Abstract
Transplantation-related mortality (TRM) is high after HLA-mismatched umbilical cord blood (UCB) transplantation (UCBT). In utero, exposure to noninherited maternal antigen (NIMA) is recognized by the fetus, which induces Tregulator cells to that haplotype. It is plausible that UCBTs in which recipients are matched to donor NIMAs may alleviate some of the excess mortality associated with this treatment. To explore this concept, we used marginal matched-pair Cox regression analysis to compare outcomes in 48 NIMA-matched UCBTs (ie, the NIMA of the donor UCB unit matched to the patient) and in 116 non–NIMA-matched UCBTs. All patients had a hematologic malignancy and received a single UCB unit. Cases and controls were matched on age, disease, disease status, transplantation-conditioning regimen, HLA match, and infused cell dose. TRM was lower after NIMA-matched UCBTs compared with NIMA-mismatched UCBTs (relative risk, 0.48; P=.05; 18% versus 32% at 5 years posttransplantation). Consequently, overall survival was higher after NIMA-matched UCBT. The 5-year probability of overall survival was 55% after NIMA-matched UCBTs versus 38% after NIMA-mismatched UCBTs (P=.04). When faced with the choice of multiple HLA-mismatched UCB units containing adequate cell doses, selecting an NIMA-matched UCB unit may improve survival after mismatched UCBT.
Keywords: Permissive match, Regulatory T cells, Fetal immune response
INTRODUCTION
Umbilical cord blood (UCB) is an acceptable graft choice when considering unrelated donor transplantation for patients with hematologic malignancies. UCB grafts are used for approximately 20% of unrelated donor transplantations for hematologic malignancies in the United States and approximately 12% in Europe. We and others have reported similar leukemia-free survival despite higher transplantation-related mortality (TRM) after UCB transplantation (UCBT) compared with transplantation of HLA-matched bone marrow or peripheral blood progenitor cells from unrelated adult donors in children and adults with leukemia [1,2].
High TRM after UCBT remains a significant limitation and can be attributed to multiple factors. Some of the excess TRM after UCBT results from infusion of units containing relatively low total nucleated cell (TNC) doses. The accepted standard now is to use a UCB unit containing a minimum precryopreserved TNC of 3 × 107/kg patient body weight, with some recommending an incremental increase in TNC to overcome the HLA barrier [3,4]. When such a UCB unit is not available, the coinfusion of 2 unmanipulated UCB units is used to deliver higher TNC doses [4,5]. Infusion of expanded hematopoietic progenitor cells with a single UCB unit is also used to deliver higher TNC doses [6,7]. Avoiding UCB units with donor-specific anti-HLA antibodies present in the recipient decreases the risk of graft failure and mortality [8–10]. The importance of better donor–recipient HLA matching for unrelated adult donor transplantation is clear [11]. The best results are obtained with an unrelated adult donor allele-matched to the recipient at HLA-A, -B, -C, and -DRB1. Matching the UCB unit to the recipient at the HLA-C locus is associated with lower TRM [12]. The role of allelic HLA matching at HLA-A, -B, and -C remains to be determined in the setting of UCBT.
Two independent clinical studies conducted a decade apart [13,14] found tolerance to noninherited maternal antigens (NIMAs) in renal transplant recipients, implying that fetal exposure to NIMAs may promote lasting tolerance in humans. As the fetal immune system develops, T cells develop tolerance to self-antigens and recognize and react against foreign antigens. The placental circulation permits the crossing of maternal cells to the fetus and vice versa. Mold et al. [15] recently reported that the human fetal immune system generates regulatory T cells (CD4+CD25highFoxP3+ Tregs) that suppress fetal immune responses to maternal antigens, and that this tolerance persists at least until early adulthood. In a recent study reported by the New York Blood Center, HLA-mismatched UCBTs in which the mismatched antigen in the recipient matched the NIMA of the UCB donor (NIMA-matched transplantation) were associated with greater neutrophil recovery and lower mortality [16]. However, in another recent report from the same group, NIMA-matched UCBTs were not associated with TRM or overall mortality, even though both analyses were performed on largely the same cohort of donor–recipient pairs [17]. Given that the majority of UCBTs are mismatched and TRM presents a barrier to successful outcome, the present analysis was undertaken in an independent cohort of patients to determine whether matching the recipient to the UCB unit’s noninherited maternal antigen (ie, NIMA) [16] would indeed decrease the mortality associated with mismatched UCBT.
