Abstract
Rationale: Alcohol use disorders cause oxidative stress in the lower airways and increase susceptibility to pneumonia and lung injury. Currently, no therapeutic options exist to mitigate the pulmonary consequences of alcoholism.
Objectives: We recently determined in an animal model that alcohol ingestion impairs pulmonary zinc metabolism and causes alveolar macrophage immune dysfunction. The objective of this research is to determine the effects of alcoholism on zinc bioavailability and alveolar macrophage function in human subjects.
Methods: We recruited otherwise healthy alcoholics (n = 17) and matched control subjects (n = 17) who underwent bronchoscopy for isolation of alveolar macrophages, which were analyzed for intracellular zinc, phagocytic function, and surface expression of granulocyte-macrophage colony–stimulating factor receptor; all three of these indices are decreased in experimental models.
Measurements and Main Results: Alcoholic subjects had normal serum zinc, but significantly decreased alveolar macrophage intracellular zinc levels (adjusted means [SE], 718 [41] vs. 948 [25] RFU/cell; P < 0.0001); bacterial phagocytosis (adjusted means [SE], 1,027 [48] vs. 1,509 [76] RFU/cell; P < 0.0001); and expression of granulocyte-macrophage colony–stimulating factor receptor β subunit (adjusted means [SE], 1,471 [42] vs. 2,114 [35] RFU/cell; P < 0.0001]. Treating alveolar macrophages with zinc acetate and glutathione in vitro increased intracellular zinc levels and improved their phagocytic function.
Conclusions: These novel clinical findings provide evidence that alcohol abuse is associated with significant zinc deficiency and immune dysfunction within the alveolar space and suggest that dietary supplementation with zinc and glutathione precursors could enhance airway innate immunity and decrease the risk for pneumonia or lung injury in these vulnerable individuals.
Keywords: alcohol abuse, alveolar macrophage, zinc, immunity, glutathione
At a Glance Commentary
Scientific Knowledge on the Subject
Alcohol abuse causes many derangements in the lung and predisposes individuals to the development of pulmonary infections and lung injury. Animal models of chronic alcohol ingestion have implicated pulmonary zinc deficiency as an important mediator of alveolar macrophage immune dysfunction, which results in increased susceptibility to, and severity, of pneumonia. However, human studies are lacking.
What This Study Adds to the Field
Our study sheds light on the effects of chronic alcohol exposure on alveolar macrophages from human alcoholics. We show that alcoholism results in intracellular zinc deficiency and immune dysfunction of alveolar macrophages compared with nonalcoholics. Treating alveolar macrophages with zinc and/or glutathione in vitro reverses these defects, providing important proof-of-concept data to evaluate the role of these potential therapeutic strategies.
Alcohol use and abuse are prevalent and impose significant health burdens in society. A recent national survey suggests that more than half of the population in the United States older than the age of 12 consumes alcohol (1) and the lifetime prevalence of alcohol abuse is 18% (2). Tragically, alcohol abuse is the third leading cause of preventable death in the United States (3), and alcoholism is associated with numerous chronic, nonfatal health implications. The overall annual cost of alcohol abuse to American society has been estimated to be upward of $200 billion (4).
The consequences of alcoholism span multiple organ systems, including the heart, liver, brain, and skeletal muscle (5, 6). Among these widespread effects, alcohol abuse renders individuals susceptible to pulmonary infections and acute lung injury (7–10). We have explored potential mechanisms in experimental animal models and determined that chronic alcohol ingestion causes previously unrecognized cellular dysfunction and oxidative stress, as best reflected by significant decreases in glutathione (GSH) levels, within the alveolar space (11–14). Alveolar macrophages, which are the primary immune cell in the lower airways, exhibit impaired phagocytosis and respiratory burst generation in animal models of chronic alcohol ingestion (15, 16), and treatment with antioxidants reverses these defects (17, 18). In addition, we established that decreased signaling of granulocyte-macrophage colony–stimulating factor (GM-CSF) mediates alcohol-induced alveolar macrophage immune deficiency (13, 19). GM-CSF is vital for the alveolar macrophage to achieve appropriate maturity, to complete terminal differentiation, and to maintain normal functioning (20).
More recently, animal models implicate zinc deficiency as a fundamental mechanism that drives the oxidative stress and impaired GM-CSF signaling in the alcoholic lung (21). Zinc is a trace metal that is an important cofactor for numerous enzymes in the body and plays a key role in the immune response (22). Zinc deficiency has been implicated in the development of pneumonia in children of third world countries (23, 24), and dietary zinc supplementation in these children decreases susceptibility to infection and improves overall health outcomes (25, 26). Importantly, recent experimental studies have shown that alcohol-fed animals have lower zinc levels in the lung compared with control-fed animals, and dietary zinc supplementation can reverse the pulmonary immune dysfunction seen with chronic alcohol ingestion (21, 27).
Although these and other experimental models are important to the understanding of the alcohol lung phenotype, human studies are lacking. For this reason, we undertook this study to establish if alveolar macrophages from otherwise healthy alcoholics have significantly reduced intracellular zinc levels and phagocytic function compared with nonalcoholics, and to determine the effect of treating these alveolar macrophages in vitro with zinc and/or GSH precursors. To answer these questions, we obtained alveolar macrophages by bronchoalveolar lavage (BAL) from otherwise healthy alcoholic and nonalcoholic individuals and compared their relative intracellular levels of zinc, surface expression of the GM-CSF receptor, and phagocytic function. In parallel, we determined whether we could enhance the functional status of alveolar macrophages from alcoholics by treating them with zinc and GSH in vitro as a preclinical proof-of-principle that dietary supplementation could enhance lung health in these vulnerable individuals. Some of the results of these studies have been previously reported in the form of an abstract (28).
Methods
Study Population
The target population was otherwise healthy adults (18–55 yr of age) who were diagnosed with an alcohol use disorder (AUD) at the time of enrollment. An AUD was defined as having a positive Short Michigan Alcohol Screening Test (SMAST) (29) and this was later confirmed with the Alcohol Use Disorders Identification Test (30). All subjects were recruited from the Substance Abuse Treatment Program at the Atlanta Veterans Affairs Medical Center in Decatur, Georgia. Nonalcoholic subjects were matched by age, sex, race, and smoking status. Greater detail regarding the study population and enrollment procedures is available in the online supplement.
Bronchoscopy Procedure and BAL Fluid Processing
All study subjects underwent a bronchoscopy and BAL for collection of alveolar macrophages using standard techniques that have been previously described (31). Further procedural details are provided in the online supplement. BAL fluid was passed through sterile gauze and centrifuged at 8,000 rpm for 5 minutes. The cell pellets contained predominantly alveolar macrophages with approximately 90% purity as measured by Diff-Quik (Dade Behring, Deerfield, IL) and were resuspended at a concentration of 1 × 106 cells/ml in RPMI-1640 medium containing 2% fetal bovine serum and 1% penicillin-streptomycin and then cultured for 2 hours as previously described (32). Some alveolar macrophages were then treated for 24 hours with 10 μM zinc acetate (Zn) (JT Baker, Phillipsburg, NJ); 500 μM GSH (ICN Nutritional Biochemicals, Cleveland, OH); or Zn and GSH combined.
