Abstract
This review reports details on the natural products isolated from Taiwan soft corals during the period 2008–2012 focusing on their in vitro and/or in vivo anti-inflammatory activities. Chemical structures, names, and literature references are also reported. This review provides useful and specific information on potent anti-inflammatory marine metabolites for future development of immune-modulatory therapeutics.
Keywords: soft coral, anti-inflammatory activity, iNOS, COX-2, superoxide anion, elastase
1. Introduction
Marine natural products, especially those from stationary or slow moving marine organisms, are used naturally as a chemical defense to protect the organisms from dangerous predators, stressful local environments, and/or the encroachment of competitors. Due to the biological and chemical diversity of marine habitats, and the identification and greater understanding of marine secondary metabolites with unique chemical structures and biological activities, natural products from marine organisms are increasingly being considered as a major source of new therapeutics [1,2,3]. More than 20,000 novel compounds have been isolated and identified from marine organisms since the 1960s [4]. At least two current drugs and a series of anti-tumor drug candidates in preclinical or clinical trials have been developed from marine natural products [2,3,4]. The soft corals or Alcyonacea, an order of Anthozoa widely distributed in warm seawaters, have been a particular focus of attention. An abundance of unique secondary metabolites including sesquiterpenoids, diterpenoids, steroids and other chemical compounds have been isolated and identified from various species of soft corals [5,6,7]. It has been estimated that the percentage of new metabolites discovered from soft corals represents up to 22% of the total new marine natural products reported from 2010 to 2011 [5,6]. Importantly, many of the natural products discovered from soft corals have been demonstrated to exhibit a spectrum of biological activities such as anti-tumor, antiviral, antifouling and anti-inflammatory [5,6,7,8].
Inflammation processes often constitute an initial activation of the mammalian immune system, and the body’s normal defense or protective mechanisms in response to microbial infection or irritation or injury of tissues/organs. Increasing evidence suggests a critical link between inflammation and the chronic promotion/progression of various human diseases, including atherosclerosis, diabetes, arthritis, inflammatory bowel disease, cancer and Alzheimer. Proinflammatory enzymes, particularly the inducible nitric oxide synthase (iNOS) for nitric oxide production and cyclooxygenase (COX-2) for prostaglandin production, have been demonstrated to play central roles in the development of inflammatory diseases. In addition, it is also known that during the initial phase of acute inflammation, neutrophils are one of the first leukocyte populations to migrate towards the damaged tissue sites [9]. Neutrophils play a key role in the pathogenesis of various chronic inflammation diseases such as rheumatoid arthritis [10,11]. Activated neutrophils can secrete the superoxide anion, reactive oxygen species (ROS) and enzymes that are associated with the killing of invading pathogens [12]. Furthermore, elastase secreted by stimulated neutrophils has been recognized to play a key contribution in the demolition of tissues affected by chronic inflammatory disease [13]. Therefore, evaluation of the inhibition of iNOS and COX-2 expression, the production of superoxide anion, and the release of elastase in inflammatory cells/tissues by various natural products have been extensively employed in a spectrum of in vitro preliminary screening systems for lead compound or drug discovery. Recently, a number of marine biology and chemistry researchers in Taiwan (including our laboratory) have systematically screened several marine natural products isolated from soft corals for such in vitro anti-inflammatory activities, mainly by measuring the inhibition of iNOS, COX-2, superoxide anion or elastase in murine immune cells. Animal models were further used to evaluate the potential therapeutic activities of candidate compounds in specific disease models. This report reviews some recent representative studies and examples of marine natural products with anti-inflammatory and other related bioactivities that have been isolated from soft corals of Taiwan. Soft corals are abundant in the off-shore environment of the island of Taiwan, and have hence become a focus of local studies of marine nature products. We hope that this review will provide a useful data for the further study of marine natural products.
2. Results and Discussion
In the reports reviewed here, anti-inflammatory activities of natural products from the soft corals of Taiwan were generally determined in vitro by their inhibition of LPS-induced expression of iNOS and COX-2 in murine macrophage cells (RAW264.7) or by their inhibition of the production of superoxide anion and the release on the elastase from human neutrophils in response to FMLP/CB.
2.1. Sesquiterpenoids
2.1.1. Triquinane-Type Sesquiterpenoids
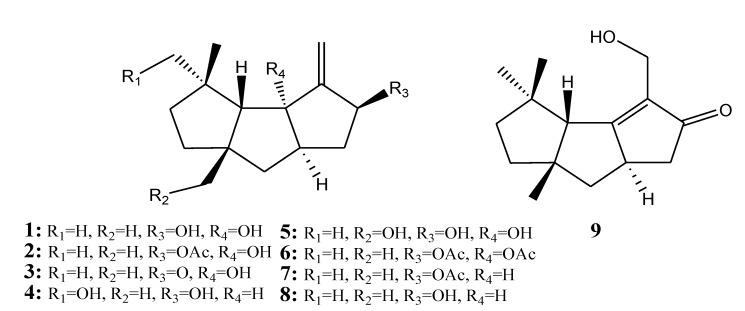
Table 1 summarizes nine triquinane-type sesquiterpenoids (1–9) evaluated for in vitro anti-inflammatory activity in literature published from 2008 to 2012. The corresponding chemical structures are reported in Figure 1.
Table 1.
Chemical constituents of triquinane-type sesquiterpenoids from soft corals of Taiwan.
| No. | Name | Sources | Activities * | Reference |
|---|---|---|---|---|
| 1 | Δ9(12)-Capnellene-8β,10α-diol | Capnella imbricata | I,C | [14] |
| 2 | 8α-Acetoxy-Δ9(12)-capnellene-10α-ol | Capnella imbricata | I,C | [14] |
| 3 | Δ9(12)-Capnellene-10α-ol-8-one | Capnella imbricata | I | [14] |
| 4 | Δ9(12)-Capnellene-8β,15-diol | Capnella imbricata | [14] | |
| 5 | Δ9(12)-Capnellene-8β,10α,13-triol | Capnella imbricata | [14] | |
| 6 | 8β,10α-Diacetoxy-Δ9(12)-capnellene | Capnella imbricata | [14] | |
| 7 | 8β-Acetoxy-Δ9(12)-capnellene | Capnella imbricata | [14] | |
| 8 | Δ9(12)-Capnellene-8β-ol | Capnella imbricata | [14] | |
| 9 | Δ9(12)-Capnellene-12-ol-8-one | Capnella imbricata | I,C | [14] |
* Inhibition of iNOS (I) and COX-2 (C).
Figure 1.
The structures of triquinane-type sesquiterpenoids (1–9).
2.1.2. Nardosinane-Type Sesquiterpenoids
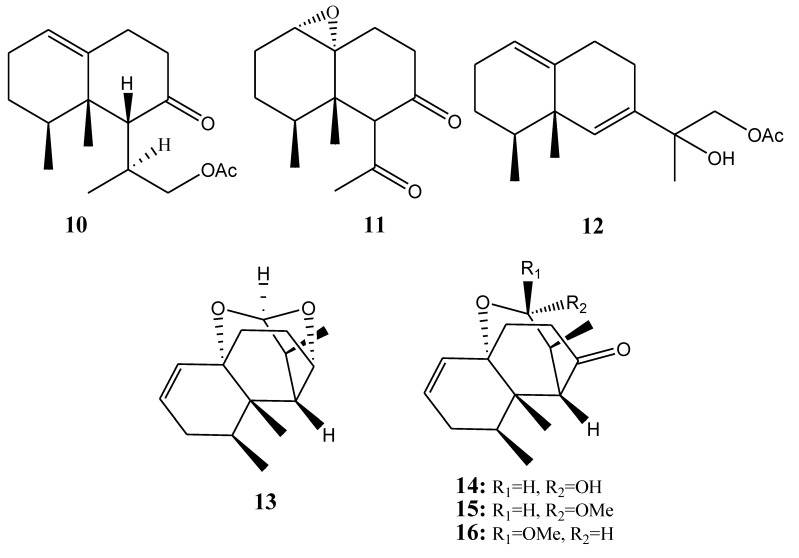
Table 2 summarizes seven nardosinane-type sesquiterpenoids (10–16) evaluated for in vitro anti-inflammatory activity in literature published from 2008 to 2012. The corresponding chemical structures are reported in Figure 2.
Table 2.
Chemical constituents of nardosinane-type sesquiterpenoids from soft corals of Taiwan.
| No. | Name | Sources | Activities * | Reference |
|---|---|---|---|---|
| 10 | Paralemnolin J | Paralemnalia thyrsoides | [15] | |
| 11 | Paralemnolin K | Paralemnalia thyrsoides | [15] | |
| 12 | Paralemnolin L | Paralemnalia thyrsoides | [15] | |
| 13 | Flavalin A | Lemnalia flava | I,C | [16] |
| 14 | Flavalin B | Lemnalia flava | [16] | |
| 15 | Flavalin C | Lemnalia flava | [16] | |
| 16 | Flavalin D | Lemnalia flava | [16] |
* Inhibition of iNOS (I) and COX-2 (C).
Figure 2.
The structures of nardosinane-type sesquiterpenoids (10–16).
2.1.3. Aromadendrane-Type Sesquiterpenoids
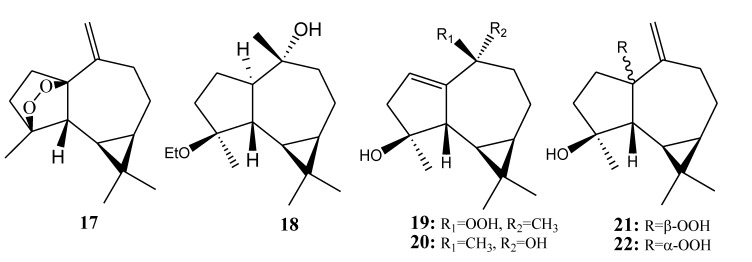
Table 3 summarizes six aromadendrane-type sesquiterpenoids (17–22) evaluated for in vitro anti-inflammatory activity in literature published from 2008 to 2012. The corresponding chemical structures are reported in Figure 3.
Table 3.
Chemical constituents of aromadendrane-type sesquiterpenoids from soft corals of Taiwan.
| No. | Name | Sources | Activities * | Reference |
|---|---|---|---|---|
| 17 | Lochmolin A | Sinularia lochmodes | C | [17] |
| 18 | Lochmolin B | Sinularia lochmodes | C | [17] |
| 19 | Lochmolin C | Sinularia lochmodes | [17] | |
| 20 | Lochmolin D | Sinularia lochmodes | [17] | |
| 21 | Lochmolin E | Sinularia lochmodes | C | [17] |
| 22 | Lochmolin F | Sinularia lochmodes | C | [17] |
* Inhibition of COX-2 (C).
Figure 3.
The structures of aromadendrane-type sesquiterpenoids (17–22).
2.1.4. Selinane- and Oppositane-Type Sesquiterpenoids
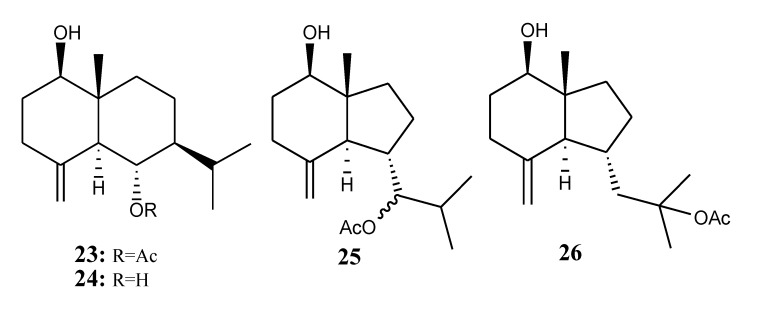
Table 4 summarizes four selinane- and oppositane-type sesquiterpenoids (23–26) evaluated for in vitro anti-inflammatory activity in literature published from 2008 to 2012. The corresponding chemical structures are reported in Figure 4.
Table 4.
Chemical constituents of selinane- and oppositane-type sesquiterpenoids from soft corals of Taiwan.
| No. | Name | Sources | Activities * | Reference |
|---|---|---|---|---|
| 23 | 1β-Hydroxy-6α-acetoxyeudesm-4(15)-ene | Sinularia leptoclados | [18] | |
| 24 | 1β,6α-Dihydroxyeudesm-4(15)-ene | Sinularia leptoclados | I | [18] |
| 25 | Leptocladolin A | Sinularia leptoclados | [18] | |
| 26 | Leptocladolin B | Sinularia leptoclados | [18] |
* Inhibition of iNOS (I).
Figure 4.
The structures of selinane- and oppositane-type sesquiterpenoids (23–26).
2.1.5. Ylangene-Type Sesquiterpenoids
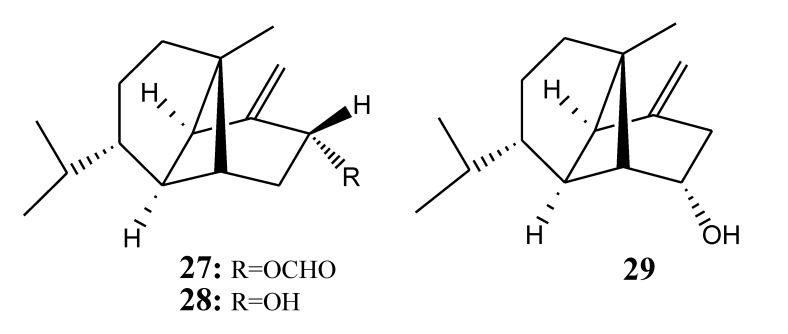
Table 5 summarizes three ylangene-type sesquiterpenoids (27–29) evaluated for in vitro anti-inflammatory activity in literature published from 2008 to 2012. The corresponding chemical structures are reported in Figure 5.
Table 5.
