Abstract
The thymic medulla represents a key site for the induction of T cell tolerance. In particular, autoimmune regulator (Aire)-expressing medullary thymic epithelial cells (mTECs) provide a spectrum of tissue-restricted Ags that, through both direct presentation and cross-presentation by dendritic cells, purge the developing T cell repertoire of autoimmune specificities. Despite this role, the mechanisms of Aire+ mTEC development remain unclear, particularly those stages that occur post-Aire expression and represent mTEC terminal differentiation. In this study, in mouse thymus, we analyze late-stage mTEC development in relation to the timing and requirements for Aire and involucrin expression, the latter a marker of terminally differentiated epithelium including Hassall’s corpuscles. We show that Aire expression and terminal differentiation within the mTEC lineage are temporally separable events that are controlled by distinct mechanisms. We find that whereas mature thymocytes are not essential for Aire+ mTEC development, use of an inducible ZAP70 transgenic mouse line—in which positive selection can be temporally controlled—demonstrates that the emergence of involucrin+ mTECs critically depends upon the presence of mature single positive thymocytes. Finally, although initial formation of Aire+ mTECs depends upon RANK signaling, continued mTEC development to the involucrin+ stage maps to activation of the LTα–LTβR axis by mature thymocytes. Collectively, our results reveal further complexity in the mechanisms regulating thymus medulla development and highlight the role of distinct TNFRs in initial and terminal differentiation stages in mTECs.
After their recruitment to the thymus, T cell precursors proliferate and differentiate to produce a large pool of immature CD4+8+ thymocytes that resides within the thymic cortex. As a result of the random recombination of gene segments at TCR-α and TCR-β loci, CD4+8+ thymocytes express a diverse repertoire of αβTCRs (αβTCRs), and so are required to undergo selection events based on αβTCRs specificity. Positive selection rescues thymocytes capable of self-MHC recognition from cell death, with the lineage commitment mechanism ensuring that changes in coreceptor expression result in the generation of CD4+ and CD8+ cells recognizing MHC class II and MHC class I, respectively (1, 2). Newly selected thymocytes are then screened further for their ability to recognize self-peptide/MHC complexes, with negative selection purging the αβTCRs repertoire of potentially autoreactive specificities (3). Such combined selection events ensure that T cell production in the thymus leads to the generation of a naive T cell pool that recognizes self-MHC molecules but is able to discriminate between self and nonself Ags.
Positive and negative selection events occur within specialized intrathymic environments defined by distinct stromal components (4). Positive selection signals are provided by cortical thymic epithelial cells (cTECs), a function that is at least in part due to their expression of a unique MHC-bound self-peptide repertoire generated by cTEC-specific expression of Prss16 (the gene encoding thymus-specific serine protease) and the β5t thymoproteosomal subunit (5-7). In contrast, the medulla provides a microenvironment where self-tolerance is imposed, through both negative selection and FoxP3+ regulatory T cell (Treg) production. Moreover, autoimmune regulator (Aire) expressing medullary thymic epithelial cells (mTECs) have been shown to play a role in both processes (8-10), highlighting their importance in the avoidance of autoimmunity. Several advances have recently been made in understanding the regulation of Aire+ mTEC development. For example, the TNFRs RANK and CD40 are key to the generation of Aire+ mTECs (11-15), a process involving lymphostromal cross-talk with RANKL+ lymphoid tissue inducer cells and RANKL+/CD40L+CD4+ thymocytes. An additional TNFR family member, LTβR, also plays a pivotal yet poorly understood role in mTEC development (16-20). Interestingly, the medulla is also recognized as a dynamic thymic compartment, with mature mTECs displaying a turnover time of 2–3 wk (21, 22). Collectively, such findings suggest that Aire+ mTEC production is a continuous process stemming from a TEC progenitor pool that is under hemopoietic cell control. Despite these advances, critical to our understanding of mTEC development and homeostasis are events that occur in mTEC post-Aire expression. Whereas initial studies suggested that Aire may induce apoptosis as a means to regulate mTEC homeostasis (23), others suggest that it plays a role in further mTEC differentiation (24, 25), with recent cell fate mapping approaches directly demonstrating that mTEC differentiation continues beyond the Aire+ stage (26). Moreover, late-stage mTEC differentiation has been shown to involve expression of involucrin (25, 26), a component of the cross-linked cornified envelope and a marker of terminal differentiation in keratinocytes (27). However, whether progression to this stage is a cell-autonomous process post-Aire expression or also requires cross-talk and regulation by hemopoietic elements is unknown. This is a key issue in understanding the regulation of mTEC homeostasis and hence the efficiency of self-Ag presentation for central tolerance.
In this study, we have analyzed cellular and molecular regulation of the mTEC lineage, including the requirements for developmental stages post-Aire expression. We show an ordered ontogenetic appearance of distinct Aire+ and involucrin+ mTEC subsets in the thymus medulla, the latter appearing postnatally at a time point correlating with the initial accumulation of positively selected thymocytes. By analyzing mTEC development in mouse models where positive selection is either absent or can selectively be restored by an inducible Zap70 transgene, we provide direct evidence that whereas the absence of positive selection does not preclude generation of Aire+ mTECs, development of terminally differentiated involucrin+ mTECs strictly depends upon the presence of mature thymocytes. Finally, we show that the requirement for positive selection in mTEC terminal differentiation cannot be met by provision of RANKL and instead maps to activation of LTβR signaling. Collectively, our data define cross-talk mechanisms in mTEC terminal differentiation, help explain the poorly understood role of LTβR in thymus medulla development, and highlight the temporal requirement for distinct TNFR signaling during initial and late-stage mTEC differentiation.
Materials and Methods
Mice
Wild-type (WT) C57BL/6 (B6) mice at 4–6 wk of age were used as indicated. Zap70−/− (28), Ltbr−/− (29), Lta−/− (30), Cd40lg−/− (31) mice have been described previously. Tetracycline-inducible Zap70 transgenic mice (Zap70Tre rtTAhuCD2 Zap70−/−; TetZap70 hereon) have been described in detail elsewhere (32). Zap70 expression in adult mice was induced by administration of tetracycline derivative doxycycline (dox) in food (3 mg/g). Mice were bred and maintained in the Biomedical Services Unit, University of Birmingham, with the exception of TetZap70 mice, which were bred and maintained at the National Institute for Medical Research (Mill Hill, London, U.K.). All experiments were carried out with institution and U.K. Home Office approval.
Human thymus samples
Fresh thymic tissue was provided by Birmingham Children’s Hospital (Birmingham, U.K.) from pediatric patients undergoing routine cardiac surgery. Tissues were obtained after informed consent and with the ethical approval of National Health Service North Staffordshire Local Research Committee.
