Abstract
Background
Previous studies suggest that obesity is associated with higher prostate cancer progression and mortality despite an association with lower prostate cancer incidence. This study aims to better understand these apparently inconsistent relationships among obese men, by combining evidence from three nationally representative cross-sectional surveys.
Methods
We evaluated relationships between obesity and (1) testosterone concentrations in the Third National Health and Nutrition Examination Survey (NHANES III; n=845), (2) prostate-specific antigen (PSA) in NHANES 2001–2004 (n=2,458) and (3) prostate biopsy rates in the National Health Interview Survey (NHIS 2000; n=4,789) population. Mean testosterone, PSA concentrations and biopsy rates were computed for body mass index (BMI) categories.
Results
Testosterone concentrations were inversely associated with obesity (p-trend<0.0001) in NHANES III. In NHANES 2001–2004 obese (BMI >35) versus lean (BMI <25) men were less likely to have PSA concentrations that reached the biopsy threshold of >4 ng/ml (3% versus 8%; p<0.0001). Among NHIS participants all BMI groups had similar rates of PSA testing (p=0.24). However, among men who had PSA tests, 11% of men with BMI >30 versus 16% with BMI <25, achieved a PSA threshold of 4 ng/ml; p=0.01. Furthermore, biopsy rates were lower among men with BMI >30 versus BMI <25 in NHIS participants (4.6% vs. 5.8%; p=0.05).
Conclusions
Obesity was associated with lower PSA-driven biopsy rates. These data support further studies to test the hypothesis that obesity affects prostate cancer detection independent of prostate cancer risk by decreasing the PSA-driven biopsy rates.
Keywords: obesity, prostate cancer, prostate-specific antigen, biopsy
INTRODUCTION
Obesity has reached epidemic proportions in the US with about 75% of men being above the desired body weight.1 Existing evidence supports an association between obesity and prostate cancer progression and mortality2, 3,4–7. In contrast, obesity is associated with a lower risk of prostate cancer.5,8, 9 The reasons for these differential findings are not yet clear.
Given that a decision to perform prostate biopsy in an individual is usually driven by prostate-specific antigen (PSA) concentrations,10–12 we hypothesize that obese men are less likely to undergo biopsy and therefore are less likely to be detected early with prostate cancer. A potential explanation of this maybe that PSA concentrations in obese men are may not reach the biopsy threshold, possibly because of lower testosterone, and increased blood-volume.13 Subsequently, lower PSA concentrations among obese men may result in lower biopsy rates. In the present study, we examine the relationship between obesity and (1) testosterone, (2) PSA concentrations, and (3) biopsy rates, using data from three large national surveys in which, relevant data were available. Because no single survey had comprehensive data on the variables of interest to examine this issue, data were combined from three surveys to help resolve the phenomenon that obesity is associated with higher prostate cancer mortality but lower prostate cancer risk, which can influence both incidence and lethality of this cancer.
METHODS
Study populations and data collection
Three nationally representative study populations (described below) were used in this study (Figure 1).
Figure 1. Design of current study.

Figure 1 shows the variables available for each of the three study populations
1. The National Health and Nutrition Examination Survey (NHANES III; 1988–94)
NHANES III, conducted by the Centers for Disease Control and Prevention (CDC), is a nationally representative sample of non-institutionalized US civilians.14 Details of the sampling strategy have been described elsewhere.14 Age and race (defined as non-Hispanic white, non-Hispanic black, Mexican American, and “Other”) were self-reported.14 Approximately 100 ml of whole blood was collected during the medical examination and frozen.15 Using immunoassay techniques, serum concentrations of testosterone were estimated from the stored blood samples. Testosterone measurements were available for males older than 12 years of age who had participated in the first phase of NHANES III (1988–1991) and had provided a morning sample of blood (n=1625).16 For these analyses, we included participants 40+ years old, who had testosterone concentrations available (n=845). Additionally, body mass index (BMI) was available and computed from height and weight that were measured during the physical examination. We categorized BMI as lean (<25), overweight (25–30) and obese (30+).17
2. The National Health and Nutrition Examination Survey (NHANES) 2001–2004
NHANES 2001–2004 is a nationally representative cross-sectional survey of the civilian non-institutionalized population of the US. Details of the procedures involved in sampling and data collection have been published elsewhere.18
Of 3,108 men 40+ years, those who underwent a recent rectal digital examination, prostate biopsy or cytoscopy, or had a history of prostate cancer or prostate inflammation (n=203) were excluded as suggested by NHANES,19 as well as those for which there was missing data (n=447), leaving 2,458 men in the analysis dataset. As described previously, the men in this dataset were younger, less likely to be black, had a greater mean BMI, and were more likely to have a PSA <4 ng/ml as compared to those excluded.20
Participant age, race/ethnicity (non-Hispanic white, non-Hispanic black, Mexican-American, and “Other”), smoking history, education, alcohol use, current prescription medication and the presence of doctor-diagnosed medical conditions including cardiovascular disease and diabetes mellitus, were self-reported in a structured personal interview. Blood was obtained for survey participants and PSA concentrations were measured using an immunoenzymatic “sandwich” assay.19 Weight and waist circumference were measured and BMI was calculated from height and weight and categorized as lean (<25), overweight (25–30) and obese (30+).17
3. The National Health Interview Survey (NHIS; 2000)
The NHIS is an annual health survey conducted by CDC. The sample was selected using a stratified multi-stage cluster design. In addition to querying the participant’s health, demographics, and use of health services, in the year 2000, the survey collected information related to cancer prevention, using a supplemental questionnaire developed by the National Cancer Institute. This questionnaire was administered concurrent with the 2000 census, which provided other population denominators in the same year. An adult randomly selected from each participating NHIS family was interviewed in-person by trained personnel.21
Race and ethnicity were self-reported by the participants and were categorized in this study as white, black and “Other”. Body mass index was determined from self-reported weight and height. To obtain information on cancer screening, males ≥40 years without existing prostate cancer were asked if they had heard of the PSA test, or had one, the frequency of the tests in the past 5 years, and when the most recent test was performed. Survey participants also reported any abnormal PSA tests and whether they underwent further tests or surgery including prostate biopsies. In the present study, those eligible for a PSA test were considered in the analyses (n=4,789).
