Abstract
Objective:
To map areas of brain activation (cFos) alongside changes in levels of brain-derived neurotrophic factor (BDNF) to provide insights into neuronal mechanisms contributing to previously observed sex differences in behavioral measures of ethanol withdrawal (EW).
Materials and Methods:
Immunohistochemical analysis of cFos and BDNF levels using protein-specific antibodies and visualization with nickel-enhanced DAB staining in 3 cortical and 4 hippocampal regions was used to assess EW-induced expression of these proteins.
Results:
EW male and female rats showed significantly higher levels of cFos expression compared to controls in the hippocampal regions whereas EW OVX rats showed higher levels compared to controls only at 1 day EW in the dentate gyrus. Males expressed higher basal levels of cFos in the CA1 subfield of the hippocampus and in the motor cortex than either intact or OVX female rats. BDNF immunoreactivity was also significantly higher in EW rats compared to that in controls, varying with sex and brain region at 1 and 3 days EW.
Conclusions:
Sex-and brain region-specific changes in expression of cFos and BDNF occurring during 1 and 3-day EW, suggest that differential activation and expression of neurotrophins may influence the observed sex differences and support the suggestion that EW is a chronic stressor, eliciting sequential neuronal activation and neurotrophin regulation.
Keywords: Brain-derived neurotrophic factor, cFos, cortex, ethanol withdrawal, hippocampus
INTRODUCTION
Ethanol withdrawal (EW) presents significant challenges to the recovering alcoholic, to the treatment of ethanol dependence, and represents a rebound hyperexcitability of the brain following termination of ethanol intake. Acute EW unmasks the brain adaptation established during chronic consumption in which excitatory glutamate receptors become more responsive while inhibitory GABAA receptors less responsive to ethanol.[1,2] CFos, an inducible immediate early gene product, has been used to map neuronal activation due to a variety of stimuli, including stress and ethanol exposure and withdrawal.[3,4,5] However, no study had assessed the relationship between cFos induction and EW-induced changes in expression of neurotrophic factors, such as brain-derived neurotrophic factor (BDNF).
In the present study our intent was to initiate mapping of the activation of brain areas involved in recovery from EW, using cFos expression, and to delineate the relationship of activation with BDNF, a growth factor produced by neurons predominantly in the hippocampus and cortex.[6,7] BDNF is involved in the control of neural proliferation and differentiation, has significant neurotrophic influences during early prenatal development that result in selective neural survival, and has potent influences on differentiation besides a guiding influence on the direction of neurite growth.[6,8] Presumably BDNF is also involved in the survival and maintenance of neurons in parts of the brain most susceptible to neurodegeneration, such as the hippocampus, and facilitates activity-dependent plasticity involving structural and functional changes; hence, it is a potent candidate molecule for regulating injury-induced circuit reorganization and modulation of remodeling,[6] such as that driven by the hyperexcitable state of EW.
A possible mechanistic link exists between gonadal steroids and the expression of both cFos and BDNF,[9] so our overall goal was to map neuronal activation and BDNF expression in pertinent brain regions of male and female rats during EW to provide insight into the potential therapeutic utility of BDNF in reducing the severity of EW during treatment of ethanol dependence in alcoholic men and women. The cortex and hippocampus were studied as they constitute the limbic system which controls cognitive functions in humans and influences cortical excitability[10] and the symptomology of acute EW.
MATERIALS AND METHODS
Animals
Male and female SD rats (Simonsen Labs, Gilroy CA) approximately 50 days old at the start of experiments were assigned to treatment or control groups by matching their weights and individually housed in standard polypropylene cages in a temperature-controlled (23 ± 1°C) research facility with a 12:12 hours light: Dark cycle (lights on at 6:00 AM). Treatment groups (male, intact female and OVX at 1 and 3 day EW) had 8 animals per sex per day.
Animal procedures
Prior to initiation of treatment, ovariectomies were performed on half of the female rats and a wash-out period of at least four days given for recovery from surgery before initiation of the experimental procedures. Intact females did not receive sham surgeries as previous studies showed no differences between sham-surgery and no-surgery responses.[11,12] Six percent ethanol in a nutritionally complete liquid diet (MP Biochemicals, Costa Mesa, CA) was given for 14 days to make the treated animals ethanol-dependent as previously described.[13] Control animals (n = 4 per sex per day) were administered the same liquid diet but with dextrose substituted isocalorically for the ethanol to ensure equivalent caloric intake. The amount of liquid diet consumed was recorded daily. Clean drinking water was provided ad libitum. Animals consumed an average of 10-12 g/kg/day, with female rats generally consuming 10% more ethanol per kg body weight than males. This level of consumption reliably produces ethanol dependence by fourteen days.[12] Blood alcohol levels were not assayed as animals drink in bouts, resulting in large fluctuations in blood alcohol levels; however, the amount consumed per day is a more reliable indicator of ethanol dependence than blood alcohol levels.[12] Furthermore, as brain tissue was not harvested before 6 hours EW, there would not be any measurable blood alcohol at this time; blood alcohol levels obtained by this liquid diet paradigm reach zero by six hours of EW.[12] The estrus cycle of female animals was monitored by daily collection of vaginal smears and histological examination; near confluence of female cycles was achieved by the end of the experiment. Intact females generally showed regular cyclicity but with a prolonged estrus. At day 15, regular lab chow replaced liquid diet for all animals. Brain harvesting was scheduled at 1 day or 3 days EW, and for when intact female rats were in estrus or diestrus 1 (low progesterone and estradiol levels). All procedures were in accordance with approved Idaho State University Animal Welfare Protocols and NIH guidelines for the humane care and use of animals and conducted in an AALAC-accredited facility.