Chemical constituents of ylangene-type sesquiterpenoids from soft corals of Taiwan.
| No. | Name | Sources | Activities * | Reference |
|---|---|---|---|---|
| 27 | (1S,2S,4R,6S,7R,8S)-4α-Formyloxy-β-ylangene | Lemnalia flava | I,C | [16] |
| 28 | Lemnalol | Lemnalia flava | [16] | |
| 29 | Isolemnalol | Lemnalia flava | [16] |
* Inhibition of NOS (I) and COX-2 (C).
Figure 5.
The structures of ylangene-type sesquiterpenoids (27–29).
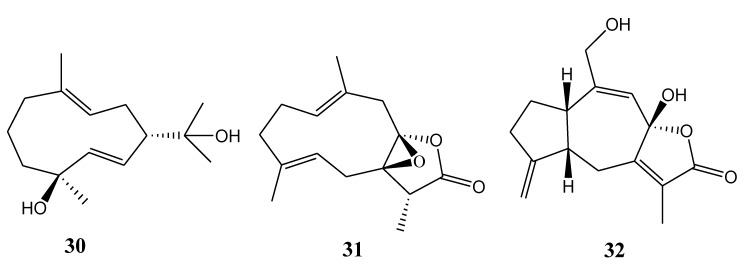
2.1.6. Germacrane-Type Sesquiterpenoids
Table 6 summarizes three germacrane-type sesquiterpenoids (30–32) evaluated for in vitro anti-inflammatory activity in literature published from 2008 to 2012. The corresponding chemical structures are reported in Figure 6.
Table 6.
Chemical constituents of germacrane-type sesquiterpenoids from soft corals of Taiwan.
| No. | Name | Sources | Activities * | Reference |
|---|---|---|---|---|
| 30 | Lochmolin G | Sinularia lochmodes | [17] | |
| 31 | Menelloide D | Menella sp. | E | [19] |
| 32 | Menelloide E | Menella sp. | [20] |
* Inhibition of elastase (E).
Figure 6.
The structures of germacrane-type sesquiterpenoids (30–32).
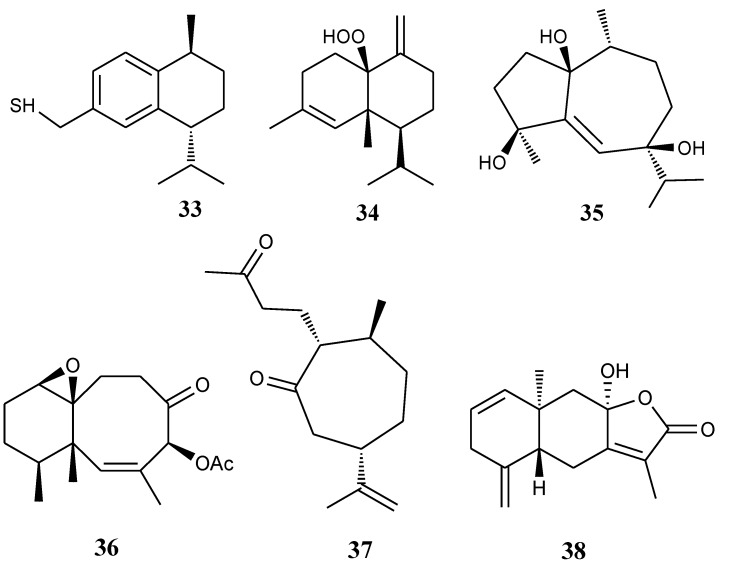
2.1.7. Other-Type Sesquiterpenoids
Table 7 summarizes six other-type sesquiterpenoids (33–38) evaluated for in vitro anti-inflammatory activity in literature published from 2008 to 2012. The corresponding chemical structures are reported in Figure 7.
Table 7.
Chemical constituents of other-type sesquiterpenoids from soft corals of Taiwan.
| No. | Name | Sources | Activities * | Reference |
|---|---|---|---|---|
| 33 | Erectathiol | Nephthea erecta | I | [21] |
| 34 | Scabralin A | Sinularia scabra | I | [22] |
| 35 | Leptocladol A | Sinularia leptoclados | [23] | |
| 36 | Paralemnolin D | Paralemnalia thyrsoides | [15] | |
| 37 | 1- epi-Chabrolidione A | Sinularia leptoclados | [23] | |
| 38 | (–)-Hydroxylindestrenolide | Menella sp. | S | [24] |
* Inhibition of iNOS (I) and superoxide anion (S).
Figure 7.
The structures of other-type sesquiterpenoids (33–38).
At a concentration of 10 µM, compounds 1–3, 13, 24, 28, 33 and 34 reduced LPS-induced expression of iNOS in murine macrophage cells [14,15,16,18,21,22]. Compounds 1, 2, 13, 17, 18, 21 and 28 suppressed LPS-induced expression of COX-2 in these cells [14,15,16,17]. At 10 µg/mL, compound 38 was shown to slightly inhibit the generation of superoxide anion in FMLP/CB-stimulated human neutrophils, and compound 31 weakly inhibited the release of elastase by activated human neutrophils [19,24]. In addition, an inflammation animal model induced by intraplantar injection of carrageenan into rat hind paws was also used to evaluate in vivo anti-inflammatory activity of lemnalol (28). Intramuscular injection of 28 (15 mg/kg) significantly inhibited the carrageenan-induced rat paw edema and thermal hyperalgesia behavior. Moreover, lemnalol significantly suppressed the carrageenan-induced expression of iNOS and COX-2 in paw tissue of test rats. Post-intrathecal injection of lemnalol provided an antinociceptive effect in carrageenan-injected rats (1 and 5 μg) [25]. Δ9(12)-capnellene-8β,10α-diol (GB9, 1) and its acetylated derivative, 8α-acetoxy-Δ9(12)-capnellene-10α-ol (GB10, 2) were reported to inhibit the expression of iNOS and COX-2 in BV2 cells post-stimulation by IFN-γ.
Intraperitoneal administration of GB9 reduced CCI-induced thermal hyperalgesia, suppressed microglial cells activation and COX-2 upregulation in the dorsal horn of the lumbar spinal cord, ipsilateral to the injury. Also, intrathecal administration of GB9 and GB10 suppressed activities of CCl-induced nociceptive sensitization and thermal hyperalgesia [26]. The above findings suggest that some of these compounds may warrant systematic investigation for future development as immune-modifiers.
2.2. Diterpenoids
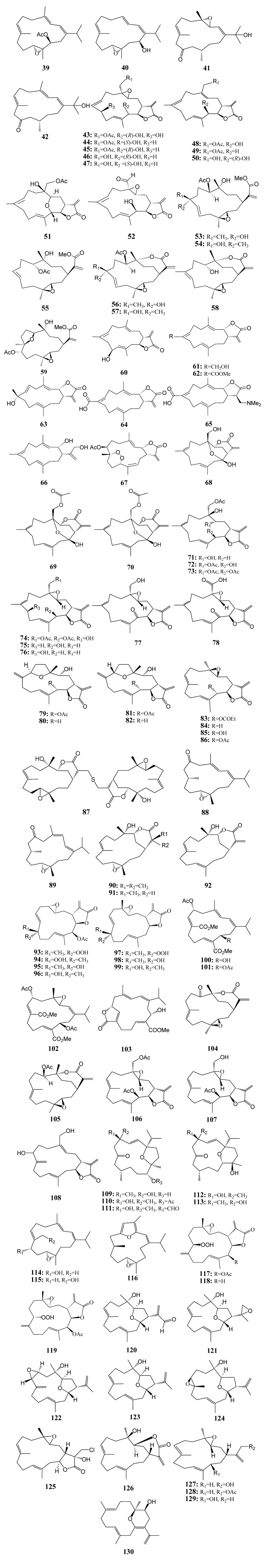
2.2.1. Cembrane-Based Diterpenoids
Table 8 summarizes 92 cembrane-based diterpenoids (39–130) evaluated for in vitro anti-inflammatory activity in literature published from 2008 to 2012. The corresponding chemical structures are reported in Figure 8.
Table 8.
Chemical constituents of cembrane-based diterpenoids from soft corals of Taiwan.
| Name | Sources | Activities * | Reference | |
|---|---|---|---|---|
| 39 | Gibberosene B | Sinularia gibberosa | I,C | [27] |
| 40 | (+)-11,12-Epoxysarcophytol A | Sinularia gibberosa | [27] | |
| 41 | Grandilobatin B | Sinularia grandilobata | [28] | |
| 42 | Grandilobatin D | Sinularia grandilobata | I | [28] |
| 43 | Durumolide A | Lobophytum durum | I,C | [29] |
| 44 | 13S-Hydroxylobolide | Lobophytum durum | I,C | [29] |
| 45 | 13R-Hydroxylobolide | Lobophytum durum | I | [29] |
| 46 | Deacetyl-13-hydroxylobolide | Lobophytum durum | I,C | [27] |
| 47 | (7E,11E)-13,18-Dihydroxy-3,4-epoxy-7,11,15(17)-cembratrien-16,14-olide | Lobophytum durum | I,C | [27] |
| 48 | Durumolide B | Lobophytum durum | I | [28] |
| 49 | (3E,7E,11E)-18-Acetoxy-3,7,11,15(17)-cembratetraen-16,14-olide | Lobophytum durum | I,C | [28] |
| 50 | Durumolide C | Lobophytum durum | I,C | [29] |
| 51 | Durumolide D | Lobophytum durum | I | [29] |
| 52 | Durumolide E | Lobophytum durum | I | [29] |
| 53 | Granosolide C | Sinularia granosa | [30] | |
| 54 | Querciformolide E | Sinularia querciformis | I | [30] |
| 55 | Granosolide D | Sinularia granosa | I | [30] |
| 56 | Flexibilisolide A | Sinularia granosa | I | [30] |
| 57 | Flexilarin | Sinularia granosa | I | [30] |
| 58 | Sinulariolide | Sinularia granosa | I | [30] |
| 59 | Sinulaflexiolide E | Sinularia granosa | [30] | |
| 60 | Crassumolide A | Lobophytum crassum | I,C | [31] |
| 61 | Crassumolide B | Lobophytum crassum | I | [31] |
| 62 | Crassumolide C | Lobophytum crassum | I,C | [31] |
| 63 | Crassumolide F | Lobophytum crassum | I | [31] |
| 64 | Lobohedleolide | Lobophytum crassum | I,C | [31] |
| 65 | 17-Dimethylaminolobohedleolide | Lobophytum crassum | I | [31] |
| 66 | Sinulariol A | Lobophytum crassum | I,C | [31] |
| 67 | Dentivulatolide | Lobophytum crassum | I,C | [31] |
| 68 | Durumhemiketalolide A | Lobophytum durum | I,C | [32] |
| 69 | Durumhemiketalolide B | Lobophytum durum | I | [32] |
| 70 | Durumhemiketalolide C | Lobophytum durum | I,C | [32] |
| 71 | Durumolide F | Lobophytum durum | I,C | [33] |
| 72 | Durumolide G | Lobophytum durum | I | [33] |
| 73 | Durumolide H | Lobophytum durum | I | [33] |
| 74 | Durumolide I | Lobophytum durum | I | [33] |
| 75 | Durumolide J | Lobophytum durum | I | [33] |
| 76 | Sinularolide D | Lobophytum durum | I | [33] |
| 77 | Durumolide K | Lobophytum durum | I,C | [33] |
| 78 | Durumolide L | Lobophytum durum | I | [33] |
| 79 | Sarcocrassocolide A | Sarcophyton crassocaule | I | [34] |
| 80 | Sarcocrassocolide C | Sarcophyton crassocaule | I | [34] |
| 81 | Sarcocrassocolide B | Sarcophyton crassocaule | I | [34] |
| 82 | Sarcocrassocolide D | Sarcophyton crassocaule | I | [34] |
| 83 | Sarcocrassocolide E | Sarcophyton crassocaule | I | [34] |
| 84 | Sarcocrassolide | Sarcophyton crassocaule | I,C | [34] |
| 85 | Sinularolide | Sarcophyton crassocaule | I | [34] |
| 86 | 13-Acetoxysarcocrassolide | Sarcophyton crassocaule | I | [34] |
| 87 | Thioflexibilolide A | Sinularia flexibilis | I,C | [35] |
| 88 | Triangulene A | Sinularia triangular | [36] | |
| 89 | Triangulene B | Sinularia triangular | [36] | |
| 90 | Sinularin | Sinularia triangular | I | [36] |
| 91 | Dihydrosinularin | Sinularia triangular | I,C | [36] |
| 92 | (−)14-Deoxycrassin | Sinularia triangular | I,C | [36] |
| 93 | Sarcocrassocolide F | Sarcophyton crassocaule | I | [37] |
| 94 | Sarcocrassocolide G | Sarcophyton crassocaule | I | [37] |
| 95 | Sarcocrassocolide H | Sarcophyton crassocaule | I | [37] |
| 96 | Sarcocrassocolide I | Sarcophyton crassocaule | I,C | [37] |
| 97 | Sarcocrassocolide J | Sarcophyton crassocaule | I | [37] |
| 98 | Sarcocrassocolide K | Sarcophyton crassocaule | I | [37] |
| 99 | Sarcocrassocolide L | Sarcophyton crassocaule | I | [37] |
| 100 | Sarcophytolin A | Lobophytum sarcophytoides | I | [38] |
| 101 | Sarcophytolin B | Lobophytum sarcophytoides | I | [38] |
| 102 | Sarcophytolin C | Lobophytum sarcophytoides | [38] | |
| 103 | Sarcophytolin D | Lobophytum sarcophytoides | I | [38] |
| 104 | 11-Dehydrosinulariolide | Sinularia discrepans | I,C | [39] |
| 105 | 11-epi-Sinulariolide acetate | Sinularia discrepans | I,C | [39] |
| 106 | Crassumolide G | Lobophytum crassum | I | [40] |
| 107 | Crassumolide H | Lobophytum crassum | I | [40] |
| 108 | Crassumolide I | Lobophytum crassum | I | [40] |
| 109 | Crassarine A | Sinularia crassa | [41] | |
| 110 | Crassarine B | Sinularia crassa | [41] | |
| 111 | Crassarine C | Sinularia crassa | [41] | |
| 112 | Crassarine D | Sinularia crassa | [41] | |
| 113 | Crassarine E | Sinularia crassa | [41] | |
| 114 | Crassarine F | Sinularia crassa | C | [41] |
| 115 | Crassarine G | Sinularia crassa | [41] | |
| 116 | Crassarine H | Sinularia crassa | I | [41] |
| 117 | Sarcocrassocolide M | Sarcophyton crassocaule | I | [42] |
| 118 | Sarcocrassocolide N | Sarcophyton crassocaule | I | [42] |
| 119 | Sarcocrassocolide O | Sarcophyton crassocaule | I | [42] |
| 120 | Culobophylin A | Lobophytum crassum | [43] | |
| 121 | Culobophylin B | Lobophytum crassum | [43] | |
| 122 | Culobophylin C | Lobophytum crassum | [43] | |
| 123 | Lobophylin B | Lobophytum crassum | [43] | |
| 124 | Lobophylin A | Lobophytum crassum | [43] | |
| 125 | Lobocrassin A | Lobophytum crassum | [44] | |
| 126 | Lobocrassin B | Lobophytum crassum | S,E | [44] |
| 127 | Lobocrassin C | Lobophytum crassum | [44] | |
| 128 | Lobocrassin D | Lobophytum crassum | [44] | |
| 129 | Lobocrassin E | Lobophytum crassum | [44] | |
| 130 | Lobocrassin F | Lobophytum crassum | E | [20] |
* Inhibition of iNOS (I), COX-2 (C), superoxide anion (S) and elastase (E).
