Abstract
For the past 100 years, vitamin A has been implicated as an essential dietary component in host resistance to infectious disease. However, only recently have studies begun to elucidate the cellular and molecular mechanisms of how vitamin A regulates cell-mediated and humoral-mediated immunity. In this review, we present an overview of the recent discoveries of the role that vitamin A and its metabolite, retinoic acid (RA), play in the regulation of immune cells. How RA impacts on leukocyte growth, differentiation, and homing is discussed with special attention to inflammatory responses and solid tumor microenvironment.
Keywords: retinoic acid, vitamin A, inflammation
1. Introduction
Vitamin A from the diet provides the retinoids necessary for a variety of biological functions including embryonic development, vision, brain function, and many others. Retinol, the alcohol form of vitamin A, gives rise to the acid form retinoic acid (RA), which is the metabolically active form of vitamin A. All-trans- and 9-cis-RA are the potent regulators of gene expression and play an essential role in the modulation of cell proliferation and differentiation [1]. For more than four decades, the impact of vitamin A deficiency on immunity has been studied. These studies have repeatedly demonstrated the indispensable requirement of this natural product to maintain host defense to bacterial, viral, and protozoal diseases [2,3]. The findings for a role of vitamin A in the regulation of inflammatory T cells and adaptive regulatory T cells (aTreg) differentiation represent a significant advance in our understanding of the relationship of vitamin A and immunity. The current understanding as to how vitamin A impacts on the development of immune cells is discussed.
2. RA mediators
2.1. Enzymes
Vitamin A needs to be catabolized to different metabolites to exert its function. To do this, a group of enzymes, divided in three families, will act together to form the final compound RA. The first step is the conversion of vitamin A to retinal and is catalyzed by the alcohol dehydrogenase (ADH) family. ADHs have specificities for ethanol, retinoids, and other alcohols and aldehydes of physiological importance [4]. This step can also be regulated by the short-chain dehydrogenase/reductase family, which shows a wide affinity for alcohols and aldehydes [5]. Additionally, the aldehyde dehydrogenase (ALDH, also known as RALDH) family participates in the conversion of aldehydes to carboxylic acid compounds (acids) [6]. It has been shown that RALDH levels are regulated by vitamin A, such that in vitamin A-deficient animals, RALDH expression is greatly reduced [7]. As expression of RALDH has been found on immune cells, it is tempting to propose that RA may play a role in the development of immunity. One more critical element in RA biosynthesis is the major RA metabolizing enzyme, CYP26A, which has been very well established in developmental models to disrupt RA metabolism by promoting RA catabolism [8–10]. Little is known about how the expression of this enzyme may influence the development of immune responses.
2.2. Retinoid-binding proteins
Retinoids are not found free extracellularly; instead, they are associated with binding proteins for delivery to target tissue, usually over very short distances. The fact that RA acts only over a short distance and within a prescribed microenvironment is much like the activities of cytokines. Once in the cell, retinoids associate with cellular membranes or will be transported/stored coupled to intracellular proteins. There are three main families of retinoid-binding proteins: retinol-binding protein (RBP), cellular retinol (orretinal)-binding protein, and cellular retinoic acid-binding protein (CRABP). RBP binds to retinol and its protein synthesis takes place not only in the liver but also in heart, testis, eyes, spleen, and others. Because the liver stores vitamin A, the expression and release of RBP-retinol will depend on the availability of vitamin A. It has been demonstrated that under vitamin A deficiency, RBP expression is downregulated and its release to circulation inhibited [11].
2.3. RA nuclear receptors
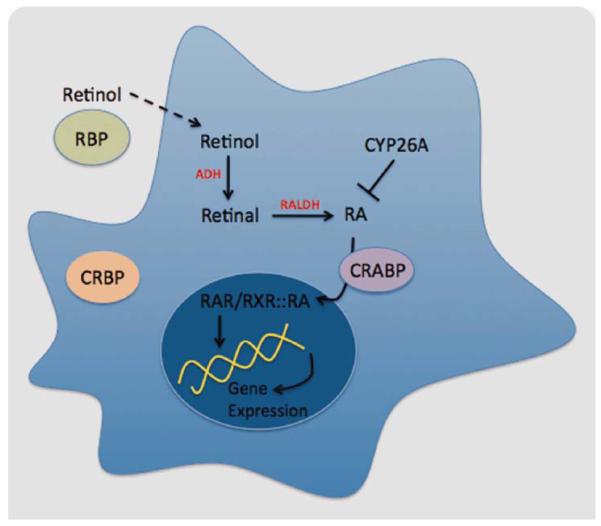
To regulate gene expression, all-trans- and 9-cis-RA bind to nuclear receptors, which act as ligand-induced transcription factors to bind to specific sequences in the DNA and modulate the transcription of target genes (Fig. 1). RA may bind to RA receptors (RARs) or to retinoic X receptors (RXRs), both of them belonging to the steroid/thyroid/vitamin D receptor super family.
Fig. 1.
