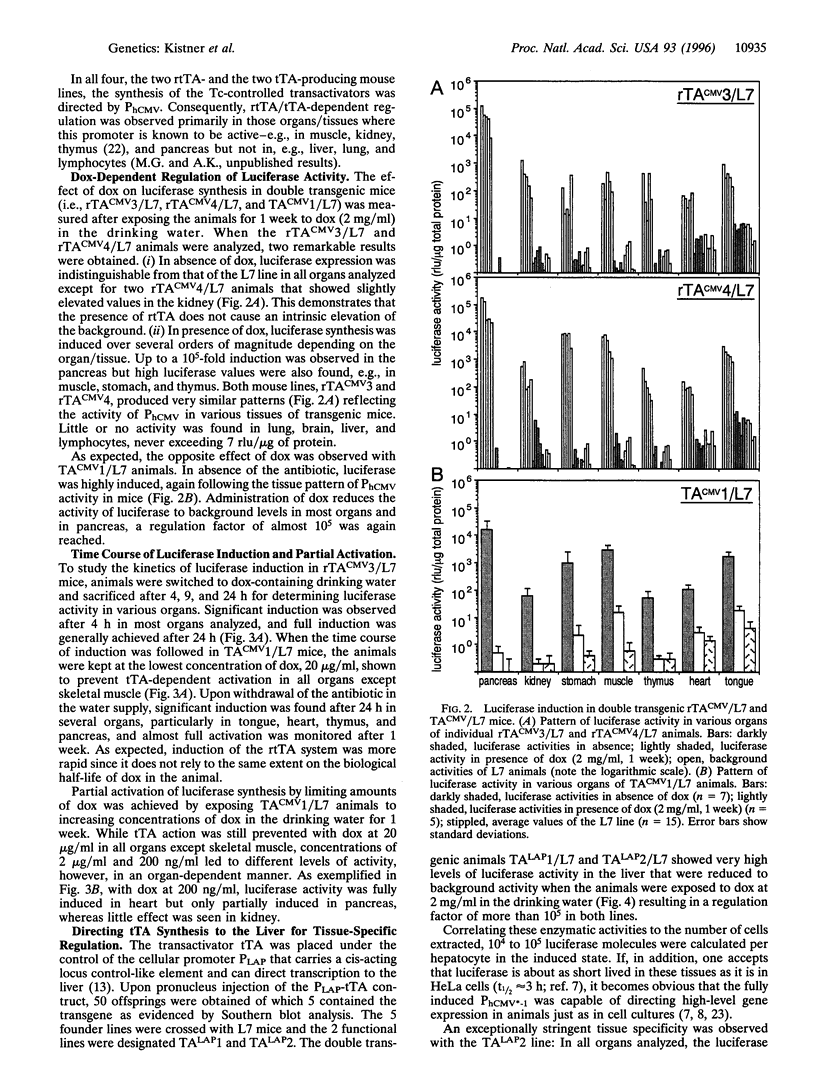
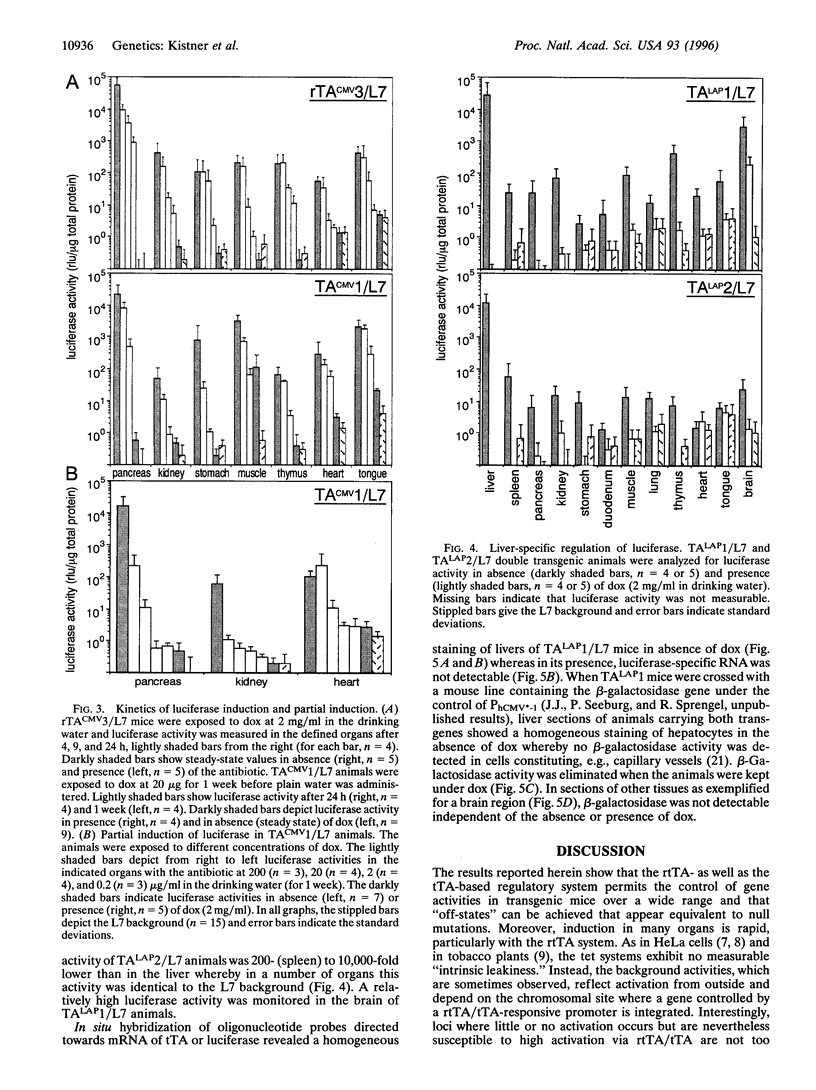
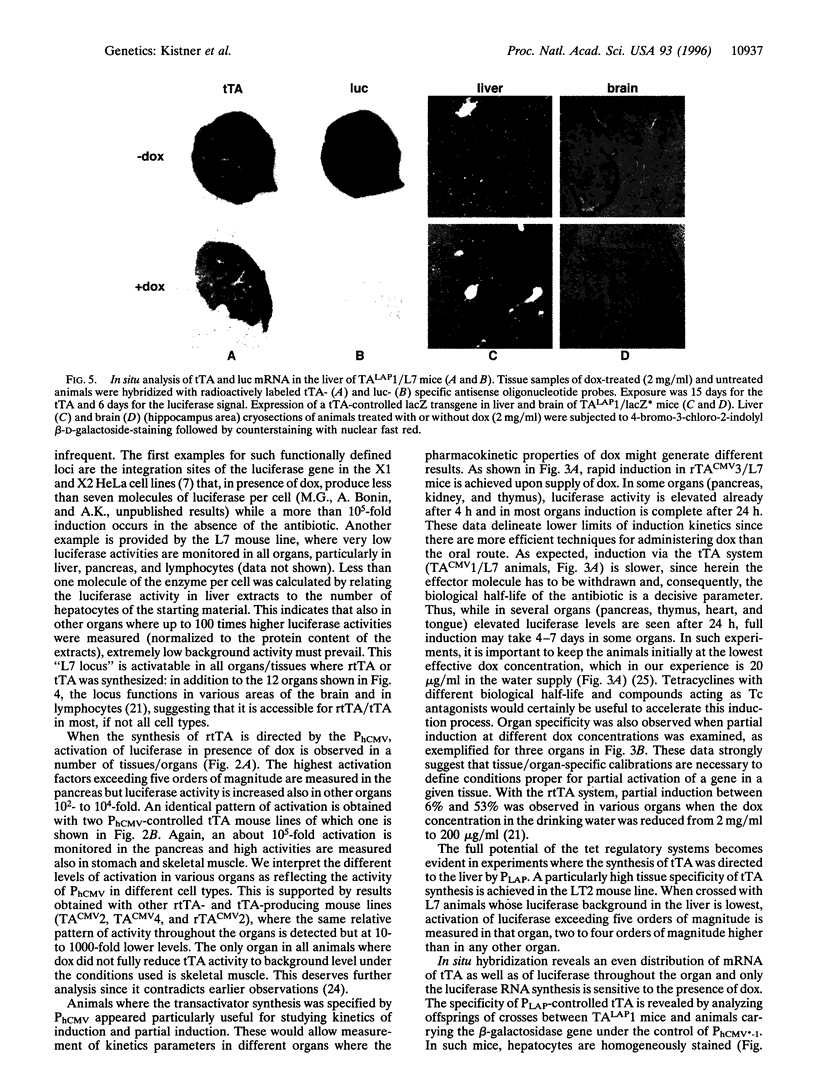
Abstract
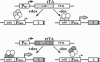
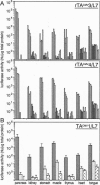
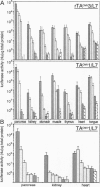
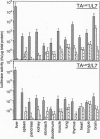
The tet regulatory system in which doxycycline (dox) acts as an inducer of specifically engineered RNA polymerase II promoters was transferred into transgenic mice. Tight control and a broad range of regulation spanning up to five orders of magnitude were monitored dependent on the dox concentration in the water supply of the animals. Administration of dox rapidly induces the synthesis of the indicator enzyme luciferase whose activity rises over several orders of magnitude within the first 4 h in some organs. Induction is complete after 24 h in most organs analyzed. A comparable regulatory potential was revealed with the tet regulatory system where dox prevents transcription activation. Directing the synthesis of the tetracycline-controlled transactivator (tTA) to the liver led to highly specific regulation in hepatocytes where, in presence of dox, less than one molecule of luciferase was detected per cell. By contrast, a more than 10(5)-fold activation of the luciferase gene was observed in the absence of the antibiotic. This regulation was homogeneous throughout but stringently restricted to hepatocytes. These results demonstrate that both tetracycline-controlled transcriptional activation systems provide genetic switches that permit the quantitative control of gene activities in transgenic mice in a tissue-specific manner and, thus, suggest possibilities for the generation of a novel type of conditional mutants.