Abstract
Background
Tiotropium has activity as an asthma controller. However, predictors of a positive response to tiotropium have not been described.
Objective
To describe individual and differential response of patients with asthma to salmeterol and tiotropium, when added to an ICS, as well as predictors of a positive clinical response.
Methods
Data from the double-blind, three-way crossover NHLBI Asthma Clinical Research Network’s TALC trial (ClinicalTrials.gov number, NCT00565266) were analyzed for individual and differential treatment responses to salmeterol and tiotropium, and predictors of a positive response to the endpoints FEV1, morning peak expiratory flow (AM PEF), and asthma control days (ACDs).
Results
While approximately equal numbers of patients showed a differential response to salmeterol and tiotropium in terms of AM PEF (90 and 78, respectively), and ACDs (49 and 53, respectively), more showed a differential response to tiotropium for FEV1 (104) than salmeterol (62). An acute response to a short-acting bronchodilator, especially albuterol, predicted a positive clinical response to tiotropium for FEV1 (OR 4.08 [CI 2.00–8.31], P < 0.001) and AM PEF (OR 2.12 [CI 1.12–4.01], P = 0.021), as did a decreased FEV1/FVC ratio (FEV1 response increased 0.39% of baseline for every 1% decrease in the FEV1/FVC ratio). Higher cholinergic tone was also a predictor, while ethnicity, gender, atopy, IgE Level, sputum eosinophils, FENO, asthma duration, and BMI were not.
Conclusion
While these results need confirmation, predictors of a positive clinical response to tiotropium include a positive response to albuterol and airway obstruction, factors which could help identify appropriate patients for this therapy.
Keywords: asthma, tiotropium, salmeterol, responder analysis, predictor of response
Multiple conflicting strategies have been proposed in attempting to obtain the best outcomes for patients with chronic diseases, including asthma. While much of the past several decades has been devoted to developing standardized treatment guidelines and attempting to improve physician and patient adherence,1,2 more recent efforts have been devoted to identifying the best treatment approaches for specific patients and subgroups of patients (i.e. “personalizing” treatment approaches).3 Such attempts have used a variety of strategies including the use of biomarkers, patient-specific and physiologic “predictors,” and genetic/genomic approaches.3–5
While investigators have explored predictors of response to a variety of drugs used to treat patients with asthma, including short-acting bronchodilators and leukotriene modifiers,6–9 more attention has been devoted to identifying variables which can be used to predict the response to glucocorticoids, particularly inhaled corticosteroids (ICS).7–10 These experiences have both provided valuable insights and approaches to move this area of investigation forward. 11
Within this framework, less information has been published concerning predictors of response of subjects to treatment with long-acting bronchodilators, such as long-acting beta-agonists (LABAs) and long-acting anti-cholinergic agents (long-acting muscarinic antagonists; LAMAs). In addition, no data are available describing the intra-subject response of asthmatics treated with both a LABA (salmeterol) and a LAMA (tiotropium bromide). This report describes the response of add-on therapy with the LABA salmeterol, or the LAMA tiotropium bromide in individual patients with asthma treated with an ICS, in the NHLBI’s Asthma Clinical Research Network’s (ACRN’s) Tiotropium Bromide as an Alternative to Increased Inhaled Glucocorticoid in Patients Inadequately Controlled on a Lower Dose of Inhaled Corticosteroid (TALC) trial (ClinicalTrials.gov number, NCT00565266).12 In addition to individual patient responses, differential responses to the two drugs, and predictors of a positive clinical response for the outcomes of morning peak expiratory flow (AM PEF), FEV1, and asthma control days (ACDs) are described.
METHODS
TALC Trial Design
The TALC trial was a double-blind, three-way crossover trial which randomized patients who were inadequately controlled on a low dose of inhaled corticosteroid (ICS) alone (80 μg beclomethasone HFA bid) to treatment with double the dose of ICS alone (160 μg beclomethasone HFA bid), single dose ICS (80 μg beclomethasone HFA bid) plus salmeterol (50 μg bid), and single dose ICS (80 μg beclomethasone HFA bid) plus tiotropium (18 μg q am via HandihalerR).12 Each treatment period lasted 14 weeks with 2 week baseline run-in/run-out periods in which patients were treated with single dose ICS, prior to each of the 3 treatment periods.