Figure 8.
The structures of cembrane-based diterpenoids (39–130).
At the concentration of 10 µM, compounds 39, 42–52, 54–58, 60–87, 90–101, 103–108 and 116–119 reduced LPS-induced expression of iNOS in murine macrophage (RAW264.7) cells [27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42]. Compounds 39, 43, 44, 46, 47, 49, 50, 62, 64, 66–68, 70, 71, 77, 84, 87, 91, 92, 96, 104, 105 and 114 suppressed LPS-induced expression of COX-2 in these cells [27,29,31,32,33,34,35,36,37,39,41]. At 10 µg/mL, compound 126 inhibited the generation of superoxide anion and the release of elastase in human neutrophils [44]. Compound 130 inhibited the release of elastase by activated human neutrophils [24]. For in vivo anti-inflammatory activities, subcutaneous (s.c.) administration of sinularin (90) (80 mg/kg) significantly inhibited carrageenan-induced nociceptive behaviors as well as carrageenan-induced activation of microglial and astrocyte, and the iNOS expression in the dorsal horn of the lumbar spinal cord [45]. Due to its promising anti-inflammatory profile, sinularin may warrant future exploration as a lead compound for immune-/inflammation-modulation.
2.2.2. Eunicellin-Based Diterpenoids
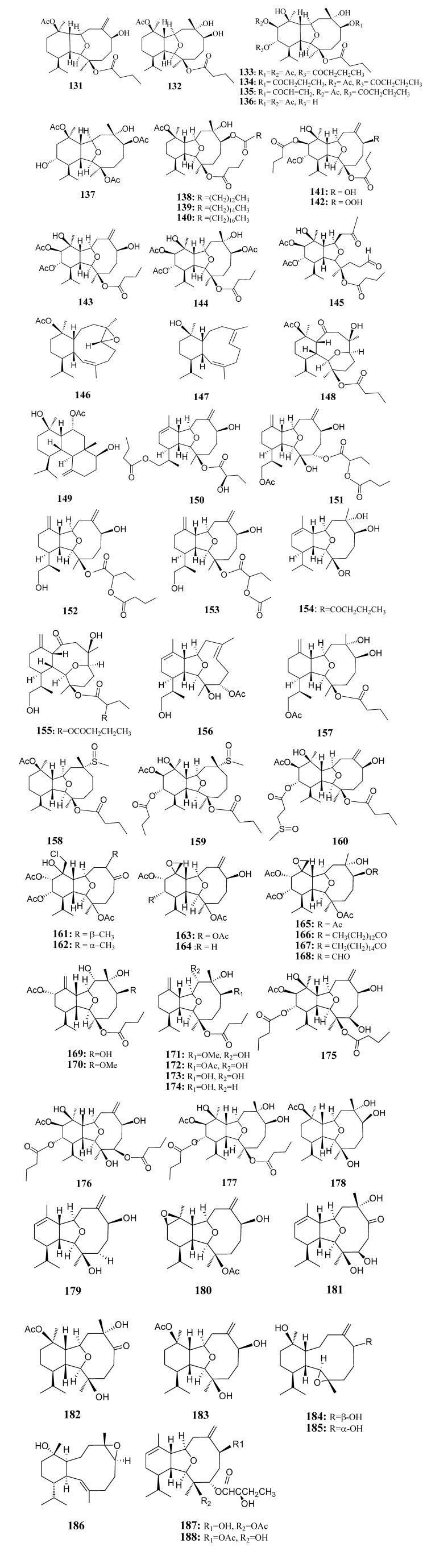
Table 9 summarizes 58 eunicellin-based diterpenoids (131–188) evaluated for in vitro anti-inflammatory activity in literature published from 2008 to 2012. The corresponding chemical structures are reported in Figure 9.
Table 9.
Chemical constituents of eunicellin-based diterpenoids from soft corals of Taiwan.
| No. | Name | Sources | Activities * | Reference |
|---|---|---|---|---|
| 131 | Simplexin A | Klyxum simplex | I | [46] |
| 132 | Simplexin B | Klyxum simplex | [46] | |
| 133 | Simplexin C | Klyxum simplex | [46] | |
| 134 | Simplexin D | Klyxum simplex | I | [46] |
| 135 | Simplexin E | Klyxum simplex | I,C | [46] |
| 136 | Simplexin F | Klyxum simplex | [46] | |
| 137 | Simplexin I | Klyxum simplex | [46] | |
| 138 | Klysimplexin I | Klyxum simplex | [47] | |
| 139 | Klysimplexin J | Klyxum simplex | I | [47] |
| 140 | Klysimplexin K | Klyxum simplex | I | [47] |
| 141 | Klysimplexin L | Klyxum simplex | I | [47] |
| 142 | Klysimplexin M | Klyxum simplex | I | [47] |
| 143 | Klysimplexin N | Klyxum simplex | I | [47] |
| 144 | Klysimplexin O | Klyxum simplex | [47] | |
| 145 | Klysimplexin P | Klyxum simplex | [47] | |
| 146 | Klysimplexin Q | Klyxum simplex | [47] | |
| 147 | Klysimplexin R | Klyxum simplex | I | [47] |
| 148 | Klysimplexin S | Klyxum simplex | I,C | [47] |
| 149 | Klysimplexin T | Klyxum simplex | [47] | |
| 150 | Hirsutalin A | Cladiella hirsuta | [48] | |
| 151 | Hirsutalin B | Cladiella hirsuta | I,C | [48] |
| 152 | Hirsutalin C | Cladiella hirsuta | I | [48] |
| 153 | Hirsutalin D | Cladiella hirsuta | I | [48] |
| 154 | Hirsutalin E | Cladiella hirsuta | [48] | |
| 155 | Hirsutalin F | Cladiella hirsuta | [48] | |
| 156 | Hirsutalin G | Cladiella hirsuta | [48] | |
| 157 | Hirsutalin H | Cladiella hirsuta | I | [48] |
| 158 | Klysimplexin sulfoxide A | Klyxum simplex | I | [49] |
| 159 | Klysimplexin sulfoxide B | Klyxum simplex | I | [49] |
| 160 | Klysimplexin sulfoxide C | Klyxum simplex | I,C | [49] |
| 161 | Lymollin A | Klyxum molle | [50] | |
| 162 | Lymollin B | Klyxum molle | I | [50] |
| 163 | Lymollin C | Klyxum molle | I,C | [50] |
| 164 | Lymollin D | Klyxum molle | I,C | [50] |
| 165 | Lymollin E | Klyxum molle | I | [50] |
| 166 | Lymollin F | Klyxum molle | I,C | [50] |
| 167 | Lymollin G | Klyxum molle | I,C | [50] |
| 168 | Lymollin H | Klyxum molle | I,C | [50] |
| 169 | Krempfielin A | Cladiella krempfi | [51] | |
| 170 | Krempfielin D | Cladiella krempfi | I | [51] |
| 171 | Krempfielin B | Cladiella krempfi | I | [51] |
| 172 | krempfielin C | Cladiella krempfi | I | [51] |
| 173 | Litophynol B | Cladiella krempfi | I | [51] |
| 174 | (1R*,2R*,3R*,6S*,7S*,9R*,10R*,14R*)3-Butanoyloxycladiell-11(17)-en-6,7-diol | Cladiella krempfi | I | [51] |
| 175 | Klysimplexin U | Klyxum simplex | [52] | |
| 176 | Klysimplexin V | Klyxum simplex | [52] | |
| 177 | Klysimplexin W | Klyxum simplex | [52] | |
| 178 | Klysimplexin X | Klyxum simplex | [52] | |
| 179 | Cladieunicellin A | Cladiella sp. | S,E | [53] |
| 180 | Cladieunicellin C | Cladiella sp. | [53] | |
| 181 | Cladieunicellin D | Cladiella sp. | [53] | |
| 182 | Cladieunicellin E | Cladiella sp. | [53] | |
| 183 | Cladieunicellin G | Cladiella sp. | S,E | [54] |
| 184 | 6-epi-Cladieunicellin F | Cladiella sp. | [54] | |
| 185 | Cladieunicellin F | Cladiella sp. | S,E | [54] |
| 186 | (–)-Solenopodin C | Cladiella sp. | [55] | |
| 187 | Cladielloide A | Cladiella sp. | [56] | |
| 188 | Cladielloide B | Cladiella sp. | S,E | [56] |
* Inhibition of iNOS (I), COX-2 (C), superoxide anion (S) and elastase (E).
Figure 9.
The structures of cembrane-based diterpenoids (131–188).
2.2.3. Briarane-based Diterpenoids
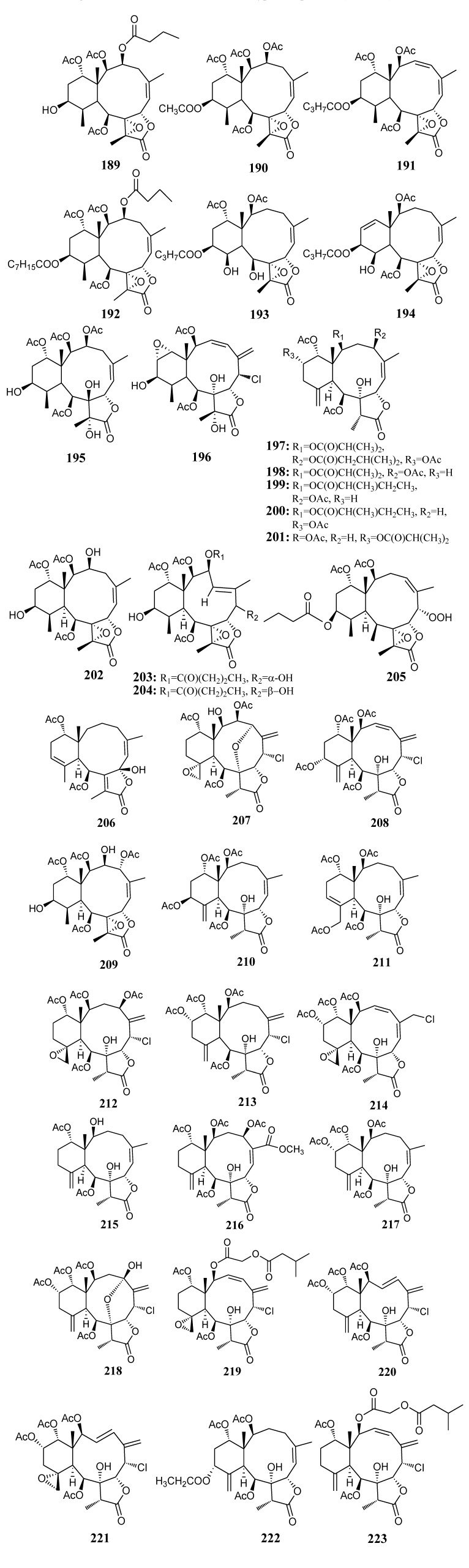
Table 10 summarizes 35 briarane-based diterpenoids (189–223) evaluated for in vitro anti-inflammatory activity in literature published from 2008 to 2012. The corresponding chemical structures are reported in Figure 10.
Table 10.
