Abstract
Purpose
Radiolabeled oligomers complementary to the 16S rRNA in bacteria were investigated as bacterial infection imaging agents.
Methods and Results
Identical sequences with backbones phosphorodiamidate morpholino (MORF), peptide nucleic acid (PNA), and phosphorothioate DNA (PS-DNA) were 99mTc-labeled and evaluated for binding to bacterial RNA. MORF binding to RNA from E. coli strains SM101 and K12 was 4-fold and 150-fold higher compared to PNA, and PS-DNA, respectively. Subsequently MORF oligomer in fluorescence in situ hybridization showed a stronger signal with study MORF compared to control in fixed preparations of two E. coli strains and K. pneumoniae. Flow cytometry analysis showed study MORF accumulation to be 8- and 80-fold higher compared to the control in live K. pneumoniae and S. aureus, respectively. Further, fluorescence microscopy showed increased accumulation of study MORF over control in live E. coli and K. pneumonia. Binding of 99mTc-study MORF to RNA from E. coli SM101 and K12 was 30.4 pmoles and 117.8 pmoles, respectively, per 1010 cells. Mice with K. pneumoniae live or heat-killed (sterile inflammation) in one thigh at 90 min for both 99mTc-study MORF and control showed higher accumulation in target thighs than in blood and all organs other than kidney and small intestines. Whereas accumulation of 99mTc-study MORF was significantly higher (p=0.009) than that of the control in the thigh with sterile inflammation.
Conclusion
A 99mTc-MORF oligomer complimentary to the bacterial 16S rRNA demonstrated binding to bacterial RNA in vitro with specific accumulation into live bacteria. Radiolabeled MORF oligomers antisense to the bacterial rRNA may be useful to image bacterial infection.
Keywords: MORF, PNA, PS-DNA, bacterial infection imaging, SPECT/CT
1. Introduction
Most radiolabeled agents for infection imaging are markers of the infection/inflammatory process and are unable to discriminate between the two conditions. Examples include gallium-67 [1], indium-111 or technetium-99m (99mTc) labeled leucocytes [2,3], cytokines [4], and chemotactic peptides [5]. Agents with specificity for binding to bacteria would seem to be an appropriate choice as a potential bacteria specific imaging agent. Already under investigation are 99mTc-infecton (antibiotic ciprofloxacin) [6] and 99mTc-ubiquicidin (UBI), an antimicrobial peptide [7]. External noninvasive imaging agents with sufficient sensitivity to distinguish between infection and sterile inflammation are still urgently needed. An attractive potential target is bacterial ribosomal RNAs that are abundant in replicating and metabolically active bacteria [8]. The use of radiolabeled oligomers with base sequences antisense to mammalian mRNAs have been successfully used to image tumors [9-11], the same approach should target bacterial RNA as well. In this investigation short oligomers complementary to the bacterial 16S ribosomal RNA (rRNA), a component of the 30S subunit of prokaryotic ribosomes, were investigated for this application. Several DNA oligomers with base sequences complementary to the bacterial 16S rRNA have been used for bacterial identification in vitro for many years [12] and both peptide nucleic acid (PNA) and phosphorodiamidate morpholinos (MORF) oligomers have been studied for the treatment of bacterial infection in mice through an antisense mechanism as alternatives to antibiotics [13-15].
In this investigation, an 18 mer oligomer sequence identified elsewhere, Eub338, has been used that is complementary to an 18 mer segment of the 16S rRNA found in most if not all bacteria [16]. Since the phosphodiester DNA is unstable to nucleases [17], and since the pharmacokinetics and binding properties of oligomers can depend on their structure [18] three different oligomer types were studied as alternatives to the native phosphodiester DNA: PNA; phosphorothioate DNA (PS-DNA) and MORF. Each oligomer type has previously been radiolabeled in this laboratory with 99mTc for various applications [9,10,19,20]. These oligomers differ in the linkages between the bases and in charge, but each is stable to nucleases and each maintains the proper structure for complementary base pairing and stable hybridization. In each case, the 18 mer base sequence was reduced to 12 mer based on findings for PNA by Good et al [13] and for MORF by Deere et al [15], that the optimum length for traversing the bacterial cell wall was 9-12 mer. The study sequence was therefore 5′ GCT GCC TCC CGT in which 6 bases were removed from the 3′ equivalent end while the control sequence was 5′ AGG GCA TCC TCA with 6 bases removed from the 5′ equivalent end to keep a similar G and C content between the two sequences.
In this report, we first compared the three oligomer types to identify MORF as the best binder to its target RNA in Gram positive and Gram negative bacteria, and thereafter using that oligomer type, demonstrated its accumulation and binding to the bacterial RNA in vitro while labeled either with a fluorophore or with 99mTc. We also evaluated the biodistribution and targeting potential of the 99mTc-MORF in mice with live Klebsiella pneumonia (K. pneumonia) or heat-killed K. pneumoniae (sterile inflammation) in one thigh.
2. Material and Methods
Bacterial cultures: Escherichia coli (E. coli) K12 and E. coli SM101 were purchased from the E. coli Genetic Stock Center (Yale University, New Haven, CT) and were grown in Luria-Bertani (LB) medium. The E. coli SM101 is deficient in the UDP-N-acetylglucosamine acyltransferase, and because of its low lipid A content, the outer membrane is more permeable than the non-mutant strain of E. coli [21]. The K. pneumonia and Staphylococcus aureus (S. aureus) were from the American Type Culture Collection (Rockville, MD) and were grown in nutrient broth or tryptic soy broth respectively. Only E. coli SM101was grown at 28°C while the remaining bacteria (E. coli K12, K. pneumonia and S. aureus) were grown at 37°C, unless stated otherwise.
The Alexa Fluor 633 carboxylic acid succinimidyl ester (AF633) and the lipophilic membrane dye FM 1-43 were from Invitrogen (Eugene, OR). The 99mTc-pertechnetate was eluted from a 99Mo-99mTc generator (Perkin-Elmer, Billerica, MA). The S-acetyl NHS-MAG3(NHS-MAG3) was synthesized in house [22]. The HPLC system was equipped with a 515 pump, an in-line dual variable UV detector and an in-line gamma-radioactivity detector under the control of Millennium 32 software (Waters, Milford, MA).
The three oligomers: PS-DNA (Integrated DNA Technologies, Coralville, IA), PNA (Biosynthesis, Lewisville, Texas) and MORF (Gene Tools, Philomath, OR) were purchased with the study and control sequences, each with a primary amine attached via a 6 carbon linker on the 3′ equivalent end for conjugation either to the fluorophore or the MAG3 chelator.