Brain perfusion and fixation
Following deep pentobarbital anesthesia, each animal's chest cavity was opened exposing the cardiac left ventricle into which a lavage needle was inserted after a small incision; a second incision was made on the right aorta to allow outflow. PBS containing heparin was run through the needle at a low flow rate for approximately 3 minutes to clear out blood, then perfusion done with 4% paraformaldehyde in PBS (pH 7.4) as fixative for 10 minutes, the brain removed from the skull, and post fixed overnight at 4°C in a 50-ml tube with fresh fixative. Brains were then sectioned on a vibrating microtome into 40μ coronal sections. Equivalent brain sections were obtained from each animal across all treatment conditions.
Immunohistochemistry
cFos Immunohistochemistry
Anti-cFos polyclonal antibody (Sigma, Saint Louis, MO) was used on free-floating sections at a 1:5000 dilution, determined previously by testing a range of dilutions.[14] Specificity of signal was verified by lack of staining in no-primary antibody and no-secondary antibody slices. Sections were washed in 1 XPBS for 15 minutes followed by 15 minutes incubation in PBS with 0.3% H2 O2 and four more 5-minute washes in PBS Triton X-100. Sections were then blocked in normal goat serum in PBS with Triton X-100 for 4 hours, and exposed to the primary antibody (1:5000) for 24 hours at 4°C. Slices were washed in PBS-Triton X three times for 5 minutes each and incubated for 1 hour in biotinylated secondary antibody at 1:200 dilutions. Following the incubation, sections were washed three times in PBS Triton X-100 for 5 minutes each. Sections were then incubated in avidin-biotin complex reagent using a kit (ABC Vectastain®, Burlingame, CA) for 1 hour at room temperature, washed 3 times (5 minutes each) in PBS Triton X-100, and nickel-enhanced diaminobenzadine (DAB) (Pierce, Rockford, IL) added to visualize primary antibody staining. Sections were washed once in water, mounted, dried and dehydrated by immersing in 70% ethanol for 10 minutes, 95% ethanol for 10 minutes, 100% ethanol for 10 minutes, and xylenes for 12 minutes. After dehydration, the mounted slides were cover slipped and photomicrographs of DAB grains taken using a Leica FireCam microscope. Grains were counted using Image-Pro Plus 5.0 software program (Media Cybernetics Inc., Silver Spring, MD). Expression of cFos was quantified in four hippocampal regions: CA1, CA2, CA3, and dentate gyrus (DG), and in three cortical areas: Cingulate cortex, piriform, and motor cortex.
BDNF Immunohistochemistry
As for cFos staining, the initial set of experiments combining previously published protocols[15] and manufacturer's insert were conducted to optimize concentration of the BDNF antibody and to verify specificity of staining of the antibody. There was essentially no staining present when either the primary antibody or the secondary antibody was omitted. Rabbit anti-human BDNF (Chemicon, Temecula, CA) was used at 1 μg/ml on free-floating sections. Sections were washed once in 1XPBS for 15 minutes followed by 1 hour incubation in 20% normal goat serum in PBS; sections were then exposed to rabbit anti-human BDNF primary antibody (1:200) in 2XPBS, 0.3% Triton-X, and 0.02% sodium azide for 72 hrs at 4°C. This time was found in a pilot study to increase the sensitivity of BDNF detection without increasing the background signals. Subsequently, sections were washed in PBS-Tween-20 three times for 15 minutes each, and incubated for 2 hours in anti-rabbit IgG-biotinylated secondary antibody at 1:200 dilution; sections were washed three times in PBS for 15 minutes each, incubated in biotin ABC (Burlingame, CA) reagent for 2 hours at room temperature, washed three times (15 minutes each) in PBS-Triton X, and nickel-enhanced DAB (Rockford, IL) added to visualize primary antibody staining. Washing, mounting, dehydration, cover slipping, photography and grain-counting were then done as described for cFos; BDNF expression was quantified in the same areas as for cFos.
Data analysis
Actual counts of visualized DAB-labeled cFos and BDNF antibodies were analyzed; each brain region was assessed independently per antibody studied and data from the different brain regions analyzed separately. Bilateral brain sections were matched at bregma levels according to the rat brain atlas[16] and numerical summaries of the data from the different bilateral sections obtained and presented graphically as means ± standard error of the mean (SEM). Further statistical analysis entailed two-way ANOVA with the independent variables being treatment (control or ethanol) and time (1 or 3 days). Two-way ANOVA was used because of the hypothesis that sex-selective differences would exist in brain activation and BDNF expression, as indicators of EW recovery, at 1 and 3 days. Significant main effects were further analyzed using Bonferroni's multiple comparison post hoc tests. All statistical analyses were done on sections processed simultaneously to control for variability in intensity of DAB staining across different experiments. Microsoft Excel® and GraphPad Prism® version 4.0 (GraphPad Software Inc., San Diego, CA) were used for graphing and data analysis.