METHODS
Data Collection
This study included patients reported to Eurocord-European Group for Blood and Marrow Transplantation and the Center for International Blood and Marrow Transplant Research. Eligibility criteria included available data on UCB unit HLA typing, UCB donor maternal HLA typing, or maternal sample and recipient HLA typing. Seven Netcord banks in Europe and 10 cord blood banks in the U.S. National Marrow Donor Program (NMDP) network provided the UCB units. Data for UCBTs in Europe were obtained from Eurocord, and data for UCBTs in the United States were obtained from the Center for International Blood and Marrow Transplant Research. All patients received a single unrelated UCB unit for treatment of leukemia, lymphoma, or myelodysplastic syndrome. Patients who received UCB units matched at HLA-A, -B, and -DRB1, coinfusion of 2 units, or expanded units were excluded. All patients (or their guardians) provided written consent for research. The Institutional Review Boards of the Medical College of Wisconsin, the Eurocord-Netcord Scientific Committee, and the NMDP approved this study.
HLA Typing and Match Assignment
Donor, donor maternal, and recipient HLA typing were considered matching at HLA-A, -B, and -DRB1. Donor–recipient match grades were assigned considering HLA-A and -B at intermediate resolution (antigen level) and HLA-DRB1 at high resolution (allele level). For UCBTs facilitated by the Netcord banks, maternal HLA typing data were available from the banks. For UCBTs facilitated by the NMDP, maternal HLA typing was obtained from the banks when available, or HLA typing of banked maternal samples was performed at a centralized laboratory using DNA-based methods. Maternal HLA typing was scored at intermediate resolution (antigen level) for HLA-A and -B and at high resolution (allele level) for HLA-DRB1. UCBTs were classified as NIMA-matched or NIMA-mismatched based on review of recipient, donor, and donor maternal HLA typing at HLA-A, -B, and -DRB1. In an NIMA-matched UCBT, the mismatched antigen of the recipient was matched to the noninherited maternal antigen of the UCB donor. In contrast, in NIMA-mismatched UCBT, the mismatched antigen of the recipient was not matched to the noninherited maternal antigen of the UCB donor. Examples of NIMA-matched and NIMA-mismatched UCBTs are provided in Table 1.
Table 1.
Examples of NIMA Matching and NIMA Mismatching in the Setting of a Single Locus Mismatched Umbilical Cord Blood Transplant
| HLA-A | HLA-B | HLA-DRB1 | |
|---|---|---|---|
| NIMA matched* | |||
| UCB unit/donor | A*02, 32 | B*18, 35 | DRB1*01:01, 11:04 |
| UCB donor mother | A*24, 32 | B*07, 35 | DRB1*01:01, 13:01 |
| Recipient | A*02, 24 | B*18, 35 | DRB1*01:01, 11:04 |
| NIMA mismatched† | |||
| UCB unit/donor | A*02, 32 | B*18, 35 | DRB1*01:01, 11:04 |
| UCB donor mother | A*24, 32 | B*07, 35 | DRB1*01:01, 13:01 |
| Recipient | A*01, 02 | B*18, 35 | DRB1*01:01, 11:04 |
HLA-A*24 is not carried by UCB donor. HLA-A*24 is carried by the UCB donor’s mother and the recipient; thus, this is an NIMA-matched UCBT.
HLA-A*01 is not carried by UCB donor or the UCB donor’s mother; thus, this is an NIMA-mismatched UCBT.
Outcomes
TRM was defined as the time from transplantation to death not related to disease recurrence or progression. Overall mortality was defined as death from any cause. Neutrophil recovery was defined as achieving an absolute neutrophil count ≥0.5 × 109/L for 3 consecutive measurements on different days. Grade II-IV acute [18] and chronic [19] graft-versus-host disease (GVHD) was based on reports using standard criteria from each transplantation center. Disease recurrence was based on morphological evaluation, supported by the reappearance of abnormalities in cytogenetic or molecular analyses.
Statistical Methods
The probabilities of TRM, recurrent disease, neutrophil recovery, and acute and chronic GVHD were calculated using the cumulative incidence estimator to accommodate competing risks [20]. The probability of overall survival was calculated using the Kaplan-Meier estimator [20]; 95% confidence intervals (CIs) were calculated with log-transformation.