Inclusion criteria included a history of asthma which was confirmed either by bronchodilator reversibility testing (≥ 12% improvement in FEV1 AND ≥ 200 ml improvement after 4 puffs, 90 μg each, of albuterol) OR bronchial hyperresponsiveness to methacholine (PC20 FEV1 for methacholine of ≤ 8 mg/ml for patient NOT on an ICS, or ≤ 16 mg/ml for patients taking an ICS); 174 qualified on the basis of methacholine hyperresponsiveness and 36 on the basis of the albuterol-reversal requirements, although many of the patients who qualified on the basis of methacholine hyperresponsiveness, would also have qualified based on the albuterol reversibility requirement, had both tests been performed for everyone
Response Analyses and Statistical Approaches
Potential predictors of response to tiotropium and salmeterol were evaluated. While this work should be considered exploratory, pre-specified hypotheses for predictors of a positive response included: 1) increased cholinergic tone (lower resting heart rate) would predict a better response to tiotropium; and 2) positive response to short-acting bronchodilator (≥ 12% and ≥ 200 ml increase in FEV1) would predict a positive response to same class long-acting bronchodilator (i.e. a positive response to albuterol would predict a positive clinical response to salmeterol, and a positive response to ipratropium would predict a positive clinical response to tiotropium).
Morning PEF and asthma-control day data were collected daily; therefore, 2-week averages before the beginning and end of a treatment were used to characterize the drug response. Responses to the morning peak expiratory flow (AM PEF), pre-bronchodilator FEV1 (at the end of the drug dosing interval for all drugs), and asthma-control days (ACDs, days with no asthma symptoms, and no rescue albuterol use) were defined as both continuous and categorical variables. A lung-function response was defined as a relative change between the end and the beginning of a treatment in the AM PEF and FEV1. A 7.5% change was used as a cut-off to create the categorical response variables, similar to a previous NHLBI Childhood Asthma Research and Education (CARE) clinical trial.7 For the average patient in TALC, a 7.5% improvement in AM PEF would translate to approximately 28 L/min, and a 7.5% improvement in FEV1 to approximately 173 ml. The asthma-control day response was defined as an absolute change between the end and the beginning of a treatment, and 0.1 change was used as a cut-off. A 0.1 change in asthma control days translates to 36.5 days on an annualized basis.
A two-dimensional (2D) response was defined as a positive response to either lung function or the asthma-control days, AND having had no asthma exacerbation while on that treatment, providing a binary response. Only patients who had complete data for all treatment periods were included in these analyses (n=166 for FEV1 and asthma control days, n=168 for morning PEF, n=160 for 2D; figures provide n’s for the various outcomes listed).
The set of potential predictors included demographic and asthma characteristics, pulmonary function and biomarkers. Several biomarkers were logarithmically transformed because of skewed distributions. Bronchodilator reversibility variables were dichotomized based on ≥ 12% and 200 mL increase over baseline in FEV1. Other continuous predictors that are normally distributed were dichotomized based on their mean values. Predictors that were not normally distributed were dichotomized based on the mean of the logarithmically transformed values. SAS statistical analysis software, version 9.2 (SAS Institute), was used for both univariate and multivariate analyses. The categorical responses were examined through PROC LOGISTIC for both categorical and continuous predictors. The continuous responses were examined through PROC MIXED for categorical predictors and for continuous predictors through PROC REG for univariate and PROC GLMSELECT for multivariate approaches. Stepwise selection processes were applied for multivariate analyses. TIBCO Spotfire SPLUS, version 8.1 for Windows, software was used for graphic displays of the results. A 2-sided p-value of less than 0.05 was considered statistically significant.