RA metabolism. Retinol gives rise to RA through the activity of different families of enzymes, including RALDH, which catalyzes the last step in an irreversible manner. Once in the cytoplasm, RA binds to CRABP and is transferred to the nucleus, where it is recognized by the nuclear receptors (RAR/RXR), and it binds to the DNA to regulate gene expression. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
RAR family contains three members: RARα (isoforms a1-2), RARβ (isoforms b1-4), and RARγ. The RXR family also contains three members (RXRα, RXRβ, and RXRγ) [12]. In attempts to dissect the role of RA signaling, genetically deficient mice were created in the early 90s. From these studies, it was shown that mice with deficiency in some of the receptor’s isoforms appear normal (RARα1, RARβ, RARβ2, and RARγ2). However, null mutants in RARα [13] or RARγ [14] show some of the defects observed in postnatal vitamin A deficiency (pre and postnatal development malformations, male sterility, photoreceptor degeneration, and others). Hence, some of these mice are not suitable to study the role of different RAR/RXRs in immune regulation due to embryonic lethality or other severe phenotypes. To bypass the lethality issue of these null mutant strains, dominant-negative tissue-restricted RARα (dnRARα) have been created [15,16]. A dnRARα still binds to DNA and dimerizes with its partner, but either it cannot bind to coactivators/suppressors or the binding to its ligand (RA) is weak, as such alters RA signaling and the final regulation of gene expression. Using this system, dnRARα expression was directed to lung epithelia and it was shown that RA signaling through this receptor is required for normal alveolar development in neonatal lungs [16]. Utilizing the same approach, Mira-y-Lopez and coworkers demonstrated that mice expressing a dnRARα under the mouse mammary tumor virus promoter, which targets the expression of the transgene to some lymphocytes and mammary epithelia, developed B cell lymphoma [15]. This observation supports the physiological role of RA in the control of leukocyte differentiation and proliferation, which has been previously suggested by other groups [17,18].
With respect to RXRs, deletion of RXRα results in early lethality [19]. Stephensen et al. created a viable hypomorphic allele of RXRα induced by random mutagenesis. The characterization of this mutant mouse showed that the immune response is skewed toward a Th1-type response [19]. This observation supports previous reports where vitamin A was shown to enhance Th2 differentiation [20], but it carries the caveat that vitamin D, an additional RXRα ligand, can also alter the phenotype of T cells toward a Th2 phenotype [21]; therefore, the T-cell phenotype observed is not exclusively dependent on RA. Lloyd and coworkers took advantage of the Cre-Lox system to disrupt RXRα in the T-cell compartment by intercrossing lck-cre with the RXRαFLOX mice. They observed a modest difference on B- and T-cell number, proliferation, cytokine production, and apoptosis ex vivo [22]. These studies suggest that the activation of RXRα signaling may contribute to T-cell differentiation, but the specific role of RA on this event needs to be specifically addressed.
3. The impact of RA on immunity
The recognition that poor nutrition positively correlated with an increased susceptibility to infectious disease dates back as far as the 18th century. The “nutritional theories” stated that milk and fat (source of vitamin A) are indispensable for the development of healthy children, and supplements of carrots could protect and ameliorate an infection. Studies on vitamin A supplementation indicated that children affected with xerophthalmia also suffered of respiratory diseases, wasting, and gastroenteritis, which were reduced when children received vitamin A supplements [23–25]. After the identification and purification of vitamin A (Frederick Hopkins, 1912), studies in which vitamin A was specifically eliminated in an experimental diet allowed scientists to assess the essentiality of this vitamin in immunity [26]. The study of host resistant to infectious disease in animals on vitamin A deficient diets began to provide the first clues as to the role of vitamin A in immunity. We will briefly touch on the impact of vitamin A on a spectrum of hematopoietic cells but focus on the impact of vitamin A on the major cellular elements of the immune system.
3.1. Monocyte/macrophages
Overall, the impact of RA on monocytes, macrophages, and macrophage cell lines suggests that RA inhibits the production of cytokines that favor the generation of Th1-type T cells and enhances the production of cytokines favoring the generation of Th2-type cells. A number of studies have shown that all-trans-RA modulates NO production, enhances interleukin (IL)-1 production, and inhibits the production of tumor necrosis factor (TNF)-α [27]. Kim et al. [28,29] evaluated the role of RA on mouse macrophages and the indirect effect on T cells. In their experiments, macrophages were pretreated with RA and subsequently activated with lipopolysaccharide (LPS). It was shown that RA inhibits IL-12 secretion by activated macrophages and these RA-treated macrophages when used as antigen presenting cells (APCs), reduced T-cell production of interferon (IFN)-γ and enhanced production of IL-4 were observed. Thus, overall RA signaling appears to establish a Th2-Treg noninflammatory environment.
3.2. Dendritic cells
Darmanin et al. explored the impact of RA in murine bone marrow-derived dendritic cells (BMDC) with the purpose of clarifying the mechanism of how RA induces the differentiation and migration of dendritic cells (DCs) [30]. In their study, they observed that when RA-treated BMDC are injected intratumorally into tumor-bearing mice, these BMDC are found in draining lymph nodes (DLN), in comparison with the lack of migration injected with nontreated BMDC. This result suggests that RA is affecting BMDC migratory properties, which was confirmed when they analyzed the mRNA expression and secretion of matrix metalloproteinases (MMPs). MMP-9 and MMP-14 were highly increased under RA exposure, together with downregulation of tissue inhibitor of MMP-1, -2, and -3 (TIMPs). These observations suggest that RA induces migration of DC from the tumor to DLN through the secretion of molecules, which permit the exit from the tumor microenvironment/matrix, allowing the DC to migrate to the appropriate place to encounter T cells and present antigen (Ag).
Geissman et al. [31] assessed many retinoids (retinol, 9-cis- and all-trans-RA) and their effects on human monocyte-derived DCs (MoDCs). They demonstrated that retinoids, together with inflammatory cytokines (but not with retinoids alone), upregulate MHC-II, and CD86 expression on MoDCs supporting enhanced allogeneic T-cell proliferation seen when retinoid-treated MoDCs were used in the cocultures. In parallel, retinoids cooperated with inflammatory signals (cytokines and CD40 signaling) to improve the ability of MoDCs to present Ag. Using specific synthetic agonist and antagonists, it was elucidated that RA modulated the pheno-type and function of immature MoDCs via RARα/RXR signaling, indicating a direct effect of RA through its pathway on this APCs.