To assess the association between clinical outcomes and NIMA matching, cases (NIMA matched) were matched to controls (NIMA mismatched). A matched-pair analysis was considered appropriate given the relatively low frequency of NIMA-matched transplantations (8.5%). Before matching cases to controls, we built a multivariate Cox regression model for TRM using patients who met the study eligibility criteria (n = 508) [21]. Results are expressed as relative risk (RR). The characteristics of this cohort are presented in Supplemental Table 1. Fifty-two donor–recipient pairs were NIMA-matched, and 456 donor–recipient pairs were NIMA-mismatched. We aimed to identify variables other than NIMA matching with a significant effect on TRM: patient age, donor–recipient HLA match, disease status at transplantation, and transplantation conditioning regimen were significantly associated with TRM (Supplemental Table 2). Cases were matched to controls for patient age, HLA match, disease status, conditioning regimen, and 2 other variables (disease type and total nucleated cell dose [TNC] ≤3 × 107/kg versus >3 × 107/kg), known to be frequently associated with UCBT outcomes.
The final study population included 48 NIMA-matched and 116 NIMA-mismatched transplant recipients. Nineteen cases were matched to 76 controls (1:4), 1 case was matched to 3 controls (1:3), 9 cases were matched to 18 controls (1:2), and 19 cases were matched to 19 controls (1:1). To assess the association between clinical outcomes and NIMA matching status, using matched pairs, we built marginal Cox regression models for neutrophil recovery, acute and chronic GVHD, TRM, disease recurrence, and overall mortality [21]. Models were built with the forward stepwise selection procedure and confirmed with the use of the backward selection procedure. All variables met the proportional hazards assumption. All P values are 2-sided, with values of ≤.05 considered statistically significant. All analyses were performed using SAS 9.2 (SAS Institute, Cary, NC).
The frequencies of NIMA-matched and NIMA-mismatched antigens in U.S. transplantations were evaluated by comparing the average antigen (HLA-A and -B) and allele (HLA-DRB1) frequencies within the overall population. HLA frequencies for the U.S. donor population provided by the NMDP were used as a reference. NIMA-matched and NIMA-mismatched antigens/alleles were aggregated by locus, and average frequency was compared between the NIMA-matched and -mismatched groups using 2-sided t tests. Analyses were performed for the overall population and the Caucasian subset.
RESULTS
Patient, Disease, and Transplant Characteristics
Characteristics of cases and controls are shown in Table 2. Some 75% of patients were age 16 years or younger at the time of transplantation; 52% were male, and 46% were cytomegalovirus-seropositive. Acute leukemia was the most common indication for transplantation, and 74% of transplantations occurred in remission. A total body irradiation–containing myeloablative conditioning regimen was used in 82% of transplantations, and cyclosporine alone or in combination with steroids, methotrexate, or mycophenolate mofetil for GVHD prophylaxis was used in 86%. Thirty-five percent of transplantations were mismatched at 1 HLA locus, and the remainder were matched at 2 HLA loci. All UCBTs were performed between 2002 and 2009; 50% of the NIMA-matched and 42% of NIMA-mismatched transplantations were performed between 2002 and 2005, and the remainder were done between 2006 and 2009. Three-quarters of recipients received >3 × 107/kg TNCs. The median follow-up of surviving patients was 42 months (range, 3–103 months) after NIMA-matched UCBT and 36 months (range, 3–93 months) after NIMA-mismatched UCBT.
Table 2.