2.1. Oligomer conjugation
The amine-derivatized PS-DNA, PNA and MORF oligomers were conjugated with NHS-MAG 3 for radiolabeling with 99mTc using methods standard in this laboratory [22]. In brief, a solution of 300 μg of oligomer in 200 μl of 0.3 M HEPES buffer (pH 8.0) was added to a vial containing 0.7-1.0 mg NHS-MAG3 and immediately mixed on a vortex to form a clear solution, and then left for 1 h at room temperature then purified as described previously [22]. Thereafter, to the solution was added 50 μl of 1 M ammonium acetate and 120 μl of freshly prepared 20 mg/ml stannous chloride (SnCl2 2H2O)/tartrate solution (100 mg/ml sodium tartrate in 0.5 M ammonium bicarbonate, 0.25 M ammonium acetate, and 0.18 M ammonium hydroxide, pH 9.2) with agitation. After heating at 95 °C for 20 min, the mixture was allowed to come to room temperature, and absolute ethanol was added to a final concentration of 20% (v/v) before purification on a 1 × 20 cm Bio-Gel P-2 size exclusion column (Bio-Rad, Hercules, CA) using 0.25 M ammonium acetate pH 7.0 as eluant. The PS-DNA and PNA concentrations were determined at 260 nM and MORF was at 265 nM.
For flow cytometry and fluorescence microscopy, the amine derivatized MORFs were conjugated with the fluorophore AF633. Briefly, 200 μg in 0.1M sodium bicarbonate buffer pH 8.4 were mixed with AF633 (at 10 mg/ml in N-methyl-2-pyrrolidone, Sigma Aldrich, St. Louis, MO) at an AF633 to MORF molar ratio of 10:1. After 45 min incubation in the dark, the mixture was purified on a 1 × 20 cm P-2 column using 0.25 M ammonium acetate buffer pH 7.0 as eluant.
2.2. Oligomer radiolabeling
The oligomers were radiolabeled with 99mTc using methods standard in this laboratory [22]. In brief, the MAG3 conjugated oligomers (about 1 μg in 4 μl) were added to a combined solution of 45 μ l 0.25 M ammonium acetate, and 15 μl 50 mg/ml tartrate solution followed by 2 μl of freshly prepared 10 mg/ml SnCl2-2H2O solution in 10 mM HCl with 1 mg/ml ascorbate. After mixing on a vortex, the 99mTc pertechnetate (2-5 μl with 200-500 μCi) was added and agitated, followed by heating at 95 °C for 20 min. Radiochemical purity was determined by size exclusion HPLC on a Superose-12 column (Amersham Pharmacia Biotech, Piscataway, NJ) with running solution of 20% acetonitrile in 0.1 M Tris-HCl pH 8.0 at a flow rate of 0.6 ml/min. Radioactivity recovery was routinely measured.
2.3. Hybridization of radiolabeled oligomers to isolated total RNA
Total RNA was isolated from E. coli strains SM101 and K12 using the TRIzol® Max™ bacterial RNA isolation kit from Invitrogen (Eugene, OR) following the manufacturer’s instructions. In brief, the bacteria were cultured as usual on a shaker until log phase, and then 1.5 ml of the culture was spun at 6,000 × g for 5 min at 4°C to pellet the cells. The medium was discarded and the pellet was resuspended in 200 μl of Max Bacterial Enhancement Reagent preheated to 95°C and the sample was incubated at 95°C for 4 min followed by addition of 1 ml TRIzol®reagent. After 5 min at room temperature, 0.2 ml cold chloroform was added, and the sample vigorously shaken and left at room temperature for another 2-3 min before the sample was spun at 12,000 × g for 15 min at 4°C to separate the aqueous and chloroform phases. The top colorless aqueous phase containing the RNA was transferred to a fresh tube, to which was added 0.5 ml cold isopropanol to precipitate the RNA. After 10 min at room temperature the sample was spun at 15,000 × g for 10 min at 4°C. The RNA containing pellet was resuspended in 1 ml 75% ethanol, mixed well and spun, now at 7,500 × g for 5 min at 4°C. The RNA pellet was air-dried and resuspended in 50 μl RNase-free diethyl pyrocarbonate treated water (MP Biomedicals LLC, Solon, OH). The RNA concentration was determined by OD at 260 nm using 25 μl/μg/cm as the RNA extinction coefficient.
Following the TRIzol® kit instructions samples containing 2.5 μg of RNA in about 1.5 μl were denatured by adding to 100 μl of 10 mM NaOH containing 1 mM EDTA before immediately transfer to wells of a 96-well Millipore Multiscreen membrane filtration plate (Multiscreen HTS, Millipore, MA). The RNA was absorbed to the membrane by applying a vacuum. The wells were then incubated with 150 μl ExpressHyb Solution (Clontech Laboratories, Mountain View, CA) with shaking at 37°C for 30 min, before the solution was replaced with fresh ExpressHyb Solution containing 21.6 ng of 99mTc-labeled study or control oligomers of PS-DNA, MORF or the study PNA oligomer each with a specific activity of about 0.375 μCi/ng. The amount of labeled oligomer used per sample was in the range recommended for hybridization with the ExpressHyb™ solution. After incubation with continuous shaking at 37°C for 1 h, the solution was removed; the wells were washed with a solution containing 0.3 M NaCl, 30 mM tri-sodium citrate dihydrate, pH 7.0, and 0.05% sodium dodecyl sulfate (SDS, Sigma Aldrich) several times with agitation. Finally the wells were washed with a solution containing 15 mM NaCl, 1.5 mM tri-sodium citrate dihydrate, pH 7.0, and 0.1% SDS with continuous shaking at room temperature for 40 min with one change of wash solution. The membranes with the absorbed RNA were removed from each well and the radioactivity counted in a gamma well counter.
2.4. Hybridization of fluorescent MORFs to total RNA in fixed cells by FISH
In preparation for fluorescence in situ hybridization (FISH), E. coli SM101, E. coli K12 and K. pneumoniae were fixed with 4% formaldehyde in Dulbecco’s PBS (D-PBS) by adding one volume of bacterial cell culture grown to log phase, to three volumes of 4% formaldehyde, followed by gentle mixing on a vortex and then incubation at room temperature for at least 3 h. The cells were separated by centrifugation at 12,000 × g for 2 min at 4°C, washed with D-PBS to remove residual formaldehyde, spun again, and the pellet resuspended at a concentration of 108 to 109 cells per ml in D-PBS. The fixed cell suspension was mixed with an equal volume of cold absolute ethanol and stored at −20°C.