Serum and Alveolar Macrophage Studies
Serum zinc levels were determined by sending samples to a commercial laboratory (Quest Diagnostics, Chantilly, VA). Isolated alveolar macrophages were incubated with FluoZin-3AM dye (200 nM; Invitrogen, Carlsbad, CA) for 30 minutes per the manufacturer’s instructions and then fixed to chamber slides with 4% paraformaldehyde. For GM-CSF receptor measurement, we evaluated expression of the α subunit (GM-CSFRα) that mediates ligand binding and the β subunit (GM-CSFRβ) that initiates the intracellular signaling cascade that ultimately induces the expression of multiple genes responsible for alveolar macrophage function, including phagocytosis. Alveolar macrophages were fixed to chamber slides with 4% paraformaldehyde and then incubated with a primary antibody against the α binding and β signaling subunit (Santa Cruz Biotechnology, Santa Cruz, CA; 1:100), followed by incubation with a fluorescent TRITC-labeled secondary antibody. Fluorescence measurements for zinc and GM-CSF were performed using FluoView (Olympus Corporation, Center Valley, PA) by quantitative digital analysis and expressed as mean relative fluorescent units (RFU) per cell. The relative phagocytic capacity of alveolar macrophages at baseline and after treatment with Zn, GSH, or Zn + GSH was assessed as previously described (32) and further details are provided in the online supplement.
Statistical Analyses
For statistical comparisons between alcoholic and nonalcoholic subjects, we used general estimating equations with repeated measures to account for the matching design by SAS software’s (version 9.3; SAS Institute Inc., Cary, NC) Genmod procedure (33) and one-step robust regression estimator weighting to reduce the influence of outliers (34). Further statistical details are in the online supplement.
Research Ethics
All aspects of this clinical project have been conducted according to Declaration of Helsinki principles and were approved by the institutional review board at Emory University and the Research and Development Committee at the Atlanta Veterans Affairs Medical Center. Written informed consent was received from each enrolled participant.
Results
Subject Enrollment
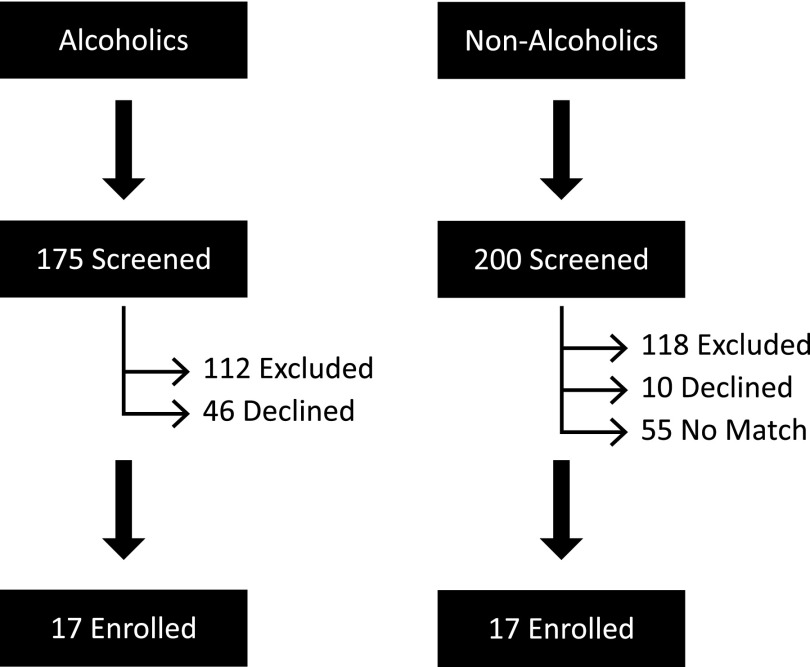
A total of 375 subjects were screened for enrollment into the study; 175 with an AUD and 200 without any history of alcohol abuse (Figure 1). Among the screened subjects with an AUD, 112 met at least one of the exclusion criteria and 46 declined to participate in the study. Among the nonalcoholics who were screened, 118 met at least one of the exclusion criteria, 10 declined to participate, and 55 did not have a match among the alcoholic subjects. Overall, 17 subjects with an active AUD and 17 matched nonalcoholics subjects were enrolled and underwent bronchoscopy. There was improper handling of one BAL sample from an alcoholic subject that rendered it unusable. All other samples were analyzed as described in the Methods section.
Figure 1.
Assessment of study participants. Nonalcoholics were matched to enrolled alcoholic subjects based on age, sex, race, and smoking status.
Subject Characteristics
Alcoholic and nonalcoholic subjects were similar for sex and race (both groups were all male and 88% were black); average age (45.2 vs. 45.8 yr); and body mass index (28.0 vs. 28.6 kg/m2) (Table 1). Eighty-eight percent of both groups smoked cigarettes; however, those in the alcoholic group smoked more heavily (median, 360 vs. 150 cigarettes in the previous 30 d). Illegal drug use was reported by seven (44%) of the alcoholic subjects but not by any of the control subjects. Per the inclusion criteria, all alcoholic subjects had a current AUD and were actively drinking at the time of enrollment (mean SMAST score [SE], 6.9 [3.4]), whereas the control subjects were either nondrinkers or drank socially but did not meet criteria for an AUD (mean SMAST score [SE], 0.9 [0.7]). The median alcohol intake (in grams per day) for nonalcoholics was zero and 126 among alcoholic subjects. The median time since the most recent alcohol drink was 1 day in the alcoholic subjects and 14 days in the control subjects.
TABLE 1.
DEMOGRAPHIC AND HEALTH CHARACTERISTICS OF STUDY SUBJECTS
| Characteristics* | Nonalcoholic (n = 17) Mean (SD), Median (Q1, Q3), or n (%) | Alcoholic (n = 17) Mean (SD), Median (Q1, Q3), or n (%) |
|---|---|---|
| Demographics |
|
|
| Male |
17 (100%) |
17 (100%) |
| Black |
15 (88%) |
15 (88%) |
| Age, yr |
45.2 (7.5) |
45.8 (8.4) |
| Body mass index, kg/m2 |
28.0 (4.0) |
28.6 (7.8) |
| Health-related habits |
|
|
| Drinking-AUDIT score |
2.2 (1.9) |
20.3 (8.6) |
| Drinking-SMAST score |
0.9 (0.7) |
6.9 (3.4) |
| Days since last drink |
14 (7, 60) |
1 (1, 3) |
| Alcohol intake, g/d |
0 (0, 3.5) |
126 (105, 154) |
| Smokes cigarettes |
15 (88%) |
15 (88%) |
| If yes, number cigarettes in last 30 d |
150 (40, 150) |
360 (120, 600) |
| Illegal drug use† | 0 (0%) | 7 (44%) |
Definition of abbreviations: AUDIT = Alcohol Use Disorders Identification Test; SMAST = Short Michigan Alcohol Screening Test.
Characteristics not related to the study design were assessed for differences between groups: body mass index (P = 0.7); number of cigarettes in last 30 d (P = 0.0003); illegal drug use (P = 0.0027).
Missing information for one alcoholic.