Statistical analyses
1. NHANES III: BMI and testosterone concentrations
First, participant characteristics were described for adult men 40+ years of age, for whom testosterone concentrations were measured. The associations of testosterone concentrations and BMI categories were computed using the analysis of covariance models. Mean testosterone concentrations, adjusted for race and age were computed for three BMI categories (<25, 25–30 and 30+) and the linear trend across the BMI categories was tested. Mean testosterone concentrations were plotted against BMI categories. Next, testosterone concentrations were divided into tertiles. The Chi-squared test was used to study the associations between the tertiles of testosterone concentrations and the BMI categories, to determine the proportion of obese versus lean men who have high concentrations of testosterone. The recommended NHANES III sample weights were used for all analyses to account for the complex survey design. SAS Proc Surveyreg was used to obtain correct standard errors due to the complex sampling strategy.
2. NHANES 2001–2004 population: BMI and PSA concentrations
Characteristics of the study population and PSA concentrations across categories of BMI were evaluated (Table 1). Chi-squared tests were used for the analyses of the association between BMI groups and the categorical variables. F-test was used for the analyses of the association between the BMI groups and the continuous variables. Non-parametric tests based on ranks were used for the analyses of the association between the serum total PSA and BMI categories. Next, the analyses of covariance models were used to study the age- and race-adjusted associations of PSA concentrations with BMI. Prostate-specific antigen was log-transformed to achieve a normal distribution. In separate models, geometric means of age-adjusted PSA concentration across BMI categories were computed for the three major racial groups: non-Hispanic whites, non-Hispanic blacks and Mexican-Americans. The linear trends of age-adjusted log PSA concentration across the BMI categories were tested. NHANES 2001–2004 weights were used in all analyses to account for the complex sampling strategy.
TABLE 1.
| NHANES III (N=845) |
NHANES 2001–2004 (N=2,458) |
NHIS 2000 (4,789) |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Variable | BMI <25 |
BMI 25–30 |
BMI 30+ |
P | Variable | BMI <25 |
BMI 25–30 |
BMI 30–35 |
BMI 35+ |
3P | Variable | BMI <25 |
BMI 25–30 |
BMI 30+ |
P |
| Non-Hispanic Whites (%) | 77 | 84 | 83 | <0.0001 | Non-Hispanic Whites (%) | 74 | 81 | 82 | 81 | <0.0001 | Whites (%) | 84 | 86 | 85 | <0.0001 |
| Non-Hispanic Blacks (%) | 8 | 8 | 6 | Non-Hispanic Blacks (%) | 11 | 7 | 8 | 11 | Blacks or African-American (%) | 8 | 9 | 10 | |||
| Mexican-Americans (%) | 2 | 4 | 5 | Mexican-Americans (%) | 5 | 5 | 5 | 4 | Mexican-American, Hispanic and other races (%) | 8 | 5 | 5 | |||
| Other races (%) | 13 | 4 | 6 | Other races | 10 | 7 | 5 | 4 | |||||||
| Age (years) | 56 (.69) | 57 (.66) | 55 (.82) | 0.2538 | Age (years) | 56 (.50) | 55 (.34) | 54 (.47) | 54 (.65) | 0.0095 | Age (years) | 60 (.35) | 58 (.25) | 56 (.32) | <0.0001 |
