Abstract
In cooperatively breeding mammals and birds, intra-sexual reproductive competition among females may often render variance in reproductive success higher among females than males, leading to the prediction that intra-sexual selection in such species may have yielded the differential exaggeration of competitive traits among females. However, evidence to date suggests that female-biased reproductive variance in such species is rarely accompanied by female-biased sexual dimorphisms. We illustrate the problem with data from wild Damaraland mole-rat, Fukomys damarensis, societies: the variance in lifetime reproductive success among females appears to be higher than that among males, yet males grow faster, are much heavier as adults and sport larger skulls and incisors (the weapons used for fighting) for their body lengths than females, suggesting that intra-sexual selection has nevertheless acted more strongly on the competitive traits of males. We then consider potentially general mechanisms that could explain these disparities by tempering the relative intensity of selection for competitive trait exaggeration among females in cooperative breeders. Key among these may be interactions with kin selection that could nevertheless render the variance in inclusive fitness lower among females than males, and fundamental aspects of the reproductive biology of females that may leave reproductive conflict among females more readily resolved without overt physical contests.
Keywords: sexual selection, reproductive skew, cooperation, cooperative breeding, sex differences, mate choice
1. Introduction
In recent years, there has been a surge of interest in the causes and evolutionary consequences of intra-sexual competition among females [1–14]. This has been born in part of recognition that, while males (generally being the lighter investor per offspring) typically compete more intensely for mates [15–18], females frequently compete strongly for the rank or resources necessary for reproduction (doubtless due in part to typically being the heavier investor) [1–6,19–21]. The historical focus of research on the consequences of intra-sexual competition for mates per se is understandable, as success in this regard is considered the target of sexual selection, as originally conceived by Darwin [15–18]. However, it is now clear that a complete understanding of the intra-sexual selection pressures that shape the evolution of competitive traits in both sexes demands that we also consider competition for the rank or resources necessary for reproduction, whose evolutionary implications fall under the broader banner of social selection [2–4,9–12,20–23].
It has recently been highlighted that intra-sexual competition among female mammals and birds for the rank and resources necessary for reproduction may be at its strongest in cooperatively breeding societies [1,2]. In many cooperative mammals and birds, a single dominant female largely monopolizes reproduction within her group [24,25], and success in this regard often entails intense intra-sexual competition over rare dominance vacancies and/or subsequent reproductive success [2,25–30]. In most mammals and birds, male–male competition over matings is thought to leave the variance in reproductive success arising from intra-sexual competition higher among males than females, leading to stronger intra-sexual selection among males for the traits that yield success in competition (typically large body size and/or weaponry; [18,31–33]). In cooperatively breeding mammals and birds, by contrast, where dominant females often monopolize reproduction to a greater extent than dominant males, and their dominance tenures are often longer than those of males (so fewer females become dominant during their lifetimes), it has been estimated that the variance in reproductive success may frequently be higher among females than males [1–3]. This observation has led to the prediction that, all other things being equal [34–36], intra-sexual selection would be expected to have led to the differential exaggeration of the traits that yield success in competition among females relative to males [1,2].
Two high profile studies have now tested the prediction that intra-sexual selection should have favoured the differential exaggeration of competitive traits among females in cooperatively breeding mammals and birds. In the first, research on cooperatively breeding meerkats, Suricata suricatta (in which females strongly contest dominance vacancies and the dominant female produces around 80% of the pups; [37]), strongly suggests that variance in lifetime reproductive success is indeed higher among females than males, and reveals that females also show higher rates of intra-sexual aggression and exhibit certain endocrine and morphological changes on dominance acquisition that appear to be reduced or absent in males [2]. Similarly, a comparative study of African starlings has found that, while in the non-cooperative species males are typically larger and have brighter plumage than females, in the cooperatively breeding species (one of which has been shown to exhibit higher variance in annual reproductive success among females than males), the extent of the male bias in body size is significantly reduced and clear plumage dimorphisms are absent [3]. The findings of both studies are therefore consistent with the view that intra-sexual selection has promoted the exaggeration of traits that yield success in competition among females in such species.
However, it is notable that, despite the variance in reproductive success arising from intra-sexual competition appearing to be higher among females than males in both of the above studies, neither provides support for the prediction that females might therefore be expected to be larger (and/or show brighter plumage) than males. Female meerkats are no larger than males (if anything, males have a tendency to be heavier and may have larger canines; [2,38–40]), which is all the more surprising as body mass advantages have a greater impact on dominance acquisition and retention among female meerkats than males [2]. Likewise in the African starlings, the cooperatively breeding species without exception show male-biased size dimorphisms, albeit weaker than those in the non-cooperative species [3]. Indeed, while female-biased size dimorphisms clearly have evolved among non-cooperative vertebrates in which males are the primary investor (e.g. species of phalarope, genus Phalaropus, and jacana, family Jacanidae, show reversed sexual size and plumage dimorphisms [17,41–43]) or in which females remain the primary investor but intensely contest resources (e.g. the spotted hyaena, Crocuta crocuta [19]), such reversals have rarely been reported in cooperatively breeding mammals and birds (see Discussion). These patterns beg the question: why are females not bigger and better armed than males in the cooperatively breeding mammals and birds that show higher variance in reproductive success among females than males?
Here, we consider this problem with a two-step approach. First, we demonstrate that this apparent discord between female-biased variance in reproductive success and male-biased sexual dimorphism is striking in a cooperative mammal that shows extreme variance in female reproductive success: the Damaraland mole-rat, Fukomys damarensis. Second, we consider potentially general explanations for this discord, both in our focal species and across cooperative mammals and birds.
2. Material and methods
(a). Study species and specific aims
Damaraland mole-rats live in groups of up to 41 individuals, in which reproduction is completely monopolized by a single dominant female (no subordinate female has ever been known to rear young; [44,45]). Complete reproductive skew among females, coupled with the low proportion of individuals estimated to become dominant during their lifetimes (just 8%), suggests that variance in reproductive success among females is extreme, having been likened to the patterns seen in some eusocial insects [46,47]. While reproduction is also highly skewed among males, it is more equitably shared, with two males sometimes co-breeding within a group and genetic evidence suggestive of extra-group paternity [44,45]. New groups are typically founded by a single dispersing female and one or two dispersing males and grow through the delayed dispersal of their young [44,45]. While dispersing females have rarely been documented entering established colonies, dispersing males are known to, both briefly and permanently, and may sire offspring and/or become dominant when doing so (N. C. Bennett & J. Jarvis 1988–2002, unpublished data; [45,48]). While intra-sexual aggression is rare in extended nuclear families housed in the laboratory (where subordinates of both sexes lack access to unrelated mates), it escalates markedly on the introduction of extra-group individuals of either sex [49–54]. In these experiments, both dominant females and males subject same-sex intruders to intense intra-sexual aggression, but may meet opposite-sex intruders with reproductive solicitations ([51] see also [52,53]). Furthermore, experimentally replacing the dominant male with an extra-group male (simulating his replacement by an immigrant) gives rise to intra-sexual aggression between the existing dominant female and her daughters, which can result in dominance usurpations and injuries that may require their separation ([49] see also [50]). Indeed, intra-sexual competition has resulted in severe wounding and death among both males [53] and females [53,54] in other laboratory studies of this species, though such severe outcomes may reflect the artificial constraints on their dispersal. Intra-sexual aggression in both sexes entails bouts of incisor-fencing (where two animals stand face to face with their formidable extrabuccal incisors locked together and may shove each other back and forth along their tunnels and rock their heads from side to side) and aggressive biting and chasing, which may proceed to the submission, exclusion or death of one party [49–54]. In both sexes, larger animals tend to dominate smaller ones, with the dominant pair typically being the largest and heaviest animals of their sex in the colony [44,52,55]. Intra-sexual selection for the traits that yield success in competition might therefore be predicted to have favoured larger body size and/or exaggerated skulls and/or incisors relative to body size. Published data reflecting the distributions of body masses in wild-caught colonies suggest that males are heavier than females [44,56], though the extent to which this arises from sex differences in growth trajectories or age remains unclear [56,57], and whether the sexes differ in the relative exaggeration of their skulls and incisors is unknown.
To investigate whether variance in lifetime reproductive success is indeed higher among females than males and how this has impacted patterns of sexual dimorphism, we use data from two longitudinal field studies, lasting 14 and 3 years, to address two main aims. First, we estimate the relative dominance tenure lengths of males and females. Because the dominant female completely monopolizes reproduction, while the dominant male does not [45], the variance in lifetime reproductive success among females is likely to be higher than that among males as long as the turnover rate of female dominants is no higher than that among males. Second, we test the prediction that, if the variance in reproductive success arising from intra-sexual competition is indeed higher among females than males, intra-sexual selection should have led to the differential exaggeration in females of the morphological traits that yield success in competition: specifically large body size (reflected as higher growth rates and/or asymptotic adult mass in an age-related analysis of body mass) and exaggerated weaponry (reflected as larger skulls and/or incisors relative to their body length).
(b). Study populations and trapping methods
This study uses data from two longitudinal field studies of Damaraland mole-rats in the Kalahari desert: a 14 year field study at Dordabis in Namibia (22°58’ S, 17°41’ E) between 1988 and 2002 [47] and a more intensive 3 year field study at Tswalu Kalahari Reserve (27°22’ S, 22°19′ E) in the Northern Cape province of South Africa between 2004 and 2006 [48,58]. At both sites, the entire study population (15–30 colonies) was trapped approximately every six to 12 months. Colonies were trapped by digging a trench across a line of mole-hills to locate the underlying tunnel and setting traps baited with sweet potato in the line of the tunnel. Traps were typically checked every 1–3 h, and any trapped animals were weighed ±1 g and then transferred to a large sand-lined box for housing with fellow colony members until the colony had been completely trapped out (gauged by an absence of triggering or sand displacement at the trap site for 36 h), at which point the entire colony was returned together to their original burrow system.
All individuals were sexed by the shape of their genitalia [44], and the single dominant female in each colony could be readily distinguished from her non-breeding subordinates by her perforate vagina and/or swollen teats ([44] as validated by [45]). Prior to release, all individuals trapped in the Tswalu study were briefly anesthetized by halothane inhalation for the taking of morphological measurements: skull width (zygomatic arch width ±0.1 mm, taken across the skull's widest point using callipers), incisor width (taken across the incisor pair's widest point using callipers ± 0.1 mm; incisor lengths were not measured as they grow continuously and are worn during tunnelling) and body length (front of the snout to the end of the short tail, using a tape measure accurate to±1 mm; the tail's length (approx. 10 mm) was not deducted as the tail has no clearly discernable base). All protocols were approved by the University of Pretoria ethics committee.