RESULTS
NHLBI’s ACRN TALC Trial Results
Two hundred and ten patients were randomized in the trial, 32.9% male, 87.5% atopic, with an average age of 42.2 ± 12.3 (mean, SD) years, an average duration of asthma of 26.1 ± 14.1 years, and an FEV1 of 2.31 ± 0.77 L (71.5 ± 14.9 predicted). The use of tiotropium resulted in a superior primary outcome, as compared with a doubling of the dose of the ICS, as assessed by measuring the morning peak expiratory flow (PEF), with a mean difference of 25.8 liters per minute (P < 0.001) and superiority in most secondary outcomes, including evening PEF, with a difference of 35.3 liters per minute (P < 0.001); the proportion of asthma control days, with a difference of 0.079 (P = 0.01); the forced expiratory volume in 1 second (FEV1) before bronchodilation, with a difference of 0.10 liters (P = 0.004); and daily symptom scores, with a difference of −0.11 points (P<0.001). The addition of tiotropium was also non-inferior to the addition of salmeterol for all assessed outcomes and increased the pre-bronchodilator FEV1 more than did salmeterol, with a difference of 0.11 liters (P = 0.003). 12
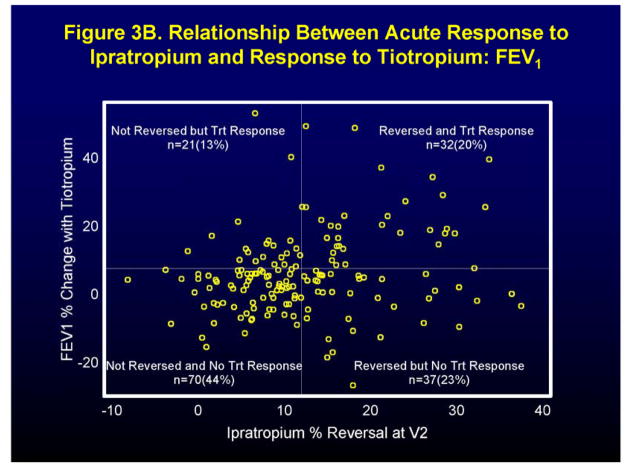
While the average reversibility after 4 puffs of bronchodilator was similar for albuterol (14.9. ± 9.8 %) and ipratropium (12.4. ± 9.5 %) (tests were performed on different days), individual patient responses to these agents showed marked variability. As shown in Figure 1, of the 202 patients who had acceptable data, 22% reversed (≥ 12% improvement in FEV1) to albuterol alone, 10% to ipratropium alone, 34% to both agents, and 34% to neither. Note that the additional criterion requiring ≥200 ml improvement in FEV1 cannot be incorporated in the definition of reversibility when examining it on a continuous scale, as presented in the figures (Figures 1, 3A, 3B). When using reversibility as a dichotomous predictor variable, this additional criterion is incorporated (Table 1). When ≥200 ml improvement is incorporated in the definition, six fewer individuals reverse to albuterol and two fewer individuals reverse to ipratropium (one of these individuals did not have tiotropium clinical response data available).
Figure 1.
Acute responses (defined as FEV1 % change) of patients in the TALC trial to 4 puffs of albuterol and 4 puffs of ipratropium, administered on different days, after bronchodilator drug withholds.
Figure 3.
Relationship between the acute response (defined as FEV1 % change) to short-acting bronchodilator and the clinical effect of long-acting bronchodilator. Figure 3A acute response to 4 puffs of albuterol and the clinical response to salmeterol in terms of FEV1. Figure 3B acute response to 4 puffs of ipratropium and the clinical response to tiotropium in terms of FEV1.
Table 1.
Odds Ratios for the Ability of an Acute Response to a Short-Acting Bronchodilator (12% and 200 ml Improvement in FEV1) to Predict a Clinical Response to a Long-Acting Bronchodilator (7.5% Improvement in FEV1, 7.5% Improvement in AM PEF, 0.1 Proportional Increase in Asthma Control Days).
