Abstract
The most significant social behaviour of the lactating mother is maternal behaviour, which comprises maternal care and maternal aggression (MA). The latter is a protective behaviour of the mother serving to defend the offspring against a potentially dangerous intruder. The extent to which the mother shows aggressive behaviour depends on extrinsic and intrinsic factors, as we have learned from studies in laboratory rodents. Among the extrinsic factors are the pups’ presence and age, as well as the intruders’ sex and age. With respect to intrinsic factors, the mothers’ innate anxiety and the prosocial brain neuropeptides oxytocin (OXT) and arginine vasopressin (AVP) play important roles. While OXT is well known as a maternal neuropeptide, AVP has only recently been described in this context. The increased activities of these neuropeptides in lactation are the result of remarkable brain adaptations peripartum and are a prerequisite for the mother to become maternal. Consequently, OXT and AVP are significantly involved in mediating the fine-tuned regulation of MA depending on the brain regions. Importantly, both neuropeptides are also modulators of anxiety, which determines the extent of MA. This review provides a detailed overview of the role of OXT and AVP in MA and the link to anxiety.
Keywords: HAB/LAB, central amygdala, intracerebroventricular, paraventricular nucleus, receptor agonist/antagonist
1. Introduction
In pregnant females, dramatic changes occur in the brain resulting in physiological and behavioural adaptations which enable the prospective mother to cope with her new situation. As the offspring represent the mother's reproductive fitness, maternal behaviour appears to be her most important social behaviour. This behaviour can be found throughout various species from fish, reptiles and birds, to mammals. The mother expresses a wide variety of maternal behaviours, which serve to ensure the well-being and proper development of the offspring. Most mothers build a nest, retrieve the young into it, provide them with food and keep them warm and clean; these behaviours can be summarized as maternal care. A further component of maternal behaviour is maternal aggression (MA). Importantly, this phrase does not refer to aggressive behaviour towards the offspring, which is suppressed in lactating mothers [1,2], but rather to the mother defending the offspring against a potentially threatening intruder. For this reason, MA should be considered a positive form of aggression.
A lactating mother has an innate drive to fight against an intruder. This innate drive to physically protect the pups is almost unique to the lactating mother; in fact, in rodents a female virgin hardly ever attacks an intruder. However, this changes dramatically from late pregnancy through early lactation, and the extent to which MA is displayed can depend on both extrinsic and intrinsic factors. Extrinsic factors include various aspects of the pups and/or the intruder. Intrinsic factors relate mainly to the substantial adaptations found in the peripartum brain, like the expression and activity of brain oxytocin (OXT) and arginine vasopressin (AVP) neuropeptide systems. Here, I will review our current knowledge on how MA is modulated by these extrinsic and intrinsic factors with particular emphasis on OXT and AVP. As the vast majority of experiments have been performed in the most common laboratory rodents, i.e. rats and mice, I will focus on these studies unless otherwise stated.
(a). Behavioural components of rodents’ maternal aggression
During the display of MA, the mother shows offensive and defensive behaviours in order to defeat the intruder [3–6]. The mother's offensive behaviours consist of fast attacks, which are sometimes paired with bites, directed toward the neck or back region of the intruder. However, bites can also occur without a previous attack. Furthermore, the mother is frequently engaged in lateral threats, also called lateral postures, where she approaches the intruder sideways, often with physical contact to force the intruder aside. Another offensive aggressive behaviour of the mother is sniffing at the intruder's genital area after an attack because the genital area is not only important for reproduction but is also a highly vulnerable body part. Indeed, highly aggressive mothers sometimes even attack this region. The defensive nature of MA is expressed in the mother's piloerection—a sign of fear and/or stress. With respect to defensive aggressive behaviour, the mother often stands in an upright posture in front of the intruder. This can be followed by the intruder taking a submissive horizontal posture, with the mother holding the intruder down with her front legs, also known as the ‘keep down’ posture.
2. Extrinsic and intrinsic factors modulate maternal aggression
MA in rodents is modulated by both extrinsic and intrinsic factors. However, when considering the impact of such aspects, the saying ‘mice are not small rats’ is advisable. Though the majority of the extrinsic and intrinsic factors have similar effects in both species, some of them differently affect MA (see below). With respect to intrinsic factors, the social decision-making network in various vertebrate lineages reveals that brain regions relevant for social behaviour are remarkably conserved. However, the spatial distribution of neuroendocrine ligands, rather than of receptors, is variable [7]. Hence, the diversity of social behaviour is based on variations within a conserved neural and gene expression network [7]. In the following, I will discuss how extrinsic (pups and intruders) and intrinsic (anxiety, OXT and AVP) factors modulate MA and highlight where such factors differ or converge in their effects.
(a). Maternal aggression is triggered by extrinsic factors: role of pups and intruders
The environment a lactating mother lives in provides numerous stimuli that can directly impact the behavioural expression of MA. In more detail, MA is strongly modulated by two extrinsic key factors: the pups, who are dependent on their mother for protection, and the intruder, who is (usually) defeated by the mother.
(i). Pups’ stimulation of the mother is required for maternal aggression
A virgin rat would not defend foster pups against an intruder, because she would not show any maternal behaviour within the first days of pup-exposure (for review, see [8]). It is even thought that a brain circuit gets activated in the virgin rat that mediates avoidance behaviour towards the pups [9]. By contrast, a virgin mouse shows spontaneous maternal care and, consequently, would defend the pups if she experienced a suckling stimulus [10]. In support of this, suckling, but not lactation per se, is necessary to initiate and maintain MA in mouse dams [11,12]. In turn, suckling increases OXT release, not only into the blood stream to stimulate the milk ejection reflex but also within distinct brain regions (as shown in rats, [13–15]) and brain OXT is capable of modulating MA (see below). In rats, the presence of the pups immediately after birth is important for the onset of MA [16]. While MA is also significantly decreased after short (2.5 h) pup separation [17], MA can be reinstated by placing cross-foster pups with the mother, which is possible up to 4, but not 8, days after separation [16]. The lack of effect 8 days after separation has been attributed to a significant amount of nipple regression in the mothers, especially because induced nipple growth in ovariectomized and maternally sensitized virgin female rats elicits fighting behaviour [16]. However, the suckling stimulus is not required for the onset or maintenance of MA [18,19]. For example, in rat mothers, removing the nipples by thelectomy does not alter MA [20], whereas ventral trunk anaesthesia does, thereby demonstrating that at least somatosensory stimulation of this region is required to evoke MA [21]. In terms of a translational aspect of such findings, a recent study in humans found that breastfeeding mothers are more verbally aggressive toward an unduly aggressive opponent than formula-feeding mothers or women who had never been pregnant before [22]. This suggests that in humans, the suckling stimulus might be required for initiating heightened aggressive behaviour in response to a threatening situation similar to what has been described in the mouse (see above).
(ii). Developmental stage of the pups affects maternal aggression
Besides these different mechanical stimulations of the mother by the pups, their age and, consequently, the progress of lactation affects MA. This is similar in both mouse and rat mothers. MA is highest in the early lactation phase but rapidly declines to the end of lactation, i.e. three weeks after parturition in most laboratories (figure 1; rat [4,6,23,24]; mouse [25–27]). The reason for this decline in MA is not fully understood. Some studies which cross-foster pups of different ages to rat mothers at various stages of lactation suggest that the decline in MA is not caused by the pups’ physical and behavioural maturation [28], whereas other studies show the opposite [29]. However, it seems that the length of cross-fostering before testing MA is an important factor [29]. Furthermore, younger pups [28,29] are less mobile, and therefore depend more on the mother compared with older pups [30]. Consequently, it has been suggested that the degree of the pups’ independence from the mother may also cause the differences in MA [29]. In conclusion, the development of the pups can indeed trigger the natural decrease of MA.
Figure 1.
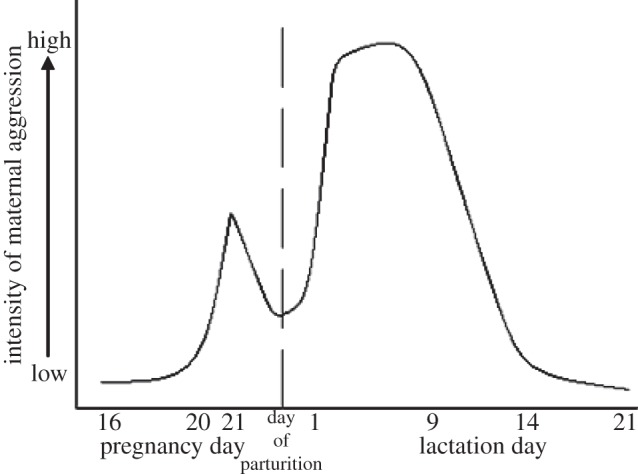
Proposed model of changes in the intensity of maternal aggression from mid-pregnancy to lactation in the rat. The highest level of maternal aggression occurs in early lactation, i.e. the first week postpartum, and declines afterwards until weaning. Adapted from [23]. © Blackwell Publishing Ltd.
(iii). The intruder's age and sex modulate maternal aggression
As the offspring represent the reproductive fitness of the mother, she is eager to fight for their survival. The killing of offspring, i.e. infanticide, is the main threat posed by intruders. Infanticide appears to be a widespread, though not common, phenomenon in mammals. However, the underlying motivations of the intruder seem to be diverse. In 1979, Hrdy [31] proposed five classes of explanation for infanticide in mammals: social pathology, exploitation, resource competition, parental manipulation and sexual selection. The latter seems to be a key factor for pup killing in rodents as it is mainly males that commit infanticide, especially when they are unrelated to the pups [32,33]. With respect to females, virgin intruder rats can be implicated in infanticide upon acute exposure to pups [1,2], whereas in virgin intruder mice this is almost absent. The biological basis for this difference is probably related to the fact that virgin mice, but not rats, display spontaneous maternal behaviour [8].