| BMI (kg/m2) | 23 (.10) | 27 (.08) | 33 (.32) | <0.0001 | BMI (kg/m2) | 23 (.07) | 28 (.04) | 32 (.06) | 39 (.34) | <0.0001 | BMI (kg/m2) | 23 (.04) | 27 (.03) | 34 (.12) | <0.0001 |
| Waist circumference (cm) | 88 (.36) | 100 (.31) | 114 (.81) | <0.0001 | Waist circumference (cm) | 88 (.29) | 101 (.20) | 112 (.27) | 129 (.76) | <0.0001 | n/a | ||||
| Testosterone ng/ml | 5.3 (.11) | 4.5 (.09) | 4 (.10) | <0.0001 | PSA > 4ng/ml (% of men) | 8 | 7 | 4 | 3 | <0.0001 | Ever had PSA (%) | 66 | 67 | 69 | 0.24 |
Population-specific sample weights were applied in all the analyses to account for individual selection probabilities, non-response and post-stratification that resulted from the complex survey design
Data represents weighted percentages for categorical variables and means (SE) for continuous variables
P-values for general association were generated by using analysis of variance
BMI categories were reassigned for each racial subgroup for all racial subgroup analyses
3. NHIS 2000 population: BMI and abnormal PSA tests and biopsy rates
We generated descriptive statistics for the NHIS study population. We divided BMI into three categories (lean: <25, overweight: 25–30 and obese: 30+) and computed the proportion of men who had a PSA test. Among men who underwent the PSA test, we computed the proportion of participants who had abnormal PSA test results within each BMI group. Additionally, we determined the proportion of men who had mentioned biopsy among those who had an abnormal PSA, and compared these across the BMI groups. Furthermore, the population biopsy rates were also calculated across BMI categories. Cochran-Armitage22 trend tests were used to test for linear trend for biopsy rates across the BMI groups. The provided NHIS sample weights were used to account for the complex study design. Analyses for all the three national databases were done using SASv9 (SAS Institute Inc, Cary, NC).
RESULTS
1. BMI and testosterone concentrations among NHANES III participants
Participant characteristics
A greater proportion of non-Hispanic white men had higher BMI, and the mean age ranged from 55–57 years across the BMI categories. Waist circumference increased and testosterone concentrations decreased significantly as BMI increased (Table 1).
Associations of testosterone and BMI
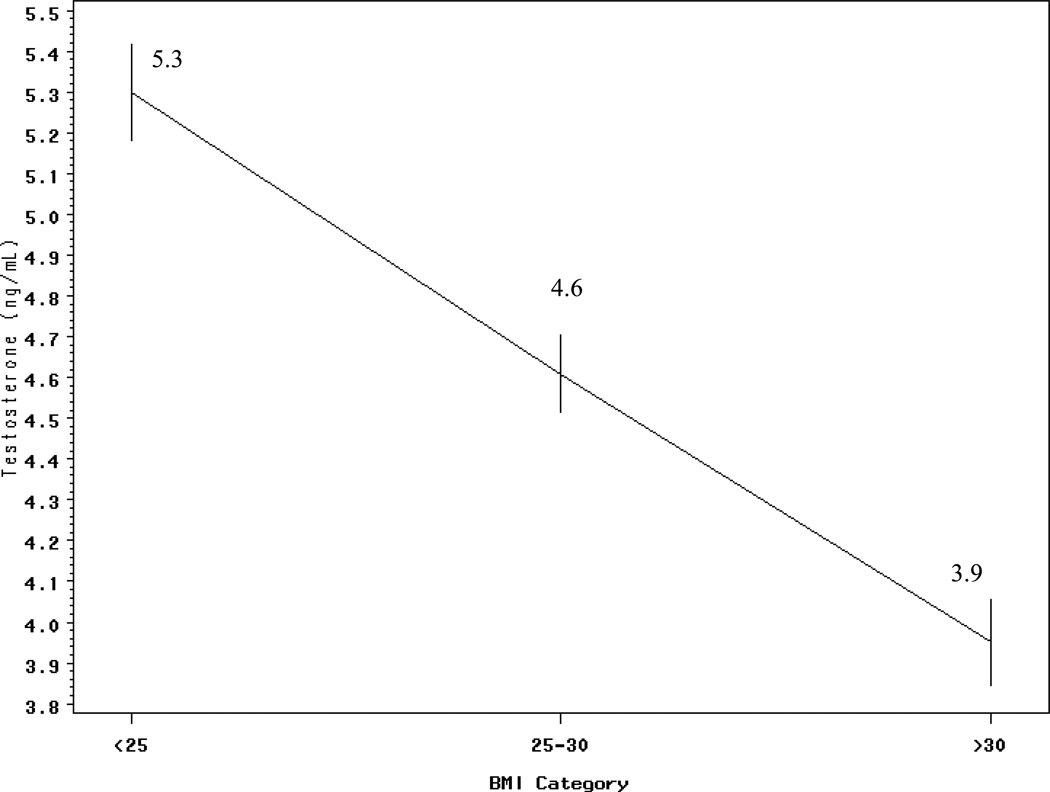
Mean testosterone concentrations were inversely and significantly associated with increasing BMI (p-trend <0.0001). The mean testosterone concentrations decreased for each BMI group (Figure 2). In addition, when the percent of men having high and low testosterone concentrations was examined across the three BMI categories, we observed that 50% of lean men whereas 12% of obese men belonged to the highest tertile of testosterone (>5.3 ng/ml). Conversely, 48% of obese men were in the lowest tertile of testosterone (<3.8 ng/ml) compared to 12% of lean men. The level of testosterone and BMI category were significantly associated (p <0.0001).
