Abstract
Female chimpanzees exhibit exceptionally slow rates of reproduction and raise their offspring without direct paternal care. Therefore, their reproductive success depends critically on long-term access to high-quality food resources over a long lifespan. Chimpanzee communities contain multiple adult males, multiple adult females and their offspring. Because males are philopatric and jointly defend the community range while most females transfer to new communities before breeding, adult females are typically surrounded by unrelated competitors. Communities are fission–fusion societies in which individuals spend time alone or in fluid subgroups, whose size depends mostly on the abundance and distribution of food. To varying extents in different populations, females avoid direct competition by foraging alone or in small groups in distinct, but overlapping core areas within the community range to which they show high fidelity. Although rates of aggression are low, females compete for space and access to food. High rank correlates with high reproductive success, and high-ranking females win direct contests for food and gain preferential access to resource-rich sites. Females are aggressive to immigrant females and even kill the newborn infants of community members. The intensity of such aggression correlates with population density. These patterns are compared to those in other species, including humans.
Keywords: resource competition, female transfer, dominance, aggression to immigrants, infanticide
1. Introduction
Because female mammals invest much more heavily than males in individual offspring through gestation and lactation, their reproductive success is usually limited by their ability to turn food resources into offspring, while male reproductive success depends more on their access to mates [1]. The difference between the sexes in the resources that limit reproduction is particularly stark in chimpanzees, where the pace of female reproduction is among the slowest of any mammal [2]. Females in the wild do not start reproducing until they are 13–15 years old; they then produce one infant every 5–7 years and die at a maximum age of 50–60 years [2,3]. To date, the maximum observed number of surviving offspring produced by any female is seven (by Fifi at Gombe National Park, Tanzania [4]) and at most sites, average female reproduction is such that populations are at or below replacement levels [2]. To maximize reproductive success females must therefore invest in their own mortality reduction as well as parental care. In chimpanzees, as in many other mammals, reproduction is accelerated by increased access to food. Age at first reproduction and interbirth intervals in captive female chimpanzees are each reduced by 2–3 years, and there is increasing evidence from the field that differences in food availability influence reproductive rates [2]. There is no direct paternal care in chimpanzees; therefore, we expect female behaviour to be shaped critically by their success in accessing high-quality food resources, their maximization of foraging efficiency and their avoidance of mortality risk. By contrast, male reproductive success depends most heavily on their access to fertile females. Breeding is not seasonal in chimpanzees, thus low rates of female reproduction produce highly skewed operational sex ratios of adult males to fertile females which result in intense male–male competition.
As in many species, early studies of competition in chimpanzees emphasized the conspicuous and dramatic competitive behaviour of males [5,6]. On a day-to-day basis, competitive interactions among females are much less obvious. For example, de Waal [6] reported stable social relationships and very low rates of aggression among captive females in a stable group at Arnhem Zoo. In the wild, Goodall [7] found that some pairs of females at Gombe spent many hours together with few social interactions of any sort, and Muller [8] reported only five attacks among adolescent and adult females in 680 h of observation at Kanyawara, Uganda. Nevertheless, studies of females at long-term field sites have gradually revealed that female behaviour is strongly shaped by competition with other females and that females occasionally exhibit intense aggression when the pay-off in terms of reduced competition is large. In this paper, we describe the unusual social organization of chimpanzees in which males remain in their natal groups, while females disperse. We discuss how competition for food shapes female grouping and spacing patterns and describe circumstances in which females engage in physical aggression. Because of recent attention to the fact that females in other species sometimes compete for mates as well as food, we consider the extent to which this is true for female chimpanzees. We conclude by summarizing female competitive strategies in chimpanzees across the lifespan and compare these to those of other species, including humans.
2. Chimpanzee social organization
Beginning with the pioneering work of Jane Goodall and Toshisada Nishida in the 1960s [7,9], chimpanzees are now the subject of more than six long-term studies across Africa (Tanzania: Gombe National Park [7], Mahale National Park [9]; Uganda: Kibale National Park: Kanyawara community [10], Ngogo community [11], Budongo Forest Reserve [12]; Cote d'Ivoire: Tai National Park [13]; Guinea: Bossou [14]). These studies have gradually elucidated the complex and unusual social organization and structure of chimpanzees [15,16]. Chimpanzees live in permanent social groups called communities, which consist of up to 200 individuals (median 46.3) [17] including multiple breeding females and their offspring, and multiple adult males. When compared with most other mammalian groups, chimpanzee communities are unusual in three respects: they are fission–fusion social units, males are philopatric while females transfer to new communities around sexual maturity, and male community members are more social and range more widely than females.
Although communities are distinct social units, all the members are rarely, if ever, observed together in one group. Instead, they exhibit a pattern of fission and fusion [18] in which members associate in temporary subgroups (parties), whose composition may last from minutes to days, and individuals can spend some time alone [7,19,20]. Party size is strongly influenced by the distribution and abundance of food, with larger parties forming during seasons or periods of high fruit abundance, or when fruit patches are aggregated [7,21–26]. Other influences include the presence of sexually receptive females, which increases the number of adult males and sometimes females in parties [7,25–27], and predation risk from leopards [28]. Parties are also larger in border areas where intergroup encounters are more likely [29]. Males form strong social bonds with other adult males and together patrol and defend the community range against members of other communities. Intercommunity encounters are often aggressive and sometimes involve lethal attacks, especially in cases where the males on one side strongly outnumber their opponents [30–33]. Males kill other adult males, infants and occasionally adult females [34], and such killing can result in expansion of the community range [35].
Unlike many other species of social mammal, in which males transfer to other groups before breeding while most or all females remain for life in the natal group, in chimpanzees, males generally stay in the community in which they are born while females leave [36]. The extent of female transfer varies between sites. At Gombe, approximately half of maturing females in the Kasekela community and one of five females in the Mitumba community remained to breed in their natal community [37] (A. E. Pusey & K. Schroepfer-Walker 2013, unpublished data). At other sites, over 90% of females leave, though at least one has been observed to remain at all long-term sites [12,13,38–40]. An expected result of such sex-biased dispersal is that community males should be more closely related than adult females. This has been confirmed by genetic analysis at Mahale [41] and Gombe [42], but the difference is less consistent at Tai, and not observed at Budongo [43,44]. Adaptive explanations for patterns of sex-biased dispersal are the subject of considerable discussion, with debate focusing on the relative importance of resource and mate competition and avoidance of inbreeding [45–48]. In chimpanzees, it has been suggested that because of the dispersed distribution of females, males gain unusual benefits from staying in their natal community and cooperating with kin to jointly defend access to multiple females, forcing females to leave the community to avoid the deleterious consequences of inbreeding from mating with relatives [5,49,50].
Within the community, the sexes differ in gregariousness and ranging patterns. Females, except when they are sexually receptive, spend more time alone or with small groups of females and offspring and less time in mixed groups than males [7,19,27,51,52]. Males also range more widely than females at all sites [10,53,54]. However, the degree of these sex differences varies between sites; females are generally less gregarious and range less widely at East African sites than West African sites [26,55].
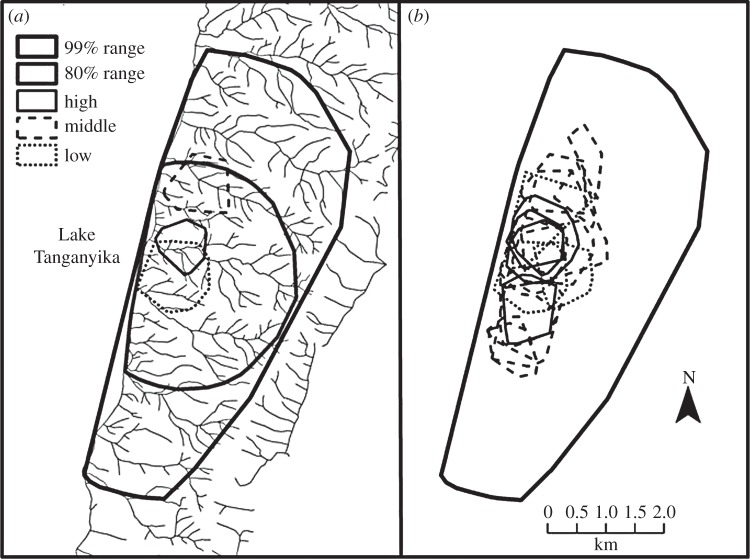
Sex differences in dispersion have been a particular focus of study at Gombe where, in the early years of the study, habituation by provisioning made it possible to follow females, even when they were alone, and hence to gain a better appreciation of their behaviour. In the early 1970s, Gombe females spent up to 65% of their time alone with just their dependent offspring, while adult males spent only 14–29% of their time alone [54,56]. In later decades, Gombe females have become more gregarious—probably because of increases in community range size [57] and a general increase in vegetation density caused by habitat protection [58]—but still spend 39–48% of their time alone [59]. Anoestrous females travel shorter daily distances than males and have more restricted ranges [7,54,60]. Females with infants also travel more slowly than males or sexually receptive females [61]. Although most females are seen in all parts of the community range as members of large parties over the course of a year [7], when they are alone, females concentrate their foraging in distinct, but overlapping core areas that are small portions of the community range (figure 1). Moreover, most females show high fidelity to these areas over many years [59,62] but sometimes shift or shrink their core areas in response to territorial shifts in the community boundary [62]. Although males generally range more widely over the whole community range, they also restrict their range use when they are alone [60,65], and show levels of fidelity similar to those of females to small core areas close to those that their mothers occupied during the male's first 9 years of life [66].
Figure 1.
