Abstract
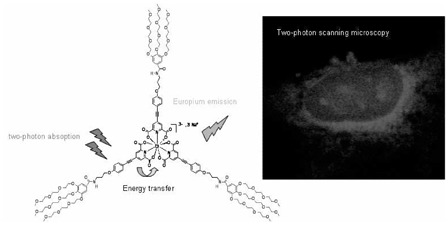
A new europium complex presenting good solubility and stability in water, intense emission in the red (616 nm), long luminescence lifetime and significant two-photon absorption cross-section in the biological window has been designed and successfully used for two-photon scanning microscopy bio-imaging experiments on fixed cancer cells.
Keywords: Europium; chemistry; Luminescent Measurements; Microscopy, Fluorescence, Multiphoton; Molecular Structure; Solubility; Water; chemistry
The unique luminescence properties of LnIII ions [1] (sharp emission, large Stockes shift, insensitivity to oxygen) and particularly their long excited state lifetime ranging from μs (Yb, Nd) to ms (Eu, Tb) triggered the development of time-resolved spectroscopy or microscopy for applications in biological, environmental or clinical analysis.[2,3] These techniques consist in the introduction of a time delay before the detection of the lanthanide luminescence in order to eliminate parasitic scattering and short-lived luminescence resulting in an enhanced signal/noise ratio. However, UV-light is needed for the metal sensitization, which is a drawback for biological applications. Indeed, these wavelengths are scattered/absorbed by the medium, which limits the investigation depth and presents some photo-toxicity for the biological samples. Two (or multi-) photon excitation, that is simultaneous absorption of two photons of half energy, is an elegant way to circumvent the use of UV-light. Following pioneering works of Webb in the 1990’s,[4] confocal biphotonic microscopy is becoming even more popular with the commercialization of tunable fs-laser source. This technique allows the use of irradiation wavelengths located in the near infrared (700–900 nm), a spectral range where the biological media are transparent. In addition, its intrinsic confocal character strongly limits the photo-damage and gives rise to 3D resolved spectroscopy and microscopy.[5] Whereas numerous works have been devoted to the optimization of the luminescence properties of lanthanide complexes for imaging or sensing applications,[2,3] their two-photon sensitization is becoming an emerging field of research. The proof-of-concept of two-photon antenna effect has already been demonstrated in biological media with complexes featuring very low (generally not measured) two-photon absorption (2PA) cross-section, σ2PA.[6] On the other hand, significant σ2PA values are reported in the cases of EuIII complexes only stable in non aqueous solvents,[7] not suitable for any practical applications as biological probes. In this article, we report the design of new functionalized tris-dipicolinate EuIII complexes (Chart 1) that are (i) soluble and stable in aqueous media, (ii) luminescent with long excited lifetime and (iii) that present significant two-photon absorption cross-section in the biological window.
Chart 1.
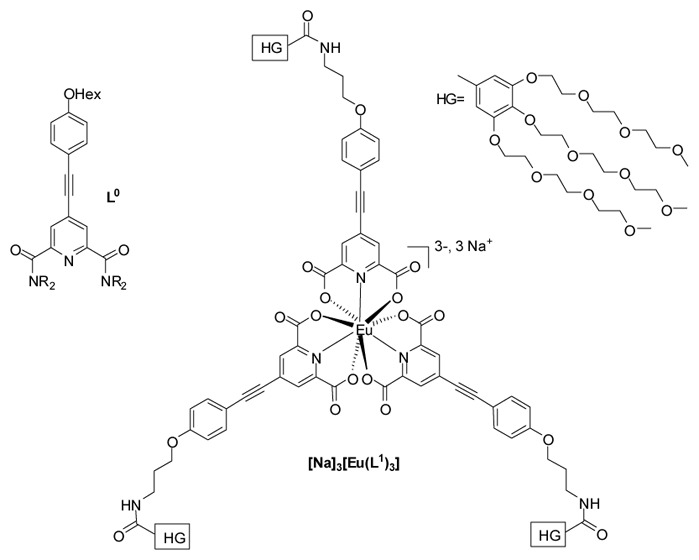
Structure of the ligands and related complex.
Recently, we described the two-photon antenna effect of tricationic [EuL03][OTf]3 complex (σ2PA = 96 GM at 720 nm), where L0 is a alkyloxyphenylacetylene functionalized pyridine-dicarboxamide ligand (Chart 1).[7a] Its very low stability prevented any use in aqueous media and prompted us to switch to pyridine-dicarboxylic acid (DPA) analogous known to present a enough stability in water for spectroscopic studies.[8] DPA was functionalized by extended π-system featuring weak electron donor alkoxy group (L1 Chart 1) and the hydrosolubility was ensured by 3,4,5-tris(triethyleneglycol)phenyl moieties (HG).[9] Ligand synthesis is reported in supporting information. The corresponding complex [Na]3[Eu(L1)3], was prepared in water by mixing three equiv. of ligand in basic media with EuCl3,6H2O and isolated by extraction with dichloromethane. The spectroscopic characterizations are in agreement with the proposed structures (SI) and the complex is stable and soluble in organic solvents and water.