Figure 2. Mean ± SE testosterone concentrations by BMI groups in men 40+ years from the NHANES III (1988–1994) population (n= 845).
The line graph above demonstrates the mean testosterone ± SE for each BMI category. There is a significant linear trend in mean testosterone with BMI categories.
2. PSA concentrations and BMI in the NHANES 2001–2004 population
Participant Characteristics
A greater proportion of non-Hispanic white men had higher BMI. The mean age of participants decreased, and waist circumference increased with across BMI categories. The proportion of obese men with PSA >4 ng/ml, the most commonly used cut-off for abnormal PSA, was about a third that of lean men (3% versus 8% respectively) (Table 1).
Associations of PSA concentration and BMI
Mean age-adjusted PSA concentrations were inversely associated with BMI (Table 2). In the whole population, we observed that PSA concentrations decreased with increasing BMI (p-trend=0.01). PSA concentrations were approximately 17% lower among men in the highest BMI category as compared to the lean men. When we examined PSA values separately by race, PSA decreased with BMI among non-Hispanic white men (p-trend <0.0001) and Mexican-American men (p-trend=0.03) with PSA values being 22% and 24% lower in the obese compared to lean men respectively. In contrast, there was no such trend in non-Hispanic blacks across BMI categories (p-trend=0.91). We observed a similar inverse trend for PSA and waist circumference, an alternate measure of obesity; however, these associations were weaker than those observed for BMI (data not shown).
TABLE 2.
Adjusted geometric adjusted mean PSA concentration (95% CI)1,2 NHANES 2001–2004 by BMI category in the overall population and by race (n=2,485)
| Whole population | Non-Hispanic whites (1,428) |
Non-Hispanic blacks (428) |
Mexican-Americans (473) |
|||||
|---|---|---|---|---|---|---|---|---|
| N | PSA | N | PSA | N | PSA | N | PSA | |
| BMI ≤ 25 | 602 | 1.04 | 333 | 1.08 | 119 | 1.07 | 100 | 0.92 |
| (0.97–1.1) | (0.98–1.2) | (0.9–1.2) | (0.8–1.1) | |||||
| BMI 25–30 | 1096 | 0.92 | 644 | 0.93 | 169 | 0.93 | 225 | 0.94 |
| (0.9–0.98) | (0.9–1.0) | (0.9–1.0) | ||||||
| BMI 30–35 | 523 | 0.90 | 312 | 0.88 | 86 | 0.99 | 111 | 0.92 |
| (0.8–0.96) | (0.8–0.9) | (0.8–1.1) | (0.7–1.1) | |||||
| BMI 35+ | 237 | 0.86 | 139 | 0.84 | 54 | 1.06 | 37 | 0.70 |
| (0.75–0.98) | (0.7–0.99) | (0.9–1.2) | (0.6–0.8) | |||||
| % diff in PSA concentrations | −17 | −22 | <1 | −24 | ||||
| BMI <25 vs. 35+ P for trend | 0.01 | <0.0001 | 0.91 | 0.03 | ||||
Adjusted for age (adjusted for age and race for the whole population category)
NHANES 2001–2004 sample weights were applied in all the analyses to account for individual selection probabilities, non-response and post-stratification that resulted from the complex survey design.
3. Abnormal PSA tests and biopsy rates in relation with BMI in the NHIS 2000 population
The proportions of white and black men increased with BMI and obese versus lean men were more likely to be younger (Table 1). Based on the results of this nationally representative study, PSA testing rate was similar among lean, overweight and obese men ranging from 66–69% of men who underwent a PSA test (p=0.24) (Table 3). However, among men who had undergone the PSA test, obese men (BMI>30) were less likely to have abnormal PSA test results as compared with men with BMI< 25 (11% versus 16%; p=0.01). Among men with abnormal PSA tests (>4 ng/ml), the rate of biopsy was similar across the three BMI categories (p=0.48). Furthermore, in the total population, prostate biopsy rate was lower among men with a BMI ≥30 as compared to biopsy rates among men with a BMI of <25 (4.6% versus 5.8%; p=0.05) as shown in Table 3.
Table 3.