Chemical constituents of briarane-type diterpenoids from soft corals of Taiwan.
| No. | Name | Sources | Activities * | Reference |
|---|---|---|---|---|
| 189 | Excavatolide B | Briareum excavatum | [57] | |
| 190 | Excavatolide K | Briareum excavatum | [57] | |
| 191 | Excavatolide F | Briareum excavatum | [57] | |
| 192 | Briaexcavatolide R | Briareum excavatum | [57] | |
| 193 | Excavatolide Z | Briareum excavatum | [57] | |
| 194 | Briaexcavatolide B | Briareum excavatum | [57] | |
| 195 | Briaexcavatolide K | Briareum excavatum | [57] | |
| 196 | Briaexcavatolide H | Briareum excavatum | [57] | |
| 197 | Junceol D | Junceella juncea | [58] | |
| 198 | Junceol E | Junceella juncea | S | [58] |
| 199 | Junceol F | Junceella juncea | S | [58] |
| 200 | Junceol G | Junceella juncea | S | [58] |
| 201 | Junceol H | Junceella juncea | S | [58] |
| 202 | Excavatoid L | Briareum excavatum | S,E | [59] |
| 203 | Excavatoid M | Briareum excavatum | S,E | [59] |
| 204 | Excavatoid N | Briareum excavatum | S,E | [59] |
| 205 | Briarenolide F | Briareum sp. | S | [60] |
| 206 | Briarenolide G | Briareum sp. | [60] | |
| 207 | Fragilide J | Ellisella robusta | E | [61] |
| 208 | Robustolide L | Ellisella robusta | S | [61] |
| 209 | Briaexcavatin P | Briareum excavatum | S | [62] |
| 210 | Frajunolide L | Junceella fragilis | S,E | [63] |
| 211 | Frajunolide M | Junceella fragilis | [63] | |
| 212 | Frajunolide N | Junceella fragilis | E | [63] |
| 213 | Frajunolide O | Junceella fragilis | S,E | [63] |
| 214 | Juncenolide M | Junceella juncea | [64] | |
| 215 | Juncenolide N | Junceella juncea | E | [64] |
| 216 | Juncenolide O | Junceella juncea | S,E | [64] |
| 217 | Frajunolide E | Junceella fragilis | S,E | [65] |
| 218 | Frajunolide F | Junceella fragilis | [65] | |
| 219 | Frajunolide G | Junceella fragilis | [65] | |
| 220 | Frajunolide H | Junceella fragilis | [65] | |
| 221 | Frajunolide I | Junceella fragilis | [65] | |
| 222 | Frajunolide J | Junceella fragilis | S,E | [65] |
| 223 | Frajunolide K | Junceella fragilis | [65] |
* Inhibition of superoxide anion (S) and elastase (E).
Figure 10.
The structures of briarane-type diterpenoids (189–223).
2.2.4. Verticillane-Based Diterpenoids
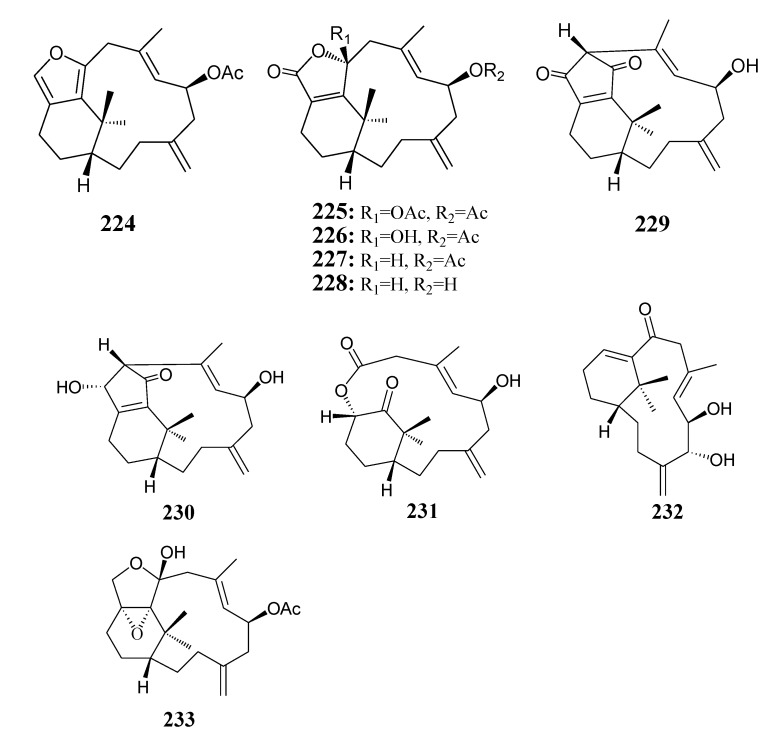
Table 11 summarizes 10 verticillane-based diterpenoids (224–233) evaluated for in vitro anti-inflammatory activity in literature published from 2008 to 2012. The corresponding chemical structures are reported in Figure 11.
Table 11.
Chemical constituents of verticillane-type diterpenoids from soft corals of Taiwan.
| No. | Name | Sources | Activities * | Reference |
|---|---|---|---|---|
| 224 | Cespitularin R | Cespitularia hypotentaculata | [66] | |
| 225 | Cespitularin S | Cespitularia hypotentaculata | I,C | [66] |
| 226 | Cespitularin J | Cespitularia hypotentaculata | [66] | |
| 227 | Cesputularin K | Cespitularia hypotentaculata | I | [66] |
| 228 | Cespitularin M | Cespitularia hypotentaculata | [66] | |
| 229 | Cespitularin I | Cespitularia hypotentaculata | I | [66] |
| 230 | Cespitularin F | Cespitularia hypotentaculata | I | [66] |
| 231 | Cespitularin Q | Cespitularia hypotentaculata | [66] | |
| 232 | Cespitulin E | Cespitularia taenuate | S,E | [67] |
| 233 | Cespitulin G | Cespitularia taenuate | S,E | [67] |
* Inhibition of iNOS (I), COX-2 (C), superoxide anion (S) and elastase (E).
Figure 11.
The structures of verticillane-based diterpenoids (224–233).
2.2.5. Norditerpenoids
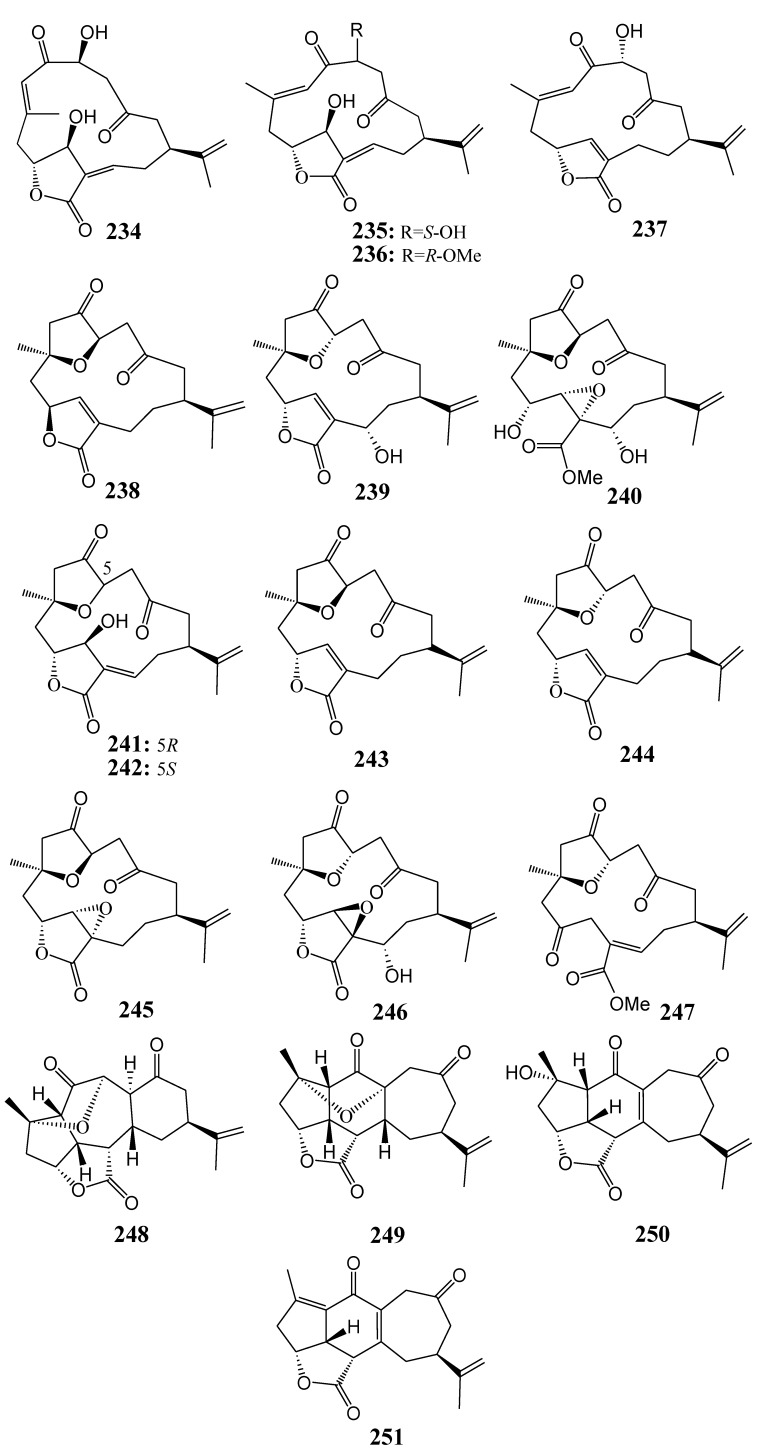
Table 12 summarizes 18 norditerpenoids (234–251) evaluated for in vitro anti-inflammatory activity in literature published from 2008 to 2012. The corresponding chemical structures are reported in Figure 12.
Table 12.
Chemical constituents of norditerpenoids from soft corals of Taiwan.
| No. | Name | Sources | Activities * | Reference |
|---|---|---|---|---|
| 234 | Gyrosanolide A | Sinularia gyrosa | [68] | |
| 235 | Gyrosanolide B | Sinularia gyrosa | I | [68] |
| 236 | Gyrosanolide C | Sinularia gyrosa | I | [68] |
| 237 | Gyrosanolide D | Sinularia gyrosa | [68] | |
| 238 | Gyrosanolide E | Sinularia gyrosa | [68] | |
| 239 | Gyrosanolide F | Sinularia gyrosa | I | [68] |
| 240 | Gyrosanin A | Sinularia gyrosa | I | [68] |
| 241 | (1 S*,5R*,8S*,10R*,11S*)-11-Hydroxyl-1-isopropenyl-8-methyl-3,6-dioxo-5,8-epoxycyclotetradec-12-ene-10,12-carbonlactone | Sinularia gyrosa | I | [68] |
| 242 | (1 S*,5S*,8S*,10R*,11S*)-11-Hydroxyl-1-isopropenyl-8-methyl-3,6-dioxo-5,8-epoxycyclotetradec-12-ene-10,12-carbonlactone | Sinularia gyrosa | I | [68] |
| 243 | Norcembrene | Sinularia gyrosa | [68] | |
| 244 | epi-Norcembrene | Sinularia gyrosa | [68] | |
| 245 | Leptocladolide B | Sinularia gyrosa | I | [68] |
| 246 | Scabrolide D | Sinularia gyrosa | I | [68] |
| 247 | Norcembrene | Sinularia gyrosa | [68] | |
| 248 | Ineleganolide | Sinularia gyrosa | [68] | |
| 249 | Sinulochemodin C | Sinularia gyrosa | [68] | |
| 250 | Scabrolide A | Sinularia gyrosa | [68] | |
| 251 | Yanarolide | Sinularia gyrosa | [68] |
* Inhibition of iNOS (I).
Figure 12.
The structures of norditerpenoids (234–251).
2.2.6. Xenicane-Type Diterpenoids
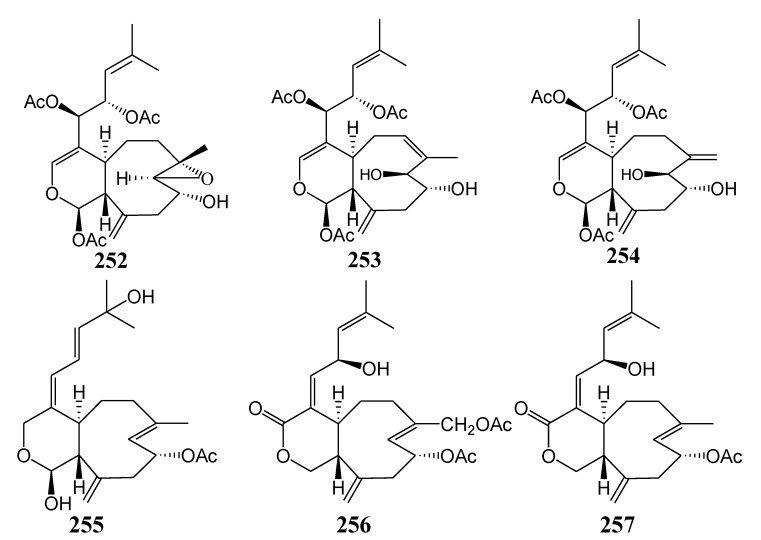
Table 13 summarizes six xenicane-type diterpenoids (252–257) evaluated for in vitro anti-inflammatory activity in literature published from 2008 to 2012. The corresponding chemical structures are reported in Figure 13.
Table 13.
Chemical constituents of xenicane-type diterpenoids from soft corals of Taiwan.
| No. | Name | Sources | Activities * | Reference |
|---|---|---|---|---|
| 252 | Asterolaurin A | Asterospicularia laurae | [69] | |
| 253 | Asterolaurin B | Asterospicularia laurae | [69] | |
| 254 | Asterolaurin C | Asterospicularia laurae | [69] | |
| 255 | Asterolaurin D | Asterospicularia laurae | S,E | [69] |
| 256 | Asterolaurin E | Asterospicularia laurae | [69] | |
| 257 | Asterolaurin F | Asterospicularia laurae | [69] |
* Inhibition of superoxide anion (S) and elastase (E).
Figure 13.
The structures of xenicane-type diterpenoids (252–257).
