Abstract
Introduction: An international clinical trial enrolled 174 ambulatory males ≥5 years old with nonsense mutation Duchenne muscular dystrophy (nmDMD). Pretreatment data provide insight into reliability, concurrent validity, and minimal clinically important differences (MCIDs) of the 6-minute walk test (6MWT) and other endpoints. Methods: Screening and baseline evaluations included the 6-minute walk distance (6MWD), timed function tests (TFTs), quantitative strength by myometry, the PedsQL, heart rate–determined energy expenditure index, and other exploratory endpoints. Results: The 6MWT proved feasible and reliable in a multicenter context. Concurrent validity with other endpoints was excellent. The MCID for 6MWD was 28.5 and 31.7 meters based on 2 statistical distribution methods. Conclusions: The ratio of MCID to baseline mean is lower for 6MWD than for other endpoints. The 6MWD is an optimal primary endpoint for Duchenne muscular dystrophy (DMD) clinical trials that are focused therapeutically on preservation of ambulation and slowing of disease progression. Muscle Nerve 48: 357–368, 2013
Keywords: 6-minute walk test, ambulation, Duchenne muscular dystrophy, energy expenditure index, muscular dystrophy, myometry, natural history, PedsQL, timed function test
Duchenne muscular dystrophy (DMD) is a disabling and life-threatening X-linked genetic disorder caused by defects in the gene for dystrophin, a protein that stabilizes muscle cell membranes.1 DMD is characterized by complete loss of dystrophin and is the most common neuromuscular disease of childhood. It affects 1 in 3800–6300 males, and there are an estimated 15,000 patients with the disease in the USA.2,3 There is no approved therapy that addresses the underlying cause of DMD.4,5 In ∽13% of boys with nonsense mutation DMD (nmDMD, which represents ∽1700 boys in the USA and ∽2400 boys in Europe), the causative defect in the dystrophin gene is a nonsense mutation that truncates dystrophin protein production by introducing a premature stop codon into dystrophin mRNA.6–8
THE NEED FOR CLINICALLY MEANINGFUL ENDPOINTS IN DMD
Given that several novel approaches to treatment of DMD have shown promise in preclinical and/or proof-of-concept clinical studies,9–12 the research community has faced the need to identify and develop clinically meaningful outcome measures for use in pivotal therapeutic trials. In boys with DMD, walking abnormalities are a major disease manifestation that has great importance to patients and families. Ambulation has been noted to be a prerequisite for normal physical functioning in humans13; the major goal of medical and physical therapy intervention during the ambulatory phase of DMD is to maintain ambulation for as long as possible.4,5,14,15 Given that ambulatory compromise is a key component of the DMD disease process and that ambulation measures the function of multiple muscle groups as well as cardiovascular activity, ambulation-related outcome measures are the most relevant endpoints in DMD patients who are still able to walk. Typically, evaluation of ambulation in DMD features short-term assessments, such as the 10-meter run/walk,14,16 which measure transient peak activities. The 10-m run/walk test is well accepted and commonly employed in assessing disease progression, but it does not measure endurance, a crucial aspect of ambulatory functioning.
DEVELOPMENT OF THE 6-MINUTE WALK TEST IN DMD
The 6-minute walk test (6MWT), a well-established outcome measure in a variety of diseases. It is accurate, reproducible, simple to administer, and well tolerated.17 It was originally developed as an integrated global assessment of cardiac, respiratory, circulatory, and muscular capacity.17 More recently, it has been used to evaluate functional capacity in neuromuscular diseases18–25 and has been the basis for regulatory registration of several drugs.19,21,24,25 Importantly, the 6MWT assesses function and endurance, which are important aspects of DMD patients' disease status. The 6MWT is a robust assessment tool for use in clinical trials given its ability to quantitatively evaluate ambulation in a controlled environment.
In advance of this study, the American Thoracic Society (ATS) version of the 6MWT was modified specifically for DMD.26 Also, an orientation video was developed to assist the pediatric subjects (some of whom have cognitive delay) in their understanding of the nature and expectations of the test. In an earlier short-term study, we reported that the modified 6MWT is feasible, safe, and reliable in boys with DMD who have not yet transitioned to full-time wheelchair use.26,27 We also documented that they have markedly compromised ambulation relative to healthy boys and correlated 6-minute walk distance (6MWD) with age, anthropometric characteristics, and measures, which change with disease progression, including stride length and cadence.26,27
In a follow-up longitudinal study,27 we documented that changes in 6MWD depended on stride length and age; improvements in 6MWD usually occurred up to 7 years of age in both healthy subjects and patients with DMD. However, the 6MWD of older DMD subjects worsened, whereas the 6MWD of older healthy subjects tended to either increase or remain stable. Subsequent studies have demonstrated that the 6MWD correlates with other clinical endpoints in DMD, such as timed function tests and the North Star Ambulatory Assessment (NSAA).28,29
MINIMAL CLINICALLY IMPORTANT DIFFERENCE OF ENDPOINTS
Interpretation of functional changes in walk tests can guide clinical management and be primary endpoints in interventional studies. It thus is important to determine whether a change in function is clinically relevant. Data from this study will allow us to determine quantitatively the minimal clinically important difference (MCID) for the test. The MCID is a concept defined as “the smallest difference in score in the domain of interest which patients perceive as beneficial and which would mandate, in the absence of troublesome side effects and excessive cost, a change in patient management.”30 The MCID is different from the minimal detectable change, which indicates the amount of change required to exceed measurement variability.31,32 When interpreting clinical measures, it is important to consider that, although small changes may be significant statistically, they may not be relevant clinically.31,33 Numerous methods to derive the MCID have been described.31,32,34–44 These include anchor-based methods,31,36,39,40 which compare a patient's change score with another measure of clinically relevant change, and distribution-based methods,36,41–43 such as the standard error of measurement (SEM), effect size, and one third the standard deviation (SD) at baseline, which are built on the statistical distribution and psychometric properties of the measure in a population. Anchor-based and distribution-based methods are seen as complementary approaches for MCID determination.44
AIMS
There are 3 aims in this report: (1) to assess safety and feasibility of the 6MWT in a large multicenter context; (2) to assess the reproducibility and concurrent validity of the 6MWT results in comparison with other commonly used clinical endpoints utilizing pretreatment results from the international multicenter, randomized, placebo-controlled study of ataluren in ambulatory boys with nmDMD (PTC124-GD-007-DMD, Study 007); and (3) to estimate the MCID using distribution-based methods. These analyses lay the groundwork for longitudinal studies of the 6MWD in DMD.
METHODS
Participants
The pretreatment data under evaluation were derived from Study 007; this study enrolled males ≥5 years old at 37 sites in 11 countries (Australia, Belgium, Canada, France, Germany, Israel, Italy, Spain, Sweden, UK, and USA). All patients had phenotypic evidence of dystrophinopathy; had a nonsense mutation in the dystrophin gene as determined by gene sequencing; and walked ≥75 m unassisted during a 6MWT at screening. There was an inclusion criterion of phenotypic evidence of more severe dystrophinopathy based on the onset of characteristic clinical symptoms (i.e., proximal muscle weakness, waddling gait, and Gower maneuver) by 9 years of age, an elevated serum creatine kinase (CK), and ongoing difficulty with ambulation. Patients on systemic glucocorticoids were required to be on a stable dose for 6 months prior to study entry. Institutional review boards/institutional ethics committees and health authorities approved the study protocol. All parents/participants provided signed informed consent/assent before study initiation.
Overall Study Design and Procedures
Study 007 was a phase 2b, international, multicenter, randomized, double-blind, placebo-controlled, dose-ranging study to evaluate the efficacy and safety of ataluren in ambulatory male patients with nmDMD ≥5 years old. Initial study evaluations (the subject of this report) were performed at screening and baseline separated by up to 6 weeks. Test–retest reliability was determined using the baseline and screening values for all endpoints. Concurrent validity and MCIDs were determined using pretreatment data.
Subject Disposition and Characteristics
There were 174 randomized patients. All patients screened and randomized were males, ranging in age from 5 to 20 years (Table 1). Approximately 56% of patients were age <9 years, 57% had a baseline 6MWD ≥350 m, and 71% were receiving glucocorticoids. Nonsense mutations were distributed across the 79 exons of the dystrophin gene, with no mutational hotspots identified, and represented all 3 types of premature stop codons.
Table 1.