Apparently, the intruder is a main extrinsic trigger of MA. In fact, the behavioural expression of MA is the sum of the action towards the intruder and the reaction to the intruder's presence and behaviour, which in turn reflect the mother's level of aggression [3,34]. For example, the age and/or size of the intruder directly affect the aggressive behaviour of lactating rat [35,36] but not mouse mothers [24]. Lactating rats fight more against younger than older male intruders [35]. As age and size/weight are related to each other, another study replicated these findings with respect to the weight difference between mother and intruder [36]. Intruders smaller than the mother are bitten by 80% of the residential dams, but larger male intruders only by 30%. The higher weight difference paired with a greater fight-back level in the older intruders might cause the lower aggression level of the mothers [35]. Older male rats also have higher serum testosterone levels, which led to the speculation that an androgen-dependent pheromone could inhibit the mother's attacks [35]. However, the authors also mention an unpublished observation with similar study design; here older rats had higher testosterone levels compared with younger ones, confirming previous findings [35], whereas both male intruder groups elicited similar aggression levels from lactating females. Hence, the effect of testosterone on MA in rats is not clear. In contrast, in mice, significantly reducing testosterone levels in male intruders by removal of their gonads at six weeks of age results in fewer attacks by lactating mothers against those intruders when adults are compared with intact ones [37]. The same study shows that gonadectomized female mice are attacked less often than intact male or female intruders, but with no difference between the latter two groups. Thus, it appears that MA does not depend on the sex of the intruder in mice ([24,37], but also see [38]), whereas in rats male intruders receive less aggression from the lactating mother when compared with female intruders [38] independent of the intruders’ reproductive status [34]. However, while we used virgin female intruders in our MA test paradigm [3,39–42], most other MA studies in rats are conducted with male intruders [5], and therefore more studies are required to investigate that conclusion.
(b). Maternal aggression is triggered by intrinsic factors: impact of anxiety, oxytocin and arginine vasopressin
Dramatic changes occur in the peripartum brain resulting in altered behaviour and emotionality of the mother. Such modifications are essential for her to cope with her new and challenging situation and to show maternal behaviour. Among the intrinsic factors are the mother's decreased innate anxiety and adaptations of her neuropeptide systems. Here, key neuropeptides involved in maternal behaviour and anxiety are OXT and AVP, which show increased activity peripartum on various levels, such as increased mRNA expression and release, as well as increased receptor mRNA expression, receptor density and/or binding (for reviews, see [8,43,44]).
At this point, I briefly introduce the OXT and AVP systems. Both neuropeptides are closely related nonapeptides, which differ from each other in only two out of nine amino acids and which are both implicated in various social behaviours [45–47]. The neuropeptide OXT is synthesized in and released from hypothalamic paraventricular (PVN) and supraoptic nuclei (SON) and their projections. When released into the periphery, OXT has essential reproductive functions in the peripartum period, i.e. promotion of labour and milk ejection. At the same time, OXT is released within the lactating brain, for example in the PVN and SON themselves but also into other brain regions like the septum, the hippocampus or the olfactory bulb ([13–15,48–52], for review see [44]). There is only one known OXT receptor (OXT-R) and it belongs to the G protein-coupled receptor family [53]. With respect to AVP, it is synthesized in the PVN and SON but—different from OXT—also in parts of the limbic system like the bed nucleus of the stria terminalis (BNST) [47]. In the periphery, AVP can act on the release of the stress hormone adrenocorticotropin from the pituitary, as well as having vasoconstrictory and antidiuretic properties. In line with the latter, AVP released within the brain is thought to maintain osmotic homoeostasis, especially in lactation [49,54,55]. In terms of receptors, there are three known types, which all belong to the G protein-coupled receptor family [56]: V1a-R (only found in the brain), V1b-R (brain and periphery) and V2-R (periphery). As the V1a-R is most abundant in the brain, most studies to date have been performed on this receptor type, at least in the maternal brain.
Each of the above mentioned intrinsic factors can modulate MA to varying extents. To begin with, I show that MA is affected by the mother's anxiety level and that it is linked to brain OXT and AVP. Thereafter, I discuss the behavioural impact of these systems on MA.
(i). The mother's innate anxiety affects maternal aggression
One of the peripartum changes associated with lactation is reduced anxiety. This is true not only for mice and rats (for reviews, see [57,58]) but similar findings have also been described in humans. Mothers that breastfeed are less anxious and more often report positive mood states compared with bottle-feeding mothers [59–62]. The reduced anxiety in lactation is likely to be a prerequisite for the mother to be able to take care of her young/offspring. Indeed, neuropeptides such as OXT and AVP, which are directly implicated in anxiety, are also modulators of MA (see below). It is feasible that a mother needs to be less anxious in order to defend the offspring, especially when confronted with a threatening situation such as the presence of an intruder. Indeed, in mice higher levels of MA are accompanied by lower anxiety [63,64]. Similarly, in studies investigating MA in lactating rats, reduced MA is paired with increased anxiety following injections of cocaine [65] or lesions of the periaqueductal grey [66]. But, there are also studies in lactating rats demonstrating the opposite. For example, injections of comparable amounts of diazepam act anxiolytically [67] but also reduce MA [68]. In addition, successful manipulation of one parameter does not necessarily result in a change of another parameter (for review, see [5]). However, especially in rats, the experimental approach of linking MA and anxiety has mainly been performed via pharmacological manipulation, which does not necessarily reflect a causal relationship [58].
We were able to contribute to this field of research by studying our rats bred for high (HAB) versus low anxiety-related behaviour (LAB; for details on selection and breeding, see [69–72]). Importantly, while HAB, but not LAB, rats become less anxious in lactation their inborn difference in anxiety is still apparent [73]. In addition, lactating HAB and LAB rats have very robust differences in maternal care, maternal motivation and MA ([3,41,42,73,74], for review see [58]). The more anxious HAB mothers display more maternal behaviour compared with the less anxious LAB, a finding which we recently replicated in a mouse model for extremes in anxiety [75]. With respect to MA in the rat breeding lines, lactating HAB mothers attack the virgin female intruder more often, with shorter attack latencies and a higher overall level of aggression compared with LABs [3,41,42,58]. This is probably linked to their overall high protective mothering style [58], and we even found that MA is positively correlated with the mothers’ innate anxiety [58]. At first sight, this might sound contrary to findings in lactating rats and mice (see above), as well as to studies on aggression in males, where high anxiety is coupled with low aggression levels ([72], mice: [76], HAB/LAB rats: [77,78]). However, in lactating HAB and LAB mothers, the high and low maternal aggressive behaviour, respectively, is apparently independent of preceding manipulations (surgery or injections). Therefore, the link between high anxiety and high MA is most probably related to the natural situation, especially when compared with pharmacological or lesion studies. In fact, it is reasonable to suggest that lactating mothers have to overcome their innate anxiety in order to defend the offspring against the potentially dangerous intruder.
One of the underlying mechanisms of reduced maternal anxiety during the display of MA is probably the brain OXT system. In the peripartum period, the activity of the OXT system is upregulated (see above) and anxiety is reduced. Indeed, OXT acts as an anxiolytic independent of sex or reproductive status in rodents ([74,79–84]; but see Campbell [85]). In females, continuous infusion of OXT into the lateral ventricle via osmotic minipumps reduces anxiety in lactating rats [74] as well as in virgin female rats non-selected for anxiety [86]. Furthermore, the same treatment/application method leads to decreased anxiety in virgin HAB rats, whereas chronic administration of a selective OXT-R antagonist (OXT-A) [87] results in increased anxiety in virgin LAB rats [88]. Regarding MA in HAB mothers, a high amount of OXT is released especially in the PVN and the central amygdala (CeA) during the display of MA (for details see below) [3]. In fact, high levels of OXT in the PVN [80,84,89] and/or the CeA [90–92] are known to have anxiolytic properties. Interestingly, only 10 min after ending the confrontation with an intruder, the HAB mothers’ OXT release within either of the brain areas and their anxiety are back to a HAB-typical level [3]. Such a mechanism, which is based on the brain adaptations peripartum, can serve to enable even highly anxious mothers to react adequately to a dangerous situation.
It is important to note that HAB and LAB rats are bred for extremes in anxiety over several years [72], and therefore also differ genetically because breeding for a trait results in homozygosity at loci conferring that trait/behaviour. In HAB rats, the naturally suppressed transcription of the AVP gene is disinhibited owing to a single nucleotide polymorphism in the promoter of this gene [93]. As AVP is anxiogenic independent of sex or physiological status [47,74,94–96], the upregulated expression and release of brain AVP in HAB rats is, consequently, the basis of their hyperanxiety, as has been shown in males [93,96,97] and in lactating females [74]. It is not surprising that the upregulated brain AVP activity is also part of the underlying neuroendocrinological mechanism for the high levels of maternal behaviour in HAB mothers [58]. For example, a single acute intracerebroventricular (icv) infusion of a selective V1a-R antagonist (V1a-A) [98] in HAB mothers decreases maternal behaviour as well as anxiety, whereas chronic infusion of synthetic AVP increases both parameters [74]; these effects were shown to be mediated via V1a-R. Importantly, in the same study we demonstrate that these findings are transferable to Wistar rats non-selected for anxiety; the same treatments have the exact same effects on maternal behaviour and anxiety. At this point, when comparing the emotional and maternal roles of AVP and OXT, it becomes clear that both neuropeptides influence maternal behaviour in the same direction but have opposite effects on anxiety. The role of anxiety as a potential modulator of maternal care and aggression may sound controversial in light of what was stated above. However, one has to keep in mind that different brain areas often have different roles in terms of emotionality and/or behaviour. In a natural situation, i.e. a non-manipulated brain, neuropeptides can be released differentially in various brain regions involved in MA (but also maternal care) or anxiety [58]. In the laboratory, such a condition can be mimicked by manipulating the neuropeptide systems in a region-specific fashion, which enables dissection of their particular role in either of these behavioural or emotional parameters. Another approach is to manipulate the OXT and AVP systems via icv injections, which results in the drugs spreading throughout almost all of the brain and thus acting on a vast number of brain areas simultaneously. However, in the latter paradigm, the resulting behavioural phenotype is the integration of the drugs’ effects in several brain areas. While this does not allow a definite conclusion on the specific role of (in this case) a neuropeptide system, it can serve as an initial approach. In the following section, I show how various experimental approaches, from knock-out models and selective breeding lines to pharmacological manipulations, helped us to understand how OXT and AVP impact on MA.