2.2.7. Other-Type Diterpenoids
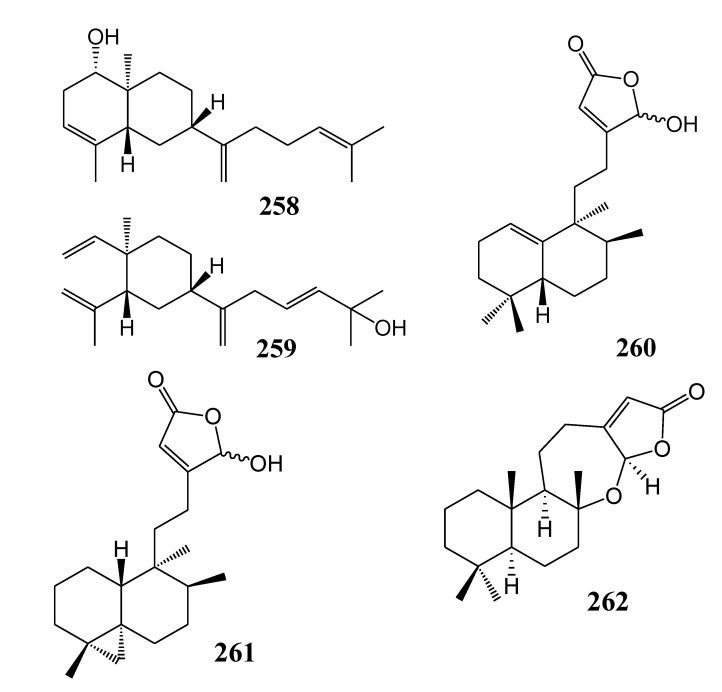
Table 14 summarizes five other-type diterpenoids (258–262) evaluated for in vitro anti-inflammatory activity in literature published from 2008 to 2012. The corresponding chemical structures are reported in Figure 14.
Table 14.
Chemical constituents of other type diterpenoids from soft corals of Taiwan.
| No. | Name | Sources | Activities * | Reference |
|---|---|---|---|---|
| 258 | Gyrosanol A | Sinularia gyrosa | C | [70] |
| 259 | Gyrosanol B | Sinularia gyrosa | C | [70] |
| 260 | Echinohalimane A | Echinomuricea sp. | E | [71] |
| 261 | Echinoclerodane A | Echinomuricea sp. | S,E | [72] |
| 262 | Echinolabdane A | Echinomuricea sp. | [73] |
* Inhibition of COX-2 (C), superoxide anion (S) and elastase (E).
Figure 14.
The structures of other type diterpenoids (258–262).
At a concentration of 10 μM, compounds 131, 133, 134, 139, 140–143, 147, 148, 151–153, 157–160, 162–168, 170 ceramide and cerebrosides 174, 225, 229, 230, 235, 236, 239–242, 244, 245, 258 and 259 reduced LPS-induced expression of iNOS in murine macrophage cells [46,47,48,49,50,51,66,68,70]. Compounds 134, 148, 151, 160, 163, 164, 166–168, 225, 258 and 259 suppressed the LPS-induced expression of COX-2 in these cells [46,47,48,49,50,66,70]. At 10 µg/mL, compounds 180, 184, 186, 188, 198–205, 208–210, 213, 216, 217, 222, 232, 233, 255 and 261 inhibited the generation of superoxide anion by activated human neutrophils [54,55,56,58,59,60,61,62,63,64,65,67,69,70,72]. Compounds 180, 184, 186, 188, 202–204, 207, 210, 212, 213, 215–217, 222, 232, 233, 255, 260 and 261 inhibited the release of elastase from these activated human neutrophils [53,54,55,56,59,61,63,65,67,69,71,72]. These results provided useful baseline information on the immune-regulatory and anti-oxidant activities of various marine diterpenoids. Compound 184, as 185 epimer at C-6, was showed to be more potent in the inhibition of the generation of superoxide anion and in inducing the release of elastase by active human neutrophils, suggesting that the stereochemistry at C-6 may play a key role in the above biological effects [54].
The briarane-type diterpenoid excavatolide B (189) has been demonstrated to significantly inhibit TPA-induced cutaneous inflammation activities in mice, including those related to vascular permeability, edema, and TPA-induced expression of iNOS, COX-2 and matrixmetalloproteinase-9. Excavatolide B also suppressed LPS-induced expression of TNF-α and IL-6 in mouse bone marrow derived dendritic cells (BMDCs) [57]. Also, excavatolide F (191), K (190) and Z (193) and briaexcavatolide B (194), H (196), K (195) and R (192) exhibited a broad spectrum of activity in inhibition of LPS-induced expression of IL-6 in BMDCs [57]. A study on the structure-activity relationship between the structures of the briarane-type diterpenoids and their inhibition of IL-6 expression in BMDCs revealed that the eight 17-epoxide of briarane-type diterpenoids may play an important role in the inhibition of IL-6 expression in specific immune cells [57]. Replacement of the C-12 hydroxyl group with long esters in briarane-type diterpenoids decreased the inhibition of IL-6 expression [57].
2.3. Steroids
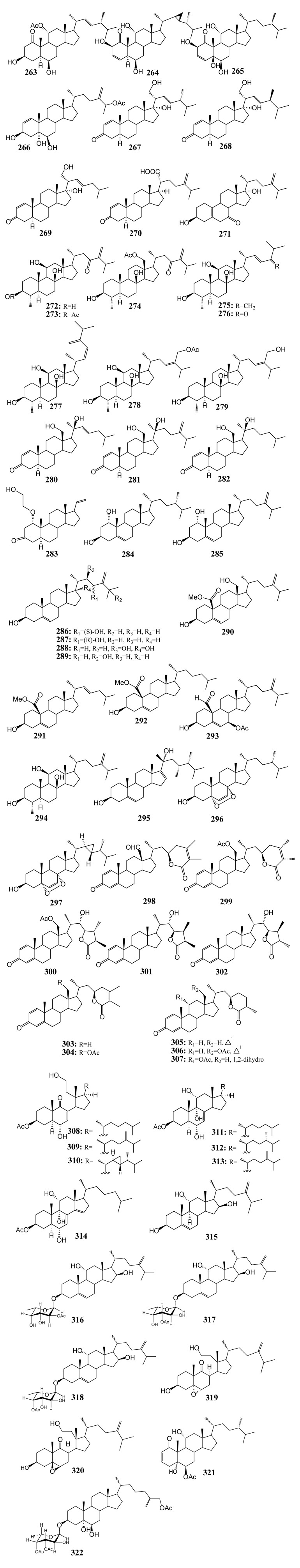
Table 15 summarizes 60 steroids (263–322) evaluated for in vitro anti-inflammatory activity in literature published from 2008 to 2012. The corresponding chemical structures are reported in Figure 15.
Table 15.
Chemical constituents of steroids from soft corals of Taiwan.
| No. | Name | Sources | Activities * | Reference |
|---|---|---|---|---|
| 263 | Stoloniferone R | Clavularia viridis | [74] | |
| 264 | Stoloniferone S | Clavularia viridis | I | [74] |
| 265 | Stoloniferone T | Clavularia viridis | I,C | [74] |
| 266 | (25S)-24-Methylenecholestane-3β,5α,6β-triol-26-acetate | Clavularia viridis | I,C | [74] |
| 267 | Griffinisterone A | Nephthea griffini | I | [75] |
| 268 | Griffinisterone B | Nephthea griffini | I | [75] |
| 269 | Griffinisterone C | Nephthea griffini | I | [75] |
| 270 | Griffinisterone D | Nephthea griffini | I | [75] |
| 271 | Chabrosterol | Nephthea chabroli | I,C | [21] |
| 272 | Nebrosteroid A | Nephthea chabroli | I | [76] |
| 273 | Nebrosteroid B | Nephthea chabroli | I | [76] |
| 274 | Nebrosteroid C | Nephthea chabroli | I | [76] |
| 275 | Nebrosteroid D | Nephthea chabroli | I,C | [76] |
| 276 | Nebrosteroid F | Nepthea chabroli | I,C | [76] |
| 277 | Nebrosteroid E | Nepthea chabroli | [76] | |
| 278 | Nebrosteroid G | Nepthea chabroli | I,C | [76] |
| 279 | Nebrosteroid H | Nepthea chabroli | I | [76] |
| 280 | Griffinisterone F | Dendronephthya griffini | I,C | [77] |
| 281 | Griffinisterone G | Dendronephthya griffini | I,C | [77] |
| 282 | Griffinisterone H | Dendronephthya griffini | I | [77] |
| 283 | Griffinipregnone | Dendronephthya griffini | I,C | [77] |
| 284 | 1α,3β-Dihydroxy-24S-methylcholesta-5-ene | Sinularia sp. | I,C | [78] |
| 285 | 1α,3β-Dihydroxy-24-methylenecholesta-5-ene | Sinularia sp. | I,C | [78] |
| 286 | 5,24(28)-Ergostadien-3β,23S-diol | Nephthea erecta | I,C | [79] |
| 287 | 5,24(28)-Ergostadien-3β,23R-diol | Nephthea erecta | I | [79] |
| 288 | (22S)-5,24(28)-Ergostadien-3β,17α,22-triol | Nephthea erecta | I,C | [79] |
| 289 | Ergostanoid | Nephthea erecta | I | [79] |
| 290 | Nebrosteroid I | Nephthea chabroli | I,C | [80] |
| 291 | Nebrosteroid J | Nephthea chabroli | I,C | [80] |
| 292 | Nebrosteroid K | Nephthea chabroli | [80] | |
| 293 | Nebrosteroid L | Nephthea chabroli | I,C | [80] |
| 294 | Nebrosteroid M | Nephthea chabroli | IC | [80] |
| 295 | Sarcophytosterol | Lobophytum sarcophytoides | [38] | |
| 296 | 5α,8α-Epidioxy-24-methylcholesta-6-en-3β-ol | Lobophytum sarcophytoides | [38] | |
| 297 | 5α,8α-Epidioxy-22,23-methylene-24-methylcholest-6-en-3β-ol | Lobophytum sarcophytoides | I | [38] |
| 298 | Paraminabeolide A | Paraminabea acronocephala | I | [81] |
| 299 | Paraminabeolide B | Paraminabea acronocephala | I | [81] |
| 300 | Paraminabeolide C | Paraminabea acronocephala | I | [81] |
| 301 | Paraminabeolide D | Paraminabea acronocephala | I | [81] |
| 302 | Paraminabeolide E | Paraminabea acronocephala | [81] | |
| 303 | Minabeolide-1 | Paraminabea acronocephala | I,C | [81] |
| 304 | Minabeolide-2 | Paraminabea acronocephala | I,C | [81] |
| 305 | Minabeolide-4 | Paraminabea acronocephala | I,C | [81] |
| 306 | Minabeolide-5 | Paraminabea acronocephala | I,C | [81] |
| 307 | Minabeolide-8 | Paraminabea acronocephala | [81] | |
| 308 | Hirsutosterol A | Cladiella hirsuta | [82] | |
| 309 | Hirsutosterol B | Cladiella hirsuta | [82] | |
| 310 | Hirsutosterol C | Cladiella hirsuta | [82] | |
| 311 | Hirsutosterol D | Cladiella hirsuta | [82] | |
| 312 | Hirsutosterol E | Cladiella hirsuta | [82] | |
| 313 | Hirsutosterol F | Cladiella hirsuta | [82] | |
| 314 | Hirsutosterol G | Cladiella hirsuta | [82] | |
| 315 | Crassarosterol A | Sinularia crassa | [83] | |
| 316 | Crassarosteroside A | Sinularia crassa | I | [83] |
| 317 | Crassarosteroside B | Sinularia crassa | I | [83] |
| 318 | Crassarosteroside C | Sinularia crassa | I | [83] |
| 319 | 8αH-3β,11-Dihydroxy-5α,6α-expoxy-24-methylene-9,11-secocholestan-9-one | Sinularia granosa | I,C | [84] |
| 320 | 3β,11-Dihydroxy-5β,6β-expoxy-24-methylene-9,11-secocholestan-9-one | Sinularia granosa | I | [84] |
| 321 | 6-epi-Yonarasterol B | Echinomuricea sp. | S,E | [73] |
| 322 | Carijoside A | Carijoa sp. | S,E | [85] |
* Inhibition of iNOS (I), COX-2 (C), superoxide anion (S) and elastase (E).
Figure 15.
The structures of steroids (263–322).
At a concentration of 10 µM, compounds 264–275, 277–291, 293, 294, 297, 303–307 and 316–320 reduced LPS-induced expression level of iNOS in murine macrophage cells (RAW264.7) [21,74,75,76,77,78,79,80,81,83,84]. Compounds 265, 266, 271, 275, 277, 278, 280, 281, 283–286, 288, 290, 291, 293 and 319 suppressed LPS-induced expression level of COX-2 in murine macrophage cells (RAW264.7) [21,74,75,76,77,78,79,80,84]. At 10 µg/mL, compounds 321 and 322 inhibited the generation of superoxide anion and the release of elastase by activated human neutrophils [73,85].
2.4. Ceramide and Cerebrosides
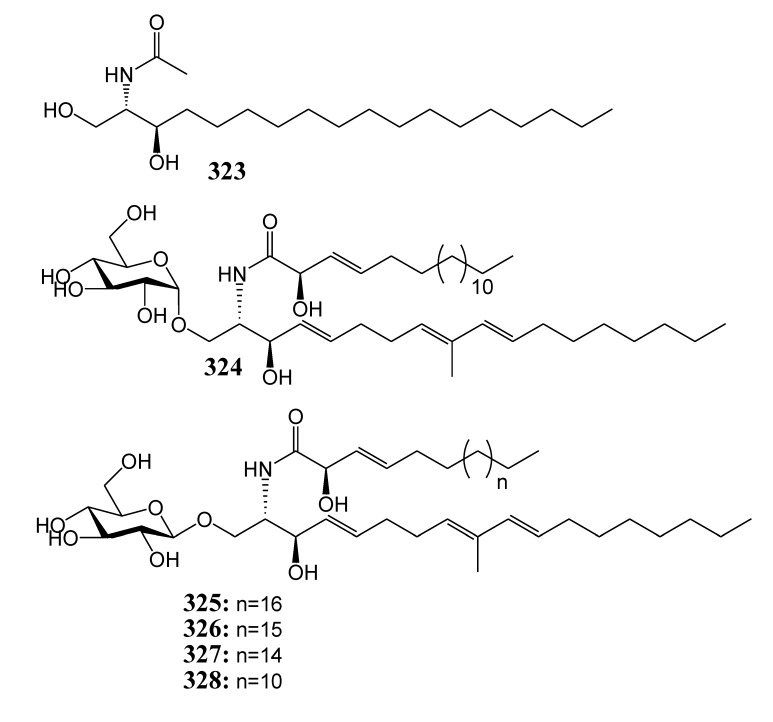
Table 16 summarizes ceramide (323) and five cerebrosides (324–328) evaluated for in vitro anti-inflammatory activity in literature published from 2008 to 2012. The corresponding chemical structures are reported in Figure 16.
