Abstract
Many clades contain ecologically and phenotypically similar species across continents, yet the processes generating this similarity are largely unstudied, leaving fundamental questions unanswered. Is similarity in morphology and performance across assemblages caused by evolutionary convergence or by biogeographic dispersal of evolutionarily conserved ecotypes? Does convergence to new ecological conditions erase evidence of past adaptation? Here, we analyse ecology, morphology and performance in frog assemblages from three continents (Asia, Australia and South America), assessing the importance of dispersal and convergent evolution in explaining similarity across regions. We find three striking results. First, species using the same microhabitat type are highly similar in morphology and performance across both clades and continents. Second, some species on different continents owe their similarity to dispersal and evolutionary conservatism (rather than evolutionary convergence), even over vast temporal and spatial scales. Third, in one case, an ecologically specialized ancestor radiated into diverse ecotypes that have converged with those on other continents, largely erasing traces of past adaptation to their ancestral ecology. Overall, our study highlights the roles of both evolutionary conservatism and convergence in explaining similarity in species traits over large spatial and temporal scales and demonstrates a statistical framework for addressing these questions in other systems.
Keywords: Anura, biogeography, ecomorphology, phenotypic diversification, phylogeny
1. Introduction
Many species are ecologically and morphologically similar to species in similar biomes on other continents. This pattern of among-continent similarity in species traits occurs across many ecological guilds, clades and biomes (e.g. placental and marsupial mammals, Mediterranean-climate plants and desert lizards [1–3]). However, the ecological and evolutionary processes underlying this similarity are not well understood, and thus many fundamental questions in ecology and evolutionary biology remain unresolved. Does similarity in ecology necessarily translate to similarity in morphology and performance? Can convergent evolution to similar ecological conditions across regions erase traces of past adaptation in morphology and performance to different conditions [4,5]? Do processes other than evolutionary change also explain similarity in species traits across regions, for example dispersal of evolutionarily conserved phenotypes among regions [6–8]? Addressing these questions is critical for understanding why certain traits are present or not in a given clade, region or community, a key issue in both evolutionary biology and ecology.
In general, the similarity among species from different regions for a given trait can result from two processes [6,7]. First, convergent evolution of similar traits in species in different regions can cause initially dissimilar species to become similar. Alternatively, similarity can result from dispersal of lineages from one region to another, if the relevant ecological trait is conserved during and after dispersal (ecologically conservative dispersal: ECD; [6–8]). Similarity at large spatial and temporal scales is often assumed to result primarily from convergent evolution rather than dispersal [9–11]. However, few studies have tested the importance of both processes [7,12], and none, to our knowledge, have done so across continents. Furthermore, most studies have focused on morphology [13,14]. Nevertheless, whole-organism performance traits (e.g. locomotion or feeding) may be more relevant for adaptation than morphology alone [15–18] and the evolution of morphology and performance may be decoupled [18–21]. Thus, analyses of similarity among regions must address both convergence and conservatism, and should preferably include ecology, morphology and performance [22].
Here, we test the processes underlying similarity in ecology, morphology and performance among frog species across continents. Frogs use many microhabitats, and arboreal, burrowing, semi-aquatic and terrestrial species occur in most tropical assemblages (figure 1a; [25–27]). However, despite previous discussions of frog ecotypes [28] and previous research on frog morphology and performance [29–32], the relationships between ecology, morphology and performance in frogs have not been studied across multiple clades, continents and traits in a phylogenetic context.
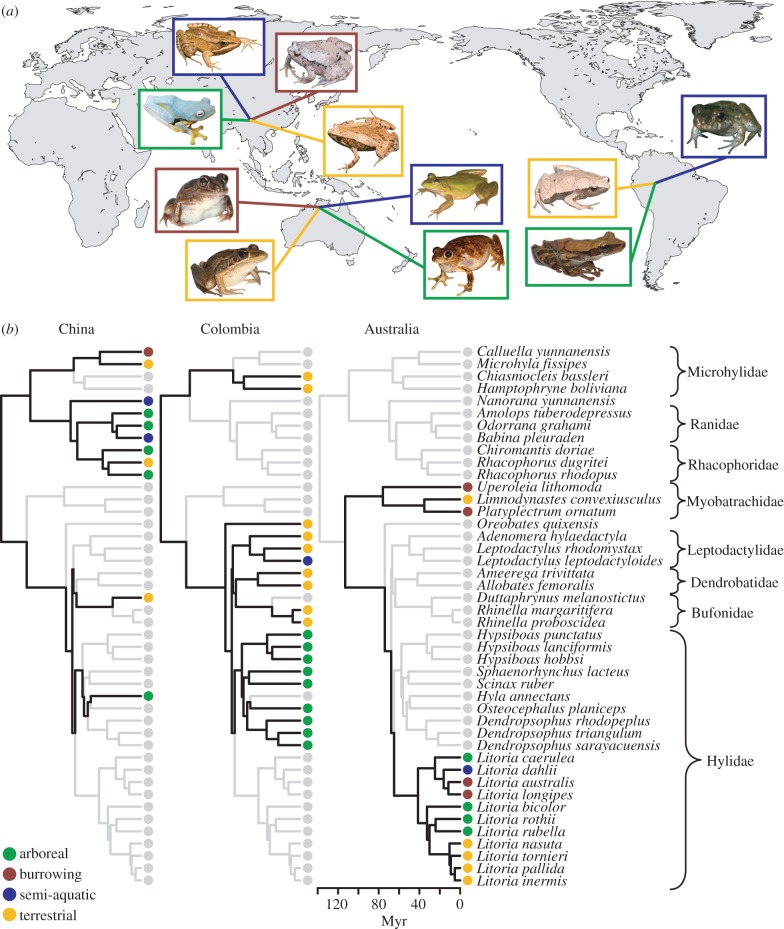
Figure 1.