(c). Sex differences in dominance tenure length
Investigating whether the sexes differ in dominance tenure length is not straightforward, as while the dominant female can be readily identified morphologically when trapped in the field, the dominant male cannot (beyond typically being the largest male in the colony). As such, to analyse differences in tenure length we restricted our attention to scenarios in our dataset from Dordabis where a group had recently been founded and so the dominant male and female could be readily identified because they were, by some considerable margin, the only individuals of their sex in excess of 100 g. To ensure that these were stable groups and not transiently interacting individuals, we restricted our attention to the 19 such cases in which the same large male and female were caught together at least twice in succession. These 19 groups were then each continuously trapped at roughly 6–12 month intervals until the disappearance of both of the original dominants (resulting in two to eight successive trapping sessions per group, where one or both dominants remained, over a total duration of 6–56 months per group). When these groups were first caught, the dominant males weighed 162–280 g (median = 207 g), whereas the dominant females weighed 117–195 g (median = 159 g). When first trapped, the majority of these groups (13 of 19) contained only the dominant pair, and the heaviest other individual present in the six groups that did have supernumeraries weighed just 86 g. We used these 19 groups to estimate the relative dominance tenure lengths of males and females, by contrasting the duration for which the dominant male and dominant female continued to be trapped within the group, considering their tenure to start when they were first trapped (having founded their group) and to have ended when they were last trapped in their group. This approach will therefore tend to underestimate tenure length for both sexes (as they were doubtless present at least briefly prior to their first trapping and after their last trapping). As such, we confirmed that any apparent sex difference in tenure length remained significant if tenures were instead deemed to have ended when the individual was first known to be absent, rather than when last found to be present. Our method also has the potential to overestimate the length of male dominance tenures relative to those of females, as while we can be certain that the founding female remained dominant as long as she was monitored (as the dominant female can be identified morphologically), the founding male could have lost his dominant breeding status but remained within the group, leading us to overestimate his tenure length (in at least two cases another large male did arrive, but we assumed that the new arrival did not take dominance). This would only have counteracted rather than confounded our findings though, as our analyses suggest that females show significantly longer dominance tenures than males.
(d). Sex differences in growth and asymptotic body mass
To investigate the nature of the sex difference in growth rates and adult body mass, we used data exclusively from individuals that were first trapped in our longer-term Dordabis study as juveniles (weighing between 25 and 50 g). Their ages were estimated for all subsequent re-traps (given the absence of precisely known birth dates) by assuming on the basis of captive data [57,59] that they were three months old when first trapped as a juvenile. Data were included for all such individuals for whom four or more repeated body mass measures were available during their lifetime (n = 31 individuals; median of five mass measures; range four to nine measures), so as to focus our analysis on those individuals for whom repeated mass measures were available for the assessment of growth (the inclusion of all available mass data only enhanced the statistical significance of our findings).
We first fitted Gompertz growth curves through the data for each sex (figure 2). As both sexes appeared to show a transition from early life growth to a later life mass asymptote at around 2 years of age, we divided the data into two at this point, using the data from individuals less than 2 years of age for the analysis of sex differences in growth (111 mass measures from 31 individuals, 17 females and 14 males, across 13 social groups) and the data from individuals greater than 2 years of age for the analysis of sex differences in asymptotic adult masses (57 mass measures from 20 individuals, 12 females and eight males, across 10 social groups). Restricting the growth rate analysis solely to the most linear phase of growth prior to 18 months of age (90 mass measures from 31 individuals, 17 females and 14 males, across 13 social groups) yielded qualitatively similar findings. To determine whether there was a sex difference in growth rate, we fitted the body mass measures of individuals less than 2 years old as the response term in a general linear mixed model (GLMM) and tested the effect of the interaction between age and sex in determining their body mass. To then determine whether there was a sex difference in asymptotic adult mass, we fitted the body mass measures of individuals greater than 2 years old as the response term in a second GLMM and tested whether there was a sex effect on body masses during this period, allowing for the possibility that body mass continued to increase with age by also fitting age and the interaction between age and sex. In both GLMMs, individual and group identity were fitted as random factors to control for repeated measures of each. For completeness, we retained dominant females in all of the analyses even though they could have been pregnant at the time of weighing (they breed year round and pregnancies are not readily detectable by eye which precluded their exclusion). This was the most conservative approach, as the analysis nevertheless revealed that males are significantly heavier than females.
Figure 2.
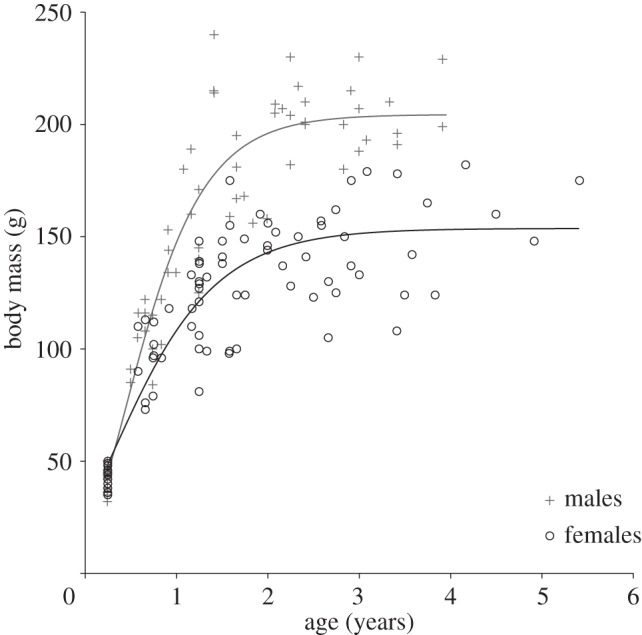
Males grew significantly faster than females and reached markedly higher asymptotic masses than females (see text for statistics; n = 168 mass measures from 31 individuals, 17 females and 14 males, across 13 different wild colonies). The lines display the best fit Gompertz growth curves for each sex.
(e). Sex differences in body shape and weaponry
To investigate the sex difference in body shape and weaponry, we used the detailed morphological data from our study at Tswalu Kalahari Reserve [48,58]. As mole-rats of both sexes fight using their skulls and incisors, we focused our attention on characterizing any sex difference in the exaggeration of skull and incisor width relative to body length. We used data from all non-juveniles (individuals > 50 g) caught during the study, which yielded 416 measures of body mass, body length, skull width and incisor width from 299 mole-rats (154 males and 145 females) from 63 different social groups.
First, we investigated whether there was a sex difference in skull width while controlling for variation in body length, by fitting skull width as the response term in a GLMM with sex as the primary predictor and body length as a covariate, and by allowing for the possibility of an interaction between sex and body length. Second, we investigated whether there was a sex difference in incisor width while controlling for variation in body length, by conducting the same analysis but with incisor width in place of skull width. Third, to investigate whether the sex difference in incisor width stemmed simply from the sex difference in skull width, we used a GLMM to investigate the relationship between skull width and incisor width and to test for any sex difference in this relationship. Finally, we investigated whether any sex difference in body mass was still apparent after controlling for variation in body length and skull width. Body mass was log-transformed for these analyses to linearize its relationship with the metrics of body size, and thereby normalize model residuals. In each GLMM, individual and colony identity were fitted as random factors to control for repeated measures of each. For completeness, we retained dominant females in all of the morphological analyses above and report the statistics and figures using this complete dataset. However, as we have previously found that females experience a significant reduction in skull width growth rate on dominance acquisition [58], we subsequently confirmed for each analysis that the exclusion of dominant females left our conclusions unchanged (which was invariably the case; see Results).
3. Results
In the 19 pairs of founding dominant males and females monitored to disappearance, the observed dominance tenure lengths of females were significantly longer than those of the males (Wilcoxon matched pairs test: p = 0.010; female median (with inter-quartile range) = 638 days (244–975 days); male median = 366 days (192–616 days); figure 1). In the two cases where the dominant male outlasted his dominant female, there was no evidence that she was replaced by another breeding female or that he subsequently bred (raising the possibility that his longer tenure did not translate into further reproductive success). In five of the 11 cases in which the dominant female outlasted her founding dominant male, recruitment patterns strongly suggest that the dominant female continued to breed after his disappearance (and in at least three cases, the original dominant male was replaced by new large immigrant males). Coupled with evidence that reproduction is entirely monopolized by the dominant female, whereas dominant males may lose paternity both to co-breeding residents and extra-group males [45], these findings suggest that the variance in lifetime reproductive success is likely to be higher among females than males.
Figure 1.
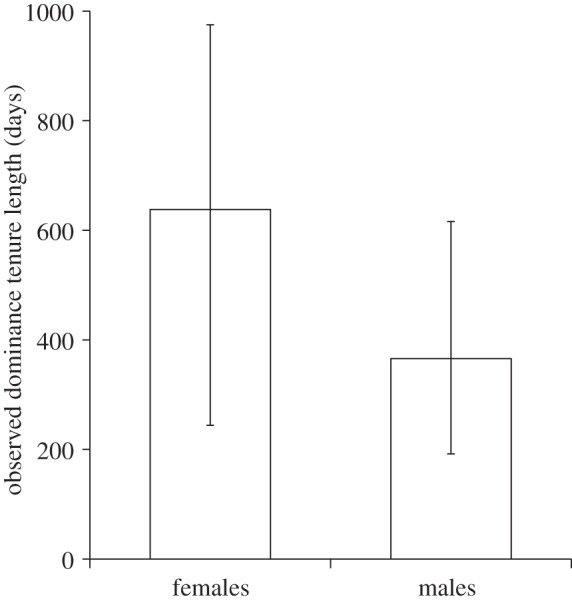
In the 19 pairs of founding dominant males and females monitored to disappearance, the observed dominance tenure lengths of females were significantly longer than those of the males (Wilcoxon test: p = 0.010). The bars show medians and inter-quartile ranges.
The growth profiles of male and female Damaraland mole-rats reveal marked sexual size dimorphism, with males being substantially heavier than females (figure 2). Dividing the body mass data around the growth curve asymptote at approximately 2 years of age revealed that, prior to this age, body mass increased significantly with increasing age (χ2 = 368.12, p < 0.001) and did so significantly faster in males than females (sex × age interaction term: χ2 = 15.92, p < 0.001; this interaction is more significant when only data from the most linear phase of growth prior to 18 months of age are used). After 2 years of age, there was no further significant increase in mass with increasing age (χ2 = 1.99, p = 0.18), and males were now significantly and substantially heavier than females (males mean±s.e. = 202.7 g±7.4 g; females mean±s.e. = 147.2 g±6.6 g; χ2 = 97.33, p < 0.001). In keeping with these results, when the 19 dominant pairs in the tenure analysis (above) were first caught, every dominant male was heavier than his dominant female, even though dominant females were most likely pregnant in many cases (dominant males mean±s.e. = 209.0 g±6.29 g; dominant females mean±s.e. = 153.7 g±5.44 g; paired t-test: t = 8.41, n = 19 pairs, p < 0.001). Interestingly, heavier dominant males were paired with heavier dominant females (Spearman rank correlation = 0.44; p = 0.015; n = 19 pairs).