Grading used during timed function tests (grades 1–6)
| Standing from supine |
|---|
| During the test for standing from a supine position, the method used by the patient was categorized and reported as follows: |
| 1. Unable to stand from supine, even with use of a chair. |
| 2. Assisted Gower―requires furniture for assistance in arising from supine to full upright posture. |
| 3. Full Gower―rolls over, stands up with both hands “climbing up” the legs to above the knees to achieve full upright posture. |
| 4. Half Gower―rolls over, stands up with 1 hand support on lower legs. |
| 5. Rolls to the side and/or stands up with one or both hands on the floor to start to rise. |
| 6. Stands up without rolling over or using hands. |
| Run/walk 10 m |
| During the test for running or walking 10 m, the method used by the patient was categorized and reported as follows: |
| 1. Unable to walk independently. |
| 2. Unable to walk independently but can walk with support from a person or with assistive device [full leg calipers (knee-ankle-foot orthoses―KAFOs) or walker]. |
| 3. Highly adapted gait, wide-based lordotic gait, cannot increase walking speed. |
| 4. Moderately adapted gait, can pick up speed but cannot run. |
| 5. Able to pick up speed but runs with a double stance phase (i.e., cannot achieve both feet off the ground). |
| 6. Runs and gets both feet off the ground (with no double stance phase). |
| 4-stair climbing |
| During the test for stair-climbing, the method used by the patient was categorized and reported as follows: |
| Ascending the stairs: |
| 1. Unable to climb up 4 standard stairs. |
| 2. Climbs 4 standard stairs “marking time” (climbs 1 foot at a time, with both feet on a step before moving to next step), using both arms on one or both handrails. |
| 3. Climbs 4 standard stairs “marking time” (climbs 1 foot at a time, with both feet on a step before moving to next step), using one arm on one handrail. |
| 4. Climbs 4 standard stairs “marking time” (climbs 1 foot at a time, with both feet on a step before moving to next step), not needing handrail. |
| 5. Climbs 4 standard stairs alternating feet, needs handrail for support. |
| 6. Climbs 4 standard stairs alternating feet, not needing handrail support. |
| 4-stair descending |
| During the test for stair-descending, the method used by the patient was categorized and reported as follows: |
| Descending the stairs: |
| 1. Unable to descend 4 standard stairs. |
| 2. Descends 4 standard stairs “marking time” (descends 1 foot at a time, with both feet on a step before moving to next step), using both arms on one or both handrails. |
| 3. Descends 4 standard stairs “marking time” (descends 1 foot at a time, with both feet on a step before moving to next step), using one arm on one handrail. |
| 4. Descends 4 standard stairs “marking time” (descends 1 foot at a time, with both feet on a step before moving to next step), not needing handrail. |
| 5. Descends 4 standard stairs alternating feet, needs handrail for support. |
| 6. Descends 4 standard stairs alternating feet, not needing handrail support. |
Outcome Measures
Before the study began, clinical evaluators from each of the 37 participating clinical sites participated in a clinical endpoint training and standardization session to harmonize the testing protocol and logistics across sites. A centralized retraining session was also held ∽1 year after study start. This report focuses on the outcome measures obtained from the patients during screening and baseline.
6-Minute Walk Test/6-Minute Walk Distance
Ambulation was assessed via the 6MWT following standardized procedures as developed at the University of California Davis,26 by measuring the 6MWD in meters. The 6MWT for this study included modifications to the method recommended by the ATS for use in adults described in Appendix 2 in the Supporting Information.17
Timed Function Tests
Timed function tests (TFTs) included time taken to stand from a supine position, time taken to run/walk 10 m, time taken to climb 4 standard-sized stairs, and time taken to descend 4 standard-sized stairs.45–51 TFTs provide a measure of functional capability in ambulatory patients that is complementary to the 6MWT. The tests are reproducible, simple to administer, and have documented response to therapeutic intervention with steroids.47,48 A stopwatch was used to time the 10-m run/walk, standing from supine, and 4-stair climb/descend. For standing from supine the velocity was calculated as 1 divided by the time to complete the task. For the total task of climbing 4 standard stairs, velocity was calculated as 1 divided by the time to complete the task. Subjects were given 30 s to complete all tasks. Use of velocities for timed function measures results in a linear pattern of decline that adequately represents the impact of the “zero velocities” of individuals who are unable to perform the evaluation.52
Timed Function Test Grades
Functional adaptations employed by patients during the TFTs were evaluated and graded by clinical evaluators according to standardized scales developed by one of the investigators (M.E.). Table 1 provides a description of the standardized scales.
Myometry
Upper and lower extremity myometry was performed using a hand-held myometer following standardized procedures.53–56 Muscle groups evaluated included knee flexors, knee extensors, elbow flexors, elbow extensors, and shoulder abductors. Bilateral assessments were done, and 3 measurements (in pounds) were recorded from each muscle group on each side.
Health-Related Quality of Life
Health-related quality of life (HRQL) was measured via the Pediatric Quality of Life Inventory (PedsQL).57–60 The generic core module comprises 23 questions. The PedsQL is available in all languages relevant for this study and was to be completed by both the patient and parent/caregiver. The appropriate age-specific version was completed. It was planned that a patient would be evaluated with the same age-specific form even if during the study an age change made him eligible for a different form. If the patient lacked the ability to complete the PedsQL, the parent/caregiver was still to complete the instrument. If possible, the same parent/caregiver was asked to complete the instrument each time. HRQL was measured by all domains of the PedsQL (Physical, Emotional, Social, and School Functioning domain scores); however, only physical scores are included in this report due to the relative insensitivity of the other domains to disease progression in DMD.61
Data Analysis
Available pretreatment data for all 174 patients from all sites were pooled for analysis of reliability (screening versus baseline performed within 6 weeks), concurrent validity (6MWD in comparison to selected secondary endpoints), and MCID determination for clinical endpoints. For the test–retest analysis in boys with DMD, subjects who had observations at both visits for the parameter of interest were included. Pearson r and intraclass correlations (ICCs) for visits 1 and 2 were recorded. For concurrent validity, either the Pearson r or Spearman rho (rs) rank order correlations were calculated. MCID was determined for clinical endpoints using 2 distribution methods: (1) the standard error of measurement method [baseline SD · √(1 − r)]; and (2) one third of SD method (baseline SD · ⅓).
Percent Predicted 6MWD
To account for maturational effects, including age, height, and associated stride length,26,27 we calculated a percent predicted 6MWD.62,63 This prediction equation has been validated in DMD62 using the same DMD modified 6MWT protocol as in this study.
Energy Expenditure Index
Mean heart rate was measured before (during 5-min rest), during, and for 3 min after the 6MWT with a Polar RS400 heart rate monitor. Using these data, a post hoc analysis of energy expenditure index (EEI) was performed. EEI is the active heart rate (beats per minute) minus resting heart rate (beats per minute) divided by walking velocity (meters per minute). Thus, EEI was measured in units of beats per meter. EEI has been documented by Rose and colleagues to be a validated measure of energy expenditure in comparison to oxygen uptake by a metabolic cart in disabled children.64–68
RESULTS
Patient Characteristics
Patient characteristics are shown in Table 2. All patients were males, ranging from 5 to 20 years of age. All 3 premature stop codon types were represented in the study population.
Table 2.
Patient characteristics (evaluated at screening and baseline)
| Characteristics | Baseline (N = 174) |
|---|---|
| Age, years | |
| Mean (SD) | 8.5 (2.6) |
| Median | 8.0 |
| Range | 5-20 |
| Race, n (%) | |
| White | 157 (90.2) |
| Black | 2 (1.1) |
| Asian | 6 (3.4) |
| Hispanic | 4 (2.3) |
| Other | 5 (2.9) |
| Body height, cm | |
| Mean (SD) | 125 (13.7) |
| Median | 123 |
| Range | 99-173 |
| Body weight, kg | |
| Mean (SD) | 31 (11.5) |
| Median | 27 |
| Range | 16-84 |
| Stop codon type, n (%) | |
| UGA | 83 (47.7) |
| UAG | 48 (27.6) |
| UAA | 43 (24.7) |
Test–Retest Reliability of Clinical Endpoints
Pretreatment screening and baseline tests were compared for test–retest reliability. The median (range) between-test interval was 42 (0–91) days. Data were available from 174 subjects enrolled at 37 study sites, as shown in Table 3. In general, test–retest reliability for most measures was high. The 6MWD had the highest test–retest reliability of any clinical endpoint (ICC = 0.92), as shown in Figure 1. ICCs for 6MWD, TFTs, and hand-held myometry were all strong (0.72–0.92), as shown in Table 3.
Table 3.
Test-retest reliability of selected clinical endpoints
| Clinical endpoints with timed dimension | ICC | Pearson r |
|---|---|---|
| 6MWD | 0.92 | 0.92 |
| 10-m run/walk | 0.85 | 0.87 |
| 4-stair climb | 0.91 | 0.91 |
| Stair descend | 0.83 | 0.83 |
| Supine to stand | 0.87 | 0.87 |
| Myometry | ICC | Pearson r | ICC | Pearson r |
|---|---|---|---|---|
| left side | left side | right side | right side | |
| Shoulder abduction | 0.76 | 0.81 | 0.74 | 0.78 |
| Elbow flexion | 0.82 | 0.86 | 0.81 | 0.95 |
| Elbow extension | 0.76 | 0.75 | 0.83 | 0.78 |
| Knee flexion | 0.72 | 0.92 | 0.77 | 0.91 |
| Knee extension | 0.91 | 0.86 | 0.89 | 0.93 |
6MWD, 6-minute walk distance; ICC, intraclass correlation coefficient. P < 0.0001 for all cases.
FIGURE 1.
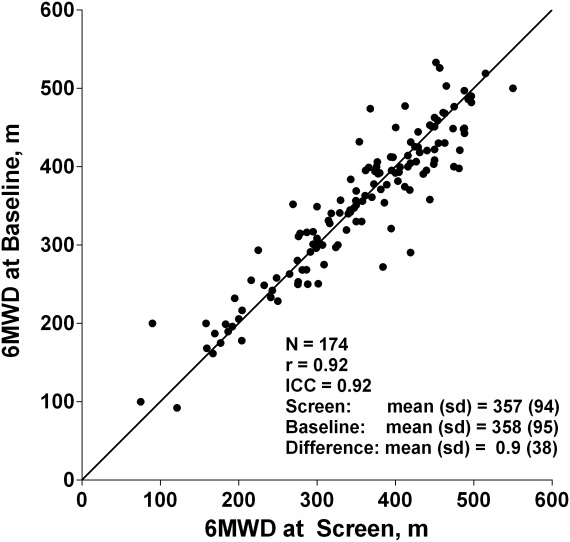
Reliability of the 6-minute walk distance (6MWD) test–retest.