For hybridization the method of Ouverney et al was followed [23], briefly, 3 μl of the fixed bacterial cell suspension prepared in ethanol-D-PBS (50:50) was deposited onto an 8-chambered cover glass slide (Lab-Tek, Rochester, NY) and air dried. The AF633 conjugated study or control MORF was added at 5 ng/μl in 150 μl buffer containing 750 mM NaCl, 100 mM Tris-Cl pH 7.8, 5 mM EDTA, 0.2% bovine serum albumin (Sigma Aldrich), 10% dextran sulfate (MW 500 kD; Calbiochem, Gibbstown, NJ), 0.01% polyadenylic acid (Sigma Aldrich) and 0.1% SDS, as described by Ouverney et al [23], and incubated at 43°C for 2 h. The chambers of the slide were then washed with distilled water at 43°C, and then washed for 30 min at 43°C with buffer containing 30 mM NaCl, 4 mM Tris-Cl pH 7.8, 0.2 mM EDTA with two changes of wash solution. To stain the cell membranes, 0.2 μl FM1-43 (Invitrogen) (5 μg/ μl) was added about 10 min before viewing the cells under oil immersion with 100× objective on an Olympus IX-70 inverted microscope (Olympus America, Inc., Center Valley, PA).
2.5. Accumulation of fluorescent and radiolabeled MORFs in live bacteria
For flow cytometry analysis, the K. pneumoniae and S. aureus bacteria from an overnight culture were diluted with media and incubated with shaking until log phase was reached (OD at 600 nm of 0.6). A 1 ml sample of the culture was spun at 12,000 × g for 2 min; the pellet was washed with 0.85% NaCl and resuspended in 1 ml of 0.85% NaCl. Then 5 μl of the AF633-conjugated study or control MORF and 10 μl of bacterial suspension were added to a tube containing 985 μl of 0.85% NaCl, and incubated for 2 h at 37°C with rocking while protected from light. After incubation, the samples were washed with 0.85% NaCl and resuspended in 500 μl 0.85% NaCl for analysis using a FACSCalibur flow cytometer (Becton Dickinson, Franklin Lakes, NJ). Control samples included bacteria alone and AF633 alone, both in 0.85% NaCl.
For fluorescence microscopy, an overnight culture of E. coli SM101, E. coli K12 and K. pneumoniae was diluted 1:50 with their respective media, and 200 μl of the diluted culture was mixed with about 15 μl of the AF633-conjugated study or control MORF to a final concentration of 15 ng/ μl and incubated for 2 h at 28 °C for E. coli SM101 and 37 °C for E. coli K12 and K. pneumonia on a lab rocker in the dark. After incubation, the samples were washed with 0.85% NaCl and resuspended in 200 μl 0.85% NaCl before 3 μl of the incubation mixture were placed into a single chamber of an 8-chamber cover glass slide followed by addition of 0.2 μl of the membrane stain FM1-43 at 5 μg/μl. The samples were then air dried, and mounted with fluorescence mounting medium (Dako, Carpintaria, CA) and viewed under oil immersion with 100 × objective on an Olympus IX-70 inverted microscope.
The accumulation and binding to RNA of the 99mTc-labeled MORFs were also evaluated in live cells. To be consistent with the fluorescence microscopy study, E. coli SM101 and E. coli K12 were used again. Overnight bacterial cultures of E. coli SM101 and K12 were diluted 1:50 with media, and 5 ml containing 10×1010 E. coli SM101 or 1.5×1010E. coli K12 were mixed with 0.5 nmole of either the 99mTc-labeled study or control MORF at a specific activity of 30 μCi/μg and incubated at the temperatures mentioned above on a lab rocker for 2 h. Thereafter, the samples were split with transfer of 1.5 ml into each of 3 microcentrifuge tubes, washed three times with 0.85% NaCl, and total RNA was isolated as before. The RNA fraction was carefully transferred to fresh tubes and measured for radioactivity in a gamma well counter and results reported as nanomoles bound per 1010 cells. To determine the number of bacteria in the incubation mixture, 100 μl of the incubation mixture was serially diluted and each dilution was spread on a separate LB agar plate and grown overnight. The following day the bacterial cell count was determined from the colony number on each plate and dilution factor.
2.6. Biodistributions of radiolabeled MORFs in mice with live or heat killed bacteria
With the approval of the UMMS Institutional Animal Care and Use Committee, biodistribution of the 99mTc-labeled study or control MORFs were determined in CD-1 mice (Charles River Laboratories International, Inc, Wilmington, MA) with live or heat killed K. pneumoniae injected in one thigh. An overnight culture of K. pneumoniae was diluted with culture medium to an OD at 600 nm of 0.6. The preparation was divided in half. One half was used for the live preparation while the remaining half was heated in a boiling water bath for 30 min to sterilize the culture and to provide a sample for injection of bacterial debris possibly including intact rRNA [24]. Then 0.1 ml of either the live or heat killed preparation of K. pneumoniae was injected subcutaneously into one thigh of CD-1 mice (n = 4). After 2 h the mice developed a minor infection or inflammation and received about 1 μg, 200 μCi, of either the 99mTc-labeled study or control MORF in 0.1 ml saline through a tail vein. The 2 h time was selected to have a mild bacterial infection with minimal inflammatory reaction. Mice were sacrificed 90 min later, and organs of interest and blood were removed, weighed, and counted in a gamma well counter.
2.7. SPECT/CT imaging of radiolabeled MORFs in mice with live bacteria
The 99mTc-labeled study MORF in mice with live K. pneumoniae injected in one thigh was imaged on a NanoSPECT/CT (Bioscan, Washington, DC) small animal camera. An overnight culture of K. pneumoniae was diluted with culture medium to an OD at 600 nm of 0.6, and 0.1 ml of the live preparation was injected subcutaneously into one thigh of CD-1 mice (n = 3). After 2 h the mice received about 3 μg, 600 μCi of the 99mTc-labeled study MORF in 0.1 ml saline through a tail vein. Immediately after injection of 99mTc-MORF, the mice were anesthetized with 1-2% isoflurane carried in oxygen and whole body scans were acquired with 24 projections and 60 sec per projection.
2.8. Statistical analysis
The Student’s t-test was used to test for significance where indicated.