Alveolar Macrophages Isolated from Subjects with an AUD Had Lower Intracellular Zinc Levels
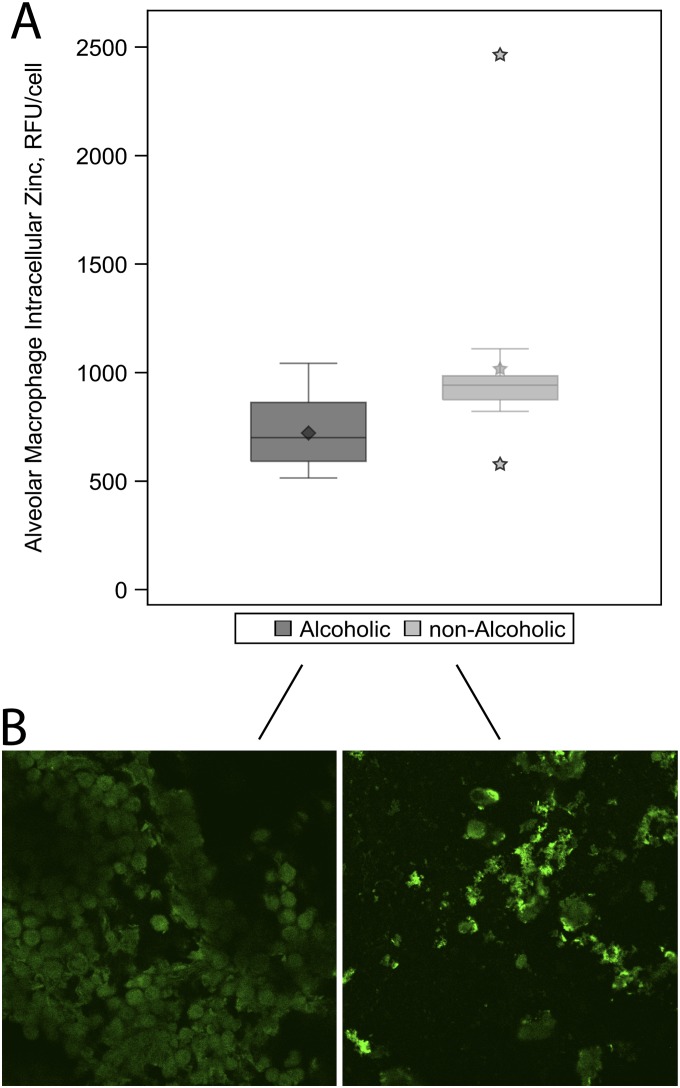
We previously determined that extracellular and intracellular zinc levels are decreased in an experimental animal model of chronic alcohol ingestion (27). In human subjects, we evaluated intracellular zinc status because extracellular zinc measurements in lavage fluid are difficult to interpret given variations in sample dilution. Alveolar macrophages isolated from alcoholic subjects had lower intracellular zinc levels compared with alveolar macrophages isolated from their matched control subjects (adjusted means [SE], 718 [41] vs. 948 [25] RFU/cell; P < 0.0001) (Figure 2A and Table 2).
Figure 2.
Intracellular zinc values in alveolar macrophages from alcoholic and nonalcoholic study participants. Intracellular zinc was measured as relative fluorescence units (RFU) per cell using confocal microscopy after macrophages were incubated with 200 nM FluoZin-3AM dye. (A) Box plots illustrate the median and interquartile ranges for intracellular zinc measurements among the alcoholic and nonalcoholic subjects. The diamond represents the mean among alcoholics and the stars represent the mean and outliers among the nonalcoholics. As shown, alcoholic subjects had approximately 30% less intracellular zinc than nonalcoholic subjects (P < 0.001). (B) Representative cell staining image from one alcoholic and one nonalcoholic subject.
TABLE 2.
ALVEOLAR MACROPHAGE MEASURES FROM ALCOHOLIC AND NONALCOHOLIC SUBJECTS, WITH AND WITHOUT ADJUSTMENT FOR THE NUMBER OF CIGARETTES SMOKED IN THE PREVIOUS MONTH*†
| |
Unadjusted Data |
Data Adjusted for Smoking Frequency |
||||
|---|---|---|---|---|---|---|
| Nonalcoholic | Alcoholic | P Value‡ | Nonalcoholic | Alcoholic | P Value‡ | |
| Alveolar macrophage intracellular zinc |
|
|
|
|
|
|
| Baseline (no treatment) |
951 (22) |
711 (33) |
<0.0001 |
948 (25) |
718 (41) |
<0.0001 |
| Zinc acetate |
1,746 (24) |
1,443 (39) |
<0.0001 |
1,752 (27) |
1,434 (43) |
<0.0001 |
| GSH |
1,941 (32) |
1,797 (49) |
0.0202 |
1,961 (28) |
1,795 (45) |
0.0021 |
| Zinc acetate + GSH |
2,513 (41) |
2,683 (47) |
0.0050 |
2,512 (37) |
2,684 (50) |
0.0031 |
| Phagocytosis |
|
|
|
|
|
|
| Baseline (no treatment) |
1,521 (82) |
988 (48) |
<0.0001 |
1,509 (76) |
1,027 (48) |
<0.0001 |
| Zinc acetate |
1,715 (35) |
1,260 (29) |
<0.0001 |
1,704 (31) |
1,273 (37) |
<0.0001 |
| GSH |
2,091 (30) |
1,775 (33) |
<0.0001 |
2,090 (28) |
1,775 (31) |
<0.0001 |
| Zinc acetate + GSH |
2,555 (27) |
2,462 (55) |
0.1090 |
2,560 (30) |
2,453 (60) |
0.1188 |
| Other |
|
|
|
|
|
|
| GM-CSF α |
2,473 (31) |
2,630 (47) |
0.0068 |
2,461 (39) |
2,642 (49) |
0.0123 |
| GM-CSF β |
2,117 (38) |
1,466 (41) |
<0.0001 |
2,114 (35) |
1,471 (42) |
<0.0001 |
| Serum zinc | 85 (2) | 88 (3) | 0.2555 | 86 (2) | 87 (2) | 0.7271 |
Definition of abbreviations: GM-CSF = granulocyte-macrophage colony–stimulating factor; GSH = glutathione.
General estimating equations were used to account for the matching of alcoholic to nonalcoholic subjects and one-step robust regression estimator weighting to reduce the influence of outliers. Each model contains the predictor alcoholic status, and the adjusted models also contain the number of cigarettes smoked in the previous month. nnonalcoholics = 17, nalcoholics = 13 for phagocytosis models, and nnonalcoholics = 17, nalcoholics = 16 for all other models.
Units are microgram per deciliter for serum zinc acetate and relative fluorescence units per cell for all others.
A family-wise type I error rate was set at 5%; a Bonferroni adjustment for 11 tests yields a significance criteria of P values less than 0.0045.