Patient, Disease, and Transplant Characteristics of the Study Population
| Variable | NIMA-Mismatched UCBT, n (%) | NIMA-Matched UCBT, n (%) |
|---|---|---|
| Number | 116 | 48 |
| Region | ||
| Europe | 37 (32) | 27 (56) |
| United States | 79 (68) | 21 (44) |
| Age | ||
| ≤16 years | 91 (78) | 30 (62) |
| >16 years | 25 (22) | 18 (38) |
| Disease | ||
| Acute myelogenous leukemia | 48 (41) | 21 (44) |
| Acute lymphoblastic leukemia | 47 (41) | 18 (38) |
| Chronic myelogenous leukemia | 1 (1) | 1 (2) |
| Myelodysplastic syndrome | 10 (9) | 4 (8) |
| Other acute leukemias | 8 (7) | 3 (6) |
| Non-Hodgkin lymphoma | 2 (2) | 1 (2) |
| Disease status | ||
| First complete remission/chronic phase | 31 (27) | 12 (25) |
| Second complete remission/chronic phase/accelerated phase | 56 (48) | 22 (46) |
| Relapse, refractory anemia with excess blasts | 29 (25) | 14 (29) |
| Conditioning regimen | ||
| Myeloablative | ||
| Total body irradiation–containing | 58 (50) | 19 (40) |
| Non–irradiation-containing | 43 (37) | 15 (31) |
| Reduced intensity | ||
| Total body irradiation–containing | 13 (11) | 10 (20) |
| Non–irradiation-containing | 2 (1) | 4 (8) |
| Infused total nucleated cell dose | ||
| ≤3 × 107/kg recipient body weight | 25 (22) | 16 (33) |
| >3 × 107/kg recipient body weight | 91 (78) | 32 (67) |
| GVHD prophylaxis | ||
| Cyclosporine alone or with steroids | 56 (48) | 23 (48) |
| Cyclosporine + methotrexate | 12 (10) | 5 (10) |
| Cyclosporine + mycophenolate mofetil | 31 (27) | 14 (29) |
| Tacrolimus + methotrexate | 7 (6) | 3 (6) |
| Tacrolimus + mycophenolate mofetil | 5 (4) | 1 (2) |
| Tacrolimus alone | 5 (4) | 2 (4) |
| Donor–recipient HLA match | ||
| 5/6 HLA match | 43 (36) | 14 (27) |
| 4/6 HLA match | 73 (63) | 34 (71) |
Neutrophil Recovery
Neutrophil recovery was similar after NIMA-matched and NIMA-mismatched transplantations (relative risk [RR], 1.18; 95% CI, 0.80–1.74; P = .42). The median time to recovery was 20 days after transplantation of NIMA-matched UCB units and 23 days after transplantation of NIMA-mismatched units. The corresponding day 28 probabilities of recovery were 71% (95% CI, 57%–81%) and 59% (95% CI, 50%–67%).
Acute and Chronic GVHD
Risks of grade II-IV acute GVHD (RR, 0.94; 95% CI, 0.56–1.59; P = .82) and chronic GVHD (RR, 0.85; 95% CI, 0.44–1.63; P = .61) were not different after NIMA-matched and NIMA-mismatched transplantations. The day 100 probabilities of grade II-IV acute GVHD were 40% (95% CI, 26%–53%) after NIMA-matched transplantations and 46% (95% CI, 37%–54%) after NIMA-mismatched transplantations. The corresponding 5-year probabilities of chronic GVHD were 26% (95% CI, 15%–39%) and 27% (95% CI, 19%–35%).
TRM and Overall Mortality
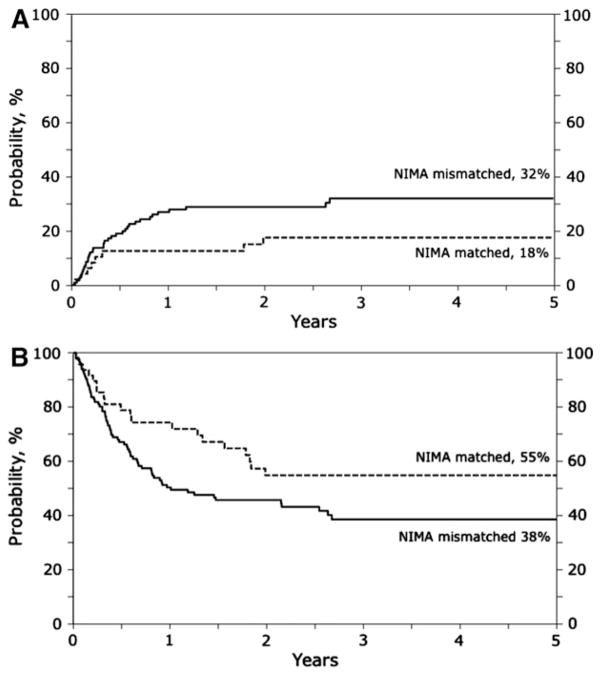
The risk of TRM was lower after NIMA-matched UCBT compared with NIMA-mismatched UCBT (RR, 0.48; 95% CI, 0.23–1.01; P = .05) (Figure 1A). Similarly, overall mortality risk was also lower after NIMA-matched UCBT (RR, 0.61; 95% CI, 0.38–0.98; P = .04) (Figure 1B). Data on infections that occurred in the first 100 days after transplantation were available for 113 of 164 (69%) transplants. The day 30 cumulative incidence of infections was 15% after NIMA-matched UCBT and 27% after NIMA-mismatched USBT (P = .24). The corresponding day 100 cumulative incidence rates were 48% and 50%. Six of 20 deaths (30%) after NIMA-matched UCBT were attributed to TRM. Four of these 6 deaths occurred within 6 months after transplantation (2 due to multiorgan failure, 1 due to infection, and 1 due to hemorrhage). Two deaths occurred beyond 6 months (1 from infection and the other from chronic GVHD). Thirty-one of 66 deaths (47%) after NIMA-mismatched transplantations were attributed to TRM. Twenty-three of the 31 deaths occurred within 6 months of transplantation, with 6 due to multiorgan failure, 8 due to infection, 4 due to adult respiratory distress syndrome/interstitial pneumonitis, 2 due to diffuse alveolar hemorrhage, and 1 due to Epstein-Barr virus posttransplantation lymphoproliferative disease; cause of death was not reported for 2 patients. Eight deaths occurred beyond 6 months, including 2 due to chronic GVHD, 3 due to infection, and 2 due to multiorgan failure; cause of death was not reported for 1 patient.