Location of female alone core areas within the Kasekela community range at Gombe National Park, Tanzania. (a) The community range is indicated by minimum convex polygons which enclose 99% and 80% of 15 min locations of all community members observed during daily, day-long focal follows in 2000–2003 (N = 1363 follows). Representative core areas are shown for a high-, middle- and low-ranking female. High-quality resources are concentrated in the valley bottoms along streams while ridges between valleys are of lower quality. Here, the high-ranking female has a small core area centred in a high-quality valley, while the middle- and low-ranking females have larger core areas centred over ridge tops. (b) Core areas of all adult females that were present in the community from 2000 to 2003. Though individual core areas are distinct, they show high degrees of overlap with other community females and most are clustered within the 80% community range. High-ranking females are concentrated in the centre of the range, in an area of high overlap, while lower ranking females settle across the community range. Core areas were calculated from female alone points following Williams et al. [62]. However, for visual purposes, they are depicted here as 50% minimum convex polygons rather than 50% adaptive kernels. Rank was determined from the direction of pant–grunts between dyads during the period. Females with a modified David's score [63] half a standard deviation above or below the mean were classified as high and low, respectively, and the rest as middle-ranking. Data are available through Dryad Digital Repository [64].
Similar differentiated space use within the community by adult, non-cycling females has been described in the Kanyawara community at Kibale, where females clustered into three groups that used different portions of the community range and fidelity to these neighbourhoods persisted over many years [67]. Also, at Mahale, individual females concentrated their ranging in different portions of the community range, particularly when they were lactating [53,68]. Females in the very large Ngogo community at Kibale could be clustered into cliques based on levels of dyadic association, with females in different cliques spending little or no time together [69]. The 100% ranges of each clique were concentrated in different parts of the community range, but showed high overlap [69]. At Tai, however, females were found to use the community range almost as extensively as the males, and individuals did not exhibit long-term, differentiated patterns of range use [55,70].
The unusual dispersion patterns of chimpanzees have been explained on the basis of costs of feeding competition to females and access to females by males [51,54,71]. Chimpanzees specialize on ripe fruit, which is often patchily distributed, leading to strong within-patch competition among party members. As party size increases, patches are depleted more quickly forcing group members to travel further to new patches [72]. Wrangham [51] proposed that female chimpanzees, with higher travel costs imposed by carrying infants, suffer more than males from scramble competition and do better to avoid competition by feeding alone or in small parties, only joining others at large food sites. They minimize travel and maximize feeding efficiency by foraging alone in small core areas, which are, however, too big to defend as exclusive territories [71]. The result is that female ranges overlap and are dispersed across the landscape. By ranging more widely, males maintain regular access to many females.
High fidelity to core areas has been argued to occur because of the advantage of intimate knowledge of resources [37,59,62]. The importance of home range fidelity and local knowledge has been demonstrated in a number of non-primate species [73]. While such an advantage has not yet been demonstrated directly in chimpanzees (by, for example, measuring feeding efficiency inside and outside core areas) the idea is indirectly supported by the fact that even alpha males, who should have first access to resources in good areas, return to forage in their familiar, natal area during times of food scarcity, even if it is of low quality [66].
3. Female competition over food resources
Because the grouping and ranging patterns of females are sensitive to the distribution and abundance of food resources and because rates of aggressive interactions between females are generally very low [7,69,74], it appears at first glance that they respond to competition chiefly by avoiding others during times of low food availability, and feeding alone or in small groups [71]. A null model in such circumstances is that competitors distribute themselves across the landscape without aggression in an ideal, free manner [75] according to the density of food resources and competitors. In a patchy environment, good areas would become more crowded but all competitors would have equal fitness. Female core areas at Gombe and Kanyawara do tend to be clustered over productive areas in this manner [62,67]. However, evidence has gradually emerged that there is considerable variation in reproductive success among females and that some females gain access to resources at the expense of others. Below we review the evidence for the importance of dominance rank at these sites and discuss how high-ranking females gain advantages.
Even in the absence of strong individual differences in access to resources within the community, all community females are likely to suffer from an increase in competitor number. For example, at Gombe, during a period when the community range shrank by half owing to encroachment by neighbouring communities, density increased, all females lost weight and reproductive rates fell [57,76]. Scramble competition should thus select for any behaviour that limits competitor number. We describe intense aggression between females in two contexts: aggression towards immigrating females and infanticide of the infants of rival competitors, which may be interpreted as efforts to reduce general scramble competition, and/or efforts to defend individual core areas.
(a). Importance of female dominance rank
Linear female dominance hierarchies based on frequent aggressive or supplant–withdrawal interactions can be discerned in many species of group-living primates, particularly those living in societies with complete female philopatry, and dominance rank frequently has consequences for reproductive success [77]. Chimpanzees give unidirectional submissive signals in the form of pant–grunts [5,6]. While linear dominance hierarchies are easily discerned among male chimpanzees by the direction of pant–grunts between dyads [7,78,79], they are less obvious among female chimpanzees. Female West African chimpanzees of Tai forest, which spend most of their time in association with other females, have higher rates of agonistic interactions than their East African counterparts and can be ranked in a linear hierarchy on the basis of pant-grunts [80]. Among East African chimpanzees, rates of agonistic interactions are lower and in some populations some females in the same community are never observed interacting [37,69,74]. Nevertheless, in most populations, it has proved possible to use the direction of pant–grunts and decided agonistic interactions to rank most females into either of the categories: high/low [10]; high, medium, low [37]; or on the basis of various measures of cardinal rank [38,74,81].
Because of the slow pace of female life histories, many years of demographic data are required to measure the effect of rank on reproductive success and such analysis has yet to be carried out in most populations. However, at Gombe, female rank was significantly correlated with the age at which daughters reached sexual maturity, interbirth interval, and infant survival [37,82]. Such effects are likely to be due at least in part to rank differences in access to food. This is supported by the fact that high-ranking females are heavier and show smaller seasonal fluctuations in body weight [76] and that low-ranking females spend longer in feeding and tend to have a diet of greater breadth and lower quality than high-ranking females [83].
There are several ways in which high-ranking females might gain access to better food resources. First, high-ranking females may win contests over food items. Direct competition over food is the most common context of female aggression at Gombe, Kanyawara and Mahale but rates of such interactions are very low [7,8,84]. At Tai, where females associate at high levels, direct contests over food items are more frequent and are won by high-ranking females [80]. Second, high-ranking females may gain access to the best food resources when feeding with females in the same patch. At Kanyawara, fruit quality and abundance is higher at the top of tree crowns [86] and high-ranking females feed higher in the trees [87]. Third, high rank may afford females preferential access to areas with productive food resources and inhibit their use by subordinates. Consistent with this idea, analyses of dyadic association rates among females at Gombe found that low-ranking females associated least often with high-ranking females in the same neighbourhood [61,83]. Similarly, at Tai, low-ranking females were found to spend less time in large groups during times of food scarcity while this had no effect on the grouping patterns of high-ranking females [88].
Finally, females may actively defend high-quality core areas and high-ranking females may do this more effectively. Evidence of active defence comes from a recent analysis of the location of female–female aggressive interactions at Gombe. Although rates of aggression were very low, females were found to direct aggression towards other females at higher rates inside than outside their core areas in non-feeding, though not in feeding, contexts [85]. Murray found correlations between dominance rank and core area location and use, and suggested that females conform to an ideal despotic, rather than an ideal free distribution [59]. In an ideal despotic distribution, like the ideal free distribution, individuals attempt to distribute themselves in accordance with habitat quality and density of competitors, but some (despotic) individuals prevent others from settling with the result that individual fitness is the highest at the best sites [89]. High-ranking females at Gombe occupy significantly higher quality core areas, as measured by the abundance of preferred foods in vegetation plots within their alone core areas [83]. The core areas of high-ranking females are also smaller, affording them higher feeding efficiency through lower travel costs, and high-ranking females show higher site fidelity to these areas, securing the benefits of intimate knowledge of resources [59]. Similar correlations between range quality and rank have been demonstrated at Kanyawara. There, females in neighbourhoods with higher quality food resources had significantly higher reproductive success [67]. These females were also of higher rank [38].
(b). Aggression to new immigrants
Given the importance of core area quality for female success, we expect females to attempt to prevent immigrant females from settling within their core areas. Even in sites where females range more widely over the whole community range, the entry of a new female to the community is likely to increase female density, to the detriment of all resident females. At many sites, resident females are aggressive to new immigrants. Dramatic cases of resident females ganging up to attack new immigrant females have been described at Gombe [7,90,91]. In one case, when a female from the Kasekela community tried to join the Mitumba community, she was repeatedly attacked by resident females over a nine-hour period, often in coalitions of two or three, and she returned to her natal community with multiple serious wounds. She made at least one further attempt when she was again attacked but eventually returned and settled in her natal community [91]. Aggression by resident females towards immigrant females has been reported at Mahale [84], Budongo [92] and Tai [13]. A detailed study at Kanyawara, Kibale, found that levels of aggression among females were correlated with the number of new immigrants present. Coalitionary aggression was rare except when immigrants were present, coalitions were formed mainly among resident mothers toward immigrant females, and females occupying the highest quality neighbourhoods demonstrated the most aggression [38,93].
(c). Infanticide by females
Infanticide is an extreme form of female aggression that results in the removal of competitors. Among mammals, females have been observed to kill the young of other females in the same group in species such as ground squirrels and prairie dogs that live in coteries and compete for burrows or feeding territories [94,95]. Also, in cooperatively breeding species such as marmosets and meerkats, where group members assist in infant care, dominant females kill the infants of subordinates whose infants would be competitors for infant care [96–98]. Among plural breeding, group-living primates, infanticide by females is generally very rare. Single cases have been reported in mountain gorillas [99], Tonkean macaques [100], black lemurs and ring-tailed lemurs [101]. Among yellow baboons, a species that has been extensively studied for decades at several sites, only a single case of female-led infanticide has been observed and another inferred [102]. In all these cases, a more dominant individual opportunistically killed the infant of a lower ranking female. A different pattern was observed in Milne–Edwards sifakas, where female transfer sometimes occurs. Over a 20-year period, four female-led infanticides were observed or inferred and all were committed by a new immigrant female, and the mother of the victim subsequently left the breeding group [103].
