Abstract
Critical to the search for an effective HIV-1 vaccine is the development of immunogens capable of inducing broadly neutralizing antibodies (BnAbs). A key first step in this process is to design immunogens that can be recognized by known BnAbs. The monoclonal antibody PG9 is a BnAb that neutralizes diverse strains of HIV-1 by targeting a conserved carbohydrate-protein epitope in the variable 1 and 2 (V1V2) region of the viral envelope. Important for recognition are two closely spaced N-glycans at Asn160 and Asn156. Glycopeptides containing this synthetically challenging bis-N-glycosylated motif were prepared by convergent assembly, and were shown to be antigenic for PG9. Synthetic glycopeptides such as these may be useful for the development of HIV-1 vaccines based on the envelope V1V2 BnAb epitope.
INTRODUCTION
Few important structures in nature are more heavily glycosylated than is the envelope spike (Env) of human immunodeficiency virus type 1 (HIV-1).1 A multitude of designed constructs that might simulate the unique architecture of Env have been considered and pursued in the context of potential HIV-1–directed vaccines. Yet, until recently, the only template for immunological recognition of this dense “glycan shield” has been the broadly neutralizing antibody (BnAb) 2G12.2 Following its discovery, many laboratories,3 including our own,4 were able to generate mimics of the oligomannose cluster that constitutes its epitope, in the hope of eliciting 2G12-like antibodies. Unfortunately, these efforts were not successful. While many factors have been cited to explain the general difficulties surrounding BnAb induction,5 the case of 2G12 is likely complicated further by the unusual domain-exchanged arrangement of its heavy chains, which is thought to be responsible for its unique mode of glycan recognition.2d
In 2009, two new and potent BnAbs, PG9 and PG16, were isolated from an HIV-1–infected donor from sub-Saharan Africa.6 These monoclonal antibodies (mAbs) were found to neutralize 70–80% of circulating HIV-1 isolates. Initial epitope mapping suggested that PG9 and PG16 were targeting a new glycan-dependent Env epitope, entirely distinct from that of 2G12. A sensitivity to quarternary structure was also noted, as these BnAbs exhibited a preference for binding fully assembled trimeric viral spike over monomeric Env. Subsequently, a co-crystal structure of PG9 with gp120 variable regions 1 and 2 (V1V2) grafted onto a mini-protein scaffold revealed that the antibody engages high mannose glycans at Asn160 and Asn156 and an adjacent β-strand (Figure 1A).7 In contrast to 2G12, which apparently does not interact with the gp120 peptide backbone, PG9 binds an epitope that contains both carbohydrate and peptide components, while possessing a normal heavy chain arrangement.
Figure 1.
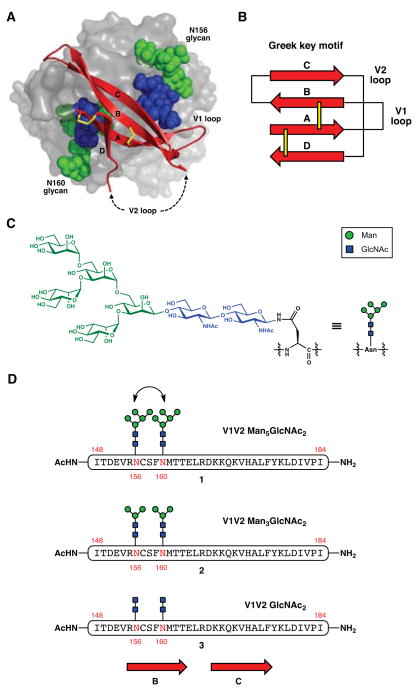
Design of gp120 V1V2 domain broadly neutralizing epitope mimics. (A) Crystal structure of a scaffolded V1V2 domain from the CAP45 strain of HIV-1 (red ribbons) in complex with PG9 Fab (gray surface) (PDB ID 3U4E with scaffold hidden). The glycans at N160 and N156 are depicted with colored spheres representing atoms of the mannose (green) and N-acetylglucosamine (blue) residues. Disulfide bonds are shown as yellow sticks. Dashed arrows indicate where the disordered region of the V2 loop would be connected. Figure was created using PyMOL. (B) Schematic of the Greek key topology of the V1V2 domain. Strands are represented as arrows and disulfide bonds as yellow bars. (C) Chemical structure of Man5GlcNAc2–Asn. (D) Structures of candidate BnAb antigens, derived from residues 148–184 of the A244 strain gp120 (HXB2 numbering), encompassing the B and C β-strands (approximate location shown with red arrows) of the V1V2 domain, and bearing two N-linked Man5GlcNAc2, Man3GlcNAc2, or GlcNAc2 oligosaccharides.
In light of our continuing involvement in the synthesis of glycoproteins and complex glycopolypeptide motifs,8 we regarded these new structural observations with particular interest. It seemed not unlikely that successful design of vaccines based on the PG9 epitope would depend crucially on close simulation of the detailed surface glycopeptide architecture of Env. Given the importance of the glycan domains in forming this conserved epitope, it seemed that access to Env constructs that are well-defined and homogeneous with respect to glycosylation state would greatly facilitate immunogen development efforts. Past work has largely relied on recombinant Env preparations, supplied as mixtures of glycoforms.9 This serious heterogeneity complicates efforts to draw precise correlations between glycan composition and immunoactivity. Absent a detailed understanding of the structural biology of the problem, informed vaccine design is, naturally, much more difficult. We were stirred by the prospect that de novo chemical synthesis could provide the complex, yet homogeneous probe substrates needed for rationally based advances in this urgent endeavor.