RESULTS
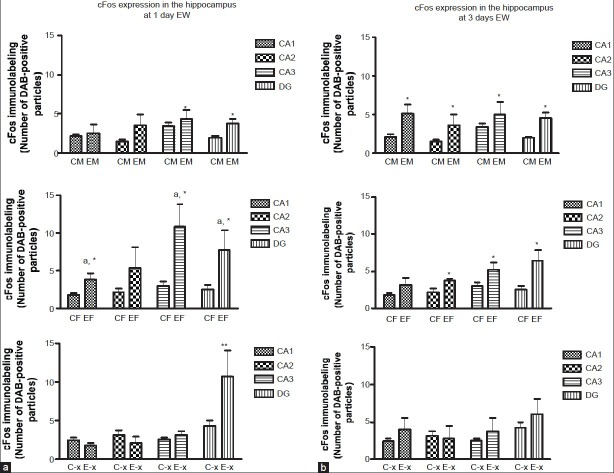
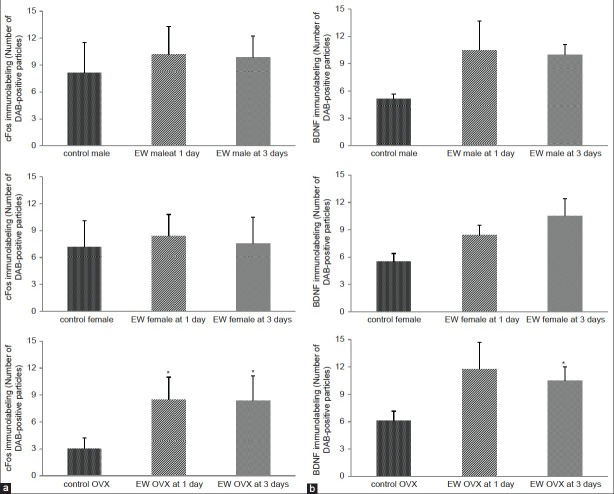
In general, comparison of cFos expression between EW and control animals showed more abundant cFos immunolabeled particles in EW than in control animals [Figure 1] at both time points of EW. There was no significant change in cFos expression in the CA1 and CA2 areas of EW males at 1 day compared to controls; however, the CA3 and DG showed significant increases in cFos expression compared to controls [Figure 2A]. At 3 days EW, there was a significant effect of hippocampal region [F(1,54) = 16.81, P = 0.0001] in the expression of cFos in males, with all four regions showing significant increases in cFos expression [Figure 2B]. In the female hippocampus, at 1 day, there was a significant effect of hippocampal region [F(3,49) = 3.18, P = 0.032] in the expression of cFos, with significantly increased cFos expression in areas CA1, CA3 and DG, while at 3 days EW there was also a significant effect of treatment [F(1,49) = 16.03, P = 0.0002] in the expression of cFos in areas CA2, CA3 and DG [Figure 2B]. In OVX females at 1 day EW, there was a significant effect of hippocampal region [F(3,46) = 3.68, P = 0.02] in the expression of cFos [Figure 2] with a significant increase in cFos expression in the DG. However at 3 days EW, OVX females in withdrawal showed cFos expression similar to that of controls [Figure 2B].
Figure 1.
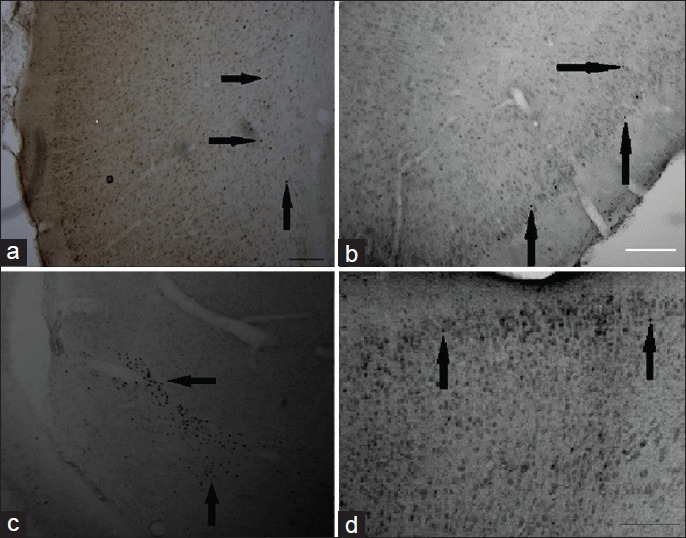
Representative photomicrographs of cFos and BDNF expression in the cortex and hippocampus at 1 and 3 days EW. Immunohistochemistry using cFos antibody showed an effect of EW on expression of cFos labeled with DAB. Generally, EW rats showed a greater expression of cFos compared to pair-fed controls. No staining was observed when either the anti-Fos antibody or the secondary antibody was omitted from the experiment. (a) Control female at 24 hour EW, piriform cortex; (b) EW female at 24 hours, piriform cortex; (c) EW OVX at 3 day, hippocampus, using cFos antibody; (d) EW female at 3 day EW, motor cortex using BDNF immuno-labeling; Magnification: ×40. Scale bars: A-C, 200 mm; D, 100 mm. Magnification: ×40. Scale bars: A, B: 200 mm
Figure 2.