3. Results
3.1. Oligomer radiolabeling
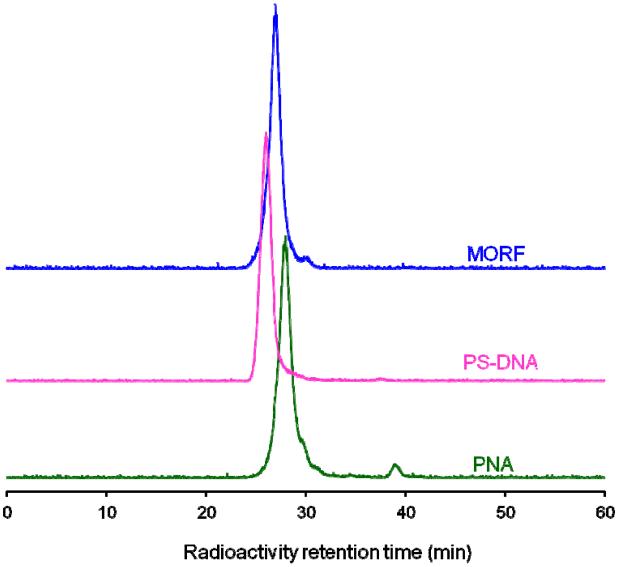
Fig. 1 presents the size exclusion HPLC radiochromatograms of the 99mTc-labeled study PNA, PS-DNA and MORF oligomers. While the retention time is slightly different among the three oligomer types, each shows essentially one major peak for the labeled oligomer. Unassociated 99mTc-MAG3 elutes at a later retention time, about 30 -32 min and is seen as the small shoulder to the right of the major peak. There is no evidence of dimerization as would be seen as a shoulder to the left of the major peak. Radioactivity recovery from the HPLC was always greater than 95% and radiochemical purity was greater than 90% for all preparations.
Fig. 1.
HPLC radiochromatograms of 99mTc-labeled study MORF, PS-DNA and PNA oligomers, in each case showing a single peak with radiochemical purity greater than 90%.
3.2. Hybridization of radiolabeled oligomers to isolated total RNA
Each study oligomer was evaluated for binding to the bacterial RNA. Total RNA isolated from E. coli strains SM101 and K12 was incubated with 99mTc-labeled study oligomers. After incubation and washing, the membranes were removed from each well and counted in a gamma well counter. Fig. 2 shows that binding to the total RNA from both E. coli strains was 4-fold higher for the 99mTc-labeled study MORF compared to the PNA, and 150-fold higher compared to the PS-DNA study oligomers. Furthermore, the binding to total RNA was statistically higher (p < 0.01) for the 99mTc-labeled study MORF compared to the labeled control MORF in both strains (data not shown). As a result of these observations showing higher binding of the study MORF to total RNA, this oligomer type was used in all subsequent studies.
Fig. 2.
Histogram showing percent of radioactivity bound following incubation of 99mTc-labeled MORF, PS-DNA and PNA oligomers with RNA isolated from E. coli strains SM101 and K12. The average percent of added radioactivity bound is presented with error bars shown representing one standard deviation (n = 4).
3.3. Hybridization of fluorescent MORFs to total RNA in fixed cells by FISH
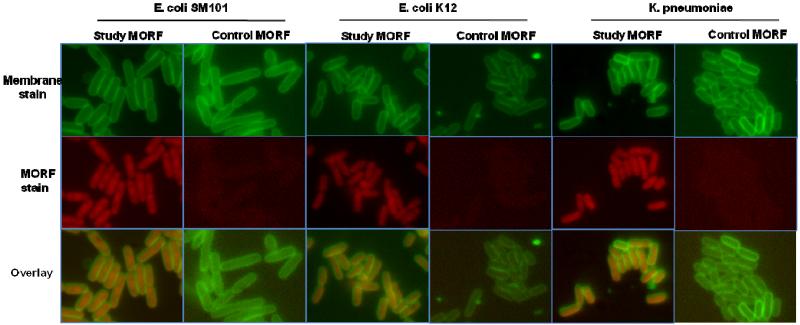
Binding of the study MORF to the total RNA from E. coli SM101, E. coli K12 and K. pneumoniae was measured in fixed cells by incubation of AF633-labeled study or control MORF. Fig. 3 presents fluorescence microscopy showing the bacterial membranes stained with FM1-43 (green, top row), the location of the MORF oligomer with AF633-MORF (red, middle row) and an overlay of both (bottom row) for E. coli SM101, E. coli K12 and K. pneumoniae. The strong red signal visibly within the bacterial cell for the study MORF in all three bacterial strains is evidence of accumulation and presumably hybridization of the study sequence to the bacterial RNA. Only weak background staining is evident for the control MORF.
Fig. 3.
Fluorescence in situ hybridization showing accumulation and presumably hybridization of the MORF oligomers to fixed E. coli SM101, E. coli K12, and K. pneumoniae. Top row shows the membrane stained with FM1-43 (green), middle row shows MORFs conjugated with AF633 (red), and bottom row shows overlay of the two colors. (Magnification 1000 ×).
3.4. Accumulation of fluorescent and radiolabeled MORFs in live bacteria
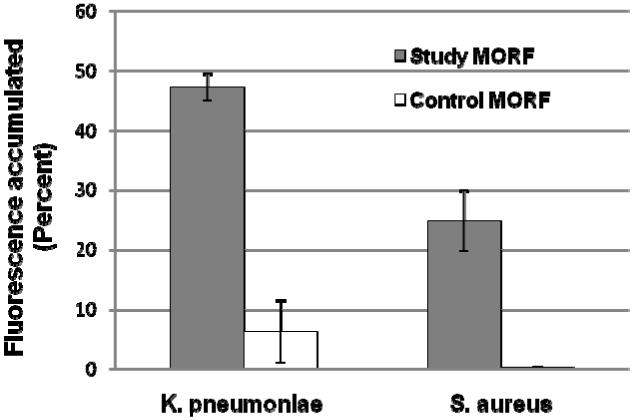
The accumulation of AF633-labeled study and control MORF oligomers in live bacteria was evaluated by flow cytometry and fluorescence microscopy. Fig. 4 presents the flow cytometry results that show the study MORF with about a 2-fold higher accumulation in K. pneumonia than S. aureus, but with an 8-fold higher binding of the study MORF to K. pneumoniae (p=0.002) and 80-fold higher binding to S. aureus (p=0.007) compared to the control MORF.
Fig. 4.
Histograms showing the results of flow cytometry analysis measuring the accumulation of study and control MORFs into live K. pneumoniae and S. aureus after incubation for 2 h at 37°C. The average percent of fluorescence accumulated is presented with error bars representing one standard deviation (n = 3).