| Acute-Albuterol To Predict Clinical-Salmeterol
|
Acute-Ipratropium To Predict Clinical-Tiotropium
|
Acute-Albuterol To Predict Clinical-Tiotropium
|
Acute-Ipratropium To Predict Clinical-Salmeterol
|
|||||
|---|---|---|---|---|---|---|---|---|
| Odds Ratio (CI)1 | P | Odds Ratio (CI) | P | Odds Ratio (CI) | P | Odds Ratio (CI) | P | |
| FEV1 | 1.56 (0.74–3.29) | 0.24 | 3.01 (1.52–5.94) | 0.002 | 4.08 (2.00–8.31) | <0.001 | 1.49 (0.72–3.10) | 0.29 |
| AM PEF | 2.81 (1.46–5.40) | 0.002 | 2.07 (1.09–3.92) | 0.026 | 2.12 (1.12–4.01) | 0.021 | 1.67 (0.89–3.16) | 0.11 |
| Asthma Control Days (ACDs) | 1.42 (0.77–2.65) | 0.27 | 1.07 (0.57–2.02) | 0.82 | 1.27 (0.68–2.37) | 0.46 | 0.90 (0.48–1.68) | 0.73 |
| 2D Response2 | 3.40 (1.67–6.95) | <0.001 | 1.76 (0.88–3.51) | 0.11 | 2.40 (1.23–4.69) | 0.01 | 1.28 (0.63–2.61) | 0.50 |
Odds Ratios and 95% Confidence Intervals (CI)
2D Response – 2 Dimensional Response defined as a response in FEV1, AM PEF, OR Asthma Control Days AND No asthma exacerbation
Individual and Differential Responses to Tiotropium and Salmeterol
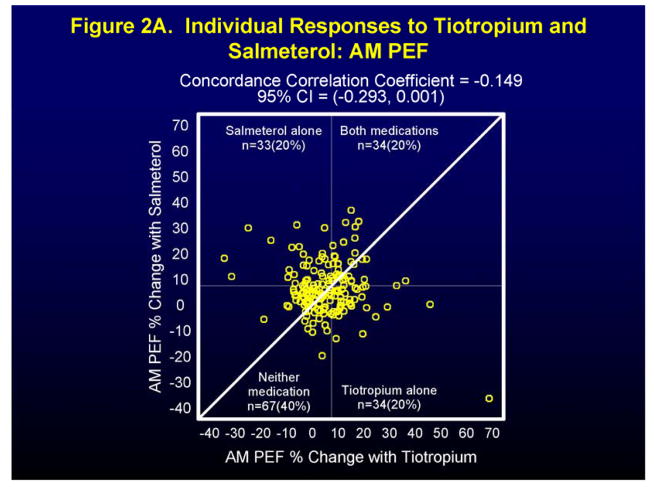
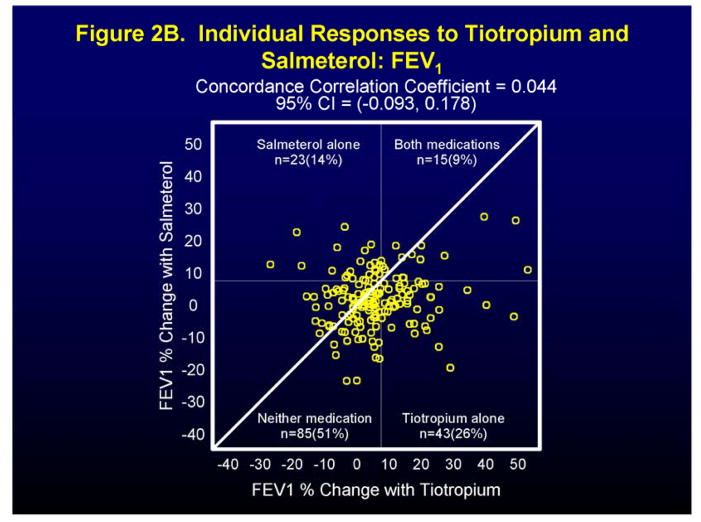
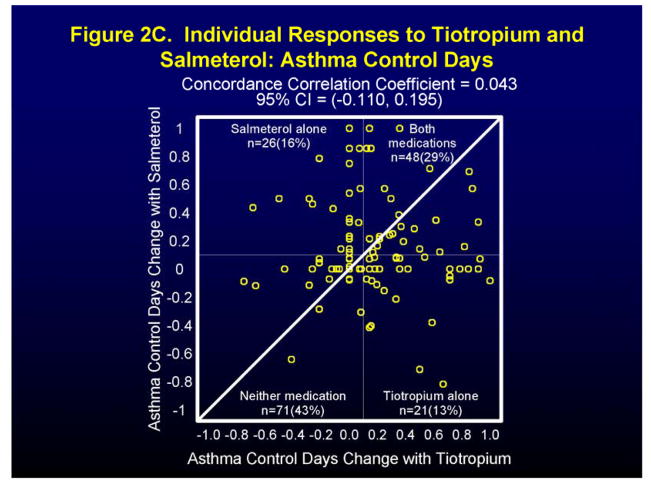
Figure 2 and Figure 1 in the Supplement show individual and differential responses of patients to both tiotropium and salmeterol for the endpoints of morning peak expiratory flow (AM PEF), FEV1 (at the end of the dosing interval for all drugs), and asthma control days (ACDs). For the endpoint AM PEF, 20% of patients showed a positive response (≥ 7.5% improvement) to tiotropium alone, to salmeterol alone, and to both medications, while 40% had a positive response to neither (Figure 2A). Not surprisingly, approximately equal number of patients showed a differential response to tiotropium (78 patients) and to salmeterol (90 patients) (Figure 1A in Supplement). For the endpoint FEV1 positive responses (≥ 7.5% improvement) were noted for tiotropium alone in 26% patients, salmeterol alone for 14% of patients, for both medications for 9% of patients with 51% showing a response to neither medication (Figure 2B). In this case, the differential response favored tiotropium (104 patients) when compared to salmeterol (62 patients) (Figure 1B in Supplement). Finally, for the endpoint of asthma control days (ACDs), a positive response (0.1 proportion increase in ACDs) was noted for tiotropium alone in 13% of patients, salmeterol alone in 16% of patients, for both medications in 29% of patients, and for neither medication in 43% of patients (Figure 2C), while differential responses were again approximately equal for tiotropium (53 patients) and salmeterol (49 patients) (Figure 1C in Supplement).
Figure 2.