More specifically, we hypothesized that fully synthetic, homogeneous gp120 V1V2 polypeptide domains, bearing defined glycosyl patterns, might be able to function as minimal mimics of the PG9 epitope. If such uniform, synthetically-derived constructs were able to simulate the conformation of the pertinent native envelope glycoproteins, they would provide a logical starting point for immunogen design. Moreover, a minimal construct could, in theory, present the desired BnAb epitope without interference from other potentially more immunogenic Env determinants.5
Herein, we describe the chemical synthesis of gp120 V1V2 glycopeptides as single glycoforms10 that were found to bind the BnAb PG9 with surprisingly high affinities. During the course of this work, we had to deal with and overcome the fundamental synthetic challenge arising from the close spacing of large glycans along the peptide backbone. In engaging this challenge, we would be pressing against the limits of the prior art we had developed in the realm of glycopeptide ligations.11
RESULTS AND DISCUSSION
Design and Strategy
The structure of the gp120 V1V2 domain in the context of a bound PG9 mAb Fab consisted of four anti-parallel β-strands (A–D) that folded into what is known as a Greek key motif (Figure 1B).7 Based on these x-ray crystallographic data, PG9 makes contacts with the C β-strand, and with the Man5GlcNAc2 glycans12 (Figure 1C) at Asn160 and Asn156, which reside on strand B. Since most of the structural features recognized by PG9 appear to be localized on the B and C strands,13 we reasoned that an epitope mimic should, at the very least, encompass this region. Our initial prototype is shown in Figure 1D. The 35-amino acid peptide corresponds to positions 148–184 of gp120 (HXB2 numbering) derived from the A244 sequence,14 an Env variant that is known to bind PG9 in monomeric form (i.e., without requiring trimerization).15 With regard to the glycan structure, Man5GlcNAc2 was thought to be the best candidate on the basis of prior studies involving perturbations of glycan processing.16,17 The primary target that emerged from this analysis was glycopeptide 1 with Man5GlcNAc2 units installed at the two glycosylation sites, Asn160 and Asn156; we also planned to gain access to simpler glycoforms 2 and 3 bearing Man3GlcNAc2 and chitobiose (GlcNAc2), respectively.18 These could be used to probe the importance of the outer mannose residues for recognition.
We term the general synthetic approach that our laboratory has applied to complex glycoprotein targets as convergent assembly.11 In our usual modus operandi, N-linked sugars are installed via aspartylation of unprotected glycosyl amines, drawing from the precedent of Lansbury,19 which we20 and others21 have extended in substantive ways. As we examined goal structures 1–3 in particular, we noted that the close spacing of the two glycans, especially with larger oligosaccharides, could present a difficult challenge for their incorporation. We anticipated that application of our methods in this demanding context would afford valuable teachings regarding the synthesis of the required clustered N-glycan motifs. As for the sugars themselves, Man3GlcNAc2 constitutes the common pentasaccharide core of all N-glycans; it has been synthesized previously by our laboratory and others.22 By contrast, Man5GlcNAc2 seems to have received less attention as a synthetic target.23,24 We start by describing our route to the desired glycans.
Synthesis of Man5GlcNAc2 and Man3GlcNAc2 glycans
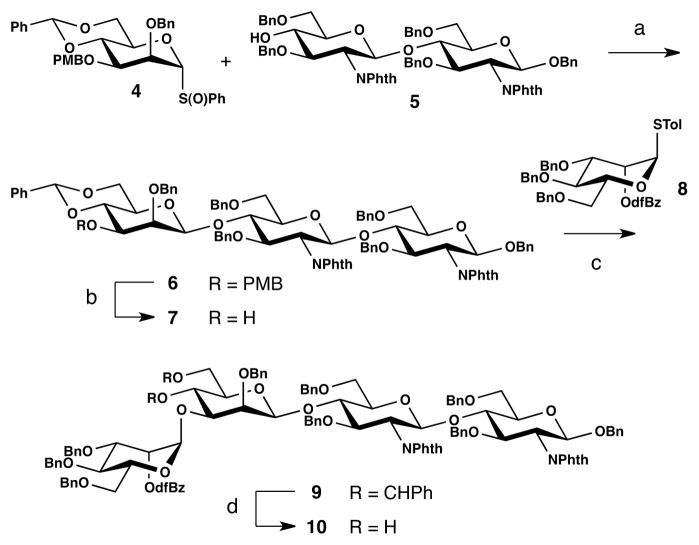
Our studies commenced with experiments directed to the synthesis of the Man5GlcNAc2 heptasaccharide. The β-mannosyl linkage of the core trisaccharide 6 was constructed as we have in prior contexts25 by uniting Crich donor 426,27 with chitobiose acceptor 525b (Scheme 1). Fortunately, the minor quantities of undesired a-isomer formed (<10%) could be separated by careful chromatography to afford trisaccharide 6 as a single diastereomer in 86% yield. The PMB group was removed in 83% yield. Coupling of the resulting acceptor 7 with thioglycoside donor 8 was accomplished under NIS/TMSOTf activation conditions, yielding tetrasaccharide 9. Cleavage of the benzylidene acetal with aqueous acetic acid afforded diol 10 in 63% overall yield from 7.
Scheme 1.
Synthesis of tetrasaccharide 10.a
aReagents and conditions: (a) Tf2O, DTBP, MS AW-300, CH2Cl2, −78 °C, 86%; (b) DDQ, CH2Cl2, H2O, 83%; (c) thioglycoside 8, NIS/TMSOTf, MS AW-300, CH2Cl2, 0 °C→r.t., (d) AcOH, H2O, 63% (2 steps).