Invalid 6MWT Values due to Musculoskeletal Injuries
Two patients had baseline 6MWD values that deviated markedly from their values at screening (up to 6 weeks earlier) and their first on-treatment values (at week 6). Both patients were documented to have sustained lower limb injuries prior to the baseline test (sprained ankle and right knee injury), which negatively affected their performances at baseline. Their 6MWD values at screening, baseline, and week 6 were 303, 125, and 309 for the first patient and 395, 309, and 481 m for the second patient, respectively. Lower limb injuries are a known source of 6MWD variability. Through a comprehensive analysis, it was verified that these were the only patients in whom the baseline 6MWT results were affected by lower limb injuries.
Considering the strong influence the injuries had on their baseline 6MWD values, it was considered appropriate to declare the baseline test for these patients invalid and to use the screening value as a more accurate reflection of their 6MWD at baseline.
Concurrent Validity of Clinical Endpoints
For concurrent validity, the following were evaluated:
6MWD vs. Timed Function Tests
Interpretation of time values (in seconds) obtained from timed function testing is limited to those patients who are able to complete the testing, thus creating results biased in favor of more functional individuals. As a result, time scores (in seconds) were converted into velocities. Table 4a shows correlations between 6MWD and velocities for TFTs at baseline (using Pearson r). Table 4b shows Spearman rank correlations between method of TFT and the respective time to perform the test. Figure 2 shows the relationship between the baseline velocity during the 10-m run/walk test and baseline 6MWD. It should be noted that a mean value of 358 m on the 6MWD corresponds to a velocity of 1.64 m/s on the 10-m run/walk, which is a time of 6 s for this test.
Table 4. a.
Pearson correlations between 6MWD and velocity for timed function tests
| 6MWD | 10-m run/walk | 4-stair climb | 4-stair descend | Supine to stand | |
|---|---|---|---|---|---|
| (m/s) | (stairs/s) | (stairs/s) | (1/s) | ||
| 6MWD | 1.0 | ||||
| 10-m run/walk (m/s) | 0.78 | 1.0 | |||
| 4-stair climb (stairs/s) | 0.77 | 0.85 | 1.0 | ||
| 4-stair descend (stairs/s) | 0.73 | 0.69 | 0.75 | 1.0 | |
| Supine to stand (1/s) | 0.73 | 0.86 | 0.82 | 0.59 | 1.0 |
P < 0.0001 in all cases. All other comparisons used Pearson r. 6MWD, 6-minute walk distance.
Table 4. b.
Correlations between timed function grade and 6MWD and velocity for timed function tests
| 6MWD | 10-m run/walk | 4-stair climb | 4-stair descend | Supine to stand | |
|---|---|---|---|---|---|
| (m/s) | (stairs/s) | (stairs/s) | (1/s) | ||
| 10-m run/walk grade | 0.63 | 0.79 | |||
| 4-stair climb grade | 0.73 | 0.82 | |||
| 4-stair descend grade | 0.70 | 0.74 | |||
| Supine to stand grade | 0.65 | 0.81 | |||
P < 0.0001 in all cases. Methods of timed function are only correlated with 6MWD and the time function velocity for the same functional task; comparisons were done using Spearman rho rank order correlations (rs). 6MWD, 6-minute walk distance.
FIGURE 2.
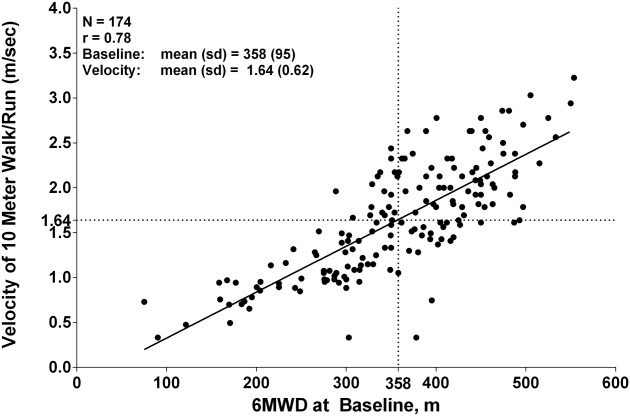
Correlation of velocity during 10-m walk/run vs. 6 minute walk distance (6MWD) at baseline. Note that a mean value of 358 m on 6MWD corresponds to a velocity of 1.64 m/s = 6 s on 10-m run/walk.
Timed Function Test Comparisons
As shown in Table 4a, all TFT velocities had a moderate to high correlation with one another and strong correlations with the specific methods for performance of the TFTs.
Myometry vs. 6MWD and Timed Function Tests
Table 5 shows the correlations between right and left myometry measures and 6MWDs, TFTs, and methods of timed function. Knee extension strength correlated better with timed function velocities and with timed function grades based on function, whereas knee flexion strength had lower correlations with timed function velocities and methods of timed function.
Table 6.
Correlations between myometry and 6MWD, velocity of timed function, and timed function grade
| Right knee extension | Left knee extension | Right knee flexion | Left knee flexion | |
|---|---|---|---|---|
| Pearson correlation | ||||
| 6MWD (m) | 0.64 | 0.68 | 0.38 | 0.42 |
| 10-m run/walk (m/s) | 0.70 | 0.69 | 0.33 | 0.34 |
| 4-stair climb (stair/s) | 0.74 | 0.73 | 0.37 | 0.36 |
| 4-stair descend (stair/s) | 0.58 | 0.58 | 0.41 | 0.43 |
| Supine to stand (1/s) | 0.70 | 0.67 | 0.28 | 0.27* |
| Spearman correlation | ||||
| 10-m run/walk grade | 0.62 | 0.60 | 0.17* | 0.21* |
| 4-stair climb grade | 0.73 | 0.69 | 0.33 | 0.31 |
| 4-stair descend grade | 0.60 | 0.61 | 0.36 | 0.33 |
| Supine to stand grade | 0.66 | 0.65 | 0.21* | 0.21* |
Comparisons with method of timed function use Spearman rho rank order correlations (rs); all other comparisons done using Pearson r. 6MWD, 6-minute walk distance.
P < 0.0001 except where noted (NS); *P < 0.05 †P < 0.01 and ‡P < 0.001.
6MWD vs. Knee Extension Strength
Figure 3a and b shows the relationships between knee extension strength and ambulatory function as measured by percent predicted 6MWD. Figure 3a depicts absolute quantitative knee extension strength (in pounds). Figure 3b depicts knee extension strength normalized to body weight. It should be noted that the relationship between these 2 variables (strength and 6MWD) is not linear but logarithmic. In DMD, at reduced 6MWD values below 50–55% predicted (based on age and height), there are substantial declines in ambulatory function as measured by the 6MWD. These declines occur despite relatively small changes in knee extension strength values, which have reached a floor effect.
FIGURE 3.
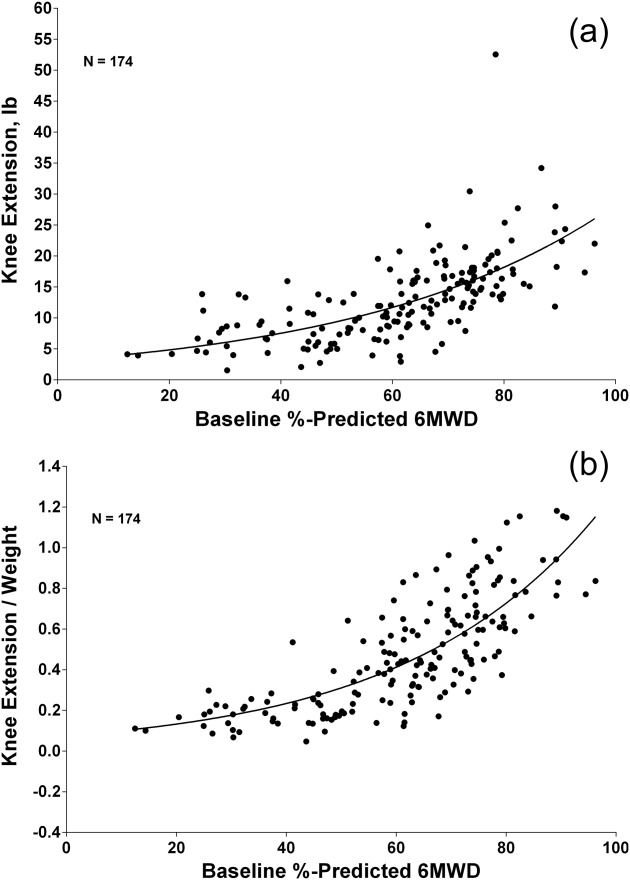
(a) Left frame: Correlation of knee extension strength (pounds) with percent predicted 6-minute walk distance (6MWD) at baseline. (b) Right frame: Correlation of knee extension strength per kilogram body weight with percent predicted 6MWD at baseline using age- and height-calculated formula.62,63
6MWD vs. Energy Expenditure Index
Figure 4 shows the relationship between heart rate–derived EEI and percent predicted 6MWD in 174 DMD subjects at baseline. The relationship between percent predicted 6MWD and heart rate–derived EEI is logarithmic. When percent predicted 6MWD approaches 50% of control values, there appears to be a precipitous increase in the energy cost of locomotion as measured by the EEI. Thus, the relationship between 6MWD and the EEI is well represented by a negative exponential model.