(ii). The brain oxytocin system mediates maternal aggression
While the initial experiments on the role of OXT in maternal care date back to the late 1970s and early 1980s [99,100], it took almost 20 more years until the first study on OXT as a modulator of MA was published. The current knowledge on OXT-mediated MA, as well as the role of relevant brain regions is summarized in detail in table 1.
Table 1.
Brain oxytocin as a regulator of maternal aggression in rats and mice. HAB/LAB, Wistar rat of the breeding line for high/low anxiety-related behaviour; MA, maternal aggression; OXT, oxytocin; OXT-A, oxytocin receptor antagonist; OXT-AS, oxytocin antisense; OXT−/−, oxytocin receptor knock-out; SD, Sprague–Dawley; ---, not different to control; ↑, increase compared with control; ↓, decrease compared with control (control refers to wild-type mice (OXT−/−) or vehicle-treated animals).
| species | brain region | strain | OXT release during MA | condition/treatment | change in MA |
|---|---|---|---|---|---|
| mouse | C57BL/6 | OXT−/− | ↓ [101] | ||
| rat | lateral ventricle | Wistar | OXT-A | --- [34] | |
| HAB | OXT-A | ↓ [44] | |||
| LAB | OXT | ↑ [44] | |||
| paraventricular nucleus | Wistar | OXT-AS | ↑ [102] | ||
| Wistar | ↑ [40] | ||||
| HAB | ↑ [3] | OXT-A | ↓ [3] | ||
| LAB | ↓ [3] | OXT | ↑ [3] | ||
| central amygdala | Wistar | --- [40] | |||
| HAB | ↑ [3] | OXT-A | ↓ [3] | ||
| LAB | ↑ [3] | OXT-A | --- [3] | ||
| SD | OXT-A | ↑ [103] | |||
| bed nucleus of the stria terminalis | Wistar | OXT | ↓ [104] | ||
| SD | ↑ [39] | ||||
| lateral septum | Wistar | --- [40] | |||
| SD | ↑ [39] |
The first study in this line of research was in OXT-knock-out mice [101], which do not have detectable levels of OXT in the PVN or SON. Homozygous OXT-knock-out mice show reduced aggressive behaviour towards an intruder when compared with the wild-type line. This result is not surprising given the fact that OXT also promotes maternal care and—together with MA—serves to secure the proper and safe development of the offspring. However, the previous finding could not be confirmed by a subsequent pharmacological study in lactating Wistar rats [34]; acute blockade of OXT-R by icv injection of OXT-A does not alter any parameter of MA. By contrast, we recently showed in the highly aggressive HAB mothers that the exact same treatment decreases MA as seen in a reduced number of attacks when compared with vehicle-treated HAB mothers [44]. Furthermore, confirming a behavioural role for the OXT system in MA, in the less aggressive LAB mothers a chronic 5-day infusion of OXT into the lateral ventricle via osmotic minipumps increases the number of attacks towards the intruder, the frequency of lateral threats and overall MA [44]. With respect to these findings, it is likely that the difference in MA in the HAB and LAB breeding lines is based on line-dependent differences in the availability of endogenous OXT [44]. Furthermore, because the results from the study in HAB and LAB rats (but also from the OXT-knock-out mice) are contrary to the findings in lactating Wistar rats, this demonstrates that the experimental approach of icv injection does not allow definite conclusions to be drawn per se as discussed above.
(iii). Brain regions involved in oxytocin-mediated maternal aggression
In addition to these icv, and thus, global studies, there are various region-targeted approaches helping us to learn more about the role of brain OXT in MA. One experimental approach is locally implanted microdialysis probes, which allow researchers to sample, for example, the neuropeptides OXT and AVP in the extracellular space from a certain brain region of freely moving and behaving rats (for review, see [105]) with no detectable behavioural interference [40]. Subsequently, the amount of neuropeptide in each sample can be measured, e.g. by radioimmunoassay [106]. Using these techniques, we were able to show that the release of OXT within the hypothalamic PVN increases during the display of MA in lactating Wistar rats [40]. In confirmation, in the more aggressive HAB mothers, OXT release increases during the maternal aggressive encounter, whereas in the less aggressive LAB mothers the release of OXT decreases even below basal levels [3]. A further indication of a link between intra-PVN OXT release and the amount of MA is the finding that both parameters are positively correlated, independent of the breeding line [3]. Furthermore, as lactating HAB and LAB rats do not differ in OXT-R mRNA expression, the OXT release pattern is thought to be responsible for the line-specific differences in MA [3]. To further demonstrate the role of OXT in MA, we altered the expression levels of this behaviour by locally manipulating OXT-R in the PVN using the technique of retrodialysis. Here, the microdialysis probe is implanted bilaterally into the target region and flushed with artificial cerebrospinal fluid enriched with the drug of interest, e.g. selective receptor agonist or antagonist, while exposing the lactating rat to the intruder. In fact, blocking OXT-R with a selective OXT-A reduces the overall offensive behaviour towards the intruder in the initially more aggressive HAB mothers, whereas in LABs bilateral infusion of synthetic OXT tends to increase their lateral threats [3]. In confirmation of our results on the role of the PVN (and hence the local OXT system) in MA, an earlier study shows that bilateral electrolytic lesion of the PVN reduces the frequency and duration of attacks towards a male intruder [107]. By contrast, the same group showed that ibotenic acid lesions, as well as acute inhibition of local OXT synthesis via antisense oligonucleotide infusion within the parvocellular region of the PVN, increase the biting frequency of the lactating mother towards a male intruder [102]. Hence, the authors speculated that the oxytocinergic parvocellular neurons of the PVN exert an inhibitory effect on MA [102]. It is important to note that, although both the parvocellular and the magnocellular part of the PVN consist of OXT-synthesizing neurons, it is likely that the majority of OXT released within the PVN derives from dendrites of magnocellular neurons [108].
In addition to the hypothalamic PVN, OXT is also released in the limbic CeA, BNST and lateral septum (LS) during the display of MA as measured by local microdialysis. Here, the CeA seems to be a highly important brain region, because the local increase of available OXT has anxiolytic properties (for more details, see above [90–92]). This is necessary to enable the mother to react adequately to the threatening situation, i.e. defending the offspring against the intruder. In support, not only is OXT-R mRNA expression increased in lactation in the CeA [3]—probably a prerequisite for the reduced anxiety at that reproductive stage—but furthermore the release of OXT within this region increases during the display of MA in both HAB and LAB mothers [3]. The release is more pronounced in the initially more anxious HAB mothers compared with the less anxious LABs. Additionally, OXT release during exposure to a virgin female intruder positively correlates with the amount of aggressive behaviour displayed. Hence, HAB mothers defeat the intruder more effectively than LAB mothers do. In detail, intruders exposed to HAB mothers show more freezing and defensive behaviours, as well as elevated anxiety 10 min after ending the MA test compared with intruders of LAB mothers. Supporting the relevance of locally released OXT for MA, bilateral retrodialysis of a selective OXT-A decreases the number of attacks and overall offensive behaviour in HAB, but not LAB, mothers [3]. Our findings in rats are supported by a study in lactating hamsters, where repeated administration of OXT in the CeA enhances aggression towards a male intruder [109]. On the other hand, it should be noted that a study in rats found the opposite relationship between intra-CeA OXT and MA [103]; infusion of a selective OXT-A into the CeA of lactating Sprague–Dawley (SD) rats 4 h before exposure to a male intruder increases the mothers’ attack frequency. Owing to the design of this study, it is possible that a local rebound effect has occurred at the time MA was tested. In detail, while initially aiming to block OXT-R, the delay between treatment injection and behavioural testing might have caused increased OXT release [44,110], which is known to enhance MA. The second explanation for the opposing results might be the use of Wistar rats (including HAB and LAB, which originate from the Wistar strain [3]) as opposed to SD rats [103]. Such a strain-related effect has already been discussed in the context of intermale aggression [46].
The BNST and the LS are further limbic brain regions which might be involved in MA. In both brain regions, OXT-R binding is not only generally increased in lactation (BNST [111,112]; LS [113]), but also is the highest at the time when MA peaks, i.e. early lactation (figure 1; [23]). At this time point, MA is even positively correlated with OXT-R binding within the LS [23]. In addition, neuronal activity within the LS, as well as in the BNST, is heightened in response to MA [114]. Indeed, we found increased local OXT release during MA in the BNST and the LS of lactating SD rats using local microdialysis [39]. However, other studies do not support a facilitating role for, or even involvement of, OXT in maternal aggressive behaviour within these brain regions. For example, local infusion of synthetic OXT into the BNST has been shown to decrease the biting frequency of lactating Wistar rats towards a male intruder [104]. With respect to the LS, we did not detect any changes in local OXT release during the display of MA compared with pretest (basal) levels in lactating Wistar rats [40], in contrast to the findings in SD rats. Therefore, further studies are needed for a definite conclusion on how these brain regions might contribute to the behavioural expression of MA, e.g. pharmacological approaches or a more precise examination of the subregions of the BNST and the LS.