All the photophysical properties of the ligand and complex were recorded in water and the stability of the complex in aqueous media is similar to that of the non functionalized parent analogous (see SI for details). L1 presents a broad transition by UV-visible spectroscopy centred at 318 nm (25600 L.mol−1 cm−1) in water at pH = 5 assigned to charge transfer (CT) transition from the alkoxy donor to the pyridinic acceptor moieties.[7a] Complexation to EuIII results in a small bathochromic shift of the CT transition (λmax = 332 nm (78700 L.mol−1 cm−1), Figure 1), the lanthanide Lewis acidity effect being partially compensated by the trianionic nature of the complex, and is accompanied by a threefold exaltation of the extinction coefficient in agreement with the 3/1 ligand-to-metal ratio. Emission spectra reveal the characteristic EuIII emission profile with the very intense 5D0→7F2 transition upon excitation in the ligand CT transition (Figure 2). The quantum yield of the complex is good, about 15.7%, the lifetime is long (1.062 ms in water, figure S2) and remains far higher than that of other organic or endogen chromophores (few ns). The two-photon excitation spectrum was recorded, in the 700–900 nm range using a femtosecond Ti:sapphire laser source.[7a] The 2PA process is unambiguously confirmed by the quadratic dependence (experimental coef. 1.9) of the 613 nm band with the laser power (λex = 720 nm, Figure S5). The 2PA spectrum matches almost perfectly the wavelength doubled one absorption spectra (1PA) with a maximum 2PA wavelength around 700 nm (Figure 1), indicating that the excited states involved in the 1PA and 2PA processes are the same. In the measured range, the maximal 2PA cross-section is significant, about 92 GM at 700 nm. It is worth underlining that this value is in the same range than that of other lanthanide compounds,[7a–c] only stable in organic solvents and far higher than that of the water stable complexes.[6,7c]
Figure 1.
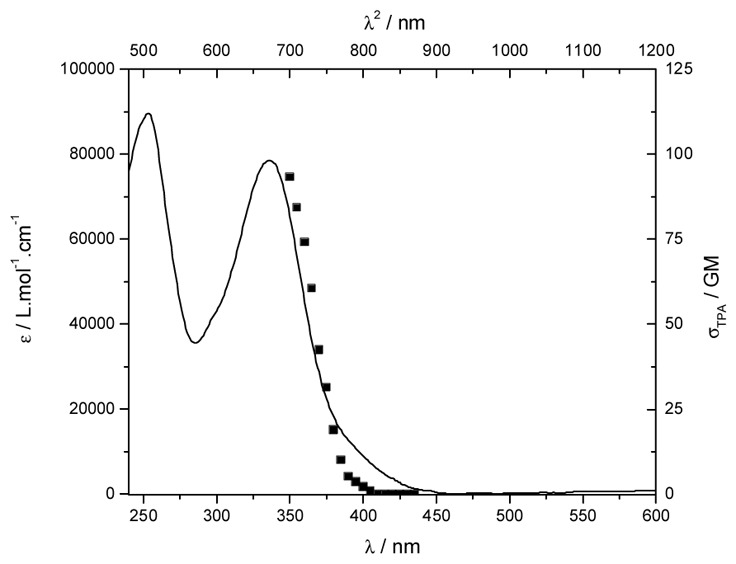
Absorption spectra of [Na]3[Eu(L1)3] (—) in water. In upper abscissa is superimposed the two-photon excitation spectra (■).
Figure 2.