Infanticide by female chimpanzees has now been observed at several sites. Female-led infanticides in chimpanzees follow a general pattern where a higher ranking resident female opportunistically attacks and kills the infant of a lower ranking or new immigrant female. In contrast to male-led infanticide, in most cases victims are newborn infants under the age of two months, suggesting that successful attacks are only possible when the victim is very small. Furthermore, successful attacks are mostly carried out by females in coalitions.
In the Kasekela community at Gombe, between 1975 and 1978, a high-ranking female and her adult daughter attacked, killed and consumed two infants of a low-ranking female, one infant of a mid-ranking female and were implicated in the death of this female's subsequent infant. They were also observed mounting unsuccessful attacks on three more infants and were suspected of killing a further five, never-observed infants that should have resulted from known full-term pregnancies during the same period [7,104,105]. In the 1990s, another high-ranking female and her adult daughter, on one occasion also aided by another adult female, made determined but unsuccessful attacks on the successive infants of a low- to middle-ranking female [91]. Most recently, in 2012, a higher ranking female killed and consumed the infant of a lower ranking female, and another high-ranking female attacked the infant of a low-ranking female but was deterred by the intervention of adult males (D. Mjungu 2012, personal communication). An inferred case of female infanticide was also recorded in the Mitumba community in 1994 where observers arrived at a group to see adult females in possession of the carcass of the newly killed infant of another female in the group [91]. Pusey et al. [91] estimate that female infanticide at Gombe accounts for a minimum of 3–5% of infant mortality to a maximum of close to 30%, if sudden disappearances of infants in their first two months and full-term pregnancies in which no infants were seen are taken into account. Females appear to be sensitive to this risk. They are significantly less likely to be observed with other chimpanzees around the time of parturition [7,91], new mothers are often submissive to and fearful of other females [7], and once they rejoin the group they often attempt to associate at higher rates with adult males and avoid other females [104].
Infanticide by females has also been observed in the Sonso community at Budungo, where coalitions of resident females attacked and killed one infant and were implicated in the deaths of two other infants, all belonging to females that had recently immigrated as part of an influx of 13 adult females into the community [92]. More recently, in 2012, a resident female attacked another resident female with a 3-day-old infant. The infant sustained mortal wounds, though it was unclear if the wounds were inflicted deliberately. Subsequently, several more infanticides have taken place but either the attack was not observed, or males rather than females were implicated in the initial attack and injury (C. Hobeiter, C. Asiimwe, K. Zuberbuhler 2013, personal communication). At Tai, females were observed eating an infant whose origin was unknown [13]; and at Kanyawara, a female was apparently coerced by a male to aid him in attacking a female and seizing her infant [106]. This latter case differed from other cases in that the infant was older (two years) than other victims, and the female resided in a different neighbourhood from her attacker. Infanticide by females has not been observed at Mahale. However, infant mortality is high in the first year [40], females also withdraw from the group around parturition [107], and new mothers are fearful of females [108].
These patterns of infanticide by female chimpanzees are reasonably interpreted as an extreme manifestation of competition for space [91,92]. By taking the opportunity to attack victims when they are most vulnerable, females remove future feeding competitors for themselves and their offspring. In addition, even unsuccessful attacks may traumatize the victim, thus cementing or increasing the attacker's dominance. At Gombe, all the victims were the offspring of resident females who often had core areas overlapping those of their attackers. We are currently investigating whether infanticidal attacks cause the mother of the victim to shift her core area away from that of her attacker(s) or permanently improve the dominance rank of the attacker relative to the victim.
4. Competition over mating
Besides reducing future resource competition through infanticide of potential competitors, females in some species of equids, marmosets and canids have been suggested to attempt to depress the reproduction of their competitors by interfering in their competitors' mating [109]. Among apes, female gorillas are known to engage in sexual behaviour at higher rates when other individuals are sexually receptive [110,111]. These patterns have also been interpreted as female competition, whereby females may deplete the sperm available to other females by mating with the single male or deflect the interest of the male in mating with a rival female, thus reducing her probability of conception [110,111].
Chimpanzees are highly promiscuous. Females exhibit sexual swellings lasting 6–18 days [112] during which time they attempt to mate with most or all the males of the community [113]. Likewise, males generally attempt to mate with all swollen females, although they prefer older females [7,114]. Several lines of evidence indicate some female competition over mating. First, at Mahale, females sometimes directly interfered in the mating attempts of their rivals by forcing themselves between a copulating pair. In some cases, the aggressive female went on to mate with the male [108]. At Gombe, during a day-long series of attacks by Mitumba females on a fully swollen new immigrant female, the most active attackers were also swollen and their behaviour was interpreted as ‘sexual jealousy’ by the observers [91]. Townsend et al. [115] found that females at Budongo suppressed copulation calls when in the presence of the dominant female, possibly to prevent direct interference in their copulations. Second, females occasionally seem to respond to the sexual swellings of others by swelling themselves. Goodall [116] described an unusual incident in which a dominant, lactating female suddenly appeared with a full swelling a day after a young oestrous female had been followed by many males. Nishida [108] described cases at Mahale in which a female would produce isolated swellings that were not part of her regular cycles when a second oestrous female was present in the group. More generally, though, female cycles have been shown to be asynchronous at Mahale, and females are suggested to benefit from asynchrony through a reduction in female competition and potentially better opportunities for female choice [117]. In summary, female chimpanzees sometimes compete over mates but the degree to which such competition affects female reproduction is unclear and requires further investigation.
5. Female competitive strategies over the lifespan
Female reproductive success in chimpanzees depends critically on access to high-quality food resources over a long lifespan. This depends, in turn, on settling in a productive, safe area, high rank and stable social relationships. With some exceptions [62,118,119], most females transfer just once and then spend the rest of their lives in the new community. In populations where adult females concentrate their ranging in restricted core areas, their fidelity to such areas is usually strong. Therefore, the quality of the community and the core area into which they initially settle is crucial for their success. The process of female transfer has seldom been observed from start to finish, both because of the low numbers of females reaching maturity at any one site and because most long-term studies concentrate observations on only one habituated community. Thus, at most long-term sites natal females are only observed until they transfer, and the prior history of immigrant females is unknown. Moreover, immigrant females are initially shy and hard to observe. However, there is some evidence that females may shop around before settling. At Mahale and Gombe, females have been observed to visit other communities before making their settling decisions [40,49] and one Gombe female transferred to a new community, gave birth and lost an infant, and then transferred to another community a few years later, where she bred successfully (as revealed by DNA analysis [119]). Dispersal is costly. At Gombe, one female was severely injured by resident females in the new community and failed to transfer, as described above, and three other females of dispersal age were observed after absences from the community with injuries consistent with similar attacks [7]. Also, age at first breeding is delayed by at least two years at Gombe in immigrant females compared with females breeding in the natal community (A. E. Pusey, K. Schroepfer-Walker 2013, unpublished data), and similar delays are seen at Mahale [40]. Such delay is likely to be caused by stress from social challenges as well as reduced foraging efficiency in a new area. Immigrant females at Kanyawara have elevated cortisol levels [93].
Females use several strategies to ameliorate these costs. Some simply remain in their natal community. At Mahale, this was more common following a marked decline in community size and density owing to disease [68]. However, while avoiding dispersal costs such females run the risk of close inbreeding. At Kanyawara, the timing of dispersal correlates with periods of high food abundance suggesting that females wait until they are in positive energy balance to transfer [120]. Most females transfer while they have a sexual swelling, which serves as a social passport [121], both reducing the probability of attack by males [57] and increasing the chance of males protecting them against aggression from resident females [93]. Once in the new community, females also associate at higher levels with adult males than do natal females of the same age [93,122]. The settling decisions of immigrant females at Gombe are sensitive to the dominance ranks of resident females. Williams found that immigrant females settled in the neighbourhood that did not contain the highest ranking female [123]. Later, Murray et al. [59] found that immigrant females settled in areas where the average rank of females was lower. Although immigrants are likely to reduce rates of immediate aggression by settling away from high-ranking females, they run the risk of settling in an area with low-quality resources, to which they are then committed (given that females seem to have strong site fidelity once they have settled). We are currently analysing female settlement decisions at Gombe in more detail, investigating the relative influence of such factors as range quality—in terms of food resources and safety from neighbours, female density and female rank.
Differences in female dominance rank are easier to discern in some populations than others, but rank clearly influences access to resources and reproductive success, raising the question of how the rank is determined. Dominance hierarchies develop in many animals that live in permanent social groups, and determinants of rank vary [124]. Often, individual resource holding power is important, with larger, stronger or older individuals dominating smaller individuals [125]. However, among primate species such as baboons and macaques, where females remain for life in their natal communities and contest competition over usurpable food resources is high, rank is not correlated with age or body size but is determined nepotistically. Young females are supported by close female relatives such that daughters rank immediately below their mothers, and one matriline dominates another [124].
In chimpanzees, some mothers do support their adolescent daughters [7]. But females that remain in their natal group at Gombe do not always assume their mother's rank (A. E. Pusey, K. Schroepfer-Walker 2013, unpublished data), and in most populations females leave their mothers to transfer to other communities with few or no close relatives. Rank increases with age at several sites [80,83,84,93], but at Kanyawara tenure in the community predicted rank better than age, with the newest immigrants ranking the lowest [93]. The most recent immigrants also tended to rank the lowest at Mahale [84]. Age and tenure could both enhance rank either through increased foraging efficiency, or through the accrual of social support, or both. Although it is hard to disentangle cause and effect, several lines of evidence suggest that rank also depends on strength and other individual characteristics. The effect of age on rank could, at least initially, be the result of a maturational increase in mass. In addition, at Gombe, female rank remained significantly correlated with body mass after controlling for age [76]. Several other observations support the importance of size and physical fitness. The female that was repelled from the Mitumba community was small in stature, while around the same time, a larger female succeeded in immigrating with little trouble [91]. In the early years at Gombe, Goodall [7] described two robust and assertive females achieving high rank at a young age. In another instance, a female in Mitumba fell in rank after she lost her hand to a snare (D. Mjungu 2013, personal communication) and while females often maintain their rank into old age [7,108], an old female fell in rank at Kanyawara [38]. Personality, however, can also be important. Nishida [68] described a small but assertive female who dominated many other females.