Besides a direct impact on MA, OXT can also promote this behaviour indirectly by affecting amino acid release, e.g. γ-aminobutyric acid and glutamate [42], or by its inhibiting effect on corticotropin-releasing factor (CRF)-containing neurons. With respect to the latter, activation of the CRF system decreases MA in lactating rats [115] and mice [116]. However, increased CRF mRNA expression in the PVN following stress exposure is attenuated by chronic icv infusion of OXT in ovariectomized virgin female rats [117], thereby counteracting the activation of the CRF system. Furthermore, an acute infusion of OXT into the PVN of virgin female [84] and male [80] rats acts anxiolytically, which might enable the initially highly anxious HAB mothers to overcome their innate fear and to react adequately to the challenging situation similar to its proposed effects in the CeA (see above).
(iv). The brain arginine vasopressin system mediates maternal aggression
Almost concurrent with OXT, the neuropeptide AVP was implicated in maternal care in the early 1980s [99]. However, regarding involvement of AVP in MA, Lonstein and Gammie in 2002 noted that ‘it is surprising … that no work has directly examined a link between vasopressin and MA in any species’ [5, p. 879]. Only a few years later, the first studies were published linking the brain AVP system to MA in lactating mice [118,119] and rats [120]. A detailed summary of our present knowledge on AVP-mediated MA and the role of relevant brain regions in mice and rats can be found in table 2.
Table 2.
Brain vasopressin as a regulator of maternal aggression in rats and mice. AVP, arginine vasopressin; HAB/LAB, Wistar rat of the breeding line for high/low anxiety-related behaviour; MA, maternal aggression; SD, Sprague–Dawley; V1a-A, V1a receptor antagonist; V1a−/−, V1a receptor knock-out; V1b−/−, V1b receptor knock-out; ---, not different to control; ↑, increase compared with control; ↓, decrease compared with control (control refers to wild-type mice (V1a−/−, V1b−/−) or vehicle-treated animals).
| species | brain region | strain | AVP release during MA | condition/treatment | change in MA |
|---|---|---|---|---|---|
| mouse | C57BL/6J | V1a−/− | --- [118] | ||
| V1b−/− | ↓ [119] | ||||
| rat | lateral ventricle | HAB | V1a-A | ↓ [41] | |
| LAB | AVP | ↑ [41] | |||
| SD | V1a-A | ↑ [121,122] | |||
| SD | AVP | ↓ [121,122] | |||
| paraventricular nucleus | HAB | --- [41] | |||
| LAB | --- [41] | ||||
| SD | ↑ [39] | ||||
| central amygdala | HAB | ↑ [41,120] | V1a-A | ↓ [41,120] | |
| LAB | --- [41,120] | AVP | ↑ [41,120] | ||
| bed nucleus of the stria terminalis | Wistar | V1a-A | ↓ [111] | ||
| SD | ↑ [39] | ||||
| lateral septum | SD | ↑ [39] |
To date, the only studies in lactating mice investigating an involvement of brain AVP in MA were performed in V1a-R- and V1b-R-knock-out lines. In V1a-R-knock-out mice, MA is not different from the behaviour in wild-type mice [118]. The authors interpreted their results in two ways; either V1a-R is not critical for MA or due to the lifelong knock-out of V1a-R other neural circuitries underlying MA have compensated for the loss. In fact, the latter might be the case when taking the findings in lactating rats into account (see below). With respect to lactating mice of the V1b-R- knock-out line, they show a significantly longer attack latency, as well as a lower number of attacks, compared with wild-type mothers [119], making this receptor type an interesting target for MA. In support, male V1b-R-knock-out mice also have impaired attack behaviour towards a conspecific [123]. Together with the fact that V1b-R are expressed in brain regions related to social behaviour [124], this finding suggests that this receptor might generally be involved in modulating aggressive behaviour. However, so far no further studies have investigated V1b-R-mediated MA in mice or rats.
To study AVP-mediated MA in lactating rats, the HAB and LAB breeding lines are particularly useful animal models. The more maternally aggressive HAB rats also have genetically determined higher AVP synthesis and release (for details see above), although V1a-R binding is similar between the breeding lines, at least in the PVN and CeA [41]. In lactating HAB rats, blocking V1a-R by acute icv injection of V1a-A reduces the number of attacks and overall offensive behaviour [41]. Accordingly, in LAB mothers, increasing the available AVP by chronic infusion of the synthetic agonist over 5 days increases these aggressive parameters. These findings are in line with studies in male rodents where AVP facilitates aggression (for review, see [54]). On the other hand, studies in lactating SD rats indicate the opposite role of AVP [121,122]. At first sight, the use of different rat strains (Wistar versus SD) and/or breeding lines (HAB/LAB) might be a key factor contributing to the opposing results (also see above [78]). However, we recently found that, in lactating SD rats, local AVP release is enhanced within distinct brain regions in response to MA (for details see below [39]). Thus, the different experimental approaches, i.e. icv versus local, are more likely to be responsible for the different results (see above [44]).
(v). Brain regions involved in arginine vasopressin-mediated maternal aggression
To reveal whether locally released AVP within the PVN is involved in the behavioural expression of MA, we performed microdialysis in lactating HAB and LAB rats [41], as well as in lactating SD rats [39]. Interestingly, lactating SD rats show high levels of MA comparable with lactating HAB rats (O. J. Bosch and S. M. Klampfl 2011, unpublished data). However, while the AVP levels within the PVN are unaltered during MA compared with pretest levels in HAB and LAB mothers, in lactating SD rats the release of AVP is increased. Whether such local AVP release in fact contributes to the display of MA requires further studies involving pharmacological manipulations, e.g. injection of a selective receptor antagonist and subsequent behavioural observation. It is also noteworthy that in male rats AVP levels within the PVN increase in response to social defeat, a social and stressful stimulus [125]. Similarly, a lactating mother's exposure to MA is also stressful as seen in the elevated plasma levels of stress hormones not only in lactating Wistar rats [34] but also in lactating HAB and LAB rats [126]. Therefore, it is surprising that in HAB and LAB mothers, we did not detect changes in AVP release in response to a maternal aggressive encounter.
Another brain region importantly involved in mediating MA is the CeA, as has been shown already for the OXT system (see above). In fact, the findings for OXT and AVP are similar. By using intracerebral microdialysis in our animal models for extremes in anxiety, we found that the high levels of MA in lactating HAB mothers are accompanied by increased release of AVP within the CeA, whereas it remains unchanged in LAB mothers [41,120]. Furthermore, locally released AVP in the CeA is positively correlated with the amount of MA displayed, independent of innate anxiety. In support, blocking V1a-R by retrodialysis of V1a-A into the CeA of lactating HAB mothers decreases MA (number of attacks as well as the overall aggressive behaviour). Furthermore, retrodialysis of synthetic AVP into the CeA of the initially less aggressive LAB mothers makes them more aggressive. Hence, the release of AVP within the CeA appears to be an important trigger of MA. Interestingly, the display of MA and/or defeat of the intruder as a stressful event for the lactating mother [34,126] are thought to be emotionally evaluated by the amygdala causing acute adaptations at behavioural and neuroendocrine levels [34,127–129].
Two further brain regions have been investigated regarding AVP-mediated MA, namely the BNST and the LS. In both these regions, V1a-R binding is increased in early lactation (LS: [23], BNST: [111]). Within the LS, V1a-R binding even positively correlates with the display of aggressive behaviour [23]. Regarding the release of AVP, we recently found that in both the BNST and the LS local AVP release increases during a maternal aggressive encounter in lactating SD rats [39]. In support of a behavioural function of intra-BNST AVP release, acute bilateral infusion of V1a-A decreases MA in lactating Wistar rats [111]. In more detail, the number and duration of attacks is decreased, and consequently the latency until the first attack is prolonged as well as the overall aggressive behaviour being decreased. A similar study for the LS establishing the relevance of locally released AVP for MA has not yet been performed. However, in male rats, local AVP release within the BNST and the LS has been correlated negatively and positively, respectively, with the display of intermale aggression [130].
3. Conclusion
The extent to which the mother displays MA is triggered by both extrinsic and intrinsic factors. Here, brain OXT and AVP systems within hypothalamic and limbic brain areas are key factors regulating/modulating MA as well as anxiety. In fact, the level of the mother's innate anxiety correlates with the release of these neuropeptides in various brain regions, which can be triggered also by extrinsic factors. Hence, the regulation of MA in rodents is complex and depends on various factors. This ensures that the mother can evaluate the situation appropriately and react accordingly when confronted with an intruder.
Acknowledgements
The author thanks Prof. Inga Neumann for her continuous support, Prof. Rainer Landgraf (RIAgnosis) for the radioimmunoassays, Prof. Maurice Manning for providing the selective receptor antagonists and Dr Daniela Beiderbeck for the reliable organization of the HAB/LAB breeding. Furthermore, I am grateful to Dr Simone Meddle for collaborations over the years, and to Prof. Anne Campbell and Dr David Slattery for their critical comments on the manuscript.
Funding statement
Supported by the DFG (BO 1958/6-1).
