman H, Cadirci E. Effects of calcium channel blockers on hyaluronidase-induced capillary vascular permeability. Arch Pharm Res. 2008;31:891–9. doi: 10.1007/s12272-001-1243-0. [DOI] [PubMed] [Google Scholar]
- 10.Goa KL, Sorkin EM. Nitrendipine. A review of its pharmacodynamic and pharmacokinetic properties and therapeutic efficacy in the treatment of hypertension. Drugs. 1987;33:123–55. doi: 10.2165/00003495-198733020-00003. [DOI] [PubMed] [Google Scholar]
- 11.Meredith PA, Elliott HL. Amlodipine: Clinical relevance of a unique pharmacokinetic profile. J Cardiovasc Pharmacol. 1993;22(Suppl A):S6–8. [PubMed] [Google Scholar]
- 12.Triggle DJ. Pharmacologic and therapeutic differences among calcium channel antagonists: Profile of mibefradil, a new calcium antagonist. Am J Cardiol. 1996;78:7–12. doi: 10.1016/s0002-9149(96)00732-1. [DOI] [PubMed] [Google Scholar]
- 13.Brohani NO, Mercuri M, Borhani PA, Buckalew VM, Canossa-Rerris M, Carr AA, et al. Final outcome results of the Multicenter Isradipine Diuretic Atherosclerosis Study (MIDAS). A randomized controlled trial. JAMA. 1996;276:785–91. [PubMed] [Google Scholar]
- 14.Barrios V, Escobar C, Navarro A, Barrios L, Navarro-cid J, Calderón A. Lercanidipine is an effective and well tolerated antihypertensive drug regardless the cardiovascular risk profile: The LAURA Study. Int J Clin Pract. 2006;60:1364–70. doi: 10.1111/j.1742-1241.2006.01176.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Borghi C. Lercanidipine in Hypertension. Ann Pharmacother. 2007;41:465–73. doi: 10.1345/aph.1H299. [DOI] [PubMed] [Google Scholar]
- 16.Rossoni G, Bernareggi M, De Gennaro Colonna V, Polvani G, Berti F. Lercanidipine protects the heart from low flow ischemia damage and antagonizes the vasopressor activity of endothelin-1. J Cardiovasc Pharmacol. 1997;29(Suppl 1):S41–7. [Google Scholar]
- 17.Bernocchi P, Ceconi C, Cargnoni A, Pedersini P, Boraso A, Curello S, et al. Effects of lercanidipine on Fe2+-induced mitochondrial lipid peroxidation. J Cardiovasc Pharmacol. 1997;29(Suppl 1):S63–8. [Google Scholar]
- 18.Costa S, Zimetti F, Pedrelli M, Cremonesi G, Bernini F. Manidipine reduces pro-inflammatory cytokines secretion in human endothelial cells and macrophages. Pharmacol Res. 2010;62:265–70. doi: 10.1016/j.phrs.2010.03.004. [DOI] [PubMed] [Google Scholar]
- 19.Buset Ríos N, Rodriguez Esparragón F, Rodriguez Pérez J. Cardio-metabolic properties of manidipine: Beyond lowering arterial pressure? Nefrologia. 2009;29:203–7. doi: 10.3265/Nefrologia.2009.29.3.5078.en.full. [DOI] [PubMed] [Google Scholar]
- 20.Martinez ML, Rizzi E, Castro MM, Fernandes K, Bendhack LM, Gerlach RF, et al. Lercanidipine decreases vascular matrix metalloproteinase-2 activity and protects against vascular dysfunction in diabetic rats. Eur J Pharmacol. 2008;599:110–6. doi: 10.1016/j.ejphar.2008.10.007. [DOI] [PubMed] [Google Scholar]
- 21.Wu JR, Liou SF, Lin SW, Chai CY, Dai ZK, Liang JC, et al. Lercanidipine inhibits vascular smooth muscle cell proliferation and neointimal formation via reducing intracellular reactive oxygen species and inactivating Ras-ERK1/2 signaling. Pharmacol Res. 2009;59:48–56. doi: 10.1016/j.phrs.2008.09.015. [DOI] [PubMed] [Google Scholar]
- 22.Saha L, Hota D, Chakrabarti A. Evaluation of lercanidipine in Paclitaxel-induced neuropathic pain model in rat: A preliminary study. Pain Res Treat 2012. 2012 doi: 10.1155/2012/143579. 143579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Winter CA, Risley EA, Nuss GW. Carrageenan-induced edema in hind paw of the rat as an assay for anti-inflammatory drugs. Proc Soc Exp Biol Med. 1962;111:545–7. doi: 10.3181/00379727-111-27849. [DOI] [PubMed] [Google Scholar]
- 24.Firestein GS, Budd RC, Harris ED, Jr, McInnes IB, Ruddy S, Sergent JS. 8th ed. Philadelphia: Elsevier; 2008. KELLEY's Textbook of Rheumatology; p. 1018. [Google Scholar]
- 25.Ebina T, Kimura K, Umemura S. Calcium antagonists: Current and future applications based on new evidence. Calcium channel blockers and JSH2009. Clin Calcium. 2010;20:16–22. [PubMed] [Google Scholar]
- 26.Khaksari M, Mahani SE, Mahmoodi M. Calcium channel blockers reduce inflammatory edema in the rat. Involvement of the hypothalamus-pituitary-adrenal axis. Indian J Pharmacol. 2004;36:351–4. [Google Scholar]
- 27.Bochner BS, Undem BJ, Lichtenstein LM. Immunological aspects of allergic asthma. Annu Rev Immunol. 1994;12:295–335. doi: 10.1146/annurev.iy.12.040194.001455. [DOI] [PubMed] [Google Scholar]
- 28.Marone G, Casolaro V, Patella V, Florio G, Triggiani M. Molecular and cellular biology of mast cells and basophils. Int Arch Allergy Immunol. 1997;114:207–17. doi: 10.1159/000237670. [DOI] [PubMed] [Google Scholar]
- 29.Yong LC. The mast cell: Origin, morphology, distribution, and function. Exp Toxicol Pathol. 1997;49:409–24. doi: 10.1016/S0940-2993(97)80129-7. [DOI] [PubMed] [Google Scholar]