Given the apparent importance of initial rank in a female's success in settling in a high-quality area, we might expect females to behave as assertively as possible against other females on their arrival in a new community. When a de novo group of chimpanzees was assembled at Detroit Zoo, females engaged in frequent aggressive dominance displays, fought at high rates, formed coalitions during attacks on other females and reconciled at high rates [126]. Their behaviour resembled the habitual behaviour of males and was quite unlike the peaceful behaviour observed in the long-established group of females at Arnhem Zoo. Baker & Smuts [126] explained these sex differences on the basis that females should fight like males when the pay-off in terms of access to a high-quality area is high, while males benefit from fighting for high rank throughout their lives, and especially for the alpha position, which affords high reproductive success [78]. Aggression by immigrating females is observed in some other primates. Immigrating female mantled howlers compete with residents in the new group and always achieve the alpha position [127], while immigrating female sifakas have occasionally been observed to kill infants of residents and evict the mothers [103]. An interesting question for future research is whether female chimpanzees of transfer age, or females in other female-dispersing species, experience any underlying hormonal changes that may enhance their aggressiveness.
Once females are settled in their new community, their reproductive success will depend on keeping female density low. Resident females are aggressive to immigrants at all sites and commit infanticide at some sites. As may be expected, such aggression appears to be more intense where population density is high. Among long-term studies, population density is the highest in the Sonso community at Budongo, where several episodes of infanticide have been observed and it is also relatively high at Gombe, where female aggression toward immigrants is sometimes particularly severe, and infanticide is prevalent (M. L. Wilson et al. 2013, unpublished data). Female aggression towards vulnerable competitors also occurs in other plural breeding species such as macaques [128,129] and baboons [130]. Yet, infanticide appears to be very rare in these species. One reason that it may be more common in chimpanzees follows from kin selection theory in that females are generally competing with non-relatives, rather than kin [131]. A related possibility is that strong social bonds and the constant presence of closely related allies in macaques and baboons [132] are effective in preventing such attacks. Also, while males often serve as protectors in species such as baboons [133], male behaviour towards resident females is more variable in chimpanzees. Males often protect females against aggression by other females [93], but males also mount unprovoked attacks on females, perhaps as part of a strategy of sexual coercion [7,134]. The infanticide at Gombe in 2012 started with an intense, unprovoked attack on the mother by a male. The mother ran from the male to a resident female who opportunistically seized and killed the infant (D. Mjungu 2012, personal communication).
The apparently low levels of aggression among resident females present something of a puzzle. Why do they not continue to strive for high rank or to shift to a higher quality core area? Several explanations have been proposed. First, the costs, in terms of injuries from aggression sustained either by the female or her infant, outweigh the benefits [38,84]. Second, to the extent that females form beneficial social bonds with their neighbours, aggression would disrupt these bonds. With increasing evidence of the importance of social bonds for female reproductive success in other species [132,135], investigation of the presence of similar kinds of bonds in chimpanzees is an area of active research. So far, the evidence is mixed. At Gombe, the strongest bonds are between mothers and their adult daughters [7], but such pairs are rare in other populations because almost all females transfer. Strongly differentiated, long-term female associations at Kanyawara occur but these appear to result from shared space use, rather than the presence of affiliative social bonds in the form of strong grooming relationships [136]. On the other hand, affiliative social bonds that extend beyond mere shared space use have been proposed to exist at Tai and Ngogo [39,69,70]. A third possibility is that low rates of daily aggression belie the existence of continual competition among resident females for space. Females are opportunistic in their interactions with their neighbours, only being intensely aggressive when they may gain a large pay-off by repelling an immigrant or removing a future competitor by infanticide. Yet they are more aggressive inside than outside their core areas and may shift to higher quality areas when the opportunity arises. Although female fidelity to core areas is generally high, range shifts do occur at Gombe and further research is underway to examine how these depend on such factors as the death of neighbours, severe attacks, rank changes or the arrival of new females.
6. Comparisons with other species
The pattern that we have described here, of female competition for long-term access to high-quality core areas within the social group's range, is unusual but has some parallels in other species. Female Bechstein's bats roost together with kin in communal roosts but forage separately in overlapping foraging areas to which they have high fidelity [137]. Spotted hyaenas live in large social groups which defend a group territory. These are fission–fusion societies in which females spend some time foraging alone in smaller areas within the group territory and, as in chimpanzees, range quality appears to be influenced by rank. High-ranking females have higher reproductive success and occupy home ranges that are smaller and closer to the communal den and further from dangerous territory boundaries than those of low-ranking females. Low-ranking females without cubs have the largest home ranges, especially during periods of food scarcity [138]. Red deer on Rhum forage together in home ranges with kin. Females with higher quality home ranges and high-ranking females have higher reproductive success, and high-ranking females suffer less from feeding interference [139]. However, unlike chimpanzees, all these species show female philopatry, and in at least the bats and red deer, females tend to occupy ranges close to their mothers.
Only a few other plural breeding species live in multi-male philopatric societies from which females must disperse. Spider monkeys resemble chimpanzees in depending heavily on ripe fruit. As in chimpanzees, males defend the group territory and are generally more social than females. Females are highly aggressive to new immigrants, who are the most frequent targets of aggressive events, and aggression can be severe [140]. In comparison with female spider monkeys, muriqui females generally show very low rates of aggression and no dominance hierarchies, but they supplant female immigrants from food at rates twice those of resident females when they first enter the group [141]. Red colobus are also characterized by female dispersal, but evidence of aggression towards immigrants is generally lacking [142]. Genetic analysis of the relatedness of females in a large and a small group found that females in the small group were more closely related [143]. Miyamoto et al. suggest that increased scramble competition in large groups drives increased female dispersal.
Bonobos resemble chimpanzees in showing male philopatry and female dispersal [144]. They differ, however, in the nature of female relationships. Unlike chimpanzees, female bonobos act together to dominate adult males in competition for food [145,146]. Whereas immigrating female chimpanzees generally seek protection from adult males against resident females, immigrating female bonobos attempt to form bonds with particular resident females [147]. Bonds among bonobos are the strongest between male–female and female–female pairs [148], facilitating cooperation to ensure access to resources.
The shared pattern of male philopatry and female dispersal in our two closest living relatives, as well as gorillas [149], and evidence for similar patterns in many human societies ([150–152] but see [153–156]), suggests that the last common ancestor of humans and African apes showed this pattern. During human evolution, females would therefore have been competing with non-relatives directly for food resources. As extractive foraging and food processing, including cooking, became more important, the resulting food products would have become increasingly usurpable [157,158]. Consequently, simply avoiding a rival may not have been sufficient and as well as forming long-term bonds with male protectors [158], females may have gained increasing benefits from forming strong, stable bonds and coalitionary relationships with unrelated females, as in bonobos. In addition, as provisioning from males and other social group members became more important [156–160], competition for mates and good providers would intensify. Enhanced cognitive ability may have led to the evolution of indirect competitive strategies where human females could avoid physical injury but, nevertheless, harm their rivals such as through gossip and social exclusion [161,162].
7. Future directions
Female chimpanzees are difficult to study. In most populations, observations have been concentrated on a subset of more easily observed females that tend to be high ranking, more gregarious and more often observed in central areas of the community range, while other females range in peripheral ranges and are seldom seen (Gombe [62,83], Kanyawara [67], Ngogo [69]). Until the behaviour of all females is studied, we will not have a complete picture of female competitive strategies. There is much still to be learned about the importance of female rank, female bonds, the extent and process of female transfer, different female strategies (peripheral and central), ranging patterns across a lifetime and under different ecological conditions and the factors that cause these to vary at different sites. The fact that female dispersal is so costly underscores the need to understand why females, rather than males, disperse. Extensions of game theoretical models [45–47] that take into account the strength of local resource competition among females, the advantages of local mate enhancement for males and the costs of inbreeding, should help explain this unusual pattern of sex-biased dispersal [48] as well as the differences in the extent of female dispersal at different sites.
Acknowledgements
Long-term research at Gombe National Park is conducted with permission from Tanzania National Parks, the Tanzania Wildlife Research Institute and the Tanzanian Council for Science and Technology. We thank Dr Jane Goodall for permission to work with the long-term data, and the staff of the Gombe Stream Research Center for maintaining data collection. We thank Joseph Feldblum, Ian Gilby, Deus Mjungu, Carson Murray, Michael Wilson and Emily Wroblewski for discussion.
Funding statement
Data collection is primarily supported by the Jane Goodall Institute, with additional support from the National Science Foundation (LTREB-1052693), the National Institutes of Health (R01 A1058715) and Duke University. Digitization and analysis of behavioural data were also supported by grants from the National Science Foundation (DBS-9021946, SBR-9319909, BCS-0452315), the University of Minnesota, the Harris Steel Group, the Windibrow Foundation and the Carnegie Corporation.