References
- 1.Peters LC, Sist TC, Kristal MB. 1991. Maintenance and decline of the suppression of infanticide in mother rats. Physiol. Behav. 50, 451–456 (doi:10.1016/0031-9384(91)90093-4) [DOI] [PubMed] [Google Scholar]
- 2.Peters LC, Kristal MB. 1983. Suppression of infanticide in mother rats. J. Comp. Psychol. 97, 167–177 (doi:10.1037/0735-7036.97.2.167) [PubMed] [Google Scholar]
- 3.Bosch OJ, Meddle SL, Beiderbeck DI, Douglas AJ, Neumann ID. 2005. Brain oxytocin correlates with maternal aggression: link to anxiety. J. Neurosci. 25, 6807–6815 (doi:10.1523/JNEUROSCI.1342-05.2005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Flannelly KJ, Flannelly L. 1987. Time course of postpartum aggression in rats (Rattus norvegicus) . J. Comp. Physiol. Psychol. 101, 101–103 (doi:10.1037/0735-7036.101.1.101) [Google Scholar]
- 5.Lonstein JS, Gammie SC. 2002. Sensory, hormonal, and neural control of maternal aggression in laboratory rodents. Neurosci. Biobehav. Rev. 26, 869–888 (doi:10.1016/S0149-7634(02)00087-8) [DOI] [PubMed] [Google Scholar]
- 6.Erskine MS, Barfield RJ, Goldman BD. 1978. Intraspecific fighting during late pregnancy and lactation in rats and effects of litter removal. Behav. Biol. 23, 206–218 (doi:10.1016/S0091-6773(78)91814-X) [DOI] [PubMed] [Google Scholar]
- 7.O'Connell LA, Hofmann HA. 2012. Evolution of a vertebrate social decision-making network. Science 336, 1154–1157 (doi:10.1126/science.1218889) [DOI] [PubMed] [Google Scholar]
- 8.Numan M, Insel TR. 2003. The neurobiology of parental behaviour. New York, NY: Springer [Google Scholar]
- 9.Rosenblatt JS, Mayer AD. 1995. An analysis of approach/withdrawal processes in the initiation of maternal behavior in the laboratory rat. In Handbook of behavioral neurobiology (eds Hood KE, Greenberg G, Tobach E.), pp. 229–298 New York, NY: Plenum Press [Google Scholar]
- 10.Svare B, Gandelman R. 1976. Suckling stimulation induces aggression in virgin female mice. Nature 260, 606–608 (doi:10.1038/260606a0) [DOI] [PubMed] [Google Scholar]
- 11.Svare B, Mann M, Samuels O. 1980. Mice: suckling stimulation but not lactation important for maternal aggression. Behav. Neural. Biol. 29, 453–462 (doi:10.1016/S0163-1047(80)92654-0) [DOI] [PubMed] [Google Scholar]
- 12.Svare B, Gandelman R. 1976. Postpartum aggression in mice: the influence of suckling stimulation. Horm. Behav. 7, 407–416 (doi:10.1016/0018-506X(76)90012-X) [DOI] [PubMed] [Google Scholar]
- 13.Neumann I, Ludwig M, Engelmann M, Pittman QJ, Landgraf R. 1993. Simultaneous microdialysis in blood and brain: oxytocin and vasopressin release in response to central and peripheral osmotic stimulation and suckling in the rat. Neuroendocrinology 58, 637–645 (doi:10.1159/000126604) [DOI] [PubMed] [Google Scholar]
- 14.Neumann I, Landgraf R. 1989. Septal and hippocampal release of oxytocin, but not vasopressin, in the conscious lactating rat during suckling. J. Neuroendocrinol. 1, 305–308 (doi:10.1111/j.1365-2826.1989.tb00120.x) [DOI] [PubMed] [Google Scholar]
- 15.Neumann I, Russell JA, Landgraf R. 1993. Oxytocin and vasopressin release within the supraoptic and paraventricular nuclei of pregnant, parturient and lactating rats: a microdialysis study. Neuroscience 53, 65–75 (doi:10.1016/0306-4522(93)90285-N) [DOI] [PubMed] [Google Scholar]
- 16.Gandelman R, Simon NG. 1980. Postpartum fighting in the rat: nipple development and the presence of young. Behav. Neural. Biol. 28, 350–360 (doi:10.1016/S0163-1047(80)92357-2) [DOI] [PubMed] [Google Scholar]
- 17.Deschamps S, Woodside B, Walker CD. 2003. Pups presence eliminates the stress hyporesponsiveness of early lactating females to a psychological stress representing a threat to the pups. J. Neuroendocrinol. 15, 486–497 (doi:10.1046/j.1365-2826.2003.01022.x) [DOI] [PubMed] [Google Scholar]
- 18.Bridges RS. 1975. Long-term effects of pregnancy and parturition upon maternal responsiveness in the rat. Physiol. Behav. 14, 245–249 (doi:10.1016/0031-9384(75)90028-1) [DOI] [PubMed] [Google Scholar]
- 19.Moltz H, Geller D, Levin R. 1967. Maternal behavior in the totally mammectomized rat. J. Comp. Physiol. Psychol. 64, 225–229 (doi:10.1037/h0088025) [DOI] [PubMed] [Google Scholar]
- 20.Mayer AD, Carter L, Jorge WA, Mota MJ, Tannu S, Rosenblatt JS. 1987. Mammary stimulation and maternal aggression in rodents: thelectomy fails to reduce pre- or postpartum aggression in rats. Horm. Behav. 21, 501–510 (doi:10.1016/0018-506X(87)90008-0) [DOI] [PubMed] [Google Scholar]
- 21.Stern JM, Kolunie JM. 1993. Maternal aggression of rats is impaired by cutaneous anesthesia of the ventral trunk, but not by nipple removal. Physiol. Behav. 54, 861–868 (doi:10.1016/0031-9384(93)90293-O) [DOI] [PubMed] [Google Scholar]
- 22.Hahn-Holbrook J, Holt-Lunstad J, Holbrook C, Coyne SM, Lawson ET. 2011. Maternal defense: breast feeding increases aggression by reducing stress. Psychol. Sci. 22, 1288–1295 (doi:10.1177/0956797611420729) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Caughey SD, Klampfl SM, Bishop VR, Pfoertsch J, Neumann ID, Bosch OJ, Meddle SL. 2011. Changes in the intensity of maternal aggression and central oxytocin and vasopressin V1a receptors across the peripartum period in the rat. J. Neuroendocrinol. 23, 1113–1124 (doi:10.1111/j.1365-2826.2011.02224.x) [DOI] [PubMed] [Google Scholar]
- 24.Svare B, Betteridge C, Katz D, Samuels O. 1981. Some situational and experiential determinants of maternal aggression in mice. Physiol. Behav. 26, 253–258 (doi:10.1016/0031-9384(81)90020-2) [DOI] [PubMed] [Google Scholar]
- 25.Gandelman R. 1972. Mice: postpartum aggression elicited by the presence of an intruder. Horm. Behav. 3, 23–28 (doi:10.1016/0018-506X(72)90003-7) [DOI] [PubMed] [Google Scholar]
- 26.St John RD, Corning PA. 1973. Maternal aggression in mice. Behav. Biol. 9, 635–639 (doi:10.1016/S0091-6773(73)80058-6) [DOI] [PubMed] [Google Scholar]
- 27.Svare B, Gandelman R. 1976. A longitudinal analysis of maternal aggression in Rockland–Swiss albino mice. Dev. Psychobiol. 9, 437–446 (doi:10.1002/dev.420090506) [DOI] [PubMed] [Google Scholar]
- 28.Albert DJ, Walsh ML. 1995. Aggression in the lactating female rat: the normal decline is not dependent on the physical development of the pups. Physiol. Behav. 58, 477–481 (doi:10.1016/0031-9384(95)00033-F) [DOI] [PubMed] [Google Scholar]
- 29.Giovenardi M, Consiglio AR, Barros HM, Lucion AB. 2000. Pup age and aggressive behavior in lactating rats. Braz. J. Med. Biol. Res. 33, 1083–1088 (doi:10.1590/S0100-879X2000000900015) [DOI] [PubMed] [Google Scholar]
- 30.Deviterne D, Desor D, Krafft B. 1990. Maternal behavior variations and adaptations, and pup development within litters of various sizes in Wistar rat. Dev. Psychobiol. 23, 349–360 (doi:10.1002/dev.420230406) [DOI] [PubMed] [Google Scholar]
- 31.Hrdy SB. 1979. Infanticide among animals: a review, classification, and examination of the implications for the reproductive strategies of females. Ethol. Sociobiol. 1, 13–40 (doi:10.1016/0162-3095(79)90004-9) [Google Scholar]
- 32.Jakubowski M, Terkel J. 1985. Transition from pup killing to parental behavior in male and virgin female albino rats. Physiol. Behav. 34, 683–686 (doi:10.1016/0031-9384(85)90364-6) [DOI] [PubMed] [Google Scholar]
- 33.Huck UW, Soltis RL, Coopersmith CB. 1982. Infanticide in male laboratory mice: effects of social status, prior sexual experience, and basis for discrimination between related and unrelated young. Anim. Behav. 30, 1158–1165 (doi:10.1016/S0003-3472(82)80206-6) [Google Scholar]
- 34.Neumann ID, Toschi N, Ohl F, Torner L, Kromer SA. 2001. Maternal defence as an emotional stressor in female rats: correlation of neuroendocrine and behavioural parameters and involvement of brain oxytocin. Eur. J. Neurosci. 13, 1016–1024 (doi:10.1046/j.0953-816x.2001.01460.x) [DOI] [PubMed] [Google Scholar]
- 35.Erskine MS, Denenberg VH, Goldman BD. 1978. Aggression in the lactating rat: effects of intruder age and test arena. Behav. Biol. 23, 52–66 (doi:10.1016/S0091-6773(78)91148-3) [DOI] [PubMed] [Google Scholar]
- 36.Flannelly KJ, Flannelly L. 1985. Opponents’ size influences maternal aggression. Psychol. Rep. 57, 883–886 (doi:10.2466/pr0.1985.57.3.883) [DOI] [PubMed] [Google Scholar]
- 37.Rosenson LM, Asheroff AK. 1975. Maternal aggression in CD-l mice: influence of the hormonal condition of the intruder. Behav. Biol. 15, 219–224 (doi:10.1016/S0091-6773(75)91603-X) [DOI] [PubMed] [Google Scholar]
- 38.Haney M, DeBold JF, Miczek KA. 1989. Maternal aggression in mice and rats towards male and female conspecifics. Aggress. Behav. 15, 443–453 (doi:10.1002/1098-2337(1989)15:6<443::AID-AB2480150605>3.0.CO;2-U) [Google Scholar]
- 39.Bosch OJ, Klampfl SM, Caughey SD, Meddle SL. In preparation. Hypothalamic and limbic vasopressin and oxytocin release facilitates maternal aggression in the lactating rat.