Examining the body shapes of males and females revealed that males have significantly wider skulls for their body lengths than females (effect of sex: χ2 = 96.5, p < 0.001; controlling for the effect of body length: χ2 = 2742.7, p < 0.001) and that the extent of this sexual dimorphism in skull width increases with body length (sex × body length interaction: χ2 = 67.7, p < 0.001; figure 3a). Males also showed significantly wider incisors for their body lengths than females (effect of sex: χ2 = 40.2, p < 0.001; controlling for the effect of body length: χ2 = 1537.4, p < 0.001), and the extent of this sexual dimorphism in incisor width also increased with body length (sex × body length interaction: χ2 = 35.1, p < 0.001; figure 3b). This sex difference in incisor width appears to stem directly from the sex difference in skull width, as incisor width increased linearly with skull width (χ2 = 2400.0, p < 0.001), and there was no sex difference in this relationship (sex effect: χ2 = 0.96, p = 0.33; sex × skull width interaction: χ2 = 0.08, p = 0.78). Males were also significantly heavier than females (χ2 = 23.77, p < 0.001) even after controlling for variation in body length (χ2 = 4888.5, p < 0.001). That this sex difference in body mass for a given body length is attributable principally to the sex difference in skull size is suggested by the fact that controlling for the positive effects on body mass of both skull width (χ2 = 140.76, p < 0.001) and body length (now χ2 = 319.45, p < 0.001), eliminates the sex difference in body mass (now χ2 = 0.30, p = 0.58). None of these sex differences in morphology can be attributed simply to the previously documented reductions in skull width growth rates that female Damaraland mole-rats experience on dominance acquisition [58], as all of the significant effects above remain so (all p < 0.001) after the exclusion of all morphological data from dominant females.
Figure 3.
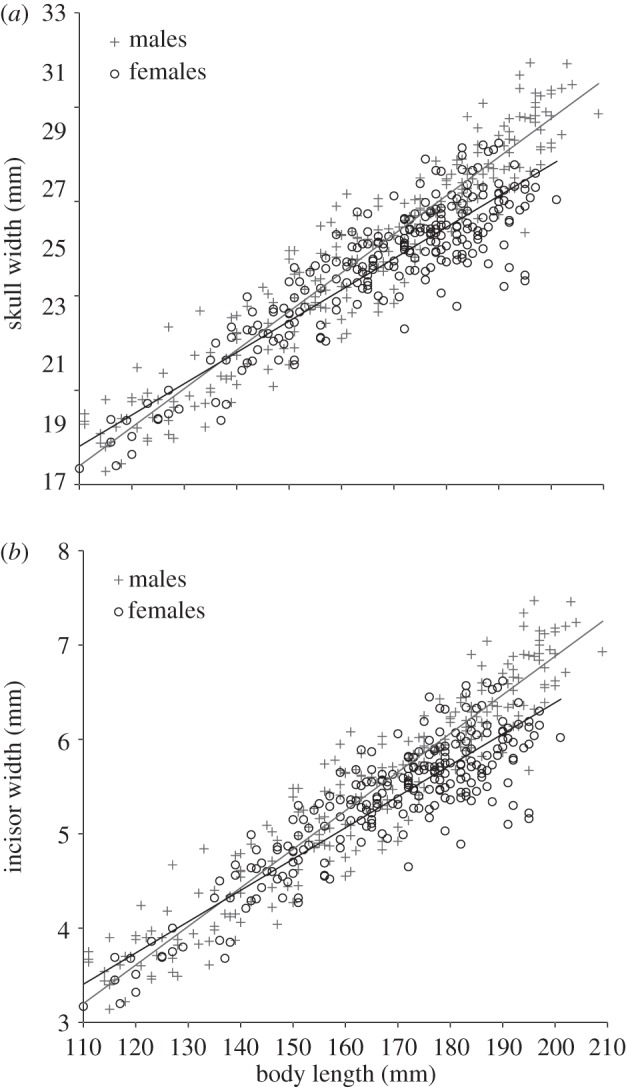
(a) Males had significantly wider skulls for their body lengths than females, and the extent of this sexual dimorphism increased with increasing body length (see text for statistics). (b) Males also had significantly wider incisors for their body lengths than females, and again the extent of this sexual dimorphism increased with increasing body length (see text for statistics). Both analyses are based on a sample of n = 416 sets of morphological measurements from 299 different mole-rats (154 males and 145 females) from 63 different wild colonies.
4. Discussion
Genetic evidence reveals that dominant female Damaraland mole-rats completely monopolize reproduction within their colonies while dominant males do not [45], and our findings suggest that the dominance tenures of females are also longer than those of males (so fewer females may become dominant during their lifetimes). Unusually among mammals, therefore, and in keeping with the patterns outlined by Hauber & Lacey [1] and reported by Clutton-Brock et al. [2], the variance in lifetime reproductive success among female Damaraland mole-rats is likely to be higher than that among males. All other things being equal, intra-sexual selection might therefore be expected to have led to the differential exaggeration among females of the traits that yield success in competition [1,2]. Contrary to expectation, however, male Damaraland mole-rats grow faster, are substantially larger and heavier in adulthood and sport proportionally larger skulls and incisors for their body lengths than females, suggesting that selection has nevertheless differentially exaggerated these traits among males. This discord between the apparent direction of the sex-bias in variance in reproductive success and the nature of sexual dimorphism highlights the paradox outlined in the introduction, and begs the question: why are females in such species not bigger and better armed? Indeed, of the 14 ‘singular breeding’ cooperatively breeding mammal and bird species used in Hauber & Lacey's [1] study in which such species were estimated to show female-biased relative variance in reproductive success, none show female-biased sexual size dimorphism: 11 show clear or slight male-biased size dimorphisms ([2,38–40,60–67]; see also the cooperative African starlings [3]), and the remaining three show no clear sexual size dimorphism [68–73]. Even the naked mole-rat, Heterocephalus glaber, a cooperative breeder that may show the highest variance in female reproductive success of any known mammal [46,70] and in which males share reproduction more equitably and have shorter reproductive tenures than females [70], shows no clear sexual size dimorphism among subordinates [70–73]. The only clear sex difference in growth in this species emerges after dominance acquisition, when dominant females undergo vertebral elongation: an adaptation that conveys fecundity advantages [71,74,75] but could also facilitate the control of other group members [72,76]. We first consider specific explanations for our findings from wild Damaraland mole-rat societies, before outlining three potentially general mechanisms that might account for a more widespread disparity between the patterns of variance in reproductive success and the outcomes of intra-sexual selection in cooperative mammals and birds.
One possible explanation for the disparity is that the seemingly widespread importance of body size and/or weaponry advantages for success in intra-sexual contests in mammals and birds [5,10,32,33,42,77,78] could for some reason either be reversed in Damaraland mole-rats (i.e. intra-sexual selection favours smaller size) or be differentially important in competition among males because the sexes fight in different ways. Both seem unlikely, however, as laboratory studies reveal that when dominance is contested both sexes do so via prolonged bouts of incisor-fencing, aggressive biting and chasing [49–54], heavier animals of both sexes tend to be dominant to lighter ones ([50,52,55] though mass advantages alone do not guarantee dominance [49]), and dominant males and females are typically the largest and heaviest animals of their sex within their colonies [44,52,55]. Indeed, if smaller size and/or weapons were advantageous in competition, then it would also be difficult to explain why the dominant male is typically dominant over the dominant female and the same is true among subordinates [44,52].
It also seems unlikely that the strongly male-biased size dimorphism documented here simply reflects evolutionary lag from a time in the species’ history when intra-sexual selection acted more strongly among males. If anything, the most recent ancestors of the Damaraland mole-rat may have had weaker male-biased body mass dimorphisms. The Damaraland mole-rat (F. damarensis) is considered the most recently derived species in the African mole-rat family [79,80], yet it and its social congener the giant Zambian mole-rat, Fukomys mechowii, show strongly male-biased size dimorphisms [81], whereas weaker or absent sexual size dimorphisms have been reported in other species of Fukomys (e.g. the Mashona mole-rat, Fukomys darlingi [44]) and the social genus Cryptomys from which Fukomys branched [56,79,80]. Across the cooperative mammals and birds, it also seems unlikely that evolutionary lag from strongly polygynous ancestors can generally account for the extant male-biased dimorphisms seen in the species that have been estimated to show higher reproductive variance among females, as recent studies suggest that cooperative breeding has evolved most readily among monogamous ancestors (in birds: [82] and in mammals: [83]). Indeed, if cooperative breeding is strongly associated with monogamy, then this might provide an alternative explanation for the finding that cooperatively breeding species (which may show higher reproductive variance among females) show weaker male-biased dimorphisms than non-cooperative species [3], as such a finding might already be expected when comparing monogamous and polygynous species [31,78,84].
One plausible explanation for the male-biased sexual dimorphism in Damaraland mole-rats is that it arises instead from a sex difference in the way that natural selection has acted on these traits [85]. For example, while both male and female mole-rats clearly do use their skulls, incisors and associated musculature when fighting, both sexes also use them throughout their lives to dig tunnels [44]. As males transfer between groups more frequently than females (N. C. Bennett & J. Jarvis, unpublished data; [44]), males might conceivably benefit more than females from enhanced tunnelling efficiency if it facilitated this mode of dispersal. It seems unlikely, however, that this alone could account for the extent of the male-biased size dimorphism observed here, as both sexes also excavate tunnels throughout their lives while foraging for tubers [44] and females also disperse long distances to found new groups [48]. Indeed, whether long-distance dispersal in this species occurs mainly above ground or below remains a matter for debate [86].
Another key force influencing sexual dimorphisms is fecundity selection, which may commonly favour the differential exaggeration of body size in females as fecundity is often more size-dependent in females than males [10,77]. While the role of fecundity selection in shaping mammalian sexual dimorphisms remains poorly understood [10,77], it might be expected to act more strongly in cooperatively breeding species, where the availability of additional post-natal care from helpers may relax a key constraint on fecundity enhancement among females [58,74,87,88]. It is all the more surprising, therefore, that female Damaraland mole-rats (and females in many other cooperative mammals and birds), who are both aided by helpers and appear to show higher variance in reproductive success than males, nevertheless remain smaller than males. Indeed, even the naked mole-rat, in which females show both high reproductive variance and high fecundity [46,70,74], shows no clear sexual size dimorphism among subordinates [70–73]; fecundity selection in this species appears to have resulted instead in morphological changes after dominance acquisition to enhance the abdominal capacity of females [71,74,75]. In species where females face a trade-off between reproduction and growth, fecundity selection has also been suggested to favour investment in reproduction at the expense of the growth of competitive traits [10]. While such a trade-off could explain the reduced growth rates of newly dominant Damaraland mole-rat females [58], it could not readily account for the emergence of clear male-biased size dimorphisms among subordinates of this species, as subordinate females are unable to reproduce until they have already won dominance [45].