References
- 1.Trivers R. 1972. Parental investment and sexual selection. In Sexual selection and the descent of man (ed. Campbell B.), pp. 136–179 Chicago, IL: Aldine [Google Scholar]
- 2.Emery Thompson ME. 2013. Reproductive ecology of female chimpanzees. Am. J. Primatol. 75, 222–237 (doi:10.1002/ajp.22084) [DOI] [PubMed] [Google Scholar]
- 3.Bronikowski AM, et al. 2011. Aging in the natural world: comparative data reveal similar mortality patterns across primates. Science 331, 1325–1328 (doi:10.1126/science.1201571) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goodall J, Pusey AE. In press The Flo family. In Encyclopedia of human sexuality (eds Whelehan P, Bolin A.). Hoboken, NJ: Wiley-Blackwell [Google Scholar]
- 5.Bygott JD. 1979. Agonistic behavior, dominance and social structure in wild chimpanzees of the Gombe National Park. In The great apes (eds Hamburg D, McCown E.), pp. 405–428 Menlo Park, CA: Benjamin Cummings Publishing Company [Google Scholar]
- 6.de Waal F. 1982. Chimpanzee politics: power and sex among the apes. London, UK: Jonathon Cape [Google Scholar]
- 7.Goodall J. 1986. The chimpanzees of Gombe. Cambridge, MA: Belknap Press [Google Scholar]
- 8.Muller M. 2002. Agonistic relations among Kanyawara chimpanzees. In Behavioural diversity in chimpanzees and bonobos (eds Boesch C, Hohmann G, Marchant L.), pp. 112–124 Cambridge, UK: Cambridge University Press [Google Scholar]
- 9.Nishida T. 1990. A quarter century of research in the Mahale mountains: an overview. In The chimpanzees of the Mahale mountains (ed. Nishida T.), pp. 3–36 Tokyo, Japan: University of Tokyo Press [Google Scholar]
- 10.Wrangham RW, Clark AP, Isabirye-Basuta G. 1992. Female social relationships and social organization of Kibale Forest chimpanzees. Top. Primatol. 1, 81–98 [Google Scholar]
- 11.Watts DP. 2012. Long-term research on chimpanzee behavioral ecology in Kibale National Park, Uganda. In Long-term field studies of primates (eds Kappeler P, Watts D.), pp. 313–338 Berlin, Germany: Springer [Google Scholar]
- 12.Reynolds V. 2005. The chimpanzees of the Budongo forest: ecology, behaviour and conservation. Oxford, UK: Oxford University Press [Google Scholar]
- 13.Boesch C, Boesch-Achermann H. 2000. The chimpanzees of the Taï forest: behavioural ecology and evolution. New York, NY: Oxford University Press [Google Scholar]
- 14.Sugiyama Y. 1984. Population dynamics of wild chimpanzees at Bossou, Guinea, between 1976 and 1983. Primates 25, 391–400 (doi:10.1007/BF02381662) [Google Scholar]
- 15.Mitani JC, Watts DP, Muller MN. 2002. Recent developments in the study of wild chimpanzee behavior. Evol. Anthropol. 11, 9–25 (doi:10.1002/evan.10008) [Google Scholar]
- 16.Mitani JC. 2009. Cooperation and competition in chimpanzees: current understanding and future challenges. Evol. Anthropol. 18, 215–227 (doi:10.1002/evan.20229) [Google Scholar]
- 17.Wrangham RW, Wilson ML, Muller MN. 2006. Comparative rates of violence in chimpanzees and humans. Primates 47, 14–26 (doi:10.1007/s10329-005-0140-1) [DOI] [PubMed] [Google Scholar]
- 18.Aureli F, et al. 2008. Fission-fusion dynamics. Curr. Anthropol. 49, 627–654 (doi:10.1086/586708) [Google Scholar]
- 19.Nishida T. 1968. The social group of wild chimpanzees in the Mahali Mountains. Primates 9, 167–224 (doi:10.1007/BF01730971) [Google Scholar]
- 20.Boesch C. 1996. Social grouping in Taï chimpanzees. In Great ape societies (eds McGrew W, Marchant L, Nishida T.), pp. 101–113 Cambridge, UK: Cambridge University Press [Google Scholar]
- 21.Wrangham RW. 1986. Social relationships in chimpanzees. In Ecological aspects of social evolution: birds and mammals (eds Rubenstein D, Wrangham R.), pp. 352–378 Princeton, NJ: Princeton University Press [Google Scholar]
- 22.White FJ, Wrangham RW. 1988. Feeding competition and patch size in the chimpanzee species Pan paniscus and Pan troglodytes. Behaviour 105, 148–164 (doi:10.1163/156853988X00494) [Google Scholar]
- 23.Chapman CA, White FJ, Wrangham RW. 1994. Party size in chimpanzees and bonobos. In Chimpanzee cultures (eds Wrangham R, McGrew W, de Waal F, Heltne P.), pp. 41–57 Cambridge, MA: Harvard University Press [Google Scholar]
- 24.Matsumoto-Oda A, Hosaka K, Huffman MA, Kawanaka K. 1998. Factors affecting party size in chimpanzees of the Mahale mountains. Int. J. Primatol. 19, 999–1011 (doi:10.1023/A:1020322203166) [Google Scholar]
- 25.Anderson DP, Nordheim EV, Boesch C, Moermond TC. 2002. Factors influencing fission-fusion grouping in chimpanzees in the Taï National Park, Côte d'Ivoire. In Behavioural diversity in chimpanzees and bonobos (eds Boesch C, Hohmann G, Marchant L.), pp. 90–101 Cambridge, UK: Cambridge University Press [Google Scholar]
- 26.Wittiger L, Boesch C. 2013. Female gregariousness in Western Chimpanzees (Pan troglodytes verus) is influenced by resource aggregation and the number of females in estrus. Behav. Ecol. Sociobiol. 67, 1–15 (doi:10.1007/s00265-013-1534-5) [Google Scholar]
- 27.Mitani JC, Watts DP, Lwanga JS. 2002. Ecological and social correlates of chimpanzee party size and composition. In Behavioural diversity in chimpanzees and bonobos (eds Boesch C, Hohmann G, Marchant L.), pp. 102–111 Cambridge, UK: Cambridge University Press [Google Scholar]
- 28.Boesch C. 1991. The effects of leopard predation on grouping patterns in forest chimpanzees. Behaviour 117, 220–242 (doi:10.1163/156853991X00544) [Google Scholar]
- 29.Wilson ML, Kahlenberg SM, Wells M, Wrangham RW. 2012. Ecological and social factors affect the occurrence and outcomes of intergroup encounters in chimpanzees. Anim. Behav. 83, 277–291 (doi:10.1016/j.anbehav.2011.11.004) [Google Scholar]
- 30.Goodall J, Bandora A, Bergmann E, Busse C, Matama H, Mpongo E, Pierce A, Riss D. 1979. Intercommunity interactions in the chimpanzee population of the Gombe National Park. In The great apes (eds Hamburg D, McCown E.), pp. 13–53 Menlo Park, CA: Benjamin Cummings [Google Scholar]
- 31.Wilson ML, Wrangham RW. 2003. Intergroup relations in chimpanzees. Annu. Rev. Anthropol. 32, 363–392 (doi:10.1146/annurev.anthro.32.061002.120046) [Google Scholar]
- 32.Watts DP, Muller M, Amsler SJ, Mbabazi G, Mitani JC. 2006. Lethal intergroup aggression by chimpanzees in Kibale National Park, Uganda. Am. J. Primatol. 68, 161–180 (doi:10.1002/ajp.20214) [DOI] [PubMed] [Google Scholar]
- 33.Boesch C, Crockford C, Herbinger I, Wittig R, Moebius Y, Normand E. 2008. Intergroup conflicts among chimpanzees in Tai National Park: lethal violence and the female perspective. Am. J. Primatol. 70, 519–532 (doi:10.1002/ajp.20524) [DOI] [PubMed] [Google Scholar]
- 34.Wilson ML, et al. 2012. Rates of lethal aggression in chimpanzees depend on the number of adult males rather than measures of human disturbance. Am. J. Phys. Anthropol. 147(S56), 305 [Google Scholar]
- 35.Mitani JC, Watts DP, Amsler SJ. 2010. Lethal intergroup aggression leads to territorial expansion in wild chimpanzees. Curr. Biol. 20, R507–R508 (doi:10.1016/j.cub.2010.04.021) [DOI] [PubMed] [Google Scholar]
- 36.Pusey AE, Packer C. 1987. Dispersal and philopatry. In Primate societies (eds Smuts B, Cheney D, Seyfarth R, Wrangham R, Struhsaker T.), pp. 250–266 Chicago, IL: University of Chicago Press [Google Scholar]
- 37.Pusey A, Williams J, Goodall J. 1997. The influence of dominance rank on the reproductive success of female chimpanzees. Science 277, 828–831 (doi:10.1126/science.277.5327.828) [DOI] [PubMed] [Google Scholar]
- 38.Kahlenberg S, Emery Thompson M, Wrangham R. 2008. Female competition over core areas in Pan troglodytes, Kibale National Park, Uganda. Int. J. Primatol. 29, 931–947 (doi:10.1007/s10764-008-9276-3) [Google Scholar]
- 39.Langergraber K, Mitani J, Vigilant L. 2009. Kinship and social bonds in female chimpanzees (Pan troglodytes). Am. J. Primatol. 71, 840–851 (doi:10.1002/ajp.20711) [DOI] [PubMed] [Google Scholar]
- 40.Nishida T, et al. 2003. Demography, female life history, and reproductive profiles among the chimpanzees of Mahale. Am. J. Primatol. 59, 99–121 (doi:10.1002/ajp.10068) [DOI] [PubMed] [Google Scholar]
- 41.Inoue E, Inoue-Murayama M, Vigilant L, Takenaka O, Nishida T. 2008. Relatedness in wild chimpanzees: influence of paternity, male philopatry, and demographic factors. Am. J. Phys. Anthropol. 137, 256–262 (doi:10.1002/ajpa.20865) [DOI] [PubMed] [Google Scholar]