3.3. T lymphocytes
Much of what we know about the impact of vitamin A on T-cell immunity comes from studies in mice maintained on a vitamin A-deficient diet [2]. Overall, Hayes and coworkers showed that RA downregulated Th1 cells (IFN-γ secretion) decreased the activation of APCs and promoted Th2-cell growth and/or differentiation. Supporting the tenet that RA can bias immune responses to Th2-type responses, Iwata et al. [32] showed that RA impaired T cell skewing toward Th1 phenotype but allowed the development and expansion of Th2-type T cells. This observation was supported by the analysis of expression of transcription factors and molecules characteristic of either Th1 (T-bet and IL-12Rβ2) or Th2 response (c-maf, IL-4Rα, and GATA3). When RA was present in the cultures, in addition to inducing the secretion of Th2 cytokines, RA also promoted the expression of c-maf, IL-4Rα, and GATA3, together with reducing T-bet and IL-12Rβ2 [32]. The use of agonists and antagonists identified RARα and RARβ as the players in the promotion of Th2-type T cells.
3.4. Homing impact of RA
In 2004, Iwata et al. presented striking results involving RA as a key mediator in T-cell homing (or trafficking). In this study, they observed that RA “imprints” T-cell homing by inducing the expression of the gut-homing receptors α4β7 and CCR9 on CD4+ T lymphocytes. Most importantly, they showed that mainly gut-resident DCs (mesenteric lymph nodes and Peyer’s patches derived) express the enzymes necessary to synthesize RA and also that on coculture with CD4+ T cells, conversion of retinol to all-trans-RA takes place indicating that gut-DCs express metabolically active enzymes. It is important to note that splenic DC also display these properties but in a less extend [33]. Later, Svensson et al. also demonstrated that not only gut-resident DC but also splenic DC were capable of producing RA and inducing gut-homing receptors on T lymphocytes [34]. The homing properties of RA were also applicable to CD4+ regulatory T lymphocytes (Treg) and CD8+ T and B lymphocytes [35,36]. These studies have made a major contribution to how nutrition may impact on immunity by elucidating how a dietary vitamin can dramatically alter gut immunity. These findings help to begin explaining how vitamin A deficiency may alter host resistance within the gut. Another significant advance in our understanding of the role of RA in immunity was the observation that RA enhanced the expression of the transcription factor FoxP3 on Tregs in a TGF-β-dependent fashion [37–39]. Tregs are one of the critical cellular subsets that control the development of inflammation and autoimmunity through suppression. The finding that RA exerts such profound effects on such important immunoregulatory subset has significant implications [40]. In addition to enhancing the differentiation of Treg, it appears that RA also induces their irreversible commitment to the Treg lineage. Hence, through its power to imprint T cells and enhance Treg function and number, RA no doubt plays a critical role in maintaining the inflammatory/anti-inflammatory balance in the gut [16].
In 2005, Harrington et al. described a new population of T lymphocytes. These IL-17-producing CD4+T lymphocytes (Th17 cells) showed inflammatory properties and the ability to mediate autoimmune diseases such as experimental autoimmune encephalomyelitis (EAE) and arthritis in mice [41]. Extensive characterization of Th17 cells also revealed that RA plays a role in Th17 generation. In this case, RA promotes Th17 differentiation at physiological concentrations (nM) but inhibits Th17 development at higher doses (42,43). Interestingly, RA besides imprinting gut-homing receptors on Th17 cells also seems to impact their activity as Th17 cells produced in the presence of RA showed enhanced inflammatory properties when assayed in a colitis mouse model [44]. These findings suggest that the levels of RA exquisitely control differentiation of different T-lymphocyte populations as it has been described for Th1, Th17, and CD4+FoxP3+ cells as well [45].
Besides Th1, Th2, and Treg subsets, Th17 cells have also been described in response to allografts (transplantation) [46], tumors [47], and other conditions where inflammation takes place. These new insights into the proinflammatory and anti-inflammatory role that RA plays in controlling T-cell function provides a rationale basis for using RA agonists and antagonists for the management of immune-related diseases.
3.5. RA and the induction of aTreg
Several populations of Treg have been identified. Briefly, natural Treg are developed in the thymus and express FoxP3 constitutively. On the other hand, Treg can also be generated in the periphery, and they are denoted adaptive Treg (aTreg). In this regard, the presence of anti-inflammatory cytokines will determine aTreg identity as TGF-β drives the induction of Th3 subset and IL-10 permits the development of Tr1 cells [48]. A number of studies [37–39,43,49] show that RA dramatically enhances the expression of Foxp3 by CD4+ T cells and greatly enhances the expansion and function of aTreg. Studies from our lab [39] demonstrated that RA greatly enhanced the expression of Foxp3 in CD4+ T cells stimulated with Ag or αCD3 and TGF-β. The results were quite striking, in which almost 100% of T cells were Foxp3+ in the presence of RA. Even more important is that RA enhanced the growth of the Foxp3+ T cells, increased their suppressor activity, and made them resistant to reversion to Foxp3− phenotype in vivo. One last important aspect of RA influence is that RA extinguishes the negative impact of costimulation on Foxp3 expression. That is, in cultures where one uses DCs as an APC source (or αCD28 as a model of high costimulation) T cells activated under these conditions (in the presence of TGF-β) do not become Foxp3+. However, the inclusion of RA allows for expansion and high levels of Foxp3 expression.