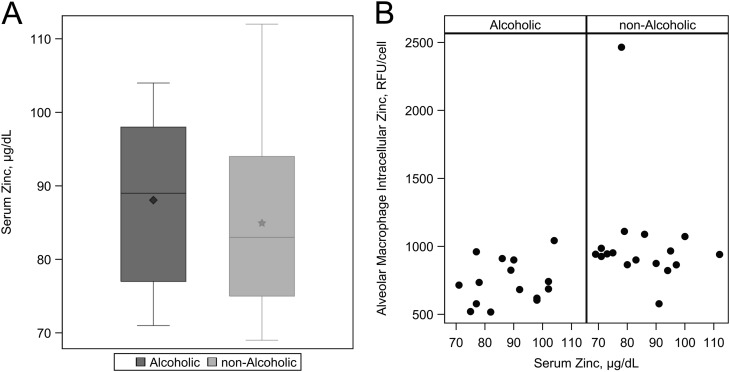
In Contrast, Serum Zinc Levels Did Not Differ between Alcoholic and Control Subjects and Did Not Correlate with Alveolar Macrophage Zinc Levels
There were no appreciable differences in serum zinc levels between the alcoholic subjects and the matched control subjects (Figure 3A and Table 2). In fact, the serum zinc levels were within the normal range in all of the subjects. Furthermore, there was no significant correlation between the serum and the alveolar macrophage zinc levels (Spearman ralcoholic = 0.24, P = 0.4 vs. rnonalcoholic = −0.26, P = 0.3) (Figure 3B).
Figure 3.
Serum zinc measurements among alcoholic and nonalcoholic subjects. (A) Median and interquartile ranges for serum zinc values. The diamond represents the mean among alcoholics and the star represents the mean among nonalcoholics. There was no significant difference between alcoholic and nonalcoholic subjects with regards to systemic zinc levels (P = 0.7271). (B) Association between serum zinc value and intracellular zinc value. There is no appreciable relationship between serum zinc levels and alveolar macrophage intracellular zinc measurements among alcoholics and nonalcoholics.
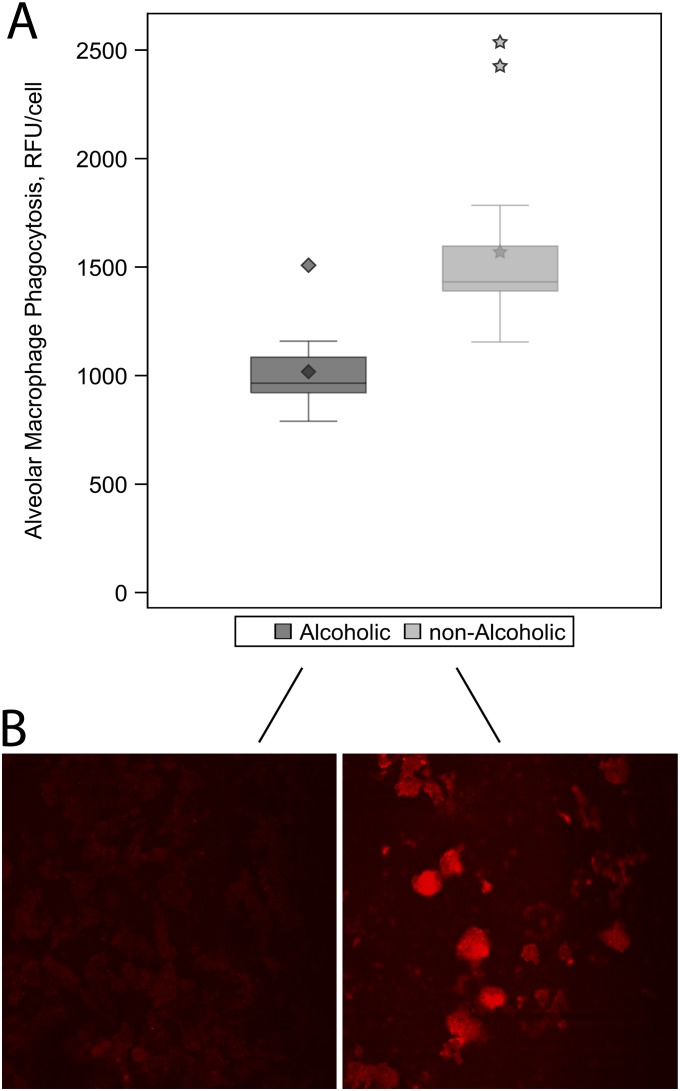
Alveolar Macrophages from Alcoholic Subjects Had Decreased Bacterial Phagocytic Capacity
Previously, we showed that alveolar macrophages isolated from alcohol-fed animals had decreased bacterial phagocytic function compared with alveolar macrophages from control-fed animals (27). To confirm the relevance of these findings, we compared the relative bacterial phagocytic capacities of alveolar macrophages isolated from human alcoholic and nonalcoholic subjects. The bacterial phagocytic function of alveolar macrophages was more than 30% lower in alcoholics compared with nonalcoholics (adjusted means [SE], 1,027 [48] vs. 1,509 [76] RFU/cell; P < 0.001) (Figure 4A and Table 2). Representative fluorescent images illustrate this decreased ability of alveolar macrophages from alcohol subjects to ingest fluorescent-labeled Staphylococcus aureus in vitro (Figure 4B).
Figure 4.
Evaluation of phagocytic capacity in alveolar macrophages from alcoholic and nonalcoholic study participants. Phagocytosis was measured as relative fluorescence units (RFU) per cell using confocal microscopy after macrophages were incubated with Staphylococcus aureus. (A) Box plots represent median and interquartile ranges for alveolar macrophage phagocytosis among the alcoholic and nonalcoholic subjects. The diamonds and stars represent mean values and outliers among alcoholics and nonalcoholics, respectively. As shown, alveolar macrophages from alcoholic subjects had approximately 30% less phagocytic capacity than nonalcoholic subjects (P < 0.001). (B) Representative cell image from one alcoholic and one nonalcoholic subject.
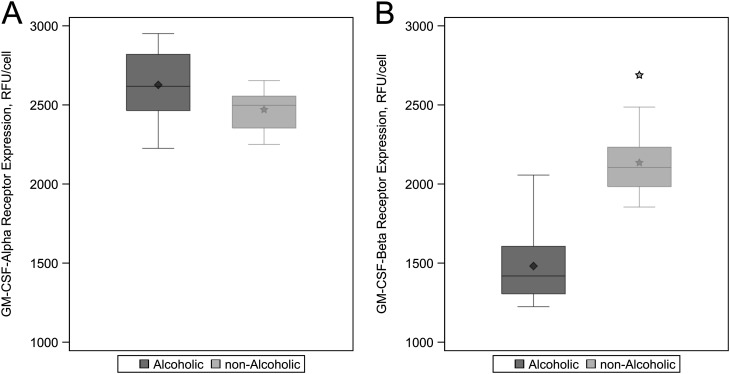
The Cell Surface Expression of the Signaling Subunit of the GM-CSF Receptor Was Decreased in the Alveolar Macrophages of Alcoholic Subjects
The GM-CSF receptor has an α subunit (GM-CSFRα) that mediates ligand binding and a β subunit (GM-CSFRβ) that initiates the intracellular signaling cascade that ultimately induces the expression of multiple genes responsible for alveolar macrophage function, including phagocytosis. Experimental models of chronic alcohol ingestion have demonstrated a decrease in GM-CSF receptor expression and signaling in alveolar macrophages (13, 19). In this study, the relative expression of GM-CSFRα in the alveolar macrophages did not differ significantly between alcoholic and nonalcoholic control subjects (Figure 5A and Table 2). However, the expression of the GM-CSFRβ subunit was decreased by more than 30% in the alveolar macrophages from the alcoholic subjects compared with control subjects (adjusted means [SE], 1,471 [42] vs. 2,114 [35] RFU/cell; P < 0.001) (Figure 5B and Table 2).