- 40.Bosch OJ, Kromer SA, Brunton PJ, Neumann ID. 2004. Release of oxytocin in the hypothalamic paraventricular nucleus, but not central amygdala or lateral septum in lactating residents and virgin intruders during maternal defence. Neuroscience 124, 439–448 (doi:10.1016/j.neuroscience.2003.11.028) [DOI] [PubMed] [Google Scholar]
- 41.Bosch OJ, Neumann ID. 2010. Vasopressin released within the central amygdala promotes maternal aggression. Eur. J. Neurosci. 31, 883–891 (doi:10.1111/j.1460-9568.2010.07115.x) [DOI] [PubMed] [Google Scholar]
- 42.Bosch OJ, Sartori SB, Singewald N, Neumann ID. 2007. Extracellular amino acid levels in the paraventricular nucleus and the central amygdala in high- and low-anxiety dams rats during maternal aggression: regulation by oxytocin. Stress 10, 261–270 (doi:10.1080/10253890701223197) [DOI] [PubMed] [Google Scholar]
- 43.Neumann ID. 2009. The advantage of social living: brain neuropeptides mediate the beneficial consequences of sex and motherhood. Front. Neuroendocrinol. 30, 483–496 (doi:10.1016/j.yfrne.2009.04.012) [DOI] [PubMed] [Google Scholar]
- 44.Bosch OJ, Neumann ID. 2012. Both oxytocin and vasopressin are mediators of maternal care and aggression in rodents: from central release to sites of action. Horm. Behav. 61, 293–303 (doi:10.1016/j.yhbeh.2011.11.002) [DOI] [PubMed] [Google Scholar]
- 45.Goodson JL. 2008. Nonapeptides and the evolutionary patterning of sociality. Prog. Brain Res. 170, 3–15 (doi:10.1016/S0079-6123(08)00401-9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Veenema AH, Neumann ID. 2008. Central vasopressin and oxytocin release: regulation of complex social behaviours. Prog. Brain Res. 170, 261–276 (doi:10.1016/S0079-6123(08)00422-6) [DOI] [PubMed] [Google Scholar]
- 47.Neumann ID, Landgraf R. 2012. Balance of brain oxytocin and vasopressin: implications for anxiety, depression, and social behaviors. Trends Neurosci. 35, 649–659 (doi:10.1016/j.tins.2012.08.004) [DOI] [PubMed] [Google Scholar]
- 48.Kendrick KM, Keverne EB, Chapman C, Baldwin BA. 1988. Intracranial dialysis measurement of oxytocin, monoamine and uric acid release from the olfactory bulb and substantia nigra of sheep during parturition, suckling, separation from lambs and eating. Brain Res. 439, 1–10 (doi:10.1016/0006-8993(88)91455-2) [DOI] [PubMed] [Google Scholar]
- 49.Landgraf R, Neumann I, Pittman QJ. 1991. Septal and hippocampal release of vasopressin and oxytocin during late pregnancy and parturition in the rat. Neuroendocrinology 54, 378–383 (doi:10.1159/000125917) [DOI] [PubMed] [Google Scholar]
- 50.Moos F, Freund-Mercier MJ, Guerne Y, Guerne JM, Stoeckel ME, Richard P. 1984. Release of oxytocin and vasopressin by magnocellular nuclei in vitro: specific facilitatory effect of oxytocin on its own release. J. Endocrinol. 102, 63–72 (doi:10.1677/joe.0.1020063) [DOI] [PubMed] [Google Scholar]
- 51.Moos F, Ingram CD, Wakerley JB, Guerne Y, Freund-Mercier MJ, Richard P. 1991. Oxytocin in the bed nucleus of the stria terminalis and lateral septum facilitates bursting of hypothalamic oxytocin neurons in suckled rats. J. Neuroendocrinol. 3, 163–171 (doi:10.1111/j.1365-2826.1991.tb00259.x) [DOI] [PubMed] [Google Scholar]
- 52.Moos F, Poulain DA, Rodriguez F, Guerne Y, Vincent JD, Richard P. 1989. Release of oxytocin within the supraoptic nucleus during the milk ejection reflex in rats. Exp. Brain Res. 76, 593–602 (doi:10.1007/BF00248916) [DOI] [PubMed] [Google Scholar]
- 53.van den Burg EH, Neumann ID. 2011. Bridging the gap between GPCR activation and behaviour: oxytocin and prolactin signalling in the hypothalamus. J. Mol. Neurosci. 43, 200–208 (doi:10.1007/s12031-010-9452-8) [DOI] [PubMed] [Google Scholar]
- 54.Caldwell HK, Lee HJ, Macbeth AH, Young WS., 3rd 2008. Vasopressin: behavioral roles of an ‘original’ neuropeptide. Prog. Neurobiol. 84, 1–24 (doi:10.1016/j.pneurobio.2007.10.007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Walker CD, Toufexis DJ, Burlet A. 2001. Hypothalamic and limbic expression of CRF and vasopressin during lactation: implications for the control of ACTH secretion and stress hyporesponsiveness. Prog. Brain Res. 133, 99–110 (doi:10.1016/S0079-6123(01)33008-X) [DOI] [PubMed] [Google Scholar]
- 56.Stoop R. 2012. Neuromodulation by oxytocin and vasopressin. Neuron 76, 142–159 (doi:10.1016/j.neuron.2012.09.025) [DOI] [PubMed] [Google Scholar]
- 57.Lonstein JS. 2007. Regulation of anxiety during the postpartum period. Front. Neuroendocrinol. 28, 115–141 (doi:10.1016/j.yfrne.2007.05.002) [DOI] [PubMed] [Google Scholar]
- 58.Bosch OJ. 2011. Maternal nurturing is dependent on her innate anxiety: the behavioral roles of brain oxytocin and vasopressin. Horm. Behav. 59, 202–212 (doi:10.1016/j.yhbeh.2010.11.012) [DOI] [PubMed] [Google Scholar]
- 59.Altshuler LL, Hendrick V, Cohen LS. 2000. An update on mood and anxiety disorders during pregnancy and the postpartum period. Prim. Care Companion J. Clin. Psychiatry 2, 217–222 (doi:10.4088/PCC.v02n0604) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fleming A, Ruble D, Flett G, Van Wagner V. 1990. Adjustment in first-time mothers: changes in mood and mood content during the early postpartum months. Dev. Psychol. 26, 137–143 (doi:10.1037/0012-1649.26.1.137) [Google Scholar]
- 61.Groer MW. 2005. Differences between exclusive breastfeeders, formula-feeders, and controls: a study of stress, mood, and endocrine variables. Biol. Res. Nurs. 7, 106–117 (doi:10.1177/1099800405280936) [DOI] [PubMed] [Google Scholar]
- 62.Breitkopf CR, Primeau LA, Levine RE, Olson GL, Wu ZH, Berenson AB. 2006. Anxiety symptoms during pregnancy and postpartum. J. Psychosom. Obstet. Gynaecol. 27, 157–162 (doi:10.1080/01674820500523521) [DOI] [PubMed] [Google Scholar]
- 63.Maestripieri D, D'Amato FR. 1991. Anxiety and maternal aggression in house mice (Mus musculus): a look at interindividual variability. J. Comp. Psychol. 105, 295–301 (doi:10.1037/0735-7036.105.3.295) [DOI] [PubMed] [Google Scholar]
- 64.Parmigiani S, Palanza P, Rogers J, Ferrari PF. 1999. Selection, evolution of behavior and animal models in behavioral neuroscience. Neurosci. Biobehav. Rev. 23, 957–969 (doi:10.1016/S0149-7634(99)00029-9) [DOI] [PubMed] [Google Scholar]
- 65.Johns JM, Noonan LR, Zimmerman LI, Li L, Pedersen CA. 1994. Effects of chronic and acute cocaine treatment on the onset of maternal behavior and aggression in Sprague–Dawley rats. Behav. Neurosci. 108, 107–112 (doi:10.1037/0735-7044.108.1.107) [DOI] [PubMed] [Google Scholar]
- 66.Lonstein JS, Simmons DA, Stern JM. 1998. Functions of the caudal periaqueductal gray in lactating rats: kyphosis, lordosis, maternal aggression, and fearfulness. Behav. Neurosci. 112, 1502–1518 (doi:10.1037/0735-7044.112.6.1502) [DOI] [PubMed] [Google Scholar]
- 67.Fernandez-Guasti A, Ferreira A, Picazo O. 2001. Diazepam, but not buspirone, induces similar anxiolytic-like actions in lactating and ovariectomized Wistar rats. Pharmacol. Biochem. Behav. 70, 85–93 (doi:10.1016/S0091-3057(01)00586-X) [DOI] [PubMed] [Google Scholar]
- 68.Ferreira A, Picazo O, Uriarte N, Pereira M, Fernandez-Guasti A. 2000. Inhibitory effect of buspirone and diazepam, but not of 8-OH-DPAT, on maternal behavior and aggression. Pharmacol. Biochem. Behav. 66, 389–396 (doi:10.1016/S0091-3057(00)00211-2) [DOI] [PubMed] [Google Scholar]
- 69.Liebsch G, Montkowski A, Holsboer F, Landgraf R. 1998. Behavioural profiles of two Wistar rat lines selectively bred for high or low anxiety-related behaviour. Behav. Brain Res. 94, 301–310 (doi:10.1016/S0166-4328(97)00198-8) [DOI] [PubMed] [Google Scholar]
- 70.Landgraf R, Wigger A. 2002. High vs low anxiety-related behavior rats: an animal model of extremes in trait anxiety. Behav. Genet. 32, 301–314 (doi:10.1023/A:1020258104318) [DOI] [PubMed] [Google Scholar]