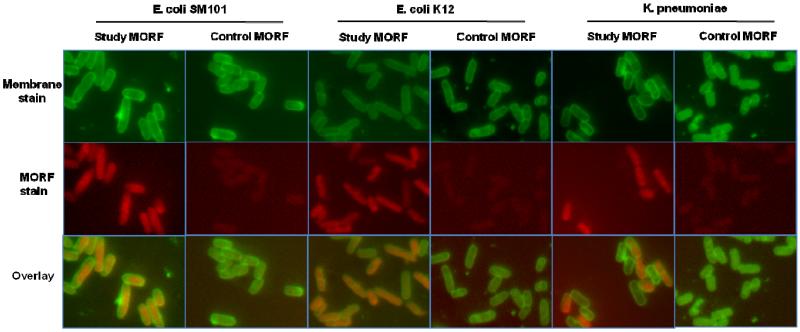
The results of fluorescence microscopy shown in Fig. 5 confirmed the incorporation of AF633-labeled MORFs into the same three live bacterial strains E. coli (SM101 and K12) and K. pneumoniae and confirmed the increased accumulations of the study MORF compared to the control MORF. The results of both flow cytometry and fluorescence microscopy demonstrate that under culture conditions, the study MORF can accumulate in live bacterial cells.
Fig. 5.
Fluorescence microscopy images showing the accumulation of study and control MORFs in live E. coli (SM101 and K12) and K. pneumoniae after 2 h. Top row shows the cell membrane stained with FM1-43, middle row shows MORFs conjugated with AF633, and bottom row shows overlay of the two colors. (Magnification 1000 ×).
To confirm further the accumulation of the study MORF into live bacteria and to provide direct evidence for the binding to bacterial RNA, the 99mTc-labeled study and control MORFs were incubated with E. coli SM101 or E. coli K12 for 2 h before RNA was isolated and counted for label bound. The amount of MORF bound to RNA from E. coli SM101 normalized per 1010 cells was 30.4 pmoles for the 99mTc-labeled study MORF with 14.5 pmoles found for the control MORF (p=0.14), likely due to weak base paring in the case of the control. Similarly the amount of MORF bound to RNA from E. coli K12 was 117.8 pmoles for the study MORF with 57.9 pmoles, for the control probe (p=0.002). In each case the specific probe was twice that observed for the control. The values observed for the control probe were likely due to non-specific sticking to surfaces and perhaps weak association of complementary bases. Nevertheless, the higher binding of the study MORF over the control MORF in both cases was most likely the results of specific binding to the RNA of each E. coli strain.
3.5. Biodistribution of radiolabeled MORFs in mice with live or heat killed bacteria
Normal mice were administered live or heat killed K. pneumoniae to evaluate whether 99mTc-labeled MORF can distinguish a live bacterial infection from a sterile inflammation as originating from the heat killed bacterial preparation. K. pneumonia was selected because this strain is multidrug resistant and a serious concern in the clinic [25]. Two hours post injection of bacteria, radiolabeled MORFs were administrated intravenously and the animals were killed 90 min later. Table 1 presents the biodistribution results in mice as percent injected dose per gram with either live or heat killed K. pneumoniae in one thigh. As we have observed previously in mice, the kidneys are the organ of greatest accumulation of 99mTc-labeled MORFs [26]. We also observed earlier that kidney accumulation in mice of 99mTc-labeled MORF oligomers increase in proportion to the number of cytosines in the sequence [26]. Presumably that will explain the higher accumulation in kidney of the study MORF with six cytosines compared to that of the control with only four. Other organs show no significant differences in accumulations between the two MORFs in either the live or heat killed bacteria models, so the biodistributions of these MORFs are similar. Apart from the intestines, the next highest accumulations were in the target thigh for both MORFs in both animal models (live and heat killed). However, in the live bacterial model the target thigh showed higher accumulation for the study MORF compared to the control MORF, but the difference was not significant (p=0.13). Although, the difference in thigh accumulations between the live versus heat killed model was significant for both the study MORF as well as the control MORF both at p=0.003. In addition, in the heat killed model alone, the accumulation in the target thigh was statistically higher (p=0.009) for the study MORF compared to the control.
Table 1.
Biodistribution 90 min post administration of 99mTc labeled study and control MORFs to mice receiving either live or heat killed K. pneumoniae 2 h earlier.
| Live | Heat killed | |||
|---|---|---|---|---|
|
|
||||
| Tissue | Study MORF | Control MORF | Study MORF | Control MORF |
| Liver | 0.19 (0.06) | 0.18 (0.07) | 0.17 (0.03) | 0.13 (0.02) |
| Heart | 0.14 (0.04) | 0.12 (0.06) | 0.15 (0.03) | 0.09 (0.01) |
| Kidney | 20.99 (2.28) | 7.02 (1.80) | 18.00 (3.02) | 6.34 (1.49) |
| Lung | 0.24 (0.05) | 0.19 (0.03) | 0.24 (0.10) | 0.17 (0.05) |
| Spleen | 0.11 (0.02) | 0.09 (0.04) | 0.11 (0.02) | 0.08 (0.02) |
| Stomach | 0.08 (0.05) | 0.08 (0.02) | 0.07 (0.01) | 0.08 (0.06) |
| Sm. Int. | 0.69 (0.12) | 0.70 (0.17) | 0.71 (0.08) | 0.71 (0.16) |
| Lg. Int. | 0.09 (0.07) | 0.08 (0.03) | 0.09 (0.00) | 0.07 (0.02) |
| Muscle | 0.17 (0.07) | 0.12 (0.06) | 0.15 (0.05) | 0.07 (0.02) |
| Target thigh | 0.57 (0.03) | 0.49 (0.07) | 0.39 (0.04) | 0.25 (0.05) |
| Normal thigli | 0.22 (0.04) | 0.18 (0.06) | 0.21 (0.04) | 0,11 (0.02) |
| Blood | 0.18 (0.08) | 0.15 (0.05) | 0.18 (0.06) | 0.11 (0.03) |
Data are percentage injected dose per gram (mean of n = 4, with SD in parentheses).
3.6. SPECT/CT imaging of radiolabeled MORFs in mice with live bacteria
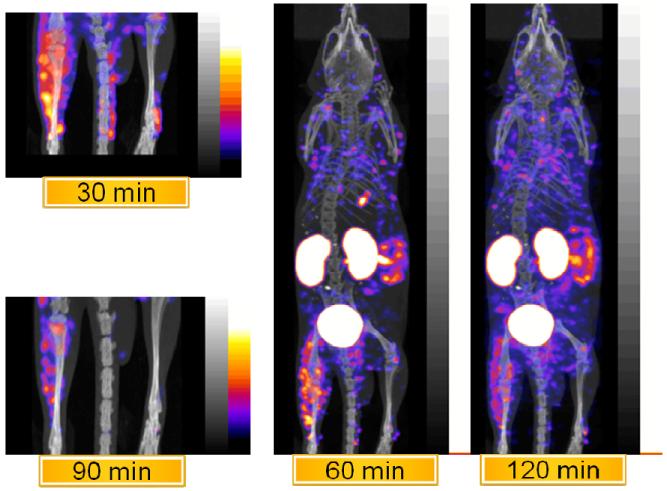
Fig. 6 presents representative SPECT/CT whole body images and spot images of the lower extremities including the infected thigh of the same mouse obtained from 30 to 120 min on a small animal camera. As in the biodistribution study, the organs of highest accumulation of activity are the kidneys and the small intestines with all other organs showing limited accumulation including skin and large intestine. The activity observed in the small intestine at 60 min changes little in the next hour and may suggest binding to endogenous bacteria in the gut, whereas and the distribution of scattered spots about the torso represents what is most probably background signal, but some areas may possibly be MORF bound to bacteria that has been carried from the target site in the leg. A further examination of these areas was not made.