(a). General explanations for male-biased size dimorphisms alongside female-biased reproductive variance in cooperative breeders
While it can be difficult to rule out a role for taxon-specific explanations for sexual dimorphism in any one clade, when taken together with the evidence suggesting that other cooperative mammals and birds may also show higher variance in reproductive success among females than males [1–3] coupled with male-biased size dimorphisms (see above), our findings suggest a need to identify more general mechanisms that could account for such a disparity. Given the extreme difficulty of determining variance in lifetime reproductive success in wild populations, it is worth first considering the evidence that females do show higher variance in lifetime reproductive success in some cooperative mammals and birds. In the societies of meerkats [2] and Damaraland mole-rats ([45] and here; and the same could be argued for naked mole-rats [46,70]), dominant females monopolize within-group reproduction to a greater extent than dominant males and appear to have longer dominance tenures than males, and calculations of the standardized variance in lifetime reproductive success in meerkats have confirmed what one might therefore predict that it is higher among females than males [2]. Naturally, potential complications remain with these approaches, born for example of the difficulty of teasing apart death and dispersal when calculating tenures or lifetimes, and of integrating extra-group paternity (EGP) in to the estimates of reproductive variance. In meerkat societies, for example, EGP can be expected to relax reproductive variance among males [2], in part because it is accrued by subordinates [89]. Whereas in Damaraland mole-rat societies, until extra-group sires are identified, it is conceivable that EGP [45] could elevate reproductive variance among males if it was accrued solely by dominants and in a highly skewed manner. Arguments that female superb starlings, Lamprotornis superbus, a plural cooperative breeder, also show higher reproductive variance than males [3,90], appear to draw on estimates of annual reproductive variance, which could presumably deviate from the patterns of lifetime reproductive variance if, for example, females had shorter reproductive tenures than males. Finally, Hauber & Lacey's [1] study, in which estimates were made of the sex-bias in reproductive variance for 14 cooperatively breeding mammal and bird species, used indirect methods that could be subject to a range of sources of error (as the authors acknowledge), though it is not clear that such error would consistently have yielded the female-biased estimates observed. As such, while robust calculations have rarely been conducted, there is certainly evidence to suggest that the variance in reproductive success is higher among females than males in a number of cooperative breeders, and as reproductive skew is frequently higher among females than males in such species [1–3,24,91–93] it seems likely that additional evidence will become available with time.
Where females do show higher reproductive variance than males, there are several reasons why this might nevertheless tend to be associated with male-biased size dimorphisms. One possibility is that female-biased size dimorphisms may simply be difficult to evolve owing to some form of constraint, but this seems unlikely as a general explanation as they clearly have evolved multiple times among non-cooperative mammals and birds [17,19,33,41–43]. More plausibly, as variance in reproductive success can of course arise through processes other than escalated intra-sexual physical contests [18,34–36,94], if such mechanisms play a differential role in generating the higher reproductive variance among females, then this could readily explain the absence of female-biased size dimorphisms. We consider two such mechanisms below (reproductive restraint and power asymmetries), but a third, in some cooperative breeders at least, could be inter-sexual mate choice [24,92]. Mate choice may generate reproductive variance in the chosen sex that selects instead for the exaggeration of attractive traits, which often differ from those that yield success in competition [4,18,94]. However, as female mammals and birds are generally expected to be the choosier sex, where mate choice does play a role it might be expected to contribute differentially to reproductive variance among males [24,92,95], leaving any association between female-biased reproductive variance and male-biased size dimorphisms if anything more perplexing.
We suggest instead three general mechanisms that could indeed leave the patterns of variance in lifetime reproductive success in cooperative breeders yielding consistent overestimates of the relative intensity of intra-sexual selection among females. (i) Fundamental sex differences in reproductive biology may leave females differentially predisposed to exercising restraint from contesting the dominant female's monopoly, thereby reducing the extent to which female reproductive variance arises from overt physical contests. (ii) In the cooperative mammals that show strong female philopatry (where females rarely transfer between existing groups), an established dominant female's only future competitors may be her daughters, generating a power asymmetry that may again facilitate reproductive monopolization and dominance retention among females without recourse to escalated physical contests. (iii) Finally, sex differences in mean relatedness among reproductive competitors could leave the patterns of variance in inclusive fitness arising from intra-sexual competition (a stronger proxy for the strength of intra-sexual selection in kin-structured populations) differing markedly from the patterns of variance in reproductive success. All three explanations could plausibly act in concert to explain the male-biased size dimorphisms shown here in Damaraland mole-rat societies, but we outline each, in turn, below in the context of its wider potential relevance to cooperative mammals and birds. Our goal is not to provide an exhaustive review of all conceivable explanations for the observed disparity, but to highlight those hypotheses that seem most plausible, with a view to stimulating the theoretical and empirical attention required to understand their effects.
(i). Reproductive conflict among females may be more readily resolved through peaceful restraint
In many cooperative breeders, much of the variance in reproductive success in both sexes may arise through mechanisms that do not entail escalated physical contests, and might thereby contribute little to the intensity of selection for competitive trait exaggeration [18,34–36,94]. One potentially general explanation for the occurrence of male-biased size dimorphisms alongside female-biased reproductive variance, therefore, is that subordinate females may be relatively predisposed to exercising peaceful reproductive restraint from challenging their dominants (whether it be restraint from breeding alongside their dominant [91,96–100], challenging her tenure [68,101] or fighting with their elders for dominance vacancies, as seen in age-based queues to inherit [2,68,102–104]). Where this is the case, more of the reproductive variance among females could arise without the need for escalated physical contests. As subordinates in many cooperative breeders have delayed dispersal from their natal group, a major driver of reproductive restraint is a lack of access to unrelated mates [49,105,106]. While such ‘inbreeding avoidance’ will frequently contribute to the reproductive variance in both sexes [49,105,106], it is not clear that it should consistently do so to a greater extent among females than males across cooperative breeders, regardless of patterns of philopatry. While the higher costs of female reproduction may more strongly favour inbreeding avoidance among females, inbreeding avoidance by one sex imposes it upon the other. We suggest instead that fundamental differences in the reproductive biology of the sexes may leave subordinate females predisposed to exercising restraint for reasons other than inbreeding avoidance per se.
Subordinates may exercise reproductive restraint whenever the costs of reproduction are no longer exceeded by the expected fitness benefits [91,96–100,107]. Reproductive restraint may therefore be more readily induced in subordinate females than males for several reasons [100]. First, simply because the costs of reproduction are typically higher among females than males [91]. Second, subordinate females may be more reluctant than subordinate males to breed alongside their dominant, because the offspring of subordinate females may suffer a cost from being reared alongside the dominant female's brood or litter (if it was already of optimal size) [91,108], whereas the offspring of subordinate males may suffer no such cost (as they may be part of the dominant female's brood or litter). Third, dominants may be better able to devalue a subordinate female's expected benefit from attempting to breed because: (i) female reproduction may be more readily detected (as it may entail conspicuous oestrus, pregnancy, nest creation and the production of additional eggs and young); (ii) female reproduction may be more readily disrupted, as it may involve prolonged oestrus, egg maturation or pregnancy phases that dominants might target [27,28] and females may be better able than males to identify and hence kill the young of their competitors [26,29,109,110]; and finally (iii) the opportunity for subordinate males to sire extra-group young [89,111] may frequently leave it impossible for dominant males to disrupt their reproduction sufficiently to induce complete restraint.
The patterns of reproduction in cooperative mammals and birds suggest that reproductive restraint may indeed have evolved more frequently among females than males. First, reproductive skew is more commonly complete among females, with subordinate females often never attempting to breed despite a lack of active interference by dominants [24,44,91,112–114]. Second, the low reproductive rates of subordinate females are more often underpinned by reproductive physiological downregulation (‘physiological suppression’) than are those of subordinate males, typically in the absence of any clear stress imposed by their dominants [91,112]. Indeed, Damaraland mole-rats exemplify such sex differences in physiological suppression: subordinate females appear to remain in a pre-pubertal anovulatory condition without elevated stress hormone levels and never attempt to breed until dominance acquisition, whereas subordinate males typically have mature testes, produce sperm and may compete for both within- and extra-group paternity [44,45,54,115]. Where subordinate females do exercise greater reproductive restraint than males, dominant females might thereby maintain their monopolies with comparatively little need for the escalated physical contests that favour competitive trait exaggeration.
(ii). Power asymmetries may also facilitate reproductive monopolization among females
In some cooperative mammals, including meerkats and Damaraland mole-rats, there is a clear dichotomy in the dispersal tactics of the sexes: females rarely if ever transfer between established groups, whereas males typically secure dominance by doing so. This creates a scenario in which a dominant female's future competitors are typically born and develop within her group, generating a power asymmetry between the dominant and her developing competitors (often her daughters) that may greatly facilitate the maintenance of her reproductive monopoly [2]. By contrast, dominant males may frequently face fully developed challengers from other groups, over whose development they have had no control. Such a scenario may, again, leave much of the variance in reproductive success among females maintained without the escalated physical contests that underpin selection for competitive traits.
Such a power asymmetry may facilitate the maintenance of the dominant female's reproductive monopoly in several ways. Inherent size and experience advantages born of her superior age may facilitate both the suppression of subordinate reproduction (e.g. via harassment [27,28,30], infanticide [26,109] or credible threats of punishment [99,100]) and their permanent eviction before they become a serious threat [28,30,116,117]. Such advantages may also allow the dominant to suppress the growth of her developing subordinates and thereby stave off future challenges (e.g. via reduced pre-natal investment [118], regular periods of harassment [28,30] or a threat of eviction [119,120]; see [2] for similar arguments). This might conceivably counter the emergence of female-biased dimorphisms not only by relaxing selection for competitive trait exaggeration among dominants, but by constraining the growth of females as subordinates. Recent work has revealed that dominance interactions can indeed modulate the growth trajectories of subordinate vertebrates [119,120] and some of the clearest evidence of socially modulated growth among subordinate mammals stems from naked and Damaraland mole-rats [57,121]. Attempts to understand the causes of sexual dimorphism in cooperative societies might therefore be well served by investigating the role that intra-sexual interactions play in regulating growth.
