Abstract
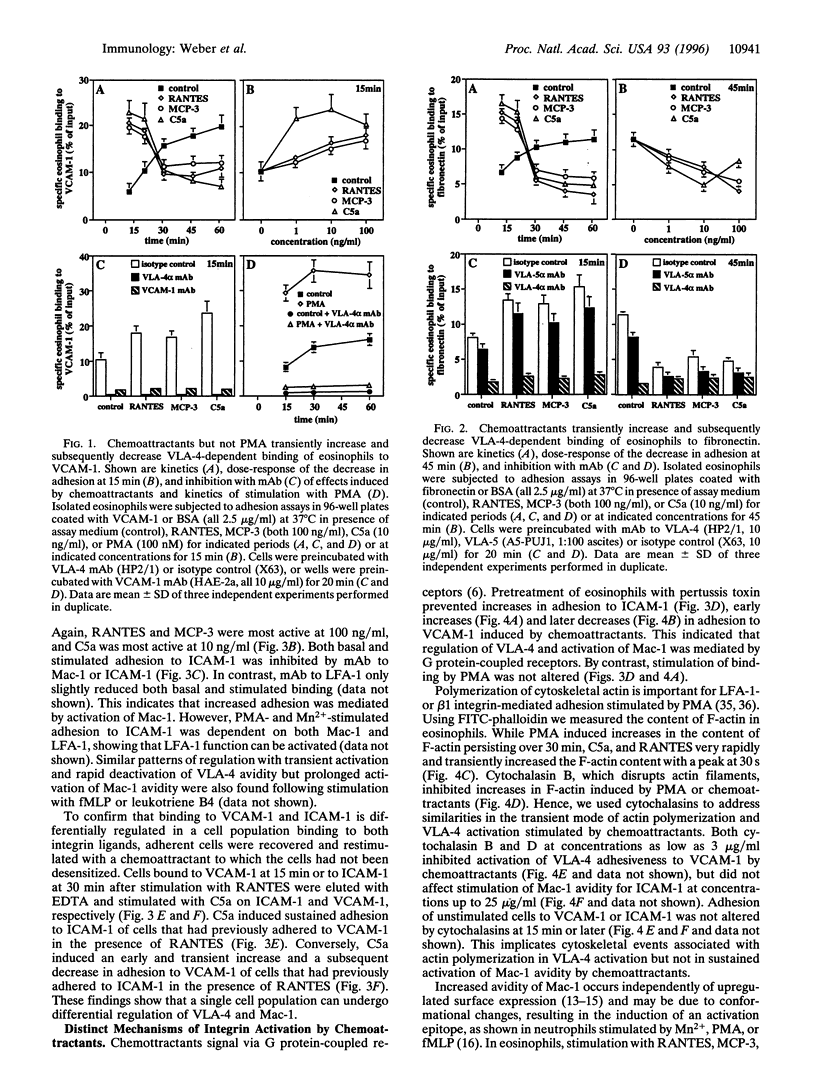
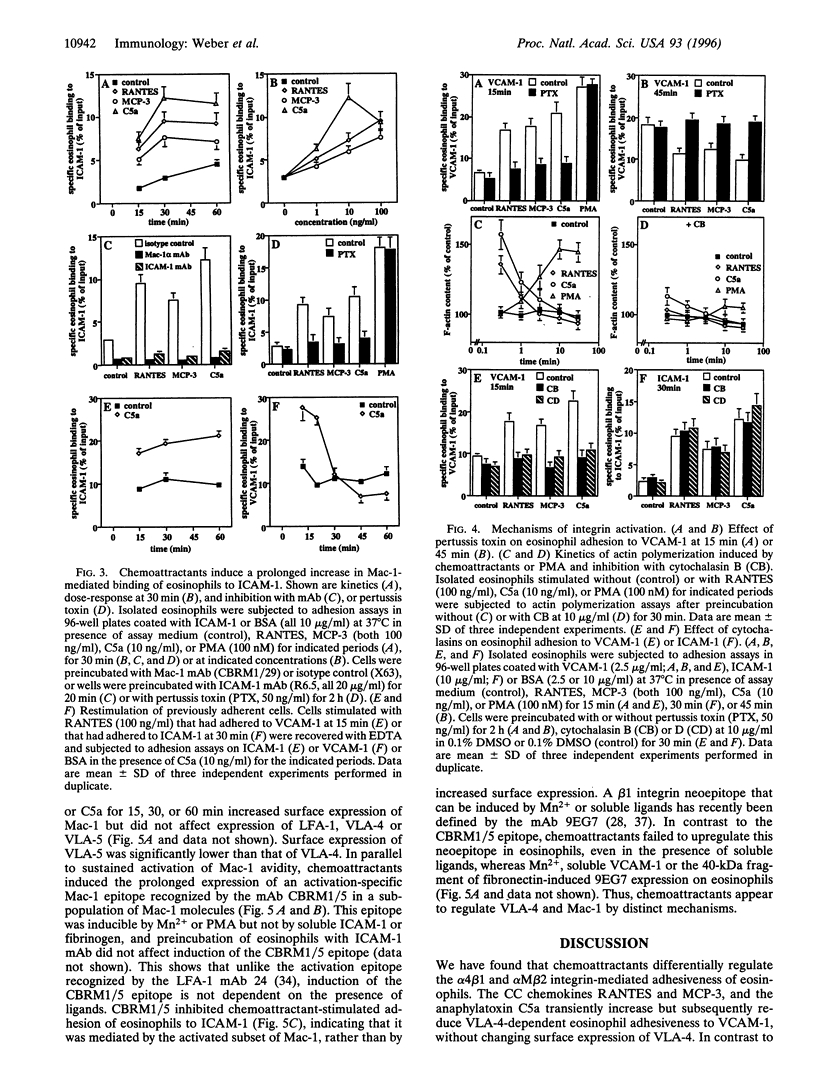
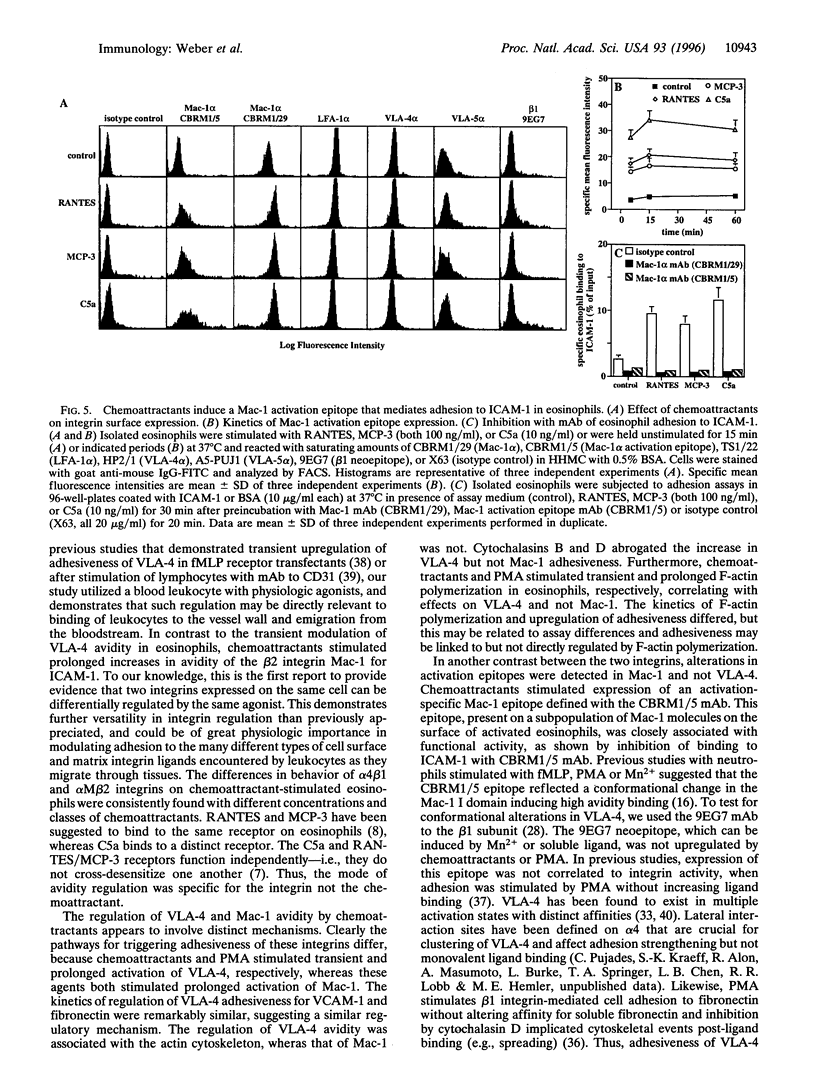
The CC chemokines regulated on activation normal T expressed and secreted (RANTES) and monocyte chemotactic protein 3 (MCP-3), and the anaphylatoxin C5a, induce activation, degranulation, chemotaxis, and transendothelial migration of eosinophils. Adhesion assays on purified ligands showed differential regulation of beta 1 and beta 2 integrin avidity in eosinophils. Adhesiveness of VLA-4 (alpha 4 beta 1, CD29/CD49d) for vascular cell adhesion molecule 1 or fibronectin was rapidly increased but subsequently reduced by RANTES, MCP-3, or C5a. The deactivation of VLA-4 lead to cell detachment, whereas phorbol 12-myristate 13-acetate induced sustained activation of VLA-4. In contrast, chemoattractants stimulated a prolonged increase in the adhesiveness of Mac-1 (alpha M beta 2, CD11b/CD18) for intercellular adhesion molecule 1. Inhibition by pertussis toxin confirmed signaling via G protein-coupled receptors. Chemoattractants induced transient, while phorbol 12-myristate 13-acetate induced sustained actin polymerization. Disruption of actin filaments by cytochalasins inhibited increases in avidity of VLA-4 but not of Mac-1. Chemoattractants did not upregulate a Mn2+-inducible beta 1 neoepitope defined by the mAb 9EG7, but induced prolonged expression of a Mac-1 activation epitope recognized by the mAb CBRM1/5. This mAb inhibited chemoattractant-stimulated adhesion of eosinophils to intercellular adhesion molecule 1. Thus, regulation of VLA-4 was dependent on the actin cytoskeleton, whereas conformational changes appeared to be crucial for activation of Mac-1. To our knowledge, this is the first demonstration that physiological agonists, such as chemoattractants, can differentially regulate the avidity of a beta 1 and a beta 2 integrin expressed on the same leukocyte.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baggiolini M., Dahinden C. A. CC chemokines in allergic inflammation. Immunol Today. 1994 Mar;15(3):127–133. doi: 10.1016/0167-5699(94)90156-2. [DOI] [PubMed] [Google Scholar]
- Baggiolini M., Dewald B., Moser B. Interleukin-8 and related chemotactic cytokines--CXC and CC chemokines. Adv Immunol. 1994;55:97–179. [PubMed] [Google Scholar]