Figure 1.
(A) The 5-year probabilities of transplant-related mortality were 18% (95% CI, 8%–29%) after NIMA-matched transplantation and 32% (95% CI, 23%–41%) after NIMA-mismatched transplantation. (B) The 5-year probabilities of overall survival were 55% (95% CI, 40%–69%) after NIMA-matched transplantation and 38% (95% CI, 29%–48%) after NIMA-mismatched transplantation.
Relapse
The relapse risk was similar after NIMA-matched and NIMA-mismatched UCBTs (RR, 0.82; 95% CI, 0.47–1.43; P=.47). The 5-year probabilities of relapse were 31% (95% CI, 18%–44%) after NIMA-matched transplantations and 33% (95% CI, 24%–42%) after NIMA-mismatched transplantations.
Influence of Antigen Frequency on NIMA Matching
The frequencies of NIMA-matched and NIMA-mismatched antigens/alleles were evaluated for the U.S. cohort (n = 429). Transplantations in Europe were excluded because information on the race of UCB and recipients was not always available. The antigen (HLA-A and -B) and allele (HLA-DRB1) frequencies recorded in the NMDP donor registry served as the reference values for the study population and were adjusted based on subject race. Overall, NIMA-matched antigens/alleles had higher population-based frequencies than NIMA-mismatched antigens/alleles (0.110 versus 0.052; P<.001). The NIMA matches were all associated with relatively common HLA antigens (frequencies >0.058), whereas the NIMA-mismatched antigens were observed across common and uncommon HLA antigens. The most frequent NIMA match (n=6; 22%)was at HLA-A*02, which is also the most common antigen in the U.S. Caucasian population, with a frequency of 0.308 [22]. To ensure that HLA-A*02 was not inordinately influencing these results, we repeated the analysis and restricted the population to non–HLA-A*02 mismatches. Consistent with the main analysis, the frequency of non–HLA-A*02 NIMA-matched antigens was higher than that of NIMA-mismatched antigens (0.107 versus 0.054; P = .008).
DISCUSSION
The primary objective of the present study was to assess the effect of tolerance to NIMA and its effect on mortality after HLA-mismatched UCBT. Although tolerance to NIMA in renal transplantation is well documented [13,14], tolerance to NIMA and its effect on survival after mismatched UCBT is by no means conclusive [16,17]. To circumvent the problems related to the relatively small sample of NIMA-matched transplantations, we used a matched-pair analysis, matching recipients for factors that influence TRM and overall survival, which allowed us to carefully control the analysis. We observed marginally lower TRM and overall mortality after NIMA-matched UCBT compared with NIMA-mismatched UCBT, consistent with the earlier report on the impact of NIMAs in UCBT [16]. We hypothesize that allowing for permissive mismatching between UCB units and recipient reduced some of the excess mortality associated with HLA-mismatched UCBT. The exact mechanism by which mortality is reduced is not easily explained, however. The higher survival after NIMA-matched UCBT was likely related to multiple factors, including better hematopoietic recovery, lower rates of acute GVHD and infections in the early posttransplantation period, and better immune reconstitution, which together contributed to the observed survival advantage.