In the many cooperative breeders that show female-biased dispersal, however, one might expect such power asymmetries to differentially facilitate the contest-free maintenance of monopolies among males, if male transfer was rare. While dispersal in avian societies, in particular, is often female-biased, the sex difference in dispersal may rarely be as stark as that in mammalian societies [122,123]. Unlike many dominant female mammals (see above), dominant males in cooperative birds with female-biased dispersal frequently must still defend both their dominant position and paternity against extra-group challengers [92,124,125]. Moreover, while power asymmetries may still facilitate the reproductive suppression or eviction of a dominant male's sons, suppressing their growth could entail severe costs if this left sons less likely to (i) win dominance in other groups, (ii) secure the local dominance position against extra-group contenders on the death of their father or (iii) sire extra-group young.
(iii). Kinship among competitors may differentially relax variance in inclusive fitness among females
Our final general explanation for the apparent discord in some cooperative breeders between female-biased reproductive variance and male-biased size dimorphism, is that selection acts upon variation in (inclusive) fitness, not variation in reproductive success [18,94,126], and the latter may be a poor proxy for the former in the kin-structured societies of cooperative breeders ([1,127,128] for similar arguments). While variance in reproductive success may frequently be higher among females than males ([1,2] and here), where females are on average more closely related to their opponents than males, the variance in inclusive fitness arising from intra-sexual competition may nevertheless remain lower among females. Theoretical models confirm that such sex differences in relatedness among interactants can indeed generate sex differences in selection for helping and harming [129], but their implications for the outcomes of sexual selection per se remain largely unexplored.
At least two processes could leave the mean relatedness among competing females higher than that among competing males. First, males frequently contest extra-group parentage (with non-relatives) [89,92,95,130,131], whereas females rarely do (though see [93]). Second, the strong female philopatry seen in many cooperative mammals [122,123,132] can be expected to exacerbate this sex difference, leaving females frequently contesting within-group reproduction near-exclusively with close relatives [28,45,133,134], whereas males disperse and contest dominance with unrelated males in other groups ([45,134,135]; though coalitional dispersal can also leave relatives competing post-dispersal). Higher average relatedness among competing females than males may therefore provide another plausible explanation (potentially acting in concert with those outlined above) for the absence of female-biased size dimorphisms in Damaraland mole-rats, meerkats and other female-philopatric species.
Among cooperatively breeding birds, the sex difference in dispersal is typically weaker than that in mammals but tends instead to be female-biased ([122,123] but see e.g. [136,137–140]) which could thereby leave males contesting dominance and within-group reproduction with relatives to a greater extent than females [93,125,141]. Even in avian societies with female-biased dispersal, however, males may nevertheless contest both extra-group paternity and dominance vacancies with unrelated males in other groups [92,124,125] (unlike females in the strongly female-philopatric mammals), potentially constraining the extent to which the mean kinship among competing males exceeds that among females. As such, it is less clear how kinship should be expected to impact the outcomes of intra-sexual selection in such societies. Nevertheless, where dispersal is strongly female-biased and extra-group paternity is rare one might indeed expect mean relatedness to be higher among competing males than females, and for this to differentially relax intra-sexual selection among males. Evidence that such a species still showed male-biased size dimorphism despite female-biased reproductive variance (as would appear to be the case in chestnut-crowned babblers, Pomatostomus ruficeps, for example; A. F. Russell 2013, personal communication) would then suggest that alternative explanations (such as those outlined above) are required to account for the male-biased dimorphisms in such species. As sex differences in relatedness to competitors are likely to be pervasive across non-cooperative organisms too (whether arising from sex differences in the spatial scale of reproductive competition or patterns of dispersal or both), we stress the wider importance of formally investigating how interactions with kin selection are likely to impact the outcomes of both intra- and inter-sexual selection [127,128].
5. Conclusion
Recent evidence suggests that cooperative mammals and birds may frequently show higher variance in reproductive success among females than males ([1–3] and here) and that this may have strengthened selection for competitive traits among females [2,3]. However, there remains little evidence to date that higher reproductive variance among females in cooperative breeders has led to the differential exaggeration of competitive morphology among females relative to males (i.e. reversed sexual dimorphisms). Instead, the dimorphisms in such species typically remain male-biased (see [2,3], our findings and above). These patterns suggest that the true relative intensity of selection for competitive trait exaggeration among females in such species may consistently be weaker than that suggested by the patterns of variance in reproductive success. We suggest that this disparity could be due to variance in reproductive success arising to a greater extent among females than males through mechanisms other than escalated intra-sexual physical contests. Key among these may be a differential propensity for females to exercise restraint from challenging their dominant, as well as power asymmetries among females in strongly female-philopatric species. Indeed, these mechanisms may explain why reproductive variance tends to be higher among females than males in singular cooperative breeders in the first place: because a reduced propensity for subordinate females to credibly challenge their dominant may frequently render reproductive skew higher and dominance tenures longer among females than males. We also suggest that sex differences in relatedness between competitors may frequently leave the patterns of variance in inclusive fitness arising from intra-sexual competition differing from the patterns of variance in reproductive success. These mechanisms could conceivably have acted in concert or isolation to yield the male-biased sexual size dimorphisms observed both in Damaraland mole-rat societies and the other cooperatively breeding mammals and birds that appear to show female-biased reproductive variance. Our findings highlight the importance of broadening our historical focus on intra-sexual competition for mates per se to encompass competition over the rank and resources necessary for reproduction (echoing [1–14,20–23]) and illustrate the challenges that may arise when doing so. We stress the need for more formal theoretical and empirical investigations into the patterns of selection on competitive traits in social species, and specifically the roles that kin selection and conflict resolution may play in modifying their intensity.
Acknowledgements
We are grateful to all those who assisted us with our fieldwork, in particular to Jennifer Jarvis for permission to use the Dordabis dataset whose collection she oversaw, to the Oppenheimer family and Mr and Mrs P. Luhl for kindly allowing us to work on their properties, to EO. & Son, all at Tswalu Kalahari Reserve and Mike and Erin Griffin for their exceptional logistical support, to the Departments of Nature Conservation in Namibia and the Northern Cape, South Africa for permission to conduct our field research, and to Sarah Hodge, Jenny Jarvis, Andy Russell and three anonymous referees for providing insightful comments on the manuscript, and Angus Buckling, Mike Cant, Tim Clutton-Brock, Sasha Dall, Dave Hosken, John Hunt, Rufus Johnstone, Olof Leimar, John McNamara, Phil Stephens and Tom Tregenza for enlightening discussions.
All protocols were approved by the University of Pretoria ethics committee.
Funding statement
This work was made possible by research grants from the National Research Foundation, the University of Pretoria (N.C.B.) and the Association for the Study of Animal Behaviour (A.Y.) and a BBSRC David Phillips research fellowship and NERC and Magdalene College, Cambridge research fellowships (A.Y.).
References
- 1.Hauber ME, Lacey EA. 2005. Bateman's principle in cooperatively breeding vertebrates: the effects of non-breeding alloparents on variability in female and male reproductive success. Integr. Comp. Biol. 45, 903–914 (doi:10.1093/icb/45.5.903) [DOI] [PubMed] [Google Scholar]
- 2.Clutton-Brock TH, Hodge SJ, Spong G, Russell AF, Jordan NR, Bennett NC, Sharpe LL, Manser MB. 2006. Intrasexual competition and sexual selection in cooperative mammals. Nature 444, 1065–1068 (doi:10.1038/nature05386) [DOI] [PubMed] [Google Scholar]
- 3.Rubenstein DR, Lovette IJ. 2009. Reproductive skew and selection on female ornamentation in social species. Nature 462, 786 (doi:10.1038/nature08614) [DOI] [PubMed] [Google Scholar]