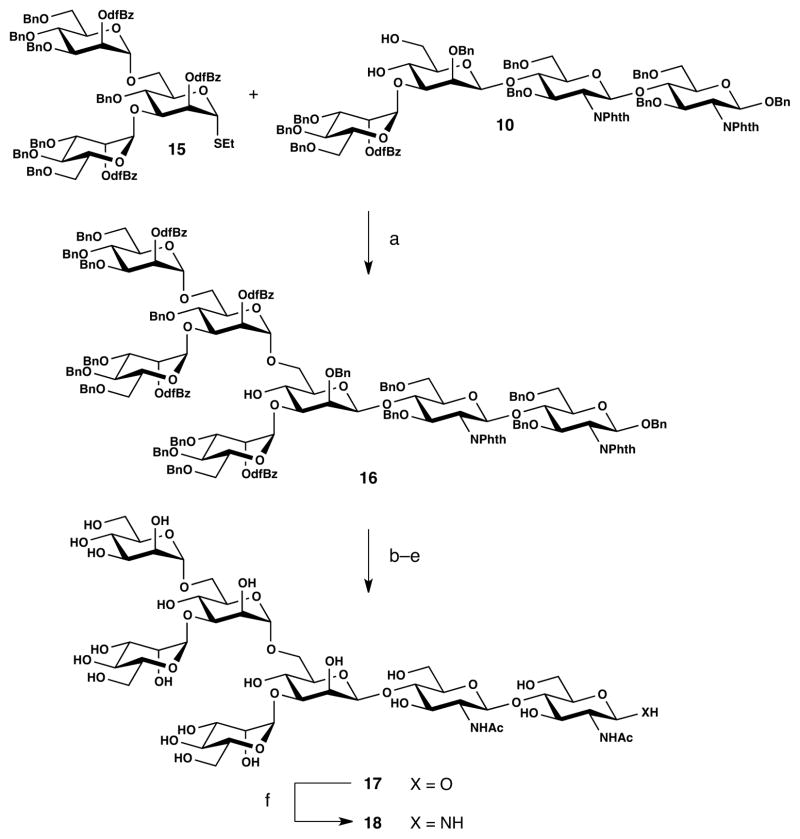
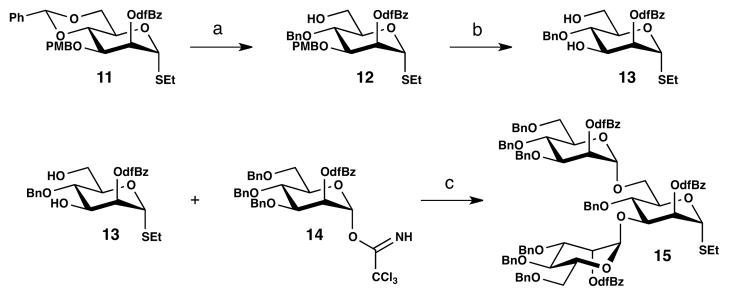
Assembly of the heptasaccharide at first in protected form was accomplished convergently by selective mannosylation at C-6 of 10 with branched donor 15. Synthesis of the requisite trisaccharide 15 was achieved by elaboration of mannosyl building block 11 (Scheme 2). Reductive ring opening was accomplished selectively with borane-THF complex in the presence of copper triflate in 96% yield.28 Cleavage of the PMB group afforded the 3,6-diol, 13, which underwent double mannosylation with imidate donor 14 to furnish the bis-a-mannosylated trisaccharide 15 in 75% yield.
Scheme 2.
Synthesis of trisaccharide donor 15.a
aReagents and conditions: (a) Cu(OTf)2, BH3·THF, THF, 0 °C, 96%; (b) DDQ, CH2Cl2, H2O, 89%; (c) TMSOTf, MS AW-300, CH2Cl2, 0 °C, 75%.
With the stage set for the key coupling, 15 was activated (NIS/TMSOTf) and joined with 10, thus providing the fully elaborated protected heptasaccharide 16 in 64% yield (Scheme 3). A four-step sequence involving ester saponification, phthalimide cleavage, N-acetylation, and hydrogenolysis proceeded smoothly to give fully deprotected heptasaccharide 17 as a mixture of anomeric alcohols in 77% yield. This compound underwent apparently quantitative conversion to the β-anomeric amine 18 under Kochetkov amination conditions.29
Scheme 3.
Synthesis of heptasaccharide 18.a
aReagents and conditions: (a) NIS/TMSOTf, MS AW-300, CH2Cl2, 0 °C→r.t., 64%; (b) NaOMe, MeOH, CH2Cl2; (c) H2NCH2CH2NH2, n-BuOH, PhMe, 90 °C; (d) Ac2O, Et3N, MeOH; (e) H2, Pd(OH)2/C, MeOH, H2O, 77% (4 steps); (f) sat. aq. NH4HCO3, 40 °C, quantitative.
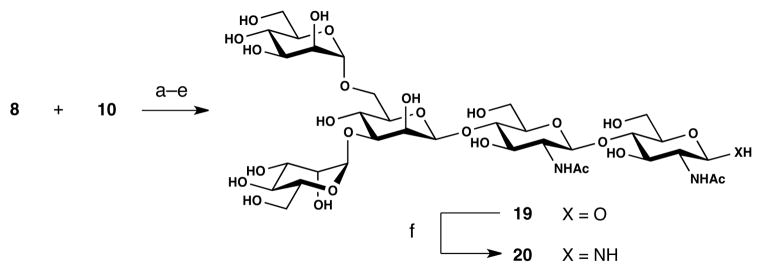
The pentasaccharide, Man3GlcNAc2, was obtained from tetrasaccharide intermediate 10 by selectively coupling donor 8 to the C-6 hydroxyl group (Scheme 4). Although this reaction was complicated by a small amount of bis-glycosylation, the protected Man3GlcNAc2 unit was isolated in 94% yield. Subjection of this material to the 4-step global deprotection protocol described above resulted in a 74% overall yield of fully deprotected pentasaccharide 19 as a mixture of anomers. The β-anomeric amine 20 was subsequently generated by application of the Kochetkov conditions.
Scheme 4.
Synthesis of pentasaccharide 20.a
aReagents and conditions: (a) NIS/TMSOTf, MS AW-300, CH2Cl2, 0 °C→r.t., 94%; (b) NaOMe, MeOH, CH2Cl2; (c) H2NCH2CH2NH2, n-BuOH, PhMe, 90 °C; (d) Ac2O, Et3N, MeOH, 95%; (e) H2, Pd(OH)2/C, MeOH, H2O, 74% (4 steps); (f) sat. aq. NH4HCO3, 40 °C, quantitative.
Convergent assembly of V1V2 glycopeptides
The most risky phase of the effort involved the assembly of the peptide domain of the targeted glycopeptide constructs, and their coupling to different oligosaccharides. Two basic strategies were considered. Our first thoughts envisioned installing both glycans simultaneously on the full-length peptide, bearing in mind our prior successes with two- and three-fold aspartylations on cyclic scaffolds.4d Pilot experiments using chitobiose as a model glycan, however, yielded only unmanageable mixtures of mono- and bis-glycosylated forms, presumably due to the steric demands imposed by the close proximity of aspartylation sites. Anticipating that driving the reaction to completion with larger oligosaccharides might require a substantial excess of precious glycosyl amine, we decided to pursue an alternative approach involving the ligation of two pre-built glycopeptide fragments. Here, the presence of Cys157 served to raise the possibility of native chemical ligation (NCL).30
We anticipated that this approach would not be without its own complications, given the close positioning of the glycans. Indeed, one of the coupling partners (Ile148–Asn156) must carry the sterically demanding oligosaccharide on its C-terminal thioester-bearing amino acid. Nevertheless, this scheme was successfully reduced to practice, as described below.