Studies by three other groups also showed a critical role for RA in Treg development, but these cases focused exclusively on its role in the gut (because of the ability of RA to induce gut homing) and also highlighted the negative impact of RA on the development of Th17. Mucida et al. [43] showed that DCs derived from the mesenteric lymph node (MLN) but not DCs from spleen-induced Foxp3 expression in T cells. Second, they showed that MLN-DCs synthesized RA. Third, they demonstrated that the in vivo administration of a RA antagonist impaired the development of Foxp3+ T cells in the gut mucosa. These important findings establish that RA, or metabolites of vitamin A, may play a pivotal role in Treg development in vivo. Another important aspect of the work from Mucida et al. is that they showed that RA impeded the development of Th17 at the cost of enhancing the development of Treg. In addition to this study, Sun et al. [37] reported that naïve CD4+ Foxp3-T cells converted to CD4+FoxP3+T cells when migrated to the gut. They identified that gut-resident DCs mediated this conversion of Treg in a TGF-β and RA-dependent fashion. In a very similar study, Coombes et al. [38] showed that conversion from naïve CD4+ T cells to Treg occurs after oral administration of Ag. They also identified CD103+ gut-resident DC as the inducers of Treg and confirmed the RA dependence. Taking together, these studies suggest that gut-derived RA may contribute to the generation and/or maintenance of Treg, which could be implicated in the control of inflammatory responses within this anatomical site.
3.6. RA in tumor immunity
RA may also play a role in regulating the immune response to tumors. Numerous studies have underscored the potent immunosuppressive impact of immature CD11b+Gr-1+ myeloid-derived suppressor cells (MDSCs) on the development of protective antitumor immunity in the host. Vitamin A-deficient mice [50] and pan-RAR antagonist-treated mice [51] have an increased number of MDSCs within the tumor microenvironment. It has also been seen that vitamin A-rich diets can enhance antitumor immunity rendered by the irradiated tumor cells [52], and that all-trans-RA can improve the antitumor protection by both tumor peptide and Ad-p53-transduced DCs in different tumor models [53]. The same effect was observed in patients with metastatic renal carcinoma [54]. Furthermore, BMDCs generated in the presence of all-trans-RA showed enhanced migration toward the DLN in B16 tumor model due to higher MMP but lower TIMP [30]. Although all-trans-RA has been widely used in “differentiation therapy” as an effective treatment for acute promyelocytic leukemia, the role of RA in regulating antitumor immunity in a solid tumor system remains to be elucidated [55]. In summary, RA may enhance protective antitumor immunity through mechanisms such as induction of cell differentiation and enhancement of migration to lymph nodes (Fig. 2). Because of the relevance in understanding the role of RA on tumor immunology, it is imperative to translate the recent discoveries in which new technologies have been used (agonists/antagonists drugs, engineered mice, etc.) to the tumor field.
Fig. 2.
Tumor cells caused by chronic inflammation lead to systemic accumulation of MDSCs and Tregs. Both Tregs and MDSCs contribute to the immunosuppressive tumor microenvironment by inhibiting both CD4+ and CD8+ T-cell response against the tumor. Although Th17 cells have not been confirmed in all tumor models, adoptive transfer of Th17 cell can eradicate large established tumors. RA treatment can induce MDSCs differentiation to nonsuppressive DCs. These mature DC cannot inhibit the CD4+ and CD8+ T-cell response against the tumor. However, the direct effect of RA on T cells in the antitumor immunity has not elucidated yet. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
3.7. RA and the response to pathogen-associated stimuli
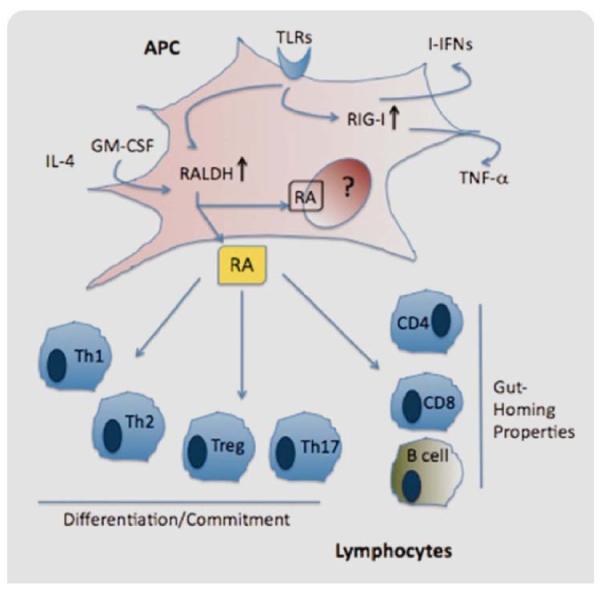
With the emergence of RA as a central regulator of immune function, how endogenous RA synthesis is controlled and which cells produce it has become an area of intense interest. As mentioned earlier, gut-resident DCs have been characterized to express RALDH enzymes and produce RA. Recently, it was described that not only gut-resident DCs are able to express RALDH but also BMDCs and splenic DCs activated with inflammatory stimuli. In this regard, proinflammatory cytokines such as GM-CSF, IL-4, and toll-like receptor (TLR) agonists upregulate RALDH expression on DCs [56]. In another report, TLR5-expressing DCs responded to flagellin (a pathogen-derived component) by upregulating the expression of RALDH, promoting the development of proinflammatory Th1 and Th17 T lymphocytes and IgA+ producing B lymphocytes [18]. This suggests that a wide spectrum of inflammatory mediators (cytokines and TLR agonists) can induce RA synthesis and this RA acts as a vital modulator of the immune system.