In contrast to studies of haploidentical transplantations [23], we failed to see significant differences in rates of acute or chronic GVHD after NIMA-matched and NIMA-mismatched UCBTs. Rates of acute and chronic GVHD after UCBT are substantially lower than after haploidentical transplantations. Given that only ~10% of mismatched UCBTs are NIMA-matched, studies involving hundreds of patients are needed to definitively identify differences in GVHD rates after NIMA-matched and NIMA-mismatched transplantations.
Better HLA matching of donors and recipients is associated with better hematopoietic recovery and thus lower early mortality. In this analysis, we found a 12% difference in the probability of neutrophil recovery after NIMA-matched and NIMA-mismatched transplantations. Our inability to detect a statistically significant difference can be explained by the small sample size and the ensuing wide confidence intervals of probability estimates. Furthermore, the use of UCB units with relatively high TNC (>3 × 107/kg) also might have diminished the importance of NIMA matching for neutrophil recovery, as has been shown to be the case with HLA-mismatched UCBTs [3,24]. Consistent with our findings, studies of tolerance to NIMAs after haploidentical transplantations in which very high cell doses were used also have failed to show an association between NIMA matching and neutrophil recovery [23,25]. Of note, most deaths from transplantation-related complications occurred within 6 months after transplantation. There may be differences in immune reconstitution after NIMA-matched and NIMA-mismatched transplantations, but we cannot test this hypothesis in our study population. We used data reported to transplantation registries, in which information on immune reconstitution was not available.
NIMA-matched transplants account for less than 10% of UCBTs. Incorporating NIMA matching in an algorithm for UCB unit selection is complex and logistically challenging. Given that maternal HLA typing data are not available for banked UCB units, NIMA-matched transplantation is more likely to occur randomly than by choice. However, approximately one-third of the cord banks in Netcord/NMDP routinely perform UCB unit maternal HLA typing, and the availability of maternal and UCB unit HLA typing data will allow physicians select UCB units that are NIMA matched to the recipient. Furthermore, based on our observations of the HLA types within the U.S. study cohort, NIMA matching is correlated with the frequency of mismatched antigens in the U.S. population. Therefore, searching for an NIMA-matched UCB unit is best facilitated by selecting a mismatched UCB unit in which the mismatch is a high-frequency HLA-antigen within the target population, such as HLA-A*02 in Caucasians [21]. However, truly understanding the probability of finding an NIMA match within a given population will require either complex mathematical models based on HLA haplotype frequencies or the addition of maternal HLA typing to UCB unit registries. Consultation with an HLA expert during the search may allow a physician to apply the surrogate approach described here to identify a potential NIMA-matched UCB unit and request donor maternal HLA typing at the time of confirmatory HLA typing of the UCB unit. The additional request for maternal HLA typing by those banks that do not routinely perform maternal HLA typing will add to their financial burden.
Taken together, our current analysis and the earlier report [16] suggest that the use of UCB units in which the recipient is matched to the donor NIMA may ameliorate some of the excess mortality associated with HLA-mismatched UCBT. Both studies are limited by modest numbers of NIMA-matched transplantations, however. Although we performed a carefully controlled analysis that considered risk factors associated with higher mortality risk, several unknown and unmeasured factors also might have influenced survival after UCBT. Nevertheless, the marginal survival advantage associated with NIMA-matched transplantation cannot be ignored. Thus, when considering mismatched UCBT for hematologic malignancy, efforts to obtain donor maternal HLA typing from cord blood banks should be encouraged. Matching recipients to donor NIMA must be considered along with other known factors associated with lowering mortality risks.