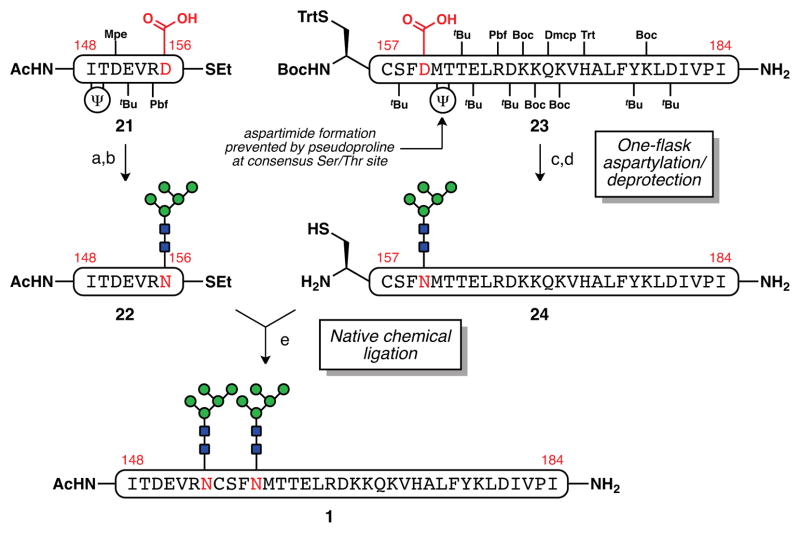
In the event, N-terminal fragment, peptide thioester 21, was obtained by Fmoc solid phase peptide synthesis (SPPS) and post-resin C-terminal functionalization procedures31 used by our laboratory in the context of other glycopeptide endeavors (Scheme 5).32 Using our recently reported one-flask aspartylation/deprotection protocol,20 the free carboxylic acid side chain at position 156 was joined to the Man5GlcNAc2 glycosyl amine 18, followed by TFA treatment to provide glycopeptide thioester 22 in 44% yield after purification by reversed-phase HPLC. The formation of a side product of identical mass was observed in small quantities (5–10%), presumably due to base-induced epimerization of the thioester during the aspartylation. Fortunately, it could be easily separated during the purification.
Scheme 5.
Synthesis of V1V2 glycopeptide 1.a
aReagents and conditions: (a) Man5GlcNAc2–NH2 (18), PyAOP, DIEA, DMSO; (b) Cocktail B = 88:5:5:2 TFA/phenol/water/triisopropylsilane, 44% (2 steps); (c) Man5GlcNAc2–NH2 (18), PyAOP, DIEA, DMSO; (d) Cocktail R = 90:5:3:2 TFA/thioanisole/ethanedithiol/anisole, 22% (2 steps); (e) 6 M Gnd·HCl, 200 mM Na2HPO4, 200 mM MPAA, 20 mM TCEP·HCl, pH 7.2, 55%.
For the C-terminal fragment, a similar one-flask sequence was used to convert protected peptide 23 to deprotected glycopeptide 24 in 22% yield. As has been previously observed, emplacement of a pseudoproline motif at Thr162 (n + 2 relative to Asp160) was helpful in suppressing undesired aspartimide formation during the aspartylation.20,21 The isolated yield for this fragment was eroded by factors that complicated the final purification of glycopeptide 24, including near overlap of the unglycosylated peptide, and the persistence of capped truncation products that had formed during the course of the SPPS (by an as yet undefined mechanism). Despite these obstacles, sufficient quantities of fragments of 22 and 24 could be synthesized and joined by NCL to afford the fully elaborated glycopeptide 1 bearing Man5GlcNAc2 units at Asn160 and Asn156 in 55% yield. The simpler glycoforms 2 and 3, possessing two Man3GlcNAc2 and two chitobiose glycans, respectively, were prepared by an analogous route (see Supporting Information for details).
In all cases, the final ligation proved to be difficult. Indeed, three equivalents of thioester were required for the reaction to progress to completion.33 Careful control of the reaction pH was needed to avoid apparent epimerization or excessive formation of succinimide (via cyclization of the asparagine side chain nitrogen onto the thioester). While certainly less than optimal, these ligations represent, to the best of our knowledge, the first examples of NCL with peptide thioesters carrying an N-glycan directly at the C-terminus. Furthermore, no other syntheses have been reported of linear glycopeptides bearing such closely spaced N-glycans, i.e., separated by three amino acids or less.34 While the yields for the overall sequence are likely to benefit from optimization,35 our concerns at first were focused more on the purity of the synthetic constructs rather than on maximizing material throughput. Fortunately, the synthesis, even in its present form, has produced sufficient quantities to initiate the biological studies now underway both in vitro and in vivo to chart a path forward to a clinically evaluable HIV-1 vaccine (vide infra).
Antigenicity studies
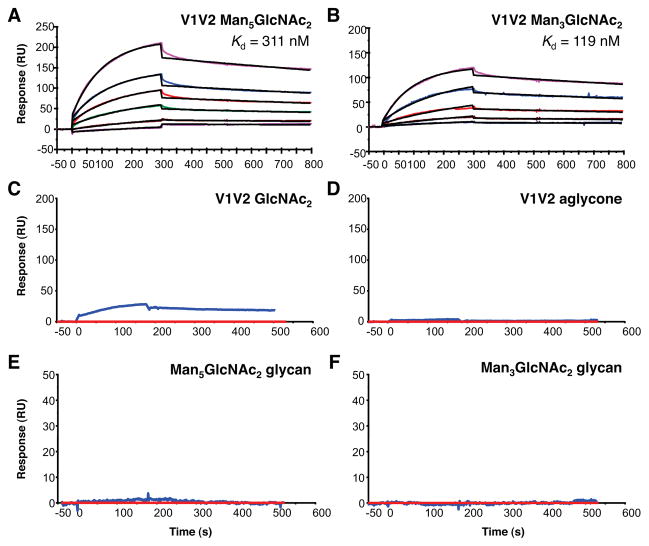
To assess the extent to which our synthetic V1V2 glycopeptides are able to recapitulate the mAb PG9 V1V2 BnAb epitope, we studied the binding of constructs 1–3 to PG9 by surface plasmon resonance (SPR) analysis (Figure 2). PG9 was captured by surface-immobilized anti-human Ig Fc, and the V1V2 glycopeptide constructs were injected as analytes on BIAcore 3000 instruments as described previously.36 We found that the Man5GlcNAc2 V1V2 (1) and Man3GlcNAc2 V1V2 (2) glycopeptides both exhibited significant affinity for mAb PG9 (Figures 2A and 2B), with Kd’s of 311 and 119 nM, respectively (obtained by using a global fit of multiple titrations to a 1:1 Langmuir model). By contrast, the chitobiose-bearing construct 3 did not bind mAb PG9 (Figure 2C), suggesting that the presence of a-linked mannose residues on the glycans is important for recognition. Furthermore, binding by the unglycosylated V1V2 peptide (i.e., “aglycone”) (Figure 2D) or the solitary protein-free Man5GlcNAc2 and Man3GlcNAc2 oligosaccharides was not detected (Figure 2E and F). Mixtures of “aglycone” and glycan similarly failed to show measurable binding (not shown).