- Bazzoni G., Shih D. T., Buck C. A., Hemler M. E. Monoclonal antibody 9EG7 defines a novel beta 1 integrin epitope induced by soluble ligand and manganese, but inhibited by calcium. J Biol Chem. 1995 Oct 27;270(43):25570–25577. doi: 10.1074/jbc.270.43.25570. [DOI] [PubMed] [Google Scholar]
- Bochner B. S., Luscinskas F. W., Gimbrone M. A., Jr, Newman W., Sterbinsky S. A., Derse-Anthony C. P., Klunk D., Schleimer R. P. Adhesion of human basophils, eosinophils, and neutrophils to interleukin 1-activated human vascular endothelial cells: contributions of endothelial cell adhesion molecules. J Exp Med. 1991 Jun 1;173(6):1553–1557. doi: 10.1084/jem.173.6.1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bousquet J., Chanez P., Lacoste J. Y., Barnéon G., Ghavanian N., Enander I., Venge P., Ahlstedt S., Simony-Lafontaine J., Godard P. Eosinophilic inflammation in asthma. N Engl J Med. 1990 Oct 11;323(15):1033–1039. doi: 10.1056/NEJM199010113231505. [DOI] [PubMed] [Google Scholar]
- Buyon J. P., Abramson S. B., Philips M. R., Slade S. G., Ross G. D., Weissmann G., Winchester R. J. Dissociation between increased surface expression of gp165/95 and homotypic neutrophil aggregation. J Immunol. 1988 May 1;140(9):3156–3160. [PubMed] [Google Scholar]
- Cabañas C., Hogg N. Ligand intercellular adhesion molecule 1 has a necessary role in activation of integrin lymphocyte function-associated molecule 1. Proc Natl Acad Sci U S A. 1993 Jun 15;90(12):5838–5842. doi: 10.1073/pnas.90.12.5838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahinden C. A., Geiser T., Brunner T., von Tscharner V., Caput D., Ferrara P., Minty A., Baggiolini M. Monocyte chemotactic protein 3 is a most effective basophil- and eosinophil-activating chemokine. J Exp Med. 1994 Feb 1;179(2):751–756. doi: 10.1084/jem.179.2.751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond M. S., Springer T. A. A subpopulation of Mac-1 (CD11b/CD18) molecules mediates neutrophil adhesion to ICAM-1 and fibrinogen. J Cell Biol. 1993 Jan;120(2):545–556. doi: 10.1083/jcb.120.2.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond M. S., Springer T. A. The dynamic regulation of integrin adhesiveness. Curr Biol. 1994 Jun 1;4(6):506–517. doi: 10.1016/s0960-9822(00)00111-1. [DOI] [PubMed] [Google Scholar]