A related effect of RA on immunity may be associated with some of the specific genes that are up regulated by RA in hematopoietic cells. RA-inducible gene-I (RIG-I) is a member of the RIG-I-like receptors (RLRs) family, which is located in the cytoplasm and recognizes viral RNA [57]. RIG-I has been involved in the innate immune response against viruses. Trottier et al. investigated the impact of RA in the infectivity of Measles virus (MeV) on the promonocytic cell line U937. In their studies, RA-treated cells showed reduced viral infection due to the upregulation of interferon-stimulated genes. Surprisingly, bystander cells exposed to the media containing the RA-treated cells were refractory to MeV infection, suggesting that soluble factors are the effectors and that the response can be transmittable. Vitamin A or RA is known as an effective treatment against acute measles, which affects ~30 millions of children a year [58]. This report suggested a mechanism by which RA exerts antiviral activity. Recently, a new mechanism of RIG-I activation has been described. Wang et al. reported that, besides viral RNA, RIG-I can also be stimulated by the TLR4 ligand LPS. In this study, LPS-stimulated macrophages upregulate the expression of RIG-I, which allowed the expression of the proinflammatory cytokine TNF-α [59]. Interestingly, the enhanced response developed by Influenza A-infected individuals is due to the recognition of the virus through RIG-I, which upregulates type-I interferons (IFN-α and IFN-β) together with the activation of NF-κB that triggers the expression of other proinflammatory modulators as IL-6 and IL-8 [60]. In Fig. 3, we summarize the current knowledge of RA on immune cells, specifically on APCs (such as DCs) and T lymphocytes.
Fig. 3.
RA modulates the immune response. An inflammatory microenvironment can impact and change the phenotype of APCs as a consequence RA production. RA is known for inducing gut-homing properties on T and B lymphocytes, and for influencing the generation and differentiation of different subpopulation of T lymphocytes, which are a crucial arm of the immune response. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
From the above, we can conclude that RA can exert both pro- and anti-inflammatory effects. No doubt, RA exerts these effects in ways to coordinate a balance of positive and negative effects to optimize host resistance.
4. Concluding remarks
The role of vitamins, specifically vitamin A, has been proven to be important to maintain a healthy state. In this review, we summarized and discussed the last discoveries regarding the involvement of vitamin A and RA in the immune system, underscoring its growing importance in regulating T-cell–mediated immunity. The fact that RA controls critical checkpoints in inflammation and tolerance makes it an attractive target to modulate immunity. However, additional studies are required to precisely understand how RA controls these events at a molecular level, the receptors involved and the way RA synthesis is regulated in vivo.
References
- [1].Noy N. Retinoid-binding proteins: mediators of retinoid action. Biochem. J. 2000;348(Part 3):481–495. [PMC free article] [PubMed] [Google Scholar]
- [2].Smith SM, Hayes CE. Contrasting impairments in IgM and IgG responses of vitamin A-deficient mice. Proc. Natl. Acad. Sci. USA. 1987;84:5878–5782. doi: 10.1073/pnas.84.16.5878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Smith SM, Levy NS, Hayes CE. Impaired immunity in vitamin A-deficient mice. J. Nutr. 1987;117:857–865. doi: 10.1093/jn/117.5.857. [DOI] [PubMed] [Google Scholar]
- [4].Duester G. Families of retinoid dehydrogenases regulating vitamin A function: production of visual pigment and retinoic acid. Eur. J. Biochem. 2000;267:4315–4324. doi: 10.1046/j.1432-1327.2000.01497.x. [DOI] [PubMed] [Google Scholar]
- [5].Baker ME. Unusual evolution of 11beta- and 17beta-hydroxysteroid and retinol dehydrogenases. Bioessays. 1996;18:63–70. doi: 10.1002/bies.950180112. [DOI] [PubMed] [Google Scholar]
- [6].Lindahl R. Aldehyde dehydrogenases and their role in carcinogenesis. Crit. Rev. Biochem. Mol. Biol. 1992;27:283–335. doi: 10.3109/10409239209082565. [DOI] [PubMed] [Google Scholar]
- [7].Bhat PV. Retinal dehydrogenase gene expression in stomach and small intestine of rats during postnatal development and in vitamin A deficiency. FEBS Lett. 1998;426:260–262. doi: 10.1016/s0014-5793(98)00355-x. [DOI] [PubMed] [Google Scholar]
- [8].Taimi M, Helvig C, Wisniewski J, Ramshaw H, White J, Amad M, Korczak B, Petkovich M. A novel human cytochrome P450, CYP26C1, involved in metabolism of 9-cis and all-trans isomers of retinoic acid. J. Biol. Chem. 2004;279:77–85. doi: 10.1074/jbc.M308337200. [DOI] [PubMed] [Google Scholar]