Individual (Figures 2A, 2B, 2C) patient responses to salmeterol and tiotropium in terms of AM PEF (Figure 2A), FEV1 (Figure 2B), and Asthma Control Days (Figure 2C).
Pre-Specified Predictors of a Positive Response to Tiotropium and Salmeterol
Higher Cholinergic Tone Predicting Response to Tiotropium
Increased cholinergic tone was inferred from a lower resting heart rate. When comparing patients with ≤ 25th percentile resting heart rate to patients with ≥ 75 percentile resting heart rate, significant odds ratios (OR) for a positive response to tiotropium were noted for asthma control days, OR 3.0 (95% CI 1.13–7.94, P = 0.027) and a 2D response, OR 4.17 (95% CI 1.52–11.43, P = 0.006).
Acute Response to Short-Acting Bronchodilator Predicting Response to Long-Acting Bronchodilator
Figure 3 shows the relationship between the acute FEV1 response to 4 puffs of the short-acting bronchodilators, albuterol (Figure 3A) and ipratropium (Figure 3B), to the analogous long-acting bronchodilators, salmeterol (Figure 3A) and tiotropium (Figure 3B) during the TALC trial. An acute response to albuterol (≥ 12% improvement in FEV1), was associated with a positive response to salmeterol (7.5% improvement in FEV1 during the trial) in 28% of patients (Figure 3A). (Sixteen percent of the total population both reversed and had a treatment response.) An acute response to ipratropium (≥ 12% improvement in FEV1), was associated with a positive response to tiotropium (7.5% improvement in FEV1 during the trial) in 46% of patients (Figure 3B). (Twenty percent of the total population both reversed and had a treatment response.) The odds ratios, confidence intervals, and P values for the ability of a positive response to a short-acting bronchodilator (≥12% and ≥200 ml improvement in FEV1) to predict a positive clinical response in the clinical trial for the three major clinical outcomes, FEV1, AM PEF and asthma control days, are shown in Table 1. A positive response to albuterol predicted a positive response to salmeterol only in terms of AM PEF (OR 2.81 [CI 1.46–5.40], P = 0.002), while a positive response to ipratropium predicted a positive response to tiotropium in terms of both FEV1 (OR 3.01 [CI 1.52–5.94], P = 0.002) and AM PEF (OR 2.07 [CI 1.09–3.92], P = 0.026). Interestingly, the acute response to albuterol was a better predictor of a positive response to tiotropium than the acute response to ipratropium: FEV1 (OR 4.08 [CI 2.00–8.31], P < 0.001) and AM PEF (OR 2.12 [CI 1.12–4.01], P = 0.021). Two dimensional (2D) responses were only noted for albuterol which predicted positive responses to both salmeterol (OR 3.40 [CI 1.67–6.95], P < 0.001) and tiotropium (OR 2.40 [CI 1.23–4.69], P = 0.01). For no outcome measure did the acute response to ipratropium predict a positive response to salmeterol (Table 1).