FIGURE 4.
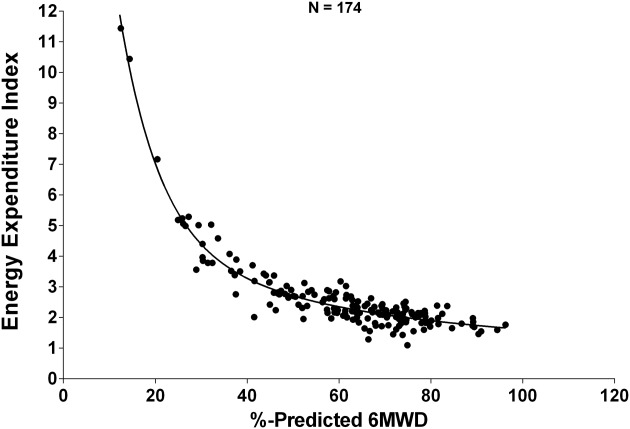
Relationship between energy expenditure index (EEI expressed in units of beats per meter) and percent predicted 6MWD in 174 DMD subjects at baseline.
6MWD vs. PedsQL Physical Function Scale
Figure 5 shows the correlation between 6MWD and the PedsQL Physical Function Scale in 174 DMD subjects evaluated at baseline. There was a moderate association between 6MWD and the patient-derived or parent-proxy PedsQL Physical Function Scale (r = 0.47).
FIGURE 5.
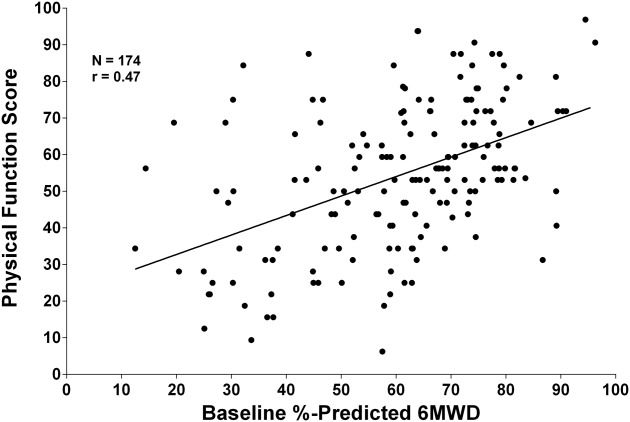
Correlation between 6MWD and PedsQL Physical Function Scale (n = 174 DMD evaluated at baseline).
Minimal Clinical Important Differences
Distribution-based methods were applied to pretreatment data on all subjects to generate a DMD-specific estimate of the MCID for the following clinical endpoints:
6MWD: Based on the standard error of measurement [defined as baseline SD · √(1 − r) where r is test–retest reliability], the MCID for 6MWD in DMD is estimated to be 28.5 m (Table 6). Based on a definition of one-third of the standard deviation at baseline, the estimated MCID for 6MWD in DMD is 31.7 m (Table 6). These MCID values represent 8.0% and 8.9% of the mean baseline 6MWD.
Table 6.
Estimates of MCID for 6MWD and other endpoints in DMD based on pretreatment baseline data
| Endpoint/method | N | Mean | SD | Correlation* | MCID | MCID/mean |
|---|---|---|---|---|---|---|
| 6MWD (m) | ||||||
| Standard error of measurement method (SD • √(1 – r)) | 174 | 358 | 95 | 0.91 | 28.5 | 8.0% |
| One third of SD method (SD • ⅓) | 31.7 | 8.9% | ||||
| Supine to stand (s) | ||||||
| Standard error of measurement method (SD • √(1 – r)) | 174 | 11.5 | 10.8 | 0.88 | 3.7 | 32.2% |
| One third of SD method (SD • ⅓) | 3.6 | 31.3% | ||||
| Climb 4 stairs (s) | ||||||
| Standard error of measurement method (SD • √(1 – r)) | 174 | 6.9 | 6.6 | 0.90 | 2.1 | 30.4% |
| One third of SD method (SD • ⅓) | 2.2 | 31.9% | ||||
| Run/walk 10 m (s) | ||||||
| Standard error of measurement method (SD * √(1 – r)) | 174 | 7.4 | 4.3 | 0.71 | 2.3 | 31.1% |
| One third of SD method (SD • ⅓) | 1.4 | 18.9% | ||||
| Knee extension strength by myometry (lbs.) | ||||||
| Standard error of measurement method (SD * √(1 – r)) | 174 | 13.4 | 7.1 | 0.91 | 2.1 | 15.7% |
| One third of SD method (SD • ⅓) | 2.4 | 17.9% | ||||
Table 9.
MCID for 6MWD in pulmonary and coronary diseases
| Disease | Method(s) | MCID (m) | Mean | MCID/ mean baseline | Reference |
|---|---|---|---|---|---|
| baseline | 6MWD | ||||
| 6MWD (m) | |||||
| Interstitial pulmonary fibrosis | Criterion referencing1 | 24 | 392 | 6.1% | Du Bois et al.74 (2011) |
| Effect size | 31 | 7.9% | |||
| SEM | 45 | 11.5% | |||
| Parenchymal lung disease | Criterion referencing2 | 29 | 403 | 7.2% | Holland et al.75 (2009) |
| SEM | 34 | 8.4% | |||
| COPD | Effect size | 29-42 | 361 | 8.0-11.6% | Puhan et al.76 (2008) |
| SEM | 35 | 9.7% | |||
| Coronary artery disease | Criterion referencing‡ | 25 | 490 | 5.1% | Gremeaux et al.77 (2011) |
| SEM | 23 | 490 | 4.7% |
6MWD, 6-minute walk distance; COPD, chronic obstructive pulmonary disease; MCID, minimal clinically important difference; SEM, standard error of measurement.
Comparison of baseline 6MWD with occurrence of hospitalization or death during subsequent 48-week period.
Table 8.
6MWT results in prior controlled registration studies
| Drug | Indication | N | Therapy duration (weeks) | Mean baseline 6MWD (m) | Mean 6MWD improvement1 (m) (SD) | % Change in 6MWD | Reference |
|---|---|---|---|---|---|---|---|
| Bosentan | PPH | 213 | 16 | 335 | 44 (NA) | 13% | Rubin et al.19 (2002) |
| Laronidase | MPS I | 45 | 26 | 344 | 38 (68) | 11% | Wraith et al.24 (2004) |
| Idursulfase | MPS II | 96 | 52 | 395 | 30 (61) | 8% | Muenzer et al.21 (2006) |
| Alglucosidase-α | Pompe disease | 90 | 78 | 327 | 28 (56) | 9% | van der Ploeg et al.25 (2010) |
6MWD, 6-minute walk distance; 6MWT, 6-minute walk test; MPS, mucopolysaccharidosis; NA, not available; PPH, primary pulmonary hypertension; SD, standard deviation.
Indicates difference between active drug and placebo group over the designated duration of therapy.
TFT: Using similar distribution-based methods for all baseline data for time to stand from supine, time to climb 4 stairs, and time to run/walk 10 m (Table 6), the MCID values represent 18.9–33.9% of the mean baseline TFT values.
Knee extension: Using these same distribution-based methods, Table 6 shows the MCID values for knee extension strength by hand-held myometry to be 15.7% and 17.9% of mean baseline knee extension strength.
DISCUSSION
The findings from this report reflect an evaluation of the largest data set collected to date in a multicenter context for determination of reliability and concurrent validity of 6MWD and other clinical endpoints in DMD. In addition, the investigation has addressed the distribution-based MCID for commonly employed endpoints, including TFTs and quantitative knee extension strength measures, as well as the 6MWD, which is the most common primary endpoint for ambulatory DMD clinical trials.
Test–Retest Reliability of Measures
The 6MWT showed the highest test–retest reliability of any endpoint used in the study. TFTs, such as the 10-m run/walk test, are easy to administer and conveniently applied in a clinical setting. However, these tests have inherent disadvantages for clinical trials, including slightly reduced test–retest reliability relative to the 6MWT. Advantages of the TFTs as secondary endpoints for DMD trials include previous steroid-naive natural history data16 and contemporary long-term natural history data available through the Cooperative International Neuromuscular Research Group (CINRG) Duchenne Natural History Study,52 relating the measures to loss of ambulation, the ease of utilizing the measures in the clinic in everyday clinical practice, and their potential inclusion as core measures for registries. Approximately 20% of patients, however, were unable to perform stand from supine at study entry.
To maximize reproducibility, the 6MWT should not be performed in the setting of an acute condition (e.g., musculoskeletal injury) that affects walking ability. In addition, future inclusion criteria can reduce variability by requiring that screening and baseline 6MWD values be within a certain percentage of one another (e.g., 20%).
Concurrent Validity of Clinical Endpoints
With regard to concurrent validity, the 6MWD was shown to be associated with other measures of disease progression in DMD, but in general it showed closer correlation with TFTs when compared with quantitative strength measures. Time to climb 4 stairs was the timed function measure most highly correlated with knee extension strength, so this may be a particularly useful secondary endpoint for DMD trials. Concurrent validity between the NSAA and 6MWD has been demonstrated previously.28,29 In this multicenter study the 6MWD correlated highly with the graded methods of performing TFTs, which are evaluator-derived DMD-specific measures of disease progression analogous to several components of the NSAA.