- [9].Abu-Abed SS, Beckett BR, Chiba H, Chithalen JV, Jones G, Metzger D, Chambon P, Petkovich M. Mouse P450RAIexpression and retinoic acid-inducible retinoic acid metabolism in F9 cells are regulated by retinoic acid receptor gamma and retinoid X receptor alpha. J. Biol. Chem. 1998;273:2409–2415. doi: 10.1074/jbc.273.4.2409. [DOI] [PubMed] [Google Scholar]
- [10].Ribes V, Otto DM, Dickmann L, Schmidt K, Schuhbaur B, Henderson C, Blomhoff R, Wolf CR, Tickle C, Dolle P. Rescue of cytochrome P450 oxidoreductase (Por) mouse mutants reveals functions in vasculogenesis, brain and limb patterning linked to retinoic acid homeostasis. Dev. Biol. 2007;303:66–81. doi: 10.1016/j.ydbio.2006.10.032. [DOI] [PubMed] [Google Scholar]
- [11].Napoli JL. Retinoic acid: its biosynthesis and metabolism. Prog. Nucleic Acid Res. Mol. Biol. 1999;63:139–188. doi: 10.1016/s0079-6603(08)60722-9. [DOI] [PubMed] [Google Scholar]
- [12].Soprano DR, Qin P, Soprano KJ. Retinoic acid receptors and cancers. Annu. Rev. Nutr. 2004;24:201–221. doi: 10.1146/annurev.nutr.24.012003.132407. [DOI] [PubMed] [Google Scholar]
- [13].Lufkin T, Lohnes D, Mark M, Dierich A, Gorry P, Gaub MP, LeMeur M, Chambon P. High postnatal lethality and testis degeneration in retinoic acid receptor alpha mutant mice. Proc. Natl. Acad. Sci. USA. 1993;90:7225–7229. doi: 10.1073/pnas.90.15.7225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Lohnes D, Kastner P, Dierich A, Mark M, LeMeur M, Chambon P. Function of retinoic acid receptor gamma in the mouse. Cell. 1993;73:643–658. doi: 10.1016/0092-8674(93)90246-m. [DOI] [PubMed] [Google Scholar]
- [15].Kupumbati TS, Cattoretti G, Marzan C, Farias EF, Taneja R, Mira-y-Lopez R. Dominant negative retinoic acid receptor initiates tumor formation in mice. Mol. Cancer. 2006;5:12. doi: 10.1186/1476-4598-5-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Yang L, Naltner A, Yan C. Overexpression of dominant negative retinoic acid receptor alpha causes alveolar abnormality in transgenic neonatal lungs. Endocrinology. 2003;144:3004–3011. doi: 10.1210/en.2002-0121. [DOI] [PubMed] [Google Scholar]
- [17].Niles RM. Recent advances in the use of vitamin A (retinoids) in the prevention and treatment of cancer. Nutrition. 2000;16:1084–1089. doi: 10.1016/s0899-9007(00)00436-6. [DOI] [PubMed] [Google Scholar]
- [18].Flynn PJ, Miller WJ, Weisdorf DJ, Arthur DC, Brunning R, Branda RF. Retinoic acid treatment of acute promyelocytic leukemia: in vitro and in vivo observations. Blood. 1983;62:1211–1217. [PubMed] [Google Scholar]
- [19].Mangelsdorf DJ, Evans RM. The RXR heterodimers and orphan receptors. Cell. 1995;83:841–850. doi: 10.1016/0092-8674(95)90200-7. [DOI] [PubMed] [Google Scholar]
- [20].Stephensen CB, Rasooly R, Jiang X, Ceddia MA, Weaver CT, Chandraratna RA, Bucy RP. Vitamin A enhances in vitro Th2 development via retinoid X receptor pathway. J. Immunol. 2002;168:4495–503. doi: 10.4049/jimmunol.168.9.4495. [DOI] [PubMed] [Google Scholar]
- [21].Mora JR, Iwata M, von Andrian UH. Vitamin effects on the immune system: vitamins A and D take centre stage. Nat. Rev. Immunol. 2008;8:685–698. doi: 10.1038/nri2378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Stephensen CB, Borowsky AD, Lloyd KC. Disruption of Rxra gene in thymocytes and T lymphocytes modestly alters lymphocyte frequencies, proliferation, survival and T helper type 1/type 2 balance. Immunology. 2007;121:484–498. doi: 10.1111/j.1365-2567.2007.02595.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Sommer A, Tarwotjo I, Djunaedi E, West KP, Jr., Loeden AA, Tilden R, Mele L. Impact of vitamin A supplementation on childhood mortality. A randomised controlled community trial. Lancet. 1986;1:1169–1173. doi: 10.1016/s0140-6736(86)91157-8. [DOI] [PubMed] [Google Scholar]
- [24].Sommer A, Tarwotjo I, Hussaini G, Susanto D. Increased mortality in children with mild vitamin A deficiency. Lancet. 1983;2:585–588. doi: 10.1016/s0140-6736(83)90677-3. [DOI] [PubMed] [Google Scholar]
- [25].West KP, Jr., Pokhrel RP, Katz J, LeClerq SC, Khatry SK, Shrestha SR, Pradhan EK, Tielsch JM, Pandey MR, Sommer A. Efficacy of vitamin A in reducing preschool child mortality in Nepal. Lancet. 1991;338:67–71. doi: 10.1016/0140-6736(91)90070-6. [DOI] [PubMed] [Google Scholar]
- [26].Semba RD. Vitamin A and immunity to viral, bacterial and protozoan infections. Proc. Nutr. Soc. 1999;58:719–727. doi: 10.1017/s0029665199000944. [DOI] [PubMed] [Google Scholar]