Immunofluorescent confocal analysis and mTEC quantitation
Freshly isolated mouse and human thymus tissue was embedded in OCT compound (Sakura Fintek UK, Thatcham, U.K.) and frozen on dry ice. Sections were cut at 5-μm thickness, fixed in acetone, and stained with Abs as described previously (12). For analysis of mouse tissue, the following reagents were used: AlexaFluor 647 anti-CD4 (L3TA; eBioscience, Hatfield, U.K.), biotinylated anti-CD8 (ebioH35-17.2; eBioscience), rabbit anti-involucrin (Covance, Maidenhead, U.K.), rabbit anti-Aire (M-300; Santa Cruz Biotechnology, Santa Cruz, CA), AlexaFluor 647 EpCAM-1 (G8.8; kind gift from A. Farr, University of Washington, Seattle, WA), biotinylated anti-CD80 (clone Ly53; eBioscience), anti-IAb MHC class II alloantigen (clone AF6-120.1; Becton Dickinson, San Jose, CA), and mouse anti-keratin 10 (clone SPM261; Covance). Biotinylated Abs were detected using streptavidin conjugated to either AlexaFluor 555 or AlexaFluor 646 (Invitrogen, Paisley, U.K.). Involucrin and Aire Abs were detected using donkey anti-rabbit AlexaFluor 488 (Invitrogen), whereas keratin 10 Abs were detected with anti-mouse AlexaFluor 594 (Invitrogen), and anti-IAb MHC class II alloantigen was detected using an FITC amplification method described later. Simultaneous two-color staining of Aire and involucrin was performed using rabbit anti-involucrin and goat anti-Aire (clone D-17; Santa Cruz Biotechnology). Human thymic sections were stained with FITC anti-CD4 (RPA-T4; eBioscience), biotinylated anti-CD8 (HIT8α; eBioscience), mouse anti-involucrin (SY5; Abcam, Cambridge, U.K.), and goat anti-Aire (D-17; Santa Cruz Biotechnology). Anti-CD4 was detected using rabbit anti-FITC (Invitrogen) then goat anti-rabbit FITC (Southern Biotechnology, Birmingham, AL), and anti-CD8 was detected using Streptavidin-AlexaFluor 646 (Invitrogen). Anti-involucrin was detected using goat anti-mouse AlexaFluor 594 (Invitrogen), and anti-Aire was detected using donkey anti-goat AlexaFluor 594 (Invitrogen). Sections were mounted using 1,4-Diazabicyclo[2.2.2]octane solution (Sigma Aldrich, Dorset, U.K.), and all images were obtained using an LSM 510 Meta microscope, with image analysis performed using Zeiss LSM software (Zeiss, Welwyn Garden City, U.K.). To quantitate the frequency of Aire+ and involucrin+ mTECs, medullary areas were initially identified on the basis of CD4, CD8 staining to identify regions containing CD4+8− and CD4−8+ cells but not CD4+8+ cortical thymocytes. For each mouse, a minimum of three separate thymus sections at least 10 sections apart were analyzed. From each section, medullary areas of up to 200,000 μm2 were imaged, and the number of involucrin+ and Aire+ cells was determined to give the number of involucrin+ and Aire+ cells per square millimeter.
Statistical analysis
Analysis was performed using a two-tailed unpaired Student t test with Prism software (GraphPad, San Diego, CA). Significance is denoted with asterisks (i.e., *p < 0.05; **p < 0.01; ***p < 0.001).
Retroviral transduction and bone marrow chimeras
To generate RANKL-expressing retrovirus, PCR-cloned cDNA fragments of murine RANKL, using the primers 5′-CTCGAGGAAGGGAGAGAACGATC-3′ and 5′-CTCGAGTCAGTCTATGTCCTGAACTTTG-3′, were cloned into XhoI site of MIGR1 (a gift from Dr. W.S. Pear, University of Pennsylvania, Philadelphia, PA). Retroviral supernatants were prepared as described (33) using the packaging cell line Plat-E (34). To generate bone marrow (BM) chimeras, adult WT B6 or Zap70−/− mice were treated with 150 mg/kg 5-fluorouracil, and 4 d later, Sca1+ progenitors were isolated from femurs and tibias by magnetic sorting (Miltenyi Biotec, Auburn, CA). Retrovirus infection of sorted Sca1+ cells was performed as described (13). Briefly, freshly sorted BM cells were cultured in IMDM supplemented with 20% FCS, l-glutamine, sodium pyruvate, nonessential amino acids, penicillin, streptomycin, 50 ng/ml stem cell factor, 50 ng/ml IL-6, and 10 ng/ml IL-3. After 48, 72, and 96 h, cells were spin-infected with retrovirus by centrifuging the culture plates in the presence of 10 μg/ml polybrene for 90 min. Following infection, total cells were intravenously injected into irradiated recipients (900 rad for WT B6 and 700/800 rad for Zap70−/− hosts), and mice were analyzed 4–5 wk after injection.
Flow cytometry
Freshly prepared WT neonatal and adult thymocyte samples were centrifuged and then analyzed by flow cytometry as described (12). Cells were acquired using CellQuest software on a Becton Dickinson LSR flow cytometer. For analysis of TetZap70 mice, thymocyte suspensions were analyzed on a FACSCanto II (Becton Dickinson) using FACS diva software v6.1.2. All samples were then analyzed postacquisition with Flo-Jo software (Tree Star, Ashland, OR).