(a) World map showing field site locations and representatives of the frog ecotypes that occur there. Field sites (clockwise from right) are Leticia, Department of Amazonas, Colombia; Middle Point, Northern Territory, Australia and Baoshan, Yunnan Province, China. Microhabitat use of each species is indicated by the colour of the border of its photo: arboreal (green), burrowing (red), semi-aquatic (blue) and terrestrial (yellow). Frog species, clockwise starting from the semi-aquatic species in each location, are: Leptodactylus leptodactyloides, Osteocephalus planiceps and Hamptophryne boliviana (Colombia); Litoria dahlii, Litoria rothii, Litoria nasuta and Litoria longipes (Australia); and Babina pleuraden, Calluella yunnanensis, Microhyla fissipes and Rhacophorus rhodopus (China). Photos from Australia and Colombia were taken by D.S.M., while those from China were taken by Jing Che. Photos are not to scale. (b) Phylogeny, biogeography and microhabitat use of the 44 frog species included in this study. All three phylogenies are the same, with branch lengths (in millions of years; Myr) estimated using the Bayesian uncorrelated lognormal approach in BEAST ([23,24]; see the electronic supplementary material). Families are indicated to the right of species names. Species at a given site are shown with black branches and coloured microhabitat character states, while others are shown in grey. The branch-length scale bar applies equally to all three trees. Three clades show ECD between Colombia and China, as they have species in both regions that share the same microhabitat (Microhylidae, Bufonidae, Hylidae). By contrast, in situ diversification of microhabitat use can be seen in Australia in Litoria, which has species that use each of the four microhabitats despite an ancestor that was seemingly an arboreal specialist.
We obtained new data on microhabitat use, morphology and performance (jumping, swimming and clinging) from frog species at three mesic, tropical sites (in Australia, China and Colombia). We then focused on three major questions and developed statistical phylogenetic methods to address them. First, do species using a given microhabitat show similar morphology and performance across continents and clades? Second, can this trait similarity be a consequence of evolutionary conservatism and biogeographic dispersal across continents? Third, can trait similarity alternatively result from convergence in morphology and performance, even when a clade has diversified from an ancestor that was putatively adapted to a single microhabitat [4,5]?
2. Material and methods
(a). Fieldwork and data collection
We examined similar numbers of frog species at each of three mesic, tropical field sites (n = 11 in Baoshan, China; n = 19 in Leticia, Colombia and n = 14 in Middle Point, Australia). Though overall anuran species richness differs among locations (n ∼ 25 in Baoshan [33], n > 97 in Leticia [34] and n = 17 at Middle Point [35]), our sample is probably representative of the overall ecological and phenotypic diversity of anurans for each assemblage, given that non-included species were closely related and phenotypically similar to included species.
Methods were similar in each location (see the electronic supplementary material for complete methods and justifications). We collected adult, mostly male frogs in the field during the breeding season in each location. Mean within-species sample size was 4.98 (see the electronic supplementary material for exact numbers for each species). Performance variables were selected as those that are important for predator avoidance and general microhabitat use [36,37] and likely to differ across species using different microhabitats (see the electronic supplementary material). Specifically, we focused on clinging ability (for arboreal locomotion), swimming ability (for aquatic locomotion) and jumping ability (arguably important for all types [32]). High-speed videos were taken of jumping take-off and burst swimming efforts of each frog, which we induced by gently tapping the back of each frog. Trials were conducted at ambient temperature (21.8–27.6°C), which was within the range of active field temperatures at which we captured frogs. Trials were conducted three to five times over the course of one week to capture each individual's maximum performance (see the electronic supplementary material for detailed methods and justification). Videos were analysed by first digitizing the position of the snout tip to estimate distance travelled versus time during take-off or swimming. Distance–time plots were then uploaded into QuickSAND [38] to smooth the plots and subsequently calculate velocity and acceleration profiles via numerical derivatives. Mass-specific power was calculated as the product of the instantaneous velocity and acceleration curves [39]. Jumping angle was measured directly from videos using ImageJ (v. 1.42 [40]). Clinging trials involved placing frogs on a flat, non-stick pan and rotating the pan on a hinge until the frog fell off the surface (following [30]); the pan's angle then represented the frog's maximum clinging angle. This procedure was done three times for each individual. Overall, we used only the maximum value for each individual for each performance variable taken from the video in which the individual gave its maximum effort, which we defined by the highest peak velocity across all videos [39,41]. For subsequent comparative analyses, each species was represented by the mean of maximum values among individuals for each performance variable (reported in the electronic supplementary material, table S2).
After performance trials, each individual was sacrificed, preserved and measured for morphological data. Morphological variables were those directly relevant to performance variables based on previous studies [29–32], including measurements of body length, limb lengths, head shape, interdigit webbing area, toe-tip area, area of inner metatarsal tubercle and leg muscle mass (see the electronic supplementary material for full description of variables). Morphological data (means across individuals for each species) are available in the electronic supplementary material, table S3. We used data on microhabitat use from the literature to characterize species as arboreal, burrowing, semi-aquatic or terrestrial (see the electronic supplementary material, table S1). These data were consistent with our field observations. For detailed characterization of these categories and justification, see the electronic supplementary material.
(b). Phylogenetic framework
We examined trait evolution using a time-calibrated phylogeny (see the electronic supplementary material for details). We started with a broad-scale dataset [42], then excluded all but 44 species that represented those in the ecology–morphology-performance dataset. We then estimated divergence times in BEAST [23,24] using the maximum-likelihood topology of Pyron & Wiens [42] to reduce any potential topological errors associated with limited taxon sampling.
(c). Data analysis
We first conducted principal components analysis (PCA) on both the performance and morphological data across all species in the study. We did both standard PCA and phylogenetic PCA [43], using R v. 2.15 [44] and the package phytools [45]. Both gave similar results, and we used scores from phylogenetic PCA in subsequent analyses (see the electronic supplementary material, table S4).