(a) CFos levels were significantly elevated in subfields of the hippocampus and in the dentate gyrus at 1 day EW compared to hippocampal subfields and dentate gyrus of controls. Data are presented as means ± SEM counts per 15mm2; *significantly different from controls, P < 0.05; asignificantly different within the hippocampal subfields of the same EW treatment, N = 4-8 animals per treatment condition. (b) CFos levels were significantly elevated in subfields of the hippocampus and in the dentate gyrus of EW male and female, but not EW OVX rats at 3 days EW, compared to pair-fed controls. Data are presented as means ± SEM counts per 15mm2; *significantly different from controls, P < 0.05, N = 4-8 animals per treatment condition. CM: Control male, EM: EW male, CF: Control female, EF: EW female, C-x: control OVX, E-x: EW OVX
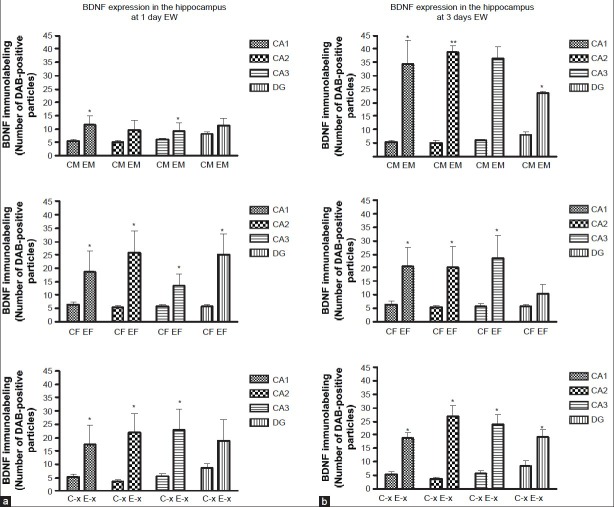
To assess the relationship in induction of cFos and BDNF, measurement of BDNF expression was done in the same regions of the hippocampus and the cortex as for cFos expression [Figure 1]. CA1, a parvocellular hippocampal region, has been reported to show potentiation of synaptic transmission after exposure to neurotrophins, whereas area CA3, a magnocellular zone bordering the dentate gyrus (DG) has granule cells from the DG project into it and in turn, CA3 neurons project into area CA1. Mossy fiber axons of granule cells are located in CA3/DG regions and contain high concentrations of BDNF. At 1 day EW, there was a significant effect of treatment [F(1,40) = 6.92, P = 0.01] on BDNF expression in the hippocampus of male rats [Figure 3A] with a significant increase in cFos expression in CA1 and CA3 regions. There was also a significant effect of treatment in the hippocampus of intact female rats [F(1,43) = 33.58, P < 0.0001] and OVX rats [F(1,40) = 33.17, P < 0.0001] on BDNF expression at 1 day EW with significant increases in cFos expression in all four hippocampal regions.
Figure 3.
(a) BDNF levels were significantly elevated in subfields of the hippocampus and in the dentate gyrus at 1 day EW compared to hippocampal subfields of pair-fed controls. Data are presented as means ± SEM counts per 15mm2; *significantly different from controls, P < 0.05, N = 4-8 animals per treatment condition. (b) BDNF levels were significantly elevated in subfields of the hippocampus and in the dentate gyrus at 3 days EW compared to pair-fed controls. Data are presented as means ± SEM counts per 15mm2; *significantly different from controls, P < 0.0001, N = 4-8 animals per treatment condition. CM: control male, EM: EW male, CF: control female, EF: EW female, C-x: control OVX, E-x: EW OVX
At 3 days EW in males there was a significant effect of treatment [F(1,34) = 174.56, P < 0.0001] with all four hippocampal regions showing significant increases in cFos expression compared to controls. There was also a significant interaction of treatment X hippocampal region [F(3, 34) = 3.73, P = 0.02] on BDNF expression in the hippocampus [Figure 3B]. In the intact female hippocampus at 3 days EW, there was also a significant effect of treatment [F(1,46) = 22.8, P < 0.0001] on BDNF expression, with significant increases in CA1, CA2 and CA3 hippocampal subfields compared to controls. Similar to males, OVX females at 3 days EW showed a significant effect of treatment [F(1,48) = 121.0, P < 0.0001] with all four hippocampal regions showing significant increases in cFos expression compared to controls. OVX females also showed a significant interaction of treatment X hippocampal region [F(3,48) = 3.48, P = 0.02] on BDNF expression [Figure 3B].
The more peripheral brain region, the cingulate cortex, showed no significant differences in cFos expression between EW male or female and control animals at both 1 and 3 days EW, except in OVX females. In EW OVX females, there was a significant effect of treatment [F(1, 23) = 8.78, P = 0.007] on cFos expression at both 1 and 3 days [Figure 4A]. However, in BDNF expression the cingulate cortex showed a significant effect of treatment in males [F(1,21) = 13.84, P < 0.0017] at 3 days EW, and in intact females [F(1,17) = 11.07, P < 0.004] and OVX female rats [F(1,20) = 10.55, P < 0.004] at both 1 and 3 days EW [Figure 4B].