Results
Analysis of Aire and involucrin expression identifies temporal regulation in the development of distinct mTEC compartments
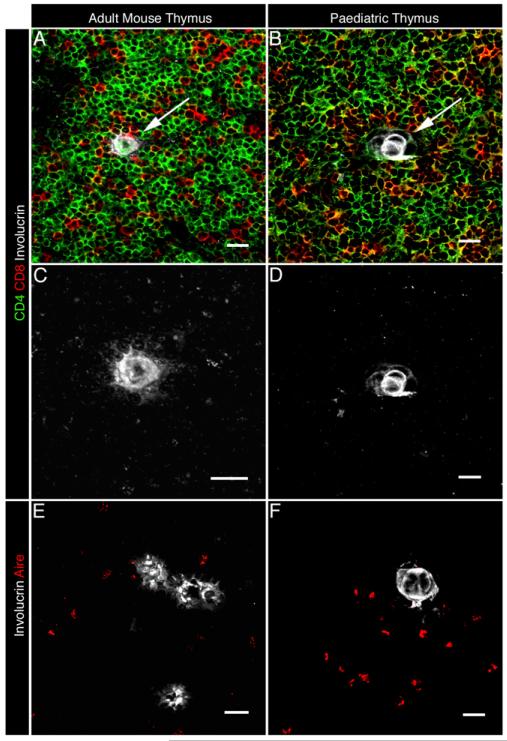
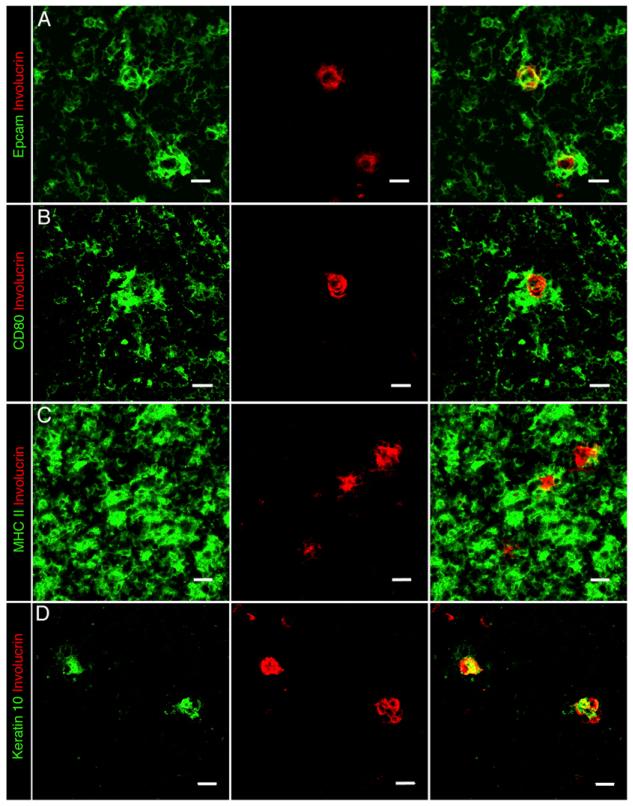
The keratinocyte terminal differentiation marker involucrin, a component of the cornified envelope in skin, has recently been reported to identify a subset of mTEC in adult mouse thymus (25, 26). Importantly, cell fate mapping studies show that mTEC maturation extends beyond the Aire+ stage and further suggest that involucrin expression may follow Aire expression, a relationship that is suggestive of a terminal differentiation program in mTECs (26). To investigate further these poorly defined stages in thymus medulla development, we initially analyzed the phenotype and ontogenetic timing of the appearance of Aire+ and involucrin+ mTECs in both adult murine and human pediatric thymus. In human thymus (Fig. 1B, 1D, 1F), involucrin identifies Hassall’s corpuscles (HCs), representing swirled epithelial structures formed from terminally differentiated epithelial cells (25, 35). In agreement with a recent study (25), analysis of adult mouse thymus (Fig. 1A, 1C, 1E) shows a similar staining pattern, with small, medullary-located involucrin+ cells displaying an organization reminiscent of HC-like structures in human thymus. Interestingly, in both mouse (Fig. 1E) and human (Fig. 1F) thymus, Aire and involucrin expression were consistently found to be nonoverlapping. However, although we failed to detect mTECs that coexpressed involucrin and Aire, Aire+involucrin− mTECs and Aire−involucrin+ mTECs were often found in close proximity to one another (Fig. 1E, 1F). Further phenotypic analysis (Fig. 2) with a panel of epithelial markers demonstrated that in contrast with Aire+ mTECs, which are enriched for CD80+MHCII+ keratin10− cells, involucrin+ mTECs are uniformly CD80−MHCII− and express keratin10+, an additional marker of epithelial differentiation in skin (36). Thus, our data suggest that Aire+ mTECs and terminally differentiated involucrin+ mTECs represent distinct subsets of epithelial cells within the thymic medulla.
FIGURE 1.
Involucrin expression defines terminally differentiated mTECs that are distinct from the Aire+ subset. A–D, Frozen tissue sections of adult mouse and neonatal human thymus were stained with Abs to CD4, CD8, and the epithelial terminal differentiation marker involucrin using an LSM 510 Meta microscope, and images were analyzed using Zeiss LSM software. C and D show higher-power magnification to demonstrate the characteristic swirled morphology of involucrin+ mTEC in both murine and human thymus. Dual analysis of Aire and involucrin expression in mouse (E) and human (F) thymus identifies nonoverlapping mTEC subsets that are often found in close anatomical association. Original magnification ×40; scale bars, 20 μm. Data shown are representative of at least three separate experiments.
FIGURE 2.
Phenotypic characterization of involucrin+ mTECs. Frozen tissue sections of adult mouse thymus were stained with Abs to involucrin and either EpCAM1 (A), CD80 (B), MHC class II (C), or keratin 10 (D). The images show typical examples of medullary areas within thymic sections. Original magnification ×40; scale bars, 20 μm, and data are representative of at least three separate experiments.
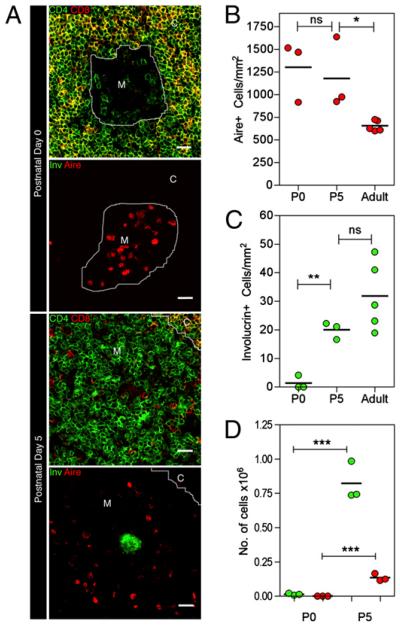
We next investigated the timing of appearance of Aire+ and involucrin+ mTECs in mouse thymus development. By quantitative confocal microscopy, we found that Aire+ mTECs, which were more abundant than involucrin+ mTECs at all stages analyzed, were readily detectable at postnatal day 0 (Fig. 3A, 3B), a finding that is in agreement with the initial emergence of Aire+ cells at E16 of gestation (12). In contrast, involucrin+ mTECs were found to be absent in embryonic thymus (data not shown) and immediately after birth, being first detected at postnatal day 5 (Fig. 3A, 3C), a time point coinciding with the appearance of positively selected CD4+αβTCRshigh and CD8+αβTCRshigh thymocytes (Fig. 3D). Collectively, these findings identify an ordered appearance of distinct Aire+involucrin− and Aire−involucrin+ mTEC subsets during thymus development, with the emergence of the latter correlating with the accumulation of mature thymocytes within thymic medullary areas.
FIGURE 3.
Ordered appearance of involucrin+ and Aire+ mTECs in thymus ontogeny identifies a postnatal phase of terminal differentiation. A, Thymus sections from newly born postnatal day (P) 0 and 5 mice were analyzed for expression of combinations of either CD4 and CD8 or Aire and involucrin. Original magnification ×40; scale bars, 20 μm. Quantitative analysis of Aire+ and involucrin+ mTECs at the indicated ages is shown in B and C, respectively. Ontogeny of thymocyte development in P0 and P5 thymus was analyzed by flow cytometry using Abs to CD4, CD8, and αβTCRs. D, Quantitative analysis of CD4+8−αβTCRshigh (green circles) and CD4−8+αβTCRshigh (red circles) subsets. In the graphs shown in B–D, each point represents a single mouse. Data are representative of three separate experiments. *p < 0.05; **p < 0.01; ***p < 0.001.