We next conducted a phylogenetic MANOVA on PC scores for both morphology and performance to test whether microhabitat use was associated with particular morphological or performance traits. All MANOVA models were estimated in R and PC scores were phylogenetically transformed (using R code from [46]). Preliminary univariate ANOVAs showed that different microhabitat specialists on average were similar in PC1 for both morphology and performance (i.e. size and an axis of overall performance, respectively), so MANOVAs were only conducted on all PCs beyond PC1 to focus on axes that distinguish microhabitat specialists. This was also done for our analyses of conservatism and convergence (see below). We also tested relationships (see Results) between our morphological and performance variables, because ecomorphological studies that do not demonstrate a clear link between morphology and function risk overinterpreting variation in morphology [18,20,21]. We used phylogenetic generalized least-squares analyses [47] assuming Brownian motion (see the electronic supplementary material for justification of this model) and all size-independent (i.e. ‘relative’) variables were estimated using phylogenetic size-correction [43] in phytools [45].
To understand the importance of biogeographic context for species similarity across assemblages, we first traced the microhabitat states in each assemblage to either in situ evolution (ISE) or ECD, using our data on phylogeny, microhabitat and geographical location [12]. We made biogeographic inferences based on the 44 sampled species and by incorporating other studies with broader taxon sampling (including some with thousands of species; see the electronic supplementary material for further details, including a discussion on shared similarity caused by vicariance versus dispersal).
Given that conservatism explained the presence of some ecologically similar species across regions (i.e. same microhabitat in each; see Results), we further tested whether there was also conservatism in morphology and performance. We focused on China and Colombia, which share three clades that each possesses species of the same microhabitat type in both regions (Microhylidae (terrestrial), Bufonidae (terrestrial) and Hylidae (arboreal)), and we developed a novel statistical test of conservatism. In this test, one calculates the average pairwise Euclidean distance in morphology and performance between the closely related, ecologically similar species from two locations (here, China and Colombia). These distances are then compared to those among all species with the same ecology (regardless of location), and also between all pairs of species from the two locations (regardless of ecology). Here, for each clade we first calculated the distance in morphology and performance (separately) between the single species that occurs in China and either its closest relative in Colombia (Hylidae) or the mean value of its two equally closest relatives in Colombia (Microhylidae and Bufonidae; figure 1b). We then calculated pairwise distances among all species sharing the same category of microhabitat use (136 and 153 comparisons among terrestrial and arboreal species, respectively), as well as all 209 comparisons comparing all species from China and Colombia. This allowed us to ask, for example: how similar are the terrestrial microhylid frogs found in China and Colombia compared to the similarity among all terrestrial frogs or among all pairs of species from China and Colombia?
Finally, we developed new tests to examine possible residual effects of previous evolutionary history on convergence and adaptation within an in situ radiation. It is increasingly recognized that previous evolutionary history may lead to a lack of convergence in the same environment [22], perhaps owing to inherent differences among adapting lineages, such as different genetic or developmental systems or different adaptive landscapes. Of particular interest is the possibility that species adapting to novel environments are not very different from closely related species, reflecting a historical footprint on evolution, but the evolutionary change could still be in the expected direction, reflecting adaptive evolution [5].
In contrast to the overall conservatism in microhabitat use in some clades in China and Colombia, a single clade in Australia (Litoria) has evolved from an arboreal ancestor into a clade including all four microhabitat types (figure 1b; [48]). Thus, we compared Litoria species in novel microhabitats (i.e. burrowing, semi-aquatic and terrestrial) to distantly related species that share the same microhabitat on other continents to test whether the previous history of Litoria as an arboreal specialist (see the electronic supplementary material, figure S3; [48]) has limited convergence in morphology and performance with species in other clades using other microhabitats.
We developed three tests for this and all three involved comparisons of the mean phenotypes in morphology and performance in three groups: (i) species of the focal group with the ancestral ecology (here, arboreal Litoria), which we will abbreviate as Fanc; (ii) species in the focal group with novel ecology (here, burrowing, semi-aquatic and terrestrial Litoria), abbreviated as Fnov; and (iii) species unrelated to those in the focal group that share the same ecology as species of the focal group with the novel ecology (here, the same ecology as non-arboreal Litoria), abbreviated as nonF (for non-focal). All comparisons are made for only one novel ecology at a time (e.g. arboreal Litoria, terrestrial Litoria and non-Litoria terrestrial species). For full details of this approach, see the electronic supplementary material. For brevity, we refer to these groups by their abbreviation above in the ensuing description of the tests.
The first test was a test of an effect of history. The expected trajectory of convergent evolution is from Fanc towards the phenotype of unrelated species in the novel microhabitat (nonF), but if history is important then Litoria in novel microhabitats (Fnov) may not have diverged far from the ancestral phenotype (Fanc). Thus, we simply compared two Euclidean distances in PC space (figure 2): (i) Fanc to nonF (expected distance = Dexp), and (ii) Fanc to Fnov (observed distance = Dobs). If history has limited convergent evolution, then we would expect Dobs to be smaller than Dexp. For this and subsequent tests, we compared the observed test statistic to null expectations from simulations (see below), and the tests were done individually for each novel microhabitat.
Figure 2.
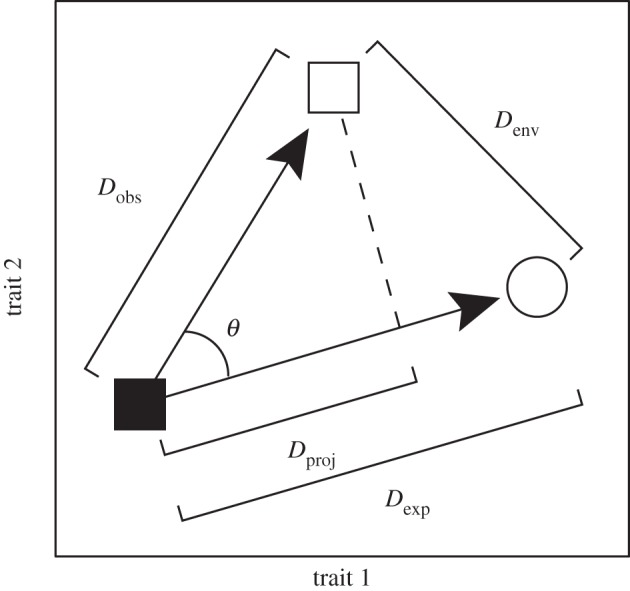
Hypothetical example of our approach for examining distances and vectors of expected and observed divergence among groups, to test for the potential imprint of past history on convergence. The black square represents the mean of species in the focal group that still have the ancestral ecology. The white square represents the mean of species of the focal group that have the novel ecology, and the white circle is the mean of non-focal species that have the same ecology as the focal group with the novel ecology. Hence, similar colour represents similar ecology and similar shape represents close phylogenetic relatedness. Dobs, Denv, Dproj, Dexp and θ are as described in the text. An effect of history would be indicated by a much shorter Dobs than Dexp, whereas convergence would be indicated by a smaller Denv than Dobs and a small θ. Note that only two traits are shown in this example for visual clarity, but this approach can be applied in any n-dimensional trait space.