- 4.Tobias JA, Montgomerie R, Lyon BE. 2012. The evolution of female ornaments and weaponry: social selection, sexual selection and ecological competition. Phil. Trans. R. Soc. B 367, 2274–2293 (doi:10.1098/rstb.2011.0280) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Robinson MR, Kruuk LEB. 2007. Function of weaponry in females: the use of horns in intrasexual competition for resources in female Soay sheep. Biol. Lett. 3, 651–654 (doi:10.1098/rsbl.2007.0278) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Watson NL, Simmons LW. 2010. Reproductive competition promotes the evolution of female weaponry. Proc. R. Soc. B 277, 2035–2040 (doi:10.1098/rspb.2009.2335) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Heinsohn R, Legge S, Endler JA. 2005. Extreme reversed sexual dichromatism in a bird without sex role reversal. Science 309, 617–619 (doi:10.1126/science.1112774) [DOI] [PubMed] [Google Scholar]
- 8.Lebas NR. 2006. Female finery is not for males. Trends Ecol. Evol. 21, 170–173 (doi:10.1016/j.tree.2006.01.007) [DOI] [PubMed] [Google Scholar]
- 9.Clutton-Brock T. 2009. Sexual selection in females. Anim. Behav. 77, 3–11 (doi:10.1016/j.anbehav.2008.08.026) [Google Scholar]
- 10.Stockley P, Bro-Jorgensen J. 2011. Female competition and its evolutionary consequences in mammals. Biol. Rev. 86, 341–366 (doi:10.1111/j.1469-185X.2010.00149.x) [DOI] [PubMed] [Google Scholar]
- 11.Rubenstein DR. 2012. Family feuds: social competition and sexual conflict in complex societies. Phil. Trans. R. Soc. B 367, 2304–2313 (doi:10.1098/rstb.2011.0283) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Clutton-Brock T, Huchard E. 2013. Social competition and its consequences in female mammals. J. Zool. 289, 151–171 (doi:10.1111/jzo.12023) [Google Scholar]
- 13.Rosvall KA. 2011. Intrasexual competition in females: evidence for sexual selection? Behav. Ecol. 22, 1131–1140 (doi:10.1093/beheco/arr106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aubin-Horth N, Desjardins JK, Martei YM, Balshine S, Hofmann HA. 2007. Masculinized dominant females in a cooperatively breeding species. Mol. Ecol. 16, 1349–1358 (doi:10.1111/j.1365-294X.2007.03249.x) [DOI] [PubMed] [Google Scholar]
- 15.Darwin C. 1871. The descent of man and selection in relation to sex. London, UK: John Murray [Google Scholar]
- 16.Bateman AJ. 1948. Intra-sexual selection in Drosophila. Heredity 2, 349–368 (doi:10.1038/hdy.1948.21) [DOI] [PubMed] [Google Scholar]
- 17.Emlen ST, Oring LW. 1977. Ecology, sexual selection, and the evolution of mating systems. Science 197, 215–223 (doi:10.1126/science.327542) [DOI] [PubMed] [Google Scholar]
- 18.Andersson M. 1994. Sexual selection. Princeton, NJ: Princeton University Press [Google Scholar]
- 19.Holekamp KE, Smale L. 2000. Feisty females and meek males: reproductive strategies in the spotted hyena. In Reproduction in context (eds Wallen K, Schneider J.), pp. 257–285 Cambridge, MA: MIT Press [Google Scholar]
- 20.West-Eberhard MJ. 1979. Sexual selection, social competition and evolution. Proc. Am. Philos. Soc. 123, 222–234 [Google Scholar]
- 21.West-Eberhard MJ. 1983. Sexual selection, social competition and speciation. Q. Rev. Biol. 55, 155–183 (doi:10.1086/413215) [Google Scholar]
- 22.Wasser SK. (ed.) 1983. Social behavior of female vertebrates. New York, NY: Academic Press [Google Scholar]
- 23.Clutton-Brock TH. 2007. Sexual selection in males and females. Science 318, 1882–1885 (doi:10.1126/science.1133311) [DOI] [PubMed] [Google Scholar]
- 24.Magrath RD, Johnstone RA, Heinsohn RG. 2004. Reproductive skew. In Ecology and evolution of cooperative breeding in birds (eds Koenig WD, Dickinson JL.), pp. 157–176 Cambridge, UK: Cambridge University Press [Google Scholar]
- 25.Koenig WD, Shen SF, Krakauer AH, Haydock J. 2009. Reproductive skew in avian societies. In Reproductive skew in vertebrate societies: proximate and ultimate causes (eds Hager R, Jones CB.), pp. 227–264 Cambridge, UK: Cambridge University Press [Google Scholar]
- 26.Mumme RL, Koenig WD, Pitelka FA. 1983. Reproductive competition in the communal acorn woodpecker—sisters destroy each others eggs. Nature 306, 583–584 (doi:10.1038/306583a0) [Google Scholar]
- 27.Hacklander K, Mostl E, Arnold W. 2003. Reproductive suppression in female alpine marmots, Marmota marmota. Anim. Behav. 65, 1133–1140 (doi:10.1006/anbe.2003.2159) [Google Scholar]
- 28.Young AJ, Carlson AA, Monfort SL, Russell AF, Bennett NC, Clutton-Brock T. 2006. Stress and the suppression of subordinate reproduction in cooperatively breeding meerkats. Proc. Natl Acad. Sci. USA 103, 12 005–12 010 (doi:10.1073/pnas.0510038103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Young AJ, Clutton-Brock TH. 2006. Infanticide by subordinates influences reproductive sharing in cooperatively breeding meerkats. Biol. Lett. 2, 385–387 (doi:10.1098/rsbl.2006.0463) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cant MA, Hodge SJ, Bell MBV, Gilchrist JS, Nichols HJ. 2010. Reproductive control via eviction (but not the threat of eviction) in banded mongooses. Proc. R. Soc. B 277, 2219–2226 (doi:10.1098/rspb.2009.2097) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Clutton-Brock TH, Harvey PH, Rudder B. 1977. Sexual dimorphism, socionomic sex ratio and body weight in primates. Nature 269, 797–800 (doi:10.1038/269797a0) [DOI] [PubMed] [Google Scholar]
- 32.Bro-Jorgensen J. 2007. The intensity of sexual selection predicts weapon size in male bovids. Evolution 61, 1316–1326 (doi:10.1111/j.1558-5646.2007.00111.x) [DOI] [PubMed] [Google Scholar]
- 33.Fairbairn DJ, Blanckenhorn WU, Szekely T. (eds) 2007. Sex, size and gender roles. Oxford, UK: Oxford University Press [Google Scholar]
- 34.Klug H, Heuschele J, Jennions MD, Kokko H. 2010. The mismeasurement of sexual selection. J. Evol. Biol. 23, 447–462 (doi:10.1111/j.1420-9101.2009.01921.x) [DOI] [PubMed] [Google Scholar]
- 35.Krakauer AH, Webster MS, Duval EH, Jones AG, Shuster SM. 2011. The opportunity for sexual selection: not mismeasured, just misunderstood. J. Evol. Biol. 24, 2064–2071 (doi:10.1111/j.1420-9101.2011.02317.x) [DOI] [PubMed] [Google Scholar]
- 36.Jennions MD, Kokko H, Klug H. 2012. The opportunity to be misled in studies of sexual selection. J. Evol. Biol. 25, 591–598 (doi:10.1111/j.1420-9101.2011.02451.x) [DOI] [PubMed] [Google Scholar]
- 37.Clutton-Brock TH, et al. 2001. Cooperation, control, and concession in meerkat groups. Science 291, 478–481 (doi:10.1126/science.291.5503.478) [DOI] [PubMed] [Google Scholar]
- 38.Clutton-Brock TH, Gaynor D, McIlrath GM, Maccoll ADC, Kansky R, Chadwick P, Manser M, Skinner JD, Brotherton PNM. 1999. Predation, group size and mortality in a cooperative mongoose, Suricata suricatta. J. Anim. Ecol. 68, 672–683 (doi:10.1046/j.1365-2656.1999.00317.x) [Google Scholar]
- 39.Clutton-Brock TH, Maccoll A, Chadwick P, Gaynor D, Kansky R, Skinner JD. 1999. Reproduction and survival of suricates (Suricata suricatta) in the southern Kalahari. Afr. J. Ecol. 37, 69–80 (doi:10.1046/j.1365-2028.1999.00160.x) [Google Scholar]
- 40.Gittleman JL, Van Valkenburgh B. 1997. Sexual dimorphism in the canines and skulls of carnivores: effects of size, phylogeny, and behavioural ecology. J. Zool. 242, 97–117 (doi:10.1111/j.1469-7998.1997.tb02932.x) [Google Scholar]
- 41.Cuervo JJ, Moller AP. 2009. The allometric pattern of sexually size dimorphic feather ornaments and factors affecting allometry. J. Evol. Biol. 22, 1503–1515 (doi:10.1111/j.1420-9101.2009.01758.x) [DOI] [PubMed] [Google Scholar]
- 42.Szekely T, Lislevand T, Figuerola J. 2007. Sexual size dimorphism in birds. In Sex, size and gender roles; evolutionary studies in sexual size dimorphism (eds Fairbairn DJ, Blanckenhorn WU, Szekely T.), pp. 27–37 Oxford, UK: Oxford University Press [Google Scholar]
- 43.Erckmann WJ. 1983. The evolution of polyandry in shorebirds: an evaluation of hypotheses. In Social behavior of female vertebrates (ed. Wasser SK.), pp. 113–168 New York, NY: Academic Press [Google Scholar]
- 44.Bennett NC, Faulkes CG. 2000. African mole-rats: ecology and eusociality. Cambridge, UK: Cambridge University Press [Google Scholar]
- 45.Burland TM, Bennett NC, Jarvis JUM, Faulkes CG. 2004. Colony structure and parentage in wild colonies of co-operatively breeding Damaraland mole-rats suggest incest avoidance alone may not maintain reproductive skew. Mol. Ecol. 13, 2371–2379 (doi:10.1111/j.1365-294X.2004.02233.x) [DOI] [PubMed] [Google Scholar]
- 46.Jarvis JUM, Oriain MJ, Bennett NC, Sherman PW. 1994. Mammalian eusociality: a family affair. Trends Ecol. Evol. 9, 47–51 (doi:10.1016/0169-5347(94)90267-4) [DOI] [PubMed] [Google Scholar]
- 47.Jarvis JUM, Bennett NC. 1993. Eusociality has evolved independently in 2 genera of Bathyergid mole-rats: but occurs in no other subterranean mammal. Behav. Ecol. Sociobiol. 33, 253–260 (doi:10.1007/BF02027122) [Google Scholar]
- 48.Young AJ, Oosthuizen MK, Lutermann H, Bennett NC. 2010. Physiological suppression eases in Damaraland mole-rat societies when ecological constraints on dispersal are relaxed. Horm. Behav. 57, 177–183 (doi:10.1016/j.yhbeh.2009.10.011) [DOI] [PubMed] [Google Scholar]
- 49.Cooney R, Bennett NC. 2000. Inbreeding avoidance and reproductive skew in a cooperative mammal. Proc. R. Soc. Lond. B 267, 801–806 (doi:10.1098/rspb.2000.1074) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rickard CA, Bennett NC. 1997. Recrudescence of sexual activity in a reproductively quiescent colony of the Damaraland mole-rat (Cryptomys damarensis), by the introduction of an unfamiliar and genetically unrelated male: a case of incest avoidance in ‘queenless’ colonies. J. Zool. 241, 185–202 (doi:10.1111/j.1469-7998.1997.tb05508.x) [Google Scholar]
- 51.Cooney R. 2002. Colony defense in Damaraland mole-rats, Cryptomys damarensis. Behav. Ecol. 13, 160–162 (doi:10.1093/beheco/13.2.160) [Google Scholar]
- 52.Jacobs DS, Bennett NC, Jarvis JUM, Crowe TM. 1991. The colony structure and dominance hierarchy of the Damaraland mole-rat, Cryptomys damarensis (Rodentia, Bathyergidae), from Namibia. J. Zool. 224, 553–576 (doi:10.1111/j.1469-7998.1991.tb03785.x) [Google Scholar]