Positive selection is essential for mTEC terminal differentiation
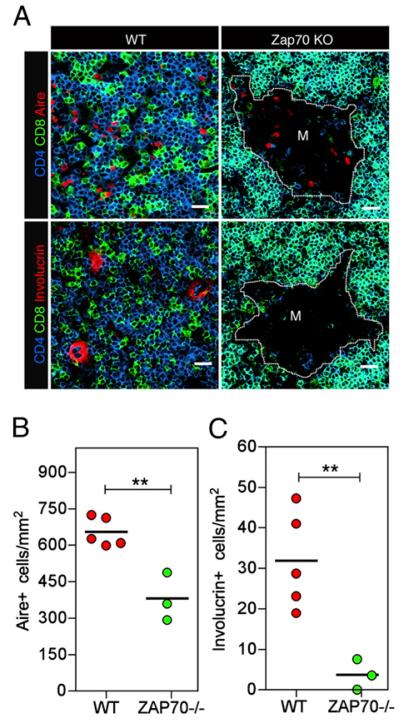
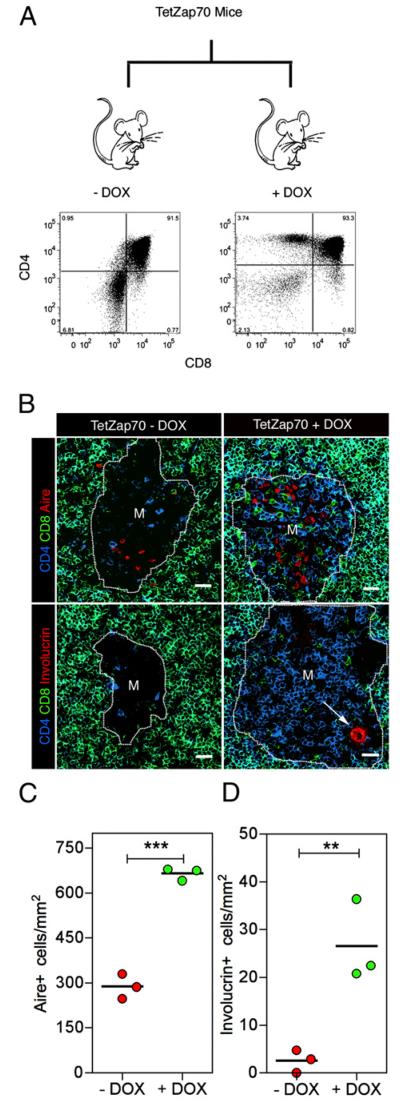
To determine whether the emergence of involucrin+ mTECs is functionally linked to the presence of positively selected thymocytes, we next analyzed Aire and involucrin expression by mTECs in Zap70−/− mice, where thymocyte development is blocked at the immature CD4+8+ stage due to an absence of αβTCRs-mediated positive selection signals (28). In Zap70−/− mice, medullary thymus areas were still readily detectable, identified as regions surrounded by cortical areas containing CD4+8+ thymocytes (Fig. 4A). Whereas Aire+ mTECs were clearly detectable in both adult WT and Zap70−/− thymic medullary areas, albeit at reduced frequency in the latter (Fig. 4A, 4B), the absence of mature CD4+ and CD8+ cells correlated with the absence of involucrin+ mTECs in Zap70−/− mice (Fig. 4A, 4C), suggesting that signals from CD4+ and/or CD8+ thymocytes are important for late stages of mTEC development. To test this possibility directly, we analyzed mTEC development in TetZap70 mice, in which thymocyte positive selection in Zap70-deficient animals can be restored by controlled expression of a tetracycline-inducible Zap70 transgene within the T cell lineage (32). Fig. 5A shows that, as reported previously, compared with untreated controls (−dox), treatment of adult TetZap70 mice with dox (+dox) for 14 d resulted in the restoration of positive selection and the appearance of mature CD4+8− and CD4−8+ thymocytes. Subsequent confocal analysis of medullary microenvironments showed that the restoration of positive selection by dox treatment was accompanied by an increase in Aire+ mTEC frequency (Fig. 5B, 5C) and the emergence of involucrin+ mTECs (Fig. 5B, 5D) at a frequency similar to that seen in unmanipulated adult WT mice (Fig. 4C). Collectively, these findings suggest that whereas mature thymocytes are not essential for the generation of Aire+ mTECs, they are an essential requirement to provide signals that drive later stages of mTEC differentiation, including generation of Aire-involucrin+ mTECs.
FIGURE 4.
Positively selected thymocytes are required for mTEC terminal differentiation. A, Adult WT and Zap70−/− thymic sections were analyzed for expression of CD4 (blue) and CD8 (green) together with either Aire (red, upper panels) or involucrin (red, lower panels). Original magnification ×40; scale bars, 20 μm. B and C, Quantitative analysis of the frequency of Aire+ (B) and involucrin+ (C) mTEC subsets in WT (red circles) and Zap70−/− (green circles) mice, with each point representing a single mouse. The ratio of Aire+/involucrin+ mTECs is 23.2:1 ± 9.6 SD and 65.1:1 ± 24.6 SD in WT and Zap70−/−, respectively. Data shown are representative of at least three separate experiments. **p < 0.01.
FIGURE 5.
Restoring positive selection rescues mTEC terminal differentiation. A, TetZap70 mice were fed dox for 14 d, resulting in the rescue of positive selection and the emergence of mature CD4+ and CD8+ thymocytes. B, Confocal analysis of untreated (left panels) or dox-treated (right panels) TetZap70 mice for CD4 (blue) and CD8 (green) together with either Aire (red, upper panels in B) or involucrin (red, lower panels). Original magnification ×40; scale bars, 20 μm. Arrow in B indicates a typical involucrin+ HC-like structure. C and D, Quantitative analysis of Aire+ and involucrin+ mTEC subsets, respectively, in untreated TetZap70 (red circles) and TetZap70 + dox (green circles). Results are representative of three separate experiments. **p < 0.01; ***p < 0.001.