- Diamond M. S., Staunton D. E., Marlin S. D., Springer T. A. Binding of the integrin Mac-1 (CD11b/CD18) to the third immunoglobulin-like domain of ICAM-1 (CD54) and its regulation by glycosylation. Cell. 1991 Jun 14;65(6):961–971. doi: 10.1016/0092-8674(91)90548-d. [DOI] [PubMed] [Google Scholar]
- Ebisawa M., Yamada T., Bickel C., Klunk D., Schleimer R. P. Eosinophil transendothelial migration induced by cytokines. III. Effect of the chemokine RANTES. J Immunol. 1994 Sep 1;153(5):2153–2160. [PubMed] [Google Scholar]
- Faull R. J., Kovach N. L., Harlan J. M., Ginsberg M. H. Stimulation of integrin-mediated adhesion of T lymphocytes and monocytes: two mechanisms with divergent biological consequences. J Exp Med. 1994 Apr 1;179(4):1307–1316. doi: 10.1084/jem.179.4.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerard C., Gerard N. P. The pro-inflammatory seven-transmembrane segment receptors of the leukocyte. Curr Opin Immunol. 1994 Feb;6(1):140–145. doi: 10.1016/0952-7915(94)90045-0. [DOI] [PubMed] [Google Scholar]
- Gleich G. J., Adolphson C. R. The eosinophilic leukocyte: structure and function. Adv Immunol. 1986;39:177–253. doi: 10.1016/s0065-2776(08)60351-x. [DOI] [PubMed] [Google Scholar]
- Hansel T. T., Pound J. D., Thompson R. A. Isolation of eosinophils from human blood. J Immunol Methods. 1990 Mar 9;127(2):153–164. doi: 10.1016/0022-1759(90)90064-3. [DOI] [PubMed] [Google Scholar]
- Hemler M. E. VLA proteins in the integrin family: structures, functions, and their role on leukocytes. Annu Rev Immunol. 1990;8:365–400. doi: 10.1146/annurev.iy.08.040190.002053. [DOI] [PubMed] [Google Scholar]
- Honda S., Campbell J. J., Andrew D. P., Engelhardt B., Butcher B. A., Warnock R. A., Ye R. D., Butcher E. C. Ligand-induced adhesion to activated endothelium and to vascular cell adhesion molecule-1 in lymphocytes transfected with the N-formyl peptide receptor. J Immunol. 1994 Apr 15;152(8):4026–4035. [PubMed] [Google Scholar]
- Hynes R. O. Integrins: versatility, modulation, and signaling in cell adhesion. Cell. 1992 Apr 3;69(1):11–25. doi: 10.1016/0092-8674(92)90115-s. [DOI] [PubMed] [Google Scholar]
- Jakubowski A., Rosa M. D., Bixler S., Lobb R., Burkly L. C. Vascular cell adhesion molecule (VCAM)-Ig fusion protein defines distinct affinity states of the very late antigen-4 (VLA-4) receptor. Cell Adhes Commun. 1995 May;3(2):131–142. doi: 10.3109/15419069509081282. [DOI] [PubMed] [Google Scholar]