Figure 2.
Binding of mAb PG9 to gp120 V1V2 glycopeptides. SPR sensorgrams showing binding of mAb PG9 to V1V2 glycopeptides derivatized with Man5GlcNAc2 (A) and Man3GlcNAc2 (B). V1V2 Man5GlcNAc2 binding curves are shown for glycopeptide concentrations at 5, 10, 20, 30 and 40 μg/mL and V1V2 Man3GlcNAc2 at 1, 2, 5, 10 and 20 μg/mL. Control SPR sensograms showing minimal to no binding of mAb PG9 to V1V2 GlcNAc2 (C), V1V2 aglycone (D), Man5GlcNAc2 glycan alone (E), and Man3GlcNAc2 glycan alone (F). V1V2 GlcNAc2 and aglycone peptides were injected at 200 μg/mL (C, D) and Man5GlcNAc2 and Man3GlcNAc2 glycans at 25 μg/mL (E, F) over PG9 captured on anti-human IgG (Fc-specific) surfaces. SPR data were derived following subtraction of non-specific signal on a control anti-RSV mAb (Synagis, red curve in C–F).
Taken together, these data demonstrate that PG9 recognition of our V1V2 constructs is critically dependent on both the peptide and carbohydrate domains. Covalent linkage between them is essential, since the apparent affinities for each individual component in isolation are very low. Indeed, NMR studies have shown that the Kd for binding of PG9 to Man5GlcNAc2–Asn alone is ~1–2 mM,6 consistent with the general trend that individual protein-carbohydrate interactions tend to be weak. The overall high “avidity” observed may be attributed to the synergies afforded by multivalency, wherein the binding to PG9 is enhanced by multiple simultaneous interactions with the C β-strand, and the Asn160 and Asn156 glycans.7 Conformational effects may also play a role, as glycosylation of the peptide backbone could have a favorable orienting influence on the involved peptide and/or sugar residues.37 Evidence of such “cross-talk” between peptido and glyco domains has been observed by our laboratory in other settings.38
In light of these findings, it seems likely that proper evaluation of the optimal glycans and peptide sequences for mimicking the V1V2 BnAb epitope—and other similar glycopeptide antigens—will require their presentation in their native N-linked context (or as some close isostere). Adopting this approach enabled us to make the unexpected discovery that the Man3GlcNAc2-based construct 2 binds PG9 just as well, and perhaps even a little better, than construct 1 (bearing Man5GlcNAc2).39 Studies are underway to better understand the robust recognition of this non-canonical40 glycan by PG9. In the meantime, we note that such fine structure preferences were not detected by previous approaches, such as glycan array analysis, that interrogated PG9 binding to isolated carbohydrates in the absence of a peptide backbone.41
More profound, perhaps, is the overall question of how the modestly sized glycopeptides 1 and 2 are able to simulate the antigenicity of native envelope glycoproteins so well. PG9 and other BnAbs that target the same V1V2 epitope (e.g., PG16 and CH01 to CH0415) are thought to be sensitive to quarternary structure, binding Env trimers better than monomeric Env.6,13 Indeed, relatively few Env sequences are known to bind PG9 in monomeric form, so it is noteworthy that comparatively small (6–7 kDa), linear 35-mer Env glycopeptide fragments like 1 and 2 are able to bind PG9 with respectable affinities, with Kd’s on the order of 10−7 M.42 Such affinities compare favorably with the published Kd’s of ~5 × 10−8 M for the full-length A244 Env monomer (from which 1 and 2 are derived),15 and ~10−8 M for a recently reported trimeric Env construct.13 We are actively investigating the nature of the binding interaction with PG9; preliminary results suggest that it cannot be fully explained by a simple “induced-fit” mode of association.
CONCLUSION
In summary, we have designed and chemically synthesized homogeneous gp120 V1V2 domain glycoforms that demonstrate robust antigenicity (Kd ~10−7) for the HIV-1 gp120 V1V2 BnAb PG9. Key to these initial successes were significant achievements at the level of chemistry, which include the development of a scalable synthetic route to the Man5GlcNAc2 heptasaccharide 17, and the execution of, arguably, some of the most ambitious glycopeptide ligations known to date. As a whole, this work represents a promising first step toward the development of experimental vaccine immunogens to be tested for the capacity to elicit gp120 V1V2 BnAb epitope-targeted antibodies. Further work is underway to characterize the antigenic properties of 1 and 2 in detail, and evaluate their immunogenicity in animal models. Similar and perhaps even more ambitious chemical synthesis strategies may be of use in preparing homogeneous glycopeptides for other HIV-1 gp120 BnAb epitopes.43 This is an ongoing program whose results will be disclosed in due course.
Supplementary Material
Acknowledgments
This work was supported by NIH grants for the Center for HIV/AIDS Vaccine Immunology, AI 067854 and the Duke Center for HIV/AIDS Vaccine Immunology-Immunogen Discovery, AI 100645. P.K.P. was supported by a postdoctoral fellowship (PF-11-014-01-CDD) from the American Cancer Society. A.F.-T. thanks the European Commission (Marie Curie International Outgoing Fellowship) for financial support. The authors thank Dr. George Sukenick, Ms. Hui Fang, and Ms. Sylvi Rusli of the NMR Analytical Core Facility at MSKCC for MS and NMR assistance. We also thank Dr. Ping Wang, Dr. Xiangyang Wu, Dr. Maciej Walczak, Dr. Gardner Creech, Dr. Steven Townsend, and Ms. Rebecca Wilson for advice and helpful discussions. We are grateful to Dr. Wayne Koff (International AIDS Vaccine Initiative, New York, NY) and Dr. Dennis Burton (The Scripps Research Institute, La Jolla, CA) for provision of mAb PG9.
