Interleukin-9 (IL-9) has emerged as a cytokine that can be produced across multiple T cell subsets. We have described that T cells expressing FoxP3, a signature transcription factor of regulatory T cells, and IL-17F, a signature cytokine of helper T (Th) 17 cells, can both produce IL-9 in addition to T cells stimulated with TGFβ and IL-4, which have been coined “Th9” cells.
The importance of TGFβ in eliciting IL-9 production by T cells was initially highlighted by Schmitt et al. in 1994. They also reported that the addition of TGFβ and IL-4 further enhanced IL-9 production while IFNγ inhibited it.1 This finding was re-examined by two groups who observed that these “Th9” cells did not express transcription factors associated with known T cell subsets, including T-bet, GATA-3, RORγt and FoxP3.2,3 However, studies from our laboratory suggest that the ability of T cells to produce IL-9 is not restricted to just T cells cultured with TGFβ and IL-4. Under conditions used to generate regulatory T cells (TGFβ and IL-2) both the FoxP3 positive and negative populations make IL-9. In a similar manner, under Th17 culture conditions (TGFβ, IL-6, anti-IL-4 and anti-IFNγ) both the IL-17F positive and negative populations can produce this cytokine as well.4 Together this suggests that IL-9 production is dependent on the presence of TGFβ during their priming and/or activation. Other factors can then further enhance this effect, such as IL-4,1 or inhibit this effect, such as retinoic acid in the context of regulatory T cells.5 Hence, IL-9 production is likely a signature of TGFβ action on T cells.
In vivo T cells produce IL-9 in both pro-inflammatory and anti-inflammatory immune environments. In the context of Th2-mediated responses observed in the lung and the gastrointestinal tract, the presence of IL-9 is associated with pro-inflammatory responses that contribute to disease pathology.6-8 However, there is still substantial controversy as to the role of IL-9 in mediating inflammation and suppression. In a model of murine multiple sclerosis (experimental autoimmune encephalomyelitis) IL-9 receptor deficiency resulted in decreased Th17 responses in the CNS and mast cell numbers in the lymph node.4 Using very similar methodologies, another group has shown that IL-9 receptor deficiency was associated with enhanced disease development, putatively due to the fact that regulatory T cells that are unresponsive to IL-9 signaling were less suppressive.9 At this time it is not possible to rectify the basis for the substantive differences between these two studies. However, one feature common throughout all of these models is that IL-9 is associated with the recruitment and/or accumulation of mast cells. As has been reviewed, mast cells have the potential to exert both pro-inflammatory and anti-inflammatory effects dependent on a myriad of factors.10 In this respect, it is not surprising that this same feature is a characteristic of IL-9.
More extensive in vivo studies are needed to substantiate whether “Th9” cells represent a unique T cell lineage or not. In part, this will occur as re-examination of what the definition of a T cell subset should be. Is it the presence of unique transcription factors or the ability of a lineage to maintain phenotype? Both regulatory T cells and Th17 cells are widely accepted as unique T cell subsets. Although both have known transcription factors associated with their differentiation and can be found during various immune responses in vivo, they also have been shown to have exhibit significant plasticity and under certain circumstances can dramatically change their phenotype. Current data obtained from in vitro differentiation of “Th9” cells suggest that their transcriptional profile does not match any of the currently accepted T cell subsets.2,3 However, more extensive characterization of these cells to determine if they can maintain their phenotype in vitro has yet to occur. In addition, in vivo examination of immune responses where TGFβ, IL-4 and IL-9 are likely to be present, such as models of Th2-driven asthma, may help to determine the extent to which this cell type may represent a T cell subset under physiological conditions.
Together, these results show that IL-9 production can be associated with multiple T cell lineages, and this effect is dependent on T cell responsiveness to TGFβ. It may then function as an autocrine factor for inflammatory T cells or regulatory T cells as well as a general recruitment and/or survival factor for mast cells to mediate inflammation or suppression (Fig. 1).
Figure 1.
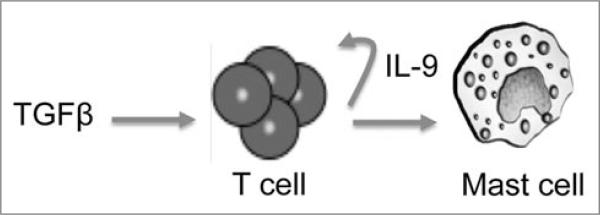
Proposed model of IL-9 production by T cells and its downstream effects. TGFβ induces the production of IL-9 by T cells, which can then act on both T cells and mast cells.
Acknowledgements
E.C.N. is supported by a postdoctoral fellowship from the National Multiple Sclerosis Society.
Abbreviations
- IL
interleukin
- Th
helper T
References
- 1.Schmitt E, et al. J Immunol. 1994;153:3989–96. [PubMed] [Google Scholar]
- 2.Dardalhon V, et al. Nat Immunol. 2008;9:1347–55. doi: 10.1038/ni.1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Veldhoen M, et al. Nat Immunol. 2008;9:1341–6. doi: 10.1038/ni.1659. [DOI] [PubMed] [Google Scholar]
- 4.Nowak EC, et al. J Exp Med. 2009;206:1653–60. doi: 10.1084/jem.20090246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hill JA, et al. Immunity. 2008;29:758–70. doi: 10.1016/j.immuni.2008.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Faulkner H, et al. Eur J Immunol. 1997;27:2536–40. doi: 10.1002/eji.1830271011. [DOI] [PubMed] [Google Scholar]
- 7.Forbes EE, et al. J Exp Med. 2008;205:897–913. doi: 10.1084/jem.20071046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Townsend JM, et al. Immunity. 2000;13:573–83. doi: 10.1016/s1074-7613(00)00056-x. [DOI] [PubMed] [Google Scholar]
- 9.Elyaman W, et al. Proc Natl Acad Sci USA. 2009;106:12885–90. doi: 10.1073/pnas.0812530106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sayed BA, et al. Annu Rev Immunol. 2008;26:705–39. doi: 10.1146/annurev.immunol.26.021607.090320. [DOI] [PubMed] [Google Scholar]