The two remaining tests were tests of convergence. The first of these was a simple comparison of the Euclidean distances in PC space between the two groups with similar microhabitats (Fnov and nonF; distance between groups sharing the same selective environment = Denv) and the two groups of closely related species (Fanc and Fnov, Dobs). Convergent evolution would be supported by finding that the two groups sharing the same microhabitat were more similar than the two groups of closely related species (figure 2).
The second test of convergence considered the vector of divergence of focal species in novel microhabitats (Fnov) from their ancestral type (Fanc), asking what proportion of that divergence has been in the direction expected given convergent evolution, with the expected direction characterized as the vector from Fanc to nonF (figure 2). This proportion is the same as the vector correlation between the observed and expected divergence vectors (Dproj divided by Dobs; figure 2), and the arccosine of this correlation is the angle between the vectors (θ; figure 2) [49].
To test the statistical significance of these three quantities, we conducted simulations of phenotypic evolution to produce null distributions against which to test our observed distances and vector correlations. Phenotypic evolution was simulated under Brownian motion as a model of neutral evolution [4,50]. We simulated 9999 replicates each for morphology and performance. For each simulation, we calculated distances and vector correlations as above. The proportion of simulated results equal to or more extreme (i.e. more in line with our predictions; see the electronic supplementary material) than our observed data was used as a p-value. All analyses were computed in R and original code is available upon request.
Finally, we note that one could use alternative approaches to define the expected phenotype given convergent evolution to a new ecology instead of using species means (‘nonF’ above). One such option is to use Ornstein–Uhlenbeck models of phenotypic evolution [4,51], which calculate phenotypic optima to ‘selective regimes’ (here, microhabitat use). Such optima could be used as the nonF values above. Therefore, we also used this approach and found nearly identical results as using mean values above (see the electronic supplementary material for full details and results). However, we mention this approach only as an option here because most current implementations of OU models necessitate an input of single values for the selective regimes at internal nodes, and in the case of high uncertainty in these estimates, implementing such OU approaches may give poor estimates of the optima.
3. Results
(a). Similarity among species in ecology, morphology and performance
Arboreal, semi-aquatic and terrestrial species occurred in all three locations, with burrowing species at only two locations (figure 1 and electronic supplementary material, table S1). Microhabitat use was strongly related to both morphology and performance (phylogenetic MANOVA; morphology: Wilks’ λ = 0.127, p < 0.001; performance: Wilks’ λ = 0.319, p = 0.003; electronic supplementary material, table S6). Thus, species generally showed distinctive morphological and performance phenotypes associated with the microhabitat in which they occurred, regardless of clade or location. Semi-aquatic species had large leg muscles and extensive foot webbing, consistent with their high velocity, acceleration and power during swimming (see the electronic supplementary material, tables S4 and S6). Arboreal species had the highest clinging ability and large finger and toe tips. Burrowing frogs had large metatarsal tubercles and short legs, both were characteristics that increase burrowing performance [52]. However, terrestrial taxa were much less distinctive than the other groups (see the electronic supplementary material, figure S1 and table S6).
The relationships between morphological and performance variables also followed biomechanical predictions ([30,31]; see the electronic supplementary material), supporting the inclusion of both types of variables in this study. These included a positive relationship between toepad size and maximum clinging angle (p < 0.001), and a positive relationship between relative muscle mass and peak acceleration and power, both in jumping and swimming (all p ≤ 0.001; see the electronic supplementary material for description of models and full results). Interestingly, we found potential evidence for many-to-one mapping of morphology on performance [19], as both higher relative leg muscle mass and longer relative leg length appeared to lead to higher peak jumping velocity (p = 0.083 and p < 0.001, respectively) but were negatively correlated among species (r = −0.530; p < 0.001).
(b). Role of conservatism
Mapping geographical regions and microhabitat evolution on the phylogeny (figure 1, electronic supplementary material, figure S3) shows that all three locations acquired their ecotypes through both ISE and ECD (see the electronic supplementary material for methods and region-by-region results). In subsequent tests of conservatism in morphology and performance between species in China and Colombia, we found that in one clade (Hylidae), the two arboreal species were not very similar, particularly when compared with other species pairs within the same microhabitat (table 1). However, in the other two clades (Microhylidae and Bufonidae), the terrestrial species in the two locations were very similar in both morphology and performance. Specifically, the species from these clades from the two regions were more similar than most pairs of terrestrial species (across all regions) and more similar than most species pairs from China and Colombia (across all microhabitats; table 1). In all three groups, results were similar for morphology and performance (table 1), though statistical results were stronger for morphology.
Table 1.