Table 16.
Chemical constituents of ceramide and cerebrosides from soft corals of Taiwan.
| No. | Name | Sources | Activities * | Reference |
|---|---|---|---|---|
| 323 | Ceramide | Sarcophyton ehrenbergi | I,C | [86] |
| 324 | Sarcoehrenoside A | Sarcophyton ehrenbergi | I | [86] |
| 325 | Sarcoehrenoside B | Sarcophyton ehrenbergi | [86] | |
| 326 | Cerebroside-3 | Sarcophyton ehrenbergi | I | [86] |
| 327 | Cerebroside-5 | Sarcophyton ehrenbergi | I | [86] |
| 328 | Cerebroside-6 | Sarcophyton ehrenbergi | I | [86] |
* Inhibition of iNOS (I) and COX-2 (C).
Figure 16.
The structures of ceramide and cerebrosides (323–328).
2.5. Other Metabolites
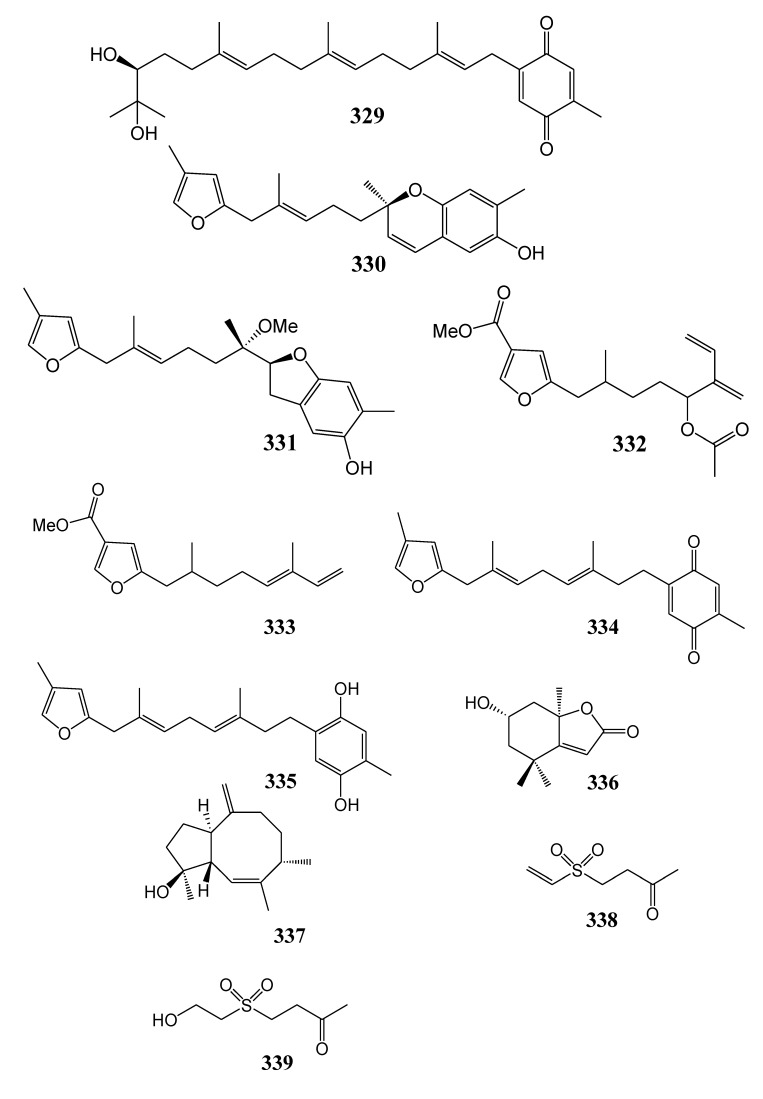
Table 17 summarizes 11 secondary metabolites of other types (329–339) evaluated for in vitro anti-inflammatory activity in literature published from 2008 to 2012. The corresponding chemical structures are reported in Figure 17.
Table 17.
Chemical constituents of other metabolites from soft corals of Taiwan.
| No. | Name | Sources | Activities * | Reference |
|---|---|---|---|---|
| 329 | Capilloquinone | Sinularia capillosa | I | [87] |
| 330 | Capillobenzopyranol | Sinularia capillosa | I | [87] |
| 331 | Capillobenzofuranol | Sinularia capillosa | [87] | |
| 332 | Capillofuranocarboxylate | Sinularia capillosa | [87] | |
| 333 | (E)-5-(2,6-Dimethylocta-5,7-dienyl)furan-3-carboxylic acid | Sinularia capillosa | [87] | |
| 334 | 2-[(2E,6E)-3,7-Dimethyl-8-(4-methylfuran-2-yl)octa-2,6-dienyl]-5-methylcyclohexa-2,5-diene-1,4-dione | Sinularia capillosa | I,C | [87] |
| 335 | 2-[(2E,6E)-3,7-Dimethyl-8-(4-methylfuran-2-yl)octa-2,6-dienyl]-5-methylbenzene-1,4-diol | Sinularia capillosa | I | [87] |
| 336 | (–)-Loliolide | Sinularia capillosa | [87] | |
| 337 | 3,4,11-Trimethyl-7-methylenebicyclo[6.3.0]undec-2-en-11R-ol | Sinularia capillosa | [87] | |
| 338 | Austrasulfone | Cladiella australis | [88] | |
| 339 | Dihydroaustrasulfone alcohol | Cladiella australis | I,C | [88] |
* Inhibition of iNOS (I) and COX-2 (C).
Figure 17.
Structures of other metabolites (329–339).
At a concentration of 10 µM, compounds 323, 324, 326–330, 334, 335 and 339 reduced LPS-induced expression level of iNOS in murine macrophage cells (RAW264.7) [86,87,88]. Compounds 323, 334 and 339 suppressed LPS-induced expression levels of COX-2 in murine macrophage cells (RAW264.7) [86,88]. Austrasulfone (338) was found to exhibit a potent neuroprotective effect in human dopaminergic neuron cells (SH-SY5Y) [89,90]. In animal disease models, the synthetic precursor of austrasulfone dihydroaustrasulfone alcohol (339) was not only demonstrated to attenuate neuropathic pain, but also to suppress the progression of multiple sclerosis and atherosclerosis [88].
3. Conclusions
Marine invertebrates, particularly octocorals, are rich potential sources of drug leads. Most of our own and other studies on anti-inflammatory activities of natural products from soft corals have been focused on “screening-like” assays using COX-2 and iNOS as target markers. These assay studies have been useful in generating small libraries of anti-oxidant and anti-inflammatory activities from a broad spectrum of soft corals. These results, however, apparently have limitations. For example, the findings are usually generic in nature, and there is often difficulty in immediate or specific application of such results to drug/pharmaceutical discovery, as compared to the existing synthetic chemicals or phytochemicals or those being developed for clinical use. We [45,57,88] and others [25,26] have recently initiated a number of cross-disciplinary studies, employing bio-organic chemistry, cellular immunology and animal disease models for systematic and in-depth studies. As a result, we believe that useful information on the possible application of specific natural products from soft corals for future clinical studies have been obtained. We consider such approaches [57] may need to be encouraged and organized at the international level, and hopefully be integrated into systematic studies, aiming to create translational research of marine natural products for pharmaceuticals/nutraceuticals. Special emphasis may need to be placed on new or specific cell biological/disease model systems.
In terms of evaluating marine natural products for future pharmaceutical application, despite the abundance of unique marine natural products identified, the extremely low quantity of a given compound of interest that can be isolated from marine organisms may be a big hurdle for evaluation of in vivo bioactivities and development for pharmaceutical applications.
Fortunately, due to the recent advancement in aquaculture technologies, aquacultural cultivation of various types of specific soft corals is becoming possible. Our team has successfully cultured a number of species of soft corals, including Klyxum simplex and Briareum excavatum [47,91]. As a result, more abundant and routine preparations of experimental materials will become available for global distribution and collaborative research purposes. Nonetheless, the vast volume of marine organisms and the small base of knowledge so far assembled on soft coral-derived marine chemicals calls for increased international cooperation in this field.
Acknowledgments
We thank Ms. Miranda Loney of the Agricultural Biotechnology Research Center, Academia Sinica, Taiwan; and Subramanian Senthilkumar of Shanmugha Arts, Science, Technology & Research Academy, India for editing the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Marris E. Marine natural products: Drugs from the deep. Nature. 2006;443:904–905. doi: 10.1038/443904a. [DOI] [PubMed] [Google Scholar]
- 2.Simmons T.L., Andrianasolo E., McPhail K., Flatt P., Gerwick W.H. Marine natural products as anticancer drugs. Mol. Cancer Ther. 2005;4:333–342. [PubMed] [Google Scholar]
- 3.Molinski T.F., Dalisay D.S., Lievens S.L., Saludes J.P. Drug development from marine natural products. Nat. Rev. Drug Discov. 2009;8:69–85. doi: 10.1038/nrd2487. [DOI] [PubMed] [Google Scholar]
- 4.Hu G.P., Yuan J., Sun L., She Z.G., Wu J.H., Lan X.J., Zhu X., Lin Y.C., Chen S.P. Statistical research on marine natural products based on data obtained between 1985 and 2008. Mar. Drugs. 2011;9:514–525. doi: 10.3390/md9040514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blunt J.W., Copp B.R., Keyzers R.A., Munro M.H., Prinsep M.R. Marine natural products. Nat. Prod. Rep. 2013;30:237–323. doi: 10.1039/c2np20112g. [DOI] [PubMed] [Google Scholar]
- 6.Blunt J.W., Copp B.R., Keyzers R.A., Munro M.H., Prinsep M.R. Marine natural products. Nat. Prod. Rep. 2012;29:144–222. doi: 10.1039/c2np00090c. [DOI] [PubMed] [Google Scholar]
- 7.Blunt J.W., Copp B.R., Munro M.H., Northcote P.T., Prinsep M.R. Marine natural products. Nat. Prod. Rep. 2011;28:196–268. doi: 10.1039/c005001f. [DOI] [PubMed] [Google Scholar]
- 8.Mayer A.M., Rodriguez A.D., Berlinck R.G., Fusetani N. Marine pharmacology in 2007–8: Marine compounds with antibacterial, anticoagulant, antifungal, anti-inflammatory, antimalarial, antiprotozoal, antituberculosis, and antiviral activities affecting the immune and nervous system, and other miscellaneous mechanisms of action. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2011;153:191–222. doi: 10.1016/j.cbpc.2010.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kaplanski G., Marin V., Montero-Julian F., Mantovani A., Farnarier C. IL-6: A regulator of the transition from neutrophil to monocyte recruitment during inflammation. Trends Immunol. 2003;24:25–29. doi: 10.1016/S1471-4906(02)00013-3. [DOI] [PubMed] [Google Scholar]
- 10.Wright H.L., Moots R.J., Bucknall R.C., Edwards S.W. Neutrophil function in inflammation and inflammatory diseases. Rheumatology. 2010;49:1618–1631. doi: 10.1093/rheumatology/keq045. [DOI] [PubMed] [Google Scholar]
- 11.Vinten-Johansen J. Involvement of neutrophils in the pathogenesis of lethal myocardial reperfusion injury. Cardiovasc. Res. 2004;61:481–497. doi: 10.1016/j.cardiores.2003.10.011. [DOI] [PubMed] [Google Scholar]
- 12.Hwang T.L., Su Y.C., Chang H.L., Leu Y.L., Chung P.J., Kuo L.M., Chang Y.J. Suppression of superoxide anion and elastase release by C18 unsaturated fatty acids in human neutrophils. Lipid Res. 2009;50:1395–1408. doi: 10.1194/jlr.M800574-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pham C.T. Neutrophil serine proteases: Specific regulators of inflammation. Nat. Rev. Immunol. 2006;6:541–550. doi: 10.1038/nri1841. [DOI] [PubMed] [Google Scholar]
- 14.Chang C.H., Wen Z.H., Wang S.K., Duh C.Y. Capnellenes from the Formosan soft coral Capnella imbricata. J. Nat. Prod. 2008;71:619–621. doi: 10.1021/np0706116. [DOI] [PubMed] [Google Scholar]
- 15.Cheng S.Y., Lin E.H., Huang J.S., Wen Z.H., Duh C.Y. Ylangene-type and nardosinane-type sesquiterpenoids from the soft corals Lemnalia flava and Paralemnalia thyrsoides. Chem. Pharm. Bull. 2010;58:381–385. doi: 10.1248/cpb.58.381. [DOI] [PubMed] [Google Scholar]
- 16.Lu Y., Li P.J., Hung W.Y., Su J.H., Wen Z.H., Hsu C.H., Dai C.F., Chiang M.Y., Sheu J.H. Nardosinane sesquiterpenoids from the Formosan soft coral Lemnalia flava. J. Nat. Prod. 2011;74:169–174. doi: 10.1021/np100541a. [DOI] [PubMed] [Google Scholar]
- 17.Tseng Y.J., Shen K.P., Lin H.L., Huang C.Y., Dai C.F., Sheu J.H. Lochmolins A–G, new sesquiterpenoids from the soft coral Sinularia lochmodes. Mar. Drugs. 2012;10:1572–1581. doi: 10.3390/md10071572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huang C.Y., Su J.H., Liu C.Y., Wen Z.H., Hsu C.H., Chiang M.Y., Sheu J.H. Oppositane-Type sesquiterpenoids from the Formosan soft coral Sinularia leptoclados. Bull. Chem. Soc. Jpn. 2010;83:678–682. doi: 10.1246/bcsj.20090263. [DOI] [PubMed] [Google Scholar]