Rate (expressed as %) of PSA testing, abnormal PSA test results and biopsy rate among men >40 years in the NHIS 2000 population (n=4,789)
| Variable | BMI categories | p-value | ||
|---|---|---|---|---|
| <25 N=1444 |
25–30 N=2256 |
30+ N=1089 |
||
| Ever had PSA test / total population | 66 | 67 | 69 | 0.24 |
| Abnormal PSA / ever had PSA test | 16 | 13 | 11 | 0.01 |
| Biopsy rate / men who had an abnormal PSA test | 58 | 59 | 62 | 0.48 |
| Population biopsy rate / total population | 5.8 | 4.9 | 4.6 | 0.05 |
DISCUSSION
In the present study, we combined evidence from three nationally representative populations, to demonstrate the associations of increasing BMI with (1) PSA, (2) testosterone concentrations and (3) biopsy rates, in order to better understand the inconsistent finding that obesity is associated with a decreased incidence of prostate cancer but an increased risk from prostate cancer progression and mortality. Using NHANES III data, we first examined BMI in relation to testosterone concentrations, and noted a significant decreasing trend of testosterone concentrations with increasing BMI (Figure 2), consistent with previous reports.23–25 In the NHANES 2001–2004 study population we noted significant inverse associations between PSA and BMI. These findings were consistent with previous studies, which reported that obese men were less likely to have PSA test greater than 4 ng/ml as compared to lean men.20, 26
Next, we examined the NHIS dataset, to evaluate the associations of BMI and biopsy rate. Consistent with the results in the NHANES 2001–2004 data, we observed that although obese men are as likely as lean men to undergo a PSA test, they were less likely to have abnormal PSA concentrations (Table 3). Furthermore, obese men had the lowest biopsy rate compared to men in the other BMI categories.
The results of the NHIS study support our hypothesis that lower biopsy rates among obese men are largely driven by their lower likelihood of reaching the PSA threshold for biopsy, although we were not directly able to evaluate PSA concentrations in this population. Existing studies have shown that biopsy rates are highly correlated with incidence rates of prostate cancer.27 Our study may explain the findings of the Health Professionals Study (n=51,529) that noted a lower incidence of prostate cancer among obese men (<60 years) as compared to men with weight within the normal range.28
Obesity is associated with more severe disease and increased mortality from prostate cancer. In a prospective study (n=16,336), incidence of aggressive prostate cancer was related to body adiposity.29 Additionally, data from previous studies demonstrate that obese men are more likely to die of prostate cancer.5, 30 For example, in a large landmark study (n=900,000), with a ~16 year follow-up, having a BMI ≥25, contributed to a 20 to 30% increased risk of mortality from prostate cancer. Another large prospective study among Swedish men noted that prostate cancer mortality was significantly higher in obese or overweight men, compared to lean men.31 Furthermore, Fesinmeyer et al. used data from NHANES and Surveillance Epidemiology and End Results (SEER) and used simulation models to quantify the impact of obesity on prostate cancer in the recent years.32 They reported that obesity was associated with an increase in high-grade prostate cancer incidence and mortality from the disease.32
The current study helps to clarify the seemingly inconsistent findings of obesity being associated with a lower risk of prostate cancer incidence but with higher mortality, case fatality and more severe disease at presentation. Data from the current study supports the hypothesis that lower incidence rate of prostate cancer among obese men may be explained by the lower likelihood of reaching the PSA threshold for biopsy (Figure 3). Indeed, some researchers question the sensitivity of the PSA test as a screening tool for prostate cancer.10 We agree with Freedland et al.33, 34 that lower detection of prostate cancer among obese individuals, specifically among non-Hispanic white men, is likely a more plausible theory as compared to the notion of lower underlying risk of prostate cancer among obese men. An understanding of the potential reason for the observed lower incidence rates among obese men is critical in clinical practice for interpreting how obesity impacts prostate cancer risk. We recognize that the mechanisms underlying the associations of obesity on PSA or biopsy rates are not well understood and may relate to other associated factors of obesity such as effects on testosterone or prostate size. Prior studies have demonstrated that PSA production is regulated by testosterone and that obesity is associated with increased prostate size, which may decrease the ability of biopsy attempts to detect cancer.33
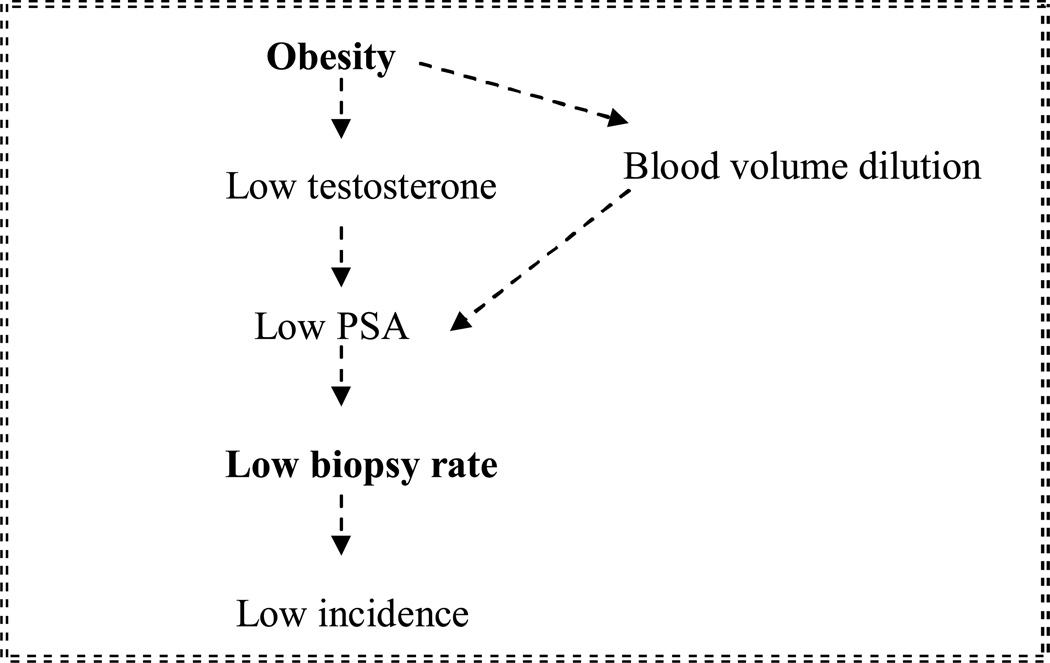
Figure 3. The hypothesized mechanism for lower prostate cancer incidence among obese men.