Statistical test for similarity (conservatism) in morphology and performance among species in clades that show ECD between Asia and South America, using two null distributions (based on all species sharing the same microhabitat and based on all species sampled from both regions). (PC axes and associated distances have no units. MDfocal refers to the mean distance among species within the focal group (e.g. between terrestrial microhylids in China and Colombia). MDall under ‘same microhabitat’ refers to mean distance among all species within the same microhabitat as the focal group in that row, regardless of location. MDall under ‘China–Colombia’ refers to mean distance among all pairs of species (across all microhabitat categories) in which one species is from China and the other from Colombia. In both cases, Psimilar refers to the proportion of species pairs (within the same microhabitat or between China and Colombia) with a smaller distance among them than within the focal group (in a given row). Overall, the results show significant (or nearly significant) similarity in morphology and performance in microhylids and bufonids, but not hylids, with generally stronger similarity in morphology than performance.)
| same microhabitat |
China–Colombia |
||||
|---|---|---|---|---|---|
| focal group | MDfocal | MDall | Psimilar | MDall | Psimilar |
| morphology | |||||
| Microhylidae | 5.27 | 12.62 | 0.037 | 14.01 | 0.038 |
| Bufonidae | 5.52 | 12.62 | 0.044 | 14.01 | 0.062 |
| Hylidae | 7.16 | 7.54 | 0.503 | 14.01 | 0.124 |
| performance | |||||
| Microhylidae | 9.60 | 17.76 | 0.103 | 19.07 | 0.062 |
| Bufonidae | 9.10 | 17.76 | 0.081 | 19.07 | 0.048 |
| Hylidae | 12.02 | 16.49 | 0.261 | 19.07 | 0.177 |
(c). Convergence and the footprint of history
We found no effect of history on adaptation to novel microhabitats in the focal clade (Litoria). All Litoria in novel microhabitats were nearly as different from arboreal Litoria in morphology as were other species in the novel microhabitat, and they were even more different in performance (figure 3 and electronic supplementary material, table S7a). We further found that all novel microhabitat specialists in Litoria were significantly closer in PC space (for both performance and morphology) to species in other clades in the same microhabitat than to Litoria in the ancestral, arboreal microhabitat (figure 3 and electronic supplementary material, table S7b). Finally, most of the total divergence of non-arboreal lineages of Litoria from the ancestral phenotype has been in the direction predicted under convergent evolution, as compared with divergence in other possible directions (figure 3 and electronic supplementary material, table S7c). These patterns were particularly clear in burrowing and semi-aquatic Litoria, though less so in terrestrial Litoria. In all cases, convergence in morphology and performance was similar in strength (figure 3 and electronic supplementary material, table S7).
Figure 3.

Visualization of convergence between Australian Litoria in derived microhabitats and other (non-Litoria) frog species that use these same microhabitats, as compared with Litoria in the ancestral microhabitat (arboreality). Plots of morphology are in the left column and plots of performance are in the right column. Plots show the mean values of Litoria species in novel microhabitats (non-green squares), other species in same microhabitat (circles) and Litoria in the ancestral habitat (arboreal; green squares). Arrows toward non-Litoria species show the expected direction of divergence from the arboreal phenotype, whereas those pointing towards Litoria in the novel microhabitat show the actual divergence. Only PC2 and PC3 are shown for ease of visualization, but note that statistical tests of convergence used all PC axes except PC1 (see the electronic supplementary material). Ellipses represent variation within each group along their principal axes of variation for PC2 and PC3 (for groups in which the number of species was greater than 2), and the radii of the ellipses represent 1 standard error along each principal axis of variation. The results show that Litoria in novel microhabitats have evolved to become highly similar in morphology and performance to unrelated frog species in the same microhabitat, with no trace of their past ecological history as arboreal specialists.
4. Discussion
In this paper, we tested fundamental questions about the processes leading to similarity among species in different assemblages around the world. We found high similarity among species in ecology, morphology and performance, and we found that this similarity can result from both evolutionary convergence and long-term evolutionary conservatism combined with dispersal (ECD). Our analyses of convergence also showed that adaptation to different ecologies can largely erase the imprint of past adaptation to an ancestral ecology. Below, we discuss in turn each of our major findings.
(a). Are species in the same microhabitats similar in both morphology and performance?
Previous studies across many organisms have shown strong relationships between ecology (e.g. microhabitat use and diet) and morphology (see reviews in [21,22]). Far fewer have considered performance [53,54], which forms the link between ecology and morphology [16]. In this study, we found that frog species using the same microhabitat had both similar morphology and performance, regardless of geographical location. Some previous studies in frogs had suggested the occurrence of morphologically similar species using similar microhabitats (i.e. ‘ecomorphs’ [28,55]). We show quantitatively that arboreal, burrowing and semi-aquatic species each show distinctive morphology and performance, with weaker results for terrestrial frogs. We also found that most of our results were similar for morphology and performance, but similarity among species in the same microhabitat was more distinctive in morphology than performance. This also occurred in both our analyses of conservatism, as microhylids and bufonids were more similar in morphology than performance (table 1). The causes of these patterns should be tested with additional studies in frogs and their generality should be tested in other groups of organisms.
(b). Role of conservatism
Dispersal has often been assumed to be unimportant relative to convergence in explaining trait similarity among species at large spatial scales [9–11], but our results show that ECD can be important even across continents. The strong conservatism in ecology, morphology and performance that we found in Microhylidae is especially remarkable, given that these species have been separated for more than 65 Myr (figure 1b). Although some similarity might still be caused by limited convergence within families coupled with dispersal, most species within this family and the other that showed strong ECD, Bufonidae, are terrestrial and have similar body forms [56,57], further suggesting that similarity is explained by common ancestry rather than convergence (see also the electronic supplementary material). Surprisingly, our study is one of only a handful to explicitly address ECD [7,12,58] and the first, to our knowledge, to test for it in ecology, morphology and performance. While selection may drive convergence in some cases (e.g. Litoria), our results suggest an alternative role for selection: similarity among species on different continents in the relationships among ecology, morphology and performance may instead be caused by strong stabilizing selection leading to long-term stasis [4].
(c). Testing the imprint of history on convergence
We developed statistical methods to address the roles of convergence and past adaptation on trait similarity. Two previous studies have considered the possible footprint of evolutionary history on converging taxa: Stayton [5] presented a method to compare sister taxa, and Revell et al. [14] presented a phylogenetic method to compare distantly related species that independently colonized the same microhabitat from different ancestral starting points. Our test examines whether taxa within a single radiation (i.e. diversification from a common ancestor) show strong convergence with distantly related taxa in the same selective environment (here, microhabitat), or if an imprint of ancestral adaptation to the environment of their common ancestor remains. The latter might be demonstrated if species in a new environment are phenotypically similar to those in the ancestral environment but have diverged in the direction expected under convergence [5]. Intriguingly, we found no imprint of history in the ecological radiation of Australian frogs (Litoria), showing that an arboreal ancestor can give rise to all the other major ecomorphs with no trace of their past adaptation on current morphology or performance. This may not be the case for other ancestral ecomorphs or in other taxa [4], but our method could be readily applied to other systems to test this.