Myometry has been found previously to be less sensitive to changes in disease status than TFTs in ambulatory DMD boys.69 Our study has shown that the 6MWT has obvious advantages over quantitative strength measures as an endpoint in DMD due to its sensitivity to detect change in children who have a decline in ambulatory function. In DMD, at reduced 6MWD values below 50–55% predicted (based on age and height), there are significant continued declines in ambulatory function as measured by the 6MWD that occur despite relatively small changes in knee extension strength values, which appear to have approached a floor effect. An alternative concept is that, once lower extremity strength reaches a critically low threshold value, a more precipitous deterioration in ambulatory function may occur over 12 months with relatively little incremental loss of strength during that time.
Although there is a moderate correlation between 6MWD and the parent-proxy–reported PedsQL Physical Function Scale, the relationship is not as strong as that reported between 6MWD or walking speed and other patient-reported outcomes, such as the Transfers/Basic Mobility scale, Sports Physical Functioning Scale, or Global scale from the POSNA/PODCI Pediatric Outcomes Instrument.61,70
6MWT Is an Integrated Global Measure of Ambulatory Function and Metabolic Efficiency
Short-term assessments in DMD that measure transient peak physical activities, such as 10-m run/walk, do not measure endurance, a crucial aspect of ambulatory functioning. Due to the combination of strength loss and cardiopulmonary involvement, children with DMD experience increases in the energy cost of locomotion (more metabolic energy consumed per distance traveled) with increasing disease progression. Other investigators have validated the use of the heart rate–determined EEI as a measure of energy cost in disabled children.64–68 Heart rate–determined EEI has been validated previously in DMD using a COSMED portable metabolic cart.71 Other studies also showed increased energy cost of locomotion in DMD.72 Our study has shown that the 6MWD can be considered a proxy measure for the energy cost of locomotion in DMD. In general, higher EEI is associated with more metabolically inefficient ambulation and more impaired endurance. A recent report demonstrated the 6MWT to also be highly correlated with an assisted 6-minute cycling test,73 which is a measure of endurance in both ambulatory and non-ambulatory patients with DMD. The 6MWT is therefore an integrated global measure of ambulatory function that is influenced by decreased lower extremity strength, biomechanical inefficiencies during gait, diminished endurance, and compromised cardiorespiratory status.
Minimal Clinically Important Differences
MCID is a construct that can be determined by statistical distribution approaches, anchor-based methods with patient-reported outcome measures, and determination of clinically meaningful changes with treatments. Prior to the acquisition of disease-specific data, the study of MCID in other diseases is instructive. As shown in Appendix 3 in the Supporting Information, data from placebo-controlled studies of laronidase for mucopolysaccharidosis type I (MPS I), idursulfase for MPS II, bosentan for primary pulmonary hypertension, and alglucosidase-alpha for Pompe disease support the clinical meaningfulness of a 30-m treatment effect for the 6MWT.19,21,24,25 In these studies, differences in mean changes in 6MWD in drug-treated patients versus placebo-treated patients ranged from 28 to 44 m, or 8–13% of baseline 6MWD (Appendix 3 in Supplementary Material). The data from the trials in MPS I, MPS II, and Pompe disease are especially relevant given that patient activity limitations in these diseases and those in DMD result from disease-related impairments in neuromuscular and pulmonary systems.
Subsequent to the initiation of the ataluren Study 007 in 2008, additional research had been conducted to define the MCID for 6MWD across multiple diseases, including interstitial pulmonary fibrosis, coronary artery disease, chronic obstructive pulmonary disease, and parenchymal lung disease.74–77 In each of these diseases, as in DMD, patients experience disease-related deficits in 6MWD relative to healthy controls. Those studies employed multiple methods to determine the MCID for 6MWD, including distribution-based methods utilizing statistical properties (e.g., effect size, standard error of measurement). In these pulmonary and cardiac diseases, estimates of the MCID for 6MWD ranged from 23 to 45 m (Appendix 3 of Supporting Information).74–77 These estimates of the MCID for 6MWD correspond to 4.7–11.6% of mean baseline 6MWD.
Distribution-based methods are commonly used for initial MCID determination. In this study, we addressed initially the question of MCID for the 6MWD—a relatively new endpoint in DMD—by applying 2 commonly utilized distribution-based methods for DMD-specific MCID determination. The clinical trials that used the 6MWD in other diseases formed the basis of the a priori choice of a 30-m 6MWD treatment effect when originally powering the ataluren trial for ambulatory DMD subjects. In the present study of placebo-treated patients, the MCIDs for 6MWD in DMD (corresponding to 8.0% and 8.9% of the mean baseline 6MWD) are both mid-range values compared with the MCIDs determined for the other diseases. Thus, the statistical distribution data provide support for a 6MWD MCID of 30 m in DMD and a targeted 30-m difference between treatment arm and placebo for DMD therapeutic trials. Future longitudinal investigations of anchor-based approaches to MCID are suggested to validate these initial statistical distribution approaches and compare specific changes in 6MWD with the occurrence of both clinically meaningful milestones and significant changes in patient-reported outcomes. These studies may support a lower MCID for 6MWD than 30 m based on distribution-based methods. In addition, more experience evaluating the 6MWD in DMD patients treated with therapeutic agents known to be efficacious will help refine the determination of the MCID for this endpoint.
In this large, multicenter, international clinical trial, the 6MWT proved to be feasible and highly reliable, and it showed excellent concurrent validity with other commonly used clinical endpoints in DMD such as timed function tests and quantitative strength measures. The 6MWD proved to be a more sensitive clinical endpoint when compared with timed function and quantitative strength measures. Statistical distribution approaches support an MCID of ∽30 m for DMD. The 6MWT is an integrated global measure of ambulatory function that is influenced by lower extremity strength, biomechanical inefficiencies, endurance, and cardiorespiratory status. This study and additional longitudinal natural history data78 from the ataluren clinical trial (Study 007) support acceptance of the 6MWT as the primary outcome measure of choice for ambulatory DMD clinical trials.
Acknowledgments
The authors thank the patients and volunteers for their time and effort. We also thank the principal investigators, supporting investigators, clinical coordinators, clinical evaluator trainers, clinical evaluators, and study coordinators (see Appendix 1), and Zejiang Yang of INC Research for statistical programming support. Finally, we thank the patient advocacy organizations (including the Muscular Dystrophy Association and the Parent Project Muscular Dystrophy) for the collaboration and support, which made this trial possible.
Glossary
- 6MWD
6-minute walk distance
- 6MWT
6-minute walk test
- ATS
American Thoracic Society
- CINRG
Cooperative International Neuromuscular Research Group
- CK
creatine kinase
- DMD
Duchenne muscular dystrophy
- EEI
energy expenditure index
- HRQL
health-related quality of life
- MCID
minimal clinically important differences
- MPS
mucopolysaccharidosis
- nmDMD
nonsense mutation DMD
- NSAA
North Star Ambulatory Assessment
- PedsQL
Pediatric Quality of Life Inventory
- TFT
timed function test
Appendix 1
PTC124-GD-007-DMD Study Group Collaborating Authors
Australia
Murdoch Chidlren's Research Institute, Australia Royal Children's Hospital and University of Melbourne, Parkville, Victoria – Monique Ryan, MBBS
Department of Clinical Genetics, Children's Hospital at Westmead, New South Wales, Australia – Kristi Jones, MBBS
Belgium
Department of Pediatrics and Child Neurology, University Hospital Leuven, Belgium – Nathalie Goemans, MD