Figure 3 illustrates the hypothesis that obese men are less likely to reach the biopsy threshold, possibly because of lower testosterone concentrations, which in turn cause PSA concentrations to be lower, ultimately resulting in lower biopsy rates and therefore lower incidence of prostate cancer
Some limitations of this study need to be considered. Although we used large nationally representative samples, the largest weakness of this study is that there was no single survey that had data on the testosterone and PSA concentrations and prostate biopsy rates. Therefore, we linked the results from three national surveys to better understand the impact of obesity on prostate cancer screening-related issues. Limitations of each individual survey must also be considered. Self-reported BMI in the NHIS survey may be subject to measurement error, biasing results towards the null. Second, in NHAES 2001–2004, the inverse associations observed between PSA and obesity were consistent for two major ethnic groups, non-Hispanic whites and Mexican-Americans but not for non-Hispanic blacks. Although it is unclear why similar associations were not observed among the non-Hispanic blacks, we speculate that the smaller sample size of blacks or other genetic variants that were not measured in NHANES may explain the lack of association.
Conclusions and Clinical implications
The present study linking evidence from three large, nationally representative samples of the US population notes that obesity is inversely associated with prostate biopsy rates, leading to a lower detection rate among obese men, possibly explained in part by lower testosterone and PSA levels. The findings of this study have important public health implications. Prostate cancer accounts for approximately 30,000 deaths per year.35 With over 75% American males 40+ years classified as obese or overweight1, progression and mortality from prostate cancer are likely to increase.2, 3 The impact of obesity on prostate cancer screening and biopsy, can influence stage of diagnosis and thus both the incidence and lethality of this cancer.
The reported inverse relationships between testosterone levels and BMI in conjunction with lower biopsy rates among obese individuals in the current study support the theory that ‘high-risk’ obese patients may not be referred for a biopsy as frequently, potentially due to their lower PSA levels. A prostate cancer risk calculator was recently designed to aid clinicians and patients to make informed decisions about prostate biopsies.36 This statistical tool that was designed based on the results of a large clinical trial, considers PSA and other related factors including race, family history, and rectal examinations. To our knowledge, this risk calculator does not incorporate BMI. Based on the findings of our study, we propose that the inclusion of BMI in this tool may improve its performance, and that it will better be able to identify individuals eligible for biopsy.
In conclusion, our study suggests that lower detection should not be interpreted as lower risk of prostate cancer among obese men. Further investigation on the influence of obesity on prostate cancer risk is warranted, specifically among African-Americans to investigate whether this phenomenon differs by ethnicity.
Supplementary Material
ACKNOWLEDGEMENTS
The authors thank Thanusha Puvananayagam and Maria Chondronikola for their assistance.
Funding sources: This study was supported in part by Department of Defense award W81XWG-05-1-0235 and P30CA072720 and research funds from Steinhardt School, New York University.
Footnotes
Competing Interests: The author(s) declare that they have no competing interests.