(d). Role of incumbency
Our results provide evidence for the importance of both convergence and conservatism, but also raise the question: why do we observe ECD in some cases and ISE in others? The large literature on ecological opportunity and adaptive radiation (recently reviewed in [11,59]) suggests that a given ecotype may be more likely to evolve in situ or disperse into a region if that ecotype is not already present. Given this, the geographical context may determine which of the two processes occurs. Relatively isolated regions (e.g. islands and mountains) may have more ISE of ecological types, whereas dispersal of ecotypes from outside the region may be important in more biogeographically connected regions (e.g. adjacent continents) [12].
Interestingly, these expectations are only partially fulfilled in frogs. As expected, we found extensive ISE in Australia (as in plants and marsupial mammals; [1,60]): all non-arboreal microhabitat ecotypes evolved within Litoria after an arboreal ancestor arrived in the region. Yet two of these ecotypes (terrestrial and burrowing) were already present in Myobatrachidae, which arrived earlier (figure 1b; [61,62]), and similar ecotypes of different origins now occur sympatrically (e.g. at our site). In China, we found that while terrestrial and arboreal lineages arrived relatively recently via ECD (Bufonidae and Hylidae, respectively), other lineages also evolved within the region to use these microhabitats (both types in Rhacophoridae, and possibly terrestriality in Microhylidae; figure 1b) and all these similar lineages can occur sympatrically. Overall, we find that ecological incumbency does not prevent the dispersal, ISE or co-occurrence of similar ecotypes in the same region.
5. Conclusion
Our study reveals three main results that together shed light on the processes that create similarity in species assemblages across continents. First, we found strong relationships among ecology, morphology and performance, both across continents and across the phylogeny of frogs, showing that frogs that use the same microhabitat have evolved similar morphology and performance characteristics. Second, we found that ECD can explain the occurrence of species in different assemblages that have maintained similar relationships among ecology, morphology and performance over tens of millions of years and across continents. Third, we found that convergence can erase traces of previous adaptation in morphology and performance to an ancestral microhabitat. We expect that both conservatism and convergence may explain patterns of trait similarity and divergence between regions in many other organisms, and we have developed new statistical tools that can be applied to other systems to address these questions.
Acknowledgements
We thank the following individuals for assistance with fieldwork: J. Che, Y.-P. Zhang and D.-Q. Rao (China); A. Crawford, A. Aguilar, E. Jiménez, R. Mesa, G. Mihajlovic, M. Peñuela and G. Rene (Colombia); and M. Greenlees, I. Bleach, G. Brown, M. Franklin and R. Shine (Australia). We also thank P. Aerts, G. Brown, E. Cabrera-Guzmán, M. Collyer, D. Futuyma, Hua X., R. McDiarmid, S. Munch, G. Zug and two anonymous reviewers for helpful discussion and suggestions. We thank Jing Che for permission to use her photos of Chinese frogs for figure 1.
All work was conducted under Stony Brook University IACUC no. 2011-1876-NF.
Funding statement
Financial support was provided by the US National Science Foundation (Graduate Research Fellowship to D.S.M., EAPSI grant OISE-0914012 to D.S.M. and DDIG award DEB-1110704 to D.S.M. and J.J.W.), a Fulbright Grant to Colombia (to D.S.M.), and a grant from the Lewis and Clark Fund of the American Philosophical Society (to D.S.M.).
References
- 1.Futuyma DJ. 1998. Evolutionary biology, 3rd edn Sunderland, MA: Sinauer Associates [Google Scholar]
- 2.Cody ML, Mooney HA. 1978. Convergence versus nonconvergence in Mediterranean climate ecosystems. Annu. Rev. Ecol. Syst. 9, 265–321 (doi:10.1146/annurev.es.09.110178.001405) [Google Scholar]
- 3.Melville J, Harmon LJ, Losos JB. 2006. Intercontinental community convergence of ecology and morphology in desert lizards. Proc. R. Soc. B 273, 557–563 (doi:10.1098/rspb.2005.3328) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hansen TF. 1997. Stabilizing selection and the comparative analysis of adaptation. Evolution 51, 1341–1351 (doi:10.2307/2411186) [DOI] [PubMed] [Google Scholar]
- 5.Stayton CT. 2006. Testing hypotheses of convergence with multivariate data: morphological and functional convergence among herbivorous lizards. Evolution 60, 824–841 (doi:10.1554/04-575.1) [PubMed] [Google Scholar]
- 6.Losos JB. 1996. Phylogenetic perspectives on community ecology. Ecology 77, 1344–1354 (doi:10.2307/2265532) [Google Scholar]
- 7.Stephens PR, Wiens JJ. 2004. Convergence, divergence, and homogenization in the ecological structure of emydid turtle communities: the effects of phylogeny and dispersal. Am. Nat. 164, 244–254 (doi:10.1086/422342) [DOI] [PubMed] [Google Scholar]
- 8.Ackerly DD. 2003. Community assembly, niche conservatism, and adaptive evolution in changing environments. Int. J. Plant Sci. 164, S165–S184 (doi:10.1086/368401) [Google Scholar]