- 42.Schroepfer-Walker KK, Pusey AE, Rudicell RS, Ramirez MA, Hahn BH, Wroblewski E. 2013. Females select mates that are less related than expected among the Gombe chimpanzees. Am. J. Phys. Anthropol. 150(S56), 246 [Google Scholar]
- 43.Vigilant L, Hofreiter M, Siedel H, Boesch C. 2001. Paternity and relatedness in wild chimpanzee communities. Proc. Natl Acad. Sci. USA 98, 12 890–12 895 (doi:10.1073/pnas.231320498) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lukas D, Reynolds V, Boesch C, Vigilant L. 2005. To what extent does living in a group mean living with kin? Mol. Ecol. 14, 2181–2196 (doi:10.1111/j.1365-294X.2005.02560.x) [DOI] [PubMed] [Google Scholar]
- 45.Perrin N, Mazalov V. 1999. Dispersal and inbreeding avoidance. Am. Nat. 154, 282–292 (doi:10.1086/303236) [DOI] [PubMed] [Google Scholar]
- 46.Perrin N, Goudet J. 2001. Inbreeding, kinship, and the evolution of natal dispersal. In Dispersal (eds Clobert J, Danchin E, Dhondt A, Nichols J.), pp. 123–142 Oxford, UK: Oxford University Press [Google Scholar]
- 47.Perrin N, Mazalov V. 2000. Local competition, inbreeding, and the evolution of sex-biased dispersal. Am. Nat. 155, 116–127 (doi:10.1086/303296) [DOI] [PubMed] [Google Scholar]
- 48.Clutton-Brock TH, Lukas D. 2012. The evolution of social philopatry and dispersal in female mammals. Mol. Ecol. 21, 472–492 (doi:10.1111/j.1365-294X.2011.05232.x) [DOI] [PubMed] [Google Scholar]
- 49.Pusey AE. 1979. Intercommunity transfer of chimpanzees in Gombe National Park. In The great apes (eds Hamburg D, McCown E.), pp. 465–479 Menlo Park, CA: Benjamin Cummings [Google Scholar]
- 50.Pusey AE. 1987. Sex-biased dispersal and inbreeding avoidance in birds and mammals. Trends Ecol. Evol. 2, 295–299 (doi:10.1016/0169-5347(87)90081-4) [DOI] [PubMed] [Google Scholar]
- 51.Wrangham RW. 2000. Why are male chimpanzees more gregarious than mothers? A scramble competition hypothesis. In Primate males (ed. Kappeler P.), pp. 278–313 Cambridge, UK: Cambridge University Press [Google Scholar]
- 52.Lehmann J, Boesch C. 2008. Sexual differences in chimpanzee sociality. Int. J. Primatol. 29, 65–81 (doi:10.1007/s10764-007-9230-9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hasegawa T. 1990. Sex differences in ranging patterns. In The chimpanzees of the Mahale mountains: sexual and life history strategies (ed. Nishida T.), pp. 99–114 Tokyo, Japan: University of Tokyo Press [Google Scholar]
- 54.Wrangham RW, Smuts BB. 1980. Sex differences in the behavioural ecology of chimpanzees in the Gombe National Park, Tanzania. J. Repro. Fertil. 28(Suppl.), 13–31 [PubMed] [Google Scholar]
- 55.Lehmann J, Boesch C. 2005. Bisexually bonded ranging in chimpanzees (Pan troglodytes verus). Behav. Ecol. Sociobiol. 57, 525–535 (doi:10.1007/s00265-004-0891-5) [Google Scholar]
- 56.Halperin SD. 1979. Temporary association patterns in free ranging chimpanzees: an assessment of individual grouping preferences. In The great apes (eds Hamburg D, McCown E.), pp. 491–499 Menlo Park, CA: Benjamin Cummings [Google Scholar]
- 57.Williams JM, Oehlert GW, Carlis JV, Pusey AE. 2004. Why do male chimpanzees defend a group range? Anim. Behav. 68, 523–532 (doi:10.1016/j.anbehav.2003.09.015) [Google Scholar]
- 58.Pusey AE, Pintea L, Wilson ML, Kamenya S, Goodall J. 2007. The contribution of long-term research at Gombe National Park to chimpanzee conservation. Conserv. Biol. 21, 623–634 (doi:10.1111/j.1523-1739.2007.00704.x) [DOI] [PubMed] [Google Scholar]
- 59.Murray CM, Mane S, Pusey AE. 2007. Dominance rank influences female space use in wild chimpanzees, Pan troglodytes: towards an ideal despotic distribution. Anim. Behav. 74, 1795–1804 (doi:10.1016/j.anbehav.2007.03.024) [Google Scholar]
- 60.Wrangham RW. 1979. Sex differences in chimpanzee dispersion. In The great apes (eds Hamburg D, McCown E.), pp. 481–490 Menlo Park, CA: Benjamin Cummings [Google Scholar]
- 61.Williams JM, Liu HY, Pusey AE. 2002. Costs and benefits of grouping for female chimpanzees at Gombe. In Behavioural diversity in chimpanzees and bonobos (eds Boesch C, Hohmann G, Marchant L.), pp. 192–205 Cambridge, UK: Cambridge University Press [Google Scholar]
- 62.Williams J, Pusey A, Carlis J, Farm B, Goodall J. 2002. Female competition and male territorial behavior influence female chimpanzees’ ranging patterns. Anim. Behav. 63, 347–360 (doi:10.1006/anbe.2001.1916) [Google Scholar]
- 63.de Vries H, Stevens JMG, Vervaecke H. 2006. Measuring and testing the steepness of dominance hierarchies. Anim. Behav. 71, 585–592 (doi:10.1016/j.anbehav.2005.05.015) [Google Scholar]
- 64.Pusey AE, Schroepfer-Walker K. 2013. Data from: female competition in chimpanzees. Dryad Digital Repository. (doi:10.5061/dryad.jg05d) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wrangham RW. 1977. Feeding behaviour of chimpanzees in Gombe National Park, Tanzania. In Primate ecology: studies of feeding and ranging behaviour in lemurs, monkeys, and apes (ed. Clutton-Brock T.), pp. 503–538 London, UK: Academic Press [Google Scholar]
- 66.Murray CM, Gilby IC, Mane SV, Pusey AE. 2008. Adult male chimpanzees inherit maternal ranging patterns. Curr. Biol. 18, 20–24 (doi:10.1016/j.cub.2007.11.044) [DOI] [PubMed] [Google Scholar]
- 67.Emery Thompson M, Kahlenberg S, Gilby IC, Wrangham R. 2007. Core area quality is associated with variance in reproductive success among female chimpanzees at Kibale National Park. Anim. Behav. 73, 501–512 (doi:10.1016/j.anbehav.2006.09.007) [Google Scholar]
- 68.Nishida T. 2012. Chimpanzees of the Lakeshore: natural history and culture at Mahale. New York, NY: Cambridge University Press [Google Scholar]
- 69.Wakefield ML. 2013. Social dynamics among females and their influence on social structure in an East African chimpanzee community. Anim. Behav. 85, 1303–1313 (doi:10.1016/j.anbehav.2013.03.019) [Google Scholar]
- 70.Lehmann J, Boesch C. 2009. Sociality of the dispersing sex: the nature of social bonds in West African female chimpanzees, Pan troglodytes. Anim. Behav. 77, 377–387 (doi:10.1016/j.anbehav.2008.09.038) [Google Scholar]
- 71.Wrangham R. 1979. On the evolution of ape social systems. Soc. Sci. Inform. 18, 336–368 (doi:10.1177/053901847901800301) [Google Scholar]
- 72.Janson CH, Goldsmith ML. 1995. Predicting group size in primates: foraging costs and predation risks. Behav. Ecol. 6, 326–336 (doi:10.1093/beheco/6.3.326) [Google Scholar]
- 73.Piper WH. 2011. Making habitat selection more ‘familiar’: a review. Behav. Ecol. Sociobiol. 65, 1329–1351 (doi:10.1007/s00265-011-1195-1) [Google Scholar]
- 74.Murray CM. 2007. Method for assigning categorical rank in female Pan troglodytes schweinfurthii via the frequency of approaches. Int. J. Primatol. 28, 853–864 (doi:10.1007/s10764-007-9164-2) [Google Scholar]
- 75.Fretwell SD, Lucas HL. 1969. On territorial behavior and other factors influencing habitat distribution in birds. Acta Biotheor. 19, 45–52 (doi:10.1007/BF01601955) [Google Scholar]
- 76.Pusey AE, Oehlert GW, Williams JM, Goodall J. 2005. Influence of ecological and social factors on body mass of wild chimpanzees. Int. J. Primatol. 26, 3–31 (doi:10.1007/s10764-005-0721-2) [Google Scholar]
- 77.Pusey A. 2012. Magnitude and sources of variation in female reproductive performance. In Evolution of primate societies (eds Mitani J, Call J, Kappeler P, Palombit R, Silk J.), pp. 343–366 Chicago, IL: University of Chicago Press [Google Scholar]
- 78.Wroblewski EE, Murray CM, Keele BF, Schumacher-Stankey JC, Hahn BH, Pusey AE. 2009. Male dominance rank and reproductive success in chimpanzees, Pan troglodytes schweinfurthii. Anim. Behav. 77, 873–885 (doi:10.1016/j.anbehav.2008.12.014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Boesch C, Kohou G, Néné H, Vigilant L. 2006. Male competition and paternity in wild chimpanzees of the Taï forest. Am. J. Phys. Anthropol. 130, 103–115 (doi:10.1002/ajpa.20341) [DOI] [PubMed] [Google Scholar]
- 80.Wittig RM, Boesch C. 2003. Food competition and linear dominance hierarchy among female chimpanzees of the Tai National Park. Int. J. Primatol. 24, 847–867 (doi:10.1023/A:1024632923180) [Google Scholar]
- 81.Newton-Fisher NE. 2006. Female coalitions against male aggression in wild chimpanzees of the Budongo Forest. Int. J. Primatol. 27, 1589–1599 (doi:10.1007/s10764-006-9087-3) [Google Scholar]