- [27].Mehta K, McQueen T, Tucker S, Pandita R, Aggarwal BB. Inhibition by all-trans-retinoic acid of tumor necrosis factor and nitric oxide production by peritoneal macrophages. J. Leukoc. Biol. 1994;55:336–342. doi: 10.1002/jlb.55.3.336. [DOI] [PubMed] [Google Scholar]
- [28].Kim BH, Kang KS, Lee YS. Effect of retinoids on LPS-induced COX-2 expression and COX-2 associated PGE(2) release from mouse peritoneal macrophages and TNF-alpha release from rat peripheral blood mononuclear cells. Toxicol. Lett. 2004;150:191–201. doi: 10.1016/j.toxlet.2004.01.010. [DOI] [PubMed] [Google Scholar]
- [29].Na SY, Kang BY, Chung SW, Han SJ, Ma X, Trinchieri G, Im SY, Lee JW, Kim TS. Retinoids inhibit interleukin-12 production in macrophages through physical associations of retinoid X receptor and NFkappaB. J. Biol. Chem. 1999;274:7674–7680. doi: 10.1074/jbc.274.12.7674. [DOI] [PubMed] [Google Scholar]
- [30].Darmanin S, Chen J, Zhao S, Cui H, Shirkoohi R, Kubo N, Kuge Y, Tamaki N, Nakagawa K, Hamada J, Moriuchi T, Kobayashi M. All-trans retinoic acid enhances murine dendritic cell migration to draining lymph nodes via the balance of matrix metalloproteinases and their inhibitors. J. Immunol. 2007;179:4616–4625. doi: 10.4049/jimmunol.179.7.4616. [DOI] [PubMed] [Google Scholar]
- [31].Geissmann F, Revy P, Brousse N, Lepelletier Y, Folli C, Durandy A, Chambon P, Dy M. Retinoids regulate survival and antigen presentation by immature dendritic cells. J. Exp. Med. 2003;198:623–634. doi: 10.1084/jem.20030390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Iwata M, Eshima Y, Kagechika H. Retinoic acids exert direct effects on T cells to suppress Th1 development and enhance Th2 development via retinoic acid receptors. Int. Immunol. 2003;15:1017–1025. doi: 10.1093/intimm/dxg101. [DOI] [PubMed] [Google Scholar]
- [33].Iwata M, Hirakiyama A, Eshima Y, Kagechika H, Kato C, Song SY. Retinoic acid imprints gut-homing specificity on T cells. Immunity. 2004;21:527–538. doi: 10.1016/j.immuni.2004.08.011. [DOI] [PubMed] [Google Scholar]
- [34].Svensson M, Johansson-Lindbom B, Zapata F, Jaensson E, Austenaa LM, Blomhoff R, Agace WW. Retinoic acid receptor signaling levels and antigen dose regulate gut homing receptor expression on CD8+ T cells. Mucosal. Immunol. 2008;1:38–48. doi: 10.1038/mi.2007.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Mora JR, Iwata M, Eksteen B, Song SY, Junt T, Senman B, Otipoby KL, Yokota A, Takeuchi H, Ricciardi-Castagnoli P, Rajewsky K, Adams DH, von Andrian UH. Generation of gut-homing IgA-secreting B cells by intestinal dendritic cells. Science. 2006;314:1157–1160. doi: 10.1126/science.1132742. [DOI] [PubMed] [Google Scholar]
- [36].Mora JR, Bono MR, Manjunath N, Weninger W, Cavanagh LL, Rosemblatt M, Von Andrian UH. Selective imprinting of gut-homing T cells by Peyer’s patch dendritic cells. Nature. 2003;424:88–93. doi: 10.1038/nature01726. [DOI] [PubMed] [Google Scholar]
- [37].Sun CM, Hall JA, Blank RB, Bouladoux N, Oukka M, Mora JR, Belkaid Y. Small intestine lamina propria dendritic cells promote de novo generation of Foxp3 T reg cells via retinoic acid. J. Exp. Med. 2007;204:1775–1785. doi: 10.1084/jem.20070602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Coombes JL, Siddiqui KR, Arancibia-Carcamo CV, Hall J, Sun CM, Belkaid Y, Powrie F. A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-beta and retinoic acid-dependent mechanism. J. Exp. Med. 2007;204:1757–1764. doi: 10.1084/jem.20070590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Benson MJ, Pino-Lagos K, Rosemblatt M, Noelle RJ. All-trans retinoic acid mediates enhanced T reg cell growth, differentiation, and gut homing in the face of high levels of co-stimulation. J. Exp. Med. 2007;204:1765–1774. doi: 10.1084/jem.20070719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Sakaguchi S, Wing K, Onishi Y, Prieto-Martin P, Yamaguchi T. Regulatory T cells: how do they suppress immune responses? Int. Immunol. 2009;21:1105–1111. doi: 10.1093/intimm/dxp095. [DOI] [PubMed] [Google Scholar]
- [41].Harrington LE, Hatton RD, Mangan PR, Turner H, Murphy TL, Murphy KM, Weaver CT. Interleukin 17-producing CD4 effector T cells develop via a lineage distinct from the T helper type+1 and 2 lineages. Nat. Immunol. 2005;6:1123–1132. doi: 10.1038/ni1254. [DOI] [PubMed] [Google Scholar]
- [42].Uematsu S, Fujimoto K, Jang MH, Yang BG, Jung YJ, Nishiyama M, Sato S, Tsujimura T, Yamamoto M, Yokota Y, Kiyono H, Miyasaka M, Ishii KJ, Akira S. Regulation of humoral and cellular gut immunity by lamina propria dendritic cells expressing Toll-like receptor 5. Nat. Immunol. 2008;9:769–776. doi: 10.1038/ni.1622. [DOI] [PubMed] [Google Scholar]