REFERENCES
- 1.Ogden CL, Carroll MD, Curtin LR, McDowell MA, Tabak CJ, Flegal KM. Prevalence of overweight and obesity in the United States, 1999–2004. JAMA. 2006 Apr 5;295(13):1549–1555. doi: 10.1001/jama.295.13.1549. [DOI] [PubMed] [Google Scholar]
- 2.Amling CL, Kane CJ, Riffenburgh RH, et al. Relationship between obesity and race in predicting adverse pathologic variables in patients undergoing radical prostatectomy. Urology. 2001 Nov;58(5):723–728. doi: 10.1016/s0090-4295(01)01373-5. [DOI] [PubMed] [Google Scholar]
- 3.Freedland SJ, Aronson WJ, Kane CJ, et al. Impact of obesity on biochemical control after radical prostatectomy for clinically localized prostate cancer: a report by the Shared Equal Access Regional Cancer Hospital database study group. J Clin Oncol. 2004 Feb 1;22(3):446–453. doi: 10.1200/JCO.2004.04.181. [DOI] [PubMed] [Google Scholar]
- 4.Giovannucci E, Liu Y, Platz EA, Stampfer MJ, Willett WC. Risk factors for prostate cancer incidence and progression in the health professionals follow-up study. Int J Cancer. 2007 Oct 1;121(7):1571–1578. doi: 10.1002/ijc.22788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wright ME, Chang SC, Schatzkin A, et al. Prospective study of adiposity and weight change in relation to prostate cancer incidence and mortality. Cancer. 2007 Feb 15;109(4):675–684. doi: 10.1002/cncr.22443. [DOI] [PubMed] [Google Scholar]
- 6.Rodriguez C, Patel AV, Calle EE, Jacobs EJ, Chao A, Thun MJ. Body mass index, height, and prostate cancer mortality in two large cohorts of adult men in the United States. Cancer Epidemiol Biomarkers Prev. 2001 Apr;10(4):345–353. [PubMed] [Google Scholar]
- 7.O'Malley RL, Taneja SS. Obesity and prostate cancer. Can J Urol. 2006 Apr;13(Suppl 2):11–17. [PubMed] [Google Scholar]
- 8.Furuya Y, Akimoto S, Akakura K, Ito H. Smoking and obesity in relation to the etiology and disease progression of prostate cancer in Japan. Int J Urol. 1998 Mar;5(2):134–137. doi: 10.1111/j.1442-2042.1998.tb00261.x. [DOI] [PubMed] [Google Scholar]
- 9.Giovannucci E, Rimm EB, Stampfer MJ, Colditz GA, Willett WC. Height, body weight, and risk of prostate cancer. Cancer Epidemiol Biomarkers Prev. 1997 Aug;6(8):557–563. [PubMed] [Google Scholar]
- 10.Shariat SF, Scardino PT, Lilja H. Screening for prostate cancer: an update. Can J Urol. 2008 Dec;15(6):4363–4374. [PMC free article] [PubMed] [Google Scholar]
- 11.Thompson IM, Pauler DK, Goodman PJ, et al. Prevalence of prostate cancer among men with a prostate-specific antigen level < or =4.0 ng per milliliter. N Engl J Med. 2004 May 27;350(22):2239–2246. doi: 10.1056/NEJMoa031918. [DOI] [PubMed] [Google Scholar]
- 12.Efstathiou JA, Chen MH, Catalona WJ, et al. Prostate-specific antigen-based serial screening may decrease prostate cancer-specific mortality. Urology. 2006 Aug;68(2):342–347. doi: 10.1016/j.urology.2006.02.030. [DOI] [PubMed] [Google Scholar]
- 13.Rundle A, Richards C, Neugut AI. Hemodilution of prostate-specific antigen levels among obese men. Cancer Epidemiol Biomarkers Prev. 2009 Aug;18(8):2343. doi: 10.1158/1055-9965.EPI-09-0441. author reply 2343–2344. [DOI] [PubMed] [Google Scholar]
- 14.Ezzati TM, Massey JT, Waksberg J, Chu A, Maurer KR. Sample design: Third National Health and Nutrition Examination Survey. Vital Health Stat 2. 1992 Sep;(113):1–35. [PubMed] [Google Scholar]
- 15.Gunter EW, Lewis BG, Koncikowski SM. Laboratory Procedures Used for the Third National Health and Nutrition Examination Survey (NHANES III), 1988–1994. Hyattsville, MD: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention; 1996. [Google Scholar]
- 16. [Accessed April 30, 2010];Third National Health and Examination Survey 1988–1994 documentation, codebook and frequencies Inhibin B, Luteinizing hormone, Testosterone. 2009 http://www.cdc.gov/nchs/data/nhanes/nh3data/htm.
- 17. [Accessed April 30, 2010];The World Health Organization Report. http://www.who.int/whr/2002/chapter4/en/index4.html.
- 18.Centers for Disease Control and Prevention (CDC) and, National Center for Health Statistics. [Accessed April 30 2010.];National Health and Nutrition Examination Survey (NHANES): Questionnaires, Examination Components, and Laboratory Components, 2003–2004. 2008 http://www.cdc.gov/nchs/nhanes/nhanes2003-2004/questexam03_04.htm.
- 19. [Accessed April 30, 2010];The National Health and Examination Survey 2003–2004 documentation on MEC Laboratory Component: Prostate Specific Antigen (PSA) 2006 http://www.cdc.gov/nchs/data/nhanes/nhanes_03_04/l11psa_c.pdf.