Fig. 6.
Representative SPECT/CT whole body and spot images of the 99mTc-study MORF in the same mouse obtained at the times indicated with a K. pneumoniae infection in one thigh (n = 3).
The infected thigh (left in each image) shows obvious accumulation through 120 min post administration of the 99mTc-labeled study MORF and minimal activity in the normal thigh.
4. Discussion
Regions within the bacterial rRNA have been conserved over time while other regions have varied as a result of random mutations, leading to the bacterial diversity that exists today. Identification of bacterial strains in vitro has been possible by use of oligomers as probes designed to target either the conserved or variant regions using methods such as in situ hybridization (ISH) or FISH [12,27,28]. Furthermore, the application of antisense oligomers against this target has been investigated as a potential treatment of bacterial infection in mice [13-15]. Geller et al [14] clearly showed inhibition of bacterial growth in the mouse peritoneum with administration of an 11 mer MORF targeted to the acpP gene in comparison to mice that received a scrambled MORF or buffer alone.
In a study designed to determine whether 99mTc-labeled oligomers with base sequences complementary to that of the rRNA can detect bacterial infection in vivo and possibly distinguish infection from sterile inflammation, we selected the sequence used by others for in vitro bacterial identification known as Eub338 but modified from 18 mer to 12 mer for facile passage through the bacterial cell wall [13,15]. The sequence is expected to be universal in that it binds to a conserved region of the bacterial rRNA and likely to be useful to detect most bacteria, but not distinguish between different bacterial types.
The important properties for radiolabeled oligomers intended for use in vivo include stability to nucleases, low normal tissue accumulation, and good pharmacokinetics. Based on the experience of this laboratory with numerous oligomer backbone chemistries [9,19,20,29], and the work of others [30,31], the three oligomer backbone chemistries selected for these studies were PS-DNA, PNA and MORF.
To identify the best oligomer backbone for this application, three 99mTc labeled oligomers with the same 12 mer sequence were compared for binding to bacterial RNA that was isolated from cells. As shown in Fig. 2, the MORF oligomer was clearly better and, as such, was used in subsequent studies. The PS-DNAs has been reported to form less stable duplexes with RNA due to its high negative charge. This may explain the lower accumulations observed compared to the uncharged MORFs and PNAs [31,32].
Despite shortening the oligomer from 18 to 12 mer, the FISH results presented in Fig. 3 demonstrate that the 12 mer sequence retained the specificity required for hybridization. The flow cytometry results presented in Fig. 4 provide further evidence of specific accumulation of the study compared to the control MORF, in two strains of live bacteria. For reasons not yet established, accumulations of both MORFs were higher in K. pneumoniae (Gram negative) than S. aureus (Gram positive) as shown in Fig. 4, and may be related to the difference in the cell envelop and different expression levels of the target RNA, which can differ between strains and phase of cell growth.
Accumulation of MORFs into live bacteria was further confirmed by fluorescence microscopy using E. coli (SM101 and K12) and K. pneumoniae. In agreement with the flow cytometry results, fluorescence microscopy showed obvious accumulations in live bacteria for the study MORF compared to the control (Fig. 5). Thus, the flow cytometry results presented in Fig. 4, and the results presented in Fig. 5 by fluorescence microscopy, both in live cells, clearly show specific accumulation, almost certainly due to hybridization binding of the fluorescent labeled study MORF compared to the control MORF in each of the three bacterial strains.
The results obtained with radiolabeled MORFs in live E. coli bacteria are similar to that presented in Fig. 4 with fluorescent MORFs in live K. pneumonia and S. aureus, and in Fig. 5 with live E. coli SM101, E. coli K12 and K. pneumonia. In these studies the uptake with the study MORF is higher than that of the control MORF. However, while the flow cytometry results of Fig. 4 only demonstrate differences in cell accumulations, the results with the radiolabeled MORFs demonstrate differences in binding of the MORFs to total RNA. Together, these results show that the increased accumulation of the radiolabeled study MORF is most likely due to binding to the RNA in these cells and that the MORFs were able to enter the bacterial cell.
Since infection due to multidrug resistant K. pneumoniae is increasing and is cause for serious concern in the clinic [25], K. pneumoniae was chosen for further study. Based on the experience in this lab that MORFs show fast clearance in mice, with most out of circulation within 30 min, 90 min post administration of the radiolabeled MORF was used to allow clearance of the non specific binding. The biodistribution at 90 min post administration of the radiolabeled MORFs to mice administered either live or heat killed K. pneumoniae presented in Table 1 shows a rapid whole body clearance and important accumulations in the kidneys. This observation is typical of multiple studies from this laboratory of naked radiolabeled PS-DNA and MORF oligomers in mice in connection with antisense imaging of tumor that all show rapid clearance. However, despite the rapid clearance, the accumulation of the MORFs in the target thigh was second only to the kidney and small intestines in accumulation and the accumulations of the study MORF was statistically higher than the control MORF in the heat killed animal model.
Nevertheless, the difference in accumulation of the study and control MORFs in the infected thigh was insignificant (p=0.13). This may be due in part to the much greater loss of the study MORF to the kidneys, leaving less available in circulation for targeting. If so, modification of the study MORF sequence to reduce the cytosine content may help to decrease the kidney accumulation while maintaining the binding specificity. However, the similar accumulation in the infected thigh of the study and control MORFs may be related less to low accumulations of the study MORF than the high nonspecific accumulations of the control MORF selected for this study. A similar observation in the case of oligomers intended for antisense imaging of tumor in mice was attributed to high nonspecific accumulations in tumor and normal tissues when, as in this case, MORFs are administered intravenous in their naked form [33]. Possibly the specific/nonspecific accumulation ratio may be improved by adjusting the period between oligomer administration and imaging and necropsy by taking advantage of the increased retention of the specific oligomer by binding to its rRNA [34]. Any study that would block specific uptake using excess unlabeled material was not performed, since it was considered that with the exponential bacterial growth and associated steady increase in the target RNA content it would be difficult to determine a reliable blocking agent concentration.