Figure 4.
(a) CFos levels were significantly elevated in the cingulate cortex of EW OVX females only, at both 1 and 3 days EW, compared to pair-fed controls. Data are presented as means ± SEM counts per 15mm2; *significantly different from controls, P < 0.05, N = 4-8 animals per treatment condition. (b) BDNF levels were significantly elevated in the cingulate cortex of EW rats compared to pair-fed controls at 3 days EW only in males, and at both 1 and 3 days EW in intact and OVX females. Data are presented as means ± SEM counts per 15mm2; *significantly different from controls, P < 0.05, N = 4-8 animals per treatment condition
In the motor cortex, there was a significant effect of treatment in cFos expression [F(1, 27) = 8.79, P = 0.0063] in males at 1 day EW [Figure 5A], while in the females there was no significant increase in cFos expression at 1 day EW. At 3 days EW, females showed expression levels similar to controls. In OVX females there was a significant effect of treatment [F(1, 21) = 10.90, P = 0.003] in cFos expression in the motor cortex of EW OVX females at 1 day [Figure 5A]. BDNF expression in the motor cortex showed a significant effect of treatment in males at 3 days [F(1,19) = 12.75, P < 0.002] and intact females [F(1,18) = 21.84, P = 0.0002] at 1 day, but no difference in BDNF expression OVX female rats at both 1 and 3 days EW compared to controls [Figure 5B].
Figure 5.
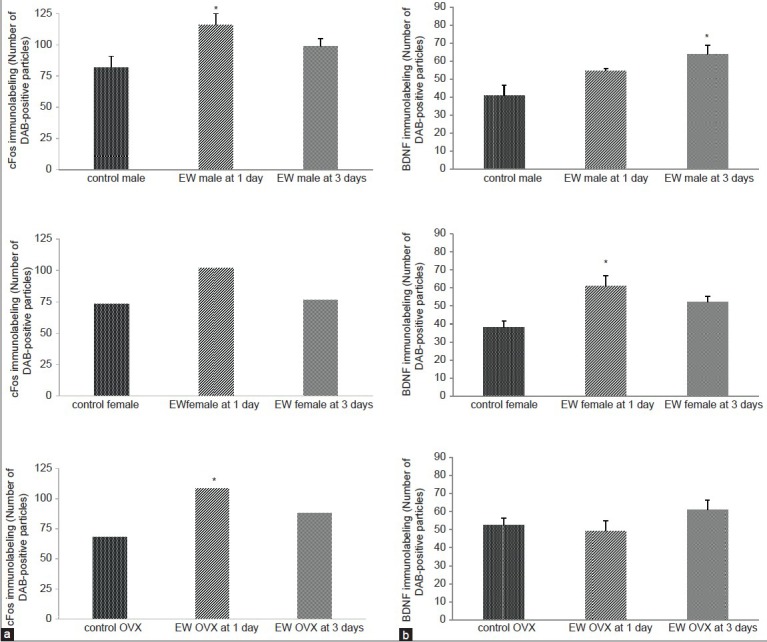
(a) CFos levels were significantly elevated in the motor cortex of EW males compared to pair-fed controls at 1 day EW; there was no significant increase in cFos levels in EW intact females at both 1 and 3 days EW. OVX females showed a significant elevation of cFos compared to controls, only at 1 day EW. Data are presented as means ± SEM counts per 15mm2; *significantly different from controls, P < 0.05, N = 4-8 animals per treatment condition. (b) BDNF levels were significantly elevated in the motor cortex of EW males compared to pair-fed controls at 3 days EW only, and in EW intact females only, compared to pair-fed controls at 1 day EW. Data are presented as means ± SEM counts per 15mm2; *significantly different from controls, P < 0.001, N = 4-8 animals per treatment condition
In the piriform cortex, there were no significant differences in cFos expression, at both 1 and 3 days EW for males compared to controls [Figure 6A]. Intact female EW rats however, showed a significant effect of treatment [F(1, 16) = 5.26, P = 0.036] on cFos expression at 3 days EW. In OVX females, there was a significant effect of treatment in the expression of cFos in EW OVX females [F(1, 18) = 14.18, P = 0.0014] compared to controls at both 1 and 3 days EW [Figure 6A].
Figure 6.