Terminal differentiation of mTEC is regulated by LTα–LTβR signaling
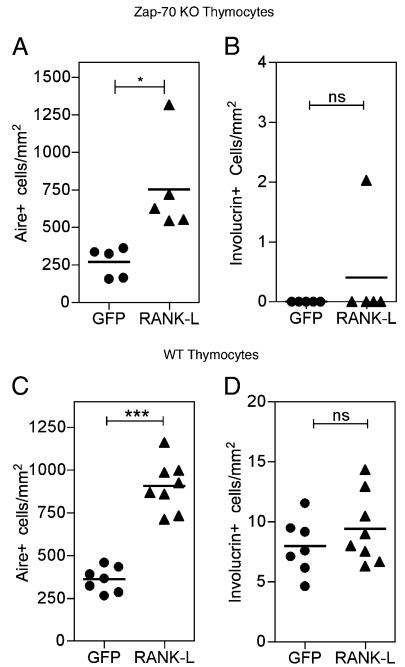
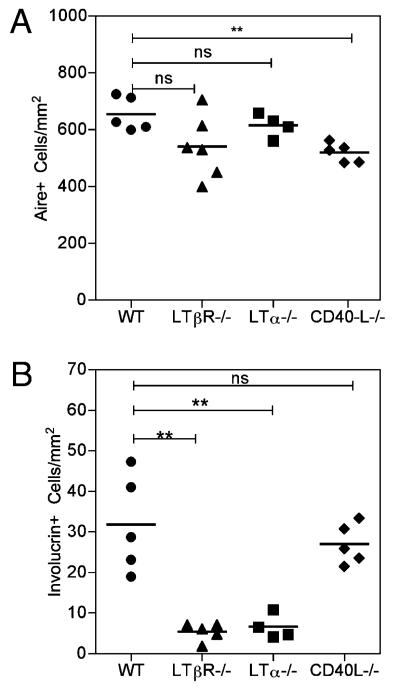
Several studies have identified a role for various TNFRs in thymus medulla development, including RANK, CD40, and LTβR. Of these, the requirement for RANK signaling during Aire+ mTEC development in adult thymus has shown to map to the presence of positively selected thymocytes (13). However, whether RANK signaling explains the requirement for single positive thymocytes in post-Aire involucrin+ mTEC development has not been addressed. Due to the dramatic reduction in Aire+ mTECs in Rank−/− thymus (11, 14), such mice cannot be readily used to determine the role of RANK in mTEC development post-Aire expression. Thus, we next determined whether RANKL could replace the requirement for single positive thymocytes in the development of involucrin+ mTECs. To this end, we established chimeras in which BM progenitors from Zap70−/− mice were transduced with retroviral constructs, resulting in constitutive expression of RANKL by thymocytes that are blocked at the CD4+8+ stage. Sca1+ BM progenitors from adult Zap70−/− mice were subjected to multiple rounds of retroviral transduction in vitro with either mouse stem cell virus (MSCV)–internal ribosomal entry site (IRES)–GFP or MSCV–RANKL–IRES–GFP biscistronic retroviral constructs. Retrovirally infected cells were then injected i.v. into sublethally irradiated Zap70−/− hosts, and thymus tissue was collected and analyzed 4 to 5 wk later. In agreement with Hikosaka et al. (13), RANKL expression by thymocytes in the absence of single positive cells (data not shown) resulted in an increase in the sizes of thymic medullary areas (data not shown) and an increase in the numbers of Aire+ mTECs (Fig. 6A), indicating that retroviral RANKL in Zap70−/− BM is expressed at a functional level. In marked contrast, however, despite these effects on the thymus medulla, RANKL expression in MSCV–RANKL–IRES–GFP Zap70−/− chimeras failed to induce the appearance of involucrin+ mTECs (Fig. 6B). Thus, despite the increased availability of RANKL, the absence of single positive thymocytes still resulted in a block in later stages of mTEC differentiation. We next made chimeras using WT BM precursors and WT hosts, in which positive selection and generation of CD4+ and CD8+ thymocytes occurred (data not shown). Flow cytometric analysis shows comparable levels of RANKL expression on CD4+ 8+ thymocytes in WT and Zap70−/− chimeras and CD4+ and CD8+ thymocytes in WT chimeras (data not shown). As expected, when RANKL was overexpressed, there was an increase in the frequency of Aire+ mTECs (Fig. 6C), again showing that retroviral RANKL expression in WT BM is expressed at a functional level. Importantly, in contrast with chimeras generated using Zap70−/− BM (Fig. 6B), involucrin+ mTECs were detected when WT BM was transduced with either MSCV–IRES–GFP or MSCV–RANKL–IRES–GFP constructs (Fig. 6D). Moreover, no difference was observed in the frequency of involucrin+ mTECs in both types of WT BM chimeras (Fig. 6D). Overall, these experiments suggest that the requirement for single positive thymocytes is a rate-limiting step in mTEC terminal differentiation and cannot be simply explained by provision of RANKL alone. Instead, these data indicate that positively selected thymocytes provide additional signals that regulate the appearance of involucrin+ mTECs. To investigate the nature of these signals, we next analyzed mTEC development in mice genetically deficient in additional TNFR ligands that are provided by mature thymocytes, namely CD40L (12, 15) and LTα (16). Although comparison of thymus medullary areas in WT and Cd40lg−/− adult mice showed no differences in the frequency of involucrin+ mTECs (Fig. 7), we found striking differences in mTEC development in mice with disrupted LTα-LTβR signaling. Thus, despite a relatively normal frequency of Aire+ mTECs, Lta−/− mice showed a marked and specific decrease in the frequency of involucrin+ mTECs, a phenotype that was also evident in Ltbr−/− adult thymus (Fig. 7). Collectively, these findings suggest that activation of the LTα–LTβR axis by LT-expressing mature thymocytes drives post-Aire mTEC development and the appearance of involucrin+ mTECs.
FIGURE 6.
RANK-RANKL signaling is insufficient to induce mTEC terminal differentiation. The frequency of Aire+ and involucrin+ mTECs was calculated in chimeras established from Zap70−/− (A, B) or WT (C, D) BM precursors, transduced with either MSCV–IRES–GFP (●) or MSCV-RANKL-IRES-GFP (▲) biscistronic retroviral constructs. Each point represents a single mouse, and data are representative of three separate experiments. *p < 0.05; ***p < 0.001.
FIGURE 7.
Terminal differentiation of mTEC is regulated by LTα-LTβR signaling. Thymus sections from adult WT, Lta−/−, Ltbr−/−, and Cd40lg−/− mice were stained with Abs to CD4, CD8, and either Aire or involucrin. The frequency of Aire+ (A) and involucrin+ (B) mTECs was determined as described in Materials and Methods, with each point representing a separate mouse. The ratio of Aire+/involucrin+ mTECs is 23.2:1 ± 9.6 SD (WT), 81.6:1 ± 19.9 SD (Ltbr−/−), 92:1 ± 38.7 SD (Lta−/−), and 19.7:1 ± 3.9 SD (Cd40lg−/−). Data shown are representative of three separate experiments. **p < 0.01.