The heat killed preparation was meant to serve as a model for sterile inflammation, but it has been reported that portions of the bacterial RNA can remain stable even after treatment at 100°C [24,35]. So it is unclear whether this preparation contained targetable RNA. If so, this may explain the significant difference between the study and control MORF in the sterile inflammation animal model. But the difference is significant for the 99mTc-study MORF in a comparison of live (infection model) versus heat killed bacteria (sterile inflammation model) with p = 0.003, which indicates that the 99mTc-study MORF may be useful in distinguishing infection from sterile inflammation.
The representative SPECT/CT images (Fig. 6) of K. pneumoniae infection in a mouse thigh using the 99mTc-study MORF showed highest accumulation at 60 min and remained high at 120 min after administration. Except for kidneys and intestine, other organs showed limited accumulations.
5. Conclusion
The study MORF oligomer of this investigation with a sequence complementary to the conserved region of the bacterial 16S rRNA accumulated specifically in live bacteria and almost certainly by specific hybridization to bacterial RNA. This study demonstrates that radiolabeled MORF oligomers with sequences complementary to the bacterial rRNA are feasible in the identification of bacterial infection and may be useful in identification of bacterial infection and may have potential in distinguishing infection from sterile inflammation by imaging.
Acknowledgements
Funding was provided by the National Institutes of Health (AI070857-01A1) to M. Rusckowski.
Abbreviations
- rRNA
ribosomal RNA
- 99mTc
technetium-99m
- MORF
phosphorodiamidate morpholino
- PNA
peptide nucleic acid
- PS-DNA
phosphorothioate DNA
- E. coli
Escherichia coli
- K. pneumonia
Klebsiella pneumonia
- S. aureus
Staphylococcus aureus
- MAG3
S-acetyl NHS-MAG3
- D-PBS
Dulbecco’s PBS
- AF633
Alexa Fluor 633 carboxylic acid succinimidyl ester
- OD
optical density
- FISH
fluorescence in situ hybridization
- SDS
sodium dodecyl sulfate
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
The authors declare that they have no conflict of interest.
References
- 1.Lavender JP, Lowe J, Barker JR, Burn JI, Chaudhri MA. Gallium 67 citrate scanning in neoplastic and inflammatory lesions. Br J Radiol. 1971;44(521):361–366. doi: 10.1259/0007-1285-44-521-361. [DOI] [PubMed] [Google Scholar]
- 2.McAfee JG, Thakur ML. Survey of radioactive agents for in vitro labeling of phagocytic leukocytes. II. Particles. J. Nucl Med. 1976;17(6):488–492. [PubMed] [Google Scholar]
- 3.Peters AM, Danpure HJ, Osman S, Hawker RJ, Henderson BL, Hodgson HJ, Kelly JD, Neirinckx RD, Lavender JP. Clinical experience with 99mTc-hexamethylpropylene-amineoxime for labelling leucocytes and imaging inflammation. Lancet. 1986;2(8513):946–949. doi: 10.1016/s0140-6736(86)90601-x. [DOI] [PubMed] [Google Scholar]
- 4.Chianelli M, Signore A, Fritzberg AR, Mather SJ. The development of technetium-99m-labelled interleukin-2: a new radiopharmaceutical for the in vivo detection of mononuclear cell infiltrates in immune-mediated diseases. Nucl Med Biol. 1997;24(6):579–586. doi: 10.1016/s0969-8051(97)00021-8. [DOI] [PubMed] [Google Scholar]
- 5.Fischman AJ, Pike MC, Kroon D, Fucello AJ, Rexinger D, ten Kate C, Wilkinson R, Rubin RH, Strauss HW. Imaging focal sites of bacterial infection in rats with indium-111-labeled chemotactic peptide analogs. J. Nucl Med. 1991;32(3):483–491. [PubMed] [Google Scholar]
- 6.Britton KE, Vinjamuri S, Hall AV, Solanki K, Siraj QH, Bomanji J, Das S. Clinical evaluation of technetium-99m infecton for the localisation of bacterial infection. Eur J Nucl Med. 1997;24(5):553–556. doi: 10.1007/BF01267688. [DOI] [PubMed] [Google Scholar]
- 7.Welling MM, Paulusma-Annema A, Balter HS, Pauwels EK, Nibbering PH. Technetium-99m labelled antimicrobial peptides discriminate between bacterial infections and sterile inflammations. Eur J Nucl Med. 2000;27(3):292–301. doi: 10.1007/s002590050036. [DOI] [PubMed] [Google Scholar]
- 8.Scheu PM, Berghof K, Stahl U. Detection of pathogenic and spoilage microorganisms in food with the polymerase chain reaction. Food Microbiol. 1998;15:13–31. [Google Scholar]
- 9.Liu X, Wang Y, Nakamura K, Kawauchi S, Akalin A, Cheng D, Chen L, Rusckowski M, Hnatowich DJ. Auger radiation-induced, antisense-mediated cytotoxicity of tumor cells using a 3-component streptavidin-delivery nanoparticle with 111In. J. Nucl Med. 2009;50(4):582–590. doi: 10.2967/jnumed.108.056366. [DOI] [PubMed] [Google Scholar]
- 10.Wang Y, Liu X, Chen L, Cheng D, Rusckowski M, Hnatowich DJ. Tumor delivery of antisense oligomer using trastuzumab within a streptavidin nanoparticle. Eur J Nucl Med Mol Imaging. 2009;36:1977–1986. doi: 10.1007/s00259-009-1201-2. [DOI] [PubMed] [Google Scholar]
- 11.Nakamura K, Wang Y, Liu X, Kubo A, Hnatowich DJ. Cell culture and xenograft-bearing animal studies of radiolabeled antisense DNA carrier nanoparticles with streptavidin as a linker. J. Nucl Med. 2007;48(11):1845–1852. doi: 10.2967/jnumed.106.039339. [DOI] [PubMed] [Google Scholar]
- 12.Moter A, Gobel UB. Fluorescence in situ hybridization (FISH) for direct visualization of microorganisms. J. Microbio Meth. 2000;41:85–112. doi: 10.1016/s0167-7012(00)00152-4. [DOI] [PubMed] [Google Scholar]
- 13.Good L, Awasthi SK, Dryselius R, Larsson O, Nielsen Bacterial antisense effects of peptide-PNA conjugates. Nat Biotechnol. 2001;19:360–364. doi: 10.1038/86753. [DOI] [PubMed] [Google Scholar]