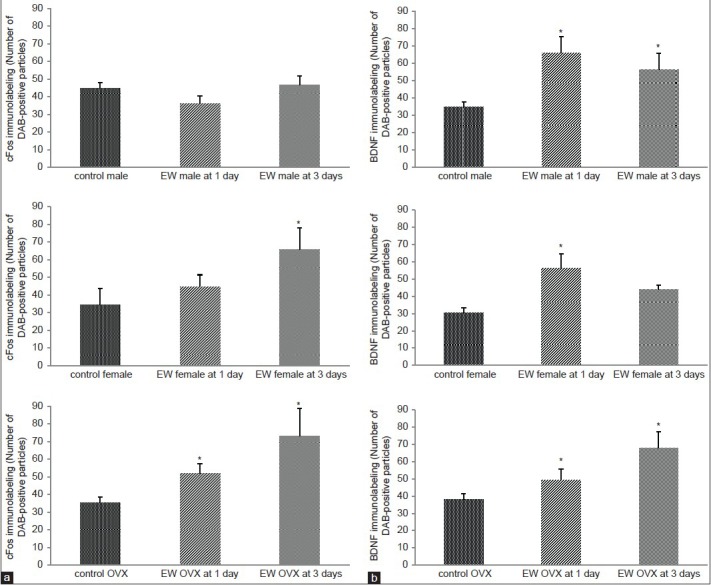
(a) CFos levels were significantly elevated in the piriform cortex of EW females compared to pair-fed controls only, at 1 day EW; cFos levels were also significantly elevated at both 1 and 3 days EW in the piriform cortex of EW OVX females, compared to pair-fed controls. Data are presented as means ± SEM counts per 15mm2; *significantly different from controls, P < 0.05, N = 4-8 animals per treatment condition. (b) BDNF levels were significantly elevated in the piriform cortex of both EW males and EW OVX animals compared to pair-fed controls at both 1 and 3 days EW; in EW intact females, BDNF levels were significantly elevated compared to pair-fed controls, at 1 day EW only. Data are presented as means ± SEM counts per 15mm2; *significantly different from controls, P < 0.001, N = 4-8 animals per treatment condition
BDNF expression in the piriform cortex showed a significant effect of treatment in males [F(1,21) = 17.26, P < 0.0004] at both 1 and 3 days EW, intact females [F(1,21) = 21.98, P < 0.0001] at 1 day EW, and OVX female rats [F(1,21) = 14.64, P < 0.001] at both 1 and 3 days EW [Figure 6B].
DISCUSSION
Previously, we found that female rats recover from EW faster than males, with females showing less severe behavioral signs of EW assessed by seizure risk at 3 days of withdrawal.[11,17] EW is a stressor, and significant sex differences were seen in the levels of plasma corticosterone, a stress marker, in male and female rats at 1 and 3 days of EW.[17] Plasma corticosterone levels had significantly decreased in female rats but persisted in males at 3 days of EW. These sex differences imply that males and females have different adaptive mechanisms to EW that can be important for treatment of withdrawal symptoms.
The present sets of studies were undertaken to initiate the identification of the cellular basis for the observations of consistent and significant sex differences in behavioral assessment of recovery from EW as well as in steroid modulation of EW seizure responses.[11,17] Bidirectional responses accompanying EW such as habituation to stress-induced elevation of corticosterone during EW,[18] but enhanced or persistent elevations in Fos expression[19] have been previously observed. In the present studies, we noted a shift in the time course for maximal induction, with elevations in cFos expression preceding elevations in BDNF expression. OVX animals tended to show patterns of changes in cFos expression more similar to females than males, especially in the hippocampus, suggesting that organizational influences have an important role in observed sex differences in brain responses to EW, rather than direct interactions between ovarian steroids and key sites of EW. Additionally, females displayed an attenuated elevation of cFos expression at 3 days EW compared to males across brain regions. This finding lends further support to our earlier evidence that males show significant behavioral signs of EW at 3 days while females appear to have recovered by this time point.[11,17]
Increasing evidence suggests a complex role of BDNF in mediating neuronal adaptations to a number of challenges. For example, BDNF modulates GABA signaling following hippocampal injury, allowing for a variety of intracellular shifts in signaling that promote survival and recovery.[20] This finding further supports the contrasting effects of BDNF that may either be beneficial or pathological, depending on the circumstances.[21] In relation to interactions between BDNF and ethanol, BDNF has been reported to play a protective role against ethanol-induced toxicity.[22] Supporting evidence suggests that a negative association exists between BDNF serum levels and ethanol withdrawal severity at 1 day EW in male patients;[23] and that chronic ethanol exposure alters BDNF expression that varies by brain region.[24] The observed pattern of EW-induced changes in expression in the present set of experiments suggests that BDNF levels were increased in response to the disruptive neuronal hyperexcitability of EW which underlies the increased seizure risk noted as a major symptom of EW. Assessment of the role of BDNF in seizure risk or protection has uncovered confounding results, with BDNF either contributing to seizure activity or protecting against seizure activity.[25,26,27]
It is likely that an outcome of the induction of BDNF by recovery from EW involves activation of circuitry involving both GABAergic and glutamatergic systems. BDNF levels increase via the excitatory neurotransmitter glutamate, and decrease via the inhibitory neurotransmitter γ-aminobutyric acid (GABA).[6] This regulation of BDNF by neuronal activity suggests that the function of trophic factors involves more than survival and differentiation of neurons. Increasing evidence for modulation of glutamatergic activity by BDNF involving both AMPA and NMDA receptor subtypes[28] suggests that in addition to promoting neuronal survival and/or neurogenesis during recovery from EW, BDNF may initiate neuronal cascades that induce alterations in GABAA and glutamate receptors, serving to reduce the neuronal hyperexcitability of EW. Our current findings suggest that induction of BDNF occurs with EW recovery, resulting in the previously observed reduction in seizure risk.