Additonal Exploratory Predictors of a Positive Response to Tiotropium
A lower FEV1/FVC ratio predicted a positive clinical response to tiotropium in terms of both FEV1 (OR 3.10 [CI 1.59–6.07], P < 0.001) and AM PEF (OR 2.32 [CI 1.24–4.37], P = 0.009). When analyzed as a continuous variable, the FEV1 response increased 0.39% of baseline for every 1% decrease in the FEV1/FVC ratio. Finally, younger patients (< 42 years old) responded better to tiotropium clinically in terms of asthma control days (OR 2.64 [CI 1.40–4.99], P = 0.003) than older patients ≥ 42 years old). Exploratory predictors NOT associated with a positive clinical response to tiotropium included ethnicity, gender, atopy (at least 1 positive skin test), IgE Level (natural logarithm), sputum eosinophils, fraction of expired nitric oxide (FENO ([natural logarithm]), asthma duration, and body mass index (BMI).
Discussion
Tiotropium has now been shown to have activity as an asthma controller when added to ICS in several well designed clinical trials.12–15 This report describes individual, differential, and predictors of response of individual patients to both salmeterol and tiotropium when added to an ICS. The cross-over design of the trial permitted an evaluation of the response of both drugs in every patient who completed the treatment periods in which salmeterol and tiotropium were assigned. Three different outcomes were examined, which could be considered to represent different aspects of the treatment response. The FEV1 at the end of the drug dosing interval could be considered a time of maximum vulnerability for patients. In TALC, similar results were obtained for both AM PEF and PM PEF; therefore AM PEF could be considered to represent lung function through the day and night. Asthma control days are a patient-centric outcome of great importance to both patient and physician, which are weakly related to lung function.
Several important observations have been made in this report. First, large numbers of patients responded either to salmeterol OR tiotropium, but not to both agents, suggesting that at the time of drug administration, different mechanisms were operative to produce airway constriction and symptoms in these two groups of patients. This observation is consistent with the data presented by Kerstjens, et al.15 who reported that when a group of patients with asthma who were inadequately controlled on a combination ICS/LABA were placed on tiotropium, they demonstrated an increase in their mean FEV1 and a decrease in asthma exacerbations. Second, patients with more cholinergic tone, as well as younger patients, displayed a better response to tiotropium in selected outcomes. Third, while the response to a short-acting bronchodilator did predict a positive response to a long-acting bronchodilator-controller of the same class, albuterol response better predicted a response to tiotropium than did ipratropium. Albuterol appeared to be a better predictor of a response to tiotropium than ipratropium because it was a more effective bronchodilator in this population (i.e. 44% of the study population showed a positive response to ipratropium while 56% of the population showed a positive response to albuterol). Finally, increased airway obstruction, as reflected in a decreased FEV1/FVC ratio, also predicted a positive response to tiotropium. Ethnicity, gender, atopy, IgE Level, sputum eosinophils, FENO, asthma duration, and BMI did not predict a clinical response.
Definitive recommendations concerning how to translate these findings into clinical practice cannot be made on the basis of these observations alone. Clearly, the findings need to be replicated in an independent study. However if the findings were replicated, we could suggest that if a patient with asthma still has suboptimal asthma control after a trial of inhaled corticosteroid alone (i.e. has asthma symptoms or rescue beta-agonist use most days of the week, and/or 2 or more awakenings per week for asthma, and/or compromised lung function [FEV1 ≤ 70% predicted]), he/she should undergo spirometry before and after the administration of 4 puffs of albuterol. Patients with airway obstruction as demonstrated by a reduced FEV1/FVC ratio and/or a positive response to albuterol should be good candidates for treatment with tiotropium as add-on therapy. Whether this strategy should be reserved for patients who “fail” combination ICS-LABA therapy, or whether physicians and patients should have the option of adding either tiotropium or a LABA to an ICS when monotherapy does not produce adequate asthma control, awaits further investigation.
Supplementary Material
Clinical Implications.
Personalized therapy for asthma requires identification of factors associated with positive responses. Predictors of positive responses to tiotropium and salmeterol add-on therapy were identified in the NHLBIs’s ACRN TALC trial.