- 20.Werny DM, Thompson T, Saraiya M, et al. Obesity is negatively associated with prostate-specific antigen in U.S. men, 2001–2004. Cancer Epidemiol Biomarkers Prev. 2007 Jan;16(1):70–76. doi: 10.1158/1055-9965.EPI-06-0588. [DOI] [PubMed] [Google Scholar]
- 21.Division of Health Interview Statistics, National Center for Health Statistics. 2000. Hyattsville, MD: National Health Interview Survey (NHIS) Public Use Data Release: NHIS Survey Description; 2000. [Google Scholar]
- 22.Agresti A. Categorical Data Analysis. Second Edition. Wiley; 2002. [Google Scholar]
- 23.Osuna JA, Gomez-Perez R, Arata-Bellabarba G, Villaroel V. Relationship between BMI, total testosterone, sex hormone-binding-globulin, leptin, insulin and insulin resistance in obese men. Arch Androl. 2006 Sep-Oct;52(5):355–361. doi: 10.1080/01485010600692017. [DOI] [PubMed] [Google Scholar]
- 24.Mohr BA, Bhasin S, Link CL, O'Donnell AB, McKinlay JB. The effect of changes in adiposity on testosterone levels in older men: longitudinal results from the Massachusetts Male Aging Study. Eur J Endocrinol. 2006 Sep;155(3):443–452. doi: 10.1530/eje.1.02241. [DOI] [PubMed] [Google Scholar]
- 25.Gapstur SM, Kopp P, Gann PH, Chiu BC, Colangelo LA, Liu K. Changes in BMI modulate age-associated changes in sex hormone binding globulin and total testosterone, but not bioavailable testosterone in young adult men: the CARDIA Male Hormone Study. Int J Obes (Lond) 2007 Apr;31(4):685–691. doi: 10.1038/sj.ijo.0803465. [DOI] [PubMed] [Google Scholar]
- 26.Culp S, Porter M. The effect of obesity and lower serum prostate-specific antigen levels on prostate-cancer screening results in American men. BJU Int. 2009 Nov;104(10):1457–1461. doi: 10.1111/j.1464-410X.2009.08646.x. [DOI] [PubMed] [Google Scholar]
- 27.Lu-Yao G, Albertsen PC, Stanford JL, Stukel TA, Walker-Corkery ES, Barry MJ. Natural experiment examining impact of aggressive screening and treatment on prostate cancer mortality in two fixed cohorts from Seattle area and Connecticut. BMJ. 2002 Oct 5;325(7367):740. doi: 10.1136/bmj.325.7367.740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Giovannucci E, Rimm EB, Liu Y, et al. Body mass index and risk of prostate cancer in U.S. health professionals. J Natl Cancer Inst. 2003 Aug 20;95(16):1240–1244. doi: 10.1093/jnci/djg009. [DOI] [PubMed] [Google Scholar]
- 29.MacInnis RJ, English DR, Gertig DM, Hopper JL, Giles GG. Body size and composition and prostate cancer risk. Cancer Epidemiol Biomarkers Prev. 2003 Dec;12(12):1417–1421. [PubMed] [Google Scholar]
- 30.Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med. 2003 Apr 24;348(17):1625–1638. doi: 10.1056/NEJMoa021423. [DOI] [PubMed] [Google Scholar]
- 31.Andersson SO, Wolk A, Bergstrom R, et al. Body size and prostate cancer: a 20-year follow-up study among 135006 Swedish construction workers. J Natl Cancer Inst. 1997 Mar 5;89(5):385–389. doi: 10.1093/jnci/89.5.385. [DOI] [PubMed] [Google Scholar]
- 32.Fesinmeyer MD, Gulati R, Zeliadt S, Weiss N, Kristal AR, Etzioni R. Effect of population trends in body mass index on prostate cancer incidence and mortality in the United States. Cancer Epidemiol Biomarkers Prev. 2009 Mar;18(3):808–815. doi: 10.1158/1055-9965.EPI-08-0784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Freedland SJ, Platz EA, Presti JC, Jr, et al. Obesity, serum prostate specific antigen and prostate size: implications for prostate cancer detection. J Urol. 2006 Feb;175(2):500–504. doi: 10.1016/S0022-5347(05)00162-X. [DOI] [PubMed] [Google Scholar]
- 34.Freedland SJ, Platz EA. Obesity and prostate cancer: making sense out of apparently conflicting data. Epidemiol Rev. 2007;29:88–97. doi: 10.1093/epirev/mxm006. [DOI] [PubMed] [Google Scholar]
- 35.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer Statistics 2009. CA Cancer J Clin. 2009 Jul 1;59(4):225–249. doi: 10.3322/caac.20006. 2009. [DOI] [PubMed] [Google Scholar]
- 36.Thompson IM, Ankerst DP, Chi C, et al. Assessing Prostate Cancer Risk: Results from the Prostate Cancer Prevention Trial. J. Natl. Cancer Inst. 2006 Apr 19;98(8):529–534. doi: 10.1093/jnci/djj131. 2006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.