Overall, the pattern of changes in BDNF levels was quite similar across the three sex conditions, rather surprisingly, because it has been reported that estrogen influences BDNF protein levels through an estrogen response element (ERE) identified in the BDNF gene in rats, resulting in increased synthesis of this growth factor.[29] Likewise, progesterone has a putative role in impacting BDNF levels in different brain regions.[10] A drawback of this study therefore, was that brains were harvested when females were in estrus or diestrus, (when progesterone and estradiol levels are low), possibly limiting direct effects on BDNF, and diminishing significant sex differences in BDNF expression during EW. Moreover, the cascade of responses elicited by the rebound hyperexcitability of EW could outweigh any direct effects of ovarian steroids. In conclusion, our results indicate that the EW-induced expression of cFos and BDNF differs from one brain region to another, with increased expression of BDNF occurring later than cFos induction, and suggest that the differential changes in expression of cFos and BDNF during EW likely involve both activation and organizational influences of steroid hormones. Future research should more thoroughly address the results of the observed increases in BDNF staining during EW; consider the activation of downstream mediators, the relationship with serum BDNF, and the utility of BDNF in abstinent ethanol-dependent patients.[30] Knowledge from identified sex differences in the neurobiological mechanisms underlying recovery from EW will contribute to developing improved therapies to optimize the management of withdrawal for men and women dependent on ethanol.
ACKNOWLEDGMENT
We gratefully acknowledge the technical assistance of Bikul Koirala and John Larkin. This work was supported by PHS RO1AA011877 (LLD) and BRIN/INBRE P20RR016454 (PEA and LLD).
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Chandler LJ. Ethanol and brain plasticity: Receptors and molecular networks of the postsynaptic density as targets of ethanol. Pharmacol Ther. 2003;99:311–26. doi: 10.1016/s0163-7258(03)00096-2. [DOI] [PubMed] [Google Scholar]
- 2.Grobin AC, Matthews DB, Devaud LL, Morrow AL. The role of GABA(A) receptors in the acute and chronic effects of ethanol. Psychopharmacology (Berl) 1998;139:2–19. doi: 10.1007/s002130050685. [DOI] [PubMed] [Google Scholar]
- 3.Chang SL, Patel NA, Romero AA. Activation and desensitization of Fos immunoreactivity in the rat brain following ethanol administration. Brain Res. 1995;679:89–98. doi: 10.1016/0006-8993(95)00210-h. [DOI] [PubMed] [Google Scholar]
- 4.Kozell LB, Hitzemann R, Buck KJ. Acute alcohol withdrawal is associated with c-Fos expression in the basal ganglia and associated circuitry: C57BL/6J and DBA/2J inbred mouse strain analyses. Alcohol Clin Exp Res. 2005;29:1939–48. doi: 10.1097/01.alc.0000187592.57853.12. [DOI] [PubMed] [Google Scholar]
- 5.Bachtell RK, Tsivkovskaia NO, Ryabinin AE. Alcohol-induced c-Fos expression in the Edinger-Wesphal nucleus: Pharmacological and signal transduction mechanisms. J Pharmacol Exp Ther. 2002;302:516–24. doi: 10.1124/jpet.102.036046. [DOI] [PubMed] [Google Scholar]
- 6.Lu B. BDNF and activity-dependent synaptic modulation. Learn Mem. 2003;10:86–98. doi: 10.1101/lm.54603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Conner JM, Lauterborn JC, Yan Q, Gall CM, Varon S. Distribution of brain-derived neurotrophic factor protein and mRNA in the normal adult rat CNS: Evidence for anterograde transport. J Neurosci. 1997;17:2295–313. doi: 10.1523/JNEUROSCI.17-07-02295.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hartmann M, Heumann R, Lessmann V. Synaptic secretion of BDNF after high-frequency stimulation of glutamatergic synapses. EMBO J. 2001;20:5887–97. doi: 10.1093/emboj/20.21.5887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gibbs RB. Treatment with estrogen and progesterone affects relative levels of brain-derived neurotrophic factor mRNA and protein in different regions of the adult brain. Brain Res. 1999;844:20–7. doi: 10.1016/s0006-8993(99)01880-6. [DOI] [PubMed] [Google Scholar]
- 10.Sandner G, Oberling P, Silviera MC, DiScala G, Rocha B, Bagri A, et al. What brain structures are active during emotions? Effects of brain stimulation-elicited aversion on cFos immunoreactivity and behavior. Behav Brain Res. 1993;58:9–18. doi: 10.1016/0166-4328(93)90086-6. [DOI] [PubMed] [Google Scholar]
- 11.Devaud LL, Chadda R. Sex differences in rats in the development of and recovery from ethanol dependence assessed by changes in seizure susceptibility. Alcohol Clin Exp Res. 2001;25:1689–96. [PubMed] [Google Scholar]
- 12.Devaud LL, Matthews DB, Morrow AL. Gender impacts behavioral and neurochemical adaptations in ethanol-dependent rats. Pharmacol Biochem Behav. 1999;64:841–9. doi: 10.1016/s0091-3057(99)00164-1. [DOI] [PubMed] [Google Scholar]