- 53.Jacobs DS, Reid S, Kuiper S. 1998. Out-breeding behaviour and xenophobia in the Damaraland mole-rat, Cryptomys damarensis. South Afr. J. Zool. 33, 189–194 [Google Scholar]
- 54.Clarke FM, Miethe GH, Bennett NC. 2001. Reproductive suppression in female Damaraland mole-rats Cryptomys damarensis: dominant control or self-restraint? Proc. R. Soc. Lond. B 268, 899–909 (doi:10.1098/rspb.2000.1426) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gaylard A, Harrison Y, Bennett NC. 1998. Temporal changes in the social structure of a captive colony of the Damaraland mole-rat, Cryptomys damarensis: the relationship of sex and age to dominance and burrow-maintenance activity. J. Zool. 244, 313–321 (doi:10.1111/j.1469-7998.1998.tb00035.x) [Google Scholar]
- 56.Bennett NC, Jarvis JUM, Wallace DB. 1990. The relative age structure and body masses of complete wild-captured colonies of 2 social mole-rats, the common mole-rat, Cryptomys hottentotus hottentotus and the Damaraland mole-rat, Cryptomys damarensis. J. Zool. 220, 469–485 (doi:10.1111/j.1469-7998.1990.tb04319.x) [Google Scholar]
- 57.Bennett NC, Navarro R. 1997. Differential growth patterns between successive litters of the eusocial Damaraland mole-rat, Cryptomys damarensis, from Namibia. J. Zool. 241, 465–473 (doi:10.1111/j.1469-7998.1997.tb04838.x) [Google Scholar]
- 58.Young AJ, Bennett NC. 2010. Morphological divergence of breeders and helpers in wild Damaraland mole-rat societies. Evolution 64, 3190–3197 (doi:10.1111/j.1558-5646.2010.01066.x) [DOI] [PubMed] [Google Scholar]
- 59.Bennett NC, Jarvis JUM, Aguilar GH, McDaid EJ. 1991. Growth and development in 6 species of African mole-rats (Rodentia, Bathyergidae). J. Zool. 225, 13–26 (doi:10.1111/j.1469-7998.1991.tb03798.x) [Google Scholar]
- 60.Creel S, Creel NM. 2002. The African wild dog: behavior, ecology, and conservation. Princeton, NJ: Princeton University Press [Google Scholar]
- 61.del Hoyo J, Elliott A, Sargatal J. (eds) 2002. Handbook of the birds of the world. vol. 7. Jacamars to woodpeckers. Barcelona, Spain: Lynx edicions [Google Scholar]
- 62.Pyle P. 1997. Identification guide to North American birds: Part I Columbidae to Ploceidae. Bolinas, CA: Slate Creek Press [Google Scholar]
- 63.Snow DJ, Perrins CM, Gillmor R, Hillcoat B, Roselaar CS, Vincent D, Wallace DIM, Wilson MG. 1998. The birds of the western palearctic. Concise edition. vol. 2 passerines. Oxford, UK: Oxford University Press [Google Scholar]
- 64.del Hoyo J, Elliott A, Christie DA. (eds) 2006. Handbook of the birds of the world. vol. 11. Old world flycatchers to old world warblers. Barcelona, Spain: Lynx Edicions [Google Scholar]
- 65.Schlotfeldt BE, Kleindorfer S. 2006. Adaptive divergence in the superb fairy-wren (Malurus cyaneus): a mainland versus island comparison of morphology and foraging behaviour. Emu 106, 309–319 (doi:10.1071/MU06004) [Google Scholar]
- 66.Schodde R, Mason IJ. 1999. The directory of Australian birds: a taxonomic and zoogeographic atlas of the biodiversity of birds in Australia and its territories. Collingwood, Australia: CSIRO Publishing [Google Scholar]
- 67.Brewer D, Mackay BK. 2001. Wrens, dippers and thrashers. New Haven, CT: Yale University Press [Google Scholar]
- 68.Creel S, Creel N, Wildt DE, Monfort SL. 1992. Behavioral and endocrine mechanisms of reproductive suppression in Serengeti dwarf mongooses. Anim. Behav. 43, 231–245 (doi:10.1016/S0003-3472(05)80219-2) [Google Scholar]
- 69.Araujo A, Arruda MF, Alencar AI, Albuquerque F, Nascimento MC, Yamamoto ME. 2000. Body weight of wild and captive common marmosets (Callithrix jacchus). Int. J. Primatol. 21, 317–324 (doi:10.1023/A:1005433722475) [Google Scholar]
- 70.Jarvis JUM. 1991. Reproduction of naked mole-rats. In The biology of the naked mole-rat (eds Sherman PW, Jarvis JUM, Alexander RD.), pp. 384–425 Princeton, NJ: Princeton University Press [Google Scholar]
- 71.Jarvis JUM, O'Riain MJ, McDaid EJ. 1991. Growth and factors affecting body size in naked mole-rats. In The biology of the naked mole-rat (eds Sherman PW, Jarvis JUM, Alexander RD.), pp. 358–383 Princeton, NJ: Princeton University Press [Google Scholar]
- 72.Lacey EA, Sherman PW. 1991. Social organization of naked mole-rat colonies: evidence of divisions of labor. In The biology of the naked mole-rat (eds Sherman PW, Jarvis JUM, Alexander RD.), pp. 275–336 Princeton, NJ: Princeton University Press [Google Scholar]
- 73.Jarvis JUM, Bennett NC. 1991. Ecology and behavior of the family Bathyerigidae. In The biology of the naked mole-rat (eds Sherman PW, Jarvis JUM, Alexander RD.), pp. 66–96 Princeton, NJ: Princeton University Press [Google Scholar]
- 74.O'Riain MJ, Jarvis JUM, Alexander R, Buffenstein R, Peeters C. 2000. Morphological castes in a vertebrate. Proc. Natl Acad. Sci. USA 97, 13 194–13 197 (doi:10.1073/pnas.97.24.13194) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Dengler-Crish CM, Catania KC. 2007. Phenotypic plasticity in female naked mole-rats after removal from reproductive suppression. J. Exp. Biol. 210, 4351–4358 (doi:10.1242/jeb.009399) [DOI] [PubMed] [Google Scholar]
- 76.Clarke FM, Faulkes CC. 2001. Intracolony aggression in the eusocial naked mole-rat, Heterocephalus glaber. Anim. Behav. 61, 311–324 (doi:10.1006/anbe.2000.1573) [Google Scholar]
- 77.Isaac JL. 2005. Potential causes and life-history consequences of sexual size dimorphism in mammals. Mammal Rev. 35, 101–115 (doi:10.1111/j.1365-2907.2005.00045.x) [Google Scholar]
- 78.Dunn PO, Whittingham LA, Pitcher TE. 2001. Mating systems, sperm competition, and the evolution of sexual dimorphism in birds. Evolution 55, 161–175 [DOI] [PubMed] [Google Scholar]
- 79.Kock D, Ingram CM, Frabotta LJ, Honeycutt RL, Burda H. 2006. On the nomenclature of Bathyergidae and Fukomys n. Gen. (Mammalia : Rodentia). Zootaxa 1142, 51–55 [Google Scholar]
- 80.Van Daele PAAG, Faulkes CG, Verheyen E, Adriaens D. 2007. African mole-rats (Bathyergidae): a complex radiation in tropical soils. In Subterranean rodents: news from the underground (eds Begall S, Burda H, Schleich CE.). Berlin, Germany: Springer [Google Scholar]
- 81.Scharff A, Locker-Grutjen O, Kawalika M, Burda H. 2001. Natural history of the giant mole-rat, Cryptomys mechowi (Rodentia : Bathyergidae), from Zambia. J. Mammal 82, 1003–1015 (doi:10.1644/1545-1542(2001)082<1003:NHOTGM>2.0.CO;2) [Google Scholar]
- 82.Cornwallis CK, West SA, Davis KE, Griffin AS. 2010. Promiscuity and the evolutionary transition to complex societies. Nature 466, 969 (doi:10.1038/nature09335) [DOI] [PubMed] [Google Scholar]
- 83.Lukas D, Clutton-Brock T. 2012. Cooperative breeding and monogamy in mammalian societies. Proc. R. Soc. B 279, 2151–2156 (doi:10.1098/rspb.2011.2468) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Crook JH. 1964. The evolution of social organisation and visual communication in the weaver birds (Ploceinae). Behavior X(Suppl.), 1–178 [Google Scholar]
- 85.Kruger O. 2005. The evolution of reversed sexual size dimorphism in hawks, falcons and owls: a comparative study. Evol. Ecol. 19, 467–486 (doi:10.1007/s10682-005-0293-9) [Google Scholar]
- 86.Hazell RWA, Bennett NC, Jarvis JUM, Griffin M. 2000. Adult dispersal in the co-operatively breeding Damaraland mole-rat (Cryptomys damarensis): a case study from the Waterberg region of Namibia. J. Zool. 252, 19–25 (doi:10.1111/j.1469-7998.2000.tb00816.x) [Google Scholar]
- 87.Creel SR, Creel NM. 1991. Energetics, reproductive suppression and obligate communal breeding in carnivores. Behav. Ecol. Sociobiol. 28, 263–270 (doi:10.1007/BF00175099) [Google Scholar]
- 88.Russell AF, Carlson AA, McIlrath GM, Jordan NR, Clutton-Brock T. 2004. Adaptive size modification by dominant female meerkats. Evolution 58, 1600–1607 [DOI] [PubMed] [Google Scholar]
- 89.Young AJ, Spong G, Clutton-Brock TH. 2007. Subordinate male meerkats prospect for extra-group paternity: alternative reproductive tactics in a cooperative mammal. Proc. R. Soc. B 274, 1603–1609 (doi:10.1098/rspb.2007.0316) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Rubenstein DR. 2007. Natural history miscellany - temporal but not spatial environmental variation drives adaptive offspring sex allocation in a plural cooperative breeder. Am. Nat. 170, 155–165 (doi:10.1086/518671) [DOI] [PubMed] [Google Scholar]
- 91.Young AJ. 2009. The causes of physiological suppression in vertebrate societies: a synthesis. In Reproductive skew in vertebrate societies: proximate and ultimate causes (eds Hager R, Jones CB.). Cambridge, UK: Cambridge University Press [Google Scholar]
- 92.Cockburn A. 2004. Mating systems and sexual conflict. In Ecology and evolution of cooperative breeding in birds (eds Koenig WD, Dickinson JL.), pp. 81–101 Cambridge, UK: Cambridge University Press [Google Scholar]
- 93.Vehrencamp SL, Quinn JS. 2004. Joint laying systems. In Ecology and evolution of cooperative breeding in birds (eds Koenig WD, Dickinson JL.), pp. 177–196 Cambridge, UK: Cambridge University Press [Google Scholar]
- 94.Shuster SM, Wade MJ. 2003. Mating systems and strategies. Princeton, NJ: Princeton University Press [Google Scholar]
- 95.Cockburn A, Osmond HL, Double MC. 2008. Swingin’ in the rain: condition dependence and sexual selection in a capricious world. Proc. R. Soc. B 275, 605–612 (doi:10.1098/rspb.2007.0916) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Johnstone RA, Cant MA. 1999. Reproductive skew and the threat of eviction: a new perspective. Proc. R. Soc. Lond. B 266, 275–279 (doi:10.1098/rspb.1999.0633) [Google Scholar]
- 97.Young AJ, Monfort SL, Clutton-Brock TH. 2008. The causes of physiological suppression among female meerkats: a role for subordinate restraint due to the threat of infanticide? Horm. Behav. 53, 131–139 (doi:10.1016/j.yhbeh.2007.09.005) [DOI] [PubMed] [Google Scholar]