- 14.Geller BL, Deere J, Tilley L, Iversen PL. Antisense phosphorodiamidate morpholino oligomer inhibits viability of Escherichia coli in pure culture and in mouse peritonitis. J. Antimicrob Chemother. 2005;55:983–988. doi: 10.1093/jac/dki129. [DOI] [PubMed] [Google Scholar]
- 15.Deere J, Iversen P, Gekker BL. Antisense phosphorodiamidate morpholino oligomer length and target position effects on gene-specific inhibition in Escherichia coli. Antimicrob Agents Chemother. 2005;49:249–255. doi: 10.1128/AAC.49.1.249-255.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Amann RI, Krumholz L, Stahl DA. Fluorescent-oligonucleotide probing of whole cells for determinative, phylogenetic, and environmental studies in microbiology. J. Bacteriol. 1990;172(2):762–770. doi: 10.1128/jb.172.2.762-770.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sands H, Gorey-Feret LJ, Cocuzza AJ, Hobbs FW, Chidester D, Trainor GL. Biodistribution and metabolism of internally 3H-labeled oligonucleotides. I. Comparison of a phosphodiester and a phosphorothioate. Mol Pharmacol. 1994;45(5):932–943. [PubMed] [Google Scholar]
- 18.Hnatowich DJ, Nakamura K. The influence of chemical structure of DNA and other oligomer radiopharmaceuticals on tumor delivery. Current Opinions Mol Therap. 2006;8:136–143. [PubMed] [Google Scholar]
- 19.Liu G, Dou S, Pretorius PH, Liu X, Chen L, Rusckowski M, Hnatowich DJ. Tumor pretargeting in mice using MORF conjugated CC49 antibody and radiolabeled complimentary cMORF effector. Q J Nucl Med Mol Imaging. 2009;53:1–8. [PMC free article] [PubMed] [Google Scholar]
- 20.Liang M, Liu X, Cheng D, Nakamura K, Wang Y, Dou S, Liu G, Rusckowski M, Hnatowich DJ. Optical antisense tumor targeting in vivo with an improved fluorescent DNA duplex probe. Bioconjug Chem. 2009;20(6):1223–1227. doi: 10.1021/bc9000933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tan XX, Actor JK, Chen Y. Peptide nucleic acid antisense oligomer as a therapeutic strategy against bacterial infection: proof of principle using mouse intraperitoneal infection. Antimicrob Agents Chemother. 2005;49(8):3203–3207. doi: 10.1128/AAC.49.8.3203-3207.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang Y, Liu G, Hnatowich DJ. Methods for MAG3 conjugation and 99mTc radiolabeling of biomolecules. Nat Protocols. 2006;1:1477–1480. doi: 10.1038/nprot.2006.262. [DOI] [PubMed] [Google Scholar]
- 23.Ouverney CC, Fuhrman JA. Increase in fluorescence intensity of 16S rRNA in situ hybridization in natural samples treated with chloramphenicol. Appl Environ Microb. 1997;63(7):2735–2740. doi: 10.1128/aem.63.7.2735-2740.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sheridan GEC, Masters CI, Shallcross JA, Machey BM. Detection of mRNA by reverse transcription-PCR as an indicator of viability in Escherichia coli cells. Appl Environ Microb. 1998;64(4):1313–1318. doi: 10.1128/aem.64.4.1313-1318.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kallen AJ, Hidron AI, Patel J, Srinivasan A. Multidrug resistance among Gram-negative pathogens that caused healthcare-associated infections reported to the national healthcare safety network, 2006-2008. Infect Control Hosp Epidemiol. 2010;31(5):528–531. doi: 10.1086/652152. [DOI] [PubMed] [Google Scholar]
- 26.Liu G, He J, Zhang S, Liu C, Rusckowski M, Hnatowich DJ. Cytosine residues influence kidney accumulations of 99mTc-labeled morpholino oligomers. Antisense Nucleic Acid Drug Dev. 2002;12(6):393–398. doi: 10.1089/108729002321082465. [DOI] [PubMed] [Google Scholar]
- 27.Göbel UB, Geiser A, Stanbridge EJ. Oligonucleotide probes complementary to variable regions of ribosomal RNA discriminate between Mycoplasma species. J. Gen Microbiol. 1987;133(7):1969–1974. doi: 10.1099/00221287-133-7-1969. [DOI] [PubMed] [Google Scholar]
- 28.Giovannoni SJ, DeLong EF, Olsen GJ, Pace NR. Phylogenetic group-specific oligodeoxynucleotide probes for identification of single microbial cells. J Bacteriol. 1988;170(2):720–726. doi: 10.1128/jb.170.2.720-726.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hnatowich DJ, Mardirossian G, Fogarasi M, et al. Comparative properties of a technetium-99m-labeled single-stranded natural DNA and a phosphorothioate derivative in vitro and in mice. J. Pharmacol Exp Ther. 1996;276(1):326–334. [PubMed] [Google Scholar]
- 30.Summerton J, Weller D. Morpholine antisense oligomers: design, preparation and properties. Antisense Nucleic Acid Drug Dev. 1997;7:187–195. doi: 10.1089/oli.1.1997.7.187. [DOI] [PubMed] [Google Scholar]
- 31.Egholm M, Buchardt O, Christensen L, Behrens C, Freier SM, Driver DA, Berg RH, Kim SK, Norden B, Nielsen PE. PNA hybridizes to complementary oligonucleotides obeying the Watson-Crick hydrogen-bonding rules. Nature. 1993;365(6446):566–568. doi: 10.1038/365566a0. [DOI] [PubMed] [Google Scholar]
- 32.Wages JM, Jr, Wages GM, Matthews P, Weller D, Summerton J. Affinity purification of RNA: sequence-specific capture by nonionic morpholino probes. Biotechniques. 1997;23(6):1116–1121. doi: 10.2144/97236pf02. [DOI] [PubMed] [Google Scholar]
- 33.Nakamura K, Fan C, Liu G, Gupta S, He J, Dou S, Kubo A, Rusckowski M, Hnatowich D,J. Evidence of antisense tumor targeting in mice. Bioconj Chem. 2004;15:1475–1480. doi: 10.1021/bc0499073. [DOI] [PubMed] [Google Scholar]
- 34.Zhang YM, Wang Y, Liu N, Zhu ZH, Rusckowski M, Hnatowich DJ. In vitro investigations of tumor targeting with 99mTc-labeled antisense DNA. J. Nucl Med. 2001;42:1660–1669. [PubMed] [Google Scholar]
- 35.Uyttendaele M, Bastiaansen A, Debevere J. Evaluation of the NASBA nucleic acid amplification system for assessment of the viability of Campylobacter jejuni. Int J Food Microbiol. 1997;37:13–20. doi: 10.1016/s0168-1605(97)00039-1. [DOI] [PubMed] [Google Scholar]