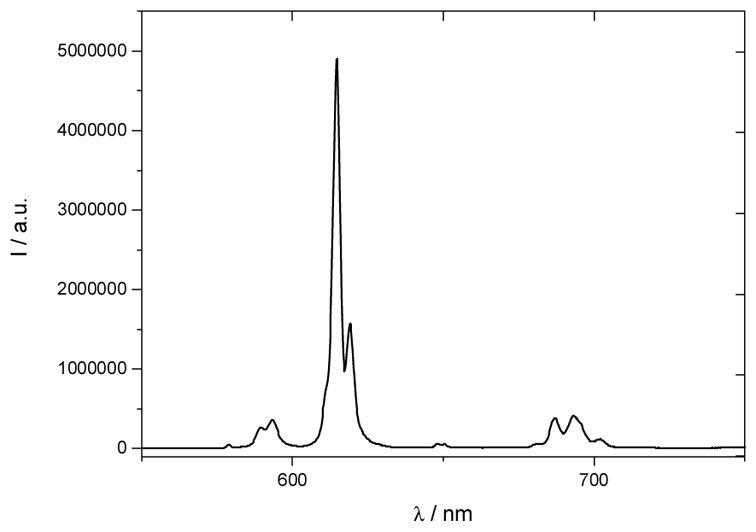
Emission spectra of [Na]3[Eu(L1)3] in water (λex = 340 nm, conc = 1.31.10−5 mol.L−1)
Finally, two-photon scanning microscopy experiments were carried out using T24 cancer cells fixed with ethanol at −20°C and loaded with [Na]3[Eu(L1)3] in PBS solution (concentration in the sample about 2.10−5 mol.L−1.cm−1, see SI for details). The images shown in figure 3 were taken on a biphotonic laser scanning microscope upon fs-760 nm irradiation (σ2PA (760) = 19 GM). The red Eu luminescence was integrated in 503–695 nm spectral range. Comparison with phase contrast image clearly indicates that the complex is mainly localised in a perinuclear region and its distribution is similar to that of the endoplasmic reticulum. In addition, bright spots are observed in the nucleus indicating that the complex preferentially targeted small organelles called nucleoli, similarly to a recent study of Parker and co-coworkers.[3a]
Figure 3.
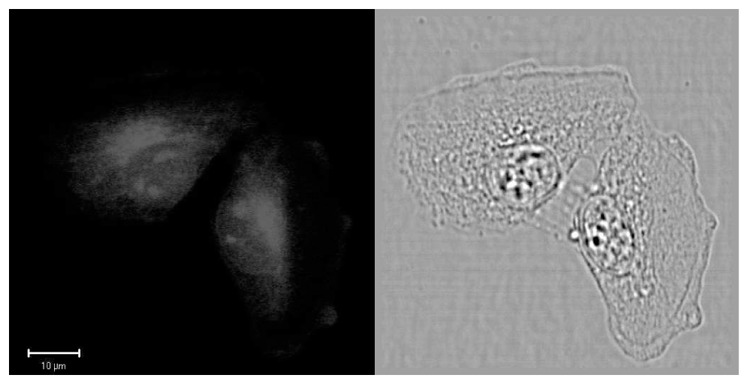
Two-photon excited luminescence (left, λex = 760 nm) and phase contrast (right) images of T24 cancer cell fixed in ethanol and loaded with [Na]3[Eu(L1)3]. Image were taken on a Zeiss LSM510 NLO META confocal microscope equipped with a fs-Ti:sapphire laser.
In conclusion, we report the design of a new europium complex that fulfills all the requirements for bio-imaging applications: (i) good solubility and stability in water, (ii) intense emission in the red (616 nm), (iii) long luminescence lifetime and (iv) significant two-photon absorption cross-section induced by two-photon antenna effect in the biological window. Furthermore, we describe the first two-photon scanning microscopy bio-imaging experiments using a lanthanide complex that can be considered as a new generation of molecular probes. Further studies are currently carried out (i) to study localization of the complex in the cells, (ii) to increase the molecular two-photon cross-section and water stability of the complexes and (iii) to develop biological imaging using biphotonic time-resolved microscopy.
Acknowledgments
The authors thank the ANR LnOnL NT05-3_42676) for financial support. Two photon microscopy facilities were partially supported by ARC and NANOBIO program.
Footnotes
Supporting Information Available. Experimental data, figures S1-5 are available free of charge via the Internet at http://pub.acs.org.
References
- 1.For reviews see: Bünzli JC. Acc Chem Res. 2006;39:53–61. doi: 10.1021/ar0400894.Pandya S, Yu J, Parker Dalton Trans. 2006:2757–2766. doi: 10.1039/b514637b.Gunnlaugsson T, Leonard JP. Chem Commun. 2005:3114–3131. doi: 10.1039/b418196d.Faulkner A, Pope SJA, Burton-Pye BP. Appl Spec Rev. 2004;39:1–35.Parker D. Chem Soc Rev. 2004;33:156–165. doi: 10.1039/b311001j.