- 82.Jones JH, Wilson ML, Murray C, Pusey A. 2010. Phenotypic quality influences fertility in Gombe chimpanzees. J. Anim. Ecol. 79, 1262–1269 (doi:10.1111/j.1365-2656.2010.01687.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Murray CM, Eberly LE, Pusey AE. 2006. Foraging strategies as a function of season and rank among wild female chimpanzees (Pan troglodytes). Behav. Ecol. 17, 1020–1028 (doi:10.1093/beheco/arl042) [Google Scholar]
- 84.Nishida T. 1989. Social interactions between resident and immigrant female chimpanzees. In Understanding chimpanzees (eds Heltne P, Marquardt L.), pp. 68–89 Cambridge, MA: Harvard University Press [Google Scholar]
- 85.Miller JA, Gilby IC, Schroepfer-Walker KK, Pusey AE, Murray CM. In press Competing for space: wild female chimpanzees (Pan troglodytes) are more aggressive inside their core areas than outside. Animal Behaviour. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Houle A, Chapman CA, Vickery WL. 2007. Intratree variation in fruit production and implications for primate foraging. Int. J. Primatol. 28, 1197–1217 (doi:10.1007/s10764-007-9214-9) [Google Scholar]
- 87.Kahlenberg S. 2006. Female–female competition and male sexual coercion in Kanyawara chimpanzees. PhD thesis, Harvard University, MA, USA [Google Scholar]
- 88.Riedel J, Franz M, Boesch C. 2011. How feeding competition determines female chimpanzee gregariousness and ranging in the Taï National Park, Côte d'Ivoire. Am. J. Primatol. 73, 305–313 (doi:10.1002/ajp.20897) [DOI] [PubMed] [Google Scholar]
- 89.Sutherland W, Parker G. 1985. Distribution of unequal competitors. In Behavioural Ecology (eds Sibly RM, Smith RH.), pp. 255–273 Oxford, UK: Blackwell Scientific [Google Scholar]
- 90.Pusey AE. 1980. Inbreeding avoidance in chimpanzees. Anim. Behav. 28, 543–552 (doi:10.1016/S0003-3472(80)80063-7) [Google Scholar]
- 91.Pusey AE, Murray C, Wallauer B, Wilson M, Wroblewski E, Goodall J. 2008. Severe aggression among female Pan troglodytes schweinfurthii at Gombe National Park, Tanzania. Int. J. Primatol. 29, 949–973 (doi:10.1007/s10764-008-9281-6) [Google Scholar]
- 92.Townsend SW, Slocombe ME, Emery Thompson ME, Zuberbuhler K. 2007. Female-led infanticide in wild chimpanzees. Curr. Biol. 17, R355–R356 (doi:10.1016/j.cub.2007.03.020) [DOI] [PubMed] [Google Scholar]
- 93.Kahlenberg S, Emery Thompson M, Muller M, Wrangham R. 2008. Immigration costs for female chimpanzees and male protection as an immigrant counterstrategy to intrasexual aggression. Anim. Behav. 76, 1497–1509 (doi:10.1016/j.anbehav.2008.05.029) [Google Scholar]
- 94.Sherman PW. 1982. Infanticide in ground squirrels. Anim. Behav. 30, 938–939 (doi:10.1016/S0003-3472(82)80174-7) [Google Scholar]
- 95.Hoogland JL. 1985. Infanticide in prairie dogs: lactating females kill offspring of close kin. Science 230, 1037–1040 (doi:10.1126/science.230.4729.1037) [DOI] [PubMed] [Google Scholar]
- 96.Digby L. 1995. Infant care, infanticide, and female reproductive strategies in polygynous groups of common marmosets (Callithrix jacchus). Behav. Ecol. Sociobiol. 37, 51–61 (doi:10.1007/BF00173899) [Google Scholar]
- 97.Bezerra BM, Da Silva Souto A, Schiel N. 2007. Infanticide and cannibalism in a free-ranging plurally breeding group of common marmosets (Callithrix jacchus). Am. J. Primatol. 69, 945–952 (doi:10.1002/ajp.20394) [DOI] [PubMed] [Google Scholar]
- 98.Clutton-Brock TH, Brotherton PNM, Smith R, McIlrath GM, Kansky R, Gaynor D, O'Riain MJ, Skinner JD. 1998. Infanticide and expulsion of females in a cooperative mammal. Proc. R. Soc. Lond. B 265, 2291–2295 (doi:10.1098/rspb.1998.0573) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Fossey D. 1984. Infanticide in mountain gorillas (Gorilla gorilla beringei) with comparative notes on chimpanzees. In Infanticide: comparative and evolutionary perspectives (eds Hausfater G, Hrdy SB.), pp. 217–236 Oxford, UK: Aldine Press [Google Scholar]
- 100.Muroyama Y, Thierry B. 1996. Fatal attack on an infant by an adult female Tonkean macaque. Int. J. Primatol. 17, 219–227 (doi:10.1007/BF02735449) [Google Scholar]
- 101.Andrews J. 1998. Infanticide by a female black lemur, Eulemur macaco, in disturbed habitat on Nosy Be, North-Western Madagascar. Folia Primatol. 69(Suppl. 1), 14–17 (doi:10.1159/000052694) [DOI] [PubMed] [Google Scholar]
- 102.Wasser SK, Starling AK. 1986. Reproductive competition among female yellow baboons. In Primate ontogeny, cognition and social behavior (eds Lee P, Else J.), pp. 343–354 Cambridge, UK: Cambridge University Press [Google Scholar]
- 103.Morelli TL, King SJ, Pochron ST, Wright PC. 2009. The rules of disengagement: takeovers, infanticide, and dispersal in a rainforest lemur, Propithecus edwardsi. Behaviour 146, 4–5 (doi:10.1163/15683908X399554) [Google Scholar]
- 104.Goodall J. 1977. Infant killing and cannibalism in free-living chimpanzees. Folia Primatol. 28, 259–282 (doi:10.1159/000155817) [DOI] [PubMed] [Google Scholar]
- 105.Goodall J. 1990. Through a window: my thirty years with the chimpanzees of Gombe. New York, NY: Mariner Books [Google Scholar]
- 106.Arcadi AC, Wrangham RW. 1999. Infanticide in chimpanzees: review of cases and a new within-group observation from the Kanyawara study group in Kibale National Park. Primates 40, 337–351 (doi:10.1007/BF02557557) [Google Scholar]
- 107.Nishida T, Takasaki H, Takahata Y. 1990. Demography and reproductive profiles. In The chimpanzees of the Mahale Mountains (ed. Nishida T.). Tokyo, Japan: Tokyo University Press [Google Scholar]
- 108.Nishida T. 1979. The social structure of chimpanzees of the Mahale Mountains. In The great apes (eds Hamburg D, McCown E.), pp. 73–121 Menlo Park, CA: Benjamin Cummings [Google Scholar]
- 109.Stockley P, Bro-Jørgensen J. 2011. Female competition and its evolutionary consequences in mammals. Biol. Rev. 86, 341–366 (doi:10.1111/j.1469-185X.2010.00149.x) [DOI] [PubMed] [Google Scholar]
- 110.Stoinski TS, Perdue BM, Legg AM. 2009. Sexual behavior in female western lowland gorillas (Gorilla gorilla gorilla): evidence for sexual competition. Am. J. Primatol. 71, 587–593 (doi:10.1002/ajp.20692) [DOI] [PubMed] [Google Scholar]
- 111.Doran-Sheehy DM, Fernandez D, Borries C. 2009. The strategic use of sex in wild female western gorillas. Am. J. Primatol. 71, 1011–1020 (doi:10.1002/ajp.20743) [DOI] [PubMed] [Google Scholar]
- 112.Deschner T, Heistermann M, Hodges K, Boesch C. 2003. Timing and probability of ovulation in relation to sex skin swelling in wild West African chimpanzees, Pan troglodytes verus . Anim. Behav. 66, 551–560 (doi:10.1006/anbe.2003.2210) [Google Scholar]
- 113.Stumpf RM, Boesch C. 2010. Male aggression and sexual coercion in wild West African chimpanzees, Pan troglodytes verus. Anim. Behav. 79, 333–342 (doi:10.1016/j.anbehav.2009.11.008) [Google Scholar]
- 114.Muller MN, Thompson ME, Wrangham RW. 2006. Male chimpanzees prefer mating with old females. Curr. Biol. 16, 2234–2238 (doi:10.1016/j.cub.2006.09.042) [DOI] [PubMed] [Google Scholar]
- 115.Townsend SW, Deschner T, Zuberbühler K. 2008. Female chimpanzees use copulation calls flexibly to prevent social competition. PLoS ONE 3, e2431 (doi:10.1371/journal.pone.0002431) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Goodall J. 1971. In the shadow of man. New York, NY: Mariner Books [Google Scholar]
- 117.Matsumoto-Oda A, et al. 2007. Estrus cycle asynchrony in wild female chimpanzees, Pan troglodytes schweinfurthii . Behav. Ecol. Sociobiol. 61, 661–668 (doi:10.1007/s00265-006-0287-9) [Google Scholar]
- 118.Emery Thompson ME, Newton-Fisher NE, Reynolds V. 2006. Probable community transfer of parous adult female chimpanzees in the Budongo Forest, Uganda. Int. J. Primatol. 27, 1601–1617 (doi:10.1007/s10764-006-9098-0) [Google Scholar]
- 119.Rudicell RS, et al. 2010. Impact of Simian Immunodeficiency Virus infection on chimpanzee population dynamics. PLoS Pathog. 6, e1001116 (doi:10.1371/journal.ppat.1001116) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Stumpf RM, Emery Thompson M, Muller MN, Wrangham RW. 2009. The context of female dispersal in Kanyawara chimpanzees. Behaviour 146, 629–656 (doi:10.1163/156853909X413853) [Google Scholar]