Footnotes
The authors declare no competing financial interest.
Supporting Information. Detailed experimental procedures, including spectroscopic and analytical data for new compounds. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Myers G, Lenroot R. AIDS Res Hum Retroviruses. 1992;8:1459–1460. doi: 10.1089/aid.1992.8.1459. [DOI] [PubMed] [Google Scholar]
- 2.(a) Trkola A, Purtscher M, Muster T, Ballaun C, Buchacher A, Sullivan N, Srinivasan K, Sodroski J, Moore JP, Katinger H. J Virology. 1996;70:1100–1108. doi: 10.1128/jvi.70.2.1100-1108.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Sanders RW, Venturi M, Schiffner L, Kalyanaraman R, Katinger H, Lloyd KO, Kwong PD, Moore JP. J Virol. 2002;76:7293–7305. doi: 10.1128/JVI.76.14.7293-7305.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Scanlan CN, Pantophlet R, Wormald MR, Ollmann Saphire E, Stanfield R, Wilson IA, Katinger H, Dwek RA, Rudd PM, Burton DR. J Virology. 2002;76:7306–7321. doi: 10.1128/JVI.76.14.7306-7321.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Calarese DA, Scanlan CN, Zwick MB, Deechongkit S, Mimura Y, Kunert R, Zhu P, Wormald MR, Stanfield RL, Roux KH, Kelly JW, Rudd PM, Dwek RA, Katinger H, Burton DR, Wilson IA. Science. 2003;300:2065–2071. doi: 10.1126/science.1083182. [DOI] [PubMed] [Google Scholar]
- 3.For recent reviews, see: Morelli L, Poletti L, Lay L. Eur J Org Chem. 2011;2011:5723–5777.Wang L-X. Carbohydrate-Based Vaccines Against HIV/AIDS. In: Klyosov AA, editor. Glycobiology and Drug Design. American Chemical Society; Washington, DC: 2012. pp. 157–186. ACS Symposium Series 1102.
- 4.(a) Mandal M, Dudkin VY, Geng X, Danishefsky SJ. Angew Chem Int Ed. 2004;43:2557–2561. doi: 10.1002/anie.200353625. [DOI] [PubMed] [Google Scholar]; (b) Geng X, Dudkin VY, Mandal M, Danishefsky SJ. Angew Chem Int Ed. 2004;43:2562–2565. doi: 10.1002/anie.200353626. [DOI] [PubMed] [Google Scholar]; (c) Dudkin VY, Orlova M, Geng X, Mandal M, Olson WC, Danishefsky SJ. J Am Chem Soc. 2004;126:9560–9562. doi: 10.1021/ja047720g. [DOI] [PubMed] [Google Scholar]; (d) Krauss IJ, Joyce JG, Finnefrock AC, Song HC, Dudkin VY, Geng X, Warren JD, Chastain M, Shiver JW, Danishefsky SJ. J Am Chem Soc. 2007;129:11042–11044. doi: 10.1021/ja074804r. [DOI] [PubMed] [Google Scholar]; (e) Joyce JG, Krauss IJ, Song HC, Opalka DW, Grimm KM, Nahas DD, Esser MT, Hrin R, Feng M, Dudkin VY, Chastain M, Shiver JW, Danishefsky SJ. Proc Natl Acad Sci U S A. 2008;105:15684–15689. doi: 10.1073/pnas.0807837105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Haynes BF, Kelsoe G, Harrison SC, Kepler TB. Nat Biotechnol. 2012;30:423–433. doi: 10.1038/nbt.2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Walker LM, Phogat SK, Chan-Hui P-Y, Wagner D, Phung P, Goss JL, Wrin T, Simek MD, Fling S, Mitcham JL, Lehrman JK, Priddy FH, Olsen OA, Frey SM, Hammond PW, Protocol G, Kaminsky S, Zamb T, Moyle M, Koff WC, Poignard P, Burton DR Principal Investigators. Science. 2009;326:285–289. doi: 10.1126/science.1178746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McLellan JS, Pancera M, Carrico C, Gorman J, Julien J-P, Khayat R, Louder R, Pejchal R, Sastry M, Dai K, O/’Dell S, Patel N, Shahzad-ul-Hussan S, Yang Y, Zhang B, Zhou T, Zhu J, Boyington JC, Chuang G-Y, Diwanji D, Georgiev I, Do Kwon Y, Lee D, Louder MK, Moquin S, Schmidt SD, Yang Z-Y, Bonsignori M, Crump JA, Kapiga SH, Sam NE, Haynes BF, Burton DR, Koff WC, Walker LM, Phogat S, Wyatt R, Orwenyo J, Wang L-X, Arthos J, Bewley CA, Mascola JR, Nabel GJ, Schief WR, Ward AB, Wilson IA, Kwong PD. Nature. 2011;480:336–343. doi: 10.1038/nature10696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.For recent reviews, see: Yuan Y, Chen J, Wan Q, Wilson RM, Danishefsky SJ. Biopolymers. 2010;94:373–384. doi: 10.1002/bip.21374.Wilson RM, Dong S, Wang P, Danishefsky SJ. Angew Chem Int Ed. 2013;52:7646–7665. doi: 10.1002/anie.201301666.
- 9.A recent analysis of the bivalent gp120 subunit vaccine AIDSVAX B/E found 16 isoforms of the clade B immunogen and 24 isoforms of the clade E immunogen: Yu B, Morales JF, O’Rourke SM, Tatsuno GP, Berman PW. PLoS ONE. 2012;7:e43903. doi: 10.1371/journal.pone.0043903.
- 10.During the preparation of this manuscript, an abstract was published by Wang referring to work on homogeneous gp120 V1V2 glycopeptides: Wang L-X. Abstracts of Papers, 245th National Meeting of the American Chemical Society; New Orleans. Apr 7–11, 2013; Washington, DC: American Chemical Society; 2013. p. CARB–13.The Wang constructs were prepared by chemoenzymatic assembly using semisynthesis-derived glycans, as detailed in a subsequent publication, which was disclosed while this manuscript was undergoing review: Amin MN, McLellan JS, Huang W, Orwenyo J, Burton DR, Koff WC, Kwong PD, Wang LX. Nat Chem Biol. 2013;9:521–526. doi: 10.1038/nchembio.1288.