- Kassner P. D., Alon R., Springer T. A., Hemler M. E. Specialized functional properties of the integrin alpha 4 cytoplasmic domain. Mol Biol Cell. 1995 Jun;6(6):661–674. doi: 10.1091/mbc.6.6.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuijpers T. W., Mul E. P., Blom M., Kovach N. L., Gaeta F. C., Tollefson V., Elices M. J., Harlan J. M. Freezing adhesion molecules in a state of high-avidity binding blocks eosinophil migration. J Exp Med. 1993 Jul 1;178(1):279–284. doi: 10.1084/jem.178.1.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenter M., Uhlig H., Hamann A., Jenö P., Imhof B., Vestweber D. A monoclonal antibody against an activation epitope on mouse integrin chain beta 1 blocks adhesion of lymphocytes to the endothelial integrin alpha 6 beta 1. Proc Natl Acad Sci U S A. 1993 Oct 1;90(19):9051–9055. doi: 10.1073/pnas.90.19.9051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo S. K., Detmers P. A., Levin S. M., Wright S. D. Transient adhesion of neutrophils to endothelium. J Exp Med. 1989 May 1;169(5):1779–1793. doi: 10.1084/jem.169.5.1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loetscher P., Seitz M., Clark-Lewis I., Baggiolini M., Moser B. Monocyte chemotactic proteins MCP-1, MCP-2, and MCP-3 are major attractants for human CD4+ and CD8+ T lymphocytes. FASEB J. 1994 Oct;8(13):1055–1060. doi: 10.1096/fasebj.8.13.7926371. [DOI] [PubMed] [Google Scholar]
- Marlin S. D., Staunton D. E., Springer T. A., Stratowa C., Sommergruber W., Merluzzi V. J. A soluble form of intercellular adhesion molecule-1 inhibits rhinovirus infection. Nature. 1990 Mar 1;344(6261):70–72. doi: 10.1038/344070a0. [DOI] [PubMed] [Google Scholar]
- Masumoto A., Hemler M. E. Multiple activation states of VLA-4. Mechanistic differences between adhesion to CS1/fibronectin and to vascular cell adhesion molecule-1. J Biol Chem. 1993 Jan 5;268(1):228–234. [PubMed] [Google Scholar]
- Pepinsky B., Hession C., Chen L. L., Moy P., Burkly L., Jakubowski A., Chow E. P., Benjamin C., Chi-Rosso G., Luhowskyj S. Structure/function studies on vascular cell adhesion molecule-1. J Biol Chem. 1992 Sep 5;267(25):17820–17826. [PubMed] [Google Scholar]