Acknowledgments
Tiotropium and placebo were supplied by Boehringer-Ingelheim Pharmaceuticals, Inc. in support of this study and this research was supported by a grant from Boehringer-Ingelheim Pharmaceuticals, Inc. We wish to thank our colleagues at TEVA for their gift of beclomethasone dipropionate-HFA which was used in this trial. We also wish to thank all of the patients who took part in the TALC and BASALT trials as well as the outstanding work of our study coordinators who made this work possible.
Supported by grants from the National Heart, Lung, and Blood Institute (U10 HL074225, U10 HL074227, U10 HL074231, U10 HL074204, U10 HL074212, U10 HL074073, U10 HL074206, U10 HL074208 and U10 HL074218).
Abbreviations
- ICS
inhaled corticosteroids
- TALC trial
National Heart, Lung and Blood Institute’s (NHLBI’s) Asthma Clinical Research Network’s (ACRN’s) Tiotropium Bromide as an Alternative to Increased Inhaled Glucocorticoid in Patients Inadequately Controlled on a Lower Dose of Inhaled Corticosteroid (ClinicalTrials.gov number, NCT00565266) (TALC) trial
- NHLBI
National Heart, Lung and Blood Institute
- ACRN
Asthma Clinical Research Network
- FEV1
forced expiratory volume in 1 second
- AM PEF
morning peak expiratory flow
- ACDs
asthma control days (days with no asthma symptoms and no need for rescue albuterol use for symptoms [excluding pre-medication for exercise])
- FENO
expired fraction of nitric oxide
- BMI
body mass index
- LABA
long-acting beta-agonist
- LAMA
long-acting muscarinic antagonist
- HFA
hydrofluoroalkane
- SD
standard deviation
- OR
odds ratio
- CARE network
National Heart, Lung and Blood Institute’s (NHLBI’s) Childhood Asthma Research and Education network
Footnotes
From the NHLBI’s Asthma Clinical Research Network (ACRN), and its Tiotropium Bromide as an Alternative to Increased Inhaled Corticosteroid in Patients Inadequately Controlled on a Lower Dose of Inhaled Corticosteroid (TALC) trial
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errorsmaybe discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.National Asthma Education and Prevention Program. Expert Panel Report 3, Guidelines for the Diagnosis and Management of Asthma. Department of Health and Human Services, National Institutes of Health, National Heart, Lung, and Blood Institute; Bethesda, MD: 2007. [Google Scholar]
- 2.Global Strategy for Asthma Management and Prevention. Global Initiative for Asthma (GINA); Available at: http://www.ginasthma.org. [Google Scholar]
- 3.Weiss ST. New approaches to personalized medicine for asthma: where are we? J Allergy Clin Immunol. 2012;129:327–334. doi: 10.1016/j.jaci.2011.12.971. [DOI] [PubMed] [Google Scholar]
- 4.Green RH, Brightling CE, McKenna S, Hargadon B, Parker D, Bradding P, Wardlaw AJ, Pavord ID. Asthma exacerbations and sputum eosinophil counts: a randomised controlled trial. Lancet. 2002;360:1715–1721. doi: 10.1016/S0140-6736(02)11679-5. [DOI] [PubMed] [Google Scholar]
- 5.Greenberger PA. Personalized medicine for patients with asthma. J Allergy Clin Immunol. 2010;125:305–306. doi: 10.1016/j.jaci.2009.11.038. [DOI] [PubMed] [Google Scholar]
- 6.Sharma S, Litonjua AA, Tantisira KG, Fuhlbrigge AL, Szefler SJ, Strunk RC, Zeiger RS, Murphy AJ, Weiss ST Childhood Asthma Management Program Research Group. Clinical predictors and outcomes of consistent bronchodilator response in the childhood asthma management program. J Allergy Clin Immunol. 2008;122:921–928. doi: 10.1016/j.jaci.2008.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Szefler SJ, Phillips BR, Martinez FD, Chinchilli VM, Lemanske RF, Strunk RC, Zeiger RS, Larsen G, Spahn JD, Bacharier LB, Bloomberg GR, Guilbert TW, Heldt G, Morgan WJ, Moss MH, Sorkness CA, Taussig LM. Characterization of within-subject responses to fluticasone and montelukast in childhood asthma. J Allergy Clin Immunol. 2005;115:233–242. doi: 10.1016/j.jaci.2004.11.014. [DOI] [PubMed] [Google Scholar]