Figure 5.
Assessment of granulocyte-macrophage colony–stimulating factor (GM-CSF) receptor expression in alveolar macrophages from alcoholic and nonalcoholic study subjects. GM-CSF receptor expression was measured as relative fluorescence units (RFU) per cell using confocal microscopy after alveolar macrophages were incubated with the primary antibody to the binding α-subunit and signaling β-subunit. Box plots represent the median and interquartile ranges for GM-CSF receptor subunit measurements. The diamonds represent the mean among alcoholics and the stars represent the mean and outlier among nonalcoholics. (A) There was no significant difference between alcoholics and nonalcoholics in α-subunit expression on alveolar macrophages (P = 0.0123; P < 0.0045 considered significant after Bonferroni adjustment). (B) Significant 30% decrease in expression of the signaling β-subunit in alveolar macrophages from alcoholic subjects compared with nonalcoholics (P < 0.0001).
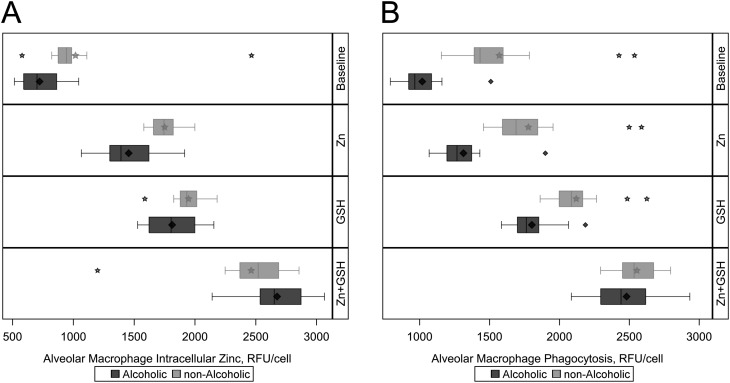
Treating Isolated Alveolar Macrophages with Zn and/or GSH In Vitro Increased Intracellular Zinc Levels and Phagocytic Capacity, and These Salutary Effects Were Most Pronounced in Response to Combination Treatment
Previously we determined that treating alveolar macrophages isolated from alcohol-fed experimental animals with either GSH precursors (i.e., procysteine) or zinc increased their phagocytic function (17, 27). More recently, we determined that dietary zinc supplementation in alcohol-fed animals normalized alveolar redox balance and restored lung bacterial clearance (21). Taken together, these experimental findings revealed a dynamic dependence between zinc bioavailability and oxidative stress within the alveolar macrophage. However, these experimental findings have not been extended to humans. Therefore, we treated alveolar macrophages from alcoholic and control subjects with Zn, GSH, or both in vitro and compared their intracellular zinc levels and bacterial phagocytic capacities after these treatments.
Treatment with either Zn or GSH increased the intracellular zinc levels in alveolar macrophages from both alcoholic and control subjects (Figure 6A). Interestingly, there was an even greater increase in the intracellular zinc levels when zinc and GSH were used in combination. Under this treatment the intracellular zinc levels were slightly higher in the alveolar macrophages from the alcoholic subjects compared with the control subjects (adjusted means [SE], 2,684 [47] vs. 2,512 [41] RFU/cell; P = 0.0031) (Table 2).
Figure 6.
Evaluation of intracellular zinc and phagocytic capacity in alveolar macrophages from alcoholic and nonalcoholic study participants after treatment ex vivo with zinc acetate (Zn; 10 μM), glutathione (GSH; 500 μM), or both zinc and GSH. Intracellular zinc and phagocytosis was measured as relative fluorescence units (RFU) per cell using confocal microscopy. (A) Median and interquartile ranges for post-treatment intracellular zinc among alcoholic and nonalcoholic subjects, with the baseline values displayed in the top bar. Intracellular zinc increases with zinc and GSH treatment alone, and combination treatment with both zinc and GSH results in intracellular zinc levels that are modestly higher in alcoholics (P = 0.0031). (B) Median and interquartile ranges for post-treatment phagocytosis among alcoholic and nonalcoholic subjects. Phagocytosis increases with zinc and GSH treatment alone, and combination treatment with both zinc and GSH also results in alveolar macrophage phagocytosis that is similar between alcoholics and nonalcoholics (P = 0.1188). The diamonds and stars represent mean values and outliers among alcoholics and nonalcoholics, respectively.
In parallel, increased intracellular zinc levels were associated with salutary effects on bacterial phagocytic function (Figure 6B). Treatment with either Zn or GSH increased phagocytic capacity in the alveolar macrophages from alcoholic and control subjects. Also consistent with intracellular zinc levels, the combination treatment produced the largest improvement in alveolar macrophage phagocytic function, and phagocytosis was not significantly different between the alveolar macrophages from alcoholic and control subjects after this treatment (adjusted means [SE], 2,453 [60] vs. 2,560 [30] RFU/cell; P = 0.1188) (Table 2).
Discussion
In this study we determined that alveolar macrophages of otherwise healthy alcoholic subjects have significantly decreased intracellular zinc levels compared with macrophages of nonalcoholic control subjects even in the presence of normal serum zinc levels. Furthermore, we confirmed that features of the alcoholic lung phenotype identified in the animal model, namely alveolar macrophage immune dysfunction and decreased GM-CSF receptor expression, also occur in human subjects. Finally, treatment of isolated alveolar macrophages from alcoholic subjects with either zinc or GSH in vitro increased intracellular zinc levels and alveolar macrophage phagocytic function. Taken together, these results provide new evidence that chronic alcohol abuse, even in the absence of clinically apparent zinc deficiency or end-organ damage, causes significant zinc depletion and immune dysfunction within the alveolar space. Therefore, this study extends the basic understanding of the association between alcoholism and pulmonary outcomes, such as pneumonia (35, 36), and suggests that increasing zinc and GSH bioavailability within the alveolar space with dietary supplements could mitigate these pathophysiologic consequences.
Zinc deficiency has been established in alcoholic liver disease for more than 50 years (37, 38). These individuals have significantly lower serum zinc levels compared with nonalcoholic control subjects (39), and both human studies and animal models of chronic alcohol ingestion demonstrate the presence of zinc deficiency in hepatocytes (40–42). However, much less is known about zinc balance in alcoholics without liver disease, and to our knowledge, the pulmonary zinc status in these individuals has never been examined. This is an important area for research because zinc therapy remains absent from clinical practice guidelines involving the management of alcohol-related disorders including alcohol withdrawal (43). In this study, our alcoholic and control subjects all had normal blood zinc levels, suggesting that these serum measurements are unreliable surrogate markers for zinc metabolism at the organ level. Specifically, alveolar macrophage intracellular zinc was about 30% lower in alcoholic subjects compared with matched nonalcoholics in this study. Although the clinical relevance of this finding requires additional investigation, this degree of intracellular zinc depletion has been shown to cause significant cellular and clinical derangements in other studies (44–46). Furthermore, our clinical findings in this study are remarkably consistent with our published findings in an animal model of chronic alcohol ingestion in which we identified a similar 30% decrease in lung zinc levels, and where correction of this deficiency reversed phagocytic dysfunction in the alveolar macrophage and restored lung bacterial clearance (21, 27).