Discussion
The importance of intrathymic medullary microenvironments in the establishment of T cell tolerance is well established. In particular, mTECs expressing Aire have been linked to both negative selection and the induction of Treg development (3). Recent studies have begun to uncover the developmental program of mTEC maturation, which begins with bipotent cTEC/mTEC progenitors (37, 38) and leads to the generation of postmitotic Aire+ mature mTECs via a RANK-dependent mTEC progenitor stage (11). In addition, several studies have now addressed the mechanisms regulating the generation of Aire+ mTECs (reviewed in Ref. 39). Several models of mTEC development have been proposed, including those that are postulated to involve either a “terminal differentiation” or a “progressive restriction” model (reviewed in Ref. 40). While the former model involves a linear differentiation program in which Aire+ mTECs expressing the most tissue-restricted Ags represent the most mature cells, the progressive restriction model suggests that expression of Aire and Aire-dependent tissue-restricted Ags occurs within immature mTECs, which under the influence of Aire become progressively restricted to stages that mimic gene expression patterns seen in other epithelia. Whereas evidence for both scenarios exists, little is known about the program of mTEC differentiation post-Aire expression. Relevant to this, the role of Aire itself in mTEC differentiation is not clear, and several models have been proposed in which Aire is involved in either the initial (41) or later stages (23, 25, 26) of terminal differentiation. In our study, we provide evidence that the generation of Aire+ mTECs is followed by a program of terminal differentiation that is associated with the loss of Aire expression and the acquisition of the terminal differentiation marker involucrin. Thus, the data presented in this study support a linear terminal differentiation program of Aire+ mTEC development and provide clues about the regulation of mTEC development post-Aire expression. First, the ontogenetic appearance initially of Aire+ mTECs and then involucrin+ mTECs is strongly suggestive of a precursor-product relationship between these cells, as was shown previously for CD80− and CD80+ mTEC subsets (11). Second, that the development of Aire+ and involucrin+ mTEC subsets appears to be controlled by cross-talk mechanisms involving distinct TNFR argues for a series of distinct steps in a program of differentiation. Importantly, this scenario is supported by data from recent cell-fate mapping studies demonstrating continued differentiation of Aire-lineage cells (26) and also by studies reporting the direct involvement of Aire expression in mTEC differentiation, as indicated by altered p63 and cytokeratin expression in mTECs from Aire-deficient mice (24, 41). That involucrin-expressing epithelial cells in murine thymus are reminiscent of involucrin+ HCs in the human thymic medulla is further suggestive of a terminal differentiation mTEC program that continues post-Aire expression. Interestingly, it is currently not clear whether involucrin represents a universal marker of mTEC terminal differentiation or is specifically indicative of terminal differentiation of the Aire+ mTEC lineage. Indeed, the relative paucity of involucrin+ cells in the adult mouse thymus is of interest, as it may suggest that its expression represents terminal differentiation of a distinct mTEC subset, rather than the total mTEC lineage. Moreover, this low frequency of involucrin+ clusters may indicate that they do not represent long-lived structures but are efficiently cleared as progressively apoptotic cells by the thymic phagocyte system. The functional significance of involucrin+ mTEC clusters is also currently unclear, and study of this is currently hampered by the lack of methods for their isolation. While in human thymus the generation of Tregs has been linked to HCs (42), which resemble involucrin+ mTEC clusters in the mouse, FoxP3+ Tregs develop normally in Ltbr−/− mice (17), which we show in this study to lack involucrin+ mTECs. The finding that mTECs have a turnover time of 2–3 wk may suggest an alternative functional significance of involucrin+ mTEC differentiation that is perhaps linked to epithelial homeostasis in the thymic medulla. For example, as tissue-restricted Ags can be divided into at least three distinct “gene pools,” the expression of which is linked to progressive mTEC differentiation (43), a normal program of terminal differentiation and mTEC homeostasis may help to ensure an adequate representation of “early” and “late” self-Ags within the thymic medulla.
Our data also show that Aire+ mTEC development and the appearance of involucrin+ terminally differentiated mTECs are separable depending upon the availability of signals provided by positively selected thymocytes. Interestingly, Aire-deficient mice have reduced numbers of involucrin+ mTECs (25), an observation that further suggests that Aire plays a role in mTEC development. However, our findings indicate that Aire expression per se is not sufficient to drive mTEC terminal differentiation and that additional signals are required. Indeed, in analysis of Lta−/− and Ltbr−/− mice, we find that activation of the LTα-LTβR axis is required for the generation of involucrin+ mTECs. In addition, our data show that Ltbr−/− mice have a slight and nonsignificant decrease in Aire+ mTECs (Fig. 7). Our experiments were performed on both male and female mice of 4–6 wk of age using the Ltbr−/− line on a C57BL/6 background (29). As reviewed recently by Fu and colleagues (44), the role of LTβR signaling in mTEC development is unclear. For example, disruption of LTβR signaling clearly impacts on thymus medulla organization (16), and one study reports a decrease in MHC class IIhigh Aire+ cells within the mTEC pool of Ltbr−/− mice (19). However, this study also showed significant differences in multiple mTEC subsets, including MHC class IIlow and Aire−MHC class IIlow subsets. While initial studies analyzing whole thymus samples reported decreases in Aire mRNA expression in the absence of LTβR signaling (20, 45), two more recent studies using purified mTEC preparations showed that Aire mRNA expression levels are normal in Ltbr−/− mice (16, 17). Finally, another study shows that in vitro stimulation of TECs via LTβR results in only a very modest induction of Aire mRNA expression (14). Thus, although the role of LTβR signaling in Aire+ mTEC development remains controversial, our observation that Ltbr−/− thymus lacks Aire−involucrin+ mTECs extends our understanding of the role of LTβR in thymus medulla development and fits well with previous studies suggesting that medullary abnormalities in the absence of LTβR signaling cannot be fully explained by a block in the generation of Aire+ mTECs (16-19). Instead, the requirement for LTβR signaling in late-stage mTEC development described in this study suggests that alterations in the thymus medulla of Ltbr−/− mice may occur at least in part as a result of disrupted mTEC homeostasis following a block in mTEC terminal differentiation post-Aire expression. Finally, we also find that thymic positive selection is an essential requirement for terminal differentiation and the appearance of involucrin+ mTECs. This link between positively selected thymocytes and mTEC homeostasis may then help to ensure that medullary environments expressing the appropriate spectrum of self-Ags are available to screen each newly generated cohort of mature thymocytes for potential autoreactivity. Interestingly, however, our data demonstrate that, unlike earlier stages in mTEC development, late-stage mTEC development is driven by LTα-LTβR, but not RANKL–RANK, signaling. Thus, we propose that following their involvement in the induction of Aire+ mTEC differentiation by provision of RANKL, mature thymocytes continue to influence late-stage mTEC terminal differentiation by their expression of LTαand stimulation of LTβR. Such a model further underscores the importance of lymphostromal cross-talk between mature CD4+ and CD8+ thymocytes and epithelial cells in the thymus medulla and identifies a stage-specific requirement for distinct thymocyte-expressed TNFR ligands at different stages of mTEC development.
Acknowledgments
We thank Dr. Art Weiss for Zap70 knockout mice. We thank Dr. Valerie Horsley for valuable discussion and critical review of the manuscript. We also acknowledge the help of Dr. David Barron, pediatric cardiac surgeon at the Birmingham Children’s Hospital.
This work was supported by a Medical Research Council Programme grant (to G.A. and E.J.J.) and by core facilities of the Medical Research Council Centre for Immune Regulation.
Abbreviations used in this paper
- Aire
autoimmune regulator
- BM
bone marrow
- cTEC
cortical thymic epithelial cell
- dox
doxycycline
- HC
Hassall’s corpuscle
- IRES
internal ribosomal entry site
- MSCV
murine stem cell virus
- mTEC
medullary thymic epithelial cell
- Treg
regulatory T cell
- WT
wild type
Footnotes
Disclosures
The authors have no financial conflicts of interest.