Canada
Department of Pediatrics, Children's Hospital Ontario, University of Western Ontario, London, ON, Canada – Craig Campbell, MD
Department of Neurology, Alberta Children's Hospital, University of Calgary, Alberta, Canada – Jean Mah, MD
Department of Pediatrics, Children's & Womens Health Center of British Columbial, University of British Columbia, Vancouver, BC, Canada– Kathryn Selby, B.Sc. MBChB, MRCP, FRCPC
France
Institiut de Myologie, Groupe Hospitalier Pitie-Salpetriere, Paris, France – Pr. Thomas Voit
Neurologie Pediatrique, Unite de Medecine Infantile Hopital d'Enfants, Marseilles, France – Pr. Brigitte Chabrol
Laboratoire d'Exploracion Fonctionnelles, Centre de Reference Maladies Neuromusculaires Nantez-Angers, CHU de Nantes, France – Pr. Yann Pereon
Germany
Department of Neuropediatrics, Neuromuscular Centre, University of Essen, Essen, Germany – Dr. med Ulrike Schara
Department of Pediatrics and Adolescent Medicine, University Hospital Frieberg, Germany – Dr. Janberndt Kirschner
Italy
Medicina Moleculare di Malattie Neuromuscolari e Neurodegenerative Dipartimento dei Laboratori Oespedale Pediatrico Bambino Gesu di Roma, Italy – Prof. Enrico Bertini
Dipartimento di Sciencze Pediatriche Medico-Chiurgiche e Neuroscienze dello Svilippo U.O. Complessa di Neurospichiatria Infantile Policlinica Universitario A. Gemelli, Rome, Italy – Prof. Eugenio Mercuri
Unita Operativa di Neurologia Fondazinone IRCCS, Ospedale Maggiore Policlinico, Milan, Italy – Prof. Giacomo Comi
Israel
Pediatric Neurology Unit, Hadassah Hebrew University Hospital, Jerusalem, Israel – Prof. Yoram Nevo
Spain
Departamento de Neurologia, Hospital Universitario La Fe, Valencia, Spain Hospital – Juan Vilchez, PhD,
Departamento de Neuropediatria, Hospital Sant Joan de Deu, Barcelona, Spain – Jaume Colomer, PhD
Sweden
Department of Pediatrics, Queen Sylvia Children's Hospital, University of Gothenburg, Sweden – Mar Tulinius, MD, PhD,
Department of Women's and Children's Health, Karolinksa University Hospital, Stockholm, Sweden – Thomas Sejersen, MD, PhD
United Kingdom
Institute of Genetic Medicine, Newcastle University, Newcastle Upon Tyne Great Britain – Prof. Katherine Bushby
Department of Neuroscience and Mental Health, King's College, London, UK King's College, London – Prof. Francesco Muntoni
National Hospital for Neurology and Neurosurgery, University College London Hospitals, London – Dr. Rosaline Christina Mary Quinlivan
United States
Department of Pediatrics and Neurology, Cincinnati Children's Medical Center, OH, USA – Brenda Wong, MD, MBBS
Department of Pediatrics, The Children's Hospital of Philadelphia, PA, USA – Richard S. Finkel, MD
Departments of Neurology and Pediatrics, University of Utah Medical Center, Salt Lake City, UT, USA – Jacinda B. Sampson, MD, PhD, Kevin M. Flanigan, MD, Russell Butterfield, MD
Department of Neurology, University of Minnesota, Minneapolis, MN, USA – John W. Day, MD, PhD
Department of Neurology, University of Iowa Children's Hospital, Iowa City, IA, USA – Katherine Mathews, MD
Department of Neurology, Children's Hospital Boston, MA, USA – Basil T. Darras, MD, Department of Rehabilitation Medicine, The Children's Hospital, University of Colorado, Denver, CO, USA – Susan D. Apkon, MD
Department of Neurology, The Children's Hospital, University of Colorado, Denver, CO, USA –Julie Parsons, MD
Department of Neurology, University of Kansas Medical Center, Kansas City, KS, USA – Richard Barohn, MD
Department of Neurology, Washington University School of Medicine at St. Louis, MO, USA – Anne Connolly, MD,
Department of Neurology, Children's Medical Center Dallas, University of Texas Southwestern, Dallas, TX, USA – Susan Iannaccone, MD
Department of Neurology, Columbia University Pediatric Neuromuscular Center, Columbia University Medical Center, New York, NY, USA – Douglas M. Sproule, MD, Petra Kaufman, MD, MSc
Department of Physical Medicine & Rehabilitation, Neuromuscular Medicine & Rehabilitation Research Center, University of California, Davis – Jay J. Han, MD, Nanette C. Joyce, DO
Northwest Florida Clinical Research Group, Gulf Breeze, FL, USA – J. Ben Renfroe, MD
Department of Neurology, Shriners Hospital for Children Portland, OR, USA – Barry S. Russman, MD
Department of Pediatrics and Division of Cardiology, Duke University, Durham, NC, USA – Stephanie Burns-Wechsler, MD
Department of Pathology, University of Iowa, Iowa City, IA, USA – Steven A Moore, MD, PhD
Department of Physiology, Unversity of Pennsylvania, PA, USA – H Lee Sweeney, PhD
OrthoCare Innovations, Mountlake Terrace, WA, USA – Kim Coleman, MS
Supplementary material
Additional Supporting Information may be found in the online version of this article.
REFERENCES
- 1.Hoffman EP, Brown RH, Jr, Kunkel LM. Dystrophin: the protein product of the Duchennemuscular dystrophy locus. Cell. 1987;51:919–928. doi: 10.1016/0092-8674(87)90579-4. [DOI] [PubMed] [Google Scholar]
- 2.Mendell JR, Shilling C, Leslie ND, Flanigan KM, al-Dahhak R, Gastier-Foster J, et al. Evidence-based path to newborn screening for Duchenne muscular dystrophy. Ann Neurol. 2012;71:304–313. doi: 10.1002/ana.23528. [DOI] [PubMed] [Google Scholar]
- 3.Prevalence of Duchenne/Becker muscular dystrophy among males aged 5–24 years—four states, 2007. MMWR Morb Mortal Wkly Rep. 2009;58:1119–1122. [PubMed] [Google Scholar]
- 4.Bushby K, Finkel R, Birnkrant DJ, Case LE, Clemens PR, Cripe L, et al. Diagnosis and management of Duchenne muscular dystrophy, part 1: diagnosis, and pharmacological and psychosocial management. Lancet Neurol. 2010;9:77–93. doi: 10.1016/S1474-4422(09)70271-6. [DOI] [PubMed] [Google Scholar]
- 5.Bushby K, Finkel R, Birnkrant DJ, Case LE, Clemens PR, Cripe L, et al. Diagnosis and management of Duchenne muscular dystrophy, part 2: implementation of multidisciplinary care. Lancet Neurol. 2010;9:177–189. doi: 10.1016/S1474-4422(09)70272-8. [DOI] [PubMed] [Google Scholar]
- 6.Dent KM, Dunn DM, von Niederhausern AC, Aoyagi AT, Kerr L, Bromberg MB, et al. Improved molecular diagnosis of dystrophinopathies in an unselected clinical cohort. Am J Med Genet A. 2005;134:295–298. doi: 10.1002/ajmg.a.30617. [DOI] [PubMed] [Google Scholar]
- 7.Hirawat S, Elfring GL, Leonard EM. Miller LL Request for FDA orphan drug designation for ataluren as treatment of Duchenne muscular dystrophy. PTC Therapeutics Orphan Drug Designation Application; 2004.
- 8.Hirawat S, Elfring GL, Leonard EM. Miller LL Request for EMEA orphan product designation for ataluren as treatment of Duchenne muscular dystrophy. PTC Therapeutics Orphan Product Designation Application; 2004.
- 9.Chamberlain JS, Rando TA. Duchenne muscular dystrophy: advances in therapeutics. New York: Taylor & Francis; 2006. [Google Scholar]
- 10.Welch EM, Barton ER, Zhuo J, Tomizawa Y, Friesen WJ, Trifillis P, et al. PTC124 targets genetic disorders caused by nonsense mutations. Nature. 2007;447:87–91. doi: 10.1038/nature05756. [DOI] [PubMed] [Google Scholar]
- 11.Goemans NM, Tulinius M, van den Akker JT, Burm BE, Ekhart PF, Heuvelmans N, et al. Systemic administration of PRO051 in Duchenne's muscular dystrophy. N Engl J Med. 2011;364:1513–1522. doi: 10.1056/NEJMoa1011367. [DOI] [PubMed] [Google Scholar]
- 12.Kinali M, Arechavala-Gomeza V, Feng L, Cirak S, Hunt D, Adkin C, et al. Local restoration of dystrophin expression with the morpholino oligomer AVI-4658 in Duchenne muscular dystrophy: a single-blind, placebo-controlled, dose-escalation, proof-of-concept study. Lancet Neurol. 2009;8:918–928. doi: 10.1016/S1474-4422(09)70211-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Crespo CJ, Keteyian SJ, Heath GW, Sempos CT. Leisure-time physical activity among US adults. Results from the Third National Health and Nutrition Examination Survey. Arch Intern Med. 1996;156:93–98. [PubMed] [Google Scholar]
- 14.Brooke MH, Fenichel GM, Griggs RC, Mendell JR, Moxley R, Florence J, et al. Duchenne muscular dystrophy: patterns of clinical progression and effects of supportive therapy. Neurology. 1989;39:475–481. doi: 10.1212/wnl.39.4.475. [DOI] [PubMed] [Google Scholar]
- 15.McDonald CM, Fowler WM., Jr The role of the neuromuscular medicine and physiatry specialists in the multidisciplinary management of neuromuscular disease. Phys Med Rehabil Clin N Am. 2012;23:475–493. doi: 10.1016/j.pmr.2012.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McDonald CM, Abresch RT, Carter GT, Fowler WM, Jr, Johnson ER, Kilmer DD, et al. Profiles of neuromuscular diseases. Duchenne muscular dystrophy. Am J Phys Med Rehabil. 1995;74(suppl):S70–92. doi: 10.1097/00002060-199509001-00003. [DOI] [PubMed] [Google Scholar]