- 19.Kao S.Y., Su J.H., Hwang T.L., Sheu J.H., Wen Z.H., Wu Y.C., Sung P.J. Menelloides C and D, new sesquiterpenoids from the Gorgonian coral Menella sp. Mar. Drugs. 2011;9:1534–1542. doi: 10.3390/md9091534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee C.H., Kao C.Y., Kao S.Y., Chang C.H., Su J.H., Hwang T.L., Kuo Y.H., Wen Z.H., Sung P.J. Terpenoids from the octocorals Menella sp. (Plexauridae) and Lobophytum crassum (Alcyonacea) Mar. Drugs. 2012;10:427–438. doi: 10.3390/md10020427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cheng S.Y., Huang Y.C., Wen Z.H., Chiou S.H., Wang S.K., Hsu C.H., Dai C.F., Duh C.Y. Novel sesquiterpenes and norergosterol from the soft corals Nephthea erecta and Nephthea chabroli. Tetrahedron Lett. 2009;50:802–806. doi: 10.1016/j.tetlet.2008.12.002. [DOI] [Google Scholar]
- 22.Su J.H., Huang C.Y., Li P.J., Lu Y., Wen Z.H., Kao Y.H., Sheu J.H. Bioactive cadinane-type compounds from the soft coral Sinularia scabra. Arch. Pharmacal. Res. 2012;35:779–784. doi: 10.1007/s12272-012-0503-2. [DOI] [PubMed] [Google Scholar]
- 23.Su J.H., Chiang M.Y., Wen Z.H., Dai C.F., Hsu C.H., Sheu J.H. Sesquiterpenoids from the formosan soft coral Sinularia leptoclados. Chem. Pharm. Bull. 2010;58:250–253. doi: 10.1248/cpb.58.250. [DOI] [PubMed] [Google Scholar]
- 24.Kao S.Y., Chang Y.C., Su J.H., Lu M.C., Chen Y.H., Sheu J.H., Wen Z.H., Wang W.H., Kuo Y.H., Hwang T.L., et al. (–)-Hydroxylindestrenolide, a new sesquiterpenoid from a gorgonian coral Menella sp. (Plexauridae) Chem. Pharm. Bull. 2011;59:1048–1050. doi: 10.1248/cpb.59.1048. [DOI] [PubMed] [Google Scholar]
- 25.Jean Y.H., Chen W.F., Duh C.Y., Huang S.Y., Hsu C.H., Lin C.S., Sung C.S., Chen I.M., Wen Z.H. Inducible nitric oxide synthase and cyclooxygenase-2 participate in anti-inflammatory and analgesic effects of the natural marine compound lemnalol from Formosan soft coral Lemnalia cervicorni. Eur. J. Pharmacol. 2008;578:323–331. doi: 10.1016/j.ejphar.2007.08.048. [DOI] [PubMed] [Google Scholar]
- 26.Jean Y.H., Chen W.F., Sung C.S., Duh C.Y., Huang S.Y., Lin C.S., Tai M.H., Tzeng S.F., Wen Z.H. Capnellene, a natural marine compound derived from soft coral, attenuates chronic constriction injury-induced neuropathic pain in rats. Br. J. Pharmacol. 2009;158:713–725. doi: 10.1111/j.1476-5381.2009.00323.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ahmed A.F., Wen Z.H., Su J.H., Hsieh Y.T., Wu Y.C., Hu W.P., Sheu J.H. Oxygenated cembranoids from a Formosan soft coral Sinularia gibberosa. J. Nat. Prod. 2008;71:179–185. doi: 10.1021/np070356p. [DOI] [PubMed] [Google Scholar]
- 28.Ahmed A.F., Tai S.H., Wen Z.H., Su J.H., Wu Y.C., Hu W.P., Sheu J.H. A C-3 methylated isocembranoid and 10-oxocembranoids from a Formosan soft coral, Sinularia grandilobata. J. Nat. Prod. 2008;71:946–951. doi: 10.1021/np7007335. [DOI] [PubMed] [Google Scholar]
- 29.Cheng S.Y., Wen Z.H., Chiou S.F., Hsu C.H., Wang S.K., Dai C.F., Chiang M.Y., Duh C.Y. Durumolides A–E, anti-inflammatory and antibacterial cembranolides from the soft coral Lobophytum durum. Tetrahedron. 2008;64:9698–9704. doi: 10.1016/j.tet.2008.07.104. [DOI] [Google Scholar]
- 30.Lu Y., Su J.H., Huang C.Y., Liu Y.C., Kuo Y.H., Wen Z.H., Hsu C.H., Sheu J.H. Cembranoids from the soft corals Sinularia granosa and Sinularia querciformis. Chem. Pharm. Bull. 2010;58:464–466. doi: 10.1248/cpb.58.464. [DOI] [PubMed] [Google Scholar]
- 31.Chao C.H., Wen Z.H., Wu Y.C., Yeh H.C., Sheu J.H. Cytotoxic and anti-inflammatory cembranoids from the soft coral. Lobophytum crassum. J. Nat. Prod. 2008;71:1819–1824. doi: 10.1021/np8004584. [DOI] [PubMed] [Google Scholar]
- 32.Cheng S.Y., Wen Z.H., Wang S.K., Chiou S.F., Hsu C.H., Dai C.F., Chiang M.Y., Duh C.Y. Unprecedented hemiketal cembranolides with anti-inflammatory activity from the soft coral. Lobophytum durum. J. Nat. Prod. 2009;72:152–155. doi: 10.1021/np800686k. [DOI] [PubMed] [Google Scholar]
- 33.Cheng S.Y., Wen Z.H., Wang S.K., Chiou S.F., Hsu C.H., Dai C.F., Duh C.Y. Anti-inflammatory cembranolides from the soft coral Lobophytum durum. Bioorg. Med. Chem. 2009;17:3763–3769. doi: 10.1016/j.bmc.2009.04.053. [DOI] [PubMed] [Google Scholar]
- 34.Lin W.Y., Su J.H., Lu Y., Wen Z.H., Dai C.F., Kuo Y.H., Sheu J.H. Cytotoxic and anti-inflammatory cembranoids from the Dongsha Atoll soft coral Sarcophyton crassocaule. Bioorg. Med. Chem. 2010;18:1936–1941. doi: 10.1016/j.bmc.2010.01.036. [DOI] [PubMed] [Google Scholar]
- 35.Chen B.W., Chao C.H., Su J.H., Huang C.Y., Dai C.F., Wen Z.H., Sheu J.H. A novel symmetric sulfur-containing biscembranoid from the Formosan soft coral Sinularia flexibilis. Tetrahedron Lett. 2010;51:5764–5766. doi: 10.1016/j.tetlet.2010.08.027. [DOI] [Google Scholar]
- 36.Su J.H., Wen Z.H. Bioactive cembrane-based diterpenoids from the soft coral Sinularia triangular. Mar. Drugs. 2011;9:944–951. doi: 10.3390/md9060944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lin W.Y., Lu Y., Su J.H., Wen Z.H., Dai C.F., Kuo Y.H., Sheu J.H. Bioactive cembranoids from the dongsha atoll soft coral Sarcophyton crassocaule. Mar. Drugs. 2011;9:994–1006. doi: 10.3390/md9060994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lu Y., Lin Y.C., Wen Z.H., Su J.H., Sung P.J., Hsu C.H., Kuo Y.H., Chiang M.Y., Dai C.F., Sheu J.H. Steroid and cembranoids from the Dongsha atoll soft coral Lobophytum sarcophytoides. Tetrahedron. 2010;66:7129–7135. doi: 10.1016/j.tet.2010.06.094. [DOI] [Google Scholar]
- 39.Lu Y., Su H.J., Chen Y.H., Wen Z.H., Sheu J.H., Su J.H. Anti-Inflammatory cembranoids from the Formosan soft coral Sinularia discrepans. Arch. Pharmacal. Res. 2011;34:1263–1267. doi: 10.1007/s12272-011-0804-x. [DOI] [PubMed] [Google Scholar]
- 40.Tseng Y.J., Wen Z.H., Hsu C.H., Dai C.F., Sheu J.H. Bioactive cembranoids from the Dongsha atoll soft coral Lobophytum crassum. Bull. Chem. Soc. Jpn. 2011;84:1102–1106. doi: 10.1246/bcsj.20110096. [DOI] [Google Scholar]
- 41.Chao C.H., Chou K.J., Huang C.Y., Wen Z.H., Hsu C.H., Wu Y.C., Dai C.F., Sheu J.H. Bioactive cembranoids from the soft coral Sinularia crassa. Mar. Drugs. 2011;9:1955–1968. doi: 10.3390/md9101955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lin W.Y., Lu Y., Chen B.W., Huang C.Y., Su J.H., Wen Z.H., Dai C.F., Kuo Y.H., Sheu J.H. Sarcocrassocolides M–O, bioactive cembranoids from the Dongsha atoll soft coral Sarcophyton crassocaule. Mar. Drugs. 2012;10:617–626. doi: 10.3390/md10030617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lee N.L., Su J.H. Tetrahydrofuran cembranoids from the cultured soft coral Lobophytum crassum. Mar. Drugs. 2011;9:2526–2536. doi: 10.3390/md9122526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kao C.Y., Su J.H., Lu M.C., Hwang T.L., Wang W.H., Chen J.J., Sheu J.H., Kuo Y.H., Weng C.F., Fang L.S., Wen Z.H., Sung P.J. Lobocrassins A–E: New cembrane-type diterpenoids from the soft coral. Lobophytum crassum. Mar. Drugs. 2011;9:1319–1331. doi: 10.3390/md9081319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Huang S.Y., Chen N.F., Chen W.F., Hung H.C., Lee H.P., Lin Y.Y., Wang H.M., Sung P.J., Sheu J.H., Wen Z.H. Sinularin from indigenous soft coral attenuates nociceptive responses and spinal neuroinflammation in carrageenan-induced inflammatory rat model. Mar. Drugs. 2012;10:1899–1919. doi: 10.3390/md10091899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wu S.L., Su J.H., Wen Z.H., Hsu C.H., Chen B.W., Dai C.F., Kuo Y.H., Sheu J.H. Simplexins A–I, eunicellin-based diterpenoids from the soft coral Klyxum simplex. J. Nat. Prod. 2009;72:994–1000. doi: 10.1021/np900064a. [DOI] [PubMed] [Google Scholar]
- 47.Chen B.W., Chao C.H., Su J.H., Tsai C.W., Wang W.H., Wen Z.H., Huang C.Y., Sung P.J., Wu Y.C., Sheu J.H. Klysimplexins I–T, eunicellin-based diterpenoids from the cultured soft coral Klyxum simplex. Org. Biomol. Chem. 2011;9:834–844. doi: 10.1039/c0ob00351d. [DOI] [PubMed] [Google Scholar]
- 48.Chen B.W., Chang S.M., Huang C.Y., Chao C.H., Su J.H., Wen Z.H., Hsu C.H., Dai C.F., Wu Y.C., Sheu J.H. Hirsutalins A–H, eunicellin-based diterpenoids from the soft coral Cladiella hirsuta. J. Nat. Prod. 2010;73:1785–1791. doi: 10.1021/np100401f. [DOI] [PubMed] [Google Scholar]
- 49.Chen B.W., Chao C.H., Su J.H., Wen Z.H., Sung P.J., Sheu J.H. Anti-Inflammatory eunicellin-based diterpenoids from the cultured soft coral Klyxum simplex. Org. Biomol. Chem. 2010;8:2363–2366. doi: 10.1039/b926353e. [DOI] [PubMed] [Google Scholar]
- 50.Hsu F.J., Chen B.W., Wen Z.H., Huang C.Y., Dai C.F., Su J.H., Wu Y.C., Sheu J.H. Klymollins A–H, bioactive eunicellin-based diterpenoids from the Formosan soft coral Klyxum molle. J. Nat. Prod. 2011;74:2467–2471. doi: 10.1021/np200589n. [DOI] [PubMed] [Google Scholar]
- 51.Tai C.J., Su J.H., Huang M.S., Wen Z.H., Dai C.F., Sheu J.H. Bioactive eunicellin-based diterpenoids from the soft coral Cladiella krempfi. Mar. Drugs. 2011;9:2036–2045. doi: 10.3390/md9102036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chen B.W., Huang C.Y., Wen Z.H., Su J.H., Wang W.H., Sung P.J., Wu Y.C., Sheu J.H. Klysimplexins U–X, eunicellin-based diterpenoids from the cultured soft coral Klyxum simplex. Bull. Chem. Soc. Jpn. 2011;84:1237–1242. doi: 10.1246/bcsj.20110156. [DOI] [PubMed] [Google Scholar]
- 53.Chen Y.H., Tai C.Y., Kuo Y.H., Kao C.Y., Li J.J., Hwang T.L., Fang L.S., Wang W.H., Sheu J.H., Sung P.J. Cladieunicellins A–E, new eunicellins from an Indonesian soft coral Cladiella sp. Chem. Pharm. Bull. 2011;59:353–358. doi: 10.1248/cpb.59.353. [DOI] [PubMed] [Google Scholar]
- 54.Chen Y.H., Hwang T.L., Su Y.D., Chang Y.C., Chen Y.H., Hong P.H., Hu L.C., Yen W.H., Hsu H.Y., Huang S.J., Kuo Y.H., Sung P.J. New 6-hydroxyeunicellins from a soft coral Cladiella sp. Chem. Pharm. Bull. 2012;60:160–163. doi: 10.1248/cpb.60.160. [DOI] [PubMed] [Google Scholar]