Animal models have been instrumental in characterizing what we have termed the “alcoholic lung phenotype,” which includes increased oxidative stress in the lower airways, immune dysfunction of the alveolar macrophage, and disruption of alveolar epithelial barrier function (7). Human studies are limited, but have confirmed the presence of oxidative stress in the alveolar space (47) and revealed alteration of gene expression in alveolar macrophages isolated from alcoholics (48). In this study, we show that isolated macrophages from human alcoholics have significantly decreased phagocytic capacity compared with matched control subjects. This finding is parallel to our observations in the animal model and confirms the presence of alcohol-induced immune dysfunction in the human lung. We have shown previously that decreased GM-CSF signaling secondary to alcohol exposure is at least partly responsible for alveolar macrophage immune impairment in rat lung (13, 19). Interestingly, correction of zinc deficiency improved GM-CSF signaling in this model (27), suggesting that these two pathways may be interdependent. We evaluated the role of the GM-CSF pathway in the human lung, and we found that subjects with an AUD exhibit significantly decreased alveolar macrophage GM-CSF receptor β-subunit expression compared with matched control subjects and no difference in α-subunit expression. This is a similar albeit not a complete recapitulation of our findings in the animal model in which there was decreased expression of the signaling component receptor β-subunit but also of the ligand binding receptor α-subunit. The decreased expression of the β-subunit we identified in these human subjects suggests that the dampened GM-CSF signaling we discovered in the experimental model (including decreased expression and nuclear binding of its master transcription factor, PU.1) may contribute to alcohol-induced macrophage dysfunction in humans. Admittedly, we did not identify a significant decrease in expression of the α-subunit in the macrophages from alcoholic subjects as we had in the experimental model. Although an explanation for this difference is at present unknown, our findings nevertheless support the hypothesis that alcohol abuse dampens GM-CSF signaling in human alveolar macrophages because the GM-CSF receptor requires optimal expression of the β-subunit to initiate intracellular signaling.
There are no currently available treatments to mitigate the adverse effects of chronic alcohol use on the lung. Animal models have shown that dietary supplementation with zinc and thiol antioxidants (particularly GSH precursors) may have a therapeutic role, but no human trials have been done. The use of these modalities is of particular interest because we previously showed that zinc deficiency and oxidative stress are mechanistically connected. Specifically, other investigators have described the close relationship between oxidative stress and zinc deficiency (49, 50), and we determined that correction of zinc deficiency improves redox stress in the lower airway of alcohol-fed animals (21). In this human study, treating isolated alveolar macrophages with zinc and/or GSH in vitro improved their intracellular zinc levels and phagocytic function in parallel. Understandably, this is not the same as dietary supplementation, but these experiments provide important proof-of-concept for the development of clinical trials. Interestingly, GSH seems to improve intracellular zinc levels, strengthening the argument that zinc and oxidative stress pathways are interconnected. Although these parameters also improved in alveolar macrophages isolated from control subjects, when cells are treated with both zinc and GSH there is no appreciable difference in zinc levels or phagocytic function between the two groups.
Although this study furthers knowledge of the alcoholic lung phenotype in human subjects, it has some limitations. First, this is a relatively small, single-center study, making it difficult to control for many potential confounding factors simultaneously. Because of the nature of the veteran population at our center, all enrolled subjects were male and most were black, which limits generalizability. Despite these limitations, we attempted to control for confounding by matching. Our alcoholic subjects were heavier smokers in general, and we adjusted our analysis to control for the number of cigarettes smoked in the last month even though the unadjusted analysis does not alter the conclusion reached in this study. Second, the clinical importance of measuring intracellular zinc levels is not known and such testing is not commercially available for individuals seen by health care providers. Currently, the evaluation of pulmonary zinc status is on a research basis only and requires a relatively invasive technique (i.e., bronchoscopy) to perform. However, our experimental studies show that alveolar zinc deficiency correlates with the levels of GSH, which can be measured noninvasively with exhaled breath condensate. It may be feasible in the future to use such methods as surrogate markers of alveolar health.
In summary, this study furthers the understanding of the effects of alcoholism on the human lung. Specifically, we demonstrate that intracellular zinc levels are significantly decreased in alveolar macrophages from alcoholic subjects compared with nonalcoholics even when serum zinc levels are normal. In parallel, alveolar macrophages from alcoholic subjects have impaired immune function as characterized by decreased GM-CSF receptor (β-subunit) expression and decreased phagocytic capacity. Importantly, treating these dysfunctional macrophages with zinc and GSH in vitro restored their intracellular zinc levels and phagocytic function, providing provocative evidence that it is not the alcohol per se, but rather the alcohol-induced zinc deficiency and oxidative stress that impairs their host immune function. Although many factors contribute to the alcoholic lung phenotype, experimental and clinical evidence now implicates decreased zinc bioavailability and oxidative stress within the alveolar space as fundamental mechanisms by which alcohol impairs host immunity. Therefore, these findings should pave the way for clinical trials to evaluate the impact of dietary supplementation with zinc and GSH precursors on lung health in individuals with chronic AUDs.
Footnotes
Supported by NIAAA (T32 AA 013528 and P50 013757); the Department of Veterans Affairs (Career Development Award to A.J.M. and a Merit Review Award to D.M.G.); and the National Center for Advancing Translational Sciences of the National Institutes of Health under Award Number UL1TR000454. The content is solely the responsibility of the authors and does not represent the official views of the National Institutes of Health.
Author Contributions: A.J.M. and D.M.G. designed and analyzed experiments, obtained samples from recruited subjects, and prepared manuscript. S.M.Y. and L.A.B. performed zinc and macrophage function assays and interpreted confocal microscopy measurements. L.E. performed statistical analysis and modeling.
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.201301-0061OC on June 27, 2013
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Results from the 2008 national survey on drug use and health: national findings. HHS Publication No. SMA 09-4434 ed. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2009.