- Ponath P. D., Qin S., Ringler D. J., Clark-Lewis I., Wang J., Kassam N., Smith H., Shi X., Gonzalo J. A., Newman W. Cloning of the human eosinophil chemoattractant, eotaxin. Expression, receptor binding, and functional properties suggest a mechanism for the selective recruitment of eosinophils. J Clin Invest. 1996 Feb 1;97(3):604–612. doi: 10.1172/JCI118456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rot A., Krieger M., Brunner T., Bischoff S. C., Schall T. J., Dahinden C. A. RANTES and macrophage inflammatory protein 1 alpha induce the migration and activation of normal human eosinophil granulocytes. J Exp Med. 1992 Dec 1;176(6):1489–1495. doi: 10.1084/jem.176.6.1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothenberg M. E., Luster A. D., Leder P. Murine eotaxin: an eosinophil chemoattractant inducible in endothelial cells and in interleukin 4-induced tumor suppression. Proc Natl Acad Sci U S A. 1995 Sep 12;92(19):8960–8964. doi: 10.1073/pnas.92.19.8960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothlein R., Springer T. A. The requirement for lymphocyte function-associated antigen 1 in homotypic leukocyte adhesion stimulated by phorbol ester. J Exp Med. 1986 May 1;163(5):1132–1149. doi: 10.1084/jem.163.5.1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Madrid F., Krensky A. M., Ware C. F., Robbins E., Strominger J. L., Burakoff S. J., Springer T. A. Three distinct antigens associated with human T-lymphocyte-mediated cytolysis: LFA-1, LFA-2, and LFA-3. Proc Natl Acad Sci U S A. 1982 Dec;79(23):7489–7493. doi: 10.1073/pnas.79.23.7489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schleimer R. P., Sterbinsky S. A., Kaiser J., Bickel C. A., Klunk D. A., Tomioka K., Newman W., Luscinskas F. W., Gimbrone M. A., Jr, McIntyre B. W. IL-4 induces adherence of human eosinophils and basophils but not neutrophils to endothelium. Association with expression of VCAM-1. J Immunol. 1992 Feb 15;148(4):1086–1092. [PubMed] [Google Scholar]
- Smith C. W., Marlin S. D., Rothlein R., Toman C., Anderson D. C. Cooperative interactions of LFA-1 and Mac-1 with intercellular adhesion molecule-1 in facilitating adherence and transendothelial migration of human neutrophils in vitro. J Clin Invest. 1989 Jun;83(6):2008–2017. doi: 10.1172/JCI114111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Springer T. A. Traffic signals for lymphocyte recirculation and leukocyte emigration: the multistep paradigm. Cell. 1994 Jan 28;76(2):301–314. doi: 10.1016/0092-8674(94)90337-9. [DOI] [PubMed] [Google Scholar]