- 71.Landgraf R, et al. 2007. Candidate genes of anxiety-related behavior in HAB/LAB rats and mice: focus on vasopressin and glyoxalase-I. Neurosci. Biobehav. Rev. 31, 89–102 (doi:10.1016/j.neubiorev.2006.07.003) [DOI] [PubMed] [Google Scholar]
- 72.Neumann ID, Veenema AH, Beiderbeck DI. 2010. Aggression and anxiety: social context and neurobiological links. Front. Behav. Neurosci. 4, 12–72 (doi:10.3389/Fnbeh.2010.00012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Neumann ID, Kromer SA, Bosch OJ. 2005. Effects of psycho-social stress during pregnancy on neuroendocrine and behavioural parameters in lactation depend on the genetically determined stress vulnerability. Psychoneuroendocrinology 30, 791–806 (doi:10.1016/j.psyneuen.2005.03.008) [DOI] [PubMed] [Google Scholar]
- 74.Bosch OJ, Neumann ID. 2008. Brain vasopressin is an important regulator of maternal behavior independent of dams’ trait anxiety. Proc. Natl Acad. Sci. USA 105, 17 139–17 144 (doi:10.1073/pnas.0807412105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kessler MS, Bosch OJ, Bunck M, Landgraf R, Neumann ID. 2011. Maternal care differs in mice bred for high vs. low trait anxiety: impact of brain vasopressin and cross-fostering. Soc. Neurosci. 6, 156–168 (doi:10.1080/17470919.2010.495567) [DOI] [PubMed] [Google Scholar]
- 76.Nyberg JM, Vekovischeva O, Sandnabba NK. 2003. Anxiety profiles of mice selectively bred for intermale aggression. Behav. Genet. 33, 503–511 (doi:10.1023/A:1025718531997) [DOI] [PubMed] [Google Scholar]
- 77.Beiderbeck DI, Neumann ID, Veenema AH. 2007. Differences in intermale aggression are accompanied by opposite vasopressin release patterns within the septum in rats bred for low and high anxiety. Eur. J. Neurosci. 26, 3597–3605 (doi:10.1111/j.1460-9568.2007.05974.x) [DOI] [PubMed] [Google Scholar]
- 78.Veenema AH, Torner L, Blume A, Beiderbeck DI, Neumann ID. 2007. Low inborn anxiety correlates with high intermale aggression: link to ACTH response and neuronal activation of the hypothalamic paraventricular nucleus. Horm. Behav. 51, 11–19 (doi:10.1016/j.yhbeh.2006.07.004) [DOI] [PubMed] [Google Scholar]
- 79.Bale TL, Davis AM, Auger AP, Dorsa DM, McCarthy MM. 2001. CNS region-specific oxytocin receptor expression: importance in regulation of anxiety and sex behavior. J. Neurosci. 21, 2546–2552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Blume A, Bosch OJ, Miklos S, Torner L, Wales L, Waldherr M, Neumann ID. 2008. Oxytocin reduces anxiety via ERK1/2 activation: local effect within the rat hypothalamic paraventricular nucleus. Eur. J. Neurosci. 27, 1947–1956 (doi:10.1111/j.1460-9568.2008.06184.x) [DOI] [PubMed] [Google Scholar]
- 81.McCarthy MM, McDonald CH, Brooks PJ, Goldman D. 1996. An anxiolytic action of oxytocin is enhanced by estrogen in the mouse. Physiol. Behav. 60, 1209–1215 (doi:10.1016/S0031-9384(96)00212-0) [DOI] [PubMed] [Google Scholar]
- 82.Neumann ID, Torner L, Wigger A. 2000. Brain oxytocin: differential inhibition of neuroendocrine stress responses and anxiety-related behaviour in virgin, pregnant and lactating rats. Neuroscience 95, 567–575 (doi:10.1016/S0306-4522(99)00433-9) [DOI] [PubMed] [Google Scholar]
- 83.Ring RH, et al. 2006. Anxiolytic-like activity of oxytocin in male mice: behavioral and autonomic evidence, therapeutic implications. Psychopharmacology 185, 218–225 (doi:10.1007/s00213-005-0293-z) [DOI] [PubMed] [Google Scholar]
- 84.Jurek B, Slattery DA, Maloumby R, Hillerer K, Koszinowski S, Neumann ID, van den Burg EH. 2012. Differential contribution of hypothalamic MAPK activity to anxiety-like behaviour in virgin and lactating rats. PLoS ONE 7, e37060 (doi:10.1371/journal.pone.0037060) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Campbell A. 2013. The evolutionary psychology of women's aggression. Phil. Trans. R. Soc. B 368, 20130078 (doi:10.1098/rstb.2013.0078) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Windle RJ, Shanks N, Lightman SL, Ingram CD. 1997. Central oxytocin administration reduces stress-induced corticosterone release and anxiety behavior in rats. Endocrinology 138, 2829–2834 (doi:10.1210/en.138.7.2829) [DOI] [PubMed] [Google Scholar]
- 87.Manning M, et al. 1989. Solid-phase synthesis of 16 potent (selective and nonselective) in vivo antagonists of oxytocin. J. Med. Chem. 32, 382–391 (doi:10.1021/jm00122a016) [DOI] [PubMed] [Google Scholar]
- 88.Slattery DA, Neumann ID. 2010. Chronic icv oxytocin attenuates the pathological high anxiety state of selectively bred Wistar rats. Neuropharmacology 58, 56–61 (doi:10.1016/j.neuropharm.2009.06.038) [DOI] [PubMed] [Google Scholar]
- 89.Waldherr M, Neumann ID. 2007. Centrally released oxytocin mediates mating-induced anxiolysis in male rats. Proc. Natl Acad. Sci. USA 104, 16 681–16 684 (doi:10.1073/pnas.0705860104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Huber D, Veinante P, Stoop R. 2005. Vasopressin and oxytocin excite distinct neuronal populations in the central amygdala. Science 308, 245–248 (doi:10.1126/science.1105636) [DOI] [PubMed] [Google Scholar]
- 91.Viviani D, Charlet A, van den Burg E, Robinet C, Hurni N, Abatis M, Magara F, Stoop R. 2011. Oxytocin selectively gates fear responses through distinct outputs from the central amygdala. Science 333, 104–107 (doi:10.1126/science.1201043) [DOI] [PubMed] [Google Scholar]
- 92.Knobloch HS, et al. 2012. Evoked axonal oxytocin release in the central amygdala attenuates fear response. Neuron 73, 553–566 (doi:10.1016/j.neuron.2011.11.030) [DOI] [PubMed] [Google Scholar]
- 93.Murgatroyd C, Wigger A, Frank E, Singewald N, Bunck M, Holsboer F, Landgraf R, Spengler D. 2004. Impaired repression at a vasopressin promoter polymorphism underlies overexpression of vasopressin in a rat model of trait anxiety. J. Neurosci. 24, 7762–7770 (doi:10.1523/JNEUROSCI.1614-04.2004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Landgraf R, Gerstberger R, Montkowski A, Probst JC, Wotjak CT, Holsboer F, Engelmann M. 1995. V1 vasopressin receptor antisense oligodeoxynucleotide into septum reduces vasopressin binding, social discrimination abilities, and anxiety-related behavior in rats. J. Neurosci. 15, 4250–4258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ring RH. 2005. The central vasopressinergic system: examining the opportunities for psychiatric drug development. Curr. Pharm. Des. 11, 205–225 (doi:10.2174/1381612053382241) [DOI] [PubMed] [Google Scholar]
- 96.Wigger A, et al. 2004. Alterations in central neuropeptide expression, release, and receptor binding in rats bred for high anxiety: critical role of vasopressin. Neuropsychopharmacology 29, 1–14 (doi:10.1038/sj.npp.1300290) [DOI] [PubMed] [Google Scholar]
- 97.Keck ME, Wigger A, Welt T, Muller MB, Gesing A, Reul JM, Holsboer F, Landgraf R, Neumann ID. 2002. Vasopressin mediates the response of the combined dexamethasone/CRH test in hyper-anxious rats: implications for pathogenesis of affective disorders. Neuropsychopharmacology 26, 94–105 (doi:10.1016/S0893-133X(01)00351-7) [DOI] [PubMed] [Google Scholar]
- 98.Kruszynski M, Lammek B, Manning M, Seto J, Haldar J, Sawyer WH. 1980. [1-beta-Mercapto-beta,beta-cyclopentamethylenepropionic acid),2-(O-methyl)tyrosine]argine-vasopressin and [1-beta-mercapto-beta,beta-cyclopentamethylenepropionic acid)]argine-vasopressine, two highly potent antagonists of the vasopressor response to arginine-vasopressin. J. Med. Chem. 23, 364–368 (doi:10.1021/jm00178a003) [DOI] [PubMed] [Google Scholar]
- 99.Pedersen CA, Ascher JA, Monroe YL, Prange AJ., Jr 1982. Oxytocin induces maternal behavior in virgin female rats. Science 216, 648–650 (doi:10.1126/science.7071605) [DOI] [PubMed] [Google Scholar]
- 100.Pedersen CA, Prange AJ., Jr 1979. Induction of maternal behavior in virgin rats after intracerebroventricular administration of oxytocin. Proc. Natl Acad. Sci. USA 76, 6661–6665 (doi:10.1073/pnas.76.12.6661) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Young WS, 3rd, et al. 1998. Targeted reduction of oxytocin expression provides insights into its physiological roles. Adv. Exp. Med. Biol. 449, 231–240 (doi:10.1007/978-1-4615-4871-3_30) [DOI] [PubMed] [Google Scholar]