- 11.(a) Miller JS, Dudkin VY, Lyon GJ, Muir TW, Danishefsky SJ. Angew Chem Int Ed. 2003;42:431–434. doi: 10.1002/anie.200390131. [DOI] [PubMed] [Google Scholar]; (b) Warren JD, Miller JS, Keding SJ, Danishefsky SJ. J Am Chem Soc. 2004;126:6576–6578. doi: 10.1021/ja0491836. [DOI] [PubMed] [Google Scholar]
- 12.The scaffolded V1V2 construct was expressed in HEK 293S GnTI−/− cells, which are unable to process the Man5GlcNAc2 intermediate into hybrid and complex-type glycans. The entire Man5GlcNAc2 oligosaccharide is evident in the electron density map for the Asn160 glycan, but only four mannose residues are visible for the Asn156 glycan.
- 13.In the context of the Env trimer, PG9 may engage a third glycan on Asn160 of a neighboring protomer: Julien JP, Lee JH, Cupo A, Murin CD, Derking R, Hoffenberg S, Caulfield MJ, King CR, Marozsan AJ, Klasse PJ, Sanders RW, Moore JP, Wilson IA, Ward AB. Proc Natl Acad Sci U S A. 2013;110:4351–4356. doi: 10.1073/pnas.1217537110.
- 14.An A244 strain recombinant gp120 glycoprotein (as a mixture of glycoforms) was used as part of the vaccine regimen in the recent, modestly efficacious RV144 HIV-1 vaccination clinical trial: Rerks-Ngarm S, Pitisuttithum P, Nitayaphan S, Kaewkungwal J, Chiu J, Paris R, Premsri N, Namwat C, De Souza M, Adams E, Benenson M, Gurunathan S, Tartaglia J, McNeil JG, Francis DP, Stablein D, Birx DL, Chunsuttiwat S, Khamboonruang C, Thongcharoen P, Robb ML, Michael NL, Kunasol P, Kim JH. N Engl J Med. 2009;361:2209–2220. doi: 10.1056/NEJMoa0908492.
- 15.Bonsignori M, Hwang KK, Chen X, Tsao CY, Morris L, Gray E, Marshall DJ, Crump JA, Kapiga SH, Sam NE, Sinangil F, Pancera M, Yongping Y, Zhang B, Zhu J, Kwong PD, O’Dell S, Mascola JR, Wu L, Nabel GJ, Phogat S, Seaman MS, Whitesides JF, Moody MA, Kelsoe G, Yang X, Sodroski J, Shaw GM, Montefiori DC, Kepler TB, Tomaras GD, Alam SM, Liao HX, Haynes BF. J Virology. 2011;85:9998–10009. doi: 10.1128/JVI.05045-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Doores KJ, Burton DR. J Virology. 2010;84:10510–10521. doi: 10.1128/JVI.00552-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.A recent report suggests that there may be greater promiscuity with regard to recognition of the glycan at Asn156 by PG16 and PG9: Pancera M, Shahzad-ul-Hussan S, Doria-Rose NA, McLellan JS, Bailer RT, Dai K, Loesgen S, Louder MK, Staupe RP, Yang Y, Zhang B, Parks R, Eudailey J, Lloyd KE, Blinn J, Alam SM, Haynes BF, Amin MN, Wang L-X, Burton DR, Koff WC, Nabel GJ, Mascola JR, Bewley CA, Kwong PD. Nat Struct Mol Biol. 2013;20:804–813. doi: 10.1038/nsmb.2600.
- 18.All of our V1V2 glycopeptide constructs are chemically different from the glycopeptides prepared by Amin et al. (ref. 10b). Although one of their constructs carried Man5GlcNAc2 at Asn160 and Asn156, we used an Env sequence that was derived from a different HIV-1 strain (A244) than the two variants used in their study (CAP45 and ZM109). Moreover, in our constructs, the peptide domains are longer (35 amino acids versus 24), and we retained the native A244 sequence without mutating any residues or adding any affinity tags. Amin et al. mutated Lys155 and Phe176 to Cys, allowing for cyclization of their constructs via disulfide bond formation, and they incorporated a biotin tag at the N-terminus.
- 19.(a) Anisfeld ST, Lansbury PT. J Org Chem. 1990;55:5560–5562. [Google Scholar]; (b) Cohen-Anisfeld ST, Lansbury PT. J Am Chem Soc. 1993;115:10531–10537. [Google Scholar]
- 20.Wang P, Aussedat B, Vohra Y, Danishefsky SJ. Angew Chem Int Ed. 2012;51:11571–11575. doi: 10.1002/anie.201205038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ullmann V, Rädisch M, Boos I, Freund J, Pöhner C, Schwarzinger S, Unverzagt C. Angew Chem Int Ed. 2012;51:11566–11570. doi: 10.1002/anie.201204272. [DOI] [PubMed] [Google Scholar]
- 22.For previous reports detailing the synthesis of the reducing pentasaccharide, see: Paulsen H, Lebuhn R. Carbohydr Res. 1984;130:85–101.Nakahara Y, Shibayama S, Nakahara Y, Ogawa T. Carbohydr Res. 1996;280:67–84. doi: 10.1016/0008-6215(95)00262-6.Wang ZG, Zhang X, Live D, Danishefsky SJ. Angew Chem Int Ed. 2000;39:3652–3656. doi: 10.1002/1521-3773(20001016)39:20<3652::aid-anie3652>3.0.co;2-b.Totani K, Matsuo I, Ito Y. Bioorg Med Chem Lett. 2004;14:2285–2289. doi: 10.1016/j.bmcl.2004.01.106.Hagihara S, Totani K, Matsuo I, Ito Y. J Med Chem. 2005;48:3126–3129. doi: 10.1021/jm0489511.Wang ZG, Warren JD, Dudkin VY, Zhang X, Iserloh U, Visser M, Eckhardt M, Seeberger PH, Danishefsky SJ. Tetrahedron. 2006;62:4954–4978.
- 23.Indeed, we were only able to locate a single report dealing with its preparation, not as the reducing sugar, but suitably functionalized for the construction of carbohydrate microarrays: Serna S, Etxebarria J, Ruiz N, Martin-Lomas M, Reichardt NC. Chem Eur J. 2010;16:13163–13175. doi: 10.1002/chem.201001295.