- 55.Chen Y.H., Tai C.Y., Su Y.D., Chang Y.C., Lu M.C., Weng C.F., Su J.H., Hwang T.L., Wu Y.C., Sung P.J. Discovery of new eunicellins from an Indonesian octocoral Cladiella sp. Mar. Drugs. 2011;9:934–943. doi: 10.3390/md9060934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chen Y.H., Tai C.Y., Hwang T.L., Weng C.F., Li J.J., Fang L.S., Wang W.H., Wu Y.C., Sung P.J. Cladielloides A and B: New eunicellin-type diterpenoids from an Indonesian octocoral Cladiella sp. Mar. Drugs. 2010;8:2936–2945. doi: 10.3390/md8122936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wei W.C., Lin S.Y., Chen Y.J., Wen C.C., Huang C.Y., Palanisamy A., Yang N.S., Sheu J.H. Topical application of marine briarane-type diterpenes effectively inhibits 12-O-tetradecanoylphorbol-13-acetate-induced inflammation and dermatitis in murine skin. J. Biomed. Sci. 2011;18 doi: 10.1186/1423-0127-18-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sung P.J., Pai C.H., Hwang T.L., Fan T.Y., Su J.H., Chen J.J., Fang L.S., Wang W.H., Sheu J.H. Junceols D–H, new polyoxygenated briaranes from sea whip gorgonian coral Junceella juncea (Ellisellidae) Chem. Pharm. Bull. 2008;56:1276–1281. doi: 10.1248/cpb.56.1276. [DOI] [PubMed] [Google Scholar]
- 59.Su J.H., Chen B.Y., Hwang T.L., Chen Y.H., Huang I.C., Lin M.R., Chen J.J., Fang L.S., Wang W.H., Li J.J., et al. Excavatoids L–N, new 12-hydroxybriaranes from the cultured octocoral Briareum excavatum (Briareidae) Chem. Pharm. Bull. 2010;58:662–665. doi: 10.1248/cpb.58.662. [DOI] [PubMed] [Google Scholar]
- 60.Hong P.H., Su Y.D., Su J.H., Chen Y.H., Hwang T.L., Weng C.F., Lee C.H., Wen Z.H., Sheu J.H., Lin N.C., et al. Briarenolides F and G, new briarane diterpenoids from a Briareum sp. octocoral. Mar. Drugs. 2012;10:1156–1168. doi: 10.3390/md10051156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wang S.H., Chang Y.C., Chiang M.Y., Chen Y.H., Hwang T.L., Weng C.F., Sung P.J. Chlorinated briarane diterpenoids from the sea whip gorgonian corals Junceella fragilis and Ellisella robusta (Ellisellidae) Chem. Pharm. Bull. 2010;58:928–933. doi: 10.1248/cpb.58.928. [DOI] [PubMed] [Google Scholar]
- 62.Sung P.J., Lin M.R., Hwang T.L., Fan T.Y., Su W.C., Ho C.C., Fang L.S., Wang W.H. Briaexcavatins M–P, four new briarane-related diterpenoids from cultured octocoral Briareum excavatum (Briareidae) Chem. Pharm. Bull. 2008;56:930–935. doi: 10.1248/cpb.56.930. [DOI] [PubMed] [Google Scholar]
- 63.Liaw C.C., Kuo Y.H., Lin Y.S., Hwang T.L., Shen Y.C. Frajunolides L–O, four new 8-hydroxybriarane diterpenoids from the gorgonian Junceella fragilis. Mar. Drugs. 2011;9:1477–1486. doi: 10.3390/md9091477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chang J.Y., Liaw C.C., Fazary A.E., Hwang T.L., Shen Y.C. New Briarane diterpenoids from the gorgonian coral Junceella juncea. Mar. Drugs. 2012;10:1321–1330. doi: 10.3390/md10061321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Liaw C.C., Shen Y.C., Lin Y.S., Hwang T.L., Kuo Y.H., Khalil A.T. Frajunolides E–K, briarane diterpenes from Junceella fragilis. J. Nat. Prod. 2008;71:1551–1556. doi: 10.1021/np800126f. [DOI] [PubMed] [Google Scholar]
- 66.Cheng S.Y., Lin E.H., Wen Z.H., Chiang M.Y., Duh C.Y. Two new verticillane-type diterpenoids from the Formosan soft coral Cespitularia hypotentaculata. Chem. Pharm. Bull. 2010;58:848–851. doi: 10.1248/cpb.58.848. [DOI] [PubMed] [Google Scholar]
- 67.Chang J.Y., Fazary A.E., Lin Y.C., Hwang T.L., Shen Y.C. New verticillane diterpenoids from Cespitularia taeniata. Chem. Biodivers. 2012;9:654–661. doi: 10.1002/cbdv.201100122. [DOI] [PubMed] [Google Scholar]
- 68.Cheng S.Y., Chuang C.T., Wen Z.H., Wang S.K., Chiou S.F., Hsu C.H., Dai C.F., Duh C.Y. Bioactive norditerpenoids from the soft coral Sinularia gyrosa. Bioorg. Med. Chem. 2010;18:3379–3386. doi: 10.1016/j.bmc.2010.04.012. [DOI] [PubMed] [Google Scholar]
- 69.Lin Y.C., Abd El-Razek M.H., Hwang T.L., Chiang M.Y., Kuo Y.H., Dai C.F., Shen Y.C. Asterolaurins A–F, xenicane diterpenoids from the Taiwanese soft coral Asterospicularia laurae. J. Nat. Prod. 2009;72:1911–1916. doi: 10.1021/np900231e. [DOI] [PubMed] [Google Scholar]
- 70.Cheng S.Y., Chuang C.T., Wang S.K., Wen Z.H., Chiou S.F., Hsu C.H., Dai C.F., Duh C.Y. Antiviral and anti-inflammatory diterpenoids from the soft coral Sinularia gyrosa. J. Nat. Prod. 2010;73:1184–1187. doi: 10.1021/np100185a. [DOI] [PubMed] [Google Scholar]
- 71.Chung H.M., Hu L.C., Yen W.H., Su J.H., Lu M.C., Hwang T.L., Wang W.H., Sung P.J. Echinohalimane A, a bioactive halimane-type diterpenoid from a Formosan gorgonian Echinomuricea sp. (Plexauridae) Mar. Drugs. 2012;10:2246–2253. doi: 10.3390/md10102246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cheng C.H., Chung H.M., Hwang T.L., Lu M.C., Wen Z.H., Kuo Y.H., Wang W.H., Sung P.J. Echinoclerodane A: A new bioactive clerodane-type diterpenoid from a gorgonian coral Echinomuricea sp. Molecules. 2012;17:9443–9450. doi: 10.3390/molecules17089443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chung H.M., Hong P.H., Su J.H., Hwang T.L., Lu M.C., Fang L.S., Wu Y.C., Li J.J., Chen J.J., Wang W.H., et al. Bioactive compounds from a gorgonian coral Echinomuricea sp. (Plexauridae) Mar. Drugs. 2012;10:1169–1179. doi: 10.3390/md10051169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chang C.H., Wen Z.H., Wang S.K., Duh C.Y. New anti-inflammatory steroids from the Formosan soft coral Clavularia viridis. Steroids. 2008;73:562–567. doi: 10.1016/j.steroids.2008.01.007. [DOI] [PubMed] [Google Scholar]
- 75.Chao C.H., Wen Z.H., Chen I.M., Su J.H., Huang H.C., Chiang M.Y., Sheu J.H. Anti-inflammatory steroids from the octocoral Dendronephthya griffini. Tetrahedron. 2008;64:3554–3560. doi: 10.1016/j.tet.2008.01.109. [DOI] [PubMed] [Google Scholar]
- 76.Huang Y.C., Wen Z.H., Wang S.K., Hsu C.H., Duh C.Y. New anti-inflammatory 4-methylated steroids from the Formosan soft coral Nephthea chabroli. Steroids. 2008;73:1181–1186. doi: 10.1016/j.steroids.2008.05.007. [DOI] [PubMed] [Google Scholar]
- 77.Chao C.H., Wen Z.H., Su J.H., Chen I.M., Huang H.C., Dai C.F., Sheu J.H. Further study on anti-inflammatory oxygenated steroids from the octocoral Dendronephthya griffini. Steroids. 2008;73:1353–1358. doi: 10.1016/j.steroids.2008.06.008. [DOI] [PubMed] [Google Scholar]
- 78.Su J.H., Lo C.L., Lu Y., Wen Z.H., Huang C.Y., Dai C.F., Sheu J.H. Anti-Inflammatory polyoxygenated steroids from the soft coral Sinularia sp. Bull. Chem. Soc. Jpn. 2008;81:1616–1620. doi: 10.1246/bcsj.81.1616. [DOI] [Google Scholar]
- 79.Cheng S.Y., Wen Z.H., Wang S.K., Chiang M.Y., El-Gamal A.A., Dai C.F., Duh C.Y. Revision of the absolute configuration at C(23) of lanostanoids and isolation of secondary metabolites from Formosan soft coral Nephthea erecta. Chem. Biodivers. 2009;6:86–95. doi: 10.1002/cbdv.200800015. [DOI] [PubMed] [Google Scholar]
- 80.Cheng S.Y., Huang Y.C., Wen Z.H., Hsu C.H., Wang S.K., Dai C.F., Duh C.Y. New 19-oxygenated and 4-methylated steroids from the Formosan soft coral Nephthea chabroli. Steroids. 2009;74:543–547. doi: 10.1016/j.steroids.2009.02.004. [DOI] [PubMed] [Google Scholar]
- 81.Chao C.H., Chou K.J., Wen Z.H., Wang G.H., Wu Y.C., Dai C.F., Sheu J.H. Paraminabeolides A–F, cytotoxic and anti-inflammatory marine withanolides from the soft coral Paraminabea acronocephala. J. Nat. Prod. 2011;74:1132–1141. doi: 10.1021/np2000705. [DOI] [PubMed] [Google Scholar]
- 82.Chen B.W., Chang S.M., Huang C.Y., Su J.H., Wen Z.H., Wu Y.C., Sheu J.H. Hirsutosterols A–G, polyoxygenated steroids from a Formosan soft coral Cladiella hirsuta. Org. Biomol. Chem. 2011;9:3272–3278. doi: 10.1039/c1ob05106g. [DOI] [PubMed] [Google Scholar]
- 83.Chao C.H., Chou K.J., Huang C.Y., Wen Z.H., Hsu C.H., Wu Y.C., Dai C.F., Sheu J.H. Steroids from the soft coral Sinularia crassa. Mar. Drugs. 2012;10:439–450. doi: 10.3390/md10020439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Huang C.Y., Su J.H., Duh C.Y., Chen B.W., Wen Z.H., Kuo Y.H., Sheu J.H. A new 9,11-secosterol from the soft coral Sinularia granosa. Bioorg. Med. Chem. Lett. 2012;22:4373–4376. doi: 10.1016/j.bmcl.2012.05.002. [DOI] [PubMed] [Google Scholar]
- 85.Liu C.Y., Hwang T.L., Lin M.R., Chen Y.H., Chang Y.C., Fang L.S., Wang W.H., Wu Y.C., Sung P.J. Carijoside A, a bioactive sterol glycoside from an octocoral Carijoa sp. (Clavulariidae) Mar. Drugs. 2010;8:2014–2020. doi: 10.3390/md8072014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Cheng S.Y., Wen Z.H., Chiou S.F., Tsai C.W., Wang S.K., Hsu C.H., Dai C.F., Chiang M.Y., Wang W.H., Duh C.Y. Ceramide and cerebrosides from the octocoral Sarcophyton ehrenbergi. J. Nat. Prod. 2009;72:465–468. doi: 10.1021/np800362g. [DOI] [PubMed] [Google Scholar]
- 87.Cheng S.Y., Huang K.J., Wang S.K., Wen Z.H., Chen P.W., Duh C.Y. Antiviral and anti-inflammatory metabolites from the soft coral Sinularia capillosa. J. Nat. Prod. 2010;73:771–775. doi: 10.1021/np9008078. [DOI] [PubMed] [Google Scholar]
- 88.Wen Z.H., Chao C.H., Wu M.H., Sheu J.H. A neuroprotective sulfone of marine origin and the in vivo anti-inflammatory activity of an analogue. Eur. J. Med. Chem. 2010;45:5998–6004. doi: 10.1016/j.ejmech.2010.09.067. [DOI] [PubMed] [Google Scholar]
- 89.Kitamura Y., Kosaka T., Kakimura J.I., Matsuoka Y., Kohno Y., Nomura Y., Taniguchi T. Protective effects of the antiparkinsonian drugs talipexole and pramipexole against 1-methyl-4-phenylpyridinium-induced apoptotic death in human neuroblastoma SH-SY5Y cells. Mol. Pharmacol. 1998;54:1046–1054. [PubMed] [Google Scholar]
- 90.Blum D., Torch S., Lambeng N., Nissou M., Benabid A.L., Sadoul R., Verna J.M. Molecular pathways involved in the neurotoxicity of 6-OHDA, dopamine and MPTP: Contribution to the apoptotic theory in Parkinson’s disease. Prog. Neurobiol. 2001;65:137–172. doi: 10.1016/s0301-0082(01)00003-x. [DOI] [PubMed] [Google Scholar]
- 91.Sung P.J., Chen B.Y., Lin M.R., Hwang T.L., Wang W.H., Sheu J.H., Wu Y.C. Excavatoids E and F: Discovery of two new briaranes from the cultured octocoral Briareum excavatum. Mar. Drugs. 2009;7:472–482. doi: 10.3390/md7030472. [DOI] [PMC free article] [PubMed] [Google Scholar]