- 17.ATS statement. Guidelines for the six-minute walk test. Am J Respir Crit Care Med. 2002;166:111–117. doi: 10.1164/ajrccm.166.1.at1102. [DOI] [PubMed] [Google Scholar]
- 18.Takeuchi Y, Katsuno M, Banno H, Suzuki K, Kawashima M, Atsuta N, et al. Walking capacity evaluated by the 6-minute walk test in spinal and bulbar muscular atrophy. Muscle Nerve. 2008;38:964–971. doi: 10.1002/mus.21077. [DOI] [PubMed] [Google Scholar]
- 19.Rubin LJ, Badesch DB, Barst RJ, Galie N, Black CM, Keogh A, et al. Bosentan therapy for pulmonary arterial hypertension. N Engl J Med. 2002;346:896–903. doi: 10.1056/NEJMoa012212. [DOI] [PubMed] [Google Scholar]
- 20.Dumas HM, Fragala MA, Haley SM, Skrinar AM, Wraith JE, Cox GF. Physical performance testing in mucopolysaccharidosis I: a pilot study. Pediatr Rehabil. 2004;7:125–131. doi: 10.1080/13638490310001654763. [DOI] [PubMed] [Google Scholar]
- 21.Muenzer J, Wraith JE, Beck M, Giugliani R, Harmatz P, Eng CM, et al. A phase II/III clinical study of enzyme replacement therapy with idursulfase in mucopolysaccharidosis II (Hunter syndrome) Genet Med. 2006;8:465–473. doi: 10.1097/01.gim.0000232477.37660.fb. [DOI] [PubMed] [Google Scholar]
- 22.Muenzer J, Gucsavas-Calikoglu M, McCandless SE, Schuetz TJ, Kimura A. A phase I/II clinical trial of enzyme replacement therapy in mucopolysaccharidosis II (Hunter syndrome) Mol Genet Metab. 2007;90:329–337. doi: 10.1016/j.ymgme.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 23.Harmatz P, Giugliani R, Schwartz, Guffon N, Teles EL, Miranda MC, et al. Long-term follow-up of endurance and safety outcomes during enzyme replacement therapy for mucopolysaccharidosis VI: Final results of three clinical studies of recombinant human N-acetylgalactosamine 4-sulfatase. Mol Genet Metab. 2008;94:469–475. doi: 10.1016/j.ymgme.2008.04.001. [DOI] [PubMed] [Google Scholar]
- 24.Wraith JE, Clarke LA, Beck M, Kolodny EH, Pastores GM, Muenzer J, et al. Enzyme replacement therapy for mucopolysaccharidosis I: a randomized, double-blinded, placebo-controlled, multinational study of recombinant human alpha-L-iduronidase (laronidase) J Pediatr. 2004;144:581–588. doi: 10.1016/j.jpeds.2004.01.046. [DOI] [PubMed] [Google Scholar]
- 25.van der Ploeg AT, Clemens PR, Corzo D, Escolar DM, Florence J, Groeneveld GJ, et al. A randomized study of alglucosidase alfa in late-onset Pompe's disease. N Engl J Med. 2010;362:1396–1406. doi: 10.1056/NEJMoa0909859. [DOI] [PubMed] [Google Scholar]
- 26.McDonald CM, Henricson EK, Han JJ, Abresch RT, Nicorici A, Elfring GL, et al. The 6-minute walk test as a new outcome measure in Duchenne muscular dystrophy. Muscle Nerve. 2010;41:500–510. doi: 10.1002/mus.21544. [DOI] [PubMed] [Google Scholar]
- 27.McDonald CM, Henricson EK, Han JJ, Abresch RT, Nicorici A, Atkinson L, et al. The 6-minute walk test in Duchenne/Becker muscular dystrophy: longitudinal observations. Muscle Nerve. 2010;42:966–974. doi: 10.1002/mus.21808. [DOI] [PubMed] [Google Scholar]
- 28.Mazzone E, Martinelli D, Berardinelli A, Messina S, D'Amico A, Vasco G, et al. North Star Ambulatory Assessment, 6-minute walk test and timed items in ambulant boys with Duchenne muscular dystrophy. Neuromuscul Disord. 2010;20:712–716. doi: 10.1016/j.nmd.2010.06.014. [DOI] [PubMed] [Google Scholar]
- 29.Mazzone E, Vasco G, Sormani MP, Torrente Y, Berardinelli A, Messina S, et al. Functional changes in Duchenne muscular dystrophy: a 12-month longitudinal cohort study. Neurology. 2011;77:250–256. doi: 10.1212/WNL.0b013e318225ab2e. [DOI] [PubMed] [Google Scholar]
- 30.Jaeschke R, Singer J, Guyatt GH. Measurement of health status. Ascertaining the minimal clinically important difference. Control Clin Trials. 1989;10:407–415. doi: 10.1016/0197-2456(89)90005-6. [DOI] [PubMed] [Google Scholar]
- 31.Beaton DE, Bombardier C, Katz JN, Wright JG, Wells G, Boers M, et al. Looking for important change/differences in studies of responsiveness. OMERACT MCID Working Group. Outcome measures in rheumatology. Minimal clinically important difference. J Rheumatol. 2001;28:400–405. [PubMed] [Google Scholar]
- 32.de Vet HC, Terwee CB, Ostelo RW, Beckerman H, Knol DL, Bouter LM. Minimal changes in health status questionnaires: distinction between minimally detectable change and minimally important change. Health Qual Life Outcomes. 2006;4:54. doi: 10.1186/1477-7525-4-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hays RD, Woolley JM. The concept of clinically meaningful difference in health-related quality-of-life research. How meaningful is it? Pharmacoeconomics. 2000;18:419–423. doi: 10.2165/00019053-200018050-00001. [DOI] [PubMed] [Google Scholar]
- 34.Wells G, Anderson J, Beaton D, Bellamy N, Boers M, Bombardier C, et al. Minimal clinically important difference module: summary, recommendations, and research agenda. J Rheumatol. 2001;28:452–454. [PubMed] [Google Scholar]
- 35.Jacobson N, Follette WC, Revenstorf D. Toward a standard definition of clinically significant change. Behav Ther. 1986;17:308–311. [Google Scholar]
- 36.Crosby RD, Kolotkin RL, Williams GR. Defining clinically meaningful change in health-related quality of life. J Clin Epidemiol. 2003;56:395–407. doi: 10.1016/s0895-4356(03)00044-1. [DOI] [PubMed] [Google Scholar]
- 37.Crosby RD, Kolotkin RL, Williams GR. An integrated method to determine meaningful changes in health-related quality of life. J Clin Epidemiol. 2004;57:1153–1160. doi: 10.1016/j.jclinepi.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 38.Jacobson NS, Truax P. Clinical significance: a statistical approach to defining meaningful change in psychotherapy research. J Consult Clin Psychol. 1991;59:12–19. doi: 10.1037//0022-006x.59.1.12. [DOI] [PubMed] [Google Scholar]
- 39.Deyo RA, Diehr P, Patrick DL. Reproducibility and responsiveness of health status measures. Statistics and strategies for evaluation. Control Clin Trials. 1991;12(suppl):142S–158S. doi: 10.1016/s0197-2456(05)80019-4. [DOI] [PubMed] [Google Scholar]
- 40.Deyo RA, Centor RM. Assessing the responsiveness of functional scales to clinical change: an analogy to diagnostic test performance. J Chronic Dis. 1986;39:897–906. doi: 10.1016/0021-9681(86)90038-x. [DOI] [PubMed] [Google Scholar]
- 41.Wyrwich KW, Nienaber NA, Tierney WM, Wolinsky FD. Linking clinical relevance and statistical significance in evaluating intra-individual changes in health-related quality of life. Med Care. 1999;37:469–478. doi: 10.1097/00005650-199905000-00006. [DOI] [PubMed] [Google Scholar]
- 42.Kazis LE, Anderson JJ, Meenan RF. Effect sizes for interpreting changes in health status. Med Care. 1989;27(suppl):S178–189. doi: 10.1097/00005650-198903001-00015. [DOI] [PubMed] [Google Scholar]
- 43.Yost KJ, Eton DT. Combining distribution- and anchor-based approaches to determine minimally important differences: the FACIT experience. Eval Health Prof. 2005;28:172–191. doi: 10.1177/0163278705275340. [DOI] [PubMed] [Google Scholar]
- 44.Beaton DE, Boers M, Wells GA. Many faces of the minimal clinically important difference (MCID): a literature review and directions for future research. Curr Opin Rheumatol. 2002;14:109–114. doi: 10.1097/00002281-200203000-00006. [DOI] [PubMed] [Google Scholar]
- 45.Brooke MH, Griggs RC, Mendell JR, Fenichel GM, Shumate JB, Pellegrino RJ. Clinical trial in Duchenne dystrophy. I. The design of the protocol. Muscle Nerve. 1981;4:186–197. doi: 10.1002/mus.880040304. [DOI] [PubMed] [Google Scholar]
- 46.Brooke MH, Fenichel GM, Griggs RC, Mendell JR, Moxley R, Miller JP, et al. Clinical investigation in Duchenne dystrophy: 2. Determination of the “power” of therapeutic trials based on the natural history. Muscle Nerve. 1983;6:91–103. doi: 10.1002/mus.880060204. [DOI] [PubMed] [Google Scholar]
- 47.Mendell JR, Moxley RT, Griggs RC, Brooke MH, Fenichel GM, Miller JP, et al. Randomized, double-blind six-month trial of prednisone in Duchenne's muscular dystrophy. N Engl J Med. 1989;320:1592–1597. doi: 10.1056/NEJM198906153202405. [DOI] [PubMed] [Google Scholar]
- 48.Griggs RC, Moxley RT, III, Mendell JR, Fenichel GM, Brooke MH, Pestronk A, et al. Prednisone in Duchenne dystrophy. A randomized, controlled trial defining the time course and dose response. Clinical Investigation of Duchenne Dystrophy Group. Arch Neurol. 1991;48:383–388. doi: 10.1001/archneur.1991.00530160047012. [DOI] [PubMed] [Google Scholar]