- 2.Zhang Y, Guo X, Saitz R, Levy D, Sartini E, Niu J, Ellison RC. Secular trends in alcohol consumption over 50 years: the Framingham Study. Am J Med. 2008;121:695–701. doi: 10.1016/j.amjmed.2008.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mokdad AH, Marks JS, Stroup DF, Gerberding JL. Actual causes of death in the United States, 2000. JAMA. 2004;291:1238–1245. doi: 10.1001/jama.291.10.1238. [DOI] [PubMed] [Google Scholar]
- 4.Bouchery EE, Harwood HJ, Sacks JJ, Simon CJ, Brewer RD. Economic costs of excessive alcohol consumption in the US, 2006. Am J Prev Med. 2011;41:516–524. doi: 10.1016/j.amepre.2011.06.045. [DOI] [PubMed] [Google Scholar]
- 5.Otis JS, Guidot DM. Procysteine stimulates expression of key anabolic factors and reduces plantaris atrophy in alcohol-fed rats. Alcohol Clin Exp Res. 2009;33:1450–1459. doi: 10.1111/j.1530-0277.2009.00975.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lieber CS. Medical disorders of alcoholism. N Engl J Med. 1995;333:1058–1065. doi: 10.1056/NEJM199510193331607. [DOI] [PubMed] [Google Scholar]
- 7.Joshi PC, Guidot DM. The alcoholic lung: epidemiology, pathophysiology, and potential therapies. Am J Physiol Lung Cell Mol Physiol. 2007;292:L813–L823. doi: 10.1152/ajplung.00348.2006. [DOI] [PubMed] [Google Scholar]
- 8.de Roux A, Cavalcanti M, Marcos MA, Garcia E, Ewig S, Mensa J, Torres A. Impact of alcohol abuse in the etiology and severity of community-acquired pneumonia. Chest. 2006;129:1219–1225. doi: 10.1378/chest.129.5.1219. [DOI] [PubMed] [Google Scholar]
- 9.Moss M, Parsons PE, Steinberg KP, Hudson LD, Guidot DM, Burnham EL, Eaton S, Cotsonis GA. Chronic alcohol abuse is associated with an increased incidence of acute respiratory distress syndrome and severity of multiple organ dysfunction in patients with septic shock. Crit Care Med. 2003;31:869–877. doi: 10.1097/01.CCM.0000055389.64497.11. [DOI] [PubMed] [Google Scholar]
- 10.Thakur L, Kojicic M, Thakur SJ, Pieper MS, Kashyap R, Trillo-Alvarez CA, Javier F, Cartin-Ceba R, Gajic O. Alcohol consumption and development of acute respiratory distress syndrome: a population-based study. Int J Environ Res Public Health. 2009;6:2426–2435. doi: 10.3390/ijerph6092426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guidot DM, Modelska K, Lois M, Jain L, Moss IM, Pittet JF, Brown LA. Ethanol ingestion via glutathione depletion impairs alveolar epithelial barrier function in rats. Am J Physiol Lung Cell Mol Physiol. 2000;279:L127–L135. doi: 10.1152/ajplung.2000.279.1.L127. [DOI] [PubMed] [Google Scholar]
- 12.Polikandriotis JA, Rupnow HL, Elms SC, Clempus RE, Campbell DJ, Sutliff RL, Brown LA, Guidot DM, Hart CM. Chronic ethanol ingestion increases superoxide production and NADPH oxidase expression in the lung. Am J Respir Cell Mol Biol. 2006;34:314–319. doi: 10.1165/rcmb.2005-0320OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Joshi PC, Applewhite L, Ritzenthaler JD, Roman J, Fernandez AL, Eaton DC, Brown LA, Guidot DM. Chronic ethanol ingestion in rats decreases granulocyte-macrophage colony-stimulating factor receptor expression and downstream signaling in the alveolar macrophage. J Immunol. 2005;175:6837–6845. doi: 10.4049/jimmunol.175.10.6837. [DOI] [PubMed] [Google Scholar]
- 14.Bechara RI, Pelaez A, Palacio A, Joshi PC, Hart CM, Brown LA, Raynor R, Guidot DM. Angiotensin II mediates glutathione depletion, transforming growth factor-beta1 expression, and epithelial barrier dysfunction in the alcoholic rat lung. Am J Physiol Lung Cell Mol Physiol. 2005;289:L363–L370. doi: 10.1152/ajplung.00141.2005. [DOI] [PubMed] [Google Scholar]
- 15.D'Souza NB, Nelson S, Summer WR, Deaciuc IV. Alcohol modulates alveolar macrophage tumor necrosis factor-alpha, superoxide anion, and nitric oxide secretion in the rat. Alcohol Clin Exp Res. 1996;20:156–163. doi: 10.1111/j.1530-0277.1996.tb01059.x. [DOI] [PubMed] [Google Scholar]
- 16.D'Souza NB, Mandujano JF, Nelson S, Summer WR, Shellito JE. Alcohol ingestion impairs host defenses predisposing otherwise healthy mice to Pneumocystis carinii infection. Alcohol Clin Exp Res. 1995;19:1219–1225. doi: 10.1111/j.1530-0277.1995.tb01604.x. [DOI] [PubMed] [Google Scholar]
- 17.Brown LA, Ping XD, Harris FL, Gauthier TW. Glutathione availability modulates alveolar macrophage function in the chronic ethanol-fed rat. Am J Physiol Lung Cell Mol Physiol. 2007;292:L824–L832. doi: 10.1152/ajplung.00346.2006. [DOI] [PubMed] [Google Scholar]
- 18.Yeh MY, Burnham EL, Moss M, Brown LA. Chronic alcoholism alters systemic and pulmonary glutathione redox status. Am J Respir Crit Care Med. 2007;176:270–276. doi: 10.1164/rccm.200611-1722OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Joshi PC, Applewhite L, Mitchell PO, Fernainy K, Roman J, Eaton DC, Guidot DM. GM-CSF receptor expression and signaling is decreased in lungs of ethanol-fed rats. Am J Physiol Lung Cell Mol Physiol. 2006;291:L1150–L1158. doi: 10.1152/ajplung.00150.2006. [DOI] [PubMed] [Google Scholar]
- 20.Trapnell BC, Whitsett JA. GM-CSF regulates pulmonary surfactant homeostasis and alveolar macrophage-mediated innate host defense. Annu Rev Physiol. 2002;64:775–802. doi: 10.1146/annurev.physiol.64.090601.113847. [DOI] [PubMed] [Google Scholar]
- 21.Mehta AJ, Joshi PC, Fan X, Brown LA, Ritzenthaler JD, Roman J, Guidot DM. Zinc supplementation restores pu.1 and nrf2 nuclear binding in alveolar macrophages and improves redox balance and bacterial clearance in the lungs of alcohol-fed rats. Alcohol Clin Exp Res. 2011;35:1519–1528. doi: 10.1111/j.1530-0277.2011.01488.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tudor R, Zalewski PD, Ratnaike RN. Zinc in health and chronic disease. J Nutr Health Aging. 2005;9:45–51. [PubMed] [Google Scholar]
- 23.Hambidge KM. Zinc deficiency in young children. Am J Clin Nutr. 1997;65:160–161. doi: 10.1093/ajcn/65.1.160. [DOI] [PubMed] [Google Scholar]
- 24.Walravens PA, Hambidge KM. Nutritional zinc deficiency in infants and children. Prog Clin Biol Res. 1977;14:61–72. [PubMed] [Google Scholar]
- 25.Bhandari N, Bahl R, Taneja S, Strand T, Molbak K, Ulvik RJ, Sommerfelt H, Bhan MK. Effect of routine zinc supplementation on pneumonia in children aged 6 months to 3 years: randomised controlled trial in an urban slum. BMJ. 2002;324:1358. doi: 10.1136/bmj.324.7350.1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sazawal S, Black RE, Jalla S, Mazumdar S, Sinha A, Bhan MK. Zinc supplementation reduces the incidence of acute lower respiratory infections in infants and preschool children: a double-blind, controlled trial. Pediatrics. 1998;102:1–5. doi: 10.1542/peds.102.1.1. [DOI] [PubMed] [Google Scholar]