Supplementary Material
Acknowledgments
Authorship Statement: Vanderson Rocha, Stephen Spellman, Mei-Jie Zhang, and Mary Eapen contributed equally to study design and interpretation of data. Vanderson Rocha and Mary Eapen had primary responsibility for drafting the manuscript. Mei-Jie Zhang performed the statistical analysis. Stephen Spellman reviewed maternal HLA typing, donor–recipient HLA typing, and assignment and contributed to interpretation of data and manuscript preparation. Annalisa Ruggeri, Duncan Purtill, Colleen Brady, Lee Ann Baxter-Lowe, Etienne Baudoux, Paola Bergamaschi, Robert Chow, Brian Freed, Gesine Koegler, Joanne Kurtzberg, Jerome Larghero, Lucilla Lecchi, Arnon Nagler, Cristina Navarette, Vinod Prasad, Fabienne Pouthier, Thomas Price, Voravit Ratanatharathorn, Jon J. van Rood, Mary M. Horowitz, and Eliane Gluckman contributed to interpretation of data and approved the final report.
Financial disclosure: Supported by a Public Health Service grant (U24-CA76518) from the National Cancer Institute, the National Heart Lung and Blood Institute, and the National Institute of Allergy and Infectious Diseases; the Health Resources and Services Administration (grant HHSH234200637015C); and the Office of Naval Research, Department of the Navy to the National Marrow Donor Program (N00014-10-01-0204). Mary Eapen is a Scholar in Clinical Research for the Leukemia and Lymphoma Society. Opinions, findings, and conclusions or recommendations expressed herein are those of the authors and do not reflect the views of the Office of Naval Research or the National Marrow Donor Program. The authors have no conflicts of interest to disclose.
Footnotes
Supplementary data related to this article can be found online at http://dx.doi.org/10.1016/j.bbmt.2012.07.010
References
- 1.Eapen M, Rubinstein P, Zhang M-J, et al. Outcomes of transplantation of unrelated donor umbilical cord blood and bone marrow in children with acute leukemia: a comparison study. Lancet. 2007;369:1947–1954. doi: 10.1016/S0140-6736(07)60915-5. [DOI] [PubMed] [Google Scholar]
- 2.Eapen M, Rocha V, Sanz G, et al. Effect of graft source on unrelated donor hematopoietic stem-cell transplantation in adults with acute leukemia: a retrospective analysis. Lancet Oncol. 2010;11:653–660. doi: 10.1016/S1470-2045(10)70127-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gluckman E, Ruggeri A, Volt F, et al. Milestones in umbilical cord blood transplantation. Br J Haematol. 2011;154:441–447. doi: 10.1111/j.1365-2141.2011.08598.x. [DOI] [PubMed] [Google Scholar]
- 4.Delaney C, Gutman JA, Appelbaum FR. Cord blood transplantation for haematological malignancies: conditioning regimens, double cord blood transplant and infectious complications. Br J Haematol. 2009;147:207–216. doi: 10.1111/j.1365-2141.2009.07782.x. [DOI] [PubMed] [Google Scholar]
- 5.Brunstein CG, Barker JN, Weisdorf DJ, et al. Umbilical cord blood transplantation after non-myeloablative conditioning regimen: impact on transplantation outcomes in 110 adults with hematologic diseases. Blood. 2007;110:3064–3070. doi: 10.1182/blood-2007-04-067215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de Lima M, McMannis J, Gee A, et al. Transplantation of ex vivo expanded cord blood cells using the copper chelator tetraethylene pentamie: a phase I/II clinical trial. Bone Marrow Transplant. 2008;41:771–778. doi: 10.1038/sj.bmt.1705979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Delaney C, Heimfeld S, Brashem-Stein C, et al. Notch-mediated expansion of human cord blood progenitor cells capable of rapid myeloid reconstitution. Nat Med. 2010;16:232–236. doi: 10.1038/nm.2080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Spellman S, Bray R, Rosen-Bronson S, et al. The detection of donor-directed HLA-specific alloantibodies in recipients of unrelated hematopoietic cell transplantation is predictive of graft failure. Blood. 2010;115:2704–2708. doi: 10.1182/blood-2009-09-244525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Takanashi M, Atsuta Y, Fujiwara K, et al. The impact of anti-HLA antibodies on unrelated cord blood transplantations. Blood. 2010;116:2839–2846. doi: 10.1182/blood-2009-10-249219. [DOI] [PubMed] [Google Scholar]