- 102.Giovenardi M, Padoin MJ, Cadore LP, Lucion AB. 1998. Hypothalamic paraventricular nucleus modulates maternal aggression in rats: effects of ibotenic acid lesion and oxytocin antisense. Physiol. Behav. 63, 351–359 (doi:10.1016/S0031-9384(97)00434-4) [DOI] [PubMed] [Google Scholar]
- 103.Lubin DA, Elliott JC, Black MC, Johns JM. 2003. An oxytocin antagonist infused into the central nucleus of the amygdala increases maternal aggressive behavior. Behav. Neurosci. 117, 195–201 (doi:10.1037/0735-7044.117.2.195) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Consiglio AR, Borsoi A, Pereira GA, Lucion AB. 2005. Effects of oxytocin microinjected into the central amygdaloid nucleus and bed nucleus of stria terminalis on maternal aggressive behavior in rats. Physiol. Behav. 85, 354–362 (doi:10.1016/j.physbeh.2005.05.002) [DOI] [PubMed] [Google Scholar]
- 105.Wotjak CT, Landgraf R, Engelmann M. 2008. Listening to neuropeptides by microdialysis: echoes and new sounds? Pharmacol. Biochem. Behav. 90, 125–134 (doi:10.1016/j.pbb.2008.03.017) [DOI] [PubMed] [Google Scholar]
- 106.Landgraf R, Kubota M, Holsboer F, Wotjak CT. 1995. Release of vasopressin and oxytocin within the brain and into blood: microdialysis and antisense targeting. In Neurohypophysis: recent progress of vasopressin and oxytocin research (eds Saito T, Kurokawa K, Yoshida S.), pp. 243–256 Amsterdam, The Netherlands: Elsevier [Google Scholar]
- 107.Consiglio AR, Lucion AB. 1996. Lesion of hypothalamic paraventricular nucleus and maternal aggressive behavior in female rats. Physiol. Behav. 59, 591–596 (doi:10.1016/0031-9384(95)02117-5) [DOI] [PubMed] [Google Scholar]
- 108.Leng G, Onaka T, Caquineau C, Sabatier N, Tobin VA, Takayanagi Y. 2008. Oxytocin and appetite. Prog. Brain Res. 170, 137–151 (doi:10.1016/S0079-6123(08)00413-5) [DOI] [PubMed] [Google Scholar]
- 109.Ferris CF, Foote KB, Meltser HM, Plenby MG, Smith KL, Insel TR. 1992. Oxytocin in the amygdala facilitates maternal aggression. Ann. NY Acad. Sci. 652, 456–457 (doi:10.1111/j.1749-6632.1992.tb34382.x) [DOI] [PubMed] [Google Scholar]
- 110.Landgraf R, Neumann ID. 2004. Vasopressin and oxytocin release within the brain: a dynamic concept of multiple and variable modes of neuropeptide communication. Front. Neuroendocrinol. 25, 150–176 (doi:10.1016/j.yfrne.2004.05.001) [DOI] [PubMed] [Google Scholar]
- 111.Bosch OJ, Pfortsch J, Beiderbeck DI, Landgraf R, Neumann ID. 2010. Maternal behaviour is associated with vasopressin release in the medial preoptic area and bed nucleus of the stria terminalis in the rat. J. Neuroendocrinol. 22, 420–429 (doi:10.1111/j.1365-2826.2010.01984.x) [DOI] [PubMed] [Google Scholar]
- 112.Meddle SL, Bishop VR, Gkoumassi E, van Leeuwen FW, Douglas AJ. 2007. Dynamic changes in oxytocin receptor expression and activation at parturition in the rat brain. Endocrinology 148, 5095–5104 (doi:10.1210/en.2007-0615) [DOI] [PubMed] [Google Scholar]
- 113.Francis DD, Champagne FC, Meaney MJ. 2000. Variations in maternal behaviour are associated with differences in oxytocin receptor levels in the rat. J. Neuroendocrinol. 12, 1145–1148 (doi:10.1046/j.1365-2826.2000.00599.x) [DOI] [PubMed] [Google Scholar]
- 114.Gammie SC, Nelson RJ. 2001. cFOS and pCREB activation and maternal aggression in mice. Brain Res. 898, 232–241 (doi:10.1016/S0006-8993(01)02189-8) [DOI] [PubMed] [Google Scholar]
- 115.Klampfl SM, Neumann ID, Bosch OJ. 2013. Reduced brain CRF receptor activation is required for adequate maternal care and maternal aggression in lactating rats. Eur. J. Neurosci. 38, 2742–2750 (doi:10.1111/ejn.12274) [DOI] [PubMed] [Google Scholar]
- 116.Gammie SC, Negron A, Newman SM, Rhodes JS. 2004. Corticotropin-releasing factor inhibits maternal aggression in mice. Behav. Neurosci. 118, 805–814 (doi:10.1037/0735-7044.118.4.805) [DOI] [PubMed] [Google Scholar]
- 117.Windle RJ, Kershaw YM, Shanks N, Wood SA, Lightman SL, Ingram CD. 2004. Oxytocin attenuates stress-induced c-fos mRNA expression in specific forebrain regions associated with modulation of hypothalamo-pituitary-adrenal activity. J. Neurosci. 24, 2974–2982 (doi:10.1523/JNEUROSCI.3432-03.2004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Wersinger SR, Caldwell HK, Martinez L, Gold P, Hu SB, Young WS., 3rd 2007. Vasopressin 1a receptor knockout mice have a subtle olfactory deficit but normal aggression. Genes Brain Behav. 6, 540–551 (doi:10.1111/j.1601-183X.2006.00281.x) [DOI] [PubMed] [Google Scholar]
- 119.Wersinger SR, Caldwell HK, Christiansen M, Young WS., 3rd 2007. Disruption of the vasopressin 1b receptor gene impairs the attack component of aggressive behavior in mice. Genes Brain Behav. 6, 653–660 (doi:10.1111/j.1601-183X.2006.00294.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Bosch OJ, Neumann ID. 2007. Brain vasopressin regulates maternal behavior and aggression. In Ann. Meet. Soc. Neurosci., 3–7 November, San Diego, CA. Program no. 84.14. (Online) [Google Scholar]
- 121.Nephew BC, Bridges RS. 2008. Central actions of arginine vasopressin and a V1a receptor antagonist on maternal aggression, maternal behavior, and grooming in lactating rats. Pharmacol. Biochem. Behav. 91, 77–83 (doi:10.1016/j.pbb.2008.06.013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Nephew BC, Byrnes EM, Bridges RS. 2010. Vasopressin mediates enhanced offspring protection in multiparous rats. Neuropharmacology 58, 102–106 (doi:10.1016/j.neuropharm.2009.06.032) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Wersinger SR, Ginns EI, O'Carroll AM, Lolait SJ, Young WS., 3rd 2002. Vasopressin V1b receptor knockout reduces aggressive behavior in male mice. Mol. Psychiatry 7, 975–984 (doi:10.1038/sj.mp.4001195) [DOI] [PubMed] [Google Scholar]
- 124.Hernando F, Schoots O, Lolait SJ, Burbach JP. 2001. Immunohistochemical localization of the vasopressin V1b receptor in the rat brain and pituitary gland: anatomical support for its involvement in the central effects of vasopressin. Endocrinology 142, 1659–1668 (doi:10.1210/en.142.4.1659) [DOI] [PubMed] [Google Scholar]
- 125.Wotjak CT, Kubota M, Liebsch G, Montkowski A, Holsboer F, Neumann I, Landgraf R. 1996. Release of vasopressin within the rat paraventricular nucleus in response to emotional stress: a novel mechanism of regulating adrenocorticotropic hormone secretion? J. Neurosci. 16, 7725–7732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Douglas AJ, Meddle SL, Kroemer S, Muesch W, Bosch OJ, Neumann ID. 2007. Social stress induces hypothalamo–pituitary–adrenal axis responses in lactating rats bred for high trait anxiety. Eur. J. Neurosci. 25, 1599–1603 (doi:10.1111/j.1460-9568.2007.05380.x) [DOI] [PubMed] [Google Scholar]
- 127.LeDoux J. 2007. The amygdala. Curr. Biol. 17, R868–R874 (doi:10.1016/j.cub.2007.08.005) [DOI] [PubMed] [Google Scholar]
- 128.Roozendaal B, Schoorlemmer GH, Koolhaas JM, Bohus B. 1993. Cardiac, neuroendocrine, and behavioral effects of central amygdaloid vasopressinergic and oxytocinergic mechanisms under stress-free conditions in rats. Brain Res. Bull. 32, 573–579 (doi:10.1016/0361-9230(93)90157-7) [DOI] [PubMed] [Google Scholar]
- 129.Willcox BJ, Poulin P, Veale WL, Pittman QJ. 1992. Vasopressin-induced motor effects: localization of a sensitive site in the amygdala. Brain Res. 596, 58–64 (doi:10.1016/0006-8993(92)91532-J) [DOI] [PubMed] [Google Scholar]
- 130.Veenema AH, Beiderbeck DI, Lukas M, Neumann ID. 2010. Distinct correlations of vasopressin release within the lateral septum and the bed nucleus of the stria terminalis with the display of intermale aggression. Horm. Behav. 58, 273–281 (doi:10.1016/j.yhbeh.2010.03.006) [DOI] [PubMed] [Google Scholar]