- 9.Schluter D. 1986. Tests for similarity and convergence of finch communities. Ecology 67, 1073–1085 (doi:10.2307/1939830) [Google Scholar]
- 10.Lamouroux N, Poff NL, Angermeier PL. 2002. Intercontinental convergence of stream fish community traits along geomorphic and hydraulic gradients. Ecology 83, 1792–1807 (doi:10.1890/0012-9658(2002)083[1792:ICOSFC]2.0.CO;2) [Google Scholar]
- 11.Losos JB. 2010. Adaptive radiation, ecological opportunity, and evolutionary determinism. Am. Nat. 175, 623–639 (doi:10.1086/652433) [DOI] [PubMed] [Google Scholar]
- 12.Moen DS, Smith SA, Wiens JJ. 2009. Community assembly through evolutionary diversification and dispersal in Middle American treefrogs. Evolution 63, 3228–3247 (doi:10.1111/j.1558-5646.2009.00810.x) [DOI] [PubMed] [Google Scholar]
- 13.Winemiller KO. 1991. Ecomorphological diversification in lowland freshwater fish assemblages from five biotic regions. Ecol. Monogr. 61, 343–365 (doi:10.2307/2937046) [Google Scholar]
- 14.Revell LJ, Johnson MA, Schulte JA, II, Kolbe JJ, Losos JB. 2007. A phylogenetic test for convergence in rock-dwelling lizards. Evolution 61, 2898–2912 (doi:10.1111/j.1558-5646.2007.00225.x) [DOI] [PubMed] [Google Scholar]
- 15.Arnold SJ. 1983. Morphology, performance and fitness. Am. Zool. 23, 347–361 (doi:10.1093/icb/23.2.347) [Google Scholar]
- 16.Wainwright PC. 1991. Ecological morphology: experimental functional anatomy for ecological problems. Am. Zool. 31, 680–693 [Google Scholar]
- 17.Irschick DJ, Meyers JJ, Husak JF, Le Galliard JF. 2008. How does selection operate on whole-organism functional performance capacities? A review and synthesis. Evol. Ecol. Res. 10, 177–196 [Google Scholar]
- 18.Koehl MAR. 1996. When does morphology matter? Ann. Rev. Ecol. Syst. 27, 501–542 (doi:10.1146/annurev.ecolsys.27.1.501) [Google Scholar]
- 19.Wainwright PC, Alfaro ME, Bolnick DI, Hulsey CD. 2005. Many-to-one mapping of form to function: a general principle in organismal design? Integr. Comp. Biol. 45, 256–262 (doi:10.1093/icb/45.2.256) [DOI] [PubMed] [Google Scholar]
- 20.Collar DC, Wainwright PC. 2006. Discordance between morphological and mechanical diversity in the feeding mechanism of centrarchid fishes. Evolution 60, 2575–2584 (doi:10.1111/j.0014-3820.2006.tb01891.x) [PubMed] [Google Scholar]
- 21.Wainwright PC. 2007. Functional versus morphological diversity in macroevolution. Annu. Rev. Ecol. Evol. Syst. 38, 381–401 (doi:10.1146/annurev.ecolsys.38.091206.095706) [Google Scholar]
- 22.Losos JB. 2011. Convergence, adaptation, and constraint. Evolution 65, 1827–1840 (doi:10.1111/j.1558-5646.2011.01289.x) [DOI] [PubMed] [Google Scholar]
- 23.Drummond AJ, Ho SYW, Phillips MJ, Rambaut A. 2006. Relaxed phylogenetics and dating with confidence. PLoS Biol. 4, e88 (doi:10.1371/journal.pbio.0040088) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Drummond A, Rambaut A. 2007. BEAST: Bayesian evolutionary analysis by sampling trees. BMC Evol. Biol. 7, 214–221 (doi:10.1186/1471-2148-7-214) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Glaw F, Vences M. 1994. A fieldguide to the amphibians and reptiles of Madagascar, 2nd edn Bonn, Germany: Zoologisches Forschungsinstitut und Museum Alexander Koenig [Google Scholar]
- 26.Inger RF, Stuebing RB. 1997. A field guide to the frogs of Borneo. Kota Kinabalu, Malaysia: Borneo Natural History Publishers [Google Scholar]
- 27.Duellman WE. 2005. Cusco Amazónico: the lives of amphibians and reptiles in an Amazonian rainforest. Ithaca, NY, USA: Comstock Publishing Associates, Cornell University Press [Google Scholar]
- 28.Bossuyt F, Milinkovitch MC. 2000. Convergent adaptive radiations in Madagascan and Asian ranid frogs reveal covariation between larval and adult traits. Proc. Natl Acad. Sci. USA 97, 6585–6590 (doi:10.1073/pnas.97.12.6585) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zug GR. 1978. Anuran locomotion: structure and function. 2. Jumping performance of semiaquatic, terrestrial, and arboreal frogs. Smiths. Contrib. Zool. 276, 1–31 (doi:10.5479/si.00810282.276) [Google Scholar]
- 30.Emerson SB. 1991. The ecomorphology of Bornean tree frogs (family Rhacophoridae). Zool. J. Linn. Soc. 101, 337–357 (doi:10.1111/j.1096-3642.1991.tb00656.x) [Google Scholar]
- 31.Marsh RL. 1994. Jumping ability of anuran amphibians. In Advances in veterinary science and comparative medicine, vol. 38B (ed. Jones JH.), pp. 51–111 New York, NY: Academic Press; [PubMed] [Google Scholar]
- 32.Nauwelaerts S, Ramsay J, Aerts P. 2007. Morphological correlates of aquatic and terrestrial locomotion in a semi-aquatic frog, Rana esculenta: no evidence for a design conflict. J. Anat. 210, 304–317 (doi:10.1111/j.1469-7580.2007.00691.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yang DT, Rao DQ. 2008. Amphibia and Reptilia of Yunnan. Kunming, China: Yunnan Publishing Group Corporation - Yunnan Science and Technology Press [Google Scholar]
- 34.Lynch JD. 2005. Discovery of the richest frog fauna in the world: an exploration of the forests to the north of Leticia. Rev. Acad. Colomb. Cienc. 29, 581–588 [Google Scholar]
- 35.Tyler MJ, Knight F. 2009. Field guide to the frogs of Australia. Collingwood, Australia: CSIRO Publications [Google Scholar]
- 36.Gans C, Parsons TS. 1966. On the origin of the jumping mechanism in frogs. Evolution 20, 92–99 (doi:10.2307/2406151) [DOI] [PubMed] [Google Scholar]