- 27.Joshi PC, Mehta A, Jabber WS, Fan X, Guidot DM. Zinc deficiency mediates alcohol-induced alveolar epithelial and macrophage dysfunction in rats. Am J Respir Cell Mol Biol. 2009;41:207–216. doi: 10.1165/rcmb.2008-0209OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mehta AJ, Yeligar SM, Brown LA, Guidot DM. Zinc supplementation in vitro improves immune function and restores intracellular zinc levels in alveolar macrophages isolated from human alcoholics. Am J Respir Crit Care Med. 2012;185:A1369. [Google Scholar]
- 29.Selzer ML, Vinokur A, van Rooijen L. A Self-Administered Short Michigan Alcoholism Screening Test (SMAST) J Stud Alcohol. 1975;36:117–126. doi: 10.15288/jsa.1975.36.117. [DOI] [PubMed] [Google Scholar]
- 30.Saunders JB, Aasland OG, Babor TF, de la Fuente JR, Grant M. Development of the Alcohol Use Disorders Identification Test (AUDIT): WHO collaborative project on early detection of persons with harmful alcohol consumption–II. Addiction. 1993;88:791–804. doi: 10.1111/j.1360-0443.1993.tb02093.x. [DOI] [PubMed] [Google Scholar]
- 31.Hunninghake GW, Gadek JE, Kawanami O, Ferrans VJ, Crystal RG. Inflammatory and immune processes in the human lung in health and disease: evaluation by bronchoalveolar lavage. Am J Pathol. 1979;97:149–206. [PMC free article] [PubMed] [Google Scholar]
- 32.Brown SD, Gauthier TW, Brown LA. Impaired terminal differentiation of pulmonary macrophages in a guinea pig model of chronic ethanol ingestion. Alcohol Clin Exp Res. 2009;33:1782–1793. doi: 10.1111/j.1530-0277.2009.01017.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Allison PD. Cary, NC: SAS Institute; 1999. Logistic regression using the SAS system theory and application. [Google Scholar]
- 34.Bickel PJ. One-step Huber estimates in the linear model. J Am Stat Assoc. 1975;70:428–434. [Google Scholar]
- 35.Chalmers JD, Singanayagam A, Murray MP, Scally C, Fawzi A, Hill AT. Risk factors for complicated parapneumonic effusion and empyema on presentation to hospital with community-acquired pneumonia. Thorax. 2009;64:592–597. doi: 10.1136/thx.2008.105080. [DOI] [PubMed] [Google Scholar]
- 36.Gacouin A, Legay F, Camus C, Volatron AC, Barbarot N, Donnio PY, Thomas R, Le Tulzo Y. At-risk drinkers are at higher risk to acquire a bacterial infection during an intensive care unit stay than abstinent or moderate drinkers. Crit Care Med. 2008;36:1735–1741. doi: 10.1097/CCM.0b013e318174dd75. [DOI] [PubMed] [Google Scholar]
- 37.Vallee BL, Wacker WE, Bartho Lomay AF, Hoch FL. Zinc metabolism in hepatic dysfunction. Ann Intern Med. 1959;50:1077–1091. doi: 10.7326/0003-4819-50-5-1077. [DOI] [PubMed] [Google Scholar]
- 38.Bartholomay AF, Robin ED, Vallee RL, Wacker WE. Zinc metabolism in hepatic dysfunction. I. Serum zinc concentrations in Laennec's cirrhosis and their validation by sequential analysis. N Engl J Med. 1956;255:403–408. doi: 10.1056/NEJM195608302550901. [DOI] [PubMed] [Google Scholar]
- 39.Goode HF, Kelleher J, Walker BE. Relation between zinc status and hepatic functional reserve in patients with liver disease. Gut. 1990;31:694–697. doi: 10.1136/gut.31.6.694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kang X, Zhong W, Liu J, Song Z, McClain CJ, Kang YJ, Zhou Z. Zinc supplementation reverses alcohol-induced steatosis in mice through reactivating hepatocyte nuclear factor-4alpha and peroxisome proliferator-activated receptor-alpha. Hepatology. 2009;50:1241–1250. doi: 10.1002/hep.23090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhou Z, Liu J, Song Z, McClain CJ, Kang YJ. Zinc supplementation inhibits hepatic apoptosis in mice subjected to a long-term ethanol exposure. Exp Biol Med (Maywood) 2008;233:540–548. doi: 10.3181/0710-RM-265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zarski JP, Arnaud J, Labadie H, Beaugrand M, Favier A, Rachail M. Serum and tissue concentrations of zinc after oral supplementation in chronic alcoholics with or without cirrhosis. Gastroenterol Clin Biol. 1987;11:856–860. [PubMed] [Google Scholar]
- 43.Mayo-Smith MF, Beecher LH, Fischer TL, Gorelick DA, Guillaume JL, Hill A, Jara G, Kasser C, Melbourne J. Management of alcohol withdrawal delirium: an evidence-based practice guideline. Arch Intern Med. 2004;164:1405–1412. doi: 10.1001/archinte.164.13.1405. [DOI] [PubMed] [Google Scholar]
- 44.Ho E, Courtemanche C, Ames BN. Zinc deficiency induces oxidative DNA damage and increases p53 expression in human lung fibroblasts. J Nutr. 2003;133:2543–2548. doi: 10.1093/jn/133.8.2543. [DOI] [PubMed] [Google Scholar]
- 45.Oteiza PI, Clegg MS, Zago MP, Keen CL. Zinc deficiency induces oxidative stress and ap-1 activation in 3t3 cells. Free Radic Biol Med. 2000;28:1091–1099. doi: 10.1016/s0891-5849(00)00200-8. [DOI] [PubMed] [Google Scholar]
- 46.Golub MS, Gershwin ME, Hurley LS, Hendrickx AG, Saito WY. Studies of marginal zinc deprivation in rhesus monkeys: infant behavior. Am J Clin Nutr. 1985;42:1229–1239. doi: 10.1093/ajcn/42.6.1229. [DOI] [PubMed] [Google Scholar]
- 47.Moss M, Guidot DM, Wong-Lambertina M, Ten Hoor T, Perez RL, Brown LA. The effects of chronic alcohol abuse on pulmonary glutathione homeostasis. Am J Respir Crit Care Med. 2000;161:414–419. doi: 10.1164/ajrccm.161.2.9905002. [DOI] [PubMed] [Google Scholar]
- 48.Burnham EL, Phang TL, House R, Vandivier RW, Moss M, Gaydos J. Alveolar macrophage gene expression is altered in the setting of alcohol use disorders. Alcohol Clin Exp Res. 2011;35:284–294. doi: 10.1111/j.1530-0277.2010.01344.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Eide DJ. The oxidative stress of zinc deficiency. Metallomics. 2011;3:1124–1129. doi: 10.1039/c1mt00064k. [DOI] [PubMed] [Google Scholar]
- 50.Zhao Y, Tan Y, Dai J, Li B, Guo L, Cui J, Wang G, Shi X, Zhang X, Mellen N, et al. Exacerbation of diabetes-induced testicular apoptosis by zinc deficiency is most likely associated with oxidative stress, p38 MAPK activation, and p53 activation in mice. Toxicol Lett. 2011;200:100–106. doi: 10.1016/j.toxlet.2010.11.001. [DOI] [PubMed] [Google Scholar]