- 98.Saltzman W, Digby LJ, Abbott DH. 2009. Reproductive skew in female common marmosets: what can proximate mechanisms tell us about ultimate causes? Proc. R. Soc. B 276, 389–399 (doi:10.1098/rspb.2008.1374) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Cant MA. 2011. The role of threats in animal cooperation. Proc. R. Soc. B 278, 170–178 (doi:10.1098/rspb.2010.1241) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Cant MA, Young AJ. 2013. Resolving social conflict among females without overt aggression. Phil. Trans. R. Soc. B 368, 20130076 (doi:10.1098/rstb.2013.0076) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Sharp SP, Clutton-Brock TH. 2011. Reluctant challengers: why do subordinate female meerkats rarely displace their dominant mothers? Behav. Ecol. 22, 1337–1343 (doi:10.1093/beheco/arr138) [Google Scholar]
- 102.Cant MA, English S. 2006. Stable group size in cooperative breeders: the role of inheritance and reproductive skew. Behav. Ecol. 17, 560–568 (doi:10.1093/beheco/arj065) [Google Scholar]
- 103.Cronin AL, Field J. 2007. Social aggression in an age-dependent dominance hierarchy. Behaviour 144, 753–765 (doi:10.1163/156853907781476436) [Google Scholar]
- 104.Alberts SC, Watts HE, Altmann J. 2003. Queuing and queue-jumping: long-term patterns of reproductive skew in male savannah baboons, Papio cynocephalus. Anim. Behav. 65, 821–840 (doi:10.1006/anbe.2003.2106) [Google Scholar]
- 105.Koenig WD, Haydock J. 2004. Incest and incest avoidance. In Ecology and evolution of cooperative breeding in birds (eds Koenig WD, Dickinson JL.), pp. 142–156 Cambridge, UK: Cambridge University Press [Google Scholar]
- 106.Greeff JM, Bennett NC. 2000. Causes and consequences of incest avoidance in the cooperatively breeding mole-rat, Cryptomys darlingi (Bathyergidae). Ecol. Lett. 3, 318–328 (doi:10.1046/j.1461-0248.2000.00162.x) [Google Scholar]
- 107.Cant MA, Johnstone RA. 2009. How threats influence the evolutionary resolution of within-group conflict. Am. Nat. 173, 759–771 (doi:10.1086/598489) [DOI] [PubMed] [Google Scholar]
- 108.Chao L. 1997. Evolution of polyandry in a communal breeding system. Behav. Ecol. 8, 668–674 (doi:10.1093/beheco/8.6.668) [Google Scholar]
- 109.Ebensperger LA. 1998. Strategies and counterstrategies to infanticide in mammals. Biol. Rev. Camb. Philos. Soc. 73, 321–346 (doi:10.1017/S0006323198005209) [Google Scholar]
- 110.Hodge SJ, Bell MBV, Cant MA. 2011. Reproductive competition and the evolution of extreme birth synchrony in a cooperative mammal. Biol. Lett. 7, 54–56 (doi:10.1098/rsbl.2010.0555) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Double MC, Cockburn A. 2003. Subordinate superb fairy-wrens (Malurus cyaneus) parasitize the reproductive success of attractive dominant males. Proc. R. Soc. Lond. B 270, 379–384 (doi:10.1098/rspb.2002.2261) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Creel S. 2001. Social dominance and stress hormones. Trends Ecol. Evol. 16, 491–497 (doi:10.1016/S0169-5347(01)02227-3) [Google Scholar]
- 113.Schoech SJ, Reynolds SJ, Boughton RK. 2004. Endocrinology. In Ecology and evolution of cooperative breeding in birds (eds Koenig WD, Dickinson JL.), pp. 128–141 Cambridge, UK: Cambridge University Press [Google Scholar]
- 114.Harrison XA, York JE, Cram DL, Hares M, Young AJ. In press. Complete reproductive skew within white-browed sparrow weaver groups despite outbreeding opportunities for subordinates of both sexes. Behav. Ecol. Sociobiol. (doi:10.1007/s00265-013-1599-1) [Google Scholar]
- 115.Faulkes CG, Trowell SN, Jarvis JUM, Bennett NC. 1994. Investigation of numbers and motility of spermatozoa in reproductively active and socially suppressed males of 2 eusocial African mole-rats, the naked mole-rat (Heterocephalus glaber) and the Damaraland mole-rat (Cryptomys damarensis). J. Reprod. Fertil. 100, 411–416 (doi:10.1530/jrf.0.1000411) [DOI] [PubMed] [Google Scholar]
- 116.Clutton-Brock TH, Brotherton PNM, Smith R, McIlrath GM, Kansky R, Gaynor D, O'Riain MJ, Skinner JD. 1998. Infanticide and expulsion of females in a cooperative mammal. Proc. R. Soc. Lond. B 265, 2291–2295 (doi:10.1098/rspb.1998.0573) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Buston P. 2003. Forcible eviction and prevention of recruitment in the clown anemonefish. Behav. Ecol. 14, 576–582 (doi:10.1093/beheco/arg036) [Google Scholar]
- 118.Russell AF, Lummaa V. 2009. Maternal effects in cooperative breeders: from hymenopterans to humans. Phil. Trans. R. Soc. B 364, 1143–1167 (doi:10.1098/rstb.2008.0298) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Wong MYL, Buston PM, Munday PL, Jones GP. 2007. The threat of punishment enforces peaceful cooperation and stabilizes queues in a coral-reef fish. Proc. R. Soc. B 274, 1093–1099 (doi:10.1098/rspb.2006.0284) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Wong MYL, Munday PL, Buston PM, Jones GP. 2008. Monogamy when there is potential for polygyny: tests of multiple hypotheses in a group-living fish. Behav. Ecol. 19, 353–361 (doi:10.1093/beheco/arm141) [Google Scholar]
- 121.O'Riain MJ, Jarvis JUM. 1998. The dynamics of growth in naked mole-rats: the effects of litter order and changes in social structure. J. Zool. 246, 49–60 (doi:10.1111/j.1469-7998.1998.tb00131.x) [Google Scholar]
- 122.Greenwood PJ. 1980. Mating systems, philopatry and dispersal in birds and mammals. Anim. Behav. 28, 1140–1162 (doi:10.1016/S0003-3472(80)80103-5) [Google Scholar]
- 123.Dobson FS. 2013. The enduring question of sex-biased dispersal: Paul J. Greenwood's (1980) seminal contribution. Anim. Behav. 85, 299–304 (doi:10.1016/j.anbehav.2012.11.014) [Google Scholar]
- 124.Cockburn A, Osmond HL, Mulder RA, Double MC, Green DJ. 2008. Demography of male reproductive queues in cooperatively breeding superb fairy-wrens Malurus cyaneus. J. Anim. Ecol. 77, 297–304 (doi:10.1111/j.1365-2656.2007.01335.x) [DOI] [PubMed] [Google Scholar]
- 125.Koenig WD, Mumme RL, Pitelka F. 1983. Female roles in cooperatively breeding acorn woodpeckers. In Social behavior of female vertebrates (ed. Wasser SK.), pp. 235–261 New York, NY: Academic Press [Google Scholar]
- 126.Hamilton WD. 1964. The genetical evolution of social behaviour. Parts I and II. J. Theor. Biol. 7, 1–52 (doi:10.1016/0022-5193(64)90038-4) [DOI] [PubMed] [Google Scholar]
- 127.Pizzari T, Foster KR. 2008. Sperm sociality: cooperation, altruism, and spite. PLoS Biol. 6, 925–931 (doi:10.1371/journal.pbio.0060130) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Pizzari T, Gardner A. 2012. The sociobiology of sex: inclusive fitness consequences of inter-sexual interactions. Phil. Trans. R. Soc. B 367, 2314–2323 (doi:10.1098/rstb.2011.0281) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Johnstone RA, Cant MA. 2008. Sex differences in dispersal and the evolution of helping and harming. Am. Nat. 172, 318–330 (doi:10.1086/589899) [DOI] [PubMed] [Google Scholar]
- 130.Sillero-Zubiri C, Gottelli D, Macdonald DW. 1996. Male philopatry, extra pack copulations and inbreeding avoidance in Ethiopian wolves (Canis simensis). Behav. Ecol. Sociobiol. 38, 331–340 (doi:10.1007/s002650050249) [Google Scholar]
- 131.Hughes JM, Mather PB, Toon A, Ma J, Rowley I, Russell E. 2003. High levels of extra-group paternity in a population of Australian magpies Gymnorhina tibicen: evidence from microsatellite analysis. Mol. Ecol. 12, 3441–3450 (doi:10.1046/j.1365-294X.2003.01997.x) [DOI] [PubMed] [Google Scholar]
- 132.Clutton-Brock TH, Lukas D. 2012. The evolution of social philopatry and dispersal in female mammals. Mol. Ecol. 21, 472–492 (doi:10.1111/j.1365-294X.2011.05232.x) [DOI] [PubMed] [Google Scholar]
- 133.Clutton-Brock TH, Hodge SJ, Flower TP, Spong GF, Young AJ. 2010. Adaptive suppression of subordinate reproduction in cooperative mammals. Am. Nat. 176, 664–673 (doi:10.1086/656492) [DOI] [PubMed] [Google Scholar]
- 134.Rood JP. 1987. Dispersal and intergroup transfer in the dwarf mongoose. In Mammalian dispersal patterns: the effects of social structure on population genetics (eds Chepko-Sade BD, Halpin Z.), pp. 85–102 Chicago, IL: Chicago University Press [Google Scholar]
- 135.Spong GF, Hodge SJ, Young AJ, Clutton-Brock TH. 2008. Factors affecting the reproductive success of dominant male meerkats. Mol. Ecol. 17, 2287–2299 (doi:10.1111/j.1365-294X.2008.03734.x) [DOI] [PubMed] [Google Scholar]
- 136.Beck NR, Peakall R, Heinsohn R. 2008. Social constraint and an absence of sex-biased dispersal drive fine-scale genetic structure in white-winged choughs. Mol. Ecol. 17, 4346–4358 (doi:10.1111/j.1365-294X.2008.03906.x) [DOI] [PubMed] [Google Scholar]
- 137.Nelson-Flower MJ, Hockey PAR, O'Ryan C, Ridley AR. 2012. Inbreeding avoidance mechanisms: dispersal dynamics in cooperatively breeding southern pied babblers. J. Anim. Ecol. 81, 876–883 (doi:10.1111/j.1365-2656.2012.01983.x) [DOI] [PubMed] [Google Scholar]
- 138.Williams DA, Rabenold KN. 2005. Male-biased dispersal, female philopatry, and routes to fitness in a social corvid. J. Anim. Ecol. 74, 150–159 (doi:10.1111/j.1365-2656.2004.00907.x) [Google Scholar]
- 139.Berg EC, Eadie JM, Langen TA, Russell AF. 2009. Reverse sex-biased philopatry in a cooperative bird: genetic consequences and a social cause. Mol. Ecol. 18, 3486–3499 (doi:10.1111/j.1365-294X.2009.04284.x) [DOI] [PubMed] [Google Scholar]
- 140.Eikenaar C, Brouwer L, Komdeur J, Richardson DS. 2010. Sex biased natal dispersal is not a fixed trait in a stable population of Seychelles warblers. Behaviour 147, 1577–1590 (doi:10.1163/000579510X510511) [Google Scholar]
- 141.Cockburn A, Osmond HL, Mulder RA, Green DJ, Double MC. 2003. Divorce, dispersal and incest avoidance in the cooperatively breeding superb fairy-wren Malurus cyaneus. J. Anim. Ecol. 72, 189–202 (doi:10.1046/j.1365-2656.2003.00694.x) [Google Scholar]