- Spry C. J., Kay A. B., Gleich G. J. Eosinophils 1992. Immunol Today. 1992 Oct;13(10):384–387. doi: 10.1016/0167-5699(92)90085-L. [DOI] [PubMed] [Google Scholar]
- Sriramarao P., von Andrian U. H., Butcher E. C., Bourdon M. A., Broide D. H. L-selectin and very late antigen-4 integrin promote eosinophil rolling at physiological shear rates in vivo. J Immunol. 1994 Nov 1;153(9):4238–4246. [PubMed] [Google Scholar]
- Sánchez-Madrid F., De Landázuri M. O., Morago G., Cebrián M., Acevedo A., Bernabeu C. VLA-3: a novel polypeptide association within the VLA molecular complex: cell distribution and biochemical characterization. Eur J Immunol. 1986 Nov;16(11):1343–1349. doi: 10.1002/eji.1830161106. [DOI] [PubMed] [Google Scholar]
- Tanaka Y., Albelda S. M., Horgan K. J., van Seventer G. A., Shimizu Y., Newman W., Hallam J., Newman P. J., Buck C. A., Shaw S. CD31 expressed on distinctive T cell subsets is a preferential amplifier of beta 1 integrin-mediated adhesion. J Exp Med. 1992 Jul 1;176(1):245–253. doi: 10.1084/jem.176.1.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vedder N. B., Harlan J. M. Increased surface expression of CD11b/CD18 (Mac-1) is not required for stimulated neutrophil adherence to cultured endothelium. J Clin Invest. 1988 Mar;81(3):676–682. doi: 10.1172/JCI113372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vonderheide R. H., Tedder T. F., Springer T. A., Staunton D. E. Residues within a conserved amino acid motif of domains 1 and 4 of VCAM-1 are required for binding to VLA-4. J Cell Biol. 1994 Apr;125(1):215–222. doi: 10.1083/jcb.125.1.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber C., Alon R., Moser B., Springer T. A. Sequential regulation of alpha 4 beta 1 and alpha 5 beta 1 integrin avidity by CC chemokines in monocytes: implications for transendothelial chemotaxis. J Cell Biol. 1996 Aug;134(4):1063–1073. doi: 10.1083/jcb.134.4.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wegner C. D., Gundel R. H., Reilly P., Haynes N., Letts L. G., Rothlein R. Intercellular adhesion molecule-1 (ICAM-1) in the pathogenesis of asthma. Science. 1990 Jan 26;247(4941):456–459. doi: 10.1126/science.1967851. [DOI] [PubMed] [Google Scholar]