- 8.Zeiger RS, Szefler SJ, Phillips BR, Schatz M, Martinez FD, Chinchilli VM, Lemanske RF, Jr, Strunk RC, Larsen G, Spahn JD, Bacharier LB, Bloomberg GR, Guilbert TW, Heldt G, Morgan WJ, Moss MH, Sorkness CA, Taussig LM Childhood Asthma Research and Education Network of the National Heart, Lung, and Blood Institute. Response profiles to fluticasone and montelukast in mild-to-moderate persistent childhood asthma. J Allergy Clin Immunol. 2006;117:45–52. doi: 10.1016/j.jaci.2005.10.012. [DOI] [PubMed] [Google Scholar]
- 9.Knuffman JE, Sorkness CA, Lemanske RF, Jr, Mauger DT, Boehmer SJ, Martinez FD, Bacharier LB, Strunk RC, Szefler SJ, Zeiger RS, Taussig LM Childhood Asthma Research and Education Network of the National Heart, Lung, and Blood Institute. Phenotypic predictors of long-term response to inhaled corticosteroid and leukotriene modifier therapies in pediatric asthma. J Allergy Clin Immunol. 2009;123:411–416. doi: 10.1016/j.jaci.2008.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Martin RJ, Szefler SJ, King TS, Kraft M, Boushey HA, Chinchilli VM, Craig TJ, Dimango EA, Deykin A, Fahy JV, Israel E, Lazarus SC, Lemanske RF, Jr, Leone FT, Pesola GR, Peters SP, Sorkness CA, Szwejbka LA, Wechsler ME National Heart, Lung, and Blood Institute’s Asthma Clinical Research Center. The Predicting Response to Inhaled Corticosteroid Efficacy (PRICE) trial. J Allergy Clin Immunol. 2007;119:73–80. doi: 10.1016/j.jaci.2006.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Szefler SJ, Martin RJ. Lessons learned from variation in response to therapy in clinical trials. J Allergy Clin Immunol. 2010;125:285–292. doi: 10.1016/j.jaci.2009.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Peters SP, Kunselman SJ, Icitovic N, Moore WC, Pascual R, Ameredes BT, Boushey HA, Calhoun WJ, Castro M, Cherniack RM, Craig T, Denlinger L, Engle LL, DiMango EA, Fahy JV, Israel E, Jarjour N, Kazani SD, Kraft M, Lazarus SC, Lemanske RF, Jr, Lugogo N, Martin RJ, Meyers DA, Ramsdell J, Sorkness CA, Sutherland ER, Szefler SJ, Wasserman SI, Walter MJ, Wechsler ME, Chinchilli VM, Bleecker ER National Heart, Lung, and Blood Institute Asthma Clinical Research Network. Tiotropium bromide step-up therapy for adults with uncontrolled asthma. N Engl J Med. 2010;363:1715–1726. doi: 10.1056/NEJMoa1008770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bateman ED, Kornmann O, Schmidt P, Pivovarova A, Engel M, Fabbri LM. Tiotropium is noninferior to salmeterol in maintaining improved lung function in B16-Arg/Arg patients with asthma. J Allergy Clin Immunol. 2011;128:315–322. doi: 10.1016/j.jaci.2011.06.004. [DOI] [PubMed] [Google Scholar]
- 14.Kerstjens HA, Disse B, Schroder-Babo W, Bantje TA, Gahlemann M, Sigmund R, Engel M, van Noord JA. Tiotropium improves lung function in patients with severe uncontrolled asthma: a randomized controlled trial. J Allergy Clin Immunol. 2011;128:308–314. doi: 10.1016/j.jaci.2011.04.039. [DOI] [PubMed] [Google Scholar]
- 15.Kerstjens HA, Engel M, Dahl R, Paggiaro P, Beck E, Vandewalker M, Sigmund R, Seibold W, Moroni-Zentgraf P, Bateman ED. Tiotropium in asthma poorly controlled with standard combination therapy. N Engl J Med. 2012;367:1198–1207. doi: 10.1056/NEJMoa1208606. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.