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baskar J. F., Smith P. P., Nilaver G., Jupp R. A., Hoffmann S., Peffer N. J., Tenney D. J., Colberg-Poley A. M., Ghazal P., Nelson J. A. The enhancer domain of the human cytomegalovirus major immediate-early promoter determines cell type-specific expression in transgenic mice. J Virol. 1996 May;70(5):3207–3214. doi: 10.1128/jvi.70.5.3207-3214.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boshart M., Weber F., Jahn G., Dorsch-Häsler K., Fleckenstein B., Schaffner W. A very strong enhancer is located upstream of an immediate early gene of human cytomegalovirus. Cell. 1985 Jun;41(2):521–530. doi: 10.1016/s0092-8674(85)80025-8. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Byrne G. W., Ruddle F. H. Multiplex gene regulation: a two-tiered approach to transgene regulation in transgenic mice. Proc Natl Acad Sci U S A. 1989 Jul;86(14):5473–5477. doi: 10.1073/pnas.86.14.5473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrast-Balz J., Hooft van Huijsduijnen R. Bi-directional gene switching with the tetracycline repressor and a novel tetracycline antagonist. Nucleic Acids Res. 1996 Aug 1;24(15):2900–2904. doi: 10.1093/nar/24.15.2900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhawan J., Rando T. A., Elson S. L., Bujard H., Blau H. M. Tetracycline-regulated gene expression following direct gene transfer into mouse skeletal muscle. Somat Cell Mol Genet. 1995 Jul;21(4):233–240. doi: 10.1007/BF02255778. [DOI] [PubMed] [Google Scholar]
- Efrat S., Fusco-DeMane D., Lemberg H., al Emran O., Wang X. Conditional transformation of a pancreatic beta-cell line derived from transgenic mice expressing a tetracycline-regulated oncogene. Proc Natl Acad Sci U S A. 1995 Apr 11;92(8):3576–3580. doi: 10.1073/pnas.92.8.3576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furth P. A., Hennighausen L., Baker C., Beatty B., Woychick R. The variability in activity of the universally expressed human cytomegalovirus immediate early gene 1 enhancer/promoter in transgenic