- 2.(a) Mariott G, Clegg RM, Arndt-Jovin DJ, Jovin TM. Biophys J. 1991;60:1374–1386. doi: 10.1016/S0006-3495(91)82175-0. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Hemmilä I, Webb S. Drug Discovery Today. 1997;2:373–381. [Google Scholar]
- 3.(a) Yu J, Parker D, Pal R, Poole RA, Cann MJ. J Am Chem Soc. 2006;128:2294–2299. doi: 10.1021/ja056303g. [DOI] [PubMed] [Google Scholar]; (b) Charbonnière LJ, Hildebrandt N, Ziessel R, Löhmannsröben HG. J Am Chem Soc. 2006;128:12800–12809. doi: 10.1021/ja062693a. [DOI] [PubMed] [Google Scholar]; (c) Aarons RJ, Notta JK, Meloni MM, Feng J, Vidyasagar R, Narvainen J, Allan S, Spencer N, Kauppinen RA, Snaith JS, Faulkner S. Chem Commun. 2006:909–911. doi: 10.1039/b515160k. [DOI] [PubMed] [Google Scholar]; (d) Weibel N, Charbonnière LJ, Guardigli M, Roda A, Ziessel R. J Am Chem Soc. 2004;126:4888–4896. doi: 10.1021/ja031886k. [DOI] [PubMed] [Google Scholar]; (e) Charbonnière LJ, Weibel N, Estournes C, Leuvrey C, Ziessel R. New J Chem. 2004;28:777–781. [Google Scholar]; (f) Beeby A, Botchway SW, Clarkson IM, Faulkner S, Parker AW, Parker D, Williams JAG. J Photochem Photobiol B. 2000;57:83–89. doi: 10.1016/s1011-1344(00)00070-1. [DOI] [PubMed] [Google Scholar]; (g) Vereb G, Jares-Reijman E, Selvin PR, Jovin TM. Biophys J. 1998;74:2210–2222. doi: 10.1016/S0006-3495(98)77930-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Denk W, Strickler JH, Webb WW. Science. 1990;248:73. doi: 10.1126/science.2321027. [DOI] [PubMed] [Google Scholar]
- 5.(a) Yuste R. Nat Methods. 2005;2:902–904. doi: 10.1038/nmeth1205-902. [DOI] [PubMed] [Google Scholar]; (b) Campagnola PJ, Loew LM. Nat Biotechnol. 2003;21:1356–1360. doi: 10.1038/nbt894. [DOI] [PubMed] [Google Scholar]; (c) Zipfel WR, Williams RM, Web WW. Nat Biotechnol. 2003;21:1356–1360. doi: 10.1038/nbt899. [DOI] [PubMed] [Google Scholar]
- 6.(a) White GF, Litvinenko KL, Meech SR, Andrews DL, Thomson AJ. Photochem Photobiol Sci. 2004;3:47–55. doi: 10.1039/b306760b. [DOI] [PubMed] [Google Scholar]; (b) Piszczek G, Maliwal BP, Gryczynski I, Dattelbaum J, Lakowicz JR. J Fluoresc. 2001;11:101–107. doi: 10.1023/A:1016673300913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.(a) Picot A, Malvolti F, Le Guennic B, Baldeck PL, Williams JAG, Andraud C, Maury O. Inorg Chem. 2007;46:2659–2665. doi: 10.1021/ic062181x. [DOI] [PubMed] [Google Scholar]; (b) Fu LM, Wen XF, Ai XC, Sun Y, Wu YS, Zhang JP, Wang Y. Angew Chem Int Ed. 2005;44:747–750. doi: 10.1002/anie.200462382. [DOI] [PubMed] [Google Scholar]; (c) Werts MHV, Nerambourg N, Pélégry D, Le Grand Y, Blanchard-Desce M. Photochem Photobiol Sci. 2005;4:531–538. doi: 10.1039/b504495b. [DOI] [PubMed] [Google Scholar]
- 8.The stability of [Ln(DPA]3]3− complexes in water was studied by NMR and spectroscopic experiments. In the 10−4/10−5 concentration range, the dissociation can be estimated about 30%: Ouali N, Bocquet B, Rigault S, Morgantini PY, Weber J, Piguet C. Inorg Chem. 2002;41:1436–1445. doi: 10.1021/ic010801i.Chauvin A-S, Gumy F, Imbert D, Bünzli J-CG. Spetro Lett. 2004;37:517–532.Lessmann JJ, Horrocks W, Jr, De W. Inorg Chem. 2000;39:3114–3124. doi: 10.1021/ic990698l.Values from critical stability constant (vol 1) by Martell AE, Smith RM. Plenum Press; New York: 1974.
- 9.(a) Hayek A, Bolze F, Nicoud JF, Baldeck PL, Mély Y. Photochem Photobiol Sci. 2006;5:102–106. doi: 10.1039/b509843b. [DOI] [PubMed] [Google Scholar]; (c) Oar MA, Serin JM, Dichtel WR, Fréchet JM. Chem Mater. 2005;17:2267–2275. [Google Scholar]