- 10.Cutler C, Kim HT, Sun L, et al. Donor-specific anti-HLA antibodies predict outcome in double umbilical cord blood transplantation. Blood. 2011;118:6691–6697. doi: 10.1182/blood-2011-05-355263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee SJ, Klein J, Haagenson M, et al. High-resolution donor–recipient HLA matching contributes to the success of unrelated donor transplantation. Blood. 2007;110:4576–4583. doi: 10.1182/blood-2007-06-097386. [DOI] [PubMed] [Google Scholar]
- 12.Eapen M, Klein JP, Sanz G, et al. Effect of donor–recipient HLA matching at HLA- A, -B, -C, and -DRB1 on outcomes after umbilical-cord blood transplantation for leukemia and myelodysplastic syndrome: a retrospective analysis. Lancet Oncol. 2011;12:1214–1221. doi: 10.1016/S1470-2045(11)70260-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Claas FH, Gijbels Y, van der Velden de Munck J, et al. Induction of B cell unresponsiveness to non-inherited maternal antigens during fetal life. Science. 1988;241:1815–1817. doi: 10.1126/science.3051377. [DOI] [PubMed] [Google Scholar]
- 14.Burlingham WJ, Grailer AP, Heisey DM, et al. The effect of tolerance to non-inherited maternal HLA antigens on the survival of renal transplants from sibling donors. N Engl J Med. 1998;339:1657–1664. doi: 10.1056/NEJM199812033392302. [DOI] [PubMed] [Google Scholar]
- 15.Mold JE, Michaelsson J, Burt TD, et al. Maternal alloantigens promote the development of tolerogenic fetal regulatory T cells in utero. Science. 2008;322:1562–1565. doi: 10.1126/science.1164511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.van Rood JJ, Stevens CE, Smits J, et al. Reexpsoure of cord blood to noninherited maternal HLA antigens improves transplant outcome in hematological malignancies. Proc Natl Acad Sci USA. 2009;106:19952–19957. doi: 10.1073/pnas.0910310106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.van Rood JJ, Scaradavou A, Stevens CE. Indirect evidence that maternal microchimerism in cord blood mediates a graft-versus-leukemia effect in cord blood transplantation. Proc Natl Acad Sci USA. 2012;109:2509–2514. doi: 10.1073/pnas.1119541109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Przepiorka D, Weisdorf D, Martin P, et al. 1994 Consensus conference on acute graft-versus-host disease grading. Bone Marrow Transplant. 1995;15:825–828. [PubMed] [Google Scholar]
- 19.Flowers ME, Kansu E, Sullivan KM. Pathophysiology and treatment of graft-versus-host disease. Hematol Oncol Clin North Am. 1999;13:1091–1112. doi: 10.1016/s0889-8588(05)70111-8. [DOI] [PubMed] [Google Scholar]
- 20.Klein JP, Moeschberger ML. Survival Analysis: Statistical Method for Censored and Truncated Data. 2. New York: Springer-Verlag; 2003. [Google Scholar]
- 21.Cox DR. Regression model and life tables. J R Stat Soc B. 1972;34:187–200. [Google Scholar]
- 22.Maiers M, Gargert L, Kitz W. High-resolution HLA alleles and haplotypes in the US population. Hum Immunol. 2007;68:779–788. doi: 10.1016/j.humimm.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 23.van Rood JJ, Loberiza FR, Zhang M-J, et al. Effect of tolerance to noninherited maternal antigens on the occurrence of graft-versus-host disease after bone marrow transplantation from a parent or an HLA-halpo identical sibling. Blood. 2002;99:1572–1577. doi: 10.1182/blood.v99.5.1572. [DOI] [PubMed] [Google Scholar]
- 24.Rocha V, Gluckman E. Improving outcomes of cord blood transplantation: HLA-matching, cell dose and other graft and transplantation-related factors. Br J Haematol. 2009;147:262–274. doi: 10.1111/j.1365-2141.2009.07883.x. [DOI] [PubMed] [Google Scholar]
- 25.Ichinohe T, Uchiyama T, Shimazaki C, et al. Feasibility of HLA-haploidentical hematopoietic stem cell transplantation between non-inherited maternal antigen (NIMA) mismatched family members linked with long-term fetomaternal microchimerism. Blood. 2004;104:3821–3828. doi: 10.1182/blood-2004-03-1212. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.