mice. Nucleic Acids Res. 1991 Nov 25;19(22):6205–6208. doi: 10.1093/nar/19.22.6205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furth P. A., St Onge L., Böger H., Gruss P., Gossen M., Kistner A., Bujard H., Hennighausen L. Temporal control of gene expression in transgenic mice by a tetracycline-responsive promoter. Proc Natl Acad Sci U S A. 1994 Sep 27;91(20):9302–9306. doi: 10.1073/pnas.91.20.9302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gossen M., Bujard H. Tight control of gene expression in mammalian cells by tetracycline-responsive promoters. Proc Natl Acad Sci U S A. 1992 Jun 15;89(12):5547–5551. doi: 10.1073/pnas.89.12.5547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gossen M., Freundlieb S., Bender G., Müller G., Hillen W., Bujard H. Transcriptional activation by tetracyclines in mammalian cells. Science. 1995 Jun 23;268(5218):1766–1769. doi: 10.1126/science.7792603. [DOI] [PubMed] [Google Scholar]
- Gu H., Marth J. D., Orban P. C., Mossmann H., Rajewsky K. Deletion of a DNA polymerase beta gene segment in T cells using cell type-specific gene targeting. Science. 1994 Jul 1;265(5168):103–106. doi: 10.1126/science.8016642. [DOI] [PubMed] [Google Scholar]
- Gu H., Zou Y. R., Rajewsky K. Independent control of immunoglobulin switch recombination at individual switch regions evidenced through Cre-loxP-mediated gene targeting. Cell. 1993 Jun 18;73(6):1155–1164. doi: 10.1016/0092-8674(93)90644-6. [DOI] [PubMed] [Google Scholar]
- Lakso M., Sauer B., Mosinger B., Jr, Lee E. J., Manning R. W., Yu S. H., Mulder K. L., Westphal H. Targeted oncogene activation by site-specific recombination in transgenic mice. Proc Natl Acad Sci U S A. 1992 Jul 15;89(14):6232–6236. doi: 10.1073/pnas.89.14.6232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passman R. S., Fishman G. I. Regulated expression of foreign genes in vivo after germline transfer. J Clin Invest. 