- 13.Alele PE, Devaud LL. Differential adaptations in GABAergic and glutamatergic systems during ethanol withdrawal in male and female rats. Alcohol Clin Exp Res. 2005;29:1027–34. doi: 10.1097/01.alc.0000167743.96121.40. [DOI] [PubMed] [Google Scholar]
- 14.Khurana RC, Devaud LL. Sex differences in neurotransmission parameters and Fos expression in response to repeated restraint stress. J Neuroendocrinol. 2007;19:511–20. doi: 10.1111/j.1365-2826.2007.01557.x. [DOI] [PubMed] [Google Scholar]
- 15.Zhou J, Zhang H, Cohen RS, Pandey SC. Effects of estrogen treatment on expression of BDNF and CREB expression and phosporylation in rat amygdaloid and hippocampal structures. Neuroendocrinology. 2005;81:294–310. doi: 10.1159/000088448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Paxinos G, Watson C. 4th ed. San Diego: Academic Press; 1998. The rat brain in stereotaxic coordinates. [Google Scholar]
- 17.Alele PE, Devaud LL. Sex differences in steroid modulation of ethanol withdrawal in male and female rats. J Pharmacol Exp Ther. 2007;320:427–36. doi: 10.1124/jpet.106.107896. [DOI] [PubMed] [Google Scholar]
- 18.Janis GC, Devaud LL, Mitsuyama H, Morrow AL. Effects of chronic ethanol consumption and withdrawal on the neuroactive steroid 3α-hydroxy-5α-pregnan-20-one in male and female rats. Alcohol Clin Exp Res. 1998;22:2055–61. [PubMed] [Google Scholar]
- 19.Borlikova GG, Le Merrer J, Stephens DN. Previous experience of ethanol withdrawal increases withdrawal-induced c-fos expression in limbic areas but not withdrawal-induced anxiety and prevents withdrawal-induced elevations in plasma corticosterone. Psychopharmacology (Berl) 2006;185:188–200. doi: 10.1007/s00213-005-0301-3. [DOI] [PubMed] [Google Scholar]
- 20.Shulga A, Thoms-Crusells J, Sigl T, Blaesse A, Mestres P, Meyer M, et al. Posttraumatic GABA(A) mediated [Ca2+]i increase is essential for the induction of brain-derived neurotrophic factor dependent survival of mature central neurons. J Neurosci. 2008;28:6996–7005. doi: 10.1523/JNEUROSCI.5268-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hu Y, Russek SJ. BDNF and the diseased nervous system: A delicate balance between adaptive and pathological processes of gene regulation. J Neurochem. 2008;105:1–17. doi: 10.1111/j.1471-4159.2008.05237.x. [DOI] [PubMed] [Google Scholar]
- 22.Sakai R, Ukai W, Sohma H, Hashimoto E, Yamamato M, Ikeda H, et al. Attenuation of brain derived neurotrophic factor by ethanol and cytoprotective effect of exogenous BDNF against ethanol damage in neuronal cells. J Neural Transm. 2005;112:1005–13. doi: 10.1007/s00702-004-0246-4. [DOI] [PubMed] [Google Scholar]
- 23.Heberlein A, Muschler M, Wilhelm J, Frieling H, Lenz B, Gröschl M, et al. BDNF and GDNF serum levels in alcohol-dependent patients during withdrawal. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34:1060–4. doi: 10.1016/j.pnpbp.2010.05.025. [DOI] [PubMed] [Google Scholar]
- 24.Miller MW, Mooney SM. Chronic exposure to ethanol alters neurotrophin content in the basal forebrain-cortex system in the mature rat: Effects on autocrine-paracrine mechanisms. J Neurobiol. 2004;60:490–8. doi: 10.1002/neu.20059. [DOI] [PubMed] [Google Scholar]
- 25.Barton ME, Shannon HE. The seizure-related phenotype of brain- derived neurotrophic factor knockdown mice. Neuroscience. 2005;136:563–9. doi: 10.1016/j.neuroscience.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 26.Koyama R, Ikegaya Y. To BDNF or not to BDNF: That is the epileptic hippocampus. Neuroscience. 2005;11:282–7. doi: 10.1177/1073858405278266. [DOI] [PubMed] [Google Scholar]
- 27.Reibel S, Larmet Y, Carnahan J, Marescaux C, Depaulis A. Endogenous control of hippocampal epileptogenesis: A molecular cascade involving brain-derived neurotrophic factor and neuropeptide Y. Epilepsia. 2000;41(Suppl 6):S127–33. doi: 10.1111/j.1528-1157.2000.tb01571.x. [DOI] [PubMed] [Google Scholar]
- 28.Caldeira MV, Melo CV, Pereira DB, Carvalho RF, Carvalho AL, Duarte CB. BDNF regulates the expression and traffic of NMDA receptors in cultured hippocampal neurons. Mol Cell Neurosci. 2007;35:208–19. doi: 10.1016/j.mcn.2007.02.019. [DOI] [PubMed] [Google Scholar]
- 29.Scharfman HE, Mercurio TC, Goodman JH, Wilson MA, MacLusky NJ. Hippocampal excitability increases during the estrous cycle in the rat: A potential role for brain-derived neurotrophic factor. J Neurosci. 2003;23:11641–52. doi: 10.1523/JNEUROSCI.23-37-11641.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.D’Sa C, Dileone RJ, Anderson GM, Sinha R. Serum and plasma brain-derived neurotrophic factor in abstinent alcoholics and social drinkers. Alcohol. 2012;46:253–9. doi: 10.1016/j.alcohol.2011.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]