- 121.Deschner T, Boesch C. 2007. Can the patterns of sexual swelling cycles in female Taï chimpanzees be explained by the cost-of-sexual-attraction hypothesis? Int. J. Primatol. 28, 389–406 (doi:10.1007/s10764-007-9120-1) [Google Scholar]
- 122.Pusey AE. 1990. Behavioural changes at adolescence in chimpanzees. Behaviour 115, 203–246 (doi:10.1163/156853990X00581) [Google Scholar]
- 123.Williams JM. 2000. Female strategies and reasons for territoriality in chimpanzees: lessons from three decades of research at Gombe. PhD thesis University of Minnesota, MN, USA [Google Scholar]
- 124.Pusey AE, Packer C. 1997. The ecology of relationships. In Behavioural ecology: an evolutionary approach (eds Krebs JR, Davies NB.), pp 254–283 Malden, MA: Blackwell [Google Scholar]
- 125.Fournier F, Festa-Bianchet M. 1995. Social dominance in adult female mountain goats. Anim. Behav. 49, 1449–1459 (doi:10.1016/0003-3472(95)90066-7) [Google Scholar]
- 126.Baker KC, Smuts BB. 1994. Social relationships of female chimpanzees. In Chimpanzee cultures (eds Wrangham R, McGrew W, de Waal F, Heltne P.), pp. 227–242 Chicago, IL: Chicago Academy of Sciences [Google Scholar]
- 127.Glander KE. 1980. Reproduction and population growth in free-ranging mantled howling monkeys. Am. J. Phys. Anthropol. 53, 25–36 (doi:10.1002/ajpa.1330530106) [DOI] [PubMed] [Google Scholar]
- 128.Silk JB. 1980. Kidnapping and female competition among captive bonnet macaques. Primates 21, 100–110 (doi:10.1007/BF02383827) [Google Scholar]
- 129.Silk JB. 1989. Reproductive synchrony in captive macaques. Am. J. Primatol. 19, 137–146 (doi:10.1002/ajp.1350190302) [DOI] [PubMed] [Google Scholar]
- 130.Wasser SK, Starling AK. 1988. Proximate and ultimate causes of reproductive suppression among female yellow baboons at Mikumi National Park, Tanzania. Am. J. Primatol. 16, 97–121 (doi:10.1002/ajp.1350160202) [DOI] [PubMed] [Google Scholar]
- 131.Hamilton WD. 1964. The genetical evolution of social behaviour. I. J. Theor. Biol. 7, 1–16 (doi:10.1016/0022-5193(64)90038-4) [DOI] [PubMed] [Google Scholar]
- 132.Silk JB, Beehner JC, Bergman TJ, Crockford C, Engh AL, Moscovice LR, Wittig RM, Seyfarth RM, Cheney DL. 2009. The benefits of social capital: close social bonds among female baboons enhance offspring survival. Proc. R. Soc. B 276, 3099–3104 (doi:10.1098/rspb.2009.0681) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Palombit RA, Seyfarth RM, Cheney DL. 1997. The adaptive value of ‘friendships’ to female baboons: experimental and observational evidence. Anim. Behav. 54, 599–614 (doi:10.1006/anbe.1996.0457) [DOI] [PubMed] [Google Scholar]
- 134.Muller MN, Kahlenberg SM, Thompson ME, Wrangham RW. 2007. Male coercion and the costs of promiscuous mating for female chimpanzees. Proc. R. Soc. B. 274, 1009–1014 (doi:10.1098/rspb.2006.0206) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Cameron EZ, Setsaas TH, Linklater WL. 2009. Social bonds between unrelated females increase reproductive success in feral horses. Proc. Natl Acad. Sci. USA 106, 13 850–13 853 (doi:10.1073/pnas.0900639106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Gilby IC, Wrangham RW. 2008. Association patterns among wild chimpanzees (Pan troglodytes schweinfurthii) reflect sex differences in cooperation. Behav. Ecol. Sociobiol. 62, 1831–1842 (doi:10.1007/s00265-008-0612-6) [Google Scholar]
- 137.Kerth G, Wagner M, König B. 2001. Roosting together, foraging apart: Information transfer about food is unlikely to explain sociality in female Bechstein's bats. Behav. Ecol. Sociobiol. 50, 283–291 (doi:10.1007/s002650100352) [Google Scholar]
- 138.Boydston EE, Kapheim KM, Szykman M, Holekamp KE. 2003. Individual variation in space use by female spotted hyenas. J. Mammol. 84, 1006–1018 (doi:10.1644/BOS-038) [Google Scholar]
- 139.Clutton-Brock TH, Albon SD, Guinness FE. 1988. Reproductive success in male and female red deer. In Reproductive success: studies of individual variation in contrasting breeding systems (ed. Clutton-Brock T.), pp. 325–343 Chicago, IL: University of Chicago Press [Google Scholar]
- 140.Asensio N, Korstjens AH, Schaffner CM, Aureli F. 2008. Intragroup aggression, fission-fusion dynamics and feeding competition in spider monkeys. Behaviour 145, 983–1001 (doi:10.1163/156853908784089234) [Google Scholar]
- 141.Printes RC, Strier KB. 1999. Behavioral correlates of dispersal in female muriquis (Brachyteles arachnoides). Int. J. Primatol. 20, 941–960 (doi:10.1023/A:1020882719850) [Google Scholar]
- 142.Struhsaker T. 2010. The red colobus monkeys. Oxford, UK: Oxford University Press [Google Scholar]
- 143.Miyamoto MM, Allen JM, Gogarten JF, Chapman CA. 2013. Microsatellite DNA suggests that group size affects sex-biased dispersal patterns in red colobus monkeys. Am. J. Primatol. 75, 478–490 (doi:10.1002/ajp.22124) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Kano T. 1992. The last ape: pygmy chimpanzee behavior and ecology. Stanford, CA: Stanford University Press [Google Scholar]
- 145.Furuichi T. 1997. Agonistic interactions and matrifocal dominance rank of wild bonobos (Pan paniscus) at Wamba. Int. J. Primatol. 18, 855–875 (doi:10.1023/A:1026327627943) [Google Scholar]
- 146.Vervaecke H, de Vries H, van Elsacker L. 2000. Dominance and its behavioral measures in a captive group of bonobos (Pan paniscus). Int. J. Primatol. 21, 47–68 (doi:10.1023/A:1005471512788) [Google Scholar]
- 147.Idani GI. 1991. Social relationships between immigrant and resident bonobo (Pan paniscus) females at Wamba. Folia Primatol. 57, 83–95 (doi:10.1159/000156568) [DOI] [PubMed] [Google Scholar]
- 148.Hohmann G, Gerloff U, Tautz D, Fruth B. 1999. Social bonds and genetic ties: kinship, association and affiliation in a community of bobobos (Pan paniscus). Behaviour 136, 1219–1235 (doi:10.1163/156853999501739) [Google Scholar]
- 149.Robbins A, Stoinksi T, Fawcett K, Robbins M. 2009. Leave or conceive: natal dispersal and philopatry of female mountain gorillas in the Virunga volcano region. Anim. Behav. 77, 831–838 (doi:10.1016/j.anbehav.2008.12.005) [Google Scholar]
- 150.Murdock G. 1967. Ethnographic Atlas: a summary. Ethnology 6, 109–236 (doi:10.2307/3772751) [Google Scholar]
- 151.Ember C. 1978. Myths about hunter–gatherers. Ethnology 17, 439–448 (doi:10.2307/3773193) [Google Scholar]
- 152.Copeland SR, Sponheimer M, de Ruiter DJ, Lee-Thorp JA, Codron D, le Roux PJ, Grimes V, Richards MP. 2011. Strontium isotope evidence for landscape use by early hominins. Nature 474, 76–78 (doi:10.1038/nature10149) [DOI] [PubMed] [Google Scholar]
- 153.Marlowe F. 2004. Marital residence among foragers. Curr. Anthropol. 45, 277–283 (doi:10.1086/382256) [Google Scholar]
- 154.Wilder J, Kingan S, Mobasher Z, Pilkington M, Hammer M. 2004. Global patterns of human mitochondrial DNA and Y-chromosome structure are not influenced by higher migration rates of males versus females. Nat. Genetics 36, 1122–1125 (doi:10.1038/ng1428) [DOI] [PubMed] [Google Scholar]
- 155.Hill K, et al. 2011. Co-residence patterns in hunter–gatherer societies show unique human social structure. Science 331, 1286–1289 (doi:10.1126/science.1199071) [DOI] [PubMed] [Google Scholar]
- 156.Kaplan H, Hill K, Lancaster J, Hurtado AM. 2000. A theory of human life history evolution: diet, intelligence, and longevity. Evol. Anthropol. 9, 156–185 (doi:10.1002/1520-6505(2000)9:4<156::AID-EVAN5>3.0.CO;2-7) [Google Scholar]
- 157.Wrangham R, Jones JH, Laden G, Pilbeam D, Conklin-Brittain N. 1999. The raw and the stolen: cooking and the ecology of human origins. Curr. Anthropol. 40, 567–594 (doi:10.1086/300083) [PubMed] [Google Scholar]
- 158.Wrangham R. 2009. Catching fire: how cooking made us human. Philadelphia, PA: Basic Books [Google Scholar]
- 159.Burkart JM, Hrdy SB, Van Schaik CP. 2009. Cooperative breeding and human cognitive evolution. Evol. Anthropol. 18, 175–186 (doi:10.1002/evan.20222) [Google Scholar]
- 160.Hrdy SB. 2009. Mothers and others. Cambridge, MA: Belknap Press [Google Scholar]
- 161.Vaillancourt T. 2013. Do human females use indirect aggression as an intrasexual competition strategy? Phil. Trans. R. Soc. B 368, 20130080 (doi:10.1098/rstb.2013.0080) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Benenson JF. 2013. The development of human female competition: allies and adversaries. Phil. Trans. R. Soc. B 368, 20130079 (doi:10.1098/rstb.2013.0079) [DOI] [PMC free article] [PubMed] [Google Scholar]