- 49.Fowler WM, Jr, Abresch RT, Aitkens S, Carter GT, Johnson ER, Kilmer DD, et al. Profiles of neuromuscular diseases. Design of the protocol. Am J Phys Med Rehabil. 1995;74:S62–69. doi: 10.1097/00002060-199509001-00002. [DOI] [PubMed] [Google Scholar]
- 50.Beenakker EA, Fock JM, van Tol MJ, Maurits NM, Koopman HM, Brouwer OF, et al. Intermittent prednisone therapy in Duchenne muscular dystrophy: a randomized controlled trial. Arch Neurol. 2005;62:128–132. doi: 10.1001/archneur.62.1.128. [DOI] [PubMed] [Google Scholar]
- 51.Pradhan S, Ghosh D, Srivastava NK, Kumar A, Mittal B, Pandey CM, et al. Prednisolone in Duchenne muscular dystrophy with imminent loss of ambulation. J Neurol. 2006;253:1309–1316. doi: 10.1007/s00415-006-0212-1. [DOI] [PubMed] [Google Scholar]
- 52.McDonald CM, Henricson EK, Abresch RT, Han JJ, Escolar DM, Florence JM, et al. The CINRG Duchenne Natural History Study—a longitudinal natural history study in the era of glucocorticoid therapy: design of the protocol and methods. Muscle Nerve. 2013;48:32–54. doi: 10.1002/mus.23807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Beenakker EA, van der Hoeven JH, Fock JM, Maurits NM. Reference values of maximum isometric muscle force obtained in 270 children aged 4–16 years by hand-held dynamometry. Neuromuscul Disord. 2001;11:441–446. doi: 10.1016/s0960-8966(01)00193-6. [DOI] [PubMed] [Google Scholar]
- 54.Hyde SA, Steffensen BF, Floytrup I, et al. Longitudinal data analysis: an application to construction of a natural history profile of Duchenne muscular dystrophy. Neuromuscul Disord. 2001;11:165–170. doi: 10.1016/s0960-8966(00)00175-9. [DOI] [PubMed] [Google Scholar]
- 55.Stuberg WA, Metcalf WK. Reliability of quantitative muscle testing in healthy children and in children with Duchenne muscular dystrophy using a hand-held dynamometer. Phys Ther. 1988;68:977–982. doi: 10.1093/ptj/68.6.977. [DOI] [PubMed] [Google Scholar]
- 56.Brussock CM, Haley SM, Munsat TL, Bernhardt DB. Measurement of isometric force in children with and without Duchenne's muscular dystrophy. Phys Ther. 1992;72:105–114. doi: 10.1093/ptj/72.2.105. [DOI] [PubMed] [Google Scholar]
- 57.Varni JW, Seid M, Kurtin PS. PedsQL™ 4.0: reliability and validity of the Pediatric Quality of Life Inventory™ Version 4.0 Generic Core Scales in healthy and patient populations. Med Care. 2001;39:800–812. doi: 10.1097/00005650-200108000-00006. [DOI] [PubMed] [Google Scholar]
- 58.Varni JW, Seid M, Knight TS, Uzark K, Szer IS. The PedsQL 4.0 Generic Core Scales: sensitivity, responsiveness, and impact on clinical decision-making. J Behav Med. 2002;25:175–193. doi: 10.1023/a:1014836921812. [DOI] [PubMed] [Google Scholar]
- 59.Varni JWBT, Seid M, Skarr D. The PedsQL 4.0 as a pediatric population health measure: feasibility, reliability, and validity. Ambul Pediatr. 2003;3:329–341. doi: 10.1367/1539-4409(2003)003<0329:tpaapp>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 60.Varni JWLC, Burwinkle TM. How young can children reliably and validly self-report their health-related quality of life?: an analysis of 8,591 children across age subgroups with the PedsQLTM 4.0 Generic Core Scales. Health Qual Life Outcomes. 2007;3:1. doi: 10.1186/1477-7525-5-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.McDonald CM, McDonald DA, Bagley A, Sienko ThomasS, Buckon CE, Henricson E, et al. Relationship between clinical outcome measures and parent proxy reports of health-related quality of life in ambulatory children with Duchenne muscular dystrophy. J Child Neurol. 2010;25:1130–1144. doi: 10.1177/0883073810371509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Henricson E, Abresch R, Han JJ, Nicorici A, Goude KellerE, Elfring G, et al. Percent-predicted 6-minute walk distance in Duchenne muscular dystrophy to account for maturational influences. PLoS Curr. 2012;4:RRN1297. doi: 10.1371/currents.RRN1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Geiger R, Strasak A, Treml B, Gasser K, Kleinsasser A, Fischer V, et al. Six-minute walk test in children and adolescents. J Pediatr. 2007;150:395–399. doi: 10.1016/j.jpeds.2006.12.052. [DOI] [PubMed] [Google Scholar]
- 64.Rose J, Medeiros JM, Parker R. Energy cost index as an estimate of energy expenditure of cerebral-palsied children during assisted ambulation. Dev Med Child Neurol. 1985;27:485–490. doi: 10.1111/j.1469-8749.1985.tb04572.x. [DOI] [PubMed] [Google Scholar]
- 65.Rose J, Gamble JG, Medeiros J, Burgos A, Haskell WL. Energy cost of walking in normal children and in those with cerebral palsy: comparison of heart rate and oxygen uptake. J Pediatr Orthop. 1989;9:276–279. [PubMed] [Google Scholar]
- 66.Rose J, Gamble JG, Burgos A, Medeiros J, Haskell WL. Energy expenditure index of walking for normal children and for children with cerebral palsy. Dev Med Child Neurol. 1990;32:333–340. doi: 10.1111/j.1469-8749.1990.tb16945.x. [DOI] [PubMed] [Google Scholar]
- 67.Rose J, Gamble JG, Lee J, Lee R, Haskell WL. The energy expenditure index: a method to quantitate and compare walking energy expenditure for children and adolescents. J Pediatr Orthop. 1991;11:571–578. [PubMed] [Google Scholar]
- 68.Rose J, Haskell WL, Gamble JG, Hamilton RL, Brown DA, Rinsky L. Muscle pathology and clinical measures of disability in children with cerebral palsy. J Orthop Res. 1994;12:758–768. doi: 10.1002/jor.1100120603. [DOI] [PubMed] [Google Scholar]
- 69.Beenakker EA, Maurits NM, Fock JM, Brouwer OF, van der Hoeven JH. Functional ability and muscle force in healthy children and ambulant Duchenne muscular dystrophy patients. Eur J Paediatr Neurol. 2005;9:387–393. doi: 10.1016/j.ejpn.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 70.Henricson EK, Abresch RT, Han JJ, Nicorici A, Goude E, de Bie E, McDonald CM. The 6-minute walk test and person-reported outcomes in boys with Duchenne muscular dystrophy and typically developing controls: Longitudinal comparisons and clinically-meaningful changes over one year Presented at the Muscular Dystrophy Association National Scientific Meeting, April 21–24, 2013. Washington, D.C. Submitted for publication. [DOI] [PMC free article] [PubMed]
- 71.Ong EKMC, Walsh DD, Widman L, Walsh SA, Abresch RT, McDonald CM. Energy cost of locomotion in boys with Duchenne muscular dystrophy measured with a portable metabolic cart. Devel Med Child Neurol. 1999;41:34. [Google Scholar]
- 72.Sienko Thomas S, Buckon CE, Nicorici A, Bagley A, McDonald CM, Sussman MD. Classification of the gait patterns of boys with Duchenne muscular dystrophy and their relationship to function. J Child Neurol. 2010;25:1103–1109. doi: 10.1177/0883073810371002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Jansen M, de Jong M, Coes HM, Eggermont F, van Alfen N, de Groot IJM. The assisted 6-minute cycling test to assess endurance in children with a neuromuscular disorder. Muscle Nerve. 2012;46:520–530. doi: 10.1002/mus.23369. [DOI] [PubMed] [Google Scholar]
- 74.Du Bois RM, Weycker D, Albera C, Bradford WZ, Costabel U, Kartashov A, et al. Six-minute-walk test in idiopathic pulmonary fibrosis: test validation and minimal clinically important difference. Am J Respir Crit Care Med. 2011;183:1231–1237. doi: 10.1164/rccm.201007-1179OC. [DOI] [PubMed] [Google Scholar]
- 75.Holland AE, Hill CJ, Conron M, Munro P, McDonald CF. Small changes in six-minute walk distance are important in diffuse parenchymal lung disease. Respir Med. 2009;103:1430–1435. doi: 10.1016/j.rmed.2009.04.024. [DOI] [PubMed] [Google Scholar]
- 76.Puhan MA, Mador MJ, Held U, Goldstein R, Guyatt GH, Schünemann HJ. Interpretation of treatment changes in 6-minute walk distance in patients with COPD. Eur Respir J. 2008;32:637–643. doi: 10.1183/09031936.00140507. [DOI] [PubMed] [Google Scholar]
- 77.Gremeaux V, Troisgros O, Benaïm S, Hannequin A, Laurent Y, Casillas JM, et al. Determining the minimal clinically important difference for the six-minute walk test and the 200-meter fast-walk test during cardiac rehabilitation program in coronary artery disease patients after acute coronary syndrome. Arch Phys Med Rehabil. 2011;92:611–619. doi: 10.1016/j.apmr.2010.11.023. [DOI] [PubMed] [Google Scholar]
- 78.McDonald C, Henricson EK, Abresch RT, Florence JM, Eagle M, Gappmaier E, et al. The 6-minute walk test and other endpoints in nonsense-mutation-mediated dystrophinopathy: longitudinal natural history observations in a multicenter study. Muscle Nerve. doi: 10.1002/mus.23902. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