- 37.Heinen JT, Hammond G. 1997. Antipredator behaviors of newly metamorphosed green frogs (Rana clamitans) and leopard frogs (R. pipiens) in encounters with eastern garter snakes (Thamnophis s. sirtalis). Am. Midl. Nat. 137, 136–144 (doi:10.2307/2426762) [Google Scholar]
- 38.Walker JA. 1998. Estimating velocities and accelerations of animal locomotion: a simulation experiment comparing numerical differentiation algorithms. J. Exp. Biol. 201, 981–995 [Google Scholar]
- 39.Toro E, Herrel A, Irschick DJ. 2003. A biomechanical analysis of intra- and interspecific scaling of jumping and morphology in Caribbean Anolis lizards. J. Exp. Biol. 206, 2641–2652 (doi:10.1242/jeb.00473) [DOI] [PubMed] [Google Scholar]
- 40.Rasband WS. 1997. ImageJ. Bethesda, MD: U.S. National Institutes of Health; (http://imagej.nih.gov/ij/) [Google Scholar]
- 41.Kuo C-Y, Gillis GB, Irschick DJ. 2011. Loading effects on jump performance in green anole lizards, Anolis carolinensis. J. Exp. Biol. 214, 2073–2079 (doi:10.1242/jeb.053355) [DOI] [PubMed] [Google Scholar]
- 42.Pyron RA, Wiens JJ. 2011. A large-scale phylogeny of Amphibia including over 2800 species, and a revised classification of extant frogs, salamanders, and caecilians. Mol. Phylogenet. Evol. 61, 543–583 (doi:10.1016/j.ympev.2011.06.012) [DOI] [PubMed] [Google Scholar]
- 43.Revell LJ. 2009. Size-correction and principal components for interspecific comparative studies. Evolution 63, 3258–3268 (doi:10.1111/j.1558-5646.2009.00804.x) [DOI] [PubMed] [Google Scholar]
- 44.R Development Core Team 2012. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; (http://www.R-project.org/) [Google Scholar]
- 45.Revell LJ. 2012. phytools: an R package for phylogenetic comparative biology (and other things). Methods Ecol. Evol. 3, 217–223 (doi:10.1111/j.2041-210X.2011.00169.x) [Google Scholar]
- 46.Blankers T, Adams DC, Wiens JJ. 2012. Ecological radiation with limited morphological diversification in salamanders. J. Evol. Biol. 25, 634–646 (doi:10.1111/j.1420-9101.2012.02458.x) [DOI] [PubMed] [Google Scholar]
- 47.Martins EP, Hansen TF. 1997. Phylogenies and the comparative method: a general approach to incorporating phylogenetic information into the analysis of interspecific data. Am. Nat. 149, 646–667 (doi:10.1086/286013) [Google Scholar]
- 48.Young JE, Christian KA, Donnellan S, Tracy CR, Parry D. 2005. Comparative analysis of cutaneous evaporative water loss in frogs demonstrates correlation with ecological habits. Physiol. Biochem. Zool. 78, 847–856 (doi:10.1086/432152) [DOI] [PubMed] [Google Scholar]
- 49.Collyer ML, Adams DC. 2007. Analysis of two-state multivariate phenotypic change in ecological studies. Ecology 88, 683–692 (doi:10.1890/06-0727) [DOI] [PubMed] [Google Scholar]
- 50.Felsenstein J. 1988. Phylogenies and quantitative characters. Annu. Rev. Ecol. Syst. 19, 445–471 (doi:10.1146/annurev.es.19.110188.002305) [Google Scholar]
- 51.Butler MA, King AA. 2004. Phylogenetic comparative analysis: a modeling approach for adaptive evolution. Am. Nat. 164, 683–695 (doi:10.1086/426002) [DOI] [PubMed] [Google Scholar]
- 52.Emerson SB. 1976. Burrowing in frogs. J. Morphol. 149, 437–458 (doi:10.1002/jmor.1051490402) [DOI] [PubMed] [Google Scholar]
- 53.Losos JB. 1990. Ecomorphology, performance capability, and scaling of West Indian Anolis lizards: an evolutionary analysis. Ecol. Monogr. 60, 369–388 (doi:10.2307/1943062) [Google Scholar]
- 54.Herrel A, Vanhooydonck B, Van Damme R. 2004. Omnivory in lacertid lizards: adaptive evolution or constraint? J. Evol. Biol. 17, 974–984 (doi:10.1111/j.1420-9101.2004.00758.x) [DOI] [PubMed] [Google Scholar]
- 55.Drewes R. 2009. Habitats and morphotypes. In Ecological and environmental physiology of amphibians (eds Hillman S, Withers P, Drewes R, Hillyard S.), pp. 23–58 Oxford, UK: Oxford University Press [Google Scholar]
- 56.Duellman WE, Trueb L. 1986. Biology of amphibians. Baltimore, MD: Johns Hopkins University Press [Google Scholar]
- 57.Van Bocxlaer I, Loader SP, Roelants K, Biju SD, Menegon M, Bossuyt F. 2010. Gradual adaptation toward a range-expansion phenotype initiated the global radiation of toads. Science 327, 679–682 (doi:10.1126/science.1181707) [DOI] [PubMed] [Google Scholar]
- 58.Ackerly DD. 2004. Adaptation, niche conservatism, and convergence: comparative studies of leaf evolution in the California chaparral. Am. Nat. 163, 654–671 (doi:10.1086/383062) [DOI] [PubMed] [Google Scholar]
- 59.Yoder JB, et al. 2010. Ecological opportunity and the origin of adaptive radiations. J. Evol. Biol. 23, 1581–1596 (doi:10.1111/j.1420-9101.2010.02029.x) [DOI] [PubMed] [Google Scholar]
- 60.Lomolino MV, Riddle BR, Whittaker RJ, Brown JH. 2010. Biogeography, 4th edn Sunderland, MA: Sinauer Associates [Google Scholar]
- 61.Roelants K, Gower DJ, Wilkinson M, Loader SP, Biju SD, Guillaume K, Moriau L, Bossuyt F. 2007. Global patterns of diversification in the history of modern amphibians. Proc. Natl Acad. Sci. USA 104, 887–892 (doi:10.1073/pnas.0608378104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wiens JJ. 2007. Global patterns of species richness and diversification in amphibians. Am. Nat. 170, S86–S106 (doi:10.1086/519396) [DOI] [PubMed] [Google Scholar]