- 24.Interestingly, Cohen-Anisfeld and Lansbury did demonstrate their method using Man5GlcNAc2, but it was obtained from the urine of sheep with swainsonine-induced a-mannosidosis (see ref. 19b).
- 25.(a) Wang P, Zhu J, Yuan Y, Danishefsky SJ. J Am Chem Soc. 2009;131:16669–16671. doi: 10.1021/ja907136d. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Walczak MA, Danishefsky SJ. J Am Chem Soc. 2012;134:16430–16433. doi: 10.1021/ja307628w. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Walczak MA, Hayashida J, Danishefsky SJ. J Am Chem Soc. 2013;135:4700–4703. doi: 10.1021/ja401385v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.(a) Crich D, Sun S. J Org Chem. 1996;61:4506–4507. doi: 10.1021/jo9606517. [DOI] [PubMed] [Google Scholar]; (b) Crich D, Sun S. J Org Chem. 1997;62:1198–1199. [Google Scholar]; (c) Crich D, Sun S. J Am Chem Soc. 1998;120:435–436. [Google Scholar]; (d) Crich D, Sun S. Tetrahedron. 1998;54:8321–8348. [Google Scholar]
- 27.Crich D, Li H, Yao Q, Wink DJ, Sommer RD, Rheingold AL. J Am Chem Soc. 2001;123:5826–5828. doi: 10.1021/ja015985e. [DOI] [PubMed] [Google Scholar]
- 28.Shie C, Tzeng Z, Kulkarni SS, Uang B, Hsu C, Hung S. Angew Chem Int Ed. 2005;44:1665–1668. doi: 10.1002/anie.200462172. [DOI] [PubMed] [Google Scholar]
- 29.Likhosherstov LM, Novikova OS, Derevitskaja VA, Kochetkov NK. Carbohydr Res. 1986;146:C1–C5. [Google Scholar]
- 30.Dawson PE, Muir TW, Clark-Lewis I, Kent SB. Science. 1994;266:776–779. doi: 10.1126/science.7973629. [DOI] [PubMed] [Google Scholar]
- 31.Kuroda H, Chen Y, Kimura T, Sakakibara S. Int J Pept Prot Res. 1992;40:294–299. doi: 10.1111/j.1399-3011.1992.tb00304.x. [DOI] [PubMed] [Google Scholar]
- 32.Nagorny P, Fasching B, Li X, Chen G, Aussedat B, Danishefsky SJ. J Am Chem Soc. 2009;131:5792–5799. doi: 10.1021/ja809554x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.One equivalent (combined) of MPAA-exchanged and unreacted ethyl thioester can be recovered after the reaction.
- 34.Closely spaced N-linked glycans on cyclic peptide scaffolds have been synthesized previously. For example, see: Sprengard U, Schudok M, Schmidt W, Kretzschmar G, Kunz H. Angew Chem Int Ed. 1996;35:321–324.
- 35.Work is also in progress to evaluate alternative synthetic strategies that would yield a more “process-friendly” route.
- 36.Alam SM, McAdams M, Boren D, Rak M, Scearce RM, Gao F, Camacho ZT, Gewirth D, Kelsoe G, Chen P, Haynes BF. J Immunol. 2007;178:4424–4435. doi: 10.4049/jimmunol.178.7.4424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.The conformational consequences of N-glycosylation have been well studied. For reviews, see: Imperiali B, O’Connor SE. Curr Opin Chem Biol. 1999;3:643–649. doi: 10.1016/s1367-5931(99)00021-6.Meyer B, Möller H. Conformation of Glycopeptides and Glycoproteins. In: Wittmann V, editor. Glycopeptides and Glycoproteins: Synthesis, Structure, and Application. Vol. 267. Springer; Berlin: 2007. pp. 187–251. Topics in Current Chemistry.
- 38.(a) Live DH, Kumar RA, Beebe X, Danishefsky SJ. Proc Natl Acad Sci U S A. 1996;93:12759–12761. doi: 10.1073/pnas.93.23.12759. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Live DH, Wang ZG, Iserloh U, Danishefsky SJ. Org Lett. 2001;3:851–854. doi: 10.1021/ol0070233. [DOI] [PubMed] [Google Scholar]
- 39.Similarly, using V1V2 glycopeptide isoforms bearing natively N-linked sugars, Amin et al. (ref. 10b) detected an astonishing preference of PG9 for a sialylated complex-type glycan at Asn156.
- 40.Man3GlcNAc2, also known as “paucimannose,” is more characteristically associated with plant and invertebrate glycoproteins, although some have argued that it can be expressed in certain pathological states in mammals, such as inflammation or malignancy: Zipser B, Bello-DeOcampo D, Diestel S, Tai M-H, Schmitz B. J Carbohydr Chem. 2012;31:504–518.
- 41.Others have remarked on the limitations of glycan array analysis in delineating BnAb anti-glycan specificities: “Protein-free glycan binding by anti-HIV antibodies is not always detectable; e.g., although PG9 recognizes a gp120-associated high-mannose glycan, no binding to protein-free glycans was detected in microarrays. Thus, although a positive result in a glycan microarray implies involvement of a particular glycan in an antibody epitope, a negative result does not rule out glycan recognition.” For the full discussion, see: Mouquet H, Scharf L, Euler Z, Liu Y, Eden C, Scheid JF, Halper-Stromberg A, Gnanapragasam PNP, Spencer DIR, Seaman MS, Schuitemaker H, Feizi T, Nussenzweig MC, Bjorkman PJ. Proc Natl Acad Sci U S A. 2012;109:E3268–E3277. doi: 10.1073/pnas.1217207109.
- 42.As a point of comparison, we note that Amin et al. (ref. 10b) prepared more than 25 V1V2 glycopeptides bearing various combinations of high mannose and complex-type glycans, and yet their highest affinity constructs bound PG9 Fab with Kd’s of ~5 × 10−6 M.
- 43.Walker LM, Huber M, Doores KJ, Falkowska E, Pejchal R, Julien J-P, Wang S-K, Ramos A, Chan-Hui P-Y, Moyle M, Mitcham JL, Hammond PW, Olsen OA, Phung P, Fling S, Wong C-H, Phogat S, Wrin T, Simek MD, Koff WC, Wilson IA, Burton DR, Poignard P Principal Investigators PG. Nature. 2011;477:466–470. doi: 10.1038/nature10373. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.