1994 Dec;94(6):2421–2425. doi: 10.1172/JCI117609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shockett P., Difilippantonio M., Hellman N., Schatz D. G. A modified tetracycline-regulated system provides autoregulatory, inducible gene expression in cultured cells and transgenic mice. Proc Natl Acad Sci U S A. 1995 Jul 3;92(14):6522–6526. doi: 10.1073/pnas.92.14.6522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sternberg N., Hamilton D. Bacteriophage P1 site-specific recombination. I. Recombination between loxP sites. J Mol Biol. 1981 Aug 25;150(4):467–486. doi: 10.1016/0022-2836(81)90375-2. [DOI] [PubMed] [Google Scholar]
- Talbot D., Descombes P., Schibler U. The 5' flanking region of the rat LAP (C/EBP beta) gene can direct high-level, position-independent, copy number-dependent expression in multiple tissues in transgenic mice. Nucleic Acids Res. 1994 Mar 11;22(5):756–766. doi: 10.1093/nar/22.5.756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teitelbaum S. L., Abu-Amer Y., Ross F. P. Molecular mechanisms of bone resorption. J Cell Biochem. 1995 Sep;59(1):1–10. doi: 10.1002/jcb.240590102. [DOI] [PubMed] [Google Scholar]
- Thomas K. R., Capecchi M. R. Site-directed mutagenesis by gene targeting in mouse embryo-derived stem cells. Cell. 1987 Nov 6;51(3):503–512. doi: 10.1016/0092-8674(87)90646-5. [DOI] [PubMed] [Google Scholar]
- Weinmann P., Gossen M., Hillen W., Bujard H., Gatz C. A chimeric transactivator allows tetracycline-responsive gene expression in whole plants. Plant J. 1994 Apr;5(4):559–569. doi: 10.1046/j.1365-313x.1994.5040559.x. [DOI] [PubMed] [Google Scholar]
- Yin D. X., Zhu L., Schimke R. T. Tetracycline-controlled gene expression system achieves high-level and quantitative control of gene expression. Anal Biochem. 1996 Mar 15;235(2):195–201. doi: 10.1006/abio.1996.0112. [DOI] [PubMed] [Google Scholar]