- [43].Mucida D, Park Y, Kim G, Turovskaya O, Scott I, Kronenberg M, Cheroutre H. Reciprocal TH17 and regulatory T cell differentiation mediated by retinoic acid. Science. 2007;317:256–260. doi: 10.1126/science.1145697. [DOI] [PubMed] [Google Scholar]
- [44].Wang J, Wu S, Jin X, Li M, Chen S, Teeling JL, Perry VH, Gu J. Retinoic acid-inducible gene-I mediates late phase induction of TNF-alpha by lipopolysaccharide. J. Immunol. 2008;180:8011–8019. doi: 10.4049/jimmunol.180.12.8011. [DOI] [PubMed] [Google Scholar]
- [45].Kang SG, Wang C, Matsumoto S, Kim CH. High and low vitamin A therapies induce distinct FoxP3+ T-cell subsets and effectively control intestinal inflammation. Gastroenterology. 2009;137:1391–402. doi: 10.1053/j.gastro.2009.06.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Mitchell P, Afzali B, Lombardi G, Lechler RI. The T helper 17-regulatory T cell axis in transplant rejection and tolerance. Curr. Opin. Organ Transplant. 2009;14:326–331. doi: 10.1097/MOT.0b013e32832ce88e. [DOI] [PubMed] [Google Scholar]
- [47].Murugaiyan G, Saha B. Protumor vs antitumor functions of IL-17. J. Immunol. 2009;183:4169–4175. doi: 10.4049/jimmunol.0901017. [DOI] [PubMed] [Google Scholar]
- [48].Bluestone JA, Abbas AK. Natural versus adaptive regulatory T cells. Nat. Rev. Immunol. 2003;3:253–257. doi: 10.1038/nri1032. [DOI] [PubMed] [Google Scholar]
- [49].Schambach F, Schupp M, Lazar MA, Reiner SL. Activation of retinoic acid receptor-alpha favours regulatory T cell induction at the expense of IL-17-secreting T helper cell differentiation. Eur. J. Immunol. 2007;37:2396–2399. doi: 10.1002/eji.200737621. [DOI] [PubMed] [Google Scholar]
- [50].Kuwata T, Wang I-M, Tamura T, Ponnamperuma RM, Levine R, Holmes KL, Ill HCM, Luca LMD, Ozato K. Vitamin A deficiency in mice causes a systemic expansion of myeloid cells. Blood. 2000;95:3349–3356. [PubMed] [Google Scholar]
- [51].Walkley CR, Yuan Y-D, Chandraratna RAS, McArthur GA. Retinoic acid receptor antagonism in vivo expands the numbers of precursor cells during granulopoiesis. Leukemia. 2002;16:1763–1772. doi: 10.1038/sj.leu.2402625. [DOI] [PubMed] [Google Scholar]
- [52].Malkovský M, Doré C, Hunt R, Palmer L, Chandler P, Medawar PB. Enhancement of specific antitumor immunity in mice fed a diet enriched in vitamin A acetate. Proc. Natl. Acad. Sci. 1983;80:6322–6326. doi: 10.1073/pnas.80.20.6322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Kusmartsev S, Cheng F, Yu B, Nefedova Y, Sotomayor E, Lush R, Gabrilovich D. All-trans-retinoic acid eliminates immature myeloid cells from tumor-bearing mice and improves the effect of vaccination. Cancer Res. 2003;63:4441–4449. [PubMed] [Google Scholar]
- [54].Mirza N, Fishman M, Fricke I, Dunn M, Neuger AM, Frost TJ, Lush RM, Antonia S, Gabrilovich DI. All-trans-retinoic acid improves differentiation of myeloid cells and immune response in cancer patients. Cancer Res. 2006;66:9299–9307. doi: 10.1158/0008-5472.CAN-06-1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Nasr R, Guillemin MC, Ferhi O, Soilihi H, Peres L, Berthier C, Rousselot P, Robledo-Sarmiento M, Lallemand-Breitenbach V, Gourmel B, Vitoux D, Pandolfi PP, Rochette-Egly C, Zhu J, De The H. Eradication of acute promyelocytic leukemia-initiating cells through PML-RARA degradation. Nat. Med. 2008;14:1333–1342. doi: 10.1038/nm.1891. [DOI] [PubMed] [Google Scholar]
- [56].Yokota A, Takeuchi H, Maeda N, Ohoka Y, Kato C, Song SY, Iwata M. GM-CSF and IL-4 synergistically trigger dendritic cells to acquire retinoic acid-producing capacity. Int. Immunol. 2009;21:361–377. doi: 10.1093/intimm/dxp003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Kawai T, Akira S. The roles of TLRs, RLRs and NLRs in pathogen recognition. Int. Immunol. 2009;21:317–337. doi: 10.1093/intimm/dxp017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Trottier C, Colombo M, Mann KK, Miller WH, Jr., Ward BJ. Retinoids inhibit measles virus through a type I IFN-dependent bystander effect. FASEB J. 2009;23:3203–3212. doi: 10.1096/fj.09-129288. [DOI] [PubMed] [Google Scholar]
- [59].Wang J, Wu S, Jin X, Li M, Chen S, Teeling JL, Perry VH, Gu J. Retinoic acid-inducible gene-I mediates late phase induction of TNF-alpha by lipopolysaccharide. J. Immunol. 2008;180:8011–8019. doi: 10.4049/jimmunol.180.12.8011. [DOI] [PubMed] [Google Scholar]
- [60].Le Goffic R, Pothlichet J, Vitour D, Fujita T, Meurs E, Chignard M, Si-Tahar M. Cutting edge: influenza A virus activates TLR3-dependent inflammatory and RIG-I-dependent antiviral responses in human lung epithelial cells. J. Immunol. 2007;178:3368–3372. doi: 10.4049/jimmunol.178.6.3368. [DOI] [PubMed] [Google Scholar]