References
- 1.Boehm T. Thymus development and function. Curr. Opin. Immunol. 2008;20:178–184. doi: 10.1016/j.coi.2008.03.001. [DOI] [PubMed] [Google Scholar]
- 2.Singer A, Adoro S, Park JH. Lineage fate and intense debate: myths, models and mechanisms of CD4- versus CD8-lineage choice. Nat. Rev. Immunol. 2008;8:788–801. doi: 10.1038/nri2416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kyewski B, Klein L. A central role for central tolerance. Annu. Rev. Immunol. 2006;24:571–606. doi: 10.1146/annurev.immunol.23.021704.115601. [DOI] [PubMed] [Google Scholar]
- 4.Takahama Y. Journey through the thymus: stromal guides for T-cell development and selection. Nat. Rev. Immunol. 2006;6:127–135. doi: 10.1038/nri1781. [DOI] [PubMed] [Google Scholar]
- 5.Gommeaux J, Grégoire C, Nguessan P, Richelme M, Malissen M, Guerder S, Malissen B, Carrier A. Thymus-specific serine protease regulates positive selection of a subset of CD4+ thymocytes. Eur. J. Immunol. 2009;39:956–964. doi: 10.1002/eji.200839175. [DOI] [PubMed] [Google Scholar]
- 6.Murata S, Sasaki K, Kishimoto T, Niwa S, Hayashi H, Takahama Y, Tanaka K. Regulation of CD8+ T cell development by thymus-specific proteasomes. Science. 2007;316:1349–1353. doi: 10.1126/science.1141915. [DOI] [PubMed] [Google Scholar]
- 7.Boehm T. The adaptive phenotype of cortical thymic epithelial cells. Eur. J. Immunol. 2009;39:944–947. doi: 10.1002/eji.200939315. [DOI] [PubMed] [Google Scholar]
- 8.Anderson MS, Venanzi ES, Klein L, Chen Z, Berzins SP, Turley SJ, von Boehmer H, Bronson R, Dierich A, Benoist C, Mathis D. Projection of an immunological self shadow within the thymus by the aire protein. Science. 2002;298:1395–1401. doi: 10.1126/science.1075958. [DOI] [PubMed] [Google Scholar]
- 9.Hinterberger M, Aichinger M, da Costa OP, Voehringer D, Hoffmann R, Klein L. Autonomous role of medullary thymic epithelial cells in central CD4(+) T cell tolerance. Nat. Immunol. 2010;11:512–519. doi: 10.1038/ni.1874. [DOI] [PubMed] [Google Scholar]
- 10.Aschenbrenner K, D’Cruz LM, Vollmann EH, Hinterberger M, Emmerich J, Swee LK, Rolink A, Klein L. Selection of Foxp3+ regulatory T cells specific for self antigen expressed and presented by Aire+ medullary thymic epithelial cells. Nat. Immunol. 2007;8:351–358. doi: 10.1038/ni1444. [DOI] [PubMed] [Google Scholar]
- 11.Rossi SW, Kim MY, Leibbrandt A, Parnell SM, Jenkinson WE, Glanville SH, McConnell FM, Scott HS, Penninger JM, Jenkinson EJ, et al. RANK signals from CD4(+)3(−) inducer cells regulate development of Aire-expressing epithelial cells in the thymic medulla. J. Exp. Med. 2007;204:1267–1272. doi: 10.1084/jem.20062497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.White AJ, Withers DR, Parnell SM, Scott HS, Finke D, Lane PJ, Jenkinson EJ, Anderson G. Sequential phases in the development of Aire-expressing medullary thymic epithelial cells involve distinct cellular input. Eur. J. Immunol. 2008;38:942–947. doi: 10.1002/eji.200738052. [DOI] [PubMed] [Google Scholar]
- 13.Hikosaka Y, Nitta T, Ohigashi I, Yano K, Ishimaru N, Hayashi Y, Matsumoto M, Matsuo K, Penninger JM, Takayanagi H, et al. The cytokine RANKL produced by positively selected thymocytes fosters medullary thymic epithelial cells that express autoimmune regulator. Immunity. 2008;29:438–450. doi: 10.1016/j.immuni.2008.06.018. [DOI] [PubMed] [Google Scholar]
- 14.Akiyama T, Shimo Y, Yanai H, Qin J, Ohshima D, Maruyama Y, Asaumi Y, Kitazawa J, Takayanagi H, Penninger JM, et al. The tumor necrosis factor family receptors RANK and CD40 cooperatively establish the thymic medullary microenvironment and self-tolerance. Immunity. 2008;29:423–437. doi: 10.1016/j.immuni.2008.06.015. [DOI] [PubMed] [Google Scholar]
- 15.Irla M, Hugues S, Gill J, Nitta T, Hikosaka Y, Williams IR, Hubert FX, Scott HS, Takahama Y, Holländer GA, Reith W. Autoantigenspecific interactions with CD4+ thymocytes control mature medullary thymic epithelial cell cellularity. Immunity. 2008;29:451–463. doi: 10.1016/j.immuni.2008.08.007. [DOI] [PubMed] [Google Scholar]
- 16.Boehm T, Scheu S, Pfeffer K, Bleul CC. Thymic medullary epithelial cell differentiation, thymocyte emigration, and the control of autoimmunity require lympho-epithelial cross talk via LTbetaR. J. Exp. Med. 2003;198:757–769. doi: 10.1084/jem.20030794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Martins VC, Boehm T, Bleul CC. Ltbetar signaling does not regulate Aire-dependent transcripts in medullary thymic epithelial cells. J. Immunol. 2008;181:400–407. doi: 10.4049/jimmunol.181.1.400. [DOI] [PubMed] [Google Scholar]
- 18.Seach N, Ueno T, Fletcher AL, Lowen T, Mattesich M, Engwerda CR, Scott HS, Ware CF, Chidgey AP, Gray DH, Boyd RL. The lymphotoxin pathway regulates Aire-independent expression of ectopic genes and chemokines in thymic stromal cells. J. Immunol. 2008;180:5384–5392. doi: 10.4049/jimmunol.180.8.5384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Venanzi ES, Gray DH, Benoist C, Mathis D. Lymphotoxin pathway and Aire influences on thymic medullary epithelial cells are unconnected. J. Immunol. 2007;179:5693–5700. doi: 10.4049/jimmunol.179.9.5693. [DOI] [PubMed] [Google Scholar]
- 20.Chin RK, Zhu M, Christiansen PA, Liu W, Ware C, Peltonen L, Zhang X, Guo L, Han S, Zheng B, Fu YX. Lymphotoxin pathway-directed, autoimmune regulator-independent central tolerance to arthritogenic collagen. J. Immunol. 2006;177:290–297. doi: 10.4049/jimmunol.177.1.290. [DOI] [PubMed] [Google Scholar]
- 21.Gäbler J, Arnold J, Kyewski B. Promiscuous gene expression and the developmental dynamics of medullary thymic epithelial cells. Eur. J. Immunol. 2007;37:3363–3372. doi: 10.1002/eji.200737131. [DOI] [PubMed] [Google Scholar]
- 22.Gray DH, Seach N, Ueno T, Milton MK, Liston A, Lew AM, Goodnow CC, Boyd RL. Developmental kinetics, turnover, and stimulatory capacity of thymic epithelial cells. Blood. 2006;108:3777–3785. doi: 10.1182/blood-2006-02-004531. [DOI] [PubMed] [Google Scholar]
- 23.Gray D, Abramson J, Benoist C, Mathis D. Proliferative arrest and rapid turnover of thymic epithelial cells expressing Aire. J. Exp. Med. 2007;204:2521–2528. doi: 10.1084/jem.20070795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gillard GO, Dooley J, Erickson M, Peltonen L, Farr AG. Airedependent alterations in medullary thymic epithelium indicate a role for Aire in thymic epithelial differentiation. J. Immunol. 2007;178:3007–3015. doi: 10.4049/jimmunol.178.5.3007. [DOI] [PubMed] [Google Scholar]
- 25.Yano M, Kuroda N, Han H, Meguro-Horike M, Nishikawa Y, Kiyonari H, Maemura K, Yanagawa Y, Obata K, Takahashi S, et al. Aire controls the differentiation program of thymic epithelial cells in the medulla for the establishment of self-tolerance. J. Exp. Med. 2008;205:2827–2838. doi: 10.1084/jem.20080046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nishikawa Y, Hirota F, Yano M, Kitajima H, Miyazaki J, Kawamoto H, Mouri Y, Matsumoto M. Biphasic Aire expression in early embryos and in medullary thymic epithelial cells before end-stage terminal differentiation. J. Exp. Med. 2010;207:963–971. doi: 10.1084/jem.20092144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Candi E, Schmidt R, Melino G. The cornified envelope: a model of cell death in the skin. Nat. Rev. Mol. Cell Biol. 2005;6:328–340. doi: 10.1038/nrm1619. [DOI] [PubMed] [Google Scholar]
- 28.Kadlecek TA, van Oers NS, Lefrancois L, Olson S, Finlay D, Chu DH, Connolly K, Killeen N, Weiss A. Differential requirements for ZAP-70 in TCR signaling and T cell development. J. Immunol. 1998;161:4688–4694. [PubMed] [Google Scholar]
- 29.Fütterer A, Mink K, Luz A, Kosco-Vilbois MH, Pfeffer K. The lymphotoxin beta receptor controls organogenesis and affinity maturation in peripheral lymphoid tissues. Immunity. 1998;9:59–70. doi: 10.1016/s1074-7613(00)80588-9. [DOI] [PubMed] [Google Scholar]
- 30.Sean Riminton D, Körner H, Strickland DH, Lemckert FA, Pollard JD, Sedgwick JD. Challenging cytokine redundancy: inflammatory cell movement and clinical course of experimental autoimmune encephalomyelitis are normal in lymphotoxin-deficient, but not tumor necrosis factor-deficient, mice. J. Exp. Med. 1998;187:1517–1528. doi: 10.1084/jem.187.9.1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xu J, Foy TM, Laman JD, Elliott EA, Dunn JJ, Waldschmidt TJ, Elsemore J, Noelle RJ, Flavell RA. Mice deficient for the CD40 ligand. Immunity. 1994;1:423–431. doi: 10.1016/1074-7613(94)90073-6. [DOI] [PubMed] [Google Scholar]
- 32.Saini M, Sinclair C, Marshall D, Tolaini M, Sakaguchi S, Seddon B. Regulation of Zap70 expression during thymocyte development enables temporal separation of CD4 and CD8 repertoire selection at different signaling thresholds. Sci. Signal. 2010;3:ra23. doi: 10.1126/scisignal.2000702. [DOI] [PubMed] [Google Scholar]
- 33.Pongracz JE, Parnell SM, Jones T, Anderson G, Jenkinson EJ. Overexpression of ICAT highlights a role for catenin-mediated canonical Wnt signalling in early T cell development. Eur. J. Immunol. 2006;36:2376–2383. doi: 10.1002/eji.200535721. [DOI] [PubMed] [Google Scholar]
- 34.Morita S, Kojima T, Kitamura T. Plat-E: an efficient and stable system for transient packaging of retroviruses. Gene Ther. 2000;7:1063–1066. doi: 10.1038/sj.gt.3301206. [DOI] [PubMed] [Google Scholar]
- 35.Hale LP, Markert ML. Corticosteroids regulate epithelial cell differentiation and Hassall body formation in the human thymus. J. Immunol. 2004;172:617–624. doi: 10.4049/jimmunol.172.1.617. [DOI] [PubMed] [Google Scholar]
- 36.Jetten AM. Multi-stage program of differentiation in human epidermal keratinocytes: regulation by retinoids. J. Invest. Dermatol. 1990;95:44S–46S. doi: 10.1111/1523-1747.ep12505757. [DOI] [PubMed] [Google Scholar]
- 37.Bleul CC, Corbeaux T, Reuter A, Fisch P, Mönting JS, Boehm T. Formation of a functional thymus initiated by a postnatal epithelial progenitor cell. Nature. 2006;441:992–996. doi: 10.1038/nature04850. [DOI] [PubMed] [Google Scholar]
- 38.Rossi SW, Jenkinson WE, Anderson G, Jenkinson EJ. Clonal analysis reveals a common progenitor for thymic cortical and medullary epithelium. Nature. 2006;441:988–991. doi: 10.1038/nature04813. [DOI] [PubMed] [Google Scholar]
- 39.Zhu M, Fu YX. Coordinating development of medullary thymic epithelial cells. Immunity. 2008;29:386–388. doi: 10.1016/j.immuni.2008.09.001. [DOI] [PubMed] [Google Scholar]
- 40.Gillard GO, Farr AG. Contrasting models of promiscuous gene expression by thymic epithelium. J. Exp. Med. 2005;202:15–19. doi: 10.1084/jem.20050976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dooley J, Erickson M, Farr AG. Alterations of the medullary epithelial compartment in the Aire-deficient thymus: implications for programs of thymic epithelial differentiation. J. Immunol. 2008;181:5225–5232. doi: 10.4049/jimmunol.181.8.5225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Watanabe N, Wang YH, Lee HK, Ito T, Wang YH, Cao W, Liu YJ. Hassall’s corpuscles instruct dendritic cells to induce CD4+CD25+ regulatory T cells in human thymus. Nature. 2005;436:1181–1185. doi: 10.1038/nature03886. [DOI] [PubMed] [Google Scholar]
- 43.Derbinski J, Gäbler J, Brors B, Tierling S, Jonnakuty S, Hergenhahn M, Peltonen L, Walter J, Kyewski B. Promiscuous gene expression in thymic epithelial cells is regulated at multiple levels. J. Exp. Med. 2005;202:33–45. doi: 10.1084/jem.20050471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhu M, Brown NK, Fu YX. Direct and indirect roles of the LTbetaR pathway in central tolerance induction. Trends Immunol. 2010 doi: 10.1016/j.it.2010.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chin RK, Lo JC, Kim O, Blink SE, Christiansen PA, Peterson P, Wang Y, Ware C, Fu YX. Lymphotoxin pathway directs thymic Aire expression. Nat. Immunol. 2003;4:1121–1127. doi: 10.1038/ni982. [DOI